Abstract
Objective:
To assess the extent of pelvic hemorrhage on CT and to estimate its significance on outcome in patients with blunt high-energy pelvic trauma.
Methods:
576 patients with blunt high-energy pelvic fractures in 2005–2011 were identified in the hospital’s Trauma Registry (a major Trauma Center). 60 of these met our further inclusion criteria of initial systolic blood pressure ≤100 mmHg and pelvic-related hemorrhage. CT scans of the patients were reviewed with regard to pelvic and abdominal retroperitoneal hemorrhage, type of fracture (Tile classification) and associated injuries. Extent of hemorrhage was correlated to 30-day mortality, transfusion requirements and need of angiography. Statistical methods include Pearson’s Χ2 test and Fisher’s exact test.
Results:
Pelvic hemorrhage extended beyond the pelvis to the abdominal area in 47% of the patients, to the thighs in 25% and to the gluteal areas in 81%. The extent of hemorrhage was significantly associated with the need for blood transfusions (p = 0.011) and angiography (p < 0.001), but not with 30-day mortality.
Conclusion:
Traumatic pelvic bleeding frequently extends beyond the true pelvis. Extrapelvic hemorrhage correlates with an increased need of transfusions, but not with 30-day mortality. Further studies are needed to assess whether present techniques to control pelvic bleeding need to be modified in order to further reduce mortality in traumatic pelvic hemorrhage.
Advances in knowledge:
The study shows localization of pelvic hemorrhage in trauma patients. It may help to select patients in need of further interventions to control bleeding.
Introduction
Mortality rates in hemodynamically unstable patients after severe pelvic fractures remains high in spite of damage control interventions.1 The majority of patients with pelvic hemorrhage are hemodynamically stabilized by fluid resuscitation alone, while a minority of patients need pelvic packing or external compression by a pelvic binder or external fixation to achieve bleeding control.2
The effect of external compression on pelvic hemorrhage is based on the assumption of a closed retroperitoneal pelvic space, whereby reduction of pelvic volume also reduces pelvic bleeding. The abdominal and pelvic extraperitoneal spaces were until, recently, considered to represent different confined cavities.3 However, anatomical studies have identified a communication between these spaces.4, 5 Consequently, pelvic hemorrhage may pass from the retroperitoneal pelvic areas to the significantly larger abdominal retroperitoneal spaces. Since current procedures to control pelvic hemorrhage focus on achieving bleeding control in the true pelvis alone, the occurrence of hemorrhage extending beyond the pelvis may indicate a need to reconsider current practices.
The aim of the study was to assess the frequency and locations of pelvic hemorrhage by CT in hemodynamically unstable patients with pelvic fracture after blunt high-energy trauma. A secondary aim was to assess whether the extent of pelvic hemorrhage was associated with transfusion requirement, the need for angiography or 30-day mortality.
methods and Materials
In this retrospective cohort study, patient data was obtained from the Trauma Registry and the Prospective Blood Transfusion Database at a major Trauma Center in Stockholm, Sweden during the time period 2005–2011. Patients arriving with a pelvic ring fracture at the trauma unit after blunt trauma requiring trauma team activation were extracted from the Trauma Registry. This included isolated sacral fractures and acetabular fractures. From a total of 567 patients, those meeting the following inclusion criteria were selected: patients aged 18–75 years; pelvic fracture due to blunt high-energy trauma; hemodynamic instability defined as systolic blood pressure (SBP) ≤100 mmHg on arrival; trauma-CT performed within 24 h of arrival. Patients with open pelvic fractures and those with penetrating trauma were excluded. A total of 62 patients met the criteria, 60 of whom had pelvic-related bleeding. These patients formed the study population.
Data retrieved from the trauma and transfusion registries included: demographics, SBP on arrival, associated injuries, injury severity score (ISS),6 angiography, probability of survival,7 revised trauma score,8 transfusions within 24 h of injury, death within 30 days. Fractures were classified according to the AO-OTA (AO-Orthopaedic Trauma Association).9
A multidetector, 64-channel CT scanner (General Electric, WI) was used. CT parameters were 120 kV, 200 mA, 0.3 s per rotation and pitch 1.3. The dose–length product was in the range of 700–900 mGy cm. Images were reconstructed with a standard algorithm at 5 mm and a soft algorithm at 0.625 mm slice thickness and increment. These enabled high definition three-dimensional reconstruction and thin slice multiplanar analysis. For intravenous contrast enhancement, 90 ml of Ioversol (Optiray® 370 mg ml−1) or Iopromid (Ultravist® 370 mg ml−1) was injected at a rate of 3 ml s−1 followed by a 40 ml saline chaser. CT examination was initiated 65 s after injection start. CT in the arterial contrast enhancement phase was included at the discretion of the attending trauma surgeon or radiologist.
