Abstract
Context
Radical cystectomy continues to be associated with a significant risk of morbidity and all-cause mortality (ACM). Practice pattern data demonstrating underuse of surgery for patients with muscle-invasive and high-risk non–muscle invasive bladder cancer (BC) have been linked to the advanced age and higher comorbidity status of such patients, which suggests that rates of ACM as well as cancer-specific mortality should be incorporated into patient counseling and guideline recommendations.
Objective
To review the literature on risk assessment tools for preoperative comorbidity in BC that may aid in treatment decision-making.
Evidence acquisition
A systematic search was conducted using Ovid and Medline according to Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines to identify studies between 1970 and 2017 reporting on comorbidity risk assessment (CRA) tools for BC. Prospective and retrospective studies were included.
Evidence synthesis
There are no published randomized control trials comparing CRA tools for BC. Patients undergoing radical cystectomy with combined high-risk comorbidity and performance scores may face up to a sevenfold greater risk of other-cause mortality compared to those with low scores. The Charlson Comorbidity Index is one of the most widely studied indices for 90-d perioperative mortality and overall and cancer-specific survival, with an area under the receiver operating characteristic curve of up to 0.810. Prospective studies of CRA tools for BC have consistently shown that patients with higher comorbidity have worse outcomes. While not specific for BC, comorbidity indices provide useful assessment of competing risks. Competing-risks assessment tools are lacking, with limited studies assessing the impact of these tools on treatment decision-making by patients and providers. We provide the impetus for incorporation of comorbidity risks into practice guidelines when discussing treatment options with patients.
Conclusions
CRA tools should be incorporated into preoperative treatment counseling and the assessment of postoperative outcomes. While retrospective evidence supports the use of CRA tools for BC, further comparative studies evaluating the effectiveness of these tools and identifying the patients most likely to benefit from a treatment according to competing-risks assessment are needed.
Patient summary
In this review we explored the clinical evidence for comorbidity risk assessment tools in bladder cancer. We found evidence to support incorporation of comorbidity risks into practice guidelines when discussing treatment options with patients.
Keywords: Comorbidity, Competing risks, Bladder cancer, Models, Indices, Survival, Mortality, Review
1. Introduction
There will be an estimated 79 000 new cases and 17 000 deaths from bladder cancer in the USA in 2017 [1]. Neoadjuvant chemotherapy followed by radical cystectomy with extended pelvic lymphadenectomy is the guideline-recommended treatment for patients with muscle-invasive bladder cancer [2–4]. The European Association of Urology and the National Comprehensive Cancer Network recommend radical cystectomy for recurrent non–muscle-invasive and muscle-invasive bladder cancer, with trimodal therapy reserved for select patients [3,5,6]. Despite these longstanding guidelines, radical cystectomy is markedly underused [7], due at least in part to the advanced age and high rate of comorbidities in bladder cancer patients. Even with the apparent selection of only a minority of patients for treatment with radical cystectomy, this surgery is associated with a perioperative mortality rate between 2% and 13% [8,9].
The concern about performing this complex surgery in patients at high risk of complications and mortality has driven providers and patients to seek alternative treatments [10,11]. These patterns of practice suggest that both cancer-specific and all-cause mortality rates should be incorporated into patient counseling and guideline recommendations [7,10]. The literature suggests that risk assessment and prediction tools may enhance clinical decision-making and counseling of patients with bladder cancer [12]. Incorporation of comorbidity into preoperative assessments may improve preoperative prediction models and lead to implementation of preoperative interventions to improve outcomes [13]. The purpose of the present study was to perform a systematic review of the literature on preoperative comorbidity assessment tools for bladder cancer that may aid in treatment decision-making. Moreover, we wanted to identify which competing-risks assessment tool would be most suitable to aid in treatment counseling.
2. Evidence acquisition
A systematic literature search was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement to identify studies reporting on comorbidity risk assessment and bladder cancer between 1970 and 2017 [14]. A systematic review was conducted to identify studies of relevance for the predefined research questions. (1) Does comorbidity risk assessment improve outcomes in bladder cancer patients? (2) Does competing-risks assessment improve outcomes in bladder cancer patients? (3) Do bladder cancer patients with higher comorbidity and competing risks have greater complication rates and/or mortality compared to patients with lower comorbidity and competing risks? The Ovid interface of Medline was searched along with a free-text manual search using one or several combinations of the following items: (“bladder”) AND (“cancer” OR “carcinoma” OR “tumour” OR “tumor” OR “neoplasm” OR “malignancy” OR “mass”) AND (“localized” OR “metastatic” OR “metastasis”) AND (“comorbidity” OR “coexisting disease” OR “concomitant disease”). All selected articles were further searched to identify additional relevant articles. A total of 856 studies were initially identified. The selection process was conducted in three stages. The first stage involved initial screening of the title to identify eligible publications, including a search of publications in journals not listed in Medline to avoid missing any eligible study. In the second stage, publications were screened for eligibility according to the abstracts. The third stage comprised full-text reading of the articles. For this systematic review, we excluded: (1) non-English articles, (2) review articles (without systematic review or meta-analysis), (3) editorial reports and case reports, and (4) repeated publications to avoid publication bias. We decided to exclude review articles as the interpretation of published results without systematic assessment or meta-analysis of data does not offer significant novel insights into comorbidity risk assessment in relation to bladder cancer.
A total of 36 papers were finally considered for evidence synthesis (Table 1). Notably, these studies are retrospective and therefore inevitably inherit the risk of selection bias for which this review cannot control. A Consolidated Standards of Reporting Trials diagram is provided in Figure 1.
Table 1.
Overview of comorbidity indices used to predict complications
| Study | Sample size | Median | CI | Hazard ratio | Odds ratio for complications | AUC | |||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Total | RC | FU | 90-d mortality | OCM | CSM | ||||
| Malavaud 2001 [27] | 161 | 161 | NR | ASA | NR | NR | NR | 5.7 | NR |
| Novotny 2012 [28] | 830 | 830 | NR | ASA | NR | NR | NR | NR | 0.609 |
| Bostrom 2009 [29] | 258 | 258 | NR | ASA | NR | NR | NR | 3.25 [1.08–9.74] | NR |
| Roghmann 2014 [30] | 535 | 535 | NR | CCI ASA |
NR | NR | NR | Overall complications: CCI 1.93 High grade complications: CCI 1.86; ASA 1.92 |
NR |
| Fairey, 2008 [9] | 314 | 314 | NR | ACE-27 | ACE-27 severe: 6.4 | NR | NR | Overall complications: 5.2–7.0 High-grade complications: 11.4–15.2 |
NR |
ACE-27 = Adult Comorbidity Evaluation 27 score; ASA = American Society of Anesthesiology score; AUC = area under the receiver operating characteristic curve; CI = comorbidity index; CCI = Charlson CI; CSM = cancer-specific mortality; FU = follow-up; NR = not recorded; OCM = other-cause mortality; RC = radical cystectomy.
