Abstract
Age is, by far, the greatest risk factor for Alzheimer's disease (AD), yet few AD drug candidates have been generated that target pathways specifically associated with the aging process itself. Two ubiquitous features of the aging brain are the intracellular accumulation of aggregated proteins and inflammation. As intraneuronal amyloid protein is detected before markers of inflammation, we argue that old, age-associated, aggregated proteins in neurons can induce inflammation, resulting in multiple forms of brain toxicities. The consequence is the increased risk of old, age-associated, neurodegenerative diseases. As most of these diseases are associated with the accumulation of aggregated proteins, it is possible that any therapeutic that reduces intracellular protein aggregation will benefit all.—Currais, A., Fischer, W., Maher, P., Schubert, D. Intraneuronal protein aggregation as a trigger for inflammation and neurodegeneration in the aging brain.
Keywords: neurodegenerative diseases, Alzheimer’s disease, cell division
Alzheimer's disease (AD) and most other neurodegenerative diseases are associated primarily with old age. We have argued previously that any development of efficient treatments for these diseases will require an understanding of the pathologic changes that take place during aging that precede the disease (1). Although these events are varied and complex, there is now strong evidence that the activation of inflammatory processes is a hallmark of all neurodegenerative diseases. Elevated levels of proinflammatory eicosanoids, cytokines, and chemokines, as well as activated microglia and astrocytes, are found in the AD brain, even at very early stages of the disease (2–8). In fact, low-grade chronic inflammation positively correlates with age, even in the absence of overt disease (8–11). For example, systemic levels of the proinflammatory mediators IL-6, C-reactive protein (CRP), and TNF-α increase 2- to 4-fold in the healthy elderly compared with young individuals (12, 13).
Several mechanisms have been proposed to explain the origins of this age-related, low-grade inflammation. The most prominent include the following: 1) endogenous inducers of inflammation that increase with aging, such as molecules and breakdown products of the extracellular matrix released as a consequence of tissue damage or malfunction [damage-associated molecular patterns (DAMPs)] (14); 2) cellular senescence, via the expression of the proinflammatory senescence-associated secretory phenotype (15, 16); 3) increases in the production of pro-oxidant molecules and concomitant decreases in antioxidant enzymes (17), perhaps via the activation of TLRs (18); 4) a decrease in the ability to resolve (terminate) acute-phase immune responses in the elderly compared with the young (9, 19), thus contributing to a chronic state of low-grade inflammation; one cause for this could be the reduction in anti-inflammatory endocannabinoids that occurs with aging (20); 5) abnormalities in mitochondrial function (1, 21–23); and finally, 6) alterations in lipid metabolism (24, 25).
Recently, we have shown that the intracellular accumulation of aggregated amyloid β (Aβ) in nerve cells themselves initiates an inflammatory cascade, mediated, in part, by the inflammasome. This sequence can lead directly to nerve cell death in culture and potentially contribute to overall inflammation-induced toxicity in the CNS (26). Our results add to published data showing that the expression of intraneuronal Aβ and proinflammatory cytokines precedes the appearance of extracellular plaques in transgenic AD rats, with some colocalization in neurons (27). These findings are important, as the loss of protein homeostasis is considered one of the hallmarks of aging (28), and alterations in pathways that control protein synthesis, folding, trafficking, aggregation, disaggregation, and degradation are ubiquitous in the old brain (29). The fact that both the intracellular accumulation of misfolded and aggregated proteins and CNS inflammation are elevated with age, and more so in neurodegenerative diseases, raises the possibility that there is a direct connection among aging, protein aggregation, inflammation, and neurodegeneration. Here, we will discuss how the systemic proteotoxicity characteristic of aging may be responsible for inducing the expression of proinflammatory molecules that contribute to toxicity in AD. We believe that understanding these interactions holds great potential for the development of new therapeutic interventions to treat neurodegenerative diseases.
THE ACCUMULATION OF MISFOLDED PROTEINS WITH AGING
In 1963, Leslie Orgel (30) proposed the error catastrophe hypothesis, a model describing the contribution of stochastic errors intrinsic to the protein synthesis machinery to the process of aging. According to this hypothesis, errors that lead to reduced specificity of information-handling enzymes cause a cumulative increase in error frequency that ultimately affects cell viability. The idea that cumulative damage to cellular components takes place with time is central to the study of aging, as cellular damage can be removed not only by specific maintenance mechanisms, but also, importantly, it can be diluted when cells divide and renew (1, 31). Therefore, when cell division slows down or halts, cellular damage is prone to increase.
Interestingly, there is evidence showing that asymmetric inheritance of damaged cellular components during cell division represents an evolutionarily conserved mechanism that favors a specific daughter cell by increasing its lifetime fitness (32, 33). The strategies underlying segregation appear to be well-regulated processes that often involve intracellular organelles (for reviews, see refs. 34–37). This idea of asymmetry giving rise to cells of unequal mortality stems from observations that specific, age-related factors segregate during cell division. This is the case in both the budding yeast Saccharomyces cerevisiae, with the accumulation of extrachromosomal ribosomal DNA circles (38) and the segregation of carbonylated proteins (39), and in higher eukaryotic cells, with the discovery of aggresomes. Aggresomes are pericentriolar inclusions of aggregated proteins that are asymmetrically inherited during mitosis in multiple cell types (40, 41). Moreover, increased protein carbonylation load in cells is associated with the loss of reproductive capability in bacteria (42), and old spindle cell pole segregation in yeast can determine aging traits (43).
However, not only can cell division dilute aspects of aging by physically distributing cellular material, such as damaged and misfolded proteins between two daughter cells, but it also leads to the synthesis of new biologic components in those two cells (44). Renewal is a critically important aspect in cell biology, as all cells are constantly eliminating and resynthesizing their components. Therefore, proteins with longer half-lives may be more susceptible to the accumulation of damage with age (45), and pulse-chase labeling experiments have indeed shown that some proteins are stable for a large number of years. These include extracellular matrix proteins, such as elastin and collagen, as well as intracellular proteins associated with the nucleus, such as histones and nucleoporins (45, 46). Interestingly, nucleoporins only disassemble from the nuclear pore complexes at the time of mitosis when the nuclear envelope breaks down, which ties protein renewal directly to cell division (45, 46).
The aggregation and accumulation of proteins during aging are ubiquitous to human tissues and prominent in age-related neurodegenerative diseases (47). The misfolding of proteins that precedes the aggregation and leads to insolubility is often a consequence of an age-dependent decrease in the activities of systems responsible for protein quality control. These systems include the molecular refolding machinery (e.g., molecular chaperones) and protein degradation processes (e.g., via proteasome or autophagy) (48). Damage in proteins caused by secondary modifications (e.g., glycation and oxidation) can also reduce their affinity for the maintenance systems and thereby, affect their aggregation (49).
β-Sheets, motifs of regular secondary structure governed by hydrogen bonds, are considered one of the most common structural elements of the aggregates. The level of organization of these β-sheets varies from unstructured, disordered aggregates to prefibrillar species to highly ordered β-sheet-rich amyloid fibrils (49). Most neurodegenerative diseases are associated with protein misfolding that leads to amyloid aggregation (50). In fact, the formation of amyloid structures reflects a well-defined structural form of the protein that is an alternative to its native state. In theory, this form can be acquired by many, if not all, polypeptide sequences (50).
PROTEIN ACCUMULATION IN THE BRAIN AFFECTS LIFESPAN AND CONTRIBUTES TO NEURODEGENERATIVE DISEASES
Perhaps the first indication of a direct correlation between protein aggregation in the brain and lifespan was based on the observation that flies accumulate detergent-insoluble, aggregated, and ubiquitinated proteins in their brains as a function of aging (51). It was then asked whether this small subset of all brain proteins contributes to lifespan by deleting them from the brain or allowing them to overaccumulate. It was shown that autophagy is responsible for the removal of the aggregated proteins and that by manipulating the expression of autophagy genes specifically in the brain, the amounts of these proteins could be reduced or enhanced. When the aggregated proteins were removed, the flies lived significantly longer, and when they were allowed to accumulate by reducing autophagy, the flies died sooner (51). This observation clearly shows that what happens in the brain can regulate lifespan. The accumulation of aggregated proteins in the brain with age could be explained by the observations that the efficiency of autophagy and the proteasome declines with age and that neurons do not divide (52, 53).
We have identified AD drug candidates that prevent the accumulation of aggregated proteins in cell cultures of human neurons, in flies, and in mice and extend the lifespan of both flies and mice (refs. 26, 54, and unpublshed results). Therefore, the accumulation of insoluble, ubiquitinated proteins in the brain correlates with both cell viability and lifespan.
A decreased lifespan also correlates with many neurologic diseases, of which, diseases associated with protein aggregation in nondividing cells or the extracellular space are among the most frequent. AD is perhaps the best studied. Other CNS diseases associated with protein aggregation include Parkinson's disease (PD), Huntington's disease, and Creutzfeldt-Jakob disease (reviewed in ref. 47). However, age-related protein aggregation is associated with many other tissues besides the CNS. One of these is sporadic inclusion-body myositis (s-IBM) (55). Like many CNS diseases, s-IBM is associated with inclusions containing Aβ and phosphorylated τ, as well as other proteins within nondividing myotubes. Importantly, s-IBM, as well as all of the conditions listed above, leads to a strong proinflammatory response that is enhanced over that of nonaffected individuals.
Because of the physiologic damage caused by the intracellular accumulation of unusual or nonfunctional proteins, cells have evolved a number of mechanisms to reduce this form of stress. One of the first protein stresses to be identified was heat shock, which induces the expression of a wide variety of chaperones to facilitate protein refolding (56). Associated with the induction of chaperones is the activation of the unfolded protein response (UPR), a complex process that both enhances endoplasmic reticulum (ER) function and reduces protein synthesis, facilitating the availability of chaperones (57, 58). Associated with the UPR is the integrated stress response, initiated by the phosphorylation of eukaryotic initiation factor 2α (eIF2α) (59). Phospho-eIF2α reduces protein synthesis and the accumulation of aggregated proteins and is neuroprotective (54). Similar to the ER, mitochondria also adapt to stress by mounting their own UPR that involves mitochondria-to-nucleus signaling (60). Finally, in parallel to the above responses to stress, cells also are able to increase the expression of the proteasome and induce autophagy, both of which clear damaged proteins (61). All of the above mechanisms for reducing the accumulation of damaged proteins decline with age (62), and it should be noted that the canonical antiaging pathway mediated by mammalian target of rapamycin inhibition also selectively reduces protein synthesis and induces autophagy (63).
In the context of disease, a key question is why normally soluble proteins misfold and aggregate late in life? In the case of heritable disease proteins, there is likely a simple mass action effect in which the gradual, old, age-associated accumulation of aggregated proteins interacts with the pool of overexpressed, aggregation-prone, disease-specific protein, leading to the cell's demise. In the case of nonheritable diseases, all alternatives that lead to impaired protein homeostasis should be considered, given that damaged proteins can be removed, not only by maintenance mechanisms, but also diluted when cells divide and renew (1, 31). It is possible that when cell division slows down or halts, as happens during the differentiation of complex organisms at the time of embryogenesis (reviewed in ref. 1), misfolded proteins become more prone to accumulate with time, eventually leading to disease when an extreme condition is reached. This situation could be more critical for postmitotic cells, such as differentiated, mature neurons.
PROTEOTOXICITY CAUSES INFLAMMATION
Essentially, all diseases of aging that are associated with protein aggregation and proteotoxicity coexpress a proinflammatory component. In both human disease and animal models, brain inflammation is usually defined in terms of gliosis and the infiltration of peripheral immune cells. Although traditionally regarded as an immediate, protective response to infections and injury, inflammation is also induced by tissue stress or malfunction (64, 65). It has been argued that extracellular Aβ in AD activates microglia and astrocytes that in turn release TNF-α and other cytokines. These molecules then bind to neurons, leading to their eventual elimination from the brain (66). However, as discussed previously (1), the activation of inflammatory pathways by disease-associated stress can occur in a cell-autonomous manner in a wide range of nonimmune cells, including neurons.
It is now well established that inflammation can lead to transient cognitive decline. A good example is the observation that older patients can have severe cognitive problems when they have a high fever and/or are suffering from postoperative inflammation (67). Therefore, whereas it is clear that inflammation can drive cognitive decline, it is less clear what is driving inflammation in the context of aging. There is a variety of mechanisms by which proteins and other molecules can trigger an inflammatory response. When an inflammatory pathway is activated in excess or out of context, it has the ability to cause disease. The primary function of the innate immune system and inflammation is to fend off pathogens. Activation of pattern recognition receptors (PRRs) leads to an inflammatory response, and among PRRs, the TLRs are the best described. The initiating event is the interaction of these receptors with molecules having either pathogen-associated molecular patterns or DAMPs. An example of the latter is free mitochondrial DNA that interacts with TLR9, NLR family, pyrin domain- containing 3, and stimulator of IFN genes (68).
In an insightful review, Clark and Vissel (69) argued that soluble Aβ can act as a DAMP to activate TLR4, leading to the expression of proinflammatory cytokines, as well as other DAMPs, such as S100B and high-mobility group box 1, resulting in an autocatalytic process that mediates AD progression. Aβ is also recognized by a variety of other TLRs (26, 70–72). In addition, TNF-α and IL-1β are both released from nerve terminals under physiologic conditions and may regulate synaptic strength (73, 74). These would also be expected to initiate an immune response in surrounding cells (69).
Whereas it is usually assumed that the interaction of extracellular Aβ with neurons is the toxic event in AD (66), not all patients with dementia have significant levels of extracellular Aβ in their brains (75). Therefore, alternative sources of toxic molecules need to be considered. As described in the next section, we have shown that intraneuronal Aβ causes a large, proinflammatory response in nerve cells themselves that could initiate CNS inflammation and perhaps AD itself (26).
INTRACELLULAR AMYLOID ACCUMULATION AND INFLAMMATION ARE EARLY EVENTS IN AD
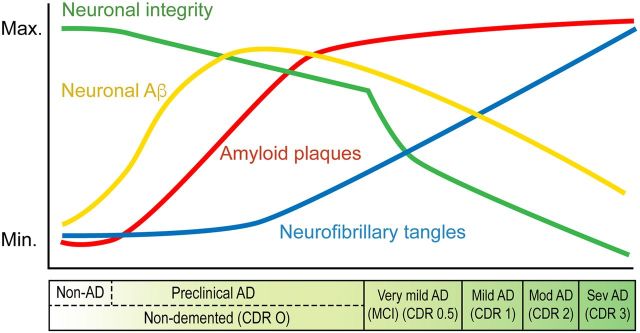
Although debated (76, 77), the accumulation of intracellular Aβ and other dysfunctional proteins in AD is likely to be a very early event in disease progression. This conclusion is supported by a number of observations, and a schematic of its place in the temporal sequence of AD pathology is shown in Fig. 1. The observations that support this schematic are as follows: 1) in humans, intracellular Aβ accumulation may be the origin of extracellular plaques (78); 2) the accumulation of polymerized Aβ occurs preferentially within the cholinergic forebrain with aging and AD (79); 3) nerve cell death occurs in mutant PS1 transgenic mice that have extensive intraneuronal Aβ but no plaques (80); 4) there is intraneuronal Aβ accumulation well before extracellular amyloid in several different AD animal models (81–84); 5) a reduction in intracellular Aβ also reduces soluble Aβ in vivo (85); 6) the removal of intracellular Aβ by apomorphine in 3 familial AD (FAD) mutation mice before the appearance of plaques improves memory and reduces subsequent AD pathology (86); and 7) both apomorphine and the 5-lipoxygenase inhibitor CNB-001 remove intracellular Aβ from cultured nerve cells, prevent cell death, and lower aggregated proteins in AD mouse brains (54).
Figure 1.
Approximate time course for the appearance of AD pathology biomarkers in the brain. The drawing is based on published data from a variety of sources and redrawn, in part, from Himeno et al. (89). max., maximum; min., minimum; CDR, clinical dementia rating (3 is the worst); MCI, mild cognitive impairment; mod, moderate; sev, severe.
As intracellular Aβ is observed in AD before or concomitant with the detection of inflammatory markers, we asked whether intraneuronal Aβ causes an inflammatory response within neurons (26). It was shown that the expression of intracellular Aβ in a human nerve cell line causes the expression of numerous proinflammatory genes and the increased synthesis of leukotrienes that are neurotoxic (26). Importantly, protein sequence does not necessarily predict the ability of a protein or peptide to form amyloids (87, 88) nor does protein sequence predict the ability of a protein to activate PRRs. The latter process is strictly conformation dependent (50). Therefore, a wide variety of proteins may aggregate and activate PRRs, initiating an inflammatory cascade that eventually culminates with disease. This process is likely involved in other neurodegenerative diseases where aggregation and accumulation of proteins other than Aβ are also a hallmark.
THE HYPOTHESIS AND FINAL CONSIDERATIONS
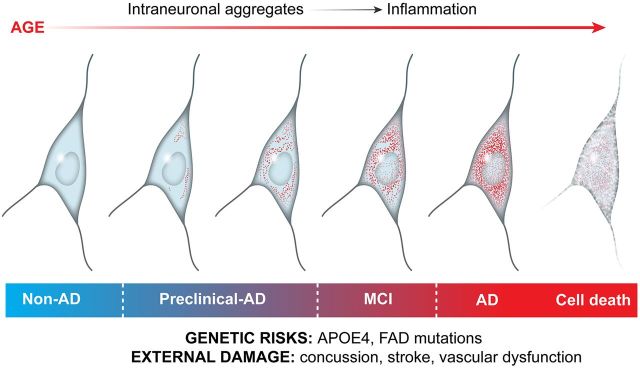
Given the observations that 1) aggregated intracellular proteins accumulate with age, 2) inflammation increases with age in a parallel manner, 3) a cause-and-effect relationship has been documented between the two, and 4) likely, a very large number of intracellular aggregated proteins interact with PRRs to generate a proinflammatory response, we hypothesize here that the accumulation of aggregated proteins over time is a major driver for the chronic, low-grade inflammation that is observed with aging and for the more aggressive forms of inflammation seen in neurologic diseases that often culminate in neurodegeneration (Fig. 2). As neurons do not divide, and therefore cannot dilute out aggregated proteins, they may be more exposed to proteotoxicity than populations of dividing cells where damaged proteins are diluted out. Whether this assumption is correct will require studies that determine how the level of aggregated protein accumulation in diverse cell types in the same organism correlates with the activation of inflammatory pathways. The molecular machinery that links systemic protein aggregation to the activation of inflammatory pathways remains to be determined.
Figure 2.
Schematic drawing of the aging aggregate hypothesis proposed in the text. During the aging process, there is a sequential accumulation of non-disease-related aggregated, insoluble proteins within CNS neurons. As aggregated proteins accumulate, they activate an inflammatory response within the cells. Under some circumstances, the neurons may receive additional insults in the form of trauma, as with a concussion, or exposure to genetic risk factors such as higher than normal levels of disease-associated proteins such as Aβ. This results in further increases in the inflammatory response and nerve cell damage and eventually death. APOE4, apolipoprotein E4.
In extreme cases, an excess of a specific protein, such as Aβ or α-synuclein, is superimposed on the aging-dependent background of aggregated proteins, resulting in a disease state, such as AD or PD. Therefore, the issue of toxicity is not whether a specific, unique DAMP, such as Aβ, is present, but rather, what the sum total is of all DAMP-like molecules present in the cell. In this context, aging can be viewed, in part, as a persistent, self-propagating, inflammatory condition, directly coupled to impaired protein translation, maintenance, and clearance conditions that damage or otherwise stress brain cells. Conditions, such as trauma or systemic inflammation, both of which are risk factors for AD and cognitive decline, would accelerate the toxicity, as well as disease pathology itself.
In summary, all cells accumulate dysfunctional proteins that tend to aggregate as a function of temporal aging. Normally, these proteins are degraded or stored in harmless inclusions by multiple processes, but these processes become less efficient as cells age. In dividing cells, they are also diluted into daughter cells, and new proteins are made. However, in nondividing cells, such as neurons, there is a temporal accumulation of many aggregated proteins that likely induces a proinflammatory response. If either genetic or environmental factors increase the coexpression of additional aggregation-prone proteins, then a distinctive, old, age-associated pathology, such as AD, will be initiated. Therefore, a more viable approach to AD drug discovery and perhaps slowing aging itself may be to target the removal of the generic aggregated proteins (not just Aβ) or the pathological molecular pathways that are initiated by the accumulation of protein aggregates.
AUTHOR CONTRIBUTIONS
All authors contributed to writing the manuscript and experimental work described in the text.
ACKNOWLEDGMENTS
This study was supported by a Salk Nomis Fellowship Award (to A.C.), a grant from the U.S. National Institutes of Health (RO1 AG046153; to P.M. and D.S.), an Edward N. and Della L. Thome Memorial Foundation Award in Alzheimer’s Disease Drug Discovery Research (to P.M.), and a California Institute of Regenerative Medicine grant (to D.S. and W.F.).
Glossary
- Aβ
amyloid β
- AD
Alzheimer's disease
- DAMP
damage-associated molecular pattern
- ER
endoplasmic reticulum
- FAD
familial Alzheimer's disease
- PD
Parkinson's disease
- PRR
pattern recognition receptor
- s-IBM
sporadic inclusion-body myositis
- UPR
unfolded protein response
REFERENCES
- 1.Currais A. (2015) Ageing and inflammation—a central role for mitochondria in brain health and disease. Ageing Res. Rev. , 30–42 [DOI] [PubMed] [Google Scholar]
- 2.Wyss-Coray T., Mucke L. (2002) Inflammation in neurode-generative disease—a double-edged sword. Neuron , 419–432 [DOI] [PubMed] [Google Scholar]
- 3.Wyss-Coray T. (2006) Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat. Med. , 1005–1015 [DOI] [PubMed] [Google Scholar]
- 4.Glass C. K., Saijo K., Winner B., Marchetto M. C., Gage F. H. (2010) Mechanisms underlying inflammation in neurodegeneration. Cell , 918–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee Y. J., Han S. B., Nam S. Y., Oh K. W., Hong J. T. (2010) Inflammation and Alzheimer’s disease. Arch. Pharm. Res. , 1539–1556 [DOI] [PubMed] [Google Scholar]
- 6.Gentile M. T., Reccia M. G., Sorrentino P. P., Vitale E., Sorrentino G., Puca A. A., Colucci-D’Amato L. (2012) Role of cytosolic calcium-dependent phospholipase A2 in Alzheimer’s disease pathogenesis. Mol. Neurobiol. , 596–604 [DOI] [PubMed] [Google Scholar]
- 7.Wyss-Coray T., Rogers J. (2012) Inflammation in Alzheimer disease—a brief review of the basic science and clinical literature. Cold Spring Harb. Perspect. Med. , a006346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howcroft T. K., Campisi J., Louis G. B., Smith M. T., Wise B., Wyss-Coray T., Augustine A. D., McElhaney J. E., Kohanski R., Sierra F. (2013) The role of inflammation in age-related disease. Aging (Albany, N.Y.) , 84–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franceschi C., Capri M., Monti D., Giunta S., Olivieri F., Sevini F., Panourgia M. P., Invidia L., Celani L., Scurti M., Cevenini E., Castellani G. C., Salvioli S. (2007) Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech. Ageing Dev. , 92–105 [DOI] [PubMed] [Google Scholar]
- 10.Chung H. Y., Cesari M., Anton S., Marzetti E., Giovannini S., Seo A. Y., Carter C., Yu B. P., Leeuwenburgh C. (2009) Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res. Rev. , 18–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pawelec G., Goldeck D., Derhovanessian E. (2014) Inflammation, ageing and chronic disease. Curr. Opin. Immunol. , 23–28 [DOI] [PubMed] [Google Scholar]
- 12.Wei J., Xu H., Davies J. L., Hemmings G. P. (1992) Increase of plasma IL-6 concentration with age in healthy subjects. Life Sci. , 1953–1956 [DOI] [PubMed] [Google Scholar]
- 13.Brüünsgaard H., Pedersen B. K. (2003) Age-related inflammatory cytokines and disease. Immunol. Allergy Clin. North Am. , 15–39 [DOI] [PubMed] [Google Scholar]
- 14.Shaw A. C., Goldstein D. R., Montgomery R. R. (2013) Age-dependent dysregulation of innate immunity. Nat. Rev. Immunol. , 875–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campisi J., d’Adda di Fagagna F. (2007) Cellular senescence: when bad things happen to good cells. Nat. Rev. Mol. Cell Biol. , 729–740 [DOI] [PubMed] [Google Scholar]
- 16.Coppé J. P., Desprez P. Y., Krtolica A., Campisi J. (2010) The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol. , 99–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kregel K. C., Zhang H. J. (2007) An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. Am. J. Physiol. Regul. Integr. Comp. Physiol. , R18–R36 [DOI] [PubMed] [Google Scholar]
- 18.Gill R., Tsung A., Billiar T. (2010) Linking oxidative stress to inflammation: Toll-like receptors. Free Radic. Biol. Med. , 1121–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Starr M. E., Saito H. (2014) Sepsis in old age: review of human and animal studies. Aging Dis. , 126–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Marzo V., Stella N., Zimmer A. (2015) Endocannabinoid signalling and the deteriorating brain. Nat. Rev. Neurosci. , 30–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tschopp J. (2011) Mitochondria: sovereign of inflammation? Eur. J. Immunol. , 1196–1202 [DOI] [PubMed] [Google Scholar]
- 22.Green D. R., Galluzzi L., Kroemer G. (2011) Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science , 1109–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.López-Armada M. J., Riveiro-Naveira R. R., Vaamonde-García C., Valcárcel-Ares M. N. (2013) Mitochondrial dysfunction and the inflammatory response. Mitochondrion , 106–118 [DOI] [PubMed] [Google Scholar]
- 24.Castello M. A., Soriano S. (2013) Rational heterodoxy: cholesterol reformation of the amyloid doctrine. Ageing Res. Rev. , 282–288 [DOI] [PubMed] [Google Scholar]
- 25.Hermann P. M., Watson S. N., Wildering W. C. (2014) Phospholipase A2—nexus of aging, oxidative stress, neuronal excitability, and functional decline of the aging nervous system? Insights from a snail model system of neuronal aging and age-associated memory impairment. Front. Genet. , 419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Currais A., Quehenberger Q., Armando A., Daugherty D., Maher P., Schubert D. (2016) Amyloid proteotoxicity initiates an inflammatory response blocked by cannabinoids. NPJ Aging Mech. Dis. , 16012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanzel C. E., Pichet-Binette A., Pimentel L. S., Iulita M. F., Allard S., Ducatenzeiler A., Do Carmo S., Cuello A. C. (2014) Neuronal driven pre-plaque inflammation in a transgenic rat model of Alzheimer’s disease. Neurobiol. Aging , 2249–2262 [DOI] [PubMed] [Google Scholar]
- 28.López-Otín C., Blasco M. A., Partridge L., Serrano M., Kroemer G. (2013) The hallmarks of aging. Cell , 1194–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Powers E. T., Morimoto R. I., Dillin A., Kelly J. W., Balch W. E. (2009) Biological and chemical approaches to diseases of proteostasis deficiency. Annu. Rev. Biochem. , 959–991 [DOI] [PubMed] [Google Scholar]
- 30.Orgel L. E. (1963) The maintenance of the accuracy of protein synthesis and its relevance to ageing. Proc. Natl. Acad. Sci. USA , 517–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gladyshev V. N. (2013) The origin of aging: imperfectness-driven non-random damage defines the aging process and control of lifespan. Trends Genet. , 506–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Evans S. N., Steinsaltz D. (2007) Damage segregation at fissioning may increase growth rates: a superprocess model. Theor. Popul. Biol. , 473–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rang C. U., Peng A. Y., Chao L. (2011) Temporal dynamics of bacterial aging and rejuvenation. Curr. Biol. , 1813–1816 [DOI] [PubMed] [Google Scholar]
- 34.Nyström T. (2007) A bacterial kind of aging. PLoS Genet. , e224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steinkraus K. A., Kaeberlein M., Kennedy B. K. (2008) Replicative aging in yeast: the means to the end. Annu. Rev. Cell Dev. Biol. , 29–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nyström T., Liu B. (2014) The mystery of aging and rejuvenation—a budding topic. Curr. Opin. Microbiol. , 61–67 [DOI] [PubMed] [Google Scholar]
- 37.Coelho M., Tolić I. M. (2015) Asymmetric damage segregation at cell division via protein aggregate fusion and attachment to organelles. BioEssays , 740–747 [DOI] [PubMed] [Google Scholar]
- 38.Sinclair D. A., Guarente L. (1997) Extrachromosomal rDNA circles—a cause of aging in yeast. Cell , 1033–1042 [DOI] [PubMed] [Google Scholar]
- 39.Aguilaniu H., Gustafsson L., Rigoulet M., Nyström T. (2003) Asymmetric inheritance of oxidatively damaged proteins during cytokinesis. Science , 1751–1753 [DOI] [PubMed] [Google Scholar]
- 40.Johnston J. A., Ward C. L., Kopito R. R. (1998) Aggresomes: a cellular response to misfolded proteins. J. Cell Biol. , 1883–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rujano M. A., Bosveld F., Salomons F. A., Dijk F., van Waarde M. A., van der Want J. J., de Vos R. A., Brunt E. R., Sibon O. C., Kampinga H. H. (2006) Polarised asymmetric inheritance of accumulated protein damage in higher eukaryotes. PLoS Biol. , e417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Desnues B., Cuny C., Grégori G., Dukan S., Aguilaniu H., Nyström T. (2003) Differential oxidative damage and expression of stress defence regulons in culturable and non-culturable Escherichia coli cells. EMBO Rep. , 400–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stewart E. J., Madden R., Paul G., Taddei F. (2005) Aging and death in an organism that reproduces by morphologically symmetric division. PLoS Biol. , e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Terman A. (2001) Garbage catastrophe theory of aging: imperfect removal of oxidative damage? Redox Rep. , 15–26 [DOI] [PubMed] [Google Scholar]
- 45.Toyama B. H., Hetzer M. W. (2013) Protein homeostasis: live long, won’t prosper. Nat. Rev. