Abstract
Fatty liver diseases, non-alcoholic fatty liver disease (NAFLD) and alcoholic liver disease (ALD) are the most common causes of chronic liver disease around the world. NAFLD and ALD can progress towards a more severe form of the disease, including as non-alcoholic steatohepatitis (NASH) and alcoholic steatohepatitis (ASH). In both instances central pathogenic events include hepatocyte death, liver inflammation, pathological angiogenesis, and fibrosis, followed by cirrhosis and cancer. Over the last few years, extracellular vesicles (EVs) have been identified as effective cell-to-cell communicators that contain a cell- and stress-specific cargo from the cell of origin and are capable of transferring this cargo to a target or acceptor cell. In this review, we focus on the growing evidence supporting a role for EVs in the pathophysiology of NASH and ASH as well as their potential roles as targets for novel biomarkers for these conditions.
Keywords: Extracellular vesicles, exosomes, microparticles, fatty liver diseases, Lipotoxicity, non-alcoholic steatohepatitis (NASH), alcoholic steatohepatitis (ASH)
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) and Alcoholic liver disease (ALD) are two of the most common causes of chronic liver disease worldwide. NAFLD is tightly associated with obesity and encompasses a wide spectrum of conditions associated with the over-accumulation of fat in the hepatocytes (isolated steatosis), to non-alcoholic steatohepatitis (NASH) characterized by steatosis accompanied by hepatocellular injury, lobular and portal inflammatory infiltrates of monocyte-derived macrophages, neutrophils and lymphocytes, and different degrees of fibrosis.1 NASH is a serious condition, since about 5 to 25 % of patients may progress to cirrhosis and end-stage liver disease with its associated complications of portal hypertension, liver failure and hepatocellular carcinoma.2 Indeed, NASH has become the second leading cause of liver transplant in the United States with projections suggesting it will become the leading cause by 2020.3 ALD is another common form of chronic liver disease in the United States, as well as many other countries.4,5 As with NAFLD, ALD represents a wide spectrum of liver damage ranging from steatosis to alcoholic steatohepatitis (ASH), to cirrhosis resulting in liver failure and hepatocellular carcinoma (HCC).6,7 During progression of liver damage in both NAFLD and ALD, inflammation, dysregulated angiogenesis, and fibrosis are key events and are closely interconnected.8 Furthermore, activated hepatic stellate cells (HSCs) sustain the extracellular matrix remodeling and collagen deposition9 following by advanced fibrosis associating with increased risk of HCC development.10 Emerging evidence suggests that multiple-hits, rather that the previously thought two hit hypothesis, are involved in the progression of these two chronic liver diseases. Indeed, accumulating evidence indicates that hepatocyte stress via lipotoxicity following by hepatocyte death, plays a central role in inflammation and hepatic injury during the progression of NASH11 and ALD.12–14 We recently reported that hepatocyte-derived extracellular vesicles (EVs) (Hep-EVs) release from damaged/stressed hepatocytes in experimental models of NASH and ASH contribute to the progression of liver diseases through activation of non-parenchymal cells, such as liver endothelial cells (ECs), HSCs, and hepatic macrophages.15,16 Furthermore, we and others have reported that Hep-EV composition, such as proteins and microRNAs (miRNAs), can be used to identify the degree as well as the different type of liver diseases.17–21
EVs are small membrane vesicles released in a highly regulated manner from damaged or activated cells. EVs are mainly categorized as exosomes and microparticles (MPs) based on size, less than 100 nm and 100–1000 nm, respectively. Exosomes are enclosed in the multi vesicular body and released from the cells via exocytosis, whereas MPs are form and release via budding from the plasma membrane. Based on the different releasing processes between exosomes and MPs, some of their composition are different, CD63, CD81, CD9, TSG101, etc. for exosomes and annexin V for MPs, which can be used for identification.22,23 Various cells release EVs, which circulate in the plasma of healthy humans and act as natural delivery systems under physiological conditions.24 Notably, EVs are efficiently internalized into target cells, and the transferring of their cargo, such as different non-coding RNAs (microRNA, longRNA, mitochondrial associated tRNA, small nucleolar RNA, small nuclear RNA, Ro associated Y-RNA, vault RNA, and Y-RNA), messenger RNAs (mRNAs), DNAs, proteins, and lipids, is a key mechanism by which EVs modulate cell signaling in target cells25–27, so called cell-to-cell communicator. For instance, ligands on EVs bind to the receptor on the target cells changing cell signal pathway or encapsulated miRNAs in EVs binding to the three prime untranslated region (3′UTR) of mRNA in the target cells following translational suppression.28 These EVs may contribute to maintenance of homeostasis during physiological conditions while they may be important triggers of pathophysiological responses through modulation of gene expression and phenotype of various target or acceptor cells.29 In this review, we focus on the growing evidence for a central contribution of EVs in fatty liver diseases, in particular in the development and progression of NASH and ASH, as well as their potential roles as novel targets for non-invasive biomarkers for these conditions.
