Abstract
Previous evidence indicates that adiponectin possesses antifibrogenic activity in inhibiting liver fibrosis. Therapeutic strategies, however, are limited by adiponectin quaternary structure and effective concentrations in circulation. Here we postulate a novel molecular mechanism, whereby adiponectin targets focal adhesion kinase (FAK) activity and disrupts key features of the fibrogenic response. Adiponectin-null (Ad−/−) mice and wild-type littermates were exposed to either saline or carbon tetrachloride (CCl4) for 6 wk. CCl4-gavaged mice were also injected with attenuated adenoviral adiponectin (Ad-Adn) or Ad-LacZ for 2 wk. Hepatic stellate cells (HSCs) were treated with or without adiponectin to elucidate signal transduction mechanisms. In vivo delivery of Ad-Adn markedly attenuates CCl4-induced expression of key integrin proteins and markers of HSC activation: αv, β3, β1, α2(I) collagen, and α-smooth muscle actin. Confocal experiments of liver tissues demonstrated that adiponectin delivery also suppressed vinculin and p-FAK activity in activated HSCs. In vitro, adiponectin induced dephosphorylation of FAK, mediated by a physical association with activated tyrosine phosphatase, Shp2. Conversely, Shp2 knockdown by siRNA significantly attenuated adiponectin-induced FAK deactivation, and expression of TIMP1 and α2(I) collagen was abolished in the presence of adiponectin and si-FAK. Finally, we documented that either adiponectin or the synthetic peptide with adiponectin properties, ADP355, suppressed p-FAK in synthetic matrices with stiffness measurements of 9 and 15 kPa, assessed by immunofluorescent imaging and quantitation. The in vivo and in vitro data presented indicate that disassembly of focal adhesion complexes in HSCs is pivotal for hepatic fibrosis therapy, now that small adiponectin-like peptides are available.—Kumar, P., Smith, T., Rahman, K., Mells, J. E., Thorn, N. E., Saxena, N. K., Anania, F. A. Adiponectin modulates focal adhesion disassembly in activated hepatic stellate cells: implication for reversing hepatic fibrosis.
Keywords: FAK, Shp2, vinculin, APPL1, myofibroblasts
Recent cell and molecular research in hepatic fibrosis, the precursor for chronic liver disease and cirrhosis, has improved our understating of mechanisms of disease progression (1–5). However, antifibrotic treatment strategies, while vitally important not just to prevent end-stage liver disease but to prevent or reverse accumulation of abnormally stiff extracellular matrix (ECM) accumulation, in general, are not available for patient care at present. Adiponectin is a 30-kDa protein adipocytokine, primarily synthesized and secreted by white adipose tissue (6). Adiponectin is best known for its protective roles against insulin resistance, glucose intolerance, and leptin-mediated metabolic syndrome. Pluripotential antifibrogenic properties of adiponectin have been well documented, not only in liver but also in different organs, such as heart, kidney, and lung (7–9). Adiponectin signaling is mainly mediated through two adiponectin receptors, AdipoR1 and AdipoR2; both of these receptors are abundantly expressed in hepatic stellate cells (HSCs; ref. 10).
Activated HSCs, herein also termed myofibroblasts, are central to the fibrogenic response (11–13). A key target on which to focus in halting fibrosis progression is to disrupt bidirectional signals sent from the ECM to the activated HSC. In this regard, a recent report indicated that disrupting such a relationship in integrin αv-knockout mice offered protection from CCl4-induced fibrosis in various organs (14). Focal adhesion (FA) complexes interact with ECM proteins via integrins, the transmembrane proteins that serve as direct sensors of the integrity and composition of the ECM environment (15). Focal adhesion kinase (FAK) orchestrates assembly of FAs within myofibroblasts. The FAs are the internal sensors of information transmitted via integrins from the ECM; hence, FAs are known to play a pivotal role in perpetuating hepatic fibrogenesis. In this report, we considered whether adiponectin could modulate the bidirectional communication pathway between HSCs and ECM by inhibiting FAK activity, and in so doing would incapacitate normal FA assembly, denying HSC cytoskeletal protein communication with transmembrane integrins, and by extension, the dense, fibrillar ECM. We postulated that disruption of FA assembly would also inhibit key molecules associated with hepatic fibrosis; reverse fibrosis in vivo and, allow us to interfere with progression of fibrosis by inhibiting FAK phosphorylation in softer gel matrices in vitro, which mimic lower-stage fibrosis scores in vivo.
MATERIALS AND METHODS
Dulbecco's modified Eagle's medium (DMEM), trypsin-EDTA, and penicillin-streptomycin were all purchased from Invitrogen (Carlsbad, CA, USA). FBS was purchased from Atlanta Biologicals (Atlanta, GA, USA). Pronase, leptin, and collagenases were purchased from Sigma-Aldrich (St. Louis, MO, USA). Recombinant high-molecular-weight (HMW) human adiponectin was purchased from Biovendor (Candler, NC, USA). Antibodies FAK, p-FAK, Src homology region2:phosphatase 2 (Shp2), p-Shp2, adenosine monophosphate-activated protein kinase (AMPK), p-AMPK, adaptor protein containing pleckstrin homology domain, phosphotyrosine-binding domain, leucine zipper motif 1 (APPL1), and Src were purchased from Cell Signaling (Danvers, MA, USA). Antibodies targeting AdipoR1, AdipoR2, and p-Src were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies against α smooth muscle actin (α-SMA), desmin, and antiactin were obtained from Sigma-Aldrich (St. Louis, MO, USA). Antibody against vinculin was purchased from Abcam (Cambridge, MA, USA).
Animals and CCl4-induced liver fibrosis in mice
Eight-week-old male wild-type (WT) C57BL/6J and adiponectin-null (Ad−/−) mice were used for animal studies. Ad−/− mice were a generous gift from the laboratory of Dr. K. Walsh (Boston University School of Medicine, Boston, MA, USA). The mice were developed on a C57BL/6J background. Animals were cared for in accordance with protocols approved by the Animal Care and Use Committee of Emory University. Animals were housed in a temperature-controlled environment with a 12-h light-dark cycle. Animals were fed ad libitum with Purina Laboratory Chow (Ralston Purina, St. Louis, MO, USA) and water. We confirmed that adiponectin is not detected in the serum of Ad−/− mice by ELISA. The study included 4 groups of mice: control mice that received olive oil by gavage and intraperitoneal injection of sterile saline, mice that received CCl4 gavage, and two further subgroups, mice gavaged with CCl4 and injected with a recombinant attenuated adenovirus vector carrying Escherichia coli β-galactosidase gene [adenoviral LacZ (Ad-LacZ)], and mice gavaged with CCl4 and administered the recombinant attenuated adenoviral vector carrying the human adiponectin cDNA under the regulation of the CMV promoter [adenoviral adiponectin (Ad-Adn)]. WT and Ad−/− mice weighing 20–25 g were administered CCl4 (2 ml/kg) with olive oil (1:1 ratio) twice weekly by gavage for 6 wk. Mice were given tail vein injection, Ad-LacZ, or Ad-Adn (1×109 viral particles) every 4 d for 2 wk following 4 wk of CCl4 gavage. We confirmed that adiponectin at baseline was not detected in the serum of Ad−/− mice by ELISA but measured significant increases in the concentration of serum adiponectin following Ad-Adn injection (mouse adiponectin ELISA kit; Millipore, Billerica, MA, USA).
