Abstract
With the march of time our bodies start to wear out: eyesight fades, skin loses its elasticity, teeth and bones become more brittle, and injuries heal more slowly. These universal features of aging can be traced back to our stem cells. Aging has a profound effect on stem cells: DNA mutations naturally accumulate over time and our bodies have evolved highly specialized mechanisms to remove these damaged cells. While obviously beneficial, this repair mechanism also reduces the pool of available stem cells and this, in turn, has a dramatic effect on tissue homeostasis, and our rate of healing. Simply put: fewer stem cells means a decline in tissue function, and slower healing.
Despite this seemingly intractable situation, research over the past decade now demonstrates that some of the effects of aging are reversible. Nobel prize-winning research demonstrates that old cells can become young again and lessons learned from these experiments-in-a-dish are now being translated into human therapies. Scientists and clinicians around the world are identifying and characterizing methods to activate stem cells to reinvigorate the body’s natural regenerative process. If this research in dental regenerative medicine pans out, the end result will be tissue homeostasis and healing back to the levels we appreciated when we were young.
Keywords: Alveolar bone, cementum, periodontal ligament, Wnt, stem cell
This review focuses primarily on how aging impacts putative stem cell populations in the components comprising the periodontium. Accumulating evidence indicates that preserving these endogenous stem cell populations is a key factor in maintaining tissue integrity and reparative potential in older patients (70). Thus, the hunt is on for the stem cell-based therapies that can safely achieve this ambitious goal.
Aging impacts every tissue in the body. In addition to structural and anatomical changes aging is also associated with a decline in the ability to maintain tissue homeostasis and a progressive loss in healing capacity (reviewed in 15, 43, 85). Both homeostasis and tissue healing are dependent upon tissue-specific stem cells. The fact that stem cells persist throughout the life of an animal- via the process of self-renewal- makes these cells particularly sensitive to DNA damage that accumulates over time. In some cases this means that the number of stem cells declines in age; in other cases, aged stem cells show a diminished responsiveness to stem cell signals in their local environment (37, 48). The ability to reprogram aged somatic cells into induced pluripotent stem cells or stem-like cells was an apt demonstration that at least some of the cellular changes that accompany aging are reversible (reviewed in 79). Age-related changes can be reversed in a cell, but whether a clinically viable rejuvenation strategy built upon induced pluripotent stem cell technology exists is not clear. Induced pluripotent stem cells clearly adopt some of the features associated with young cells- and some of the features associated with cancer cells. Clearly, any strategy that is employed to reverse cellular aging, especially for non life-threatening conditions, will have to prioritize safety.
In this review we outline how aging impacts the periodontium, with a focus on the cellular and molecular changes that occur. We focus on recent discoveries that highlight the role that Wnt signaling plays in maintaining homeostasis of the components of the periodontium and how this knowledge informs clinically relevant strategies to maintain tissue health into old age.
Alveolar bone mass declines with age
Bone undergoes constant cellular renewal, and under normal physiological conditions its integrity depends on continuous remodeling. For example, bone remodeling removes micro-damage and ensure a strong osteoid matrix (11) and for many years, the location and types of stem cells that maintain this tissue were hotly debated. Some placed the skeletal stem cell in the marrow cavity (58, 60) while others claimed the periosteum was the primary source (62, 74, 94). Pinpoint the source(s) of stem cells has direct relevance to clinical practice: for example, when placing an implant, surgeons are aware that minimal heat should be generated during osteotomy site preparation (21). The reason for this is clear: Excessive heat kills osteocytes, and this necrotic bone will eventually be resorbed (49); as a consequence, the implant can loosen and fail. Similarly, some skeletal stem cells reside within the periosteum (16); consequently efforts should be made to preserve that stem cell niche when possible. Clearly, understanding the molecular mechanisms by which stem cell proliferation and self-renewal are balanced would also provide some much-needed clues into how skeletal tissues can be maintained into old age.
Given the importance of stem cells in maintaining bone, it is obviously beneficial to understand how aging affects their behaviors. For example, if “young” skeletal stem cells are plastic but environmental changes associated with aging switch them to a program of terminal differentiation (e.g., 28, 71) then we need to invest effort into understanding how to maintain their “stemness” into advanced age. A number of laboratories (1, 8, 98), including our own (37), have focused on this question as it relates to the maintenance of bone mass and bone healing.
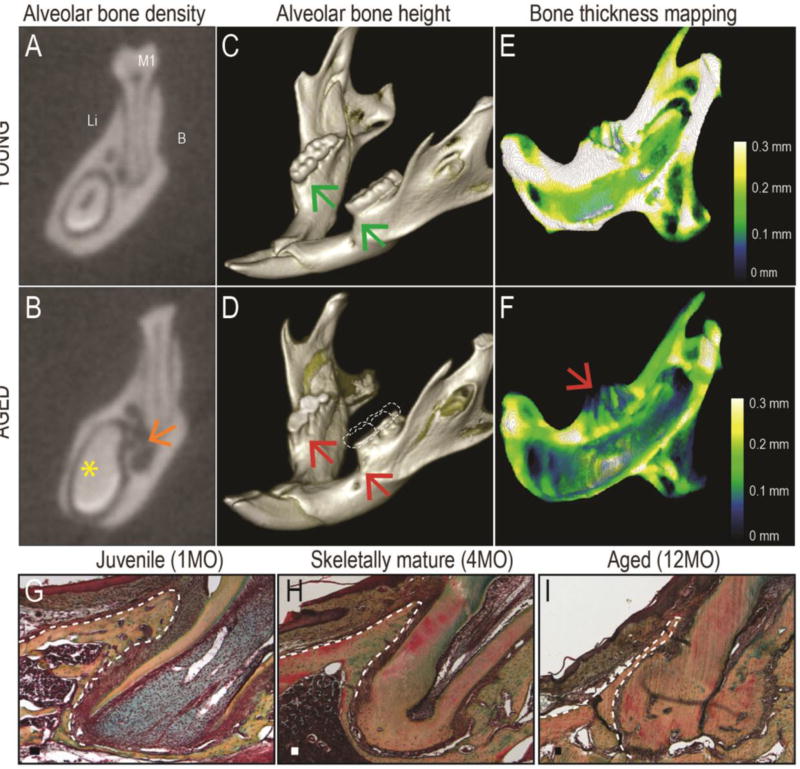
One key regulator of stem cell self-renewal, and osteogenic differentiation is regulated by Wnt proteins (reviewed in 5). In humans, Wnt signaling regulates bone mass (10, 102), which suggests that modulating Wnt levels may improve bone regeneration in elderly patients (47). Alveolar bone is an ideal target for such Wnt-based therapeutic intervention (54). Alveolar bone mass declines with age (77, 88) and this loss in alveolar bone can result in a type of chronic periodontitis (51). Micro-computed tomography documented this age-related decline in alveolar bone mass in mice (for similar results see (39, 73, 84). In these analyses young mice were between 2–3 months old, which equates with skeletally mature humans e.g., between the ages of 18–26 (31, 35). Aged mice, on the other hand, were >18 months old, which equates to 75–95 year old humans (22). Buccal-lingual micro-computed tomography sections clearly demonstrate a dramatic loss in alveolar bone density in aged mice compared to their young counterparts (Fig. 1A,B). On a single micro-computed tomography section this age-related radiolucency appears somewhat cyst-like (orange arrow, Fig. 1B), but serial sections confirm decreased trabeculation throughout the aged mandible. Volume rendering of the mandibles also demonstrates a loss in alveolar bone height: In young animals the crest of the alveolar bone is near the cementoenamel junction (green arrows, Fig. 1C). In aged animals, alveolar bone exhibits considerable recession on both buccal and lingual surfaces, sufficient to expose the mesial surfaces of the first molars as well as all the furcations (red arrows, Fig. 1D). There is also evidence of extensive occlusal attrition (compare Fig. 1C,D).