Figure 1.
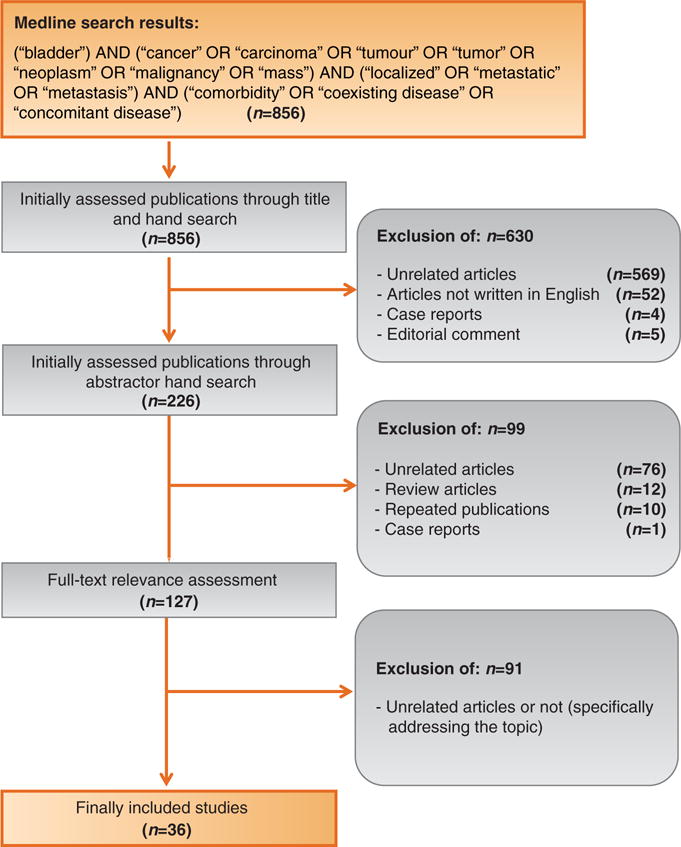
This Consolidated Standards of Reporting Trials diagram outlines the selection process of the included studies.
3. Evidence synthesis
3.1. Comorbidity and bladder cancer
3.1.1. Measures of comorbidity
Cancer patients often have comorbidities that may impact treatment decision-making, prognosis, and survival outcomes [15]. The comorbidity severity strongly influences survival in a dose-dependent fashion independent of cancer stage [15]. Bladder cancer patients present with significant competing risks, as patients are often elderly and/or have coexisting diseases that impact morbidity and mortality [7,10,16]. Radical cystectomy is associated with a significant risk of morbidity and mortality, with 5-yr overall and cancer-specific survival rates reported as approximately 50–60% and 60–70%, respectively [10,17–19]. Therefore, comorbidity and competing risks must be balanced with the benefits of treatment and inherent risks of surgery.
Individual comorbidities and combined comorbidity indices have been assessed for their ability to predict survival in bladder cancer patients undergoing radical cystectomy. Importantly, however, none of these comorbidity indices are exclusive to bladder cancer. These indices include the American Society of Anesthesiology (ASA) score, Adult Comorbidity Evaluation 27 (ACE-27) score, Charlson Comorbidity Index (CCI), Eastern Cooperative Oncology Group performance score (ECOG PS), Karnofsky Performance Status (KPS) scale, and the Elixhauser Index (EI).
The ASA score, which ranges from 1 (healthy) to 6 (brain dead), is the oldest evaluation score that assesses perioperative risk at the time of surgery. It was developed to predict mortality following general anesthesia and has been used as a method of estimating comorbidity in patients undergoing cancer surgery [19]. The ACE-27 is a 27-item comorbidity instrument validated for adult oncology patients enrolled in a hospital-based cancer registry [15]. The ACE-27 comprises 27 conditions classified into three grades according to severity, and has been extensively studied in numerous cancer types. The CCI was initially described in 1987 for general medical patients and has been converted into an age-adjusted index and the Klabunde modification using administrative claims data [20,21]. Indeed, the CCI is the most cited and most applied comorbidity index in the literature, including use in bladder cancer.
The ECOG PS is a classification of the performance status of cancer patients and runs from 0 to 5, with ECOG PS 0 describing full activity and ECOG PS 5 being equal to death [22]. Interestingly, the ECOG PS does not take any comorbidity into account, but rather only physical activity. The KPS, originally developed in 1948, was designed to measure the level of patient activity and medical care requirements among cancer patients and is a general measure of patient independence [23]. As with ECOG PS, KPS (measured on a scale from 0 = dead to 100 = normal, no complaints, no evidence of disease) does not measure comorbidity, but rather performance activity. The EI, similar to the Klabunde modification of the CCI, is a comprehensive set of 30 comorbidity measures for use with administrative data [24]. Unlike the CCI, the EI includes mental disorders, drug and alcohol abuse, obesity, coagulopathy, weight loss, and fluid and electrolyte disorders.
3.1.2 Comorbidity indices used to predict complications
It has been reported that up to 80% of patients experience a complication following radical cystectomy (open and robotic). Higher comorbidity rates have been associated with higher frequency and severity of perioperative complications [25,26]. While standardized reporting of complications using the five-grade and ten-domain modification of the Clavien-Dindo classification system is now commonplace when reporting complications, use of comorbidity indices remains heterogeneous when reporting associations and predicting which patients may be at greatest risk [26].
In one the earliest reports comparing the ASA score to complication risk, Malavaud et al [27] reported that an ASA score ≥3 was independently associated with a higher risk of major complications (odds ratio [OR] 5.7; p < 0.01; Table 1). Novotny et al [28] also found that patients with an ASA score of ≥3 versus ≤2 were significantly more likely to experience a postoperative complication following radical cystectomy (37% vs 25%; p < 0.05). Bostrom et al [29] studied 258 patients who underwent radical cystectomy and similarly identified ASA score ≥3 as an independent risk factor for major complications (OR 3.25, 95% confidence interval [CI] 1.08–9.74). Further, in a study comparing the associations of ASA score and CCI with the risk of complications, Roghmann et al [30] found that CCI ≥3 (OR 1.93) was associated with overall complications, while CCI ≥3 (OR 1.86) and ASA score ≥3 (OR 1.92) were associated with high-grade complications. In another study assessing the correlation between ACE-27 and perioperative complications, Fairey et al [9] noted that moderate and severe comorbidity were associated with any early postoperative complications (moderate: OR 5.2; p < 0.001; severe: OR 7.0; p < 0.001), major early postoperative complications (moderate: OR 11.4; p < 0.001; severe: OR 15.2; p < 0.001), and minor early postoperative complications (moderate: OR 2.1; p = 0.019; severe: OR 2.2; p = 0.038). Higher-severity comorbidity as assessed via the ACE-27 was independently associated with a higher risk of early postoperative complications after radical cystectomy.