2. Extracellular vesicles in NAFLD/NASH
2.1. Roles of hepatocytes-derived EVs
During the NAFLD progression, one of the key events is the accumulation of certain toxic lipids, such as saturated free fatty acids (SFA), free cholesterol, or ceramide and other sphingolipid in hepatocytes, leading to stressed/damaged hepatocyte by lipotoxicity.30–32 Indeed, lipotoxic lipids abundantly circulate in blood of NASH patients33,34 and stressed/damaged hepatocytes treated with lipotoxic lipids release large quantities of EVs that contribute to key processes involved in NAFLD pathogenesis, including angiogenesis, fibrosis, and inflammation, as multiple-hit mechanisms (Figure 1).15,16,35 We have reported that Hep-EVs, which are released in a caspase-3-dependent-manner, promote endothelial cell activation in a Vanin-1-dependent internalization inducing a pro-angiogenic signal15 and stimulate HSC activation in a process induced at least in part via delivery of encapsulated miR-128-3p.16 Additionally, Ibrahim and colleagues have reported that mixed lineage kinase 3 (MLK3) mediates the release of Hep-EVs induced during exposure of hepatocytes to lipotoxic lipids that carry chemokine (C-X-C motif) ligand 10 (CXCL10).36 Since CXCL10 is a macrophage chemoattractant,37 CXCL10-enriched Hep-EVs may recruit monocyte-derived macrophages and may activate hepatic macrophages (Kupffer cells) during NASH progression. While Hirsova and colleagues demonstrated that Hep-EVs from death receptor 5 (DR5) activated hepatocytes induced by lysophosphatidylcholine contained tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and they activated mouse bone marrow-derived macrophages via TRAIL-mediated activation as pro-inflammatory effects.19 They also showed that the release of Hep-EVs was decreased by inactivating mediators of the DR5 signaling pathway or Rho-associated protein kinase 1 (ROCK1) inhibition and found that ROCK1 inhibition in NASH mice led to reduction of circulating EV levels associating with reduction of liver damages, such as inflammation and fibrosis. Relating to endoplasmic reticulum (ER) stress, Kakazu and colleague reported that C16:0 ceramide-enriched Hep-EVs were released from damaged hepatocytes by lipotoxicity in an inositol requiring enzyme 1α (IRE1α)-dependent manner and they activated macrophages via formation of sphingosine-1-phosphate (S1P) from C16:0 ceramide. They also showed that C16:0 ceramide in blood was increased in mouse and human NASH.38 Interestingly, Garcia-Martinez and colleagues found that circulating encapsulated mitochondrial DNA (mtDNA) in Hep-EVs were increased in NASH patients and demonstrated that encapsulated mtDNA in Hep-EVs mediated macrophage activation through toll-like receptor 9 (TLR9) activation.39 In conclusion, Hep-EVs play crucial roles in modulation of non-parenchymal cells in the liver, including hepatic ECs, HSCs, and hepatic macrophages, as a multiple-hit mechanism, resulted in accelerating the NASH progression.
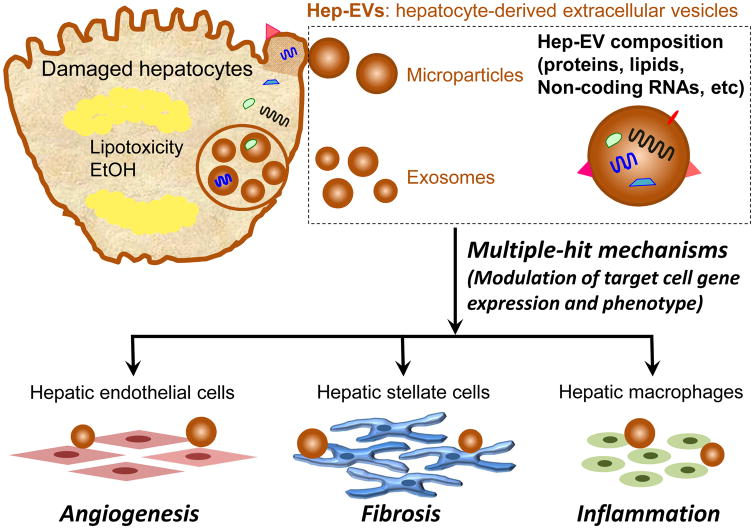
Figure 1. Hepatocyte-derived EVs contribute to the progression of fatty liver diseases.