The CT images were analyzed by two independent viewers. The following radiological data were registered: pelvic fracture location (anterior/posterior/both, left/right/both, uni-/bilateral); fracture classification;10 CT contrast enhancement phase; location of hemorrhage and contrast extravasation into the pelvis, the paravertebral area, the flanks, the thighs and the gluteal areas; associated injuries. Hemorrhage in the thigh in connection with a proximal femur fracture was excluded. A hemorrhage contiguous with pelvic hemorrhage was considered to be of pelvic origin and included in the study. A hemorrhage extending from another organ, e.g. the kidney or spine, and without continuation with the pelvic hemorrhages was not considered.
Each pelvic CT-scan divided the pelvis in three main transversal levels. Zone A extended from the ischial spine to the ischial tuberosity (Figure 1). Zone B extended from the caudal end of the sacroiliac joint to the ischial spine. Zone C extended from the iliac crest to the caudal end of the sacroiliac joint. In the axial images, each pelvic area was further divided into four compartments, where the pelvic bones defined an inner circle, while the body surface defined an outer circle (Figure 2). The compartments were further divided by a horizontal line through the center of the image into an anterior and posterior area. The outer posterior compartments (zones 7 and 8) represented the gluteal areas.
Figure 1.
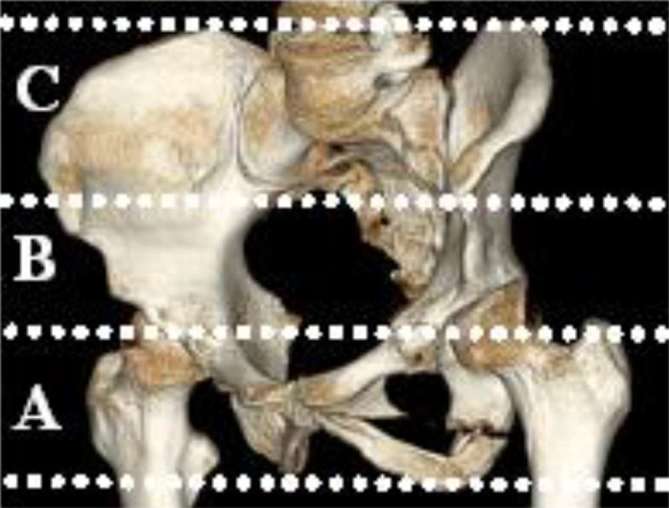
CT image of the three pelvic zones A–C.
Figure 2.
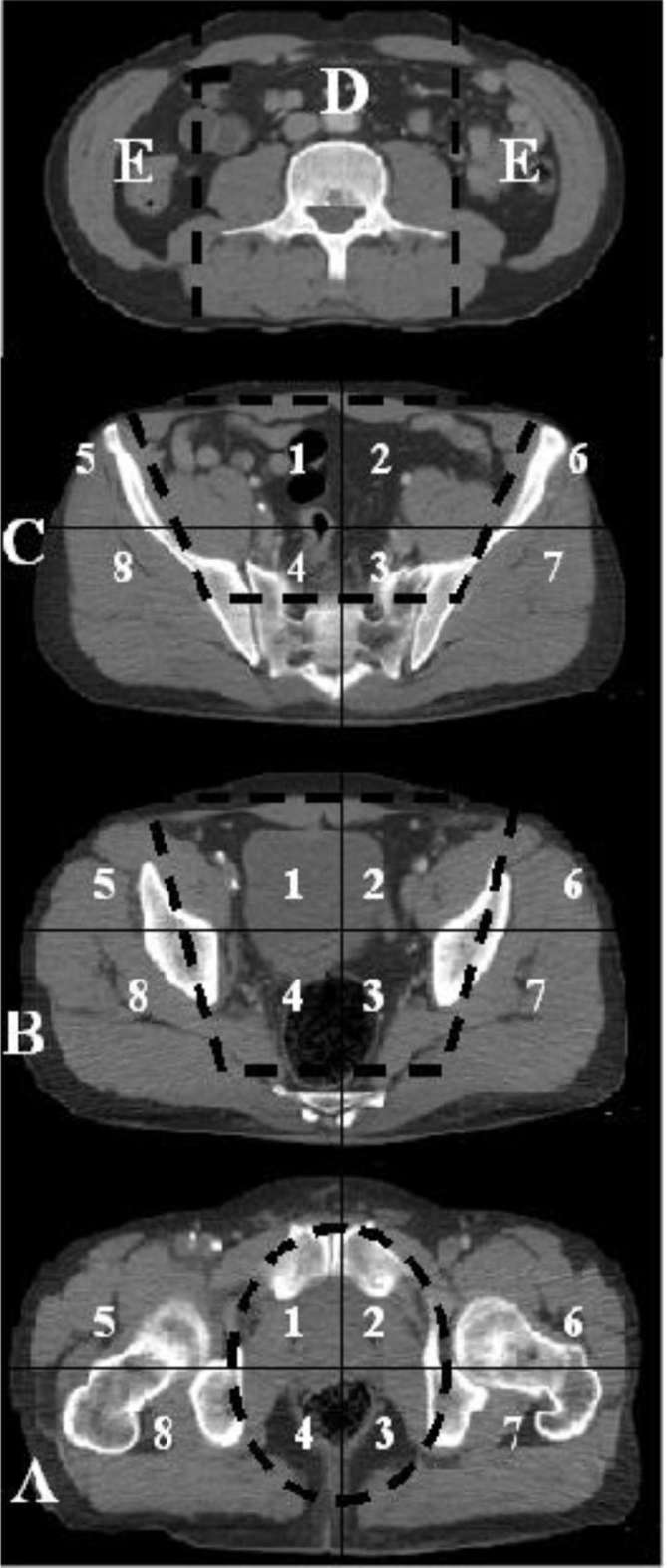
CT images of the abdominal extraperitoneal zones D, E (above) and the three pelvic zones A–C. Each pelvic zone is divided in eight regions resulting in a total of 27 CT-regions. Thigh regions (two regions) not shown.