The American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP) developed a generalizable surgical risk estimation tool (http://riskcalculator.facs.org) to provide risk estimates [31]. Variables used in the ACS NSQIP include age, sex, ASA score, functional status, and individual comorbidities. The tool exhibited excellent performance for mortality (c-statistic 0.944), morbidity (c-statistic 0.816), and complications (c-statistics >0.8) [31]. Golan et al [32] evaluated the ACS NSQIP tool in patients undergoing radical cystectomy. The universal ACS NSQIP calculator poorly predicted most postoperative complications by 10–81%, regardless of the urinary diversion type, suggesting the need for a procedure-specific risk calculator to better counsel cystectomy patients in the preoperative setting [32].
3.1.3. Comorbidity indices used to predict perioperative mortality
In one of the first studies to assess the impact of comorbidity on perioperative mortality, Fairey et al [9] evaluated the associations between age, comorbidity status according to ACE-27, and survival outcomes following radical cystectomy (Table 2). In multivariable logistic regression analysis, age was not associated with 90-d mortality. However, multivariable analysis adjusted for age and surgeon procedure volume demonstrated that severe comorbidity status (ACE-27 severe) compared to no or mild comorbidity was associated with a significantly higher risk of 90-d mortality (odds ratio [OR] 6.4, 95% CI 1.1–66.4; p = 0.03).
Table 2.
Overview of comorbidity indices used to predict perioperative mortality
| Study | Sample size
|
Median FU (yr) |
CI | Hazard ratio
|
Odds ratio for complications | AUC | |||
|---|---|---|---|---|---|---|---|---|---|
| Total | RC | 90-d mortality | OCM | CSM | |||||
| Fairey 2008 [9] | 314 | 314 | NR | ACE-27 | ACE-27 (severe): 6.4 | NR | `NR | Overall: 5.2–7.0 HG: 11.4–15.2 |
|
| Mayr 2012 [19] | 555 | 555 | NR | ACE-27, CCI, ACCI, ECOG, ASA | ACE-27: 1.72 ASA: 2.19 |
ASA 3–4: 1.59 ECOG 2–3: 2.02 ACE-27 2–3: 2.1 ACE-27 3: 1.55 CCI >2: 1.29 ACCI >5: 1.61 |
ASA 3–4: 2.70 ECOG 2–3: 3.07 ACE-27 2–3: 2.83 ACE-27 3: 2.65 CCI >2: 2.15 ACCI >5: 3.52 |
NR | ACE-27: 0.761 ASA: 0.761 |
| Boorjian 2013 [35] | 891 | 891 | 10.1 | ASA, CCI, EI, ECOG | ASA: 3.17 ECOG: 2.4 EI: 1.48 |
ASA: 1.44 ECOG: 1.97 |
NR | NR | CCI: 0.798 EI: 0.770 ECOG: 0.769 |
| Dell’Oglio 2017 [34] | 7076 | 7076 | NR | CCI, SF-CCI | NR | NR | NR | NR | CCI: 0.684 SF-CCI: 0.697 |
ACE-27 = Adult Comorbidity Evaluation 27 score; ACCI = age-adjusted CCI; ASA = American Society of Anesthesiology score; AUC = area under the receiver operating characteristic curve; CCI = Charlson CI; CI = comorbidity index; CSM = cancer-specific mortality; ECOG = Eastern Cooperative Oncology Group performance score; EI = Elixhauser Index; FU = follow-up; HG = high grade; NR = not recorded; OCM = other-cause mortality; RC = radical cystectomy; SF-CCI = short-form CCI.
Mayr et al [33] compared the associations of ACE-27, CCI, ECOG PS, and ASA score with the risk of 90-d perioperative mortality among 555 patients who underwent radical cystectomy. All four indices were significantly associated with perioperative mortality, with ACE-27 (OR 1.72; p = 0.004; area under the receiver operating characteristic curve [AUC] 0.761) and ASA score (OR 2.19; p = 0.004; AUC 0.761) having the strongest correlation. The authors concluded that the ASA score is the preferred method for assessing perioperative mortality risk because of its ease of use and incorporation at the time of surgery. Eisenberg et al [10] similarly assessed the ability of the ASA score, CCI, EI, and ECOG PS to predict 90-d perioperative mortality following radical cystectomy and noted that ASA score (OR 3.17; p = 0.001), ECOG PS (OR 2.4; p < 0.0001), and EI (OR 1.48; p = 0.002) were significantly associated with mortality.
Dell’Oglio et al [34] recently determined the feasibility of creating a short-form CCI to assess 90-d mortality following radical cystectomy. Within the development cohort, the most parsimonious and informative model resulted in inclusion of three of the 17 (17.6%) original comorbid condition groupings: congestive heart failure, cerebrovascular disease, and chronic pulmonary disease. Within the validation cohort, the accuracy was 68.4% for CCI versus 69.7% for the short-form CCI (Table 2).
3.1.4. Comorbidity indices used to predict overall and bladder cancer–specific survival
Overall and cancer-specific survival outcomes following radical cystectomy have remained unaltered over the last three decades, with 5-yr overall survival between 50% and 60% [17,19,35]. Numerous studies have confirmed the association between comorbidity status and survival outcomes following radical cystectomy [9,10,17,19,36–46]. However, no comorbidity index has demonstrated superiority in predicting survival outcomes.
Megwalu et al [47] used the ACE-27 in one of the first studies to assess the impact of comorbidity on survival outcomes (Table 3). On multivariable analysis for 675 patients with newly diagnosed bladder cancer, comorbidity (p = 0.0001), tumor stage (p = 0.0001), age (p = 0.0001), and race (p = 0.0045) were significantly associated with overall survival. On analysis of the subset of patients who underwent cystectomy, comorbidity (p = 0.0053), stage (p = 0.0001), and race (p = 0.0449) significantly predicted overall survival. Several studies have confirmed that CCI, age-adjusted CCI, and ACE-27 are independently associated with survival outcomes, suggesting that comorbidity should be considered when comparing outcomes after radical cystectomy [17,41].
Table 3.