Damaged hepatocytes by lipotoxicity or EtOH release a significant amount of extracellular vesicles (EVs), exosomes and microparticles. EVs may contain a unique cargo that reflects the cell of origin and the stress that triggered their release, including proteins, lipids, non-coding RNAs, etc. EVs and their cargo can be efficiently internalized into various target cells (HSCs, ECs, and hepatic macrophages including Kupffer cells) and the intracellular release of their cargo might represent key mechanisms to modulate gene expression and phenotype of target cells.
2.2. Role of EVs from extra-hepatic origin and non-parenchymal cells of the liver
Non-parenchymal cells and infiltrated inflammatory cells in the liver may represent another important source of EVs that contribute to liver injury. Early studies by Witek and colleagues showed that Hedgehog (Hh)-containing EVs from cholangiocytes and myofibroblastic HSCs were elevated in plasma and bile in bile duct ligated rats. Hh-EVs induced a pro-angiogenic switch in sinusoidal ECs followed by in vivo capillarization.40 Lemoinne and colleagues demonstrate that portal myofibroblasts (PMFs)-derived EVs carry the pro-angiogenic factor, vascular endothelial growth factor A (VEGF-A), and transfer into ECs resulted in endothelial cell activation and tubulogenesis via VEGF-A receptor.41 As EVs from infiltrated inflammatory cells in the liver, Kornek and colleagues demonstrate that activated CD4+ and CD8+ T cells-derived EVs transfer membrane molecules, such as CD147, into HSCs leading to the up-regulation of fibrolytic molecules.42 Cell-to-cell communication via EVs may occur via “horizontal transfer”. For instance, Charrier and colleague demonstrated that pro-fibrogenic connective tissue growth factor (CCN2)-containing EVs from activated HSCs shuttled to other HSCs as a form of paracrine pro-fibrogenic activation during liver injury.43 Furthermore, EVs act in organ-organ communication such as that between adipose tissue and liver, a crucial crosstalk in the context of steatosis and inflammation, associating with metabolic dysregulation.44,45 We have reported that hypertrophied adipocytes release large amounts of EVs that acts as chemoattractant for macrophages in the adipose tissue. These EVs are released into the circulation and can be detected in mouse models of obesity-driven fatty liver. Interestingly, the administration of EVs from obese mice into lean mice resulted in acute inflammation in the liver. Notably, adipocyte-derived EV levels were significantly increased in obese individuals showing a strong correlation with insulin levels and homeostatic model assessment for insulin resistance (HOMA-IR) and were decreased in a low calorie diet intervention for three months corresponding with improvement in metabolic complications,18 suggesting adipocyte-derived EVs (MPs) may regulate local and systemic insulin resistance. In conclusion, EV-mediated cell-to-cell and organ-to-organ communications may represent central events in multifactorial diseases like NASH.
3. Extracellular vesicles in ALD/ASH
Hepatocyte damage and cell death are two key events in the progression of ALD. Using an experimental model that closely mimics the early spectrum of human ALD going from isolated fatty liver to mild ASH, we demonstrated that circulating EV levels were increased in mice with early ASH, a time point associated with hepatocellular injury and inflammation but not those with fatty liver.18 Indeed, isolated hepatocytes and liver macrophages from mice with early ASH released large quantities of EVs with hepatocytes being the predominant source. RNA sequencing of these EVs identified a distinct miRNA profile that could accurately distinguish ASH mice from controls. More importantly as discussed in detail in the section below, this profile was present in circulating EVs from ASH mice but not in circulating EVs from other models of liver injury. In addition, similar to the findings on EVs from lipotoxic hepatocytes,18 EVs released by hepatocytes from ASH mice were capable of activate bone marrow-derived macrophages into pro-inflammatory M1 type (Eguchi et al, in preparation). Activation of caspase and pho-kinase pathways were involved in Hep-EV release. Verma and colleague demonstrated that a significant amount of Hep-EVs was released from hepatocytes overexpressing cytochrome P450 2E1 treated with EtOH in caspase-3-dependent-manner and activated macrophages with CD40 ligand (CD40L) on Hep-EVs.46 CD40L enriched circulating EVs were increased in alcoholic hepatitis patients. They also showed that mice with genetic deletion of CD40 were protected from alcohol-induced injury with suppression of macrophage activation. Recently, Cai and colleagues reported that mitochondrial DNA-enriched microparticles, which were mainly released from hepatocytes, promote neutrophilia in acute-on-chronic mouse model of ALD and human subjects with excessive alcohol use and a history of recent drinking.20 Their results indicated that mitochondrial DNA-enriched Hep-EVs were released by increase of ER stress, induced neutrophilic inflammation through TLR9 activation, and led to hepatic injury.