Fig. 1. Aging profoundly affects alveolar bone mass and density.
(A) Coronal micro-computed tomography section through the young mouse mandible at the level of the first mandibular molar (M1). The root of the incisor is visible on the lingual inferior border of the mandible. (B) Equivalent micro-computed tomography section through the aged mouse mandible; orange arrow indicates decreased bone density. Yellow asterisk indicates the lack of a pulp cavity in the incisor of the aged animal. (C) Volume rendering of a representative mandible from a young mouse; green arrows indicate the height of the alveolar bone crest. (D) Equivalent volume rending of a representative mandible from an aged mouse; red arrows indicate alveolar bone recession that exposes the root surfaces. The occlusal surfaces of the molars show significant wear; dotted lines suggest an approximate amount of occlusal wear. (E) Color mapping of alveolar bone thickness in a representative mandible from a young mouse, where the dentition has been digitally removed. (F) Equivalent color mapping of a representative mandible from an aged mouse; red arrow indicates the very thin alveolar bone. (G) Pentachrome staining of a representative tissue section from the mandible of a young mouse; alveolar bone around the mesial root of M1 is indicated with a dotted white line. (H) Similar tissue section from the mandible of a 4-month-old mouse; note that the alveolar bone is already denser and thinner. The mesial root also exhibits evidence of cellular cementum. (I) Similar tissue section from the mandible of a 12-month-old mouse; note thin alveolar bone, absence of a marrow space, and abundant cellular cementum. Abbreviations: B, buccal; Li, lingual; M1, first molar. Scale bars: 50µm.
Digitally removing the dentition allows for a detailed evaluation of how aging impacts alveolar bone thickness. In young mice color mapping illustrates that the mandible is between 0.2 and 0.3mm thick (Fig. 1E) whereas in aged mice it is on the order of 0.1mm or less (Fig. 1F); this reflects an overall age-related loss in bone thickness of ~30%. Of particular note is the extremely thin crestal bone in the aged cohort (red arrow, Fig. 1F). This pattern of bone loss in aged mice reflects the same pattern of bone loss seen in aging humans (86).
From a clinical perspective, thinning of facial alveolar bone is an inherently difficult problem to manage. Histologically, alveolar bone is a highly vascularized tissue in juveniles (Fig. 1G) and in a very young human patient population its ability to regenerate following trauma is well-documented (45, 89). In skeletally mature animals alveolar bone becomes denser and its highly vascularized status is reduced (Fig. 1H) and the bone becomes reduced to a knife-edge of mineralized tissue surrounding the dentition (Fig. 1I).
Periodontists are particularly aware that even a small amount of bone resorption in older patients with such thin alveolar bone plates has a dramatic affect on alveolar bone height (29, 68, 96). Accompanying this loss in alveolar bone height is exposure of the root surface or, if a dental implant is in place, exposure of the implant threads. In the former case there is no method that reliably restores root coverage. In the latter case, exposure of implant threads is not only esthetically unacceptable; it also poses a risk for bacterial infection that can necessitate removal of the implant (3, 14, 44).
Collectively these data document the age-related phenomenon of decreased alveolar bone mass; they do not, however, explain why bone deposition declines with age. At least a partial answer to that question has come from analyses of humans with genetic diseases resulting in diminished bone mass (for example, see 26, 75); these studies conclusively demonstrate that a reduction in endogenous Wnt signaling lies at the heart of age-related bone loss in humans (33, 66, 75, 97). In addition to its function as a stem cell activator, Wnt signals directly inhibit osteoclast activity (4, 25) and directly accelerate the commitment of osteoprogenitor cells to an osteogenic lineage (30, 40, 103).
The therapeutic potential of Wnt signal modification is now being developed for human use (41). One strategy to amplify endogenous Wnt signaling is to inhibit an inhibitor of the Wnt pathway, such as sclerostin (55). An anti-sclerostin antibody is being tested as a means to reduce age-related osteoporosis (12), with encouraging initial clinical results (72). Others (95) and we (61, 69) have demonstrated that a liposome-reconstituted version of a WNT3A protein (20) enhances Wnt signaling around implants, and improves osseointegration. This formulation of liposomal WNT (L-WNT3A) activates endogenous mesenchymal and skeletal stem cells, leading to their faster differentiation into bone-forming osteoblasts (37). As a consequence, skeletal and dental injuries treated with L-WNT show significantly better healing (37, 48, 59).
There remain a number of important questions. For example, we still need to have a better understanding of where stem cells reside within bone. Are they within the marrow cavity, or primarily the endosteum or the periosteum (16)? What about alleged “circulating skeletal stem cells” (46); do they actually contribute to bone volume in a meaningful way? And what about parts of the skeleton that don’t have a periosteum- such as the tooth extraction socket- where do the skeletal progenitors come from that lead to new bone formation? If we had answers to these questions then we would be able to focus our inquiries on whether aging represents a deficit in stem cell function- which could be reversed by stem cell activation- or an actual decline in their numbers, which would perhaps necessitate the actual introduction of stem cells as a therapeutic approach.
Cementum volume increases with age
Cementum is produced throughout life (91, 99) but unlike bone, its volume increases with age (18, 36, 42). This is a particularly puzzling phenomenon because bone and cementum have often viewed as comparable tissues (6). The obvious question becomes, why does the mass of one mineralized tissue (bone) decline with age while the mass of another, very similar mineralized tissue (cementum) increase with age?
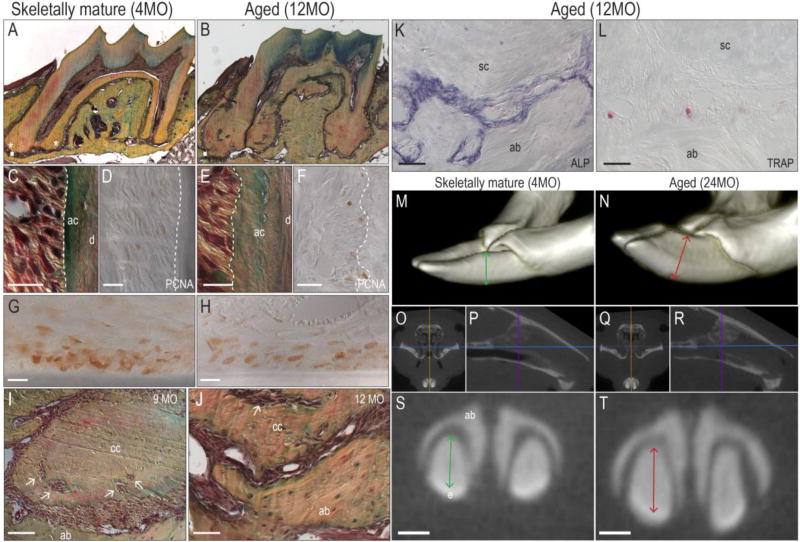
The murine cementum was examined to confirm such age-related changes. The root surfaces of murine molars were compared, starting before tooth eruption, and continuing into advanced age. At postnatal day 21 (P21) the molar teeth have erupted into the oral cavity but mastication of hard foods has not yet begun. At this early stage, a thin layer of primary (acellular) cementum covers the dentin surface and because the apex is still open, no cellular cementum is detectable. When mice are skeletally mature (e.g., 4 months of age), secondary (cellular) cementum is visible at the apex (Fig. 2A). In advanced age (e.g., 12 months), the accumulation of cellular cementum is dramatic (Fig. 2B). The same accumulation of cementum is noted in humans (6, 83), even without masticatory loading (65).
Fig. 2. Cementum volume increases with age.