The abdominal space was divided into three areas including a paravertebral area (Zone D) and the flanks (Zone E) (Figure 2). In the craniocaudal view, the paravertebral zone extended from the diaphragm to the sacral promontory. Laterally, the paravertebral zone was limited by the lateral surface of the psoas muscles. The flanks were defined as the areas extending from the diaphragm to the iliac crest in the craniocaudal view. Laterally, the flanks extended from the lateral surface of the psoas muscles to the body surface.
Hemorrhage into the thighs was registered in the area extending from the ischial tuberosity to the distal end of the CT image.
A total sum of 29 areas was, thus, analyzed on CT (24 areas in the pelvic region; 1 paravertebral; 2 flanks; 2 thighs).
Statistical methods
Results were extracted using IBM® SPSS® Statistics v. 20 and presented as number (n) and percentage (%) for qualitative variables and as means ± SD or median (min–max) for quantitative variables. p < 0.05 was considered significant. Quantitative variables were analyzed using the t-test, or a non-parametric test, depending on the distribution of the variables. For analysis of qualitative variables, we used either Pearson’s Χ2 or Fisher’s exact test. Correlation between two quantitative variables was performed using Spearman’s coefficient.
Results
Among the 60 patients included in the study, 35 (58%) were males, 25 (42%) females. Mean age was 43 ± 16 years. ISS was 41 ± 18. SBP on hospital arrival was non-measurable in 18 (30%) patients. Patient characteristics are presented in detail in Table 1.
Table 1.
Patient characteristics in the total study population, both survivors and non-survivors
| Total n = 60 | Survivors n = 46 | Non-survivors n = 14 | pa | |
| Gender (male) | 35 (58%) | 27 (59%) | 8 (57%) | 0.918 |
| Age (years) | 43 ± 16 | 42 ± 16 | 47 ± 17 | 0.479 |
| SBP, measurable at hospital arrival | 42 (70%) | 36 (78%) | 6 (43%) | 0.011 |
| Injury mechanism | ||||
| Fall from height | 29 (48%) | 24 (52%) | 5 (36%) | 0.281 |
| MVC | 15 (25%) | 11 (24%) | 4 (29%) | 0.724 |
| Pedestrian | 11 (18%) | 7 (15%) | 4 (29%) | 0.258 |
| Cyclist | 3 (5%) | 2 (4%) | 1 (7%) | 0.674 |
| Other | 2 (3%) | 2 (4%) | 0 (0%) | 1.000 |
| ISS (0–75) | 41 ± 18 | 37 ± 16 | 53 ± 19 | 0.006 |
| RTS (0–8) | 5.1 ± 2.0 | 5.6 ± 1.9 | 3.4 ± 1.7 | 0.001 |
| TRISS (0–100%) | 45.9 (0.4–98.9) | 65.9 (2.2–98.9) | 5.9 (0.4–57.6) | <0.001 |
| Time to CT (minutes) | 50 (14–327) | 50 (14–327) | 50 (30–101) | 0.965 |
| Pelvic fracture pattern | ||||
| Type A | 8 (13%) | 7 (15%) | 1 (7%) | 0.667 |
| Type B | 20 (33%) | 17 (37%) | 3 (21%) | 0.347 |
| Type C | 32 (53%) | 22 (48%) | 10 (71%) | 0.140 |
| Acetabular fracture | 13 (22%) | 11 (24%) | 2 (14%) | 0.444 |
| Associated injuries | ||||
| Head | 29 (48%) | 19 (41%) | 10 (71%) | 0.056 |
| Spine | 50 (83%) | 38 (83%) | 12 (86%) | 1.000 |
| Chest | 50 (83%) | 36 (78%) | 14 (100%) | 0.098 |
| Liver | 17 (28%) | 14 (30%) | 3 (21%) | 0.737 |
| Spleen | 12 (20%) | 10 (22%) | 2 (14%) | 0.713 |
| Kidney | 17 (28%) | 12 (26%) | 5 (36%) | 0.511 |
| Other | 39 (65%) | 29 (63%) | 10 (71%) | 0.565 |
| Angiography | 16 (27%) | 13 (28%) | 3 (21%) | 0.740 |
| 30-day mortality | 14 (23%) | |||
| Blood transfusion within 24 h (units) | ||||
| RBC | 10 (0–96) | 7 (0–96) | 22 (0–40) | 0.011 |
| Plasma | 10 (0–110) | 8 (0–110) | 19 (0–44) | 0.076 |
| Platelets | 0 (0–20) | 0 (0–20) | 2 (0–6) | 0.036 |
ISS, injury severity score; MVC, motor vehicle collision; RBC, red blood cells; RTS, revised trauma score; SBP, systolic blood pressure; TRISS, trauma score-injury severity score (probability of survival).
