Abstract
We have previously shown that estrogen receptor β (ERβ)-mediated up-regulation of quinone reductase (QR) is involved in the protection against estrogen-induced mammary tumorigenesis. Our present study provides evidence that the ERβ agonist, 2,3-bis-(4-hydroxy-phenyl)-propionitrile (DPN), and the selective estrogen receptor modulator tamoxifen (Tam), inhibit estrogen-induced DNA damage and mammary tumorigenesis in the aromatase transgenic (Arom) mouse model. We also show that either DPN or Tam treatment increases QR levels and results in a decrease in ductal hyperplasia, proliferation, oxidative DNA damage (ODD), and an increase in apoptosis. To corroborate the role of QR, we provide additional evidence in triple transgenic MMTV/QR/Arom mice, wherein the QR expression is induced in the mammary glands via doxycycline, causing a decrease in ductal hyperplasia and ODD. Overall, we provide evidence that up-regulation of QR through induction by Tam or DPN can inhibit estrogen-induced ODD and mammary cell tumorigenesis, representing a novel mechanism of prevention against breast cancer. Thus, our data have important clinical implications in the management of breast cancer; our findings bring forth potentially new therapeutic strategies involving ERβ agonists. Krishnamurthy, N., Hu, Y., Siedlak, S., Doughman, Y. Q., Watanabe, M., Montano, M. M. Induction of quinone reductase by tamoxifen or DPN protects against mammary tumorigenesis.
Keywords: aromatase, hyperplasia, oxidative DNA damage
Breast cancer, a hormone-driven malignancy, is one of the most prevalent types of cancer observed in women. It is believed that cumulative, excessive exposure to endogenous estrogen across a woman's life span may be a causal factor in breast cancer (1, 2). Estrogens are biosynthesized from androgens by aromatase, a member of the cytochrome P-450 superfamily (3). Previous work has demonstrated that aromatase expression and activity in tumors are higher than those in normal breast tissue (4, 5). Studies in aromatase (Arom) transgenic mice models have shown that local estrogen is directly involved in the initiation of preneoplastic changes in the mammary epithelium (6). Overexpression of aromatase in the mammary glands of Arom mice leads to hyperplasia and changes in the expression of genes involved in apoptosis, cell cycle, and tumor suppression functions (6, 7).
Estrogen exerts its mitogenic effect on mammary tissue through its interaction with the estrogen receptor (ER). Antiestrogens targeting the estrogenic actions via the estrogen receptor α (ERα) or aromatase inhibitors, which block estrogen biosynthesis, are currently the most effective therapeutic strategies to treat ER-positive breast cancer (8, 9). About 2/3 of breast tumors express ERα, and the selective estrogen receptor modulator (SERM) tamoxifen (Tam) is the most widely prescribed adjuvant therapy for breast cancer (10, 11). In recent years, aromatase inhibitors have also become widely used for hormone receptor-positive patients with advanced breast cancer (9).
The role of ERα as a mediator of estrogen actions in breast cancer has been well characterized; however, the role of estrogen receptor β (ERβ) is still unclear. Recent studies have shown that ERβ expression can be correlated with Tam sensitivity and disease-free/overall survival (12, 13). Studies correlating ERβ expression with breast tumor grade predict good prognosis for ERβ-positive tumors (14, 15). In addition, several in vitro studies suggest that an increase in ERβ expression decreases cellular proliferation (16–18). All of these indicate that ERβ plays a protective role against breast cancer and as a result, drugs that activate ERβ can be an attractive breast cancer prophylactic.
In recent years, much effort has been invested in the development of selective ERβ agonists. One of these, 2,3-bis-(4-hydroxy-phenyl)-propionitrile (DPN), binds to ERβ with an affinity ∼70-fold higher than ERα (19). Since the discovery and characterization of DPN as a potent and selective ERβ agonist, it has been used in numerous studies aimed at determining ERβ's biological function. Studies in breast cancer cell lines have shown that, like Tam, DPN results in a decrease in cellular proliferation and an increase in apoptosis (20, 21).
We have previously shown that antiestrogen-liganded ERβ activates the detoxifying enzyme quinone reductase (QR) in breast epithelial cells (22). Our studies on the functional role of ERβ-mediated up-regulation of antioxidative enzymes indicated protective effects against estrogen-induced oxidative DNA damage (ODD; ref. 23). Our in vivo studies suggest that prevention of mammary gland tumorigenesis by Tam was accompanied by decreased ODD and increased QR levels (24).
In this work, we determined whether up-regulation of ERβ through the ERβ agonist DPN can induce QR gene expression and inhibit genotoxicity in the context of increased mammary estrogens. Our studies show that DPN is effective in reducing estrogen-induced DNA damage in Arom mice models. We also observed a correlative decrease in mammary hyperplasia on treatment of Arom mice with DPN or expression of the QR transgene. Thus, our data have important clinical implications in the management of breast cancer and brings forth potentially new therapeutic strategies involving ERβ agonists.
MATERIALS AND METHODS
Mouse models
MMTV-aromatase (Arom) transgenic mice were obtained from Dr. Rajeswar Tekmal (University of Texas Health Science Center, San Antonio, TX, USA). The Arom transgene was detected using the primers Arom fwd, 5′-GTAGTAGTTGCAGGCATTCCA-3′; and Arom rev, 5′-TCTCCGCTCGTCACTTATCCT-3′. As phytoestrogens regulate QR expression (25), Arom transgenic mice were kept on a phytoestrogen-free diet (AIN-76A-purified rodent diet; Harlan Teklad, Madison, WI, USA). Mice were implanted with either a placebo, DPN, or Tam as a 90-d continuous-release drug delivery pellet (Innovative Research, Sarasota, FL, USA) at 6 mo of age. At the end of 3 mo, the mice were reimplanted with a new drug pellet and sacrificed at the end of the experimental period of 6 mo.
To examine the role of QR in protection against mammary tumorigenesis, QR expression was induced in mammary epithelial cells of the Arom mice. To do so, we first generated the bitransgenic MMTV/QR mice, which resulted from the mating of the MMTV mice (carrying a transgene with the reverse tetracycline-dependent transcriptional activator under the control of MMTV) and tetO-QR mice (carrying a transgene with QR coding sequence under the control of the tetracycline-dependent minimal promoter). MMTV/QR mice were mated with Arom mice to create the triple transgenic MMTV/QR/Arom (AQM) mice, as verified by PCR screening of genomic DNA from tails. The MMTV transgene was detected using the primers MMTV fwd, 5′-ATCCGCACCCTTGATACTCCG-3′; and MMTV rev, 5′-GGCTATCAACCAACACACTGCCAC-3′. The QR transgene was detected using the primers QR fwd, 5′-GCAAGTCCATCCCAACTGACAACC-3′; and QR rev, 5′-GCAGACACTCTATGCCTGTGTGGAG-3′. QR expression was induced by supplementing drinking water of mice with doxycycline at a final concentration of 2 mg/ml starting at 4 mo of age, and the MMTV/QR/Arom mice (with or without doxycycline) were sacrificed after 10 mo.