Production of recombinant adenovirus
The full-length cDNA encoding adiponectin (NM_004797) was subcloned in a Shuttle vector (pShuttle-CMV, AdEasy XL adenoviral vector system; Stratagene, La Jolla, CA, USA), and linearized by restriction enzyme PmeI digestion. Gel-purified linearized vector was transformed to pretransformed with the pAdEasy-1 plasmid in BJ5183-AD-1 competent cells (AdEasy XL adenoviral vector system; Stratagene). The AdEasy adenoviral vector is the attenuated human adenovirus serotype 5, which is replication defective by deletion of both the E1 and E3 genes. Hence, both the Ad-LacZ and Ad-Adn shared identical biological properties except for the adiponectin cDNA. Adenoviruses were propagated in AD293 cells purchased from Stratagene. Adenoviruses were purified with an Adeno-X virus purification kit (Clontech Laboratories, Mountain View, CA, USA), and the viral titers were determined with the Adeno-XMT rapid titer kit (Clontech Laboratories). We monitored potential injury from adenoviral injection by measuring serum transaminases.
Picro-Sirius red staining for collagen
Liver sections (5 μm) were deparaffinized and washed with double-distilled water. Deparaffinized sections were incubated for 60 min with picro-Sirius red solution (Abcam, Cambridge, MA, USA) followed by brief rinsing with acetic acid (0.05%). Sections were dehydrated by washing with absolute alcohol. Sections were observed under a light microscope. The collagen staining was quantified by using ImageJ software (U.S. National Institutes of Health, Bethesda, MD, USA).
RNA extraction and quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) analysis
Total RNA was extracted from liver tissue or HSCs using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA), and first-strand cDNA synthesis was performed using the Bio-Rad iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA), all according to the manufacturer's instructions. Equal amounts of cDNA were used for qRT-PCR. Primers used in the experiments were Col1α2: forward 5′-CTAGCCAACCGTGCTTCTCA-3′ and reverse 5′-TTGGTCAGCACCACCAATGT-3′; integrin β1: forward 5′-TGAAGAGCCGCCAGACCCG-3′ and reverse 5′-CATCTTTTCGCAGCGTCCGCC-3′; integrin αv: forward 5′-AAGCGCACAGCACAGCTCGG-3′ and reverse 5′-AACATCCTGGAGGACGTGCTGG-3′; integrin β3: forward 5′-TCCCTCCCCTCCGCAGGAAAA-3′ and reverse 5′-CCAACAACAACGCCCGCCAG-3′; vinculin: forward 5′-CGTCCGGGTTGGAAAAGAGA-3′ and reverse 5′-AAGGATTCCCCTAGAGCCGT-3′; FAK: forward 5′-GGCAGCTGCTTATCTTGACC-3′ and reverse 5′-TGATGCCCCTGACATCAGTA-3′; α-SMA: forward 5′-CCACCATGTACCCAGGCATT-3′ and reverse 5′-TGGAAGGTAGACAGCGAAGC-3′. qRT-PCR was performed using IQ SYBR Green Supermix (Bio-Rad), according to standard protocol. PCR cycle conditions were set at 95°C for 15 s, 55°C for 15 s, and 72°C for 20 s, for a total of 40 cycles. qRT-PCR assays were performed in triplicate using the Mastercycler ep realplex (Eppendorf, Hamburg, Germany), with internal controls (GAPDH or 18S).
Confocal microscopy for in vivo studies
Cryosections of 5-μm thickness were obtained from liver of WT and Ad−/− mice. Immunofluorescence staining was performed with antibodies against vinculin, p-FAK, and desmin. Briefly, frozen liver sections were air dried for 30 min at room temperature and fixed with 4% paraformaldehyde for 15 min, followed by permeabilization with 0.1% Triton X-100. To avoid nonspecific protein binding, liver sections were blocked with 5% normal goat serum. Primary antibodies vinculin (1:250) and desmin (1:400), or p-FAK Tyr576/577 (1:200) and desmin, were simultaneously incubated with individual sections overnight at 4°C. Immunostaining was detected with Alexa Fluor-conjugated secondary antibodies (Alexa Fluor 488 and 546 raised in two different hosts) for 1 h at 4°C. Slides were counterstained with To-Pro (Life Technologies, Grand Island, NY, USA) and mounted with ProLong Gold Antifade Reagent (Life Technologies). Images were acquired with a Zeiss Meta Confocal Laser Scanning Microscope System equipped with 488-nm Ar and 568-nm HeNe lasers (LSM510; Carl Zeiss, Bonn, Germany).
Isolation of rat hepatic stellate cells
Male Sprague-Dawley rats (400 g) were purchased from Charles River (Boston, MA, USA). All rats received humane care, and the Emory University Institutional Animal Care and Use Committee approved the protocol for HSC isolation from rats. Quiescent rat HSCs were isolated, as described previously, using a single-step density gradient following sterile portal vein perfusion of Pronase and collagenase. Fresh, primarily obtained quiescent HSCs were plated onto tissue culture plastic; after 5–7 d, they were monitored for activation and used only once for experimentation (10).
Protein lysates and Western blot procedure
HSCs, under treatment conditions for respective experiments, were washed with PBS and 200 μl of ice-cold RIPA buffer containing protease inhibitor (PI) cocktail (PhosStop; Roche Diagnostics, Indianapolis, IN, USA) on ice. Cells were scraped with a rubber spatula, and collected extracts were cleared by centrifugation at 14,000 g for 10 min at 4°C. Protein concentrations were determined by the Bradford reagent (Sigma-Aldrich) using BSA as a standard. Proteins were resolved by SDS-PAGE and transblotted onto PVDF membrane, and an immunoblot assay was performed by using antibodies specific to different experiments. Protein bands were detected using a HyGlo chemiluminescent HRP antibody detection reagent (Denville Scientific, South Plainfield, NJ, USA) and exposure to X-ray film (Kodak, Rochester, NY, USA). Densitometric analysis of resolved proteins was performed to quantify protein band intensity with αEaseFC 4.0.1 software (Alpha Innotech, San Leandro, CA, USA).