(A) Pentachrome staining of a representative tissue section from the maxillary M1 of a 4-month-old mouse; cellular cementum around the apex is detectable (asterisks). (B) Equivalent tissue section from the maxillary M1 of a 12-month-old mouse; note the abundant cementum around the entire roots, the decreased volume of the pulp cavity, and the abraded crown. (C) High magnification of pentachrome-stained acellular cementum from the maxillary M1 of a 4-month-old mouse; the dotted white line showed the smooth interface of the periodontal ligament and cementum; a thin blue-green stained acellular cementum layer is obvious between the periodontal ligament and dentin. (D) Proliferating cell nuclear antigen immunohistochemistry staining shows proliferating cells in the periodontal ligament space; dotted white line as in C. (E) High magnification of an equivalent tissue section from the maxillary M1 of a 12-month-old mouse; the dotted white line showed the irregular periodontal ligament-cementum interface; note the thick acellular cementum layer between the periodontal ligament and dentin. (F) proliferating cell nuclear antigen immunostaining from the maxillary M1 of a 12-month-old mouse; dotted white line as in E. (G) Robust proliferating cell nuclear antigen immunostaining from the middle root of the maxillary incisor of a 4-month-old mouse and (H) a 9-month-old mouse. (I) A 9-month-old and (J) a 12-month-old mouse, showing accumulated cellular cementum; arrows indicate trapped periodontal ligament cells in the extracellular matrix of the cellular cementum. (K) Alkaline phosphatase staining from apical area of the maxillary M1 of a 12-month-old mouse; note strong alkaline phosphatase activity on the alveolar bone surface and cementum surface within the narrow periodontal ligament. (L) TRAP staining from the same apical area of a 12-month-old mouse; minimum TRAP positive cells exist on the alveolar bone surface. (M) Three-dimensional reconstruction from the micro-computed tomography of mandible incisors of a 4-month-old mouse; note the labial-lingual diameter of the incisor (green arrow). (N) Three-dimensional reconstruction from the micro-computed tomography of the mandible incisors of a 24-month-old mouse; note the widened the labial-lingual diameter of the incisor (red arrow), and the incisor crown wear. (O) Coronal micro-computed tomography section through the young mouse mandible at the level the purple line indicated in (P). (P) Sagittal micro-computed tomography section of the young mouse. Purple line indicates the level of (O). (Q) Similar coronal micro-computed tomography section through the aged mouse mandible at the level the purple line indicated in (R). (R) Sagittal micro-computed tomography section of the aged mouse. Purple line indicated the level of (Q). (S) High magnification of the coronal section of the level as (O) showed; note the thickness of the labial-lingual diameter (green arrow). (T) High magnification of the same coronal level; note increased thickness of the incisor (red arrow). Abbreviations: ab, alveolar bone; ac, acellular cementum; cc, cellular cementum; M1, first molar; PDL, periodontal ligament; proliferating cell nuclear antigen, proliferating cell nuclear antigen; ALP, alkaline phosphatase; TRAP, tartrate-resistant acid phosphatase. Scale bars: 50µm in the tissue sections; 0.5mm in the micro-computed tomography sections.
To gain some insights into the underlying molecular mechanisms behind this age-related accumulation of cementum immunostaining for proliferating cell nuclear antigen was used to label mitotically active cells. In young samples, pentachrome staining identified the acellular cementum and cells immediately adjacent to it (Fig. 2C); on an adjacent section, very few cells near the acellular cementum were positive for proliferating cell nuclear antigen immunostaining (Fig. 2D). In aged mice, the acellular cementum was thicker (Fig. 2E) but again there were few cells immunopositive for proliferating cell nuclear antigen (Fig. 2F).
Maybe cell proliferation is inherently very low adjacent to acellular cementum? To address this question proliferating cell nuclear antigen immunostaining was performed on tissue sections through the incisors of young and old mice. In these animals maxillary incisors grow ~300µm/day and mandibular incisors grow ~400µm/day (101). In both cases exuberant proliferating cell nuclear antigen immunostaining was observed (Fig. 2G,H).
Some investigators regard the cementum as a calcified component of the periodontal ligament (56), and the next analyses lent credence to this interpretation: In aged animals periodontal ligament cells were observed to be trapped in the extracellular matrix of the cementum (arrows, Fig. 2I,J). Extensive alkaline phosphatase activity was detectable in the space between the alveolar bone and cellular cementum (Fig. 2K); because alkaline phosphatase activity is an indicator of active mineralization, its widespread distribution in the aged periodontal ligament space strongly suggested that the tissue was in the process of being calcified. Adjacent tissue sections showed minimal osteoclast activity bone (Fig. 2L).
Collectively, these observations indicate a robust age-related increase in cellular cementum volume. The question is, where are the cells that are responsible for this age-related increase in cementum? The only mitotically active cells detected were in the periodontal ligament space. Do these periodontal ligament cells differentiate into both osteoblasts and cementoblasts? Or is there a separate population of cementoblasts, whose activity- remarkably- increases with age? This latter point seems improbable, especially since a closely related cell, the osteoblast, shows an age-related decline in function (37, 48). Whether there is actually a stem/progenitor cell population responsible for producing cementum is, at this juncture, pure speculation. Therefore the next analyses focused on another tooth in the murine mouth with a well-documented pool of stem cells that continuously produce cementum throughout the life of the animal.
Continual eruption is attributable to a population of mitotically active stem cells residing at the incisor apex (63, 92). Incisors grow continuously and cementum accumulates with age; the question was whether this translated into an increase in the size of the murine incisor. Micro-computed tomography suggested this was the case (Fig. 2M,N). Frontal (Fig. 2O,Q) and sagittal (Fig. 2P,R) micro-computed tomography sections were used to identify anatomical landmarks (e.g., the skull base, blue line; the mid-sagittal plane, orange line; and the anterior cortex, purple line), which served as references to ensure that the same region of the incisor was being evaluated in young and aged mice. These equivalent micro-computed tomography sections clearly illustrate a 25% increase in the thickness of the dentin + cementum layers in aged versus young mice (compare green and red lines, Fig. 2S,T). Dentin apposition continues throughout life but dentin apposition is limited to the pulp surface, which explains why there is an age-related decrease in the volume of the pulp cavity (Fig. 1A,B, asterisk and see 19).
Thus, cementum accumulates with age on all murine teeth- and on human teeth- and for as-yet-unknown reasons, is resistant to the osteoclast-mediated resorptive process that causes a decline in bone mass. Like its function in bone homeostasis, Wnt signaling plays a role in cementum accumulation as shown by the fact that mice carrying a loss-of-Wnt mutation have thin acellular cementum and develop spontaneous root resorption (53). These data suggest that Wnt signals are required for continued cementum deposition.
What remains to be clarified is the relationship between endogenous Wnt signal and aging. If, as the data suggest, aging is associated with a decline in Wnt signaling then why does cementum accumulate over time? Obviously there is much work to be done here but a first, important step will be to identify the location of cementoblast stem/progenitor cell population and demonstrate that it is responsible for the deposition of cellular cementum. The following step will be to determine which signaling pathway(s) regulate cementoblast behavior, a question that orthodontists and periodontists will most surely be interested in having answered.
Age-related changes in the periodontal ligament
The architecture of the periodontal ligament is ideally suited for the transmission of tensile load. In other parts of the body, structures like this are referred to entheses; i.e., tissues that form at the sites of stress concentration and typically attach mineralized tissues to one another (24, 67, 100). An enthesis is characterized as a ligamentous tissue that gradually transitions into a calcified tissue. The periodontal ligament has been evaluated as an enthesis (32).