Data are presented as n (%), means ± SD or median (min–max).
Statistical significant results are marked with bold text.
Comparison between survivors and non-survivors.
The dominating injury mechanism was fall from height, observed in 29 (48%) patients. 15 patients (25%) were injured in motor vehicle collision (MVC). 11 (18%) were pedestrians and 3 (5%) were cyclists hit by a motor vehicle. The remaining two (3%) patients were subjected to crush injuries.
Associated injuries were present in 59 (98%) patients. Injuries to the chest and spine were the most frequent associated injuries, occurring in 50 (83%) and 50 (83%) patients respectively. Kidney injury was seen in 17 (28%) patients.
Death within 30 days occurred in 14 (23%) patients. The median probability of survival (Ps), as calculated by trauma injury severity score, was 45.9% (0.4–98.9%). In the non-survivors, a significantly higher ISS and lower probability of survival was observed, compared to the survivors (p = 0.006 and <0.001 respectively).
All included patients had pelvic fracture-related hemorrhage as seen on CT. The extent of pelvic hemorrhage on CT as measured by areas involved was significantly larger in patients with Tile Type C fractures, compared to those with Tile Type A or B fractures (p < 0.001 and p = 0.003 respectively) (Table 2). A higher number of affected areas of hemorrhage was observed in patients who underwent angiography (p < 0.001).
Table 2. .
Extent of pelvic hemorrhage and gender, survival, measurable SBP, pelvic fracture pattern and angiography
| Compared groups | p | |||
| Extent of pelvic hemorrhage(number of areas with pelvic hemorrhage of the total 29 areas) | Male | Female | ||
| 13 ± 7 | 14 ± 6 | 0.482 | ||
| Survivors | Non-survivors | |||
| 13 ± 6 | 14 ± 7 | 0.772 | ||
| Measurable SBP | No measurable SBP | |||
| 13 (2–26) | 17 (1–24) | 0.068 | ||
| Type A fracture | Type B fracture | Type C fracture | ||
| 6 (1–13) | 11 (2–23) | 17 (3–26) | <0.001a | |
| No angiography | Angiography | |||
| 12 ± 6 | 18 ± 4 | <0.001 | ||
ISS, injury severity score; MVC, motor vehicle collision; RBC, red blood cells; RTS, revised trauma score; SBP, systolic blood pressure; TRISS, trauma score-injury severity score (probability of survival).
p = 0.003 for type B fracture compared to type C fracture.
p < 0.001 for type A fracture compared to type C fracture.
Data are presented as means ± SD or median (min–max).
Statistical significant results are marked with bold text.
p = 0.248 for type A fracture compared to type B fracture
In the pelvis, hemorrhage appeared on CT mainly within compartments 1–4 (Figure 3). In only 3 of the 60 patients, bleeding was limited to the true pelvis (Zone A–B, compartments 1–4); 4 including the false pelvis (Zone A–C, compartments 1–4). In 50 patients (83%), hemorrhage extended to the gluteal areas, including isolated hemorrhages. Pelvic hemorrhage extended cranially to the abdominal areas (Zones D and/or E) in 35 of the patients (58% of all); in 25 of these patients (42% of all) to the paravertebral area (Zone D); in 33 patients (55% of all) to the flanks (Zone E). In 23 patients (38% of all), bleeding involved both the paravertebral and the flank-areas (Zones D and E). Hemorrhage was observed in the right flank in 21 patients (35% of all) and the left flank in 29 patients (48% of all). Bleeding to the thighs was observed in 15 patients (25%) (Figure 3).
Figure 3.