Immunoprecipitation (IP)
Whole-cell lysates (200 μg of protein) were precleared with 30 μl of protein A/G agarose beads (Santa Cruz Biotechnology) for 30 min at 4°C. Precleared supernatants were incubated overnight with 2 μg of antibody. Immune complexes were subsequently incubated with 30 μl of protein A/G agarose for 2 h at 4°C on a rocking platform. Agarose beads were washed 3 times with 1 ml of ice-cold RIPA lysis buffer, resuspended in 30 μl of 2× Laemmli sample buffer, and subjected to SDS-PAGE by Western blot assay using specific antibodies for various experiments.
siRNA down-regulation of Shp2 and FAK
Commercially available siRNAs for Shp2 and FAK were purchased (Sigma-Aldrich), with a minimum of three siRNAs pooled for transfection experiments. HSCs were seeded onto tissue culture plastic, grown to attain 30–40% confluence, and transfected with the pooled siRNAs for Shp2 using Lipofectamine RNAiMAX transfection reagent (Invitrogen), as per the manufacturer instructions. Scrambled siRNAs were transfected and served as controls. At 24 h after transfection, cells were serum starved (2% FBS) overnight, followed by HMW adiponectin (10 μg/ml) for 30 min. Untreated cells served as a control. Cell lysates were subjected to Western blot analysis using appropriate antibodies, as described previously.
Adiponectin receptors and knockdown in rat HSCs
AdipoR1, AdipoR2, and nontargeting shRNA plasmids were purchased from Sigma-Aldrich and were used to dissect the path by which adiponectin resulted in FAK dephosphorylation. Lentivirus was produced in house by using a standard protocol Briefly, lentivirus was produced by cotransfecting HEK-293FT cells with lentivirus packaging vectors (pCMV-dr891, VSV-G) and vectors encoding the gene of interest (pLKO.1-AdipoR1-puro or pLKO.1-AdipoR1-puro or pLKO.1-nonmammalian-puro) (Sigma-Aldrich) using Lipofectamine (Invitrogen) following the manufacturer's instructions. After 48 h of incubation, supernatants were collected, cell debris was pelleted by low-speed centrifugation (200 g for 5 min), and clarified supernatant was filtered by using a 0.45-μM filter (Millipore, Billerica, MA, USA). Viruses were concentrated by ultracentrifugation for 2 h, and the pellet was dissolved in PBS. Lentiviruses were stored at −80°C until use. Rat HSCs were seeded at 1 × 106 cells/plate in 100 mm2 tissue culture dishes. Cells at 50–70% confluence were incubated for 16 h with lentiviral particles (MOI=2) in the presence of polybrene (5 μg/ml). The infected cells were cultured in complete growth medium for 48 h, and stable clones were selected by culture for several days in complete growth medium containing 2 μg/ml puromycin. Receptor knockdown was confirmed by Western blot analysis.
In vitro kinase assay
Primary HSCs were lysed and immunoprecipitated with 2 μg of APPL1 antibody. Recombinant Shp2 (2 μg; Novus, Littleton, CO, USA) was added to the APPL1 immunoprecipitated lysate in 1× kinase buffer (Cell Signaling, Danvers, MA, USA) with 10 μM of ATP, followed by incubation at 30°C for 30 min. The kinase assay was terminated by adding 50 μl of 2× Laemmli sample buffer (16). Samples were analyzed by Western blot analysis with p-Shp2 Tyr542, total Shp2, and APPL1 antibodies.
Single-cell liver stiffness analysis
HSCs were cultured on glass coverslips with different stiffness of polyacrylamide gel matrix, as described previously by Wells and colleagues (17). Briefly, polyacrylamide gels were prepared with varying concentrations of bis-acrylamide (0.01-0.3%) and mixed with 7.5% acrylamide. The polyacrylamide was cross-linked with Sulfo-SANPAH (Pierce, Rockford, IL, USA) by 2-cycle (60 s) exposure to UV light. HSCs were seeded onto polyacrylamide gels overnight, followed by HMW adiponectin or ADP355 treatment for 6 h. ADP355 was a kind gift of Dr. Eva Surmacz (Temple University, Philadelphia, PA, USA; ref. 18) HSCs seeded onto glass coverslips coated with fibronectin served as a positive control for maximum stiffness. Quantification of p-FAK signal intensity was performed by scanning 8 groups of fields.
Statistical analyses
All statistical analyses were conducted using GraphPad Prism 5.04 for Windows (GraphPad Software, San Diego CA, USA). Data represent means ± sem of ≥3 independent experiments. Statistical significance was determined using the Student's t test. Values of P < 0.05 were considered statistically significant.
RESULTS
Ad-Adn rescue modulates FA complexes in Ad−/− mice
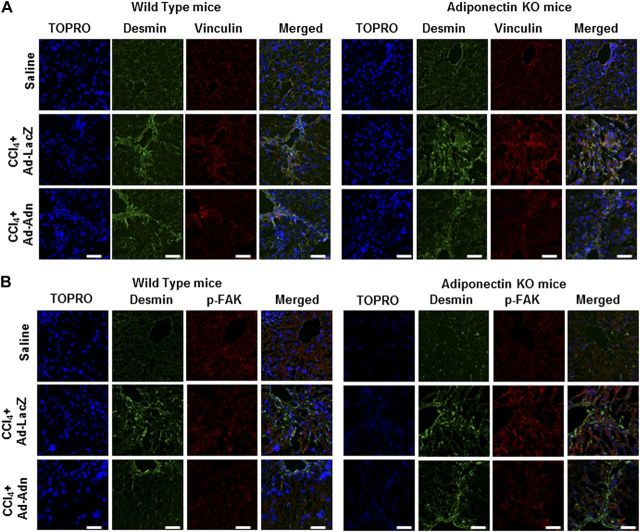
To investigate a therapeutic role for adiponectin in the context of disruption of FA complexes, we utilized WT C57BL/6J and littermate Ad−/− mice gavaged with CCl4 for 6 wk to induce liver fibrosis. We also transduced recombinant-deficient Ad-Adn, carrying full-length cDNA of adiponectin, and control Ad-LacZ with CCl4 at wk 4 for 2 wk. Virus injections occurred 2×/wk. Adenovirus-mediated adiponectin expression in WT and Ad−/− mice was monitored by serum ELISA. Preexperimental levels of adiponectin, as determined by ELISA, were not detectable in the Ad−/− mice, but significantly increased following Ad-Adn injections (WT/Ad-Adn 15.7±4.3 μg/ml vs. WT/Ad-LacZ 9.2±2.4 μg/ml; Ad−/−/Ad-Adn 8.4±5.3 μg/ml vs. Ad−/−/Ad-LacZ<0.05 μg/ml). As is evident in Fig. 1A, Sirius red staining shows significantly attenuated collagen fibers in mice gavaged with CCl4 and injected with Ad-Adn compared to mice only gavaged with CCl4 and mice gavaged with CCl4 and injected with Ad-LacZ; quantitative analysis is presented in Fig. 1B. There was no significant difference in aspartate aminotransferase values or microscopic injuries in the CCL4 + Ad-LacZ mice compared to the CCl4-gavaged mice but, these were significantly reduced after Ad-Adn injection (Fig. 1C). Following liver harvest, we performed qRT-PCR and Western blot analysis to determine the expression of integrin αv, β1, β3, vinculin, α-smooth muscle actin (α-SMA), and α2(I) collagen in the livers of the mouse cohorts (Fig. 1D, E). In CCl4-gavaged Ad−/− mice, we observed a significant increase in mRNA expression for integrins αv, β1, β3, and vinculin, as well as α-SMA and α2(I) collagen, compared to untreated Ad−/− mice (Fig. 1D). Conversely, Ad-Adn rescue in CCl4-gavaged mice significantly reduced mRNA expression for all integrins examined, vinculin, desmin, and α2(I) collagen (Fig. 1D). We performed confocal microscopy of liver sections stained with anti-vinculin from the respective mouse cohorts to determine whether vinculin activation was also inhibited by adiponectin rescue (Fig. 2A); furthermore, vinculin staining colocalized with desmin-positive cells, indicating that FA disassembly was specific to myofibroblasts (Fig. 2A). Although we observed enhanced vinculin staining in Ad−/− mice gavaged with CCl4, such staining was markedly suppressed by Ad-Adn rescue (Fig. 2A). Similar microscopy results were observed for activated p-FAK (p-FAK Tyr576/577), in liver tissues obtained from the different treatment groups; with significantly reduced staining from livers of Ad−/− mice following adiponectin rescue (Fig. 2B).