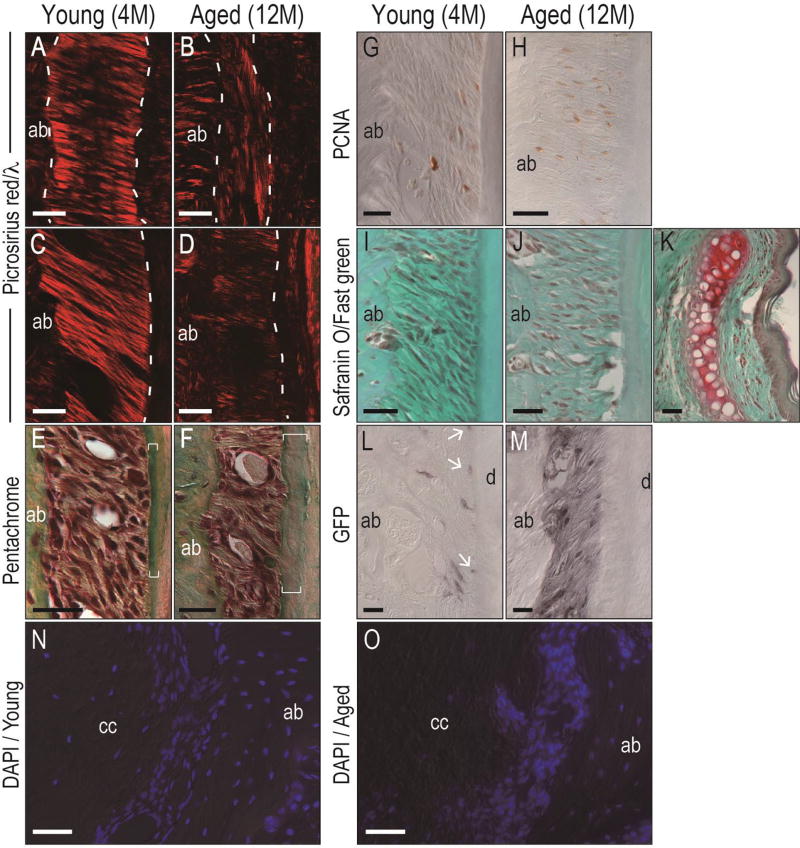
In some entheses, the calcified portion becomes enlarged and the fibrous component becomes smaller with age (7); this increase in the calcified portion of the enthesis has been associated with simultaneous thinning of the bone into which the enthesis is attached (93). Because the calcified portion of the enthesis is avascular, the age-related increase in calcification is associated with a decrease in the overall strength of the tissue (87). Whether the periodontal ligament undergoes similar kinds of age-related changes as do other enthuses is unknown. Severson and colleagues first reported that the aged periodontal ligament had a lower cellular content and exhibited uneven and irregular Sharpey’s fibers insertions (83). These histologic changes have been verified over time (2, 80, 81), and analyses shown here demonstrate the reduced collagen content and thinning of the ligamentous portion of the periodontal ligament in aged mice (Fig. 3A–D). An increase in the calcified portion of the periodontal ligament enthesis was also evident (brackets, Fig. 3E,F). One surprise was that cell proliferation was also increased in the aged periodontal ligament space (Fig. 3G,H). Some entheses have a intervening fibrocartilage; Safranin O/fast green staining failed to identify a proteoglycan-rich cartilage intermediate (Fig. 3I–K). Nonetheless, these data from a mouse model are in agreement with age-related changes observed in other entheses in the human body.
Fig. 3. Age-related changes in the periodontal ligament.
(A, C) Picrosirius red staining of the apical area, and the middle of the root of the M1 of a young mouse, respectively; note collagenous fibrils between the bone and cementum. (B, D) Picrosirius red staining of the equivalent apical area and the middle of the root the M1 of the aged mouse, respectively; note decreased collagen content and narrowed space of the periodontal ligament. The dotted white line indicates the bone-periodontal ligament or the periodontal ligament-cementum interfaces. (E) High magnification of pentachrome staining of the M1 periodontal ligament of a young mouse. White brackets indicate thinning cementum. (F) High magnification of an equivalent section of the M1 periodontal ligament of an aged mouse. White brackets indicate thicker cementum. (G) Proliferating cell nuclear antigen immunostaining in the periodontal ligament from the maxillary M1 of a young mouse and (F) an aged mouse. (I) Safranin O/fast green staining of the maxillary M1 of a young mouse and (J) an aged mouse. (K) Positive control of safranin O/fast green staining of the nasal cartilage, where the red coloration results from a proteoglycan-rich matrix. (L) Representative green fluorescent protein immunostaining from the M1 of a 4-month-old Axin2CreERT2/+;R26RmTmG/+ mouse shows positive cells in the periodontal ligament space; white arrows indicate immunopositive cells. (M) Equivalent green fluorescent protein immunostaining from the M1 of an 12-month-old Axin2CreERT2/+;R26RmTmG/+ mouse showing green fluorescent protein+ve cells and the matrix. (N) Representative DAPI staining from the apical area of M1 of the young mouse indicates the cell density of cellular cementum, periodontal ligament, and alveolar bone. (O) DAPI staining from the equivalent apical area of M1 of the aged mouse indicate that cell density is decreased in the aged cementum and increased in the aged periodontal ligament space. Abbreviations: ab, alveolar bone; cc, cellular cementum; d, dentin; M1, first molar; PDL, periodontal ligament; PCNA, proliferating cell nuclear antigen; GFP, green fluorescent protein; DAPI, 4’,6-diamidino-2-phenylindole. Scale bars: 50µm.
Wnt signaling is also evident in the periodontal ligament. In previous experiments the use of the Axin2LacZ/+ reporter strain of mice allowed mapping of the pattern of Wnt responsiveness in the periodontal ligament. Robust Xgal staining identifies Wnt responsive periodontal ligament cells around both the molars and incisors (76), but because the LacZ gene product, Xgal, has an extended half-life in vivo it was difficult to distinguish whether Wnt signaling was actively occurring. The use of an inducible lineage-tracer to identify Wnt responsive cells and their progeny avoided this problem: In Axin2CreERT2/+;R26RmTmG/+ mice, green fluorescent protein expression is under control of the endogenous Axin2 promoter, and can only be induced after of tamoxifen delivery. Young and aged mice were given tamoxifen for 5 days then sacrificed. Immunostaining for green fluorescent protein was performed, which revealed positive staining in the periodontal ligament space of young animals (Fig. 3L). By their location these were periodontal ligament cells; other immunopositive cells were located close to the cementum surface (arrow, Fig. 3L). These data were therefore in agreement with previous results obtained using Axin2LacZ/+ mice (52, 76).
Aged mice also showed significantly stronger and more widespread green fluorescent protein immunostaining (Fig. 3M). Although the periodontal ligament space was narrower, there were more immunopositive cells, and the matrix produced by the green fluorescent protein-expressing cells was also labeled (Fig. 3M). These preliminary data raise the possibility that Wnt signaling is elevated in the aged periodontal ligament, coincident with its age-related transition to a more calcified tissue. Precisely why Wnt signaling would be elevated in this locale, relative to its decline in adjacent bone, remains to be determined. Others and we have speculated that the periodontal ligament space functions as a stem cell niche (9, 17, 23, 27, 38). Certainly, cells in the periodontal ligament space are multi-potential: at least in vitro, these cells can differentiate in osteoblasts that produce bone (50, 64) and cementoblasts that produce cementum (34, 57, 82). Perhaps this cell fate choice is mediated in part by a Wnt signal? This concept is not without precedence: in other niches like the marrow cavity, stem cells can differentiate into either osteoblasts or adiopoblasts, and the choice is regulated in part by a Wnt signal (13, 48, 66, 78, 90). If this bi-potential cell fate relationship holds true then cementogenesis would occur at the expense of osteogenesis. Since the source of the putative Wnt signal remains unknown, this theory remains purely speculative.