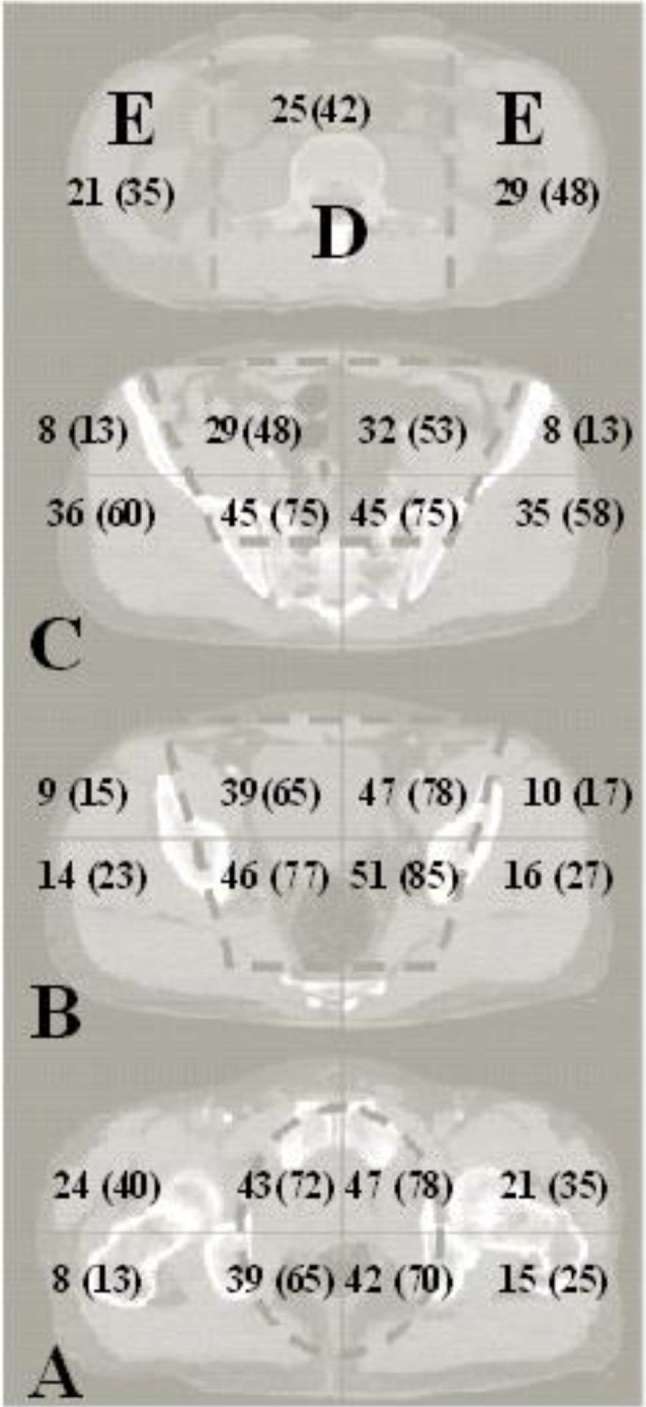
N (%) of patients with hemorrhage in each of the abdominal and pelvic CT regions.
The extent of pelvic hemorrhage was significantly larger in patients with Tile Type C-fractures, compared to those with Tile Type A- or B-fractures (p = 0.001) (Table 2). Larger extent of hemorrhage was observed in patients who underwent angiography (p < 0.001).
There was no significant association between 30-day mortality and pelvic hemorrhage extending to the thighs, the gluteal areas or any of the abdominal extraperitoneal spaces (Table 3). Also, there was no significant difference between males and females or between survivors and non-survivors in the extent of pelvic hemorrhage as documented at CT.
Table 3.
Comparison between survivors and non-survivors in the frequency, n (%), of pelvic hemorrhage in the paravertebral zone, the flanks, the thighs and the gluteal areas
| Survivors n = 46 | Non-survivors n = 14 | p | |
| Paravertebral zone | 18 (39) | 7 (50) | 0.543 |
| Right flank | 15 (33) | 6 (43) | 0.532 |
| Left flank | 21 (46) | 8 (57) | 0.547 |
| Thighs | 12 (26) | 3 (21) | 0.694 |
| Gluteal areas | 39 (85) | 11 (79) | 0.585 |
The majority of patients (n = 49) were transfused within 24 h of hospital arrival. Median transfusion requirements within 24 h from injury was 10 (0–96) units of red blood cells, 10 (0–110) units of plasma and 0 (0–20) units of platelets. Non-survivors received significantly higher volumes of red blood cells and platelets compared to survivors (p = 0.011 and p = 0.036 respectively). Correlation between extent of pelvic hemorrhage and transfusion requirement showed a fair but significant relationship for all blood components.
Time to CT examination from hospital arrival was significantly longer in patients with non-measurable SBP at hospital arrival compared to patients in whom the SBP was measurable (p = 0.010).