Figure 1.

Adiponectin attenuates CCl4-induced hepatic fibrosis in vivo. A) Sirius red staining of collagen in liver sections from mice gavaged with CCl4 followed by Ad-Adn delivery via tail vein. B) Staining was quantified, and densitometric analysis was performed (original view ×40). C) Serum aspartate aminotransferase (AST) levels from all 8 groups of mice. D) Hepatic mRNA expression of integrin αv, β3, β1, vinculin, α-SMA, and α2(I) collagen by qRT-PCR. E) Representative Western blot analysis of lysates obtained from liver tissues of WT and Ad−/− mice. *P < 0.05.
Figure 2.
Adiponectin modulates FA assembly in vivo. A) Desmin and vinculin staining of liver sections from WT and Ad−/− mice. Confocal images reveal increased vinculin and desmin staining in liver sections from CCl4-treated mice. Ad-LacZ fails to reduce staining intensity; however, Ad-Adn in CCl4-gavaged mice markedly attenuates vinculin and desmin staining. B) Confocal images of p-FAK and desmin from liver sections of WT and Ad−/− mice. In CCl4-gavaged mice, p-FAK and desmin staining increased, but following Ad-Adn rescue, staining intensity was markedly reduced. Scale bars = 50 μm (n≥4 mice/cohort).
Adiponectin suppressed vinculin expression in activated HSCs in vitro
Vinculin, which is a marker for cytoskeletal protein in the FA assembly (19), was also examined by an in vitro approach. We found that vinculin expression was markedly attenuated following HMW adiponectin treatment in myofibroblasts (Fig. 3A). In this experiment, leptin-treated myofibroblasts served as a positive control, and leptin robustly increased vinculin expression compared to cells left untreated. By contrast adiponectin-treated myofibroblast staining for vinculin was markedly attenuated (Fig. 3A). To provide physical evidence linking diminished FAK activity with vinculin, we determined whether adiponectin abolished the noncovalent association between FAK and vinculin. IP studies with anti-FAK antibody followed by Western blot analysis with anti-vinculin antibody revealed a significant reduction in the FAK-vinculin association following adiponectin treatment when compared to immunoprecipitated control extracts (Fig. 3B).
Figure 3.

Adiponectin disrupts FA assembly by inhibiting vinculin expression and dephosphorylation of FAK in activated rat HSCs in vitro. A) Activated HSCs were treated with adiponectin, followed by immunofluorescent staining (green, vinculin; red, F-actin). Control, plated primary activated HSCs; leptin, positive control. Scale bar = 20 μm. B) Western blot (WB) of vinculin after IP with anti-FAK from adiponectin-treated cells. C) Western blot with densitometric analysis of activated HSCs treated with adiponectin, demonstrating reduction in tyrosine phosphorylation of FAK. D) Adiponectin-induced Shp2 phosphorylation of Tyr542. E) Adiponectin-treated activated HSCs were subjected to IP for FAK, followed by Western blot analysis with anti-p-Shp2 and anti-FAK, demonstrating a physical association between FAK and Shp2. F) Lipofectamine transfection of HSCs with scrambled siRNA or pooled siRNA against Shp2 (si-Shp2). G) siShp2 knockdown restored FAK phosphorylation, despite the presence of adiponectin. H) mRNA and protein expression of TIMP1 and α2(I) collagen by qRT-PCR and Western blot analysis. Primary rat HSCs were transfected with scrambled siRNA or siFAK, followed by total RNA extraction, and mRNA expression was quantified. Densitometric analysis of band intensity for phosphorylated species was relative to total intensity, shown as relative density units (RDU). In siRNA experiments, β-actin was used as an internal control. Results are representative means ± se of experiments performed 3 times in triplicate. *P < 0.05.
Adiponectin-induced FAK dephosphorylation in activated HSCs in vitro
Western blot analyses from lysates of adiponectin-treated myofibroblasts revealed a marked reduction in p-FAK when compared with controls. To explore a potential mechanism for FAK dephosphorylation, we first determined the role c-Src could play to account for this observation. Since c-Src binds FAK and is permissive for FAK autophosphorylation through conformational change, we predicted that adiponectin would prevent FAK phosphorylation by suppressing c-Src expression. We then determined which FAK tyrosine residues adiponectin prevented from maintaining phosphorylation status, starting with the autophosphorylation residue Tyr397, followed by Tyr residues 925 and 576/577. Although we observed that adiponectin did invoke a decrease in autophosphorylation at FAK Tyr397, this was a transient effect, since Tyr397 phosphorylation was fully restored at 60 min (Fig. 3C). Furthermore, we found no change in c-Src protein expression during this time course (Fig. 3C). Taken together, these data suggested that prohibition of FAK autophosphorylation by c-Src could not fully explain our findings, since FAK Tyr925 and Tyr576/577, and therefore full FAK activity, were still permissive despite adiponectin treatment.
Adiponectin induced Shp2 phosphorylation
Previous studies have shown that Shp2 plays a direct role in dephosphorylation of FAK in different cell types (20, 21). Shp2 is a ubiquitously expressed tyrosine-specific protein phosphatase primarily responsible for downstream signaling of receptors for growth factors, cytokines, hormones, and extracellular matrices (22, 23). We found that adiponectin-treated myofibroblast lysates contained an abundance of activated Shp2, as assessed by the phosphorylation status of Shp2 at Tyr542 (p-Shp2; Fig. 3D), suggesting that Shp2 activation could be a relatively early event in adiponectin signaling. To analyze whether FAK dephosphorylation in myofibroblasts was a direct consequence of Shp2 activation, we used several approaches. First, we performed IP to demonstrate whether a physical association existed between p-Shp2 and FAK (Fig. 3E). We found that the FAK-p-Shp2 complex was markedly increased from lysates derived from adiponectin-treated cells (Fig. 3E) when compared to untreated control myofibroblast extracts.