Concluding remarks
Data presented here aptly demonstrate that aging has a profound impact on putative stem cell populations in the periodontium. Aging leads to a general decline in stem cell function, which manifests as a loss of alveolar bone volume and atrophy of the periodontal ligament. In one tissue, bone, a stem/progenitor population has been identified and shown to be responsive to a Wnt signal. Clinical trials are underway that seek to amplify endogenous Wnt signaling in aged patients, with an end goal of restoring bone mass back to levels appreciated in youth. Wnt signals are also important in maintaining cementum, but whether there is a method to safely manipulate this pathway during orthodontic tooth movement to curtail root resorption, or during periodontal therapy to stimulate regeneration is not yet clear. Because these conditions are not life threatening, such stem cell-based therapies will necessarily have to exhibit strong efficacy coupled with minimal risk to become the standard of care.
Acknowledgments
This work was supported by NIH grant 5R01DE024000-11 to J.A.H., and the National Natural Science Foundation of China to L.H. (Grant No. 81300914).
Footnotes
The authors declare that they have no conflicts of interest with the work presented herein.
References
- 1.Almeida M, O'Brien CA. Basic biology of skeletal aging: role of stress response pathways. J Gerontol A Biol Sci Med Sci. 2013;68:1197–1208. doi: 10.1093/gerona/glt079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andreescu CF, Mihai LL, Raescu M, Tuculina MJ, Cumpata CN, Ghergic DL. Age influence on periodontal tissues: a histological study. Rom J Morphol Embryol. 2013;54:811–815. [PubMed] [Google Scholar]
- 3.Artzi Z, Tal H, Chweidan H. Bone regeneration for reintegration in peri-implant destruction. Compend Contin Educ Dent. 1998;19:17–20. 22–13, 26–18. quiz 30. [PubMed] [Google Scholar]
- 4.Bajada S, Marshall MJ, Wright KT, Richardson JB, Johnson WE. Decreased osteogenesis, increased cell senescence and elevated Dickkopf-1 secretion in human fracture non union stromal cells. Bone. 2009;45:726–735. doi: 10.1016/j.bone.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 5.Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013;19:179–192. doi: 10.1038/nm.3074. [DOI] [PubMed] [Google Scholar]
- 6.Berkovitz BKB, Holland GR, Moxham BJ, Berkovitz BKB. Oral anatomy, embryology and histology. Edinburgh; New York: Mosby: 2002. p. xii.p. 378. [Google Scholar]
- 7.Bloebaum RD, Kopp DV. Remodeling capacity of calcified fibrocartilage of the hip. Anat Rec A Discov Mol Cell Evol Biol. 2004;279:736–739. doi: 10.1002/ar.a.20066. [DOI] [PubMed] [Google Scholar]
- 8.Borzi RM, Guidotti S, Minguzzi M, Facchini A, Platano D, Trisolino G, Filardo G, Cetrullo S, D'Adamo S, Stefanelli C, Facchini A, Flamigni F. Polyamine delivery as a tool to modulate stem cell differentiation in skeletal tissue engineering. Amino Acids. 2014;46:717–728. doi: 10.1007/s00726-013-1607-9. [DOI] [PubMed] [Google Scholar]
- 9.Bosshardt DD, Stadlinger B, Terheyden H. Cell-to-cell communication--periodontal regeneration. Clin Oral Implants Res. 2015;26:229–239. doi: 10.1111/clr.12543. [DOI] [PubMed] [Google Scholar]
- 10.Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, Wu D, Insogna K, Lifton RP. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;346:1513–1521. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- 11.Burr DB, Martin RB, Schaffler MB, Radin EL. Bone remodeling in response to in vivo fatigue microdamage. J Biomech. 1985;18:189–200. doi: 10.1016/0021-9290(85)90204-0. [DOI] [PubMed] [Google Scholar]
- 12.Canalis E. Wnt signalling in osteoporosis: mechanisms and novel therapeutic approaches. Nat Rev Endocrinol. 2013;9:575–583. doi: 10.1038/nrendo.2013.154. [DOI] [PubMed] [Google Scholar]
- 13.Cawthorn WP, Bree AJ, Yao Y, Du B, Hemati N, Martinez-Santibanez G, Macdougald OA. Wnt6, Wnt10a and Wnt10b inhibit adipogenesis and stimulate osteoblastogenesis through a beta-catenin-dependent mechanism. Bone. 2012;50:477–489. doi: 10.1016/j.bone.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chow YC, Wang HL. Factors and techniques influencing peri-implant papillae. Implant Dent. 2010;19:208–219. doi: 10.1097/ID.0b013e3181d43bd6. [DOI] [PubMed] [Google Scholar]
- 15.Collins CA, Zammit PS, Ruiz AP, Morgan JE, Partridge TA. A population of myogenic stem cells that survives skeletal muscle aging. Stem Cells. 2007;25:885–894. doi: 10.1634/stemcells.2006-0372. [DOI] [PubMed] [Google Scholar]
- 16.Colnot C. Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J Bone Miner Res. 2009;24:274–282. doi: 10.1359/jbmr.081003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coura GS, Garcez RC, de Aguiar CB, Alvarez-Silva M, Magini RS, Trentin AG. Human periodontal ligament: a niche of neural crest stem cells. J Periodontal Res. 2008;43:531–536. doi: 10.1111/j.1600-0765.2007.01065.x. [DOI] [PubMed] [Google Scholar]
- 18.Craca R, Romagnoli P, Cambi S, Orlando S. The evolution of human periodontal tissues with ageing. Arch Ital Anat Embriol. 1991;96:81–92. [PubMed] [Google Scholar]
- 19.De Angelis D, Gaudio D, Guercini N, Cipriani F, Gibelli D, Caputi S, Cattaneo C. Age estimation from canine volumes. Radiol Med. 2015 doi: 10.1007/s11547-015-0521-5. [DOI] [PubMed] [Google Scholar]
- 20.Dhamdhere GR, Fang MY, Jiang J, Lee K, Cheng D, Olveda RC, Liu B, Mulligan KA, Carlson JC, Ransom RC, Weis WI, Helms JA. Drugging a stem cell compartment using Wnt3a protein as a therapeutic. PLoS ONE. 2014;9:e83650. doi: 10.1371/journal.pone.0083650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eriksson RA, Albrektsson T. The effect of heat on bone regeneration: an experimental study in the rabbit using the bone growth chamber. J Oral Maxillofac Surg. 1984;42:705–711. doi: 10.1016/0278-2391(84)90417-8. [DOI] [PubMed] [Google Scholar]
- 22.Frost HM. On our age-related bone loss: insights from a new paradigm. J Bone Miner Res. 1997;12:1539–1546. doi: 10.1359/jbmr.1997.12.10.1539. [DOI] [PubMed] [Google Scholar]
- 23.Gay IC, Chen S, MacDougall M. Isolation and characterization of multipotent human periodontal ligament stem cells. Orthod Craniofac Res. 2007;10:149–160. doi: 10.1111/j.1601-6343.2007.00399.x. [DOI] [PubMed] [Google Scholar]
