Abstract
Background:
Targeted donor screening for strongyloidiasis performed at the time of organ procurement can prevent this life-threatening donor-derived infection.
Method:
The Association of Organ Procurement Organizations surveyed members to determine the number of US organ procurement organizations (OPOs) performing donor screening for Strongyloides infection and their screening practices.
Results:
All 58 OPOs responded to the survey. Only 6 (10%) currently screen donors for strongyloidiasis; most OPOs started 6–36 months before the survey and one started 6 years prior. All used risk-based criteria to determine which donors to screen, though the criteria varied among OPOs. A median of 56 donors have been screened at each OPO since initiating their screening programs, with a median of 2 infected donors (range 0–13) identified. Overall, 53 organs have been transplanted from 22 infected donors, including hearts, lungs, kidneys, and livers. Of 52 OPOs not currently screening, 20 had considered screening and one plans to start screening in the near future. Of those considering risk-based screening, most had not decided on the criteria. Uncertainty about the benefits of and guidelines for screening and misconceptions about the interpretation of test results were concerns shared by non-screening OPOs.
Conclusion:
Continued education and advocacy on the importance of targeted donor screening are needed.
Keywords: donor screening, donor-derived infection, parasitic infections, strongyloidiasis, transplant-associated infection
1 |. INTRODUCTION
Strongyloides stercoralis is an intestinal nematode of humans that is classified as a soil-transmitted helminth and infects over 100 million people worldwide.1 Strongyloides stercoralis is commonly found in many tropical and subtropical regions, especially in countries with poor sanitation, conducive to its transmission.1,2 Temperate areas may also have endemic levels of infection, including the Appalachian region of the United States.2,3
Strongyloides is unique in that the parasite is able to complete its life cycle within a single host, leading to an auto-infective cycle, which allows for lifelong infection in the host. In immunocompromised hosts, such as solid organ transplant recipients, the use of corticosteroids and immunosuppressive drugs, which are known risk factors in these recipients, contribute to the development of overwhelming infection (hyperinfection and disseminated disease), which may lead to death. Infection in these recipients may be due to the reactivation of latent infection in the recipient or transmission from the donor organ, both of which can be prevented when pre-transplant screening practices are employed.
In 2013, American Society of Transplantation (AST) provided guidelines recommending that evaluation for Strongyloides infection be strongly considered in transplant candidates and donors with epidemiologic risk factors or unexplained eosinophilia.4 From 2009 to 2013, CDC conducted investigations of 7 clusters of donor-derived strongyloidiasis involving 11 infected recipients, 2 of whom died from overwhelming infection. Donor screening was not performed prior to organ transplantation in any of these investigations.5 In contrast, 3 years before the AST screening recommendations were published, LiveOnNY (formerly New York Organ Donor Network) initiated targeted screening of potential donors for Strongyloides infection based on the recommendation of their infectious diseases workgroup. Their screening process includes the following: (i) interview of the authorized parties (eg, next-of-kin, relationship partner, etc.) of consented deceased donors to assess risks for infectious diseases, including the donor’s travel history, (ii) Strongyloides serologic testing for at-risk donors, (iii) informing transplant centers of the pending test result without delaying organ procurement, (iv) sharing test results with all recipient centers as soon as available, and (v) recommending prophylactic therapy to prevent infection in recipients receiving organs from infected donors. The LiveOnNY experience has provided evidence that targeted donor screening can prevent the morbidity and mortality associated with donor-derived Strongyloides infection.5 Using their protocol, LiveOnNY has not reported any cases of donor-derived infection in recipients of organs from infected donors.5
To determine the number of organ procurement organizations (OPOs) that had initiated donor screening programs for Strongyloides based on the recommendations outlined in the 2013 AST guidelines and to better understand their screening practices, the Association of Organ Procurement Organizations (AOPO) conducted a survey of all OPOs in the United States. This manuscript provides the results of the AOPO survey and highlights the strategies used by screening OPOs and considerations around implementation of non-screening OPOs. Understanding the practices of screening OPOs may help determine the application of the AST guidelines. In addition, identifying the concerns of non-screening OPOs may assist in targeting educational efforts for these OPOs.