Shp2 silencing attenuated adiponectin-mediated FAK dephosphorylation
We used pooled siShp2 constructs to knock down endogenous Shp2 phosphatase activity. Shp2 siRNA significantly decreased Shp2 expression (Fig. 3F), and also markedly increased FAK phosphorylation at Tyr576/577 compared to siRNA controls despite the presence of adiponectin (Fig. 3G). We also performed chemical inhibition of Shp2 activity with sodium stibogluconate (10 μM) and demonstrated in myofibroblasts that FAK Tyr576/577 phosphorylation was sustained in the presence of adiponectin, and neither mRNA or protein expression for FAK or Shp2 was affected (data not shown).
Finally, we developed siFAK molecules with a method similar to the pooled siShp2 constructs used in prior experiments to determine whether established markers related to hepatic fibrosis could be suppressed, as shown in Fig. 3G. Knockdown of FAK significantly reduced the expression of both tissue inhibitor of metalloproteinase 1 (TIMP1) and α2(I) collagen (Fig. 3H). Taken together, we established a link between adiponectin down-regulation of FAK and FA disassembly, as well as key molecules typically up-regulated in response to fibrogenic stimuli.
AdipoR1 plays a key role in adiponectin signaling associated with diminished FAK activity
To determine the upstream adiponectin-related signal transduction elements responsible for the endpoints observed, we performed IP studies to pull down either AdipoR1 or AdipoR2, since both AdipoR1 and AdipoR2 have biological capabilities to impart adiponectin signal transduction in the liver (24, 25). We also probed receptor lysates with an antibody against adapter protein APPL1, since APPL1 binds, without preference, to both receptors with equal affinity (Fig. 4A, B). In myofibroblast lysates, however, we found that adiponectin preferentially enhanced APPL1 activity via AdipoR1 (Fig. 4A). We then determined whether Shp2 and APPL1 were also noncovalently linked by performing an IP assay of myofibroblast lysates exposed to adiponectin for 30 min in culture, demonstrating adiponectin-enhanced APPL1 and p-Shp2 association (Fig. 4C), which was not observed in control extracts. Since adaptor proteins have pluripotent functions, we determined whether APPL1 could serve as a kinase that could directly phosphorylate Shp2. We performed an in vitro kinase assay by immunoprecipitating APPL1, followed by use of a recombinant Shp2 protein. Phosphorylated species of Shp2 were subsequently identified with anti-p-Shp2 (Fig. 4D) and demonstrated significant p-Shp2, indicating that APPL1 possessed ample kinase activity and phosphorylated Shp2 at Tyr542. Finally, to prove receptor initiation of the signaling cascade in adiponectin-mediated FAK deactivation, we performed in vitro knockdown of both AdipoR1 and AdipoR2 by lentiviral shRNA transfer in myofibroblasts (Fig. 4E, F). We achieved successful knockdown of both receptors and found that AdipoR1 knockdown significantly attenuated Shp2 phosphorylation (Fig. 4G), but AdipoR2 knockdown did not (Fig. 4G).
Figure 4.
APPL1 serves as a kinase for Shp2 activation driven by AdipoR1. A) Adiponectin-treated myofibroblast cell lysates were subjected to IP with anti-AdipoR1, followed by Western blot using anti-APPL1 and anti-AdipoR1. B) IP with anti-AdipoR2 and subsequent Western blot analysis with anti-APPL1 and anti-AdipoR2. C) Cell lysates were subjected to IP with anti-APPL1 followed by Western blot analysis with antip-Shp2 Tyr542, anti-Shp2, and anti-APPL1. D) In vitro kinase assay was performed to demonstrate APPL1 serves as kinase, capable of activating Shp2. Results shown with IP of APPL1 incubated with recombinant Shp2. Western blot antibodies included anti-Shp2 Tyr542, anti-Shp2, and anti-APPL1. E, F) Western blot analysis of shRNA knockdown of AdipoR1 and AdipoR2 by lentiviral vectors compared to nontargeted shRNA. G) Activated HSCs exposed to adiponectin followed by Western blot for p-FAK, p-Shp2, total FAK, and total Shp2. The knockdown of AdipoR1 restored FAK phosphorylation, as assessed by anti-p-FAK in Western blot analysis, which detects phosphorylated tyrosine residues 576/577. H) AICAR fails to induce Shp2 phosphorylation and dephosphorylation of p-FAK. Results represent means ± se of experiments performed 3 times in triplicate. *P < 0.05.
Since AMPK is thought to be the major upstream effector of adiponectin signal transduction, we used the potent agonist for AMPK (1 mM AICAR) to determine whether we could activate Shp2 and, in turn, deactivate FAK. As shown in Fig. 4H, however, AICAR activation of AMPK failed to alter the phosphorylation status of either Shp2 or p-FAK; that is, we could not deactivate FAK by the most potent AMPK activator in vitro.
Adiponectin diminished p-FAK activity in softer matrices than fibronectin
Rat HSCs were plated on glass coverslips with matrices of various stiffness, as described elsewhere by Wells and colleagues (17). Immunofluorescent imaging of individual HSCs grown on matrices with increased stiffness revealed HSC spreading with activation into myofibroblasts, and, as anticipated, p-FAK Tyr576/577 staining of individual HSCs intensified on increasingly stiffer matrices (Fig. 5A). On the other hand, treatment with adiponectin, or ADP355, the synthesized peptide agonist, suppressed FAK activity in myofibroblasts on matrices that were not as stiff as tissue culture plastic (in this experiment represented by glass coverslips coated with fibronectin). We found that adiponectin or ADP355 could substantially reduce p-FAK staining in myofibroblasts grown on softer matrices (9 and 15 kPa; Fig. 5A). We quantified these data as the ratio of p-FAK staining signal intensity in the presence and absence of adiponectin or ADP355 staining, as shown in Fig. 5B, demonstrating that adiponectin can abolish FAK activation in myofibroblasts grown on matrices corresponding to noncirrhotic (more compliant) liver tissue.
Figure 5.
Adiponectin prevents stiffness-associated FAK phosphorylation. Following HSC procurement from rat liver, cells were plated onto tissue culture plastic coated with gel matrices of increasing stiffness (0.4–15 kPa), as well as fibronectin. A) Untreated HSCs (top panels) or HSCs treated with ADP355 (middle panels) and adiponectin (bottom panels) for 6 h were analyzed by immunofluorescence microscopy (blue, DAPI nuclear staining; green, p-FAK Tyr576/577 staining) to determine presence of p-FAK. As can be seen, there was decreased staining intensity for p-FAK in adiponectin-treated HSCs, particularly at 9 and 15 kPa. Here, fibronectin served as positive control for maximal stiffness equivalent to tissue culture plastic. Original view was ×400. B) Densitometry of relative intensity of p-FAK staining following ADP355 or adiponectin treatment (middle and bottom panels in A) compared to controls (top panels in A). Images are representative of multiple independent experiments. *P < 0.05.