- 24.Genin GM, Birman V. Micromechanics and Structural Response of Functionally Graded, Particulate-Matrix, Fiber-Reinforced Composites. Int J Solids Struct. 2009;46:2136–2150. doi: 10.1016/j.ijsolstr.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glass DA, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA, Karsenty G. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell. 2005;8:751–764. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 26.Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, Zacharin M, Oexle K, Marcelino J, Suwairi W, Heeger S, Sabatakos G, Apte S, Adkins WN, Allgrove J, Arslan-Kirchner M, Batch JA, Beighton P, Black GC, Boles RG, Boon LM, Borrone C, Brunner HG, Carle GF, Dallapiccola B, De Paepe A, Floege B, Halfhide ML, Hall B, Hennekam RC, Hirose T, Jans A, Juppner H, Kim CA, Keppler-Noreuil K, Kohlschuetter A, LaCombe D, Lambert M, Lemyre E, Letteboer T, Peltonen L, Ramesar RS, Romanengo M, Somer H, Steichen-Gersdorf E, Steinmann B, Sullivan B, Superti-Furga A, Swoboda W, van den Boogaard MJ, Van Hul W, Vikkula M, Votruba M, Zabel B, Garcia T, Baron R, Olsen BR, Warman ML. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- 27.Gronthos S, Mrozik K, Shi S, Bartold PM. Ovine periodontal ligament stem cells: isolation, characterization, and differentiation potential. Calcif Tissue Int. 2006;79:310–317. doi: 10.1007/s00223-006-0040-4. [DOI] [PubMed] [Google Scholar]
- 28.Hadjiargyrou M, O'Keefe RJ. The convergence of fracture repair and stem cells: interplay of genes, aging, environmental factors and disease. J Bone Miner Res. 2014;29:2307–2322. doi: 10.1002/jbmr.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halling A, Bjorn AL. Periodontal status in relation to age of dentate middle aged women. A 12 year longitudinal and a cross-sectional population study. Swed Dent J. 1986;10:233–242. [PubMed] [Google Scholar]
- 30.Hartmann C. A Wnt canon orchestrating osteoblastogenesis. Trends Cell Biol. 2006;16:151–158. doi: 10.1016/j.tcb.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Henry YM, Fatayerji D, Eastell R. Attainment of peak bone mass at the lumbar spine, femoral neck and radius in men and women: relative contributions of bone size and volumetric bone mineral density. Osteoporos Int. 2004;15:263–273. doi: 10.1007/s00198-003-1542-9. [DOI] [PubMed] [Google Scholar]
- 32.Ho SP, Marshall SJ, Ryder MI, Marshall GW. The tooth attachment mechanism defined by structure, chemical composition and mechanical properties of collagen fibers in the periodontium. Biomaterials. 2007;28:5238–5245. doi: 10.1016/j.biomaterials.2007.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holmen SL, Zylstra CR, Mukherjee A, Sigler RE, Faugere MC, Bouxsein ML, Deng L, Clemens TL, Williams BO. Essential role of beta-catenin in postnatal bone acquisition. J Biol Chem. 2005;280:21162–21168. doi: 10.1074/jbc.M501900200. [DOI] [PubMed] [Google Scholar]
- 34.Hoz L, Romo E, Zeichner-David M, Sanz M, Nunez J, Gaitan L, Mercado G, Arzate H. Cementum protein 1 (CEMP1) induces differentiation by human periodontal ligament cells under three-dimensional culture conditions. Cell Biol Int. 2012;36:129–136. doi: 10.1042/CBI20110168. [DOI] [PubMed] [Google Scholar]
- 35.Iscan MY, Loth SR, Wright RK. Metamorphosis at the sternal rib end: a new method to estimate age at death in white males. Am J Phys Anthropol. 1984;65:147–156. doi: 10.1002/ajpa.1330650206. [DOI] [PubMed] [Google Scholar]
- 36.Jang AT, Lin JD, Choi RM, Choi EM, Seto ML, Ryder MI, Gansky SA, Curtis DA, Ho SP. Adaptive properties of human cementum and cementum dentin junction with age. J Mech Behav Biomed Mater. 2014;39:184–196. doi: 10.1016/j.jmbbm.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jing W, Smith AA, Liu B, Li J, Hunter DJ, Dhamdhere G, Salmon B, Jiang J, Cheng D, Johnson CA, Chen S, Lee K, Singh G, Helms JA. Reengineering autologous bone grafts with the stem cell activator WNT3A. Biomaterials. 2015;47:29–40. doi: 10.1016/j.biomaterials.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 38.Jo YY, Lee HJ, Kook SY, Choung HW, Park JY, Chung JH, Choung YH, Kim ES, Yang HC, Choung PH. Isolation and characterization of postnatal stem cells from human dental tissues. Tissue Eng. 2007;13:767–773. doi: 10.1089/ten.2006.0192. [DOI] [PubMed] [Google Scholar]
- 39.Kabasawa M, Ejiri S, Hanada K, Ozawa H. Effect of age on physiologic and mechanically stressed rat alveolar bone: a cytologic and histochemical study. Int J Adult Orthodon Orthognath Surg. 1996;11:313–327. [PubMed] [Google Scholar]
- 40.Kang S, Bennett CN, Gerin I, Rapp LA, Hankenson KD, Macdougald OA. Wnt signaling stimulates osteoblastogenesis of mesenchymal precursors by suppressing CCAAT/enhancer-binding protein alpha and peroxisome proliferator-activated receptor gamma. J Biol Chem. 2007;282:14515–14524. doi: 10.1074/jbc.M700030200. [DOI] [PubMed] [Google Scholar]
- 41.Ke HZ, Richards WG, Li X, Ominsky MS. Sclerostin and Dickkopf-1 as therapeutic targets in bone diseases. Endocr Rev. 2012;33:747–783. doi: 10.1210/er.2011-1060. [DOI] [PubMed] [Google Scholar]
- 42.Ketterl W. Age-induced changes in the teeth and their attachment apparatus. Int Dent J. 1983;33:262–271. [PubMed] [Google Scholar]
- 43.Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120:437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 44.Kitamura E, Stegaroiu R, Nomura S, Miyakawa O. Biomechanical aspects of marginal bone resorption around osseointegrated implants: considerations based on a three-dimensional finite element analysis. Clin Oral Implants Res. 2004;15:401–412. doi: 10.1111/j.1600-0501.2004.01022.x. [DOI] [PubMed] [Google Scholar]
- 45.Kiyokawa K, Kiyokawa M, Takagi M, Rikimaru H, Fukaya T. New regenerative treatment for tooth and periodontal bone defect associated with posttraumatic alveolar bone crush fracture. J Craniofac Surg. 2009;20:780–783. doi: 10.1097/SCS.0b013e3181a14b7b. [DOI] [PubMed] [Google Scholar]
- 46.Kuznetsov SA, Mankani MH, Gronthos S, Satomura K, Bianco P, Robey PG. Circulating skeletal stem cells. J Cell Biol. 2001;153:1133–1140. doi: 10.1083/jcb.153.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leucht P, Helms JA. Wnt signaling: an emerging target for bone regeneration. J Am Acad Orthop Surg. 2015;23:67–68. doi: 10.5435/JAAOS-23-01-67. [DOI] [PubMed] [Google Scholar]
- 48.Leucht P, Jiang J, Cheng D, Liu B, Dhamdhere G, Fang MY, Monica SD, Urena JJ, Cole W, Smith LR, Castillo AB, Longaker MT, Helms JA. Wnt3a reestablishes osteogenic capacity to bone grafts from aged animals. J Bone Joint Surg Am. 2013;95:1278–1288. doi: 10.2106/JBJS.L.01502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leucht P, Lam K, Kim JB, Mackanos MA, Simanovskii DM, Longaker MT, Contag CH, Schwettman HA, Helms JA. Accelerated bone repair after plasma laser corticotomies. Ann Surg. 2007;246:140–150. doi: 10.1097/01.sla.0000258559.07435.b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Y, Li SQ, Gao YM, Li J, Zhang B. Crucial role of Notch signaling in osteogenic differentiation of periodontal ligament stem cells in osteoporotic rats. Cell Biol Int. 2014;38:729–736. doi: 10.1002/cbin.10257. [DOI] [PubMed] [Google Scholar]
- 51.Liang S, Hosur KB, Domon H, Hajishengallis G. Periodontal inflammation and bone loss in aged mice. J Periodontal Res. 2010;45:574–578. doi: 10.1111/j.1600-0765.2009.01245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lim WH, Liu B, Cheng D, Williams BO, Mah SJ, Helms JA. Wnt signaling regulates homeostasis of the periodontal ligament. J Periodontal Res. 2014;49:751–759. doi: 10.1111/jre.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lim WH, Liu B, Hunter DJ, Cheng D, Mah SJ, Helms JA. Downregulation of Wnt causes root resorption. Am J Orthod Dentofacial Orthop. 2014;146:337–345. doi: 10.1016/j.ajodo.2014.05.027. [DOI] [PubMed] [Google Scholar]
- 54.Lim WH, Liu B, Mah SJ, Yin X, Helms JA. Alveolar Bone Turnover and Periodontal Ligament Width are Controlled by Wnt. J Periodontol. 2015;86:319–326. doi: 10.1902/jop.2014.140286. [DOI] [PubMed] [Google Scholar]
- 55.Lin C, Jiang X, Dai Z, Guo X, Weng T, Wang J, Li Y, Feng G, Gao X, He L. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. J Bone Miner Res. 2009;24:1651–1661. doi: 10.1359/jbmr.090411. [DOI] [PubMed] [Google Scholar]
- 56.Luan X, Walker C, Dangaria S, Ito Y, Druzinsky R, Jarosius K, Lesot H, Rieppel O. The mosasaur tooth attachment apparatus as paradigm for the evolution of the gnathostome periodontium. Evol Dev. 2009;11:247–259. doi: 10.1111/j.1525-142X.2009.00327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ma Z, Li S, Song Y, Tang L, Ma D, Liu B, Jin Y. The biological effect of dentin noncollagenous proteins (DNCPs) on the human periodontal ligament stem cells (HPDLSCs) in vitro and in vivo. Tissue Eng Part A. 2008;14:2059–2068. doi: 10.1089/ten.tea.2008.0021. [DOI] [PubMed] [Google Scholar]
- 58.Minear S, Leucht P, Jiang J, Liu B, Zeng A, Fuerer C, Nusse R, Helms JA. Wnt proteins promote bone regeneration. Sci Transl Med. 2010;2:29ra30. doi: 10.1126/scitranslmed.3000231. [DOI] [PubMed] [Google Scholar]
- 59.Minear S, Leucht P, Miller S, Helms JA. rBMP represses Wnt signaling and influences skeletal progenitor cell fate specification during bone repair. J Bone Miner Res. 2010;25:1196–1207. doi: 10.1002/jbmr.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Modder UI, Khosla S. Skeletal stem/osteoprogenitor cells: current concepts, alternate hypotheses, and relationship to the bone remodeling compartment. J Cell Biochem. 2008;103:393–400. doi: 10.1002/jcb.21423. [DOI] [PubMed] [Google Scholar]
- 61.Mouraret S, Hunter DJ, Bardet C, Popelut A, Brunski JB, Chaussain C, Bouchard P, Helms JA. Improving oral implant osseointegration in a murine model via Wnt signal amplification. J Clin Periodontol. 2014;41:172–180. doi: 10.1111/jcpe.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mouraret S, Von Kaeppler E, Bardet C, Hunter DJ, Chaussain C, Bouchard P, Helms JA. The potential for vertical bone regeneration via maxillary periosteal elevation. J Clin Periodontol. 2014;41:1170–1177. doi: 10.1111/jcpe.12310. [DOI] [PubMed] [Google Scholar]
- 63.Ness AR, Smale DE. The distribution of mitoses and cells in the tissues bounded by the socket wall of the rabbit mandibular incisor. Proceedings of the Royal Society of London. Series B, Biological Sciences. 1959;151:106–126. [Google Scholar]
- 64.Ninomiya T, Hiraga T, Hosoya A, Ohnuma K, Ito Y, Takahashi M, Ito S, Asashima M, Nakamura H. Enhanced bone-forming activity of side population cells in the periodontal ligament. Cell Transplant. 2014;23:691–701. doi: 10.3727/096368913X663587. [DOI] [PubMed] [Google Scholar]
- 65.Nitzan DW, Michaeli Y, Weinreb M, Azaz B. The effect of aging on tooth morphology: a study on impacted teeth. Oral Surg Oral Med Oral Pathol. 1986;61:54–60. doi: 10.1016/0030-4220(86)90203-3. [DOI] [PubMed] [Google Scholar]
- 66.Nuttall ME, Gimble JM. Controlling the balance between osteoblastogenesis and adipogenesis and the consequent therapeutic implications. Curr Opin Pharmacol. 2004;4:290–294. doi: 10.1016/j.coph.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 67.Onuora S. Spondyloarthritis: Evidence from animal studies supports the 'entheseal stress' hypothesis of ankylosing spondylitis. Nat Rev Rheumatol. 2012;8:248. doi: 10.1038/nrrheum.2012.52. [DOI] [PubMed] [Google Scholar]
- 68.Payne JB, Stoner JA, Lee HM, Nummikoski PV, Reinhardt RA, Golub LM. Serum bone biomarkers and oral/systemic bone loss in humans. J Dent Res. 2011;90:747–751. doi: 10.1177/0022034511402993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Popelut A, Rooker SM, Leucht P, Medio M, Brunski JB, Helms JA. The acceleration of implant osseointegration by liposomal Wnt3a. Biomaterials. 2010;31:9173–9181. doi: 10.1016/j.biomaterials.2010.08.045. [DOI] [PubMed] [Google Scholar]
- 70.Rando TA, Wyss-Coray T. Stem cells as vehicles for youthful regeneration of aged tissues. J Gerontol A Biol Sci Med Sci. 2014;69(Suppl 1):S39–42. doi: 10.1093/gerona/glu043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Raveh-Amit H, Berzsenyi S, Vas V, Ye D, Dinnyes A. Tissue resident stem cells: till death do us part. Biogerontology. 2013;14:573–590. doi: 10.1007/s10522-013-9469-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Recker RR, Benson CT, Matsumoto T, Bolognese MA, Robins DA, Alam J, Chiang AY, Hu L, Krege JH, Sowa H, Mitlak BH, Myers SL. A randomized, double-blind phase 2 clinical trial of blosozumab, a sclerostin antibody, in postmenopausal women with low bone mineral density. J Bone Miner Res. 2015;30:216–224. doi: 10.1002/jbmr.2351. [DOI] [PubMed] [Google Scholar]
- 73.Rivaldo EG, Padilha DP, Hugo FN. Alveolar bone loss and aging: a model for the study in mice. J Periodontol. 2005;76:1966–1971. doi: 10.1902/jop.2005.76.11.1966. [DOI] [PubMed] [Google Scholar]
- 74.Roberts SJ, van Gastel N, Carmeliet G, Luyten FP. Uncovering the periosteum for skeletal regeneration: the stem cell that lies beneath. Bone. 2015;70:10–18. doi: 10.1016/j.bone.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 75.Roforth MM, Fujita K, McGregor UI, Kirmani S, McCready LK, Peterson JM, Drake MT, Monroe DG, Khosla S. Effects of age on bone mRNA levels of sclerostin and other genes relevant to bone metabolism in humans. Bone. 2014;59:1–6. doi: 10.1016/j.bone.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rooker SM, Liu B, Helms JA. Role of Wnt signaling in the biology of the periodontium. Dev Dyn. 2010;239:140–147. doi: 10.1002/dvdy.22003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ruegsegger P, Durand EP, Dambacher MA. Differential effects of aging and disease on trabecular and compact bone density of the radius. Bone. 1991;12:99–105. doi: 10.1016/8756-3282(91)90007-6. [DOI] [PubMed] [Google Scholar]