Immunohistochemistry
Mammary glands were fixed in 10% formalin and embedded with paraffin and sectioned. Prior to immunostaining, paraffin sections were deparaffinized, rehydrated, and then boiled in 10 mM citrate buffer (pH 6.0) to retrieve antigenicity. Sections were incubated in 3% H2O2 in PBS for 10 min at room temperature to quench endogenous peroxidase, blocked with horse serum, and immunostained with anti-8-OHdG antibody (QED Bioscience, San Diego, CA, USA) at 4°C overnight. Following washes, sections were treated with secondary antibody for 1 h at room temperature. The Vectastain ABC kit (Vector Laboratories, Burlingame, CA, USA) was then used according to the manufacturer's instructions. Peroxidase activity was visualized with 3,3′-diaminobenzidine, and the sections were counterstained with hematoxylin. Negative controls were incubated with nonspecific IgG. Digital images were taken with an Axiocam camera using the ×20 objective, and quantification was done using the Axiovison software (Carl Zeiss Vision, Munchen-Hallbergmoss, Germany) as before (26).
Hematoxylin and eosin (H&E) staining
Mammary sections from the Arom or the AQM mice were stained with H&E and observed under a light microscope. Normal and hyperplastic luminal epithelia from each treatment group were counted in 5 different fields of vision in the stained mammary glands. The ratio was calculated and normalized to the placebo-treated mouse from the same litter to calculate fold change.
Terminal deoxyribonucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay
Paraffin-fixed tissue sections were stained using TUNEL assay with the ApopTag Plus peroxidase in situ apoptosis detection kit for immunoperoxidase staining (Millipore, Temecula, CA, USA), according to the manufacturer's instructions. Stained sections were examined under a Nikon Diaphot 200 microscope (Nikon, Tokyo, Japan). Images were captured with the digital camera and QCapture Pro software (QImaging, Surrey, BC, Canada). Quantification was performed by counting the apoptotic nuclei and the total number of epithelial cells in the mammary glands from each treatment group in five different fields of vision. The ratio of apoptotic cells to total number of epithelial cells from all five fields of vision was calculated and normalized to the ratio in the placebo-treated mouse from the same litter.
Western blot analysis
Total protein from mammary glands was extracted using M-PER mammalian protein extraction reagent (Thermo Fisher Scientific, Rockford, IL, USA). Aromatase was detected using an antibody from Biovision Research Products (Mountain View, CA, USA), and the antibody against cyclin D1 was obtained from Neomarkers (Fremont, CA, USA). The recombinant ERβ protein was obtained from Panvera (Invitrogen, Carlsbad, CA, USA), and the ERβ antibodies were obtained from Santa Cruz Biotechnology (ERβ-H150; Santa Cruz Biotechnology, Santa Cruz, CA, USA) or from GeneTex (ERβ-7B10.7; GeneTex, Irvine, CA, USA). Antibodies against the following were obtained from Santa Cruz Biotechnology: ERα, cyclin E, p21, p27, Bcl-2, Bax, phospho-histone 3 (pH3), and cytokeratin 18 (marker for luminal epithelial cells). The QR antibody was generated in the M.M.M. laboratory (25). The signal in each case was detected with either Super Femto reagents (Thermo Fisher Scientific) or ECL (GE Healthcare, Piscataway, NJ, USA), and signal intensities were normalized to their respective cytokeratin 18 loading controls. The chemiluminescence in each case was quantified by using AlphaImager software (Proteinsimple, Santa Clara, CA, USA).
Statistical analyses
Statistical analyses for Western blot and immunohistochemistry of mouse studies were performed by calculating fold change between placebo- and DPN- or Tam-treated Arom mice or between control and doxycycline-treated AQM mice. To limit variability, each placebo-treated or control mouse was from the same litter as the corresponding DPN- or Tam-treated Arom mouse or doxycycline-treated AQM mouse. For all comparisons, the value from the placebo/control mouse in the pair was given an arbitrary value of 1, and the change in the mammary glands from the treated mouse was expressed as a relative value. Average fold changes were represented using scatterplots, with vertical bars representing 2 × se. A paired t test was used to evaluate differences between control and treated mice. A value of P < 0.05 was accepted as an appropriate level of significance.
RESULTS
DPN or Tam treatment attenuates hyperplasia in Arom mice without affecting aromatase levels
Studies using the aromatase transgenic mice support direct involvement of estrogen in mammary tumorigenesis (7, 27). We have previously shown that Tam-liganded ERβ-mediated up-regulation of QR is involved in the protection against estrogen-induced mammary tumorigenesis (24). As a result, we determined whether up-regulation of ERβ receptor activity through the ERβ agonist DPN can induce antioxidative gene expression and inhibit genotoxicity in the context of increased mammary estrogens.
Arom transgenic mice were kept on a phytoestrogen-free diet and implanted with placebo, Tam, or DPN as a 90-d continuous-release drug delivery pellet. At the end of 3 mo, the mice were reimplanted with a new drug delivery pellet. A 50-mg Tam pellet designed to deliver over 90 d was used, and this dosage was selected on the basis of its effectiveness in previous studies (28, 29). The DPN pellet was constructed to deliver 0.25 mg, and this is based on the effectiveness of DPN at such doses in a previous in vivo study (30). The mice were sacrificed at the end of the experimental period of 6 mo, and mammary glands from the Arom mice were collected and processed for histology, immunochemistry, or Western blot analyses.
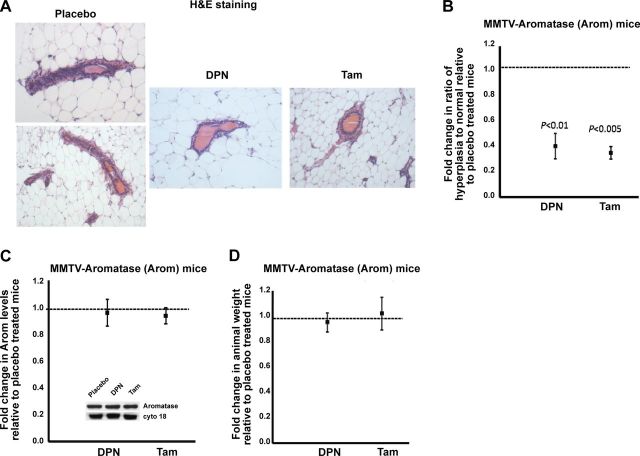
Previous work has shown that overexpression of aromatase in the mammary gland, and the ensuing increase in local mammary estrogens results in the development of premalignant lesions (6, 7). To determine whether the development of premalignant lesions can be attenuated by DPN or Tam treatment, mammary sections from the Arom mice were stained with H&E and observed under a light microscope. Normal and hyperplastic luminal epithelia from each treatment group were counted in 5 different fields of vision in the stained mammary gland sections. The ratio was calculated and normalized to the placebo-treated mouse from the same litter. The results revealed that, as expected, the mammary glands of Arom mice exhibited considerable number of ductal hyperplasia (Fig. 1A). However, the mammary glands of DPN- or Tam-treated mice showed a significantly lower number of ductal hyperplasia when compared to the placebo-treated mice (Fig. 1A, B).
Figure 1.
DPN or Tam treatment attenuates hyperplasia in Arom mice without affecting aromatase levels. A) Mammary sections from Arom mice treated with placebo, DPN, or Tam for 6 mo were stained with H&E and observed under a light microscope. B) Normal and hyperplastic luminal epithelia from each treatment were counted in 5 different fields of vision in the stained mammary glands, and the ratio was calculated and normalized to the placebo treatment. C) Western blot analyses for aromatase expression in mammary glands from placebo-, DPN-, or Tam-treated Arom mice. Blots were probed with cytokeratin 18 as a loading control. Arom expression in the mammary glands of placebo-treated mice was given an arbitrary value of 1, and Arom expression in the mammary glands of DPN- or Tam-treated mice was plotted relative to the placebo. Inset: representative Western blots of aromatase and cytokeratin 18 levels in mammary glands of Arom mice treated with placebo, DPN, or Tam. D) Effect of DPN or Tam treatment on weight of Arom mice. Weight of the placebo-treated mice was given an arbitrary value of 1, and the weight of DPN- or Tam-treated mice was plotted relative to the placebo. Error bars represent means ± 2 se from ≥4 mice/treatment.
To ensure that treatment did not affect the levels of aromatase in the transgenic mice, mammary glands collected from the Arom mice were processed for Western blot analyses of aromatase levels and normalized to cytokeratin 18. The placebo-treated mouse was assigned an arbitrary value of 1, and levels for DPN or Tam-treated mouse from the same litter were plotted relative to placebo. The results revealed that the levels of aromatase in the mammary glands of DPN or Tam-treated mice were not significantly different from the placebo-treated mice, indicating that treatment does not affect the levels of aromatase expression (Fig. 1C and inset). In addition, the Arom mice treated with placebo, DPN, or Tam were weighed before sacrificing, and the results showed that treatment with the drug did not affect the weight of the animal (Fig. 1D).
DPN or Tam treatment changes expression of genes involved in apoptosis in the mammary gland
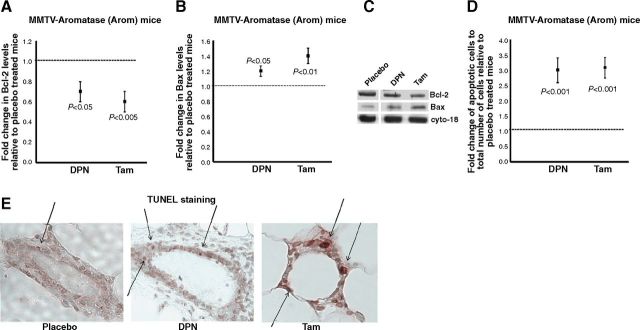
Estrogens are known to be involved in the regulation of genes involved in apoptosis (31). To study the effect of DPN or Tam treatment on apoptosis, we performed Western blot analyses for the anti- and proapoptotic proteins Bcl-2 and Bax, respectively. In addition, we stained Arom mammary glands for apoptosis using TUNEL.
Western blot analyses of mammary glands from Arom mice showed that the expression levels of the antiapoptotic protein Bcl-2 were lower in the mammary glands of DPN- or Tam-treated mice when compared to the placebo (Fig. 2A, C). However, the levels of the proapoptotic protein Bax were higher in the mammary glands of DPN or Tam mice when compared to the placebo group (Fig. 2B, C). Overall, the ratio of Bcl-2/Bax was lower in the mammary glands of DPN- or Tam-treated mice, indicating that DPN or Tam treatment results in apoptosis. This was corroborated by the TUNEL assay, which revealed that the mammary glands of DPN- or Tam-treated mice have significantly higher numbers of apoptotic nuclei when compared to the placebo (Fig. 2D, E).
Figure 2.
DPN or Tam treatment changes expression of genes involved in apoptosis in the mammary gland. Mammary glands from placebo-, DPN- or Tam-treated mice were processed for Western blot analyses or were immunostained for apoptotic nuclei by TUNEL assay. A, B) Western blot analyses of Bcl-2 (A) and Bax (B) expression from mammary glands of Arom mice treated with placebo, DPN, or Tam. Blots were probed with cytokeratin 18 as a loading control. Expression in the mammary glands of placebo-treated mice was assigned an arbitrary value of 1, and expression in the mammary glands of DPN- or Tam-treated mice was plotted relative to the placebo. C) Representative Western blots of Bcl-2, Bax, and cytokeratin 18 levels in mammary glands of Arom mice treated with placebo, DPN, or Tam. D) Quantification of TUNEL staining in mammary glands of Arom mice treated with placebo, DPN, or Tam. Apoptotic nuclei and total number of epithelial cells in the mammary glands from each treatment group were counted in 5 different fields of vision; ratio of apoptotic cells to total number of cells was calculated and normalized to the ratio in placebo-treated groups. Error bars represent means ± 2 se from ≥4 mice/treatment. E) Representative images of immunohistochemical detection of TUNEL staining in mammary glands from placebo-, DPN-, and Tam-treated mice. Arrows indicate the apoptotic nuclei.
DPN or Tam treatment decreases proliferation and affects expression levels of genes involved in cell cycle in the mammary gland
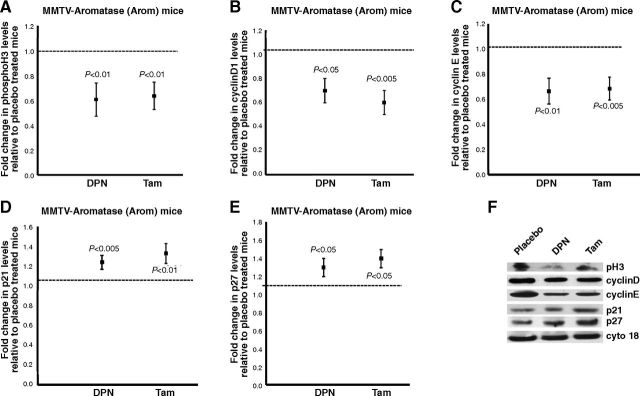
Previous studies have shown that aromatase overexpression affects the expression of genes involved in cell cycle regulation. In particular, Kirma et al. (7) observed a significant increase in cyclin D1 and cyclin E levels in the transgenic, as compared to the nontransgenic, mammary glands. To determine the effect of DPN or Tam treatment on Arom mice, we performed Western blot analyses for cellular proliferation and genes involved in cell cycle regulation.