Overview of comorbidity indices used to predict overall and bladder cancer–specific survival
| Study | Sample size
|
Median FU |
CI | Hazard ratio
|
ORC | AUC | |||
|---|---|---|---|---|---|---|---|---|---|
| Total | RC | 90-d mortality | OCM | CSM | |||||
| Megwalu 2008 [47] | 675 | 210 | 45 mo | ACE-27 | NR | Overall: 1.39 RC: 1.36 |
NR | NR | Bootstrap: 0.636 (overall) |
| Mayr 2012 [19] | 555 | 555 | 28 mo | ACE-27, CCI, ACCI, ECOG, ASA | NR | >75 yr or ASA 3–4 and ACE-27 >1: 3.34 >75 yr and ASA 3–4 and ACE-27 >1: 6.89 |
No significant predictors | NR | 0.810 |
| Boorjian 2013 [35] | 891 | 891 | 10.1 yr | ASA, CCI, EI, ECOG | ASA: 3.17 ECOG: 2.4 EI: 1.48 |
ASA: 1.44 ECOG: 1.97 |
NR | NR | CCI: 0.798 EI: 0.770 ECOG: 0.769 |
| Miller 2003 [45] | 106 | 106 | 3.2 yr | CCI | NR | 1.26 | 1.26 | NR | NR |
| Froehner 2017 [46] | 932 | 932 | 7.0 yr | ASA | NR | ASA 3–4: 1.74 | NR | NR | NR |
ACE-27 = Adult Comorbidity Evaluation 27 score; ACCI = age-adjusted CCI; ASA: American Society of Anesthesiology score; AUC = area under the receiver operating characteristic curve; CCI = Charlson CI; CI = comorbidity index; CSM = cancer-specific mortality; ECOG = Eastern Cooperative Oncology Group performance score; EI = Elixhauser Index; FU = follow-up; NR = not recorded; OCM = other-cause mortality; ORC = odds ratio for complications; RC = radical cystectomy.
Comorbidity indices have also been compared against one another to assess their relative association with survival outcomes. For example, Mayr et al [19] evaluated the correlation of ACE-27, CCI, age-adjusted CCI, ECOG PS, and ASA score with survival. All the indices were independently associated with cancer-independent but not with cancer-specific mortality. The ASA score was the only index that significantly increased the predictive accuracy of the predefined cancer independent model (+2.3%; p = 0.045), and was thereby suggested as the instrument of choice.
A separate study by Eisenberg et al [10] investigated the comparative prognostic ability of CCI, ECOG PS, EI, and ASA score with regard to 5-yr all-cause mortality following radical cystectomy. CCI (hazard ratio [HR] 1.23; p < 0.0001), EI (HR 1.28; p < 0.0001), ASA score (HR 1.44; p = 0.007), and ECOG PS (HR 1.97; p < 0.0001) were independent predictors of 5-yr all-cause mortality. Moreover, CCI (AUC 0.798; p < 0.0001), EI (AUC 0.770; p = 0.03), and ECOG PS (AUC 0.769; p = 0.03) significantly enhanced the performance of a base model that did not include comorbidity status (AUC 0.757) in predicting 5-yr all-cause mortality.
Miller et al [45] assessed the impact of concurrent medical disease on tumor control and survival following radical cystectomy. As expected, CCI was significantly associated with lower disease-specific (p = 0.049) and overall (p = 0.016) survival. Interestingly, in their multivariate model, CCI was independently associated with lower cancer-specific survival (p = 0.049) and a higher risk of extravesical disease (p = 0.033), suggesting that comorbid illness may be associated with adverse pathologic outcomes.
Froehner et al [46] identified age, angina pectoris, chronic lung disease, diabetes mellitus, current smoking, ASA score 3–4, and male sex as independent predictors of competing mortality in 932 consecutive patients who underwent radical cystectomy at a single institution. Similar to other single-institution series, bladder cancer was the cause of death when uncontrolled disease progression was present at the time of death. Deaths in the absence of uncontrolled bladder cancer were considered deaths from competing causes. The combined mortality index was superior to the age-adjusted CCI and Lee Mortality Index (LMI), which stratifies patients into risk groups, with 0% 10-yr competing mortality in the lowest and approximately 50% in the highest-risk classes [46].
3.2. Comorbidity risk assessment tools for bladder cancer
Comorbidity indices have been examined to discern associations with complications and survival outcomes. Based on this understanding, prognostic models using these indices have been developed into nomograms and calculators for predicting outcomes. While several prognostic models have been developed, these tools have largely relied on pathological characteristics [13,48,49]. The association between comorbidity and outcomes after radical cystectomy using patient, tumor, and treatment factors has been evaluated using a variety of predictive models. As highlighted by Taylor et al [50], models incorporating only age and CCI have yielded similar discrepancy in predicting survival outcomes following radical cystectomy to that of models developed from pathologic characteristics alone (Isbarn nomogram). Thus, the exclusion of important preoperative comorbidity variables probably limits the performance characteristics of nomograms designed to predict outcome after radical cystectomy. Furthermore, some nomograms have limited utility in the preoperative setting because of their dependence on postoperative pathological stage [50].
In the only study to incorporate the ACE-27, Fairey et al [17] found that severe comorbidity (ACE-27 severe) compared to no or mild comorbidity was independently associated with overall survival (moderate: HR 1.59, 95% CI 1.16–2.18; p = 0.004; severe: HR 1.83, 95% CI 1.22–2.72; p = 0.003) and bladder cancer–specific survival (moderate: HR 1.50, 95% CI 1.04–2.15; p = 0.028; severe: HR 1.65, 95% CI 1.04–2.62; p = 0.034; Table 4) [17]. Of note, the authors found higher 90-d postoperative mortality from the same center [9] which could contribute to the worse overall survival observed in this study [17]. While these results were not converted into a graphical aid, this study was one of the first to show that incorporation of a comorbidity index aids in prediction of overall and cancer-specific mortality.
Table 4.