- 78.Saidak Z, Hay E, Marty C, Barbara A, Marie PJ. Strontium ranelate rebalances bone marrow adipogenesis and osteoblastogenesis in senescent osteopenic mice through NFATc/Maf and Wnt signaling. Aging Cell. 2012;11:467–474. doi: 10.1111/j.1474-9726.2012.00804.x. [DOI] [PubMed] [Google Scholar]
- 79.Sanchez Alvarado A, Yamanaka S. Rethinking differentiation: stem cells, regeneration, and plasticity. Cell. 2014;157:110–119. doi: 10.1016/j.cell.2014.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Scardina GA, Cacioppo A, Messina P. Anatomical evaluation of oral microcirculation: capillary characteristics associated with sex or age group. Ann Anat. 2009;191:371–378. doi: 10.1016/j.aanat.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 81.Semba I, Funakoshi K, Kitano M. Histomorphometric analysis of age changes in the human inferior alveolar artery. Arch Oral Biol. 2001;46:13–21. doi: 10.1016/s0003-9969(00)00100-x. [DOI] [PubMed] [Google Scholar]
- 82.Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 83.Severson JA, Moffett BC, Kokich V, Selipsky H. A histologic study of age changes in the adult human periodontal joint (ligament) J Periodontol. 1978;49:189–200. doi: 10.1902/jop.1978.49.4.189. [DOI] [PubMed] [Google Scholar]
- 84.Shahnazari M, Dwyer D, Chu V, Asuncion F, Stolina M, Ominsky M, Kostenuik P, Halloran B. Bone turnover markers in peripheral blood and marrow plasma reflect trabecular bone loss but not endocortical expansion in aging mice. Bone. 2012;50:628–637. doi: 10.1016/j.bone.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 85.Sharpless NE, DePinho RA. How stem cells age and why this makes us grow old. Nat Rev Mol Cell Biol. 2007;8:703–713. doi: 10.1038/nrm2241. [DOI] [PubMed] [Google Scholar]
- 86.Shaw RB, Jr, Katzel EB, Koltz PF, Yaremchuk MJ, Girotto JA, Kahn DM, Langstein HN. Aging of the facial skeleton: aesthetic implications and rejuvenation strategies. Plast Reconstr Surg. 2011;127:374–383. doi: 10.1097/PRS.0b013e3181f95b2d. [DOI] [PubMed] [Google Scholar]
- 87.Shea JE, Hallows RK, Ricks S, Bloebaum RD. Microvascularization of the hypermineralized calcified fibrocartilage and cortical bone in the sheep proximal femur. Anat Rec. 2002;268:365–370. doi: 10.1002/ar.10173. [DOI] [PubMed] [Google Scholar]
- 88.Southard KA, Southard TE. Quantitative features of digitized radiographic bone profiles. Oral Surg Oral Med Oral Pathol. 1992;73:751–759. doi: 10.1016/0030-4220(92)90023-j. [DOI] [PubMed] [Google Scholar]
- 89.Taba M, Jr, Jin Q, Sugai JV, Giannobile WV. Current concepts in periodontal bioengineering. Orthod Craniofac Res. 2005;8:292–302. doi: 10.1111/j.1601-6343.2005.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Takada I, Kouzmenko AP, Kato S. Wnt and PPARgamma signaling in osteoblastogenesis and adipogenesis. Nat Rev Rheumatol. 2009;5:442–447. doi: 10.1038/nrrheum.2009.137. [DOI] [PubMed] [Google Scholar]
- 91.Tersigni-Tarrant MA, Shirley NR. Forensic anthropology: an introduction. Boca Raton, FL: CRC Press; 2013. p. xxiii.p. 462. [Google Scholar]
- 92.Thesleff I, Tummers M. StemBook. Cambridge (MA): 2008. Tooth organogenesis and regeneration. [PubMed] [Google Scholar]
- 93.Vajda EG, Bloebaum RD. Age-related hypermineralization in the female proximal human femur. Anat Rec. 1999;255:202–211. doi: 10.1002/(SICI)1097-0185(19990601)255:2<202::AID-AR10>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 94.van Gastel N, Stegen S, Stockmans I, Moermans K, Schrooten J, Graf D, Luyten FP, Carmeliet G. Expansion of murine periosteal progenitor cells with fibroblast growth factor 2 reveals an intrinsic endochondral ossification program mediated by bone morphogenetic protein 2. Stem Cells. 2014;32:2407–2418. doi: 10.1002/stem.1783. [DOI] [PubMed] [Google Scholar]
- 95.Virdi AS, Irish J, Sena K, Liu M, Ke HZ, McNulty MA, Sumner DR. Sclerostin antibody treatment improves implant fixation in a model of severe osteoporosis. J Bone Joint Surg Am. 2015;97:133–140. doi: 10.2106/JBJS.N.00654. [DOI] [PubMed] [Google Scholar]
- 96.Wactawski-Wende J, Grossi SG, Trevisan M, Genco RJ, Tezal M, Dunford RG, Ho AW, Hausmann E, Hreshchyshyn MM. The role of osteopenia in oral bone loss and periodontal disease. J Periodontol. 1996;67:1076–1084. doi: 10.1902/jop.1996.67.10s.1076. [DOI] [PubMed] [Google Scholar]
- 97.Wagner ER, Zhu G, Zhang BQ, Luo Q, Shi Q, Huang E, Gao Y, Gao JL, Kim SH, Rastegar F, Yang K, He BC, Chen L, Zuo GW, Bi Y, Su Y, Luo J, Luo X, Huang J, Deng ZL, Reid RR, Luu HH, Haydon RC, He TC. The therapeutic potential of the Wnt signaling pathway in bone disorders. Curr Mol Pharmacol. 2011;4:14–25. [PubMed] [Google Scholar]
- 98.Weng T, Xie Y, Huang J, Luo F, Yi L, He Q, Chen D, Chen L. Inactivation of Vhl in osteochondral progenitor cells causes high bone mass phenotype and protects against age-related bone loss in adult mice. J Bone Miner Res. 2014;29:820–829. doi: 10.1002/jbmr.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wittwer-Backofen U, Gampe J, Vaupel JW. Tooth cementum annulation for age estimation: results from a large known-age validation study. Am J Phys Anthropol. 2004;123:119–129. doi: 10.1002/ajpa.10303. [DOI] [PubMed] [Google Scholar]
- 100.Wopenka B, Kent A, Pasteris JD, Yoon Y, Thomopoulos S. The tendon-to-bone transition of the rotator cuff: a preliminary Raman spectroscopic study documenting the gradual mineralization across the insertion in rat tissue samples. Appl Spectrosc. 2008;62:1285–1294. doi: 10.1366/000370208786822179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zegarelli EV. Adamantoblastomas in the Slye stock of mice. Am J Pathol. 1944;20:23–87. [PMC free article] [PubMed] [Google Scholar]
- 102.Zhong Z, Zylstra-Diegel CR, Schumacher CA, Baker JJ, Carpenter AC, Rao S, Yao W, Guan M, Helms JA, Lane NE, Lang RA, Williams BO. Wntless functions in mature osteoblasts to regulate bone mass. Proc Natl Acad Sci U S A. 2012;109:E2197–2204. doi: 10.1073/pnas.1120407109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhou H, Mak W, Zheng Y, Dunstan CR, Seibel MJ. Osteoblasts directly control lineage commitment of mesenchymal progenitor cells through Wnt signaling. J Biol Chem. 2008;283:1936–1945. doi: 10.1074/jbc.M702687200. [DOI] [PubMed] [Google Scholar]