Discussion
The present study investigated the frequency and significance of pelvic hemorrhage extending beyond the pelvis, as diagnosed by CT, in hemodynamically unstable patients with blunt high-energy pelvic fractures. Pelvic hemorrhage extending beyond the pelvis to the abdominal paravertebral zone and either or both flanks was observed in 58% of the patients. The corresponding frequencies in the thighs and the gluteal areas were 25 and 83% respectively.
In the present study, hemorrhage and extravasation at CT was registered in specified compartments, including not only the pelvis but also the paravertebral zone, the flanks, the thighs and the gluteal areas. Blackmore et al6 investigated whether hemorrhage volumes could predict the requirements for angiography. They found a 45% probability for pelvic arterial injury in hemorrhage volumes exceeding 500 ml, compared to a 5% probability of arterial injury in patients with pelvic volumes below 200 ml.6 Measuring volumes at CT is, however, time-consuming and may not be feasible in the emergency clinical setting. The present CT evaluation was fast to perform due to the easily identified CT landmarks. The division of pelvic-related bleeding into pelvic and extrapelvic areas provided a rapid approximation of hemorrhage volume.
High-energy pelvic injuries carry a high potential for vascular lesions and death due to hemorrhage, if not diagnosed and treated timely. The diagnosis of pelvic hemorrhage is difficult and frequently an exclusion diagnosis after thoracic and abdominal hemorrhage have been ruled out.11 A means to verify pelvic hemorrhage is by the use of CT scans. Whole body CT examinations have been increasingly used in trauma patients, since the introduction of the faster multidetector CT scan.12 CT scans have previously not been recommended for the hemodynamically unstable patients, but the use of CT also in these patients has increased due to the rapidity and effectiveness of the technique.13
In this study, the extent of hemorrhage at CT was fairly but significantly correlated to the transfusion requirements. As early control of hemorrhage has been shown to decrease transfusion requirement,7 the CT-grading system described in this study may aid in the early identification of patients who are in need of more aggressive treatment options, including angiography or pelvic packing. Our observation of significantly larger extent of hemorrhage in patients who underwent angiography supports this theory.
The high frequency of pelvic hemorrhage observed in the abdominal extraperitoneum in the present study may be explained by the anatomical communication between the abdominal and pelvic extraperitoneal spaces.4, 5 This is also supported by previous cadaveric studies demonstrating a limited effect of external compression on intrapelvic pressures.8, 9 Since present methods of managing pelvic hemorrhage are based on the assumption that the pelvis is a confined cavity, the use of external fixation or pelvic binders to reduce pelvic volume in order to tamponade pelvic hemorrhage may therefore be questioned for effectiveness. However, the pelvic binder may still be beneficial in patients with open-book fractures to re-establish the pelvic ring structure and temporarily stabilize the fracture.14
In the present study, pelvic hemorrhage extending beyond the pelvis to the thighs was not observed as frequent as bleeding to the abdominal extraperitoneum. A possible explanation is that the pelvic floor caudally limits the pelvis restricting the passage of blood distally, unless the pelvic floor is disrupted.
In our study population, 30-day mortality was 23%, which corresponds to previous studies reporting 5–39% in-hospital mortality after severe pelvic trauma.1,15–17 The large variation in reported mortality rates may be a consequence of differences in injury mechanisms, fracture pattern, injury severity, or in the number of associated injuries. The most common mechanism of injury in the present study was fall from height. This differs from several other non-European publications reporting traffic accidents as the dominating injury mechanism in this patient category.1, 15,16,18,19 Whether the differences between trauma-mechanisms might explain the high rates of hematomas extending beyond the true pelvis remains to be assessed. However, fall from height typically result in unstable Tile Type C-fractures.10 In the present study, Type C-fractures were significantly associated with larger extension of pelvic hemorrhage.
The included patients had a high ISS (mean = 41), indicating the presence of several severe associated injuries. Particularly head and chest injuries have been associated with higher mortality in patients with high-energy pelvic fracture.1, 20 Our results support these figures, as we observed a significantly higher frequency of associated head injuries in non-survivors compared to survivors. Although no statistical significance was observed in the frequency of associated chest injuries between survivors and non-survivors, chest injuries were present in all of the non-survivors. Even if spinal or kidney injuries were seen in a substantial portion of the patients, bleeding related to these injuries was not in continuation with the pelvic bleeding. Therefore, they were considered as separate bleedings. The associated injuries are common and important confounders for mortality,1 and may explain the lack of significant association between extent of pelvic hemorrhage and 30-day mortality in the present study.