4. EVs as novel biomarkers to monitor liver injury in NASH and ASH
Liver biopsy remains the gold standard procedure to distinguish in NAFLD/NASH, ALD/ASH, fatty liver from steatohepatitis and staging the level of fibrosis. While various imaging modalities such as MR- and ultrasound-based elastography are widely used for assessment of liver fibrosis, these techniques lack sensitivity and specificity for early stages of fibrosis and are not useful for determination of inflammation and hepatocellular injury. Based on the growing evidence for a key pathophysiological role of EVs in liver injury as reviewed in the previous sections in conjunction with the fact that EVs are released into various bodily fluids and are remarkable stable leading to potential ideal targets for biomarkers development. Different approaches have been used including analyzing changes in EV types and cell-specific EVs by using selective surface markers or EV cargo as well as untargeted comprehensive approaches to assess EV composition such as protein, lipids, or RNA. Most studies up to date in NASH and ASH have focused on the two former approaches. Indeed, an early study reported the profile of blood EVs using flow cytometry analysis with selective leuko-endothelial surface markers in patients with NAFLD compared to chronic hepatitis C (CHC) and healthy controls.47 They observed an increased in MPs enriched in surface markers from monocyte and invariant natural killer T cells and a decrease in MP derived from neutrophils and ECs in NAFLD patients compared to both CHC patients and healthy controls.47 Since myeloid cell-derived EVs are released in circulation in a number of inflammatory conditions, several groups have tried to identified liver-specific markers that include cytokeratin-18, vanin-1, asialoglycoprotein receptor 1 (ASGPR1), miR-122, and miR-192.15,17,36,38,46 Notably, in diet-induced NASH mice from early- to advanced-NASH, circulating EVs with encapsulated miR-122 levels were increased over time correlating with histological features of NASH progression, such as cell death, angiogenesis, and fibrosis.17 Increased miR-122 and miR-192 were also observed in circulating EVs from an early ALD mouse model by feeding mice with the Lieber–DeCarli diet (EtOH) for 4 weeks and patients with acute alcoholic hepatitis.48 Furthermore, by untargeted profiling of miRNA composition from EV released by hepatocytes isolated from the intra-gastric infusion model of mild ASH, we recently identified that a specific miRNA signature including miR-29a, miR-340, let7f, and miR-30a could accurately identify or diagnose mice with ASH from pair-fed mice as well as mice with various other models of liver injury. This signature was present in ambulatory patients with mild ASH in a pilot proof of concept study.18,48 In summary, above early studies are pointing to potential significant roles of assessing EVs and EV composition for novel biomarker development and future studies to further interrogate and validate these approaches.
5. Conclusions
In conclusion, in this review we have summarized some of the most recent and original studies investigating the biological function of EVs, as multiple-hit mechanisms, to contribute the chronic inflammatory changes and abnormal would healing responses that are critical for the progression of fatty liver diseases to their more advance stages. In particular a number of studies have pointed to the role of EVs released by stressed hepatocytes as key modulators of these responses by their actions of various target cells such as HSCs, ECs, and macrophages, although non-parenchymal and extra-hepatic cell-derived EVs may also be involved resulted in a complex network for cell-cell and organ-organ communication during the development and progression of fatty liver diseases. In addition, the realization that these EVs are also released into the systemic circulation has generated significant interest as targets for biomarkers development. Future studies to dissect the EV mechanisms involved in their biogenesis, release and modulation of target cells as well as their potential role as liquid liver biopsies for accurate non-invasive diagnosis and monitoring of disease progression are warranted and highly awaited.