Western blot analysis for pH3, a marker for cellular proliferation, revealed that DPN or Tam treatment resulted in reduced proliferation in mammary glands when compared to the mammary glands of placebo-treated mice (Fig. 3A, F). We also performed Western blot analyses for cyclin D1 and cyclin E, genes that are known to be involved in cell cycle regulation. The results showed that there were decreases in cyclin D1 and cyclin E levels in mammary glands of DPN- or Tam-treated mice (Fig. 3B, C, F). In addition, we also tested the mammary glands for levels of cyclin D/CDK inhibitors p21 and p27. Our results showed that the mammary glands of DPN- or Tam-treated mice have higher levels of p21 and p27 when compared to the mammary glands of placebo-treated mice (Fig. 3D–F).
Figure 3.
DPN or Tam treatment decreases proliferation and affects expression levels of genes involved in cell cycle in the mammary gland. A–E) Western blot analyses of pH3 (A), cyclin D1 (B), cyclin E (C), p21 (D), and p27 (E) expression from mammary glands of Arom mice treated with placebo, DPN, or Tam. Blots were probed with cytokeratin 18 as a loading control. Expression in the mammary glands of placebo-treated group was assigned an arbitrary value of 1, and expression in the mammary glands of DPN- or Tam-treated mice was plotted relative to placebo-treated mice. Error bars represent means ± 2 se from ≥4 mice/treatment. F) Representative Western blots of pH3, cyclin D1, cyclin E, p21, p27, and cytokeratin 18 levels in mammary glands of Arom mice treated with placebo, DPN, or Tam.
DPN or Tam treatment increases QR levels and decreases ODD in the mammary gland
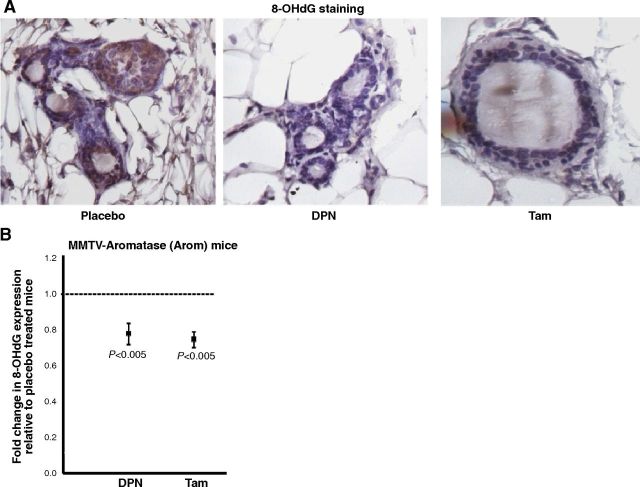
We have shown previously that an increase in ODD and a decrease in QR expression occur early in the process of estrogen-induced mammary tumorigenesis, and this effect is circumvented by Tam-liganded ERβ (24). In our present studies, the extent of ODD was studied by immunostaining the mammary glands with 8-OHdG, an oxidative marker. The results revealed that the mammary glands of Arom mice have considerable DNA damage, as seen by the brown staining in the mammary epithelium (Fig. 4A). However, the mammary glands of DPN- or Tam-treated mice showed significant reduction in ODD when compared to the placebo (Fig. 4A, B).
Figure 4.
DPN or Tam treatment decreases ODD in the mammary gland. A) Representative images of immunohistochemical detection of 8-OHdG in mammary glands of Arom mice treated with placebo, DPN, or Tam. B) Quantification of 8-OHdG staining in mammary glands for Arom mice treated with placebo, DPN, or Tam. Staining in the mammary glands of placebo-treated mice was assigned an arbitrary value of 1, and staining in the mammary glands of DPN or Tam-treated mice was plotted relative to the staining in the placebo-treated group. Error bars represent means ± 2 se from ≥4 mice/treatment.
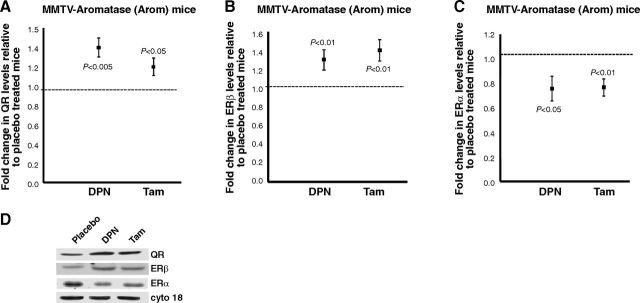
We then determined whether we could correlate the changes in DNA damage to QR expression. Western blot analyses for QR levels in the mammary glands of DPN- or Tam-treated mice showed a significant increase over the placebo, suggesting that treatment up-regulated the levels of QR, thus resulting in a decrease in ODD levels (Fig. 5A, D).
Figure 5.
DPN or Tam treatment increases QR and ERβ levels and decreases ERα levels. A–C) Western blot analyses of QR (A), ERβ (B), and ERα (C) expression in mammary glands from placebo-, DPN-, or Tam-treated Arom mice. Blots were probed with cytokeratin 18 as a loading control. Expression in the mammary glands of placebo treated mice was given an arbitrary value of 1, and expression in the DPN- or Tam-treated mice was plotted relative to the placebo. Error bars represent means ± 2 SE from ≥4 mice/treatment. D) Representative Western blots of QR, ERβ, ERα, and cytokeratin 18 levels in mammary glands of Arom mice treated with placebo, DPN, or Tam.
Work by Kirma et al. (7) has shown increased levels of ERα and ERβ in the mammary glands of Arom transgenic mice compared to the nontransgenic. In addition, work by Nair et al. (32) has shown that DPN increases ERβ levels in a letrozole-resistant xenograft tumor model. As a result, we examined the levels of ERα and ERβ in the mammary glands of Arom mice treated with placebo, DPN, or Tam. Our results showed that ERβ levels are significantly higher in the mammary glands of DPN- or Tam-treated mice when compared to the placebo group (Fig. 5B, D). However, the levels of ERα were significantly reduced in the mammary glands of DPN- or Tam-treated mice when compared to the placebo (Fig. 5C, D).
Induction of QR expression attenuates hyperplasia and ODD
Our results with the Arom mice suggest that treatment with DPN or Tam result in decreased hyperplasia, cellular proliferation, ODD, and increased levels of QR in the mammary glands. We hypothesize that treatment with the SERM Tam or the ERβ agonist DPN causes an increase in QR levels, resulting in protection against estrogen-induced damage. To confirm the role of QR in reduction of estrogen-induced mammary tumorigenesis, we generated the triple transgenic AQM mice wherein QR expression was induced using doxycycline.
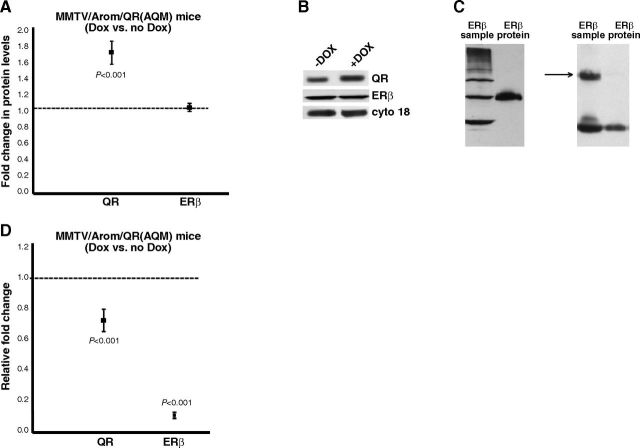
QR and ERβ expression was determined using Western blot analyses of extracts from the AQM mice mammary glands and normalized to cytokeratin 18. The levels of QR and ERβ in control mice were assigned an arbitrary value of 1, and the levels in doxycycline-treated mice from the same litter were plotted relative to control. The results revealed that doxycycline treatment resulted in an increase in QR levels in the mammary glands due to QR transgene induction (Figs. 6A, B). However, the levels of ERβ were similar for the control and the doxycycline-treated animals.
Figure 6.
Induction of QR expression attenuates hyperplasia and ODD. AQM mice were treated with doxycycline to induce QR expression for a period of 10 mo. Mammary glands were collected and processed for histology/ immunohistochemistry or Western blot analyses. A) Western blot analyses of QR and ERβ expression in mammary glands from control and doxycycline-treated AQM mice. Blots were probed with cytokeratin 18 as a loading control. Expression in control group was given an arbitrary value of 1, and expression in doxycycline-treated mice was plotted relative to the control. Error bars represent means ± 2 se from ≥5 mice/treatment. B) Representative Western blots of QR, ERβ, and cytokeratin 18 in mammary glands of control and doxycycline-treated AQM mice. C) Left panel: representative blot showing specificity of ERβ antibody from Santa Cruz Biotechnology for the recombinant ERβ protein. Right panel: representative blot using ERβ antibody from GeneTex to detect mouse mammary gland and mouse liver extract. Arrow indicates ERβ at 59 kDa. D) Mammary sections from control and doxycycline-treated AQM mice were stained with H&E and observed under a light microscope or immunostained for 8-OHdG. Quantification of the staining was performed with staining in control group given an arbitrary value of 1 and staining in doxycycline-treated mice plotted relative to the control. Error bars represent means ± 2 se from ≥4 mice/treatment.
The ERβ antibody that we used (sc-8974; Santa Cruz Biotechnology) has an epitope corresponding to aa 1-150 of the N terminus and hence can detect ERβ1, ERβ2, ERβ4, and ERβ5. However, Western blot analyses with our mammary gland samples revealed only one prominent band (∼59 kDa) between 50 and 64 kDa. The various ERβ isoforms that have been reported to be expressed at the protein level are expected to have molecular weights between 50 and 60 kDa. However, under our experimental conditions, we can only detect the 59-kDa, presumably the ERβ1, isoform. In addition, we determined the specificity of the ERβ antibody by testing it with a recombinant ERβ protein. The Western blot revealed that the antibody detects the ERβ protein as a single band at 59 kDa (Fig. 6C, left panel). As a result, we used the 59-kDa band to quantify our mammary gland samples. As a control, we also tested our samples with another ERβ antibody (from GeneTex). The Western blot revealed that the antibody detected the protein at 59 kDa (Fig. 6C, right panel, arrow). In addition, we tested this ERβ antibody with mouse liver extracts, as liver extracts have been shown to express very low levels of ERβ1 mRNA (33, 34). As expected, the antibody detected very low ERβ protein in the liver extracts (Fig. 6C, right panel).
We observed that mammary glands of Arom mice treated with DPN or Tam show decreased hyperplasia and ODD. To test our hypothesis that QR is responsible for this observed attenuation, we used the AQM mice to induce QR and performed H&E staining for hyperplasia and 8-OHdG staining for ODD. Our results showed that the levels of hyperplasia and ODD were attenuated in the mammary glands of doxycycline-treated mice (which have induced QR) when compared to the control group (Fig. 6D).
DISCUSSION
We have previously shown that ERβ-mediated up-regulation of QR is involved in the protection against estrogen-induced mammary tumorigenesis (24). Our present study provides evidence that the ERβ agonist DPN or the SERM Tam inhibit estrogen-induced DNA damage and mammary tumorigenesis in the Arom transgenic mouse model. We show that DPN or Tam treatment causes decreases in ductal hyperplasia, proliferation, ODD, and an increase in apoptosis and QR levels in the mammary glands. To corroborate the role of QR, we provide additional evidence in triple transgenic AQM mice wherein the QR expression is induced via doxycycline, causing a decrease in ductal hyperplasia and ODD in the mammary glands.
Studies using the Arom mice support the direct involvement of estrogen in mammary tumorigenesis. Overexpression of aromatase in the mammary glands leads to hyperplasia and changes in the expression of genes involved in apoptosis, cell cycle, and tumor suppression functions (6, 7). However, previous work has shown that aromatase overexpression-induced changes in mammary glands can be abrogated with very low concentrations of letrozole, an aromatase inhibitor, with no effect on normal physiology (35, 36).
Drugs targeting estrogen/ERα interaction and aromatase inhibitors that block estrogen biosynthesis have been a mainstay of hormone-based therapy for ER-positive breast cancer. However, drugs that activate ERβ are attractive breast cancer prophylactics, as ERβ is believed to play a protective role against breast cancer and has been correlated to Tam sensitivity and disease-free/overall survival (12, 13). We have previously shown through in vitro and in vivo studies that Tam-liganded ERβ-mediated up-regulation of QR resulted in protective effects against estrogen-induced ODD (23, 24). Studies in breast cancer cell lines have shown that like Tam, treatment with the ERβ agonist DPN results in decreases in cellular proliferation and increase in apoptosis (20, 21).
In this study, we provide evidence that up-regulation of QR through the ERβ agonist DPN and the SERM Tam results in reduction of estrogen-induced ODD and mammary tumorigenesis in the Arom mice. Our results show that while treatment does not affect the aromatase levels, they result in a decrease in ductal hyperplasia in the mammary gland. Although we have previously shown the up-regulation of QR by Tam, this is the first time an ERβ agonist has been shown to up-regulate an antioxidative enzyme. As QR up-regulation results in decrease of ODD, we stained the Arom mammary glands for an oxidative marker, 8-OHdG. We observe the expected decrease in 8-OHdG levels in the mammary glands of DPN- or Tam-treated mice. This suggests that the observed decrease in estrogen-induced ODD in the mammary glands of DPN- or Tam-treated mice is due to the up-regulation of QR levels.
To validate the role of QR in the attenuation of estrogen-induced DNA damage, we generated the AQM triple transgenic mice. Induction of QR transgene expression via doxycycline results in decrease of ODD, as well as in ductal hyperplasia. The levels of ERβ in the mammary glands remain unaffected, suggesting that the observed decrease in oxidative damage is due to the up-regulation of QR levels.
Our studies in the Arom mice reveal that treatment with DPN or Tam results in decrease in the levels of pH3, a proliferation marker in the mammary gland. We also observe a concomitant decrease in expression of the cell cycle genes, cyclin D1 and cyclin E, along with an increase in p21 and p27, factors that block G1/S cell cycle progression. Overall, our results suggest that the decrease in proliferative activity observed in the mammary glands of Arom mice treated with either DPN or Tam is mediated by changes in the expression of genes involved in the cell cycle.
Studies in breast cancer cell lines containing ERβ have shown that DPN or Tam treatment results in a decrease in proliferation due to the induction of proapoptotic genes (20, 21). As a result, DPN- or Tam-mediated apoptosis was studied in the mammary glands of the Arom mice by analyzing the levels of expression of Bcl-2 and Bax. Levels of Bcl-2 are lower in the mammary glands of DPN- or Tam-treated mice as compared to the placebo. However, the levels of Bax protein are higher in the mammary glands of treated mice when compared to the placebo control. Overall, we observe that the ratio of Bcl-2/Bax favors apoptosis in the mammary glands of DPN- or Tam-treated mice. This is corroborated by our results with the TUNEL assay that reveal that treatment with DPN or Tam result in an increase in apoptotic nuclei in the mammary glands.
We have previously shown that ERβ-mediated up-regulation of QR is associated with a decrease in estrogen-induced DNA damage (24). However, QR has been shown to have a more direct role in inducing apoptosis (37, 38). In addition, mice lacking QR expression not only had a decreased amount of apoptotic cells but also developed hyperplasia of the bone marrow (39, 40). It is known that ERβ and Tam associate to cause a decrease in cellular proliferation through induction of proapoptotic genes (20, 21). As a result, it is possible to speculate that this induction of apoptosis and the eventual decrease in proliferation are due to ERβ-mediated up-regulation of QR.
Overall, in this work, we provide evidence that the up-regulation of QR, through induction by Tam or DPN can inhibit estrogen-induced ODD and mammary cell tumorigenesis, representing a novel mechanism of prevention against breast cancer. Tamoxifen remains an important therapeutic agent in the treatment of women with endocrine sensitive breast cancer as it is known to effectively inhibit the proliferation-inducing effects of estrogen in ER-positive breast tumor cells. However, Tam causes hot flashes and venous thrombosis besides having stimulatory activity in the uterus (41–44). On the other hand, aromatase inhibitors have lower rates of vaginal bleeding and endometrial cancer compared to Tam but higher rates of joint and muscle pain. In addition, they often cause menopausal symptoms, such as night sweats and hot flashes (45, 46). As a result, it is essential to design new strategies to prevent or treat breast cancer. The ERβ agonist DPN is a particularly attractive reagent because ERβ inhibits the growth of breast cancer cells. While studies in breast cancer cell lines have shown that DPN decreases cellular proliferation and increases apoptosis, animal studies have shown that DPN reduces hot flashes and decreases anxiety-like and depression-like behavior in rats (47, 48). In this work, we have shown that DPN is as effective as Tam in reducing DNA damage in a model of estrogen-induced mammary tumorigenesis. Thus, our data have important clinical implications in the management of breast cancer and brings forth potentially new therapeutic strategies involving ERβ agonists.
Acknowledgments
This work was supported by a U.S. Department of Defense Breast Cancer Postdoctoral award (BC087610) to N.K.
The authors thank Dr. Rajeswar Tekmal (University of Texas Health Science Center, San Antonio, TX, USA) for providing the transgenic Arom mice.
Footnotes
- AQM
- MMTV/QR/Arom
- Arom
- MMTV-aromatase
- DPN
- 2,3-bis-(4-hydroxy-phenyl)-propionitrile
- ER
- estrogen receptor
- ERα
- estrogen receptor α
- ERβ
- estrogen receptor β
- H&E
- hematoxylin and eosin
- ODD
- oxidative DNA damage
- pH3
- phospho-histone 3
- QR
- quinone reductase
- SERM
- selective estrogen receptor modulator
- Tam
- tamoxifen
- TUNEL
- terminal deoxyribonucleotidyl transferase-mediated dUTP nick-end labeling
REFERENCES
- 1.Liehr J. G. (2000) Is estradiol a genotoxic mutagenic carcinogen? Endocr. Rev. , 40–54 [DOI] [PubMed] [Google Scholar]
- 2.Yager J. D., Davidson N. E. (2006) Estrogen carcinogenesis in breast cancer. N. Engl. J. Med. , 270–282 [DOI] [PubMed] [Google Scholar]