DISCUSSION
Significant progress has been made over the past 2 decades in elucidating molecular mechanisms related to hepatic fibrosis; however, translation of these findings into clinical practice, e.g., biomarkers and effective antifibrotic therapies, has lagged behind (11, 13, 26, 27). To date, only several phase II clinical trials with potential antifibrotic therapy are under way after surveying ClinicalTrials.gov, and only one phase I trial was found, which is using an intravenous galectin-3 antagonist (NCT01899859). Cirrhosis significantly increases a patient's risk for hepatocellular carcinoma (HCC; ref. 28), and the global epidemic of human obesity will likely result in an increased incidence of malignancy over time, including HCC (29, 30). In this report, we provide several lines of molecular and biochemical evidence concerning the role of FAs and adiponectin that are likely to be clinically relevant in the treatment of hepatic fibrosis in the near future (Fig. 6). We elucidated a molecular mechanism whereby adiponectin deactivates the critical kinase in FA assembly by inducing APPL1 kinase activity, which, in turn, phosphorylates Shp2. The activation of Shp2 then acts to dephosphorylate FAK, impairing FAK activity and ultimately preventing FA molecular assembly, as we have demonstrated both in vivo and in vitro by various approaches. Notably, FAK activity plays a critical role in enhancing profibrogenic genes in the myofibroblast, including TIMP1 and the αI collagens. We demonstrate that adiponectin down-regulates not only such genes, but also suppressed expression of the integrin αv, which Iredale and colleagues (14) recently reported, by using αv-targeted deletion mice, was also strongly protective against mammalian fibrosis, concluding that a pharmacological target should be developed against αv integrins. In a previous in vitro study, we overexpressed adiponectin in vitro, resulting in supraphysiological adiponectin concentrations that induced myofibroblast apoptosis (31). In the in vitro studies reported here, we used physiological concentrations of adiponectin added to culture in order to explore alternative mechanisms that might prohibit the fibrogenic cascade in the liver and be relevant to therapeutic studies.
Figure 6.
Summary of major findings. Adiponectin mediates disruption of FA complexes in activated HSCs. Adiponectin can bind to both receptors, but shAdiopR1 data reveal that AdipoR1 is responsible for downstream events related to FAK deactivation and FA disassembly by activating the adapter protein APPL1, which serves as a kinase, and results in the phosphorylation of Shp2. Activated Shp2, in turn, results in dephosphorylation of FAK, with deactivation of the kinase moiety, preventing or disrupting FA assembly. Adiponectin also suppresses expression of key integrins, and other proteins are critical for ECM deposition.
We also provide in vitro evidence that adiponectin prevents FAK phosphorylation. Studies performed with individually activated HSCs grown on matrices of varying stiffness indicate that administration of adiponectin offers a plausible method to deactivate FAK before maximal liver stiffness (as demonstrated in these studies with a coating of fibronectin) occurs. In addition to inhibiting the central player, FAK, in FA assembly, we demonstrated that adiponectin suppressed vinculin. Since FAK-activated vinculin is central to the scaffolding required for mature FA formation in activated HSCs, these findings, along with suppression of key integrins in vivo by adiponectin rescue, further support development of adiponectin therapy for arresting hepatic fibrosis. The absence of mature FAs would likely lead to a loss of communication between the myofibroblast and the ECM via the integrins, a process that by most accounts is required for fibrosis progression, as has been substantiated by other reports (14). Although other cell types in the liver could be responsive to the properties of adiponectin, ex vivo confocal microscopy of liver sections here demonstrate that reduced detection of key phosphorylated proteins essential for FA assembly, vinculin and p-FAK, was all associated with desmin-positive cells, specifying respective colocalization with activated HSCs (Fig. 2A, B). While the confocal images reported in Figs. 2A, 3A, and 5 are very convincing with respect to demonstrative colocalization, or clear targeting of p-FAK in individual cells (Fig. 5), we do recognize that the colocalization image of p-FAK and desmin is not as clear. We conducted the studies shown in Fig. 2A with numerous techniques. We also performed an extensive literature search of colocalization imaging of phosphorylated proteins in whole liver tissues, specifically regarding phosphorylated FAK; however, we did not find others reporting such data. While data for p-FAK colocalization images have been reported from single-cell images, the majority of studies that we reviewed used techniques to overexpress cellular p-FAK. Given the number of approaches that we provide regarding the role of adiponectin and p-FAK in our work and the balance of images displayed, we are confident that these tissue colocalization image experiments lend credence to the central hypothesis we set out to test.
We also provide evidence for a novel kinase function associated with the adaptor protein APPL1. APPL1 in adiponectin signaling typically activates AMPK (32). Here, we show that APPL1 not only binds the phosphatase Shp2, but also has a functional kinase moiety that can phosphorylate Shp2 rendering it an active phosphatase. To our knowledge, this is the first report in which APPL1 has been shown to possess active kinase activity for any substrate other than Akt (33), which adiponectin is known to phosphorylate, thereby improving insulin sensitivity (34). The fact that we could not activate Shp2 or dephosphorylate p-FAK by AICAR induction of AMPK merits further study, since recent data suggest that AMPK repressed IL-6-mediated p-Shp2 activity (35). The possibility exists that adiponectin conveys signals not associated with insulin resistance or metabolism by other classes of signaling molecules, particularly in cells that are not mature epithelial cells, in which much of these data were derived.
Our findings provide a novel mechanism with potentially important therapeutic implications targeting several molecular mechanisms. As discussed, therapies targeting integrins and ECM manipulation to inhibit the fibrogenesis are now in conceptual development or limited clinical trials. Our data raise the potential to explore adiponectin-related phosphatase activity-manipulating FA biology from an inside-out perspective of the HSC, as an alternative strategy for antifibrosis treatment. On the other hand, we recognize that synthetic adiponectin in clinical trials would be cost prohibitive, because achieving normal levels of circulating adiponectin in the healthy human would involve relatively high cost compared to other circulating adipocytokines. Also, an effective therapeutic concentration in humans has not been examined. We have recently acquired a small peptide, ADP355 (18), which we are further characterizing by ongoing in vivo and in vitro studies. As shown here, we have employed this peptide, which is inexpensive to synthesize and is relatively easy to administer, in the in vitro single-cell liver stiffness experiments. Figure 5 demonstrates nearly identical results between ADP355 and HMW adiponectin in reducing p-FAK activity in myofibroblasts grown on stiffer matrices. ADP355 was reported to have physiological characteristics of HMW adiponectin. From these data presented here, we are confident that this 10-decamer peptide could be used in future studies in conjunction with nanoparticle and by osmotic pumps for further preclinical testing. We are in the process of conducting studies in Ad−/− mice with nanoparticle conjugates to fully characterize ADP355. It is worth noting a recent report that identified a small-molecule AdipoR agonist with high receptor affinity that reversed obesity-related diseases such as type 2 diabetes mellitus by the investigators, who discovered adiponectin (36). We are also exploring whether adiponectin can down-regulate the sonic hedgehog (HH) pathway in myofibroblasts that Machado and Diehl (37) have implicated in hepatic fibrogenesis and repair. The ability to utilize a synthetic peptide targeted to liver stiffness that is clinically related to higher stages of fibrosis, but not cirrhosis, will undoubtedly be more widely available with the advent of advanced whole-liver imaging technologies. Elastography, either in conjunction with ultrasonography or magnetic resonance imaging, will make it possible for the clinician to ascertain advanced hepatic fibrosis without liver biopsy. Notably, targeted delivery of small peptides to reverse and deter fibrotic progression, in a manner similar to delivery of transarterial chemoembolization to destroy HCC, would offer a critical breakthrough. Adding to this potential therapeutic possibility, liver nanoparticle delivery may have enhanced uptake by activated HSCs, since emerging data indicate that HSCs may have phagocytic properties.
Acknowledgments
The authors thank Dr. Rebecca Wells and her laboratory staff (University of Pennsylvania, Philadelphia, PA, USA) for intellectual input with respect to experimental outcome and their assistance in helping with the glass coverslip experiments.
This work was supported by grants from the U.S. Public Health Service (DK062092), the U.S. Department of Veterans Affairs (I01BX001746), and Emory University, all awarded to F.A.A.
Footnotes
- Ad−/−
- adiponectin-null
- Ad-Adn
- adenoviral adiponectin
- AdipoR1/2
- adiponectin receptor 1/2
- Ad-LacZ
- adenoviral LacZ
- AMPK
- adenosine monophosphate-activated protein kinase
- APPL1
- adaptor protein containing pleckstrin homology domain, phosphotyrosine-binding domain, leucine zipper motif 1
- ECM
- extracellular matrix
- FA
- focal adhesion
- FAK
- focal adhesion kinase
- HCC
- hepatocellular carcinoma
- HMW
- high molecular weight
- HSC
- hepatic stellate cell
- IP
- immunoprecipitation
- qRT-PCR
- quantitative reverse transcriptase-polymerase chain reaction
- Shp2
- Src homology region2:phosphatase 2
- SMA
- smooth muscle actin
- TIMP
- tissue inhibitor of metalloproteinase
- WT
- wild type
REFERENCES
- 1.Henderson N. C., Iredale J. P. (2007) Liver fibrosis: cellular mechanisms of progression and resolution. Clin. Sci. , 265–280 [DOI] [PubMed] [Google Scholar]
- 2.Wang J., Leclercq I., Brymora J. M., Xu N., Ramezani-Moghadam M., London R. M., Brigstock D., George J. (2009) Kupffer cells mediate leptin-induced liver fibrosis. Gastroenterology , 713–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parsons C. J., Bradford B. U., Pan C. Q., Cheung E., Schauer M., Knorr A., Krebs B., Kraft S., Zahn S., Brocks B., Feirt N., Mei B., Cho M. S., Ramamoorthi R., Roldan G., Ng P., Lum P., Hirth-Dietrich C., Tomkinson A., Brenner D. A. (2004) Antifibrotic effects of a tissue inhibitor of metalloproteinase-1 antibody on established liver fibrosis in rats. Hepatology , 1106–1115 [DOI] [PubMed] [Google Scholar]
- 4.Saxena N. K., Titus M. A., Ding X., Floyd J., Srinivasan S., Sitaraman S. V., Anania F. A. (2004) Leptin as a novel profibrogenic cytokine in hepatic stellate cells: mitogenesis and inhibition of apoptosis mediated by extracellular regulated kinase (Erk) and Akt phosphorylation. FASEB J. , 1612–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qamar A., Sheikh S. Z., Masud A., Jhandier M. N., Inayat I. B., Hakim W., Mehal W. Z. (2006) In vitro and in vivo protection of stellate cells from apoptosis by leptin. Dig. Dis. Sci. , 1697–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berg A. H., Combs T. P., Scherer P. E. (2002) ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol. Metab. , 84–89 [DOI] [PubMed] [Google Scholar]
- 7.Christou G. A., Kiortsis D. N. (2014) The role of adiponectin in renal physiology and development of albuminuria. J. Endocrinol. , R49–R61 [DOI] [PubMed] [Google Scholar]
- 8.Sam F., Duhaney T. A., Sato K., Wilson R. M., Ohashi K., Sono-Romanelli S., Higuchi A., De Silva D. S., Qin F., Walsh K., Ouchi N. (2010) Adiponectin deficiency, diastolic dysfunction, and diastolic heart failure. Endocrinology , 322–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nigro E., Scudiero O., Sarnataro D., Mazzarella G., Sofia M., Bianco A., Daniele A. (2013) Adiponectin affects lung epithelial A549 cell viability counteracting TNFα and IL-1ss toxicity through AdipoR1. Int. J. Biochem. Cell. Biol. , 1145–1153 [DOI] [PubMed] [Google Scholar]
- 10.Handy J. A., Fu P. P., Kumar P., Mells J. E., Sharma S., Saxena N. K., Anania F. A. (2011) Adiponectin inhibits leptin signalling via multiple mechanisms to exert protective effects against hepatic fibrosis. Biochem. J. , 385–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman S. L. (2004) Mechanisms of disease: Mechanisms of hepatic fibrosis and therapeutic implications. Nat. Clin. Pract. Gastroenterol. Hepatol. , 98–105 [DOI] [PubMed] [Google Scholar]
- 12.Dechene A., Sowa J. P., Gieseler R. K., Jochum C., Bechmann L. P., El Fouly A., Schlattjan M., Saner F., Baba H. A., Paul A., Dries V., Odenthal M., Gerken G., Friedman S. L., Canbay A. (2010) Acute liver failure is associated with elevated liver stiffness and hepatic stellate cell activation. Hepatology , 1008–1016 [DOI] [PubMed] [Google Scholar]
- 13.Friedman S. L. (2010) Evolving challenges in hepatic fibrosis. Nat. Rev. Gastroenterol. Hepatol. , 425–436 [DOI] [PubMed] [Google Scholar]
- 14.Henderson N. C., Arnold T. D., Katamura Y., Giacomini M. M., Rodriguez J. D., McCarty J. H., Pellicoro A., Raschperger E., Betsholtz C., Ruminski P. G., Griggs D. W., Prinsen M. J., Maher J. J., Iredale J. P., Lacy-Hulbert A., Adams R. H., Sheppard D. (2013) Targeting of alpha integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat. Med. , 1617–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rovida E., Navari N., Caligiuri A., Dello Sbarba P., Marra F. (2008) ERK5 differentially regulates PDGF-induced proliferation and migration of hepatic stellate cells. J. Hepatol. , 107–115 [DOI] [PubMed] [Google Scholar]
- 16.Laemmli U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature , 680–685 [DOI] [PubMed] [Google Scholar]