Table 1.
Summary of EVs associating to the progression of NAFLD/NASH and ALD/ASH
| Source | EV type | Cargo | Effects of EVs | Ref. |
|---|---|---|---|---|
| NAFLD/NASH | ||||
| Hepatocytes | MP | Vanin-1 | EC activation | (15) |
| Hepatocytes | MP | miR-128 | HSC activation | (16) |
| Hepatocytes | No clear description (exosome and MP) | Chemokine (C-X-C motif) ligand 10 | Hepatic macrophage activation | (36) |
| Hepatocytes | Exosome and MP | TRAIL | Bone marrow-derived macrophage activation | (19) |
| Hepatocytes | Exosome and MP | Ceramide | Macrophage activation | (38) |
| Hepatocytes | MP | Mitochondrial DNA | Macrophage activation | (39) |
| Cholangiocytes and myofibroblastic HSCs | MP | Hedgehog | EC activation | (40) |
| Portal myofibroblasts | MP | VEGF-A | EC activation | (41) |
| CD4+ and CD8+ T cells | MP | CD147 | HSC activation | (42) |
| Activated HSC cells | Exosome | CCN2 | HSC activation | (43) |
| ALD/ASH | ||||
| Hepatocytes | Exosome | CD40L | Macrophage activation | (46) |
| Hepatocytes | MP | Mitochondrial DNA | Neutrophilic inflammation | (20) |
EV: extracellular vesicles, MP: microparticle, EC: Endothelial cells, HSC: hepatic stellate cells, TRAIL: tumor necrosis factor-related apoptosis-inducing ligand, VEGF-A: vascular endothelial growth factor A, CCN2: pro-fibrogenic connective tissue growth factor, CD40L: CD40 ligand,
Acknowledgments
This work was partially supported by NIH grants U01 AA022489 to A.E.F. and R21 AA023574 to A.E. and A.E.F.
Footnotes
Conflict of interest: The authors declare not to have conflict of interests related to this scientific work.
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Authors’ contribution
AE: wrote the paper and designed figure and table, AEF: designed, wrote and edited the paper, figure and table.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wree A, Broderick L, Canbay A, Hoffman HM, Feldstein AE. From NAFLD to NASH to cirrhosis-new insights into disease mechanisms. Nature reviews. Gastroenterology & hepatology. 2013;10:627–636. doi: 10.1038/nrgastro.2013.149. [DOI] [PubMed] [Google Scholar]
- 2.Angulo P. Nonalcoholic fatty liver disease. The New England journal of medicine. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 3.Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547–555. doi: 10.1053/j.gastro.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 4.Yin M, Wheeler MD, Kono H, et al. Essential role of tumor necrosis factor alpha in alcohol-induced liver injury in mice. Gastroenterology. 1999;117:942–952. doi: 10.1016/s0016-5085(99)70354-9. [DOI] [PubMed] [Google Scholar]
- 5.Mathurin P. Alcohol and the liver. Gastroenterologie clinique et biologique. 2009;33:840–849. doi: 10.1016/j.gcb.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Nanji AA, Su GL, Laposata M, French SW. Pathogenesis of alcoholic liver disease--recent advances. Alcoholism, clinical and experimental research. 2002;26:731–736. [PubMed] [Google Scholar]
- 7.Feldstein AE, Gores GJ. Apoptosis in alcoholic and nonalcoholic steatohepatitis. Frontiers in bioscience: a journal and virtual library. 2005;10:3093–3099. doi: 10.2741/1765. [DOI] [PubMed] [Google Scholar]
- 8.Bocca C, Novo E, Miglietta A, Parola M. Angiogenesis and Fibrogenesis in Chronic Liver Diseases. Cellular and molecular gastroenterology and hepatology. 2015;1:477–488. doi: 10.1016/j.jcmgh.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972–1978. doi: 10.1002/hep.23527. [DOI] [PubMed] [Google Scholar]
- 11.Hirsova P, Gores GJ. Death Receptor-Mediated Cell Death and Proinflammatory Signaling in Nonalcoholic Steatohepatitis. Cellular and molecular gastroenterology and hepatology. 2015;1:17–27. doi: 10.1016/j.jcmgh.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adachi M, Higuchi H, Miura S, et al. Bax interacts with the voltage-dependent anion channel and mediates ethanol-induced apoptosis in rat hepatocytes. American journal of physiology. Gastrointestinal and liver physiology. 2004;287:G695–705. doi: 10.1152/ajpgi.00415.2003. [DOI] [PubMed] [Google Scholar]
- 13.Lieber CS. Mechanism of ethanol induced hepatic injury. Pharmacol Ther. 1990;46:1–41. doi: 10.1016/0163-7258(90)90032-w. [DOI] [PubMed] [Google Scholar]