Retroperitoneal pelvic packing is a technique indicated in the grossly hemodynamically unstable patients with pelvic fracture who are not sufficiently stabilized by aggressive fluid resuscitation and blood transfusions.7, 19 Extraperitoneal pelvic packing is performed by placing surgical swabs bilaterally in the true pelvis in the retroperitoneal space caudal to linea terminalis.19 However, the high proportion of patients with pelvic-related paravertebral hemorrhage observed in this study indicate that packing techniques may need to be modified to include not only the pelvic retroperitoneum, but also the abdominal retroperitoneal areas so as to obtain both cranial as well as caudal bleeding control.
The strength of our study was the use of the prospective registries. A further advantage was the standardized assessment of the CT images performed by an experienced trauma radiologist to identify hemorrhage in pre-defined anatomical compartments. However, our study also had limitations. The study included only patients from a single trauma center resulting in a limited study population. Nevertheless, this trauma center is the largest trauma hospital in Sweden and serves as a referral hospital receiving trauma patients from a large area. The high number of patients with additional injuries implies that the need for transfusions was not only related to the pelvic hemorrhage. Further studies with similar study design but larger study population are, therefore, needed to corroborate our findings.
The indications for pelvic interventions to control pelvic hemorrhage vary between hospitals. Therefore, necessary interventions such as pelvic packing and angiography may be delayed due to lack of clear indications. The median time to initiation of angiography from hospital arrival is reported to be 130 min.7 Also, the performance of angiography is reported to be time-consuming.21 The indications for angiography are usually based on fracture pattern, SBP at hospital arrival, transfusion requirements and CT findings of extravasation or large hemorrhages.7, 18,22 Structured CT assessment using the zones and compartments presented in this study may provide a faster identification of patients in need of angiography and, thereby improve outcome. Although the CT examination itself is fast, the patient transport and other preparations delay the examination. Therefore, on high suspicion of arterial injury, angiography should not be preceded by CT.
Conclusions
Pelvic-related hemorrhage due to pelvic fractures frequently extends beyond the true pelvis into the abdominal extraperitoneum, the gluteals and the thigh. The extent of hemorrhage is significantly associated with blood transfusion requirements and the need for angiography. The CT classification system described in this study may aid in the rapid assessment of the severity of injury and the need for further, focused intervention.
ACKNOWLEDGMENTS
Olof Brattström, MD, for the help in obtaining patient data from the Trauma registry. Agneta Taune Wikman, MD, for providing the blood transfusion data. Elisabeth Berg for statistical support.
Contributor Information
Narin Uludag, Email: narin.uludag@outlook.com.
Anna Tötterman, Email: anna.totterman@sll.se.
Mats O Beckman, Email: mats.beckman@karolinska.se.
Anders Sundin, Email: anders.sundin@radiol.uu.se.
REFERENCES
- 1.Gabbe BJ, de Steiger R, Esser M, Bucknill A, Russ MK, Cameron PA. Predictors of mortality following severe pelvic ring fracture: results of a population-based study. Injury 2011; 42: 985–91. doi: 10.1016/j.injury.2011.06.003 [DOI] [PubMed] [Google Scholar]
- 2.Krieg JC, Mohr M, Ellis TJ, Simpson TS, Madey SM, Bottlang M. Emergent stabilization of pelvic ring injuries by controlled circumferential compression: a clinical trial. J Trauma 2005; 59: 659–64. [PubMed] [Google Scholar]
- 3.Raptopoulos V, Lei QF, Touliopoulos P, Vrachliotis TG, Marks SC. Why perirenal disease does not extend into the pelvis: the importance of closure of the cone of the renal fasciae. AJR Am J Roentgenol 1995; 164: 1179–84. doi: 10.2214/ajr.164.5.7717228 [DOI] [PubMed] [Google Scholar]
- 4.O'Connell AM, Duddy L, Lee C, Lee MJ. CT of pelvic extraperitoneal spaces: an anatomical study in cadavers. Clin Radiol 2007; 62: 432–8. doi: 10.1016/j.crad.2006.11.012 [DOI] [PubMed] [Google Scholar]