- 17.Olsen A. L., Bloomer S. A., Chan E. P., Gaca M. D., Georges P. C., Sackey B., Uemura M., Janmey P. A., Wells R. G. (2011) Hepatic stellate cells require a stiff environment for myofibroblastic differentiation. Am. J. Physiol. Gastrointest. Liver Physiol. , G110–G118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Otvos L., Haspinger E., La Russa F., Maspero F., Graziano P., Kovalszky I., Lovas S., Nama K., Hoffmann R., Knappe D., Cassone M., Wade J., Surmacz E. (2011) Design and development of a peptide-based adiponectin receptor agonist for cancer treatment. BMC Bio/Technol. , 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Rossen E., Vander Borght S., van Grunsven L. A., Reynaert H., Bruggeman V., Blomhoff R., Roskams T., Geerts A. (2009) Vinculin and cellular retinol-binding protein-1 are markers for quiescent and activated hepatic stellate cells in formalin-fixed paraffin embedded human liver. Histochem. Cell Biol. , 313–325 [DOI] [PubMed] [Google Scholar]
- 20.Huang Y. S., Cheng C. Y., Chueh S. H., Hueng D. Y., Huang Y. F., Chu C. M., Wu S. T., Tai M. C., Liang C. M., Liao M. H., Chen C. C., Shen L. H., Ma K. H. (2012) Involvement of SHP2 in focal adhesion, migration and differentiation of neural stem cells. Brain Dev. , 674–684 [DOI] [PubMed] [Google Scholar]
- 21.Rafiq K., Kolpakov M. A., Abdelfettah M., Streblow D. N., Hassid A., Dell'Italia L. J., Sabri A. (2006) Role of protein-tyrosine phosphatase SHP2 in focal adhesion kinase down-regulation during neutrophil cathepsin G-induced cardiomyocytes anoikis. J. Biol. Chem. , 19781–19792 [DOI] [PubMed] [Google Scholar]
- 22.Li S., Hsu D. D., Wang H., Feng G. S. (2012) Dual faces of SH2-containing protein-tyrosine phosphatase Shp2/PTPN11 in tumorigenesis. Front. Med. , 275–279 [DOI] [PubMed] [Google Scholar]
- 23.Grossmann K. S., Rosario M., Birchmeier C., Birchmeier W. (2010) The tyrosine phosphatase Shp2 in development and Cancer Adv. Cancer Res. , 53–89 [DOI] [PubMed] [Google Scholar]
- 24.Denzel M. S., Scimia M. C., Zumstein P. M., Walsh K., Ruiz-Lozano P., Ranscht B. (2010) T-cadherin is critical for adiponectin-mediated cardioprotection in mice. J. Clin. Invest. , 4342–4352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asada K., Yoshiji H., Noguchi R., Ikenaka Y., Kitade M., Kaji K., Yoshii J., Yanase K., Namisaki T., Yamazaki M., Tsujimoto T., Akahane T., Uemura M., Fukui H. (2007) Crosstalk between high-molecular-weight adiponectin and T-cadherin during liver fibrosis development in rats. Int. J. Mol. Med. , 725–729 [PubMed] [Google Scholar]
- 26.Friedman S. L., Roll F. J. (1987) Isolation and culture of hepatic lipocytes, Kupffer cells, and sinusoidal endothelial cells by density gradient centrifugation with Stractan. Anal. Biochem. , 207–218 [DOI] [PubMed] [Google Scholar]
- 27.Rosenbloom J., Mendoza F. A., Jimenez S. A. (2013) Strategies for anti-fibrotic therapies. Biochim. Biophys. Acta , 1088–1103 [DOI] [PubMed] [Google Scholar]
- 28.Kitade M., Yoshiji H., Kojima H., Ikenaka Y., Noguchi R., Kaji K., Yoshii J., Yanase K., Namisaki T., Asada K., Yamazaki M., Tsujimoto T., Akahane T., Uemura M., Fukui H. (2006) Leptin-mediated neovascularization is a prerequisite for progression of nonalcoholic steatohepatitis in rats. Hepatology , 983–991 [DOI] [PubMed] [Google Scholar]
- 29.Shen C., Zhao C. Y., Zhang R., Qiao L. (2012) Obesity-related hepatocellular carcinoma: roles of risk factors altered in obesity. Front. Biosci. , 2356–2370 [DOI] [PubMed] [Google Scholar]
- 30.Mair M., Blaas L., Osterreicher C. H., Casanova E., Eferl R. (2011) JAK-STAT signaling in hepatic fibrosis. Front. Biosci. , 2794–2811 [DOI] [PubMed] [Google Scholar]
- 31.Ding X., Saxena N. K., Lin S., Xu A., Srinivasan S., Anania F. A. (2005) The roles of leptin and adiponectin: a novel paradigm in adipocytokine regulation of liver fibrosis and stellate cell biology. Am. J. Pathol. , 1655–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dadson K., Chasiotis H., Wannaiampikul S., Tungtrongchitr R., Xu A., Sweeney G. (2014) Adiponectin mediated APPL1-AMPK signaling induces cell migration, MMP activation, and collagen remodeling in cardiac fibroblasts. J. Cell. Biochem. , 785–793 [DOI] [PubMed] [Google Scholar]
- 33.Liu M., Zhou L., Wei L., Villarreal R., Yang X., Hu D., Riojas R. A., Holmes B. M., Langlais P. R., Lee H., Dong L. Q. (2012) Phosphorylation of adaptor protein containing pleckstrin homology domain, phosphotyrosine binding domain, and leucine zipper motif 1 (APPL1) at Ser430 mediates endoplasmic reticulum (ER) stress-induced insulin resistance in hepatocytes. J. Biol. Chem. , 26087–26093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bard-Chapeau E. A., Li S., Ding J., Zhang S. S., Zhu H. H., Princen F., Fang D. D., Han T., Bailly-Maitre B., Poli V., Varki N. M., Wang H., Feng G. S. (2011) Ptpn11/Shp2 acts as a tumor suppressor in hepatocellular carcinogenesis. Cancer Cell , 629–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nerstedt A., Cansby E., Amrutkar M., Smith U., Mahlapuu M. (2013) Pharmacological activation of AMPK suppresses inflammatory response evoked by IL-6 signalling in mouse liver and in human hepatocytes. Mol. Cell. Endocrinol. , 68–78 [DOI] [PubMed] [Google Scholar]
- 36.Okada-Iwabu M., Yamauchi T., Iwabu M., Honma T., Hamagami K., Matsuda K., Yamaguchi M., Tanabe H., Kimura-Someya T., Shirouzu M., Ogata H., Tokuyama K., Ueki K., Nagano T., Tanaka A., Yokoyama S., Kadowaki T. (2013) A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature , 493–499 [DOI] [PubMed] [Google Scholar]
- 37.Machado M. V., Diehl A. M. (2014) Liver renewal: detecting misrepair and optimizing regeneration. Mayo Clin. Proc. , 120–130 [DOI] [PubMed] [Google Scholar]