- 3.Simpson E. R., Mahendroo M. S., Means G. D., Kilgore M. W., Hinshelwood M. M., Graham-Lorence S., Amarneh B., Ito Y., Fisher C. R., Michael M. D., Mendelson C. R., Bulun S. E. (1994) Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr. Rev. , 342–355 [DOI] [PubMed] [Google Scholar]
- 4.Agarwal V. R., Bulun S. E., Leitch M., Rohrich R., Simpson E. R. (1996) Use of alternative promoters to express the aromatase cytochrome P450 (CYP19) gene in breast adipose tissues of cancer-free and breast cancer patients. J. Clin. Endocrinol. Metab. , 3843–3849 [DOI] [PubMed] [Google Scholar]
- 5.Miller W. R., Mullen P., Sourdaine P., Watson C., Dixon J. M., Telford J. (1997) Regulation of aromatase activity within the breast. J. Steroid Biochem. Mol. Biol. , 193–202 [PubMed] [Google Scholar]
- 6.Tekmal R. R., Ramachandra N., Gubba S., Durgam V. R., Mantione J., Toda K., Shizuta Y., Dillehay D. L. (1996) Overexpression of int-5/aromatase in mammary glands of transgenic mice results in the induction of hyperplasia and nuclear abnormalities. Cancer Res. , 3180–3185 [PubMed] [Google Scholar]
- 7.Kirma N., Gill K., Mandava U., Tekmal R. R. (2001) Overexpression of aromatase leads to hyperplasia and changes in the expression of genes involved in apoptosis, cell cycle, growth, and tumor suppressor functions in the mammary glands of transgenic mice. Cancer Res. , 1910–1918 [PubMed] [Google Scholar]
- 8.Santen R. J., Brodie H., Simpson E. R., Siiteri P. K., Brodie A. (2009) History of aromatase: saga of an important biological mediator and therapeutic target. Endocr. Rev. , 343–375 [DOI] [PubMed] [Google Scholar]
- 9.Smith I. E., Dowsett M. (2003) Aromatase inhibitors in breast cancer. N. Engl. J. Med. , 2431–2442 [DOI] [PubMed] [Google Scholar]
- 10.Cuzick J., Forbes J. F., Sestak I., Cawthorn S., Hamed H., Holli K., Howell A. (2007) Long-term results of tamoxifen prophylaxis for breast cancer–96-month follow-up of the randomized IBIS-I trial. J. Natl. Cancer Inst. , 272–282 [DOI] [PubMed] [Google Scholar]
- 11.Fisher B., Costantino J. P., Wickerham D. L., Redmond C. K., Kavanah M., Cronin W. M., Vogel V., Robidoux A., Dimitrov N., Atkins J., Daly M., Wieand S., Tan-Chiu E., Ford L., Wolmark N. (1998) Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J. Natl. Cancer Inst. , 1371–1388 [DOI] [PubMed] [Google Scholar]
- 12.Harris H. A. (2007) Estrogen receptor-beta: recent lessons from in vivo studies. Mol. Endocrinol. , 1–13 [DOI] [PubMed] [Google Scholar]
- 13.Hopp T. A., Weiss H. L., Parra I. S., Cui Y., Osborne C. K., Fuqua S. A. (2004) Low levels of estrogen receptor beta protein predict resistance to tamoxifen therapy in breast cancer. Clin. Cancer Res. , 7490–7499 [DOI] [PubMed] [Google Scholar]
- 14.Palmieri C., Cheng G. J., Saji S., Zelada-Hedman M., Warri A., Weihua Z., Van Noorden S., Wahlstrom T., Coombes R. C., Warner M., Gustafsson J. A. (2002) Estrogen receptor beta in breast cancer. Endocr. Relat. Cancer , 1–13 [DOI] [PubMed] [Google Scholar]
- 15.Saji S., Hirose M., Toi M. (2005) Clinical significance of estrogen receptor beta in breast cancer. Cancer Chemother. Pharmacol. (Suppl. 1), 21–26 [DOI] [PubMed] [Google Scholar]
- 16.Lazennec G., Bresson D., Lucas A., Chauveau C., Vignon F. (2001) ER beta inhibits proliferation and invasion of breast cancer cells. Endocrinology , 4120–4130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Omoto Y., Eguchi H., Yamamoto-Yamaguchi Y., Hayashi S. (2003) Estrogen receptor (ER) beta1 and ERβcx/β2 inhibit ERα function differently in breast cancer cell line MCF7. Oncogene , 5011–5020 [DOI] [PubMed] [Google Scholar]
- 18.Paruthiyil S., Parmar H., Kerekatte V., Cunha G. R., Firestone G. L., Leitman D. C. (2004) Estrogen receptor beta inhibits human breast cancer cell proliferation and tumor formation by causing a G2 cell cycle arrest. Cancer Res. , 423–428 [DOI] [PubMed] [Google Scholar]
- 19.Meyers M. J., Sun J., Carlson K. E., Marriner G. A., Katzenellenbogen B. S., Katzenellenbogen J. A. (2001) Estrogen receptor-beta potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J. Med. Chem. , 4230–4251 [DOI] [PubMed] [Google Scholar]
- 20.Helguero L. A., Faulds M. H., Gustafsson J. A., Haldosen L. A. (2005) Estrogen receptors alfa (ERα) and beta (ERβ) differentially regulate proliferation and apoptosis of the normal murine mammary epithelial cell line HC11. Oncogene , 6605–6616 [DOI] [PubMed] [Google Scholar]
- 21.Hodges-Gallagher L., Valentine C. D., El Bader S., Kushner P. J. (2008) Estrogen receptor beta increases the efficacy of antiestrogens by effects on apoptosis and cell cycling in breast cancer cells. Breast Cancer Res. Treat. , 241–250 [DOI] [PubMed] [Google Scholar]
- 22.Montano M. M., Jaiswal A. K., Katzenellenbogen B. S. (1998) Transcriptional regulation of the human quinone reductase gene by antiestrogen-liganded estrogen receptor-α and estrogen receptor-β. J. Biol. Chem. , 25443–25449 [DOI] [PubMed] [Google Scholar]
- 23.Bianco N. R., Perry G., Smith M. A., Templeton D. J., Montano M. M. (2003) Functional implications of antiestrogen induction of quinone reductase: inhibition of estrogen-induced deoxyribonucleic acid damage. Mol. Endocrinol. , 1344–1355 [DOI] [PubMed] [Google Scholar]
- 24.Montano M. M., Chaplin L. J., Deng H., Mesia-Vela S., Gaikwad N., Zahid M., Rogan E. (2007) Protective roles of quinone reductase and tamoxifen against estrogen-induced mammary tumorigenesis. Oncogene , 3587–3590 [DOI] [PubMed] [Google Scholar]
- 25.Bianco N. R., Chaplin L. J., Montano M. M. (2005) Differential induction of quinone reductase by phytoestrogens and protection against oestrogen-induced DNA damage. Biochem. J. , 279–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sripathy S. P., Chaplin L. J., Gaikwad N. W., Rogan E. G., Montano M. M. (2008) hPMC2 is required for recruiting an ERβ coactivator complex to mediate transcriptional upregulation of NQO1 and protection against oxidative DNA damage by tamoxifen. Oncogene , 6376–6384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tekmal R. R., Liu Y. G., Nair H. B., Jones J., Perla R. P., Lubahn D. B., Korach K. S., Kirma N. (2005) Estrogen receptor alpha is required for mammary development and the induction of mammary hyperplasia and epigenetic alterations in the aromatase transgenic mice. J. Steroid Biochem. Mol. Biol. , 9–15 [DOI] [PubMed] [Google Scholar]