Overview of comorbidity risk assessment tools for bladder cancer
| Study | Sample size
|
Median FU |
CI | Hazard ratio
|
ORC | AUC | |||
|---|---|---|---|---|---|---|---|---|---|
| Total | RC | 90-d mortality | OCM | CSM | |||||
| Taylor 2012 [11] | 1141 | 1141 | NR | CCI | NR | NR | NR | NR | Bootstrap: 0.702 |
| Fairey 2009 [17] | 523 | 523 | 31 mo | ACE-27 | NR | ACE-27 moderate: 1.59; severe: 1.83 | ACE-27 moderate: 1.50; severe: 1.65 | NR | NR |
| Eisenberg 2013 [10] | 2403 | 2403 | 10.5 yr | CCI, ECOG | NR | CCI: 1.73 ECOG: 1.51 |
CCI: 1.16 ECOG: 1.34 |
NR | Bootstrap: 0.75 |
| Froehner 2015 [37] | 735 | 735 | 7.8 yr | LMI | NR | 1.06 | NR | NR | NR |
| Morgan 2011 [52] | 220 | 220 | NR | CCI | 1.30 (NS) | NR | NR | NR | Model: 0.75 Bootstrap: 0.71 |
| Abdollah 2012 [53] | 12 274 | 12 274 | NR | CCI | CCI 3 vs 1: 2.86 | NR | NR | NR | 0.70 |
| Williams 2017 [54] | 6582 | 6582 | NR | CCI | NR | OS CCI 3 vs 1: 2.71 | CSS, CCI 3 v 1: 1.17 | NR | OS: 0.65 CSS: 0.66 |
ACE-27 = Adult Comorbidity Evaluation 27 score; AUC = area under the receiver operating characteristic curve; CCI = Charlson CI; CI = comorbidity index; CSM = cancer-specific mortality; CSS = cancer-specific survival; ECOG = Eastern Cooperative Oncology Group performance score; FU = follow-up; LMI = Lee Mortality Index; NR = not recorded; NS = not significant; OCM = other-cause mortality; ORC = odds ratio for complications; OS = overall survival; RC = radical cystectomy.
A majority of predictive tools have used the CCI as a proxy for assessing comorbidity using either large single-center or nationwide cancer registry data sets. Eisenberg et al [10] used clinicopathological variables, including the CCI and ECOG PS, associated with radical cystectomy outcomes to develop the SPARC (Survival Prediction After Radical Cystectomy) score to predict bladder cancer–specific mortality. On multivariate analysis, CCI (HR 1.8; p < 0.0001) and ECOG PS (HR 1.9; p < 0.0001) were significantly associated with bladder cancer–specific mortality. The cumulative scores for these variables in stratifying patients into risk groups had estimated 5-yr cancer-specific survival of 95%, 80%, 60%, 38%, and 23% for groups with the lowest to highest risk, respectively (p < 0.001). Bootstrap internal validation of the model had a concordance index of 0.75. Froehner et al [37] used the LMI [51] developed in the Health and Retirement Study to assess comorbidity for older adults undergoing radical cystectomy. The LMI applies risk points for age, male sex, current tobacco use, body mass index <25 kg/m2, diabetes mellitus, non-skin cancers, chronic lung disease, congestive heart failure, and four functional categories [51]. Beside the age-adjusted CCI, the LMI was an independent predictor of overall mortality (HR per unit increase 1.06; p = 0.042) and replaced the age-adjusted CCI as a predictor of competing mortality (HR per unit increase 1.27; p < 0.001). The authors concluded that the LMI was at least comparable to the age-adjusted CCI as a predictor of mortality after radical cystectomy. Morgan et al [52] developed a nomogram using the CCI and serum albumin to predict 90-d mortality among 220 patients following radical cystectomy (n = 28 died) at the University of Michigan. The model c-index was 0.75, and after 200 bootstrap resamples for internal validation the adjusted c-index was 0.71. This model used albumin and highlights the potential importance of using such nutritional/laboratory parameters in comorbidity assessments and risk stratification.
Comorbidity assessment tools have been developed using large population-based cancer registries. Abdollah et al [53] divided radical cystectomy patients in Nationwide Inpatient Sample (NIS) into discovery and validation cohorts to develop a model for predicting in-hospital postoperative mortality. Mortality increased with age (≤59 yr 0.6%, 60–69 yr 1.6%, 70–79 yr 3.1%, ≥80 yr 4.6%; p < 0.001) and CCI (CCI 0 1.7%, CCI 1 3.0%, CCI 2 4.2%, CCI 3 4.3%, CCI ≥4 12.1%; p < 0.001). A reference table was developed and validated using the NIS validation cohort and had an AUC of 70%. Most recently, Williams et al [54] developed and validated a nomogram predicting 3-yr and 5-yr overall and all-cause mortality following radical cystectomy using Surveillance, Epidemiology and End Results-Medicare (n = 5325) and Texas Cancer Registry-Medicare (n = 1257) linked data. Comorbidity was assessed using the Klabunde modification of the CCI [21]. The nomogram predicted 3-yr and 5-yr overall and cancer-specific survival rates with concordance indices of 0.65 and 0.66, respectively, in the validation Texas Cancer Registry-Medicare cohort. This generalizable instrument has been converted into an online instrument called the Radical Cystectomy Survival Calculator available at www.utmb.edu.libux.utmb.edu/surgery/urology/RCSC.asp (Table 1).
3.3. Is there a need for a bladder cancer–specific comorbidity index?
It has been shown that cancer-specific comorbidity indices impact the prediction of outcomes in patients with other cancers [55]. All of the current indices reported for bladder cancer patients as described above are either not specific for cancer or do not incorporate comorbidities, which may limit their impact in the bladder cancer population. Clinicians must strive to gain a more precise understanding of the comorbidity risk profile among bladder cancer patients and its corresponding impact on outcomes. Moreover, given that the majority of patients are elderly with higher comorbidity compounded by the significant risk of morbidity and mortality of treatments, a bladder cancer–specific comorbidity index may have a more profound impact when compared to other cancer-specific indices.
3.4. Other measures of comorbidity to consider
While advanced age has been associated with adverse postoperative outcomes following surgery, studies have identified sarcopenia and patient frailty as potentially more accurate predictors of adverse postoperative outcomes [56,57]. Sarcopenia is defined as severe muscle-wasting according to sex-specific skeletal muscle index definitions based on 2 × standard deviations below the norm that is reproducible on axial computed tomography imaging [58]. The presence of sarcopenia among radical cystectomy patients was independently associated with worse cancer-specific and all-cause mortality [57]. Frailty can be defined as a clinical syndrome in which three or more of the following criteria are present: unintentional weight loss (10 lbs in the previous year), self-reported exhaustion, weakness (grip strength), slow walking speed, and low physical activity [59]. Frailty incorporates functional status and medical comorbidities [60]. It has been demonstrated that patients who are frail are more likely to experience major in-hospital complications and worse survival [56,61,62]. Chappidi et al [56] found that higher frailty as measured with the modified frailty index was an independent predictor of high-grade (Clavien grade 4 or 5) complications (OR 2.58, 95% CI 1.47–4.55) among patients undergoing radical cystectomy.