2 |. METHODS
A survey to assess the screening practices of OPOs was conducted by the AOPO from June to July 2016. A 26-item questionnaire (See Supplementary Material) was developed using the SurveyMonkey® platform and disseminated electronically to the executive directors, chief operating officers, and procurement directors of all 58 US OPOs. Email reminders were sent to non-responsive OPOs until responses from all OPOs were received. The survey included a determination of current screening status followed by specific questions for OPOs currently screening donors and a distinct set of questions for OPOs that had not started screening donors for strongyloidiasis. There were 3 primary objectives of the survey: (i) to determine how many OPOs perform screening of potential donors for Strongyloides infection; (ii) to assess what specific screening criteria and practices are employed by OPOs performing donor screening; and (iii) to ascertain the screening criteria and practices that will be used by OPOs that are considering screening. A secondary objective was to determine if the screening practices of OPOs align with those outlined in the 2013 AST guidelines for screening donors for Strongyloides infection.4 Descriptive analysis was performed using Microsoft Excel 2013 (Microsoft Corporation; Seattle, WA) and SAS 9.4 (SAS Institute, Inc.; Cary, NC).
The survey was reviewed in accordance with CDC human subjects review procedures and was determined to be a non-research, program evaluation activity.
3 |. RESULTS
3.1 |. Screening strategies, rationale, and results
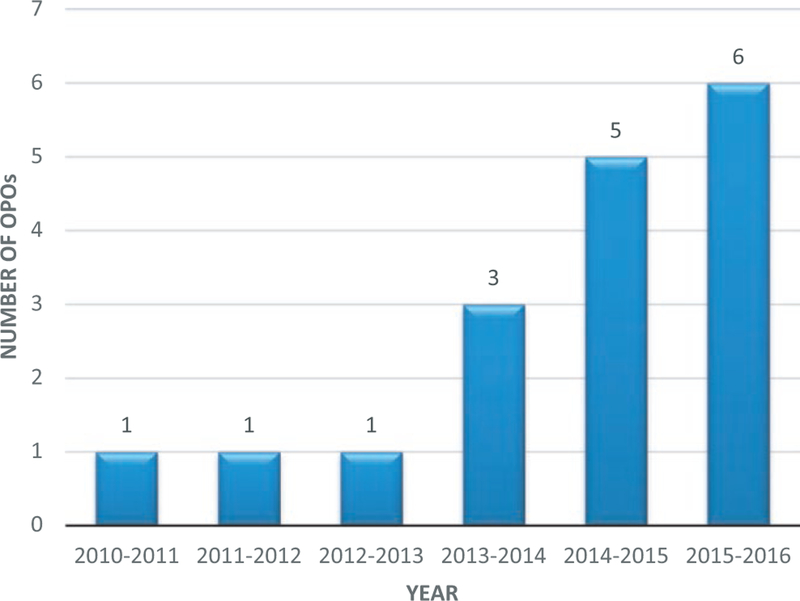
All 58 US OPOs completed the survey. Currently, 6 (10%) OPOs perform targeted donor screening for Strongyloides. LiveOnNY, which initiated screening in 2010, was the only OPO screening donors for strongyloidiasis until 2013 (Figure 1). Factors that influenced OPO decisions to initiate screening included a history of positive donor(s) from their or another OPO’s donor population and recommendation by a local working group/committee. All 6 screening OPOs used a risk-based screening strategy, citing residential or travel history to an endemic area as criteria used to determine which donors to screen. However, there was variability in the locations listed as endemic and the duration of travel to these areas that qualified donors for screening. Four of the 6 OPOs reported using a commercial laboratory for testing, including Viracor, VRL, and Quest Diagnostics; the remaining 2 OPOs used laboratories affiliated with academic hospitals.
FIGURE 1.
Number of US OPOs conducting screening of potential donors for Strongyloides infection, by year
Since initiating screening for Strongyloides, the 6 OPOs have screened 638 donors with a median of 56 donors screened per OPO (range 17–360 donors). Twenty-three (4%) donors tested positive, with a median of 2 positive donors per OPO (range 0–13 positive donors) and a total of 0%–12% positive donors per OPO. Only 2 OPOs reported occasionally sending samples for confirmatory testing; one reported sending to CDC when available, and the other only if requested by the Disease Transmission Advisory Committee (DTAC). One OPO reported always waiting for the results of screening tests before recovering organs for transplant. Overall, 53 organs were transplanted from 22 of the 23 positive donors, including hearts, livers, kidneys, and lungs.
Two OPOs reported consulting an infectious disease physician or a subject matter expert at CDC when a donor was reported to have a positive test; most OPOs (3 of 4 respondents to the question) leave patient management recommendations to the discretion of the recipient transplant center.