- 28.O'Regan R. M., Cisneros A., England G. M., MacGregor J. I., Muenzner H. D., Assikis V. J., Bilimoria M. M., Piette M., Dragan Y. P., Pitot H. C., Chatterton R., Jordan V. C. (1998) Effects of the antiestrogens tamoxifen, toremifene, and ICI 182,780 on endometrial cancer growth. J. Natl. Cancer Inst. , 1552–1558 [DOI] [PubMed] [Google Scholar]
- 29.Schafer J. M., Lee E. S., O'Regan R. M., Yao K., Jordan V. C. (2000) Rapid development of tamoxifen-stimulated mutant p53 breast tumors (T47D) in athymic mice. Clin. Cancer Res. , 4373–4380 [PubMed] [Google Scholar]
- 30.Frasor J., Barnett D. H., Danes J. M., Hess R., Parlow A. F., Katzenellenbogen B. S. (2003) Response-specific and ligand dose-dependent modulation of estrogen receptor (ER) α activity by ERβ in the uterus. Endocrinology , 3159–3166 [DOI] [PubMed] [Google Scholar]
- 31.Dong L., Wang W., Wang F., Stoner M., Reed J. C., Harigai M., Samudio I., Kladde M. P., Vyhlidal C., Safe S. (1999) Mechanisms of transcriptional activation of bcl-2 gene expression by 17β-estradiol in breast cancer cells. J. Biol. Chem. , 32099–32107 [DOI] [PubMed] [Google Scholar]
- 32.Nair H. B., Kirma N. B., Ganapathy M., Vadlamudi R. K., Tekmal R. R. (2011) Estrogen receptor-beta activation in combination with letrozole blocks the growth of breast cancer tumors resistant to letrozole therapy. Steroids , 792–796 [DOI] [PubMed] [Google Scholar]
- 33.Lu B., Leygue E., Dotzlaw H., Murphy L. J., Murphy L. C. (2000) Functional characteristics of a novel murine estrogen receptor-beta isoform, estrogen receptor-beta 2. J. Mol. Endocrinol. , 229–242 [DOI] [PubMed] [Google Scholar]
- 34.Tremblay G. B., Tremblay A., Copeland N. G., Gilbert D. J., Jenkins N. A., Labrie F., Giguere V. (1997) Cloning, chromosomal localization, and functional analysis of the murine estrogen receptor beta. Mol. Endocrinol. , 353–365 [DOI] [PubMed] [Google Scholar]
- 35.Mandava U., Kirma N., Tekmal R. R. (2001) Aromatase overexpression transgenic mice model: cell type specific expression and use of letrozole to abrogate mammary hyperplasia without affecting normal physiology. J. Steroid Biochem. Mol. Biol. , 27–34 [DOI] [PubMed] [Google Scholar]
- 36.Tekmal R. R., Kirma N., Gill K., Fowler K. (1999) Aromatase overexpression and breast hyperplasia, an in vivo model–continued overexpression of aromatase is sufficient to maintain hyperplasia without circulating estrogens, and aromatase inhibitors abrogate these preneoplastic changes in mammary glands. Endocr. Relat. Cancer , 307–314 [DOI] [PubMed] [Google Scholar]
- 37.Asher G., Lotem J., Cohen B., Sachs L., Shaul Y. (2001) Regulation of p53 stability and p53-dependent apoptosis by NADH quinone oxidoreductase 1. Proc. Natl. Acad. Sci. U. S. A. , 1188–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sollner S., Durchschlag M., Frohlich K. U., Macheroux P. (2009) The redox-sensing quinone reductase Lot6p acts as an inducer of yeast apoptosis. FEMS Yeast Res. , 885–891 [DOI] [PubMed] [Google Scholar]
- 39.Iskander K., Gaikwad A., Paquet M., Long D. J., Brayton C., Barrios R., Jaiswal A. K. (2005) Lower induction of p53 and decreased apoptosis in NQO1-null mice lead to increased sensitivity to chemical-induced skin carcinogenesis. Cancer Res. , 2054–2058 [DOI] [PubMed] [Google Scholar]
- 40.Iskander K., Jaiswal A. K. (2005) Quinone oxidoreductases in protection against myelogenous hyperplasia and benzene toxicity. Chem. Biol. Interact. , 147–157 [DOI] [PubMed] [Google Scholar]
- 41.Jaiyesimi I. A., Buzdar A. U., Decker D. A., Hortobagyi G. N. (1995) Use of tamoxifen for breast cancer: twenty-eight years later. J. Clin. Oncol. , 513–529 [DOI] [PubMed] [Google Scholar]
- 42.Shou J., Massarweh S., Osborne C. K., Wakeling A. E., Ali S., Weiss H., Schiff R. (2004) Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J. Natl. Cancer Inst. , 926–935 [DOI] [PubMed] [Google Scholar]
- 43.Cushman M., Costantino J. P., Bovill E. G., Wickerham D. L., Buckley L., Roberts J. D., Krag D. N. (2003) Effect of tamoxifen on venous thrombosis risk factors in women without cancer: the Breast Cancer Prevention Trial. Br. J. Haematol. , 109–116 [DOI] [PubMed] [Google Scholar]
- 44.Day R., Ganz P. A., Costantino J. P., Cronin W. M., Wickerham D. L., Fisher B. (1999) Health-related quality of life and tamoxifen in breast cancer prevention: a report from the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J. Clin. Oncol. , 2659–2669 [DOI] [PubMed] [Google Scholar]
- 45.Coombes R. C., Kilburn L. S., Snowdon C. F., Paridaens R., Coleman R. E., Jones S. E., Jassem J., Van de Velde C. J., Delozier T., Alvarez I., Del Mastro L., Ortmann O., Diedrich K., Coates A. S., Bajetta E., Holmberg S. B., Dodwell D., Mickiewicz E., Andersen J., Lonning P. E., Cocconi G., Forbes J., Castiglione M., Stuart N., Stewart A., Fallowfield L. J., Bertelli G., Hall E., Bogle R. G., Carpentieri M., Colajori E., Subar M., Ireland E., Bliss J. M. (2007) Survival and safety of exemestane versus tamoxifen after 2-3 years' tamoxifen treatment (Intergroup Exemestane Study): a randomised controlled trial. Lancet , 559–570 [DOI] [PubMed] [Google Scholar]
- 46.Forbes J. F., Cuzick J., Buzdar A., Howell A., Tobias J. S., Baum M. (2008) Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. , 45–53 [DOI] [PubMed] [Google Scholar]
- 47.Walf A. A., Koonce C. J., Frye C. A. (2008) Estradiol or diarylpropionitrile decrease anxiety-like behavior of wildtype, but not estrogen receptor beta knockout, mice. Behav. Neurosci. , 974–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weiser M. J., Wu T. J., Handa R. J. (2009) Estrogen receptor-β agonist diarylpropionitrile: biological activities of R- and S-enantiomers on behavior and hormonal response to stress. Endocrinology , 1817–1825 [DOI] [PMC free article] [PubMed] [Google Scholar]