- 5.Aikawa H, Tanoue S, Okino Y, Tomonari K, Miyake H. Pelvic extension of retroperitoneal fluid: analysis in vivo. AJR Am J Roentgenol 1998; 171: 671–7. doi: 10.2214/ajr.171.3.9725294 [DOI] [PubMed] [Google Scholar]
- 6.Blackmore CC, Jurkovich GJ, Linnau KF, Cummings P, Hoffer EK, Rivara FP. Assessment of volume of hemorrhage and outcome from pelvic fracture. Arch Surg 2003; 138: 504–8. doi: 10.1001/archsurg.138.5.504 [DOI] [PubMed] [Google Scholar]
- 7.Osborn PM, Smith WR, Moore EE, Cothren CC, Morgan SJ, Williams AE, et al. . Direct retroperitoneal pelvic packing versus pelvic angiography: a comparison of two management protocols for haemodynamically unstable pelvic fractures. Injury 2009; 40: 54–60. doi: 10.1016/j.injury.2008.08.038 [DOI] [PubMed] [Google Scholar]
- 8.Grimm MR, Vrahas MS, Thomas KA. Pressure-volume characteristics of the intact and disrupted pelvic retroperitoneum. J Trauma 1998; 44: 454–9. doi: 10.1097/00005373-199803000-00006 [DOI] [PubMed] [Google Scholar]
- 9.Köhler D, Sellei RM, Sop A, Tarkin IS, Pfeifer R, Garrison RL, et al. . Effects of pelvic volume changes on retroperitoneal and intra-abdominal pressure in the injured pelvic ring: a cadaveric model. J Trauma 2011; 71: 585–90. doi: 10.1097/TA.0b013e318224cd62 [DOI] [PubMed] [Google Scholar]
- 10.Tile M. Acute pelvic fractures: I. causation and classification. J Am Acad Orthop Surg 1996; 4: 143–51. doi: 10.5435/00124635-199605000-00004 [DOI] [PubMed] [Google Scholar]
- 11. American College of Surgeons Committee on Trauma. ATLS: advanced trauma life support program for doctors. 7th ed Chicago, IL: The British Institute of Radiology.; 2004. [Google Scholar]
- 12.Leidner B, Beckman MO. Standardized whole-body computed tomography as a screening tool in blunt multitrauma patients. Emerg Radiol 2001; 8: 20–8. doi: 10.1007/PL00011863 [DOI] [Google Scholar]
- 13.Huber-Wagner S, Lefering R, Qvick LM, Körner M, Kay MV, Pfeifer KJ, et al. . Effect of whole-body CT during trauma resuscitation on survival: a retrospective, multicentre study. Lancet 2009; 373: 1455–61. doi: 10.1016/S0140-6736(09)60232-4 [DOI] [PubMed] [Google Scholar]
- 14.Bottlang M, Krieg JC, Mohr M, Simpson TS, Madey SM. Emergent management of pelvic ring fractures with use of circumferential compression. J Bone Jt Surg Am 2002; 84: 43–7. doi: 10.2106/00004623-200200002-00005 [DOI] [PubMed] [Google Scholar]
- 15.Verbeek DO, Verbeek D, Sugrue M, Balogh Z, Cass D, Civil I, Harris I, et al. . Acute management of hemodynamically unstable pelvic trauma patients: time for a change? Multicenter review of recent practice. World J Surg 2008; 32: 1874–82. doi: 10.1007/s00268-008-9591-z [DOI] [PubMed] [Google Scholar]
- 16.Balogh Z, King KL, Mackay P, McDougall D, Mackenzie S, Evans JA, et al. . The epidemiology of pelvic ring fractures: a population-based study. J Trauma 2007; 63: 1066–73. doi: 10.1097/TA.0b013e3181589fa4 [DOI] [PubMed] [Google Scholar]
- 17.Hauschild O, Strohm PC, Culemann U, Pohlemann T, Suedkamp NP, Koestler W, et al. . Mortality in patients with pelvic fractures: results from the German pelvic injury register. J Trauma 2008; 64: 449–55. doi: 10.1097/TA.0b013e31815982b1 [DOI] [PubMed] [Google Scholar]
- 18.Salim A, Teixeira PG, DuBose J, Ottochian M, Inaba K, Margulies DR, et al. . Predictors of positive angiography in pelvic fractures: a prospective study. J Am Coll Surg 2008; 207: 656–62. doi: 10.1016/j.jamcollsurg.2008.05.025 [DOI] [PubMed] [Google Scholar]
- 19.Cothren CC, Osborn PM, Moore EE, Morgan SJ, Johnson JL, Smith WR. Preperitonal pelvic packing for hemodynamically unstable pelvic fractures: a paradigm shift. J Trauma 2007; 62: 834–42. doi: 10.1097/TA.0b013e31803c7632 [DOI] [PubMed] [Google Scholar]
- 20.Holstein JH, Culemann U, Pohlemann T, Working Group Mortality in Pelvic Fracture Patients. What are predictors of mortality in patients with pelvic fractures? Clin Orthop Relat Res 2012; 470: 2090–7. doi: 10.1007/s11999-012-2276-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tötterman A, Dormagen JB, Madsen JE, Kløw NE, Skaga NO, Røise O. A protocol for angiographic embolization in exsanguinating pelvic trauma: a report on 31 patients. Acta Orthop 2006; 77: 462–8. doi: 10.1080/17453670610046406 [DOI] [PubMed] [Google Scholar]
- 22.Costantini TW, Bosarge PL, Fortlage D, Bansal V, Coimbra R. Arterial embolization for pelvic fractures after blunt trauma: are we all talk? Am J Surg 2010; 200: 752–8. doi: 10.1016/j.amjsurg.2010.06.006 [DOI] [PubMed] [Google Scholar]