3.2 |. Considerations around implementing screening in the future
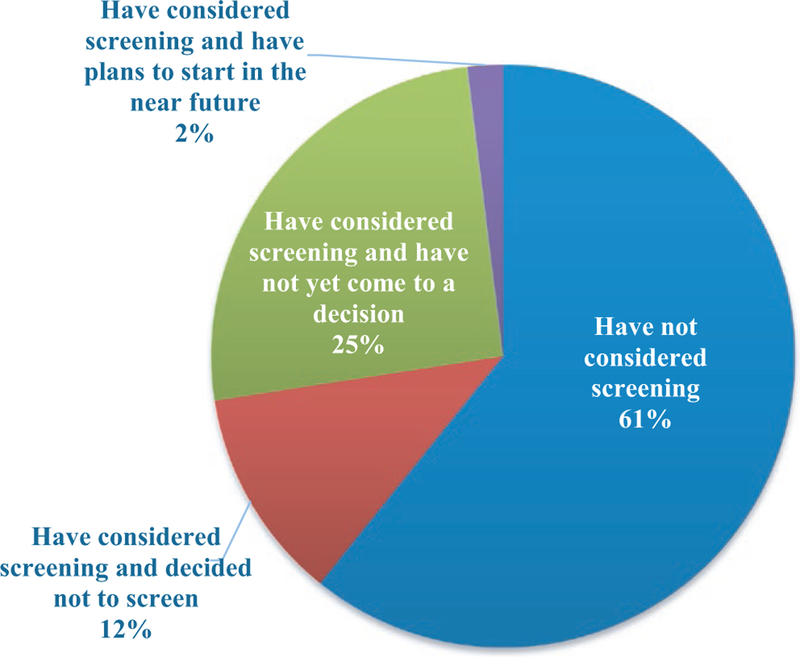
Among OPOs that do not currently screen for Strongyloides (n = 52), 31 (60%) have not considered screening, 6 (11%) considered screening and decided not to screen, 13 (25%) considered screening and have not yet come to a decision, and 1 (2%) has considered screening and has plans to start in the near future; 1 OPO did not respond to this question (Figure 2). The most common reason for considering screening was based on a lecture or information received at the AOPO conference; other reasons included history of positive donors, AST guidelines, recommendation by a local working group/committee or United Network for Organ Sharing (UNOS)/DTAC, and information from a commercial laboratory. One OPO stated that this survey conducted by AOPO prompted the consideration to screen. Of the OPOs that are considering screening (n = 14), the majority (n = 11, 79%) plan to conduct targeted screening using a risk-based strategy and 3 (21%) plan to screen all donors. Of the OPOs considering a risk-based screening strategy, only 4 (36%) reported the criteria that would be used; the remaining OPOs had not yet decided on an approach. The criteria reported by these 4 OPOs included residential/travel history and occupation. Half (n = 7) of these OPOs plan to use a commercial laboratory for testing of samples. Hesitations toward screening can be classified into 4 general categories: (i) test characteristics (n = 9), including the turnaround time to receive test results, the financial burden of testing, and the false-positive rate of the test; (ii) laboratory characteristics (n = 4), including concerns about availability of Strongyloides testing and the capability of local laboratories to perform testing; (iii) concerns about the effect on the procurement process (n = 3), specifically delays in organ procurement and the impact of a positive test on organ availability; and (iv) OPO characteristics (n = 12), including lack of familiarity with Strongyloides, concern for the added burden of including Strongyloides on the list of donor screening tests without clear benefits of testing, perceived low risk or lack of data to determine risk in donor service areas, and lack of guidelines to determine screening criteria.
FIGURE 2.
Non-screening US OPOs, attitudes toward future screening by category (N = 51*). *One non-screening OPO did not answer this question
4 |. DISCUSSION
The majority of US OPOs are currently not screening donors for Strongyloides infection; these 52 OPOs serve a population of over 258 million Americans. Those that do screen have begun to do so relatively recently, with 5 of 6 screening OPOs beginning in 2013 or later. The OPOs screening for Strongyloides infection, serving a population of 61 million Americans, all use a risk-based screening strategy, which is appropriate for this particular infection.4 However, currently implemented screening criteria differ among OPOs. Screening should be considered for potential donors who have lived in or visited any Strongyloides-endemic area2 for extended periods of time. Potential donors with unexplained eosinophilia should also be tested.4
The optimal screening process includes a risk-based assessment to determine if the donor was born in or had travelled to a Strongyloides-endemic area. LiveOnNY has been successful testing donors who have lived in the following Strongyloides-endemic areas for any period of time: southeastern United States, Mexico, Puerto Rico, the Caribbean, Latin America, South America, Sub-Saharan Africa, Asia, India, and Oceania.5 An assessment of the donor’s complete blood count to evaluate for the presence and cause of eosinophilia is also important, as unexplained eosinophilia may be a marker for strongyloidiasis. If such risks are identified, a serum sample should be collected at the same time samples are collected for other infectious diseases (eg, HIV, syphilis, CMV) testing. Unlike the results of mandated pathogen testing, the results for Strongyloides testing do not have to be known prior to organ transplantation. If a donor is determined to have evidence of strongyloidiasis, the result, typically available 1–10 days after testing, should be directly communicated to the transplant centers of all organ recipients. This direct and timely communication allows for appropriate intervention to prevent overwhelming infection in these immunocompromised hosts. In CDC’s experience to date, disease has not been reported in recipients of organs from a donor with proven or suspected evidence of strongyloidiasis who have been prophylactically treated with ivermectin.5 A treatment course of ivermectin 200 mg/kg/daily for 2 days with repeat therapy 2 weeks later (to account for one auto-infective cycle) has been successful,6 although there is no consensus recommendation.