- 14.Taieb J, Mathurin P, Poynard T, Gougerot-Pocidalo MA, Chollet-Martin S. Raised plasma soluble Fas and Fas-ligand in alcoholic liver disease. Lancet. 1998;351:1930–1931. doi: 10.1016/S0140-6736(05)78614-1. [DOI] [PubMed] [Google Scholar]
- 15.Povero D, Eguchi A, Niesman IR, et al. Lipid-induced toxicity stimulates hepatocytes to release angiogenic microparticles that require Vanin-1 for uptake by endothelial cells. Science signaling. 2013;6:ra88. doi: 10.1126/scisignal.2004512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Povero D, Panera N, Eguchi A, et al. Lipid-induced hepatocyte-derived extracellular vesicles regulate hepatic stellate cell via microRNAs targeting PPAR-gamma. Cellular and molecular gastroenterology and hepatology. 2015;1:646–663e644. doi: 10.1016/j.jcmgh.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Povero D, Eguchi A, Li H, et al. Circulating extracellular vesicles with specific proteome and liver microRNAs are potential biomarkers for liver injury in experimental fatty liver disease. PLoS One. 2014;9:e113651. doi: 10.1371/journal.pone.0113651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eguchi A, Lazaro RG, Wang J, et al. Extracellular vesicles released by hepatocytes from gastric infusion model of alcoholic liver disease contain a MicroRNA barcode that can be detected in blood. Hepatology. 2017;65:475–490. doi: 10.1002/hep.28838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirsova P, Ibrahim SH, Krishnan A, et al. Lipid-Induced Signaling Causes Release of Inflammatory Extracellular Vesicles From Hepatocytes. Gastroenterology. 2016;150:956–967. doi: 10.1053/j.gastro.2015.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cai Y, Xu MJ, Koritzinsky EH, et al. Mitochondrial DNA-enriched microparticles promote acute-on-chronic alcoholic neutrophilia and hepatotoxicity. JCI insight. 2017:2. doi: 10.1172/jci.insight.92634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bala S, Petrasek J, Mundkur S, et al. Circulating microRNAs in exosomes indicate hepatocyte injury and inflammation in alcoholic, drug-induced, and inflammatory liver diseases. Hepatology. 2012;56:1946–1957. doi: 10.1002/hep.25873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gyorgy B, Szabo TG, Pasztoi M, et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68:2667–2688. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keerthikumar S, Gangoda L, Liem M, et al. Proteogenomic analysis reveals exosomes are more oncogenic than ectosomes. Oncotarget. 2015;6:15375–15396. doi: 10.18632/oncotarget.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. The Journal of cell biology. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diehl P, Fricke A, Sander L, et al. Microparticles: major transport vehicles for distinct microRNAs in circulation. Cardiovascular research. 2012;93:633–644. doi: 10.1093/cvr/cvs007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yanez-Mo M, Siljander PR, Andreu Z, et al. Biological properties of extracellular vesicles and their physiological functions. Journal of extracellular vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lunavat TR, Cheng L, Kim DK, et al. Small RNA deep sequencing discriminates subsets of extracellular vesicles released by melanoma cells--Evidence of unique microRNA cargos. RNA Biol. 2015;12:810–823. doi: 10.1080/15476286.2015.1056975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen K, Rajewsky N. The evolution of gene regulation by transcription factors and microRNAs. Nat Rev Genet. 2007;8:93–103. doi: 10.1038/nrg1990. [DOI] [PubMed] [Google Scholar]
- 29.Rani S, Ryan AE, Griffin MD, Ritter T. Mesenchymal Stem Cell-derived Extracellular Vesicles: Toward Cell-free Therapeutic Applications. Mol Ther. 2015;23:812–823. doi: 10.1038/mt.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alkhouri N, Dixon LJ, Feldstein AE. Lipotoxicity in nonalcoholic fatty liver disease: not all lipids are created equal. Expert review of gastroenterology & hepatology. 2009;3:445–451. doi: 10.1586/egh.09.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feldstein AE, Werneburg NW, Canbay A, et al. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology. 2004;40:185–194. doi: 10.1002/hep.20283. [DOI] [PubMed] [Google Scholar]
- 32.Li Z, Berk M, McIntyre TM, Gores GJ, Feldstein AE. The lysosomal-mitochondrial axis in free fatty acid-induced hepatic lipotoxicity. Hepatology. 2008;47:1495–1503. doi: 10.1002/hep.22183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheung O, Kapoor A, Puri P, et al. The impact of fat distribution on the severity of nonalcoholic fatty liver disease and metabolic syndrome. Hepatology. 2007;46:1091–1100. doi: 10.1002/hep.21803. [DOI] [PubMed] [Google Scholar]
- 34.Puri P, Baillie RA, Wiest MM, et al. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 2007;46:1081–1090. doi: 10.1002/hep.21763. [DOI] [PubMed] [Google Scholar]
- 35.Szabo G, Momen-Heravi F. Extracellular vesicles in liver disease and potential as biomarkers and therapeutic targets. Nature reviews Gastroenterology & hepatology. 2017;14:455–466. doi: 10.1038/nrgastro.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ibrahim SH, Hirsova P, Tomita K, et al. Mixed lineage kinase 3 mediates release of C-X-C motif ligand 10-bearing chemotactic extracellular vesicles from lipotoxic hepatocytes. Hepatology. 2016;63:731–744. doi: 10.1002/hep.28252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fahey S, Dempsey E, Long A. The role of chemokines in acute and chronic hepatitis C infection. Cell Mol Immunol. 2014;11:25–40. doi: 10.1038/cmi.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kakazu E, Mauer AS, Yin M, Malhi H. Hepatocytes release ceramide-enriched pro-inflammatory extracellular vesicles in an IRE1alpha-dependent manner. J Lipid Res. 2016;57:233–245. doi: 10.1194/jlr.M063412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garcia-Martinez I, Santoro N, Chen Y, et al. Hepatocyte mitochondrial DNA drives nonalcoholic steatohepatitis by activation of TLR9. The Journal of clinical investigation. 2016;126:859–864. doi: 10.1172/JCI83885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Witek RP, Yang L, Liu R, et al. Liver cell-derived microparticles activate hedgehog signaling and alter gene expression in hepatic endothelial cells. Gastroenterology. 2009;136:320–330e322. doi: 10.1053/j.gastro.2008.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lemoinne S, Cadoret A, Rautou PE, et al. Portal myofibroblasts promote vascular remodeling underlying cirrhosis formation through the release of microparticles. Hepatology. 2015;61:1041–1055. doi: 10.1002/hep.27318. [DOI] [PubMed] [Google Scholar]
- 42.Kornek M, Popov Y, Libermann TA, Afdhal NH, Schuppan D. Human T cell microparticles circulate in blood of hepatitis patients and induce fibrolytic activation of hepatic stellate cells. Hepatology. 2011;53:230–242. doi: 10.1002/hep.23999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Charrier A, Chen R, Chen L, et al. Exosomes mediate intercellular transfer of pro-fibrogenic connective tissue growth factor (CCN2) between hepatic stellate cells, the principal fibrotic cells in the liver. Surgery. 2014;156:548–555. doi: 10.1016/j.surg.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eguchi A, Mulya A, Lazic M, et al. Microparticles release by adipocytes act as “find-me” signals to promote macrophage migration. PLoS One. 2015;10:e0123110. doi: 10.1371/journal.pone.0123110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. The Journal of clinical investigation. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verma VK, Li H, Wang R, et al. Alcohol stimulates macrophage activation through caspase-dependent hepatocyte derived release of CD40L containing extracellular vesicles. Journal of hepatology. 2016;64:651–660. doi: 10.1016/j.jhep.2015.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kornek M, Lynch M, Mehta SH, et al. Circulating microparticles as disease-specific biomarkers of severity of inflammation in patients with hepatitis C or nonalcoholic steatohepatitis. Gastroenterology. 2012;143:448–458. doi: 10.1053/j.gastro.2012.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Momen-Heravi F, Saha B, Kodys K, Catalano D, Satishchandran A, Szabo G. Increased number of circulating exosomes and their microRNA cargos are potential novel biomarkers in alcoholic hepatitis. J Transl Med. 2015;13:261. doi: 10.1186/s12967-015-0623-9. [DOI] [PMC free article] [PubMed] [Google Scholar]