It has been found that nutritional status, including preoperative serum albumin, enhances the performance of comorbidity indices in predicting survival [52]. Immunonutrition, which includes arginine-enriched supplements administered perioperatively to ameliorate complications and improve outcomes, is being extensively studied [63]. Arginine deficiency induced by surgery impairs lymphocyte proliferation and T-cell receptor integrity, which lower resistance to infection [63]. Thus, improving immune function via immunonutrition may modulate the immune response to surgery. A recent study among patients who received immunonutrition revealed reductions of 33% in postoperative complication rates (95% CI 1–64%; p = 0.060) and 39% in infection rates (95% CI 8–70%; p = 0.027) [63]. Other potential markers of comorbidity and malnutrition may include preoperative serum arginine, asymmetric dimethylarginine, and L-citrulline, which have been associated with adverse outcomes following surgery [64]. Myeloid-derived suppressor cells (MDSCs) in blood, which suppress T cells and lower resistance to infection, may serve as a marker for patients at greater risk of adverse outcomes [63]. MDSC numbers decrease following immunonutrition.
3.5. Is there a role for incorporation of comorbidity assessment into practice guidelines for bladder cancer?
There is greater awareness of comorbidity and competing-risks assessments for bladder cancer patients and their impact on prognosis and treatment decision-making. For patients with prostate cancer and many other cancers, competing-risks assessment has been incorporated into guideline recommendations [65,66], thereby increasing awareness of the important relationship between comorbidity and cancer treatment and outcomes. Bladder cancer has markedly higher mortality once it invades the muscle and some would argue that treatment regardless of comorbidity should be the mainstay. However, the delivery of different treatment modalities in a risk-adapted fashion may be a more appropriate approach when counseling patients. Thorough patient counseling that includes the risks and benefits of treatment in the context of disease characteristics and patient factors including comorbidities may aid in treatment decision-making. While prior generalizable and bladder cancer–specific tools have been developed for the postoperative setting [10,17,31,37,50,52,53], clinical applicability in the preoperative setting remains to be determined. As demonstrated with the universal ACS NSQIP tool, we need to develop bladder cancer–specific tools for better risk-adapted strategies for our patients. Moreover, as we move towards precision-based care at the molecular level, we must also recognize the potential of comorbidity indices and decision aids in improving treatment decision-making and outcomes.
4. Conclusions
To improve outcomes for our patients, we believe that comorbidity risk assessment tools should be incorporated into both preoperative treatment counseling and assessment of postoperative outcomes. Currently there are two tools: the ASA score, which is the preferred method for assessing the risk of complications and perioperative mortality because of its ease of use and incorporation at the time of surgery; and the CCI, which is the most widely studied index used to predict overall and cancer-specific survival. The CCI has been incorporated into comorbidity risk assessment tools including nomograms and calculators to aid in treatment counseling. While retrospective evidence supports the use of comorbidity risk assessment tools for bladder cancer, prospective comparative studies evaluating the effectiveness of these tools are needed and are being developed by our group.
Acknowledgments
Funding/Support and role of the sponsor: This study was conducted with the support of the Institute for Translational Sciences at the University of Texas Medical Branch, supported in part by a Clinical and Translational Science Award Mentored Career Development (KL2) Award (KL2TR001441) from the National Center for Advancing Translational Sciences, National Institutes of Health (NIH) (SBW). The study was also supported by the Herzog Foundation Scholar award (SBW). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The sponsors played a role in the design and conduct of the study; in data collection, management, analysis, and interpretation; and in preparation, review, and approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: Stephen B. Williams had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: All authors.
Acquisition of data: Williams.
Analysis and interpretation of data: All authors.
Drafting of the manuscript: All authors.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Williams.
Obtaining funding: Williams.
Administrative, technical, or material support: Williams.
Supervision: Williams, Kamat, Thalmann.
Other: None.
Financial disclosures: Stephen B. Williams certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Comorbidity risk assessment tools for bladder cancer are increasingly being explored. While retrospective evidence supports the use of comorbidity risk assessment tools for bladder cancer, further comparative studies evaluating the effectiveness of these tools and identifying patients most likely to benefit from a treatment according to competing-risks assessment are needed.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Witjes JA, Lebret T, Comperat EM, et al. Updated 2016 EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol. 2017;71:462–75. doi: 10.1016/j.eururo.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 3.Clark PE, Agarwal N, Biagioli MC, et al. Bladder cancer. J Natl Compr Cancer Netw. 2013;11:446–75. doi: 10.6004/jnccn.2013.0059. [DOI] [PubMed] [Google Scholar]
- 4.Chang SS, Bochner BH, Chou R, et al. Treatment of non-metastatic muscle-invasive bladder cancer: AUA/ASCO/ASTRO/SUO guideline. J Urol. 2017;198:552–9. doi: 10.1016/j.juro.2017.04.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gakis G, Efstathiou J, Lerner SP, et al. ICUD-EAU international consultation on bladder cancer 2012: radical cystectomy and bladder preservation for muscle-invasive urothelial carcinoma of the bladder. Eur Urol. 2013;63:45–57. doi: 10.1016/j.eururo.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 6.Mitin T, George A, Zietman AL, et al. Long-term outcomes among patients who achieve complete or near-complete responses after the induction phase of bladder-preserving combined-modality therapy for muscle-invasive bladder cancer: a pooled analysis of NRG Oncology/RTOG 9906 and 0233. Int J Radiat Oncol Biol Phys. 2016;94:67–74. doi: 10.1016/j.ijrobp.2015.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams SB, Huo J, Chamie K, et al. Underutilization of radical cystectomy among patients diagnosed with clinical stage T2 muscle-invasive bladder cancer. Eur Urol Focus. 2017;3:258–64. doi: 10.1016/j.euf.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Hollenbeck BK, Miller DC, Taub DA, et al. The effects of adjusting for case mix on mortality and length of stay following radical cystectomy. J Urol. 2006;176:1363–8. doi: 10.1016/j.juro.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 9.Fairey A, Chetner M, Metcalfe J, et al. Associations among age, comorbidity and clinical outcomes after radical cystectomy: results from the Alberta Urology Institute radical cystectomy database. J Urol. 2008;180:128–34. doi: 10.1016/j.juro.2008.03.057. [DOI] [PubMed] [Google Scholar]
- 10.Eisenberg MS, Boorjian SA, Cheville JC, et al. The SPARC score: a multifactorial outcome prediction model for patients undergoing radical cystectomy for bladder cancer. J Urol. 2013;190:2005–10. doi: 10.1016/j.juro.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 11.Taylor JM, Feifer A, Savage CJ, et al. Evaluating the utility of a preoperative nomogram for predicting 90-day mortality following radical cystectomy for bladder cancer. BJU Int. 2012;109:855–9. doi: 10.1111/j.1464-410X.2011.10391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kluth LA, Black PC, Bochner BH, et al. Prognostic and prediction tools in bladder cancer: a comprehensive review of the literature. Eur Urol. 2015;68:238–53. doi: 10.1016/j.eururo.2015.01.032. [DOI] [PubMed] [Google Scholar]
- 13.Isbarn H, Jeldres C, Zini L, et al. A population based assessment of perioperative mortality after cystectomy for bladder cancer. J Urol. 2009;182:70–7. doi: 10.1016/j.juro.2009.02.120. [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL., Jr Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291:2441–7. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- 16.Noon AP, Albertsen PC, Thomas F, Rosario DJ, Catto JW. Competing mortality in patients diagnosed with bladder cancer: evidence of undertreatment in the elderly and female patients. Br J Cancer. 2013;108:1534–40. doi: 10.1038/bjc.2013.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fairey AS, Jacobsen NE, Chetner MP, et al. Associations between comorbidity, and overall survival and bladder cancer specific survival after radical cystectomy: results from the Alberta Urology Institute Radical Cystectomy database. J Urol. 2009;182:85–92. doi: 10.1016/j.juro.2008.11.111. [DOI] [PubMed] [Google Scholar]
- 18.Mayr R, May M, Burger M, et al. The Charlson comorbidity index predicts survival after disease recurrence in patients following radical cystectomy for urothelial carcinoma of the bladder. Urol Int. 2014;93:303–10. doi: 10.1159/000362421. [DOI] [PubMed] [Google Scholar]
- 19.Mayr R, May M, Martini T, et al. Comorbidity and performance indices as predictors of cancer-independent mortality but not of cancer-specific mortality after radical cystectomy for urothelial carcinoma of the bladder. Eur Urol. 2012;62:662–70. doi: 10.1016/j.eururo.2012.03.057. [DOI] [PubMed] [Google Scholar]
- 20.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 21.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–67. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 22.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55. [PubMed] [Google Scholar]
- 23.Yates JW, Chalmer B, McKegney FP. Evaluation of patients with advanced cancer using the Karnofsky performance status. Cancer. 1980;45:2220–4. doi: 10.1002/1097-0142(19800415)45:8<2220::aid-cncr2820450835>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 24.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Shabsigh A, Korets R, Vora KC, et al. Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. Eur Urol. 2009;55:164–74. doi: 10.1016/j.eururo.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 26.Yuh BE, Nazmy M, Ruel NH, et al. Standardized analysis of frequency and severity of complications after robot-assisted radical cystectomy. Eur Urol. 2012;62:806–13. doi: 10.1016/j.eururo.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 27.Malavaud B, Vaessen C, Mouzin M, Rischmann P, Sarramon J, Schulman C. Complications for radical cystectomy. Impact of the American Society of Anesthesiologists score. Eur Urol. 2001;39:79–84. doi: 10.1159/000052416. [DOI] [PubMed] [Google Scholar]
- 28.Novotny V, Zastrow S, Koch R, Wirth MP. Radical cystectomy in patients over 70 years of age: impact of comorbidity on perioperative morbidity and mortality. World J Urol. 2012;30:769–76. doi: 10.1007/s00345-011-0782-0. [DOI] [PubMed] [Google Scholar]
- 29.Bostrom PJ, Kossi J, Laato M, Nurmi M. Risk factors for mortality and morbidity related to radical cystectomy. BJU Int. 2009;103:191–6. doi: 10.1111/j.1464-410X.2008.07889.x. [DOI] [PubMed] [Google Scholar]
- 30.Roghmann F, Trinh Q-D, Braun K, et al. Standardized assessment of complications in a contemporary series of European patients undergoing radical cystectomy. Int J Urol. 2014;21:143–9. doi: 10.1111/iju.12232. [DOI] [PubMed] [Google Scholar]
- 31.Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217:833–42. e1–3. doi: 10.1016/j.jamcollsurg.2013.07.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Golan S, Adamsky MA, Johnson SC, et al. National Surgical Quality Improvement Program surgical risk calculator poorly predicts complications in patients undergoing radical cystectomy with urinary diversion. Urol Oncol. 2018;36:77, e1–7. doi: 10.1016/j.urolonc.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 33.Mayr R, May M, Martini T, et al. Predictive capacity of four comorbidity indices estimating perioperative mortality after radical cystectomy for urothelial carcinoma of the bladder. BJU Int. 2012;110:E222–7. doi: 10.1111/j.1464-410X.2012.10938.x. [DOI] [PubMed] [Google Scholar]
- 34.Dell’Oglio P, Tian Z, Leyh-Bannurah SR, et al. Short-form Charlson comorbidity index for assessment of perioperative mortality after radical cystectomy. J Natl Compr Cancer Netw. 2017;15:327–33. doi: 10.6004/jnccn.2017.0032. [DOI] [PubMed] [Google Scholar]
- 35.Boorjian SA, Kim SP, Tollefson MK, et al. Comparative performance of comorbidity indices for estimating perioperative and 5-year all cause mortality following radical cystectomy for bladder cancer. J Urol. 2013;190:55–60. doi: 10.1016/j.juro.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 36.Hara T, Matsuyama H, Kamiryo Y, et al. Use of preoperative performance status and hemoglobin concentration to predict overall survival for patients aged ≥75 years after radical cystectomy for treatment of bladder cancer. Int J Clin Oncol. 2016;21:139–47. doi: 10.1007/s10147-015-0857-9. [DOI] [PubMed] [Google Scholar]
- 37.Froehner M, Koch R, Novotny V, et al. Lee mortality index as comorbidity measure in patients undergoing radical cystectomy. SpringerPlus. 2015;4:55. doi: 10.1186/s40064-015-0834-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Evers PD, Logan JE, Sills V, Chin AI. Karnofsky performance status predicts overall survival, cancer-specific survival, and progression-free survival following radical cystectomy for urothelial carcinoma. World J Urol. 2014;32:385–91. doi: 10.1007/s00345-013-1110-7. [DOI] [PubMed] [Google Scholar]
- 39.Lughezzani G, Sun M, Shariat SF, et al. A population-based competing-risks analysis of the survival of patients treated with radical cystectomy for bladder cancer. Cancer. 2011;117:103–9. doi: 10.1002/cncr.25345. [DOI] [PubMed] [Google Scholar]
- 40.Lund L, Jacobsen J, Clark P, Borre M, Norgaard M. Impact of comorbidity on survival of invasive bladder cancer patients, 1996–2007: a Danish population-based cohort study. Urology. 2010;75:393–8. doi: 10.1016/j.urology.2009.07.1320. [DOI] [PubMed] [Google Scholar]
- 41.Koppie TM, Serio AM, Vickers AJ, et al. Age-adjusted Charlson comorbidity score is associated with treatment decisions and clinical outcomes for patients undergoing radical cystectomy for bladder cancer. Cancer. 2008;112:2384–92. doi: 10.1002/cncr.23462. [DOI] [PubMed] [Google Scholar]
- 42.Weizer AZ, Joshi D, Daignault S, et al. Performance status is a predictor of overall survival of elderly patients with muscle invasive bladder cancer. J Urol. 2007;177:1287–93. doi: 10.1016/j.juro.2006.11.060. [DOI] [PubMed] [Google Scholar]
- 43.Prout GR, Jr, Wesley MN, Yancik R, Ries LA, Havlik RJ, Edwards BK. Age and comorbidity impact surgical therapy in older bladder carcinoma patients: a population-based study. Cancer. 2005;104:1638–47. doi: 10.1002/cncr.21354. [DOI] [PubMed] [Google Scholar]
- 44.Farnham SB, Cookson MS, Alberts G, Smith JA, Jr, Chang SS. Benefit of radical cystectomy in the elderly patient with significant co-morbidities. Urol Oncol. 2004;22:178–81. doi: 10.1016/j.urolonc.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 45.Miller DC, Taub DA, Dunn RL, Montie JE, Wei JT. The impact of co-morbid disease on cancer control and survival following radical cystectomy. J Urol. 2003;169:105–9. doi: 10.1016/S0022-5347(05)64046-3. [DOI] [PubMed] [Google Scholar]
- 46.Froehner M, Koch R, Heberling U, Novotny V, Hubler M, Wirth MP. An easily applicable single condition-based mortality index for patients undergoing radical prostatectomy or radical cystectomy. Urol Oncol. 2017;35:32, e17–23. doi: 10.1016/j.urolonc.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 47.Megwalu II, Vlahiotis A, Radwan M, Piccirillo JF, Kibel AS. Prognostic impact of comorbidity in patients with bladder cancer. Eur Urol. 2008;53:581–9. doi: 10.1016/j.eururo.2007.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sonpavde G, Khan MM, Svatek RS, et al. Prognostic risk stratification of pathological stage T2N0 bladder cancer after radical cystectomy. BJU Int. 2011;108:687–92. doi: 10.1111/j.1464-410X.2010.09902.x. [DOI] [PubMed] [Google Scholar]
- 49.Todenhofer T, Renninger M, Schwentner C, Stenzl A, Gakis G. A new prognostic model for cancer-specific survival after radical cystectomy including pretreatment thrombocytosis and standard pathological risk factors. BJU Int. 2012;110:E533–40. doi: 10.1111/j.1464-410X.2012.11231.x. [DOI] [PubMed] [Google Scholar]
- 50.Taylor JM, Feifer A, Savage CJ, et al. Evaluating the utility of a preoperative nomogram for predicting 90-day mortality following radical cystectomy for bladder cancer. BJU Int. 2012;109:855–9. doi: 10.1111/j.1464-410X.2011.10391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee SJ, Lindquist K, Segal MR, Covinsky KE. Development and validation of a prognostic index for 4-year mortality in older adults. JAMA. 2006;295:801–8. doi: 10.1001/jama.295.7.801. [DOI] [PubMed] [Google Scholar]
- 52.Morgan TM, Keegan KA, Barocas DA, et al. Predicting the probability of 90-day survival of elderly patients with bladder cancer treated with radical cystectomy. J Urol. 2011;186:829–34. doi: 10.1016/j.juro.2011.04.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abdollah F, Sun M, Schmitges J, et al. Development and validation of a reference table for prediction of postoperative mortality rate in patients treated with radical cystectomy: a population-based study. Ann Surg Oncol. 2012;19:309–17. doi: 10.1245/s10434-011-1852-7. [DOI] [PubMed] [Google Scholar]
- 54.Williams SB, Huo J, Chu Y, et al. Cancer and all-cause mortality in bladder cancer patients undergoing radical cystectomy: development and validation of a nomogram for treatment decision-making. Urology. 2017;110:76–83. doi: 10.1016/j.urology.2017.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Daskivich TJ, Kwan L, Dash A, Saigal C, Litwin MS. An age adjusted comorbidity index to predict long-term, other cause mortality in men with prostate cancer. J Urol. 2015;194:73–8. doi: 10.1016/j.juro.2015.01.081. [DOI] [PubMed] [Google Scholar]
- 56.Chappidi MR, Kates M, Patel HD, et al. Frailty as a marker of adverse outcomes in patients with bladder cancer undergoing radical cystectomy. Urol Oncol. 2016;34:256, e1–6. doi: 10.1016/j.urolonc.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Psutka SP, Carrasco A, Schmit GD, et al. Sarcopenia in patients with bladder cancer undergoing radical cystectomy: impact on cancer-specific and all-cause mortality. Cancer. 2014;120:2910–8. doi: 10.1002/cncr.28798. [DOI] [PubMed] [Google Scholar]
- 58.Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–95. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 59.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol Ser A Biol Sci Med Sci. 2001;56:M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 60.Patel HD, Ball MW, Cohen JE, Kates M, Pierorazio PM, Allaf ME. Morbidity of urologic surgical procedures: an analysis of rates, risk factors, and outcomes. Urology. 2015;85:552–9. doi: 10.1016/j.urology.2014.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pearl JA, Patil D, Filson CP, et al. Patient frailty and discharge disposition following radical cystectomy. Clin Genitourin Cancer. 2017;15:e615–21. doi: 10.1016/j.clgc.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 62.Robinson TN, Wallace JI, Wu DS, et al. Accumulated frailty characteristics predict postoperative discharge institutionalization in the geriatric patient. J Am Coll Surg. 2011;213:37–42. doi: 10.1016/j.jamcollsurg.2011.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hamilton-Reeves JM, Bechtel MD, Hand LK, et al. Effects of immunonutrition for cystectomy on immune response and infection rates: a pilot randomized controlled clinical trial. Eur Urol. 2016;69:389–92. doi: 10.1016/j.eururo.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maas R, Dentz L, Schwedhelm E, et al. Elevated plasma concentrations of the endogenous nitric oxide synthase inhibitor asymmetric dimethylarginine predict adverse events in patients undergoing noncardiac surgery. Crit Care Med. 2007;35:1876–81. doi: 10.1097/01.CCM.0000277038.11630.71. [DOI] [PubMed] [Google Scholar]
- 65.Mohler JL, Armstrong AJ, Bahnson RR, et al. Prostate cancer, version 1.2016. J Natl Compr Cancer Netw. 2016;14:19–30. doi: 10.6004/jnccn.2016.0004. [DOI] [PubMed] [Google Scholar]
- 66.Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017;71:618–29. doi: 10.1016/j.eururo.2016.08.003. [DOI] [PubMed] [Google Scholar]