A diagnosis of latent strongyloidiasis in a donor is not a contraindication to organ transplantation. Donors with positive Strongyloides serology should be routinely considered, based on the reported success of transplant of organs from antibody positive donors to recipients who receive prophylactic treatment.5 The key aspects to ensuring the safety of solid organ recipients in this scenario are the direct communication of the result of Strongyloides testing from the OPOs to the transplant centers,7 the timely use of ivermectin therapy to prevent disease in recipients, and monitoring for post-transplant infection.
Currently, Strongyloides testing is available at many commercial laboratories, as well as laboratories affiliated with academic institutions. These laboratories presently perform testing using enzyme-linked immunosorbent assays (ELISA) for immunoglobulin G (IgG) that have a sensitivity ranging from 84% to 95% and a specificity ranging from 82% to 100%, depending on the specific laboratory assay used. The turnaround time ranges from 1 to 10 days; 1 OPO reported a cost of $70 per test when using a laboratory affiliated with an academic institution. In addition, the CDC provides confirmatory testing using a crude antigen (CrAg) ELISA with a sensitivity of 96% and a specificity of 98%.
Targeted donor screening for Strongyloides, as recommended by the 2013 AST guidelines4 and supported by the LiveOnNY experience, can prevent potentially life-threatening disease in recipients of organs from donors with detectable Strongyloides antibody.5 The substantial proportion (27%) of non-screening OPOs currently considering or planning to initiate screening in the near future is encouraging for the safety of organ transplantation. It is important that OPOs follow AST’s guidelines, which recommend evaluation for Strongyloides infection be strongly considered in transplant candidates and donors with epidemiologic risk factors or unexplained eosinophilia,4 to optimize the safety of the transplant population. In addition, continued effort is needed to educate OPOs that have not considered screening for strongyloidiasis. These OPOs serve almost 60% of the population covered by the 58 OPOs and are critical to improving transplant safety for the nation.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Mark Paster, Chief Information Officer of the Association of Organ Procurement Organizations, for his assistance with conducting the survey, and all OPO staff members who completed the survey. The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Abbreviations:
- AST
American Society of Transplantation
- CDC
Centers for Disease Control and Prevention
- CMV
cytomegalovirus
- CrAg
crude antigen
- DTAC
Disease Transmission Advisory Committee
- ELISA
enzyme-linked immunosorbent assay
- OPO
organ procurement organizations
- UNOS
United Network for Organ Sharing
Footnotes
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
REFERENCES
- 1.Olsen A, van Lieshout L, Marti H, et al. Strongyloidiasis - the most neglected of the neglected tropical diseases? Trans R Soc Trop Med Hyg. 2009;103:967–972. [DOI] [PubMed] [Google Scholar]
- 2.Schär F, Trostdorf U, Giardina F, et al. Strongyloides stercoralis: global distribution and risk factors. PLoS Negl Trop Dis. 2013;7:e2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hotez PJ. Neglected infections of poverty in the United States of America. PLoS Negl Trop Dis. 2008;2:e256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartz BS, Mawhorter SD; AST Infectious Diseases Community of Practice. Parasitic infections in solid organ transplantation. Am J Transplant. 2013;13(Suppl 4):280–303. [DOI] [PubMed] [Google Scholar]
- 5.Abanyie FA, Gray EB, Delli Carpini KW, et al. Donor-derived Strongyloides stercoralis infection in solid organ transplant recipients in the United States, 2009–2013. Am J Transplant. 2015;15:1369–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roxby AC, Gottlieb GS, Limaye AP. Strongyloidiasis in transplant patients. Clin Infect Dis. 2009;49:1411–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller R, Covington S, Taranto S, et al. Communication gaps associated with donor-derived infections. Am J Transplant. 2015;15:259–264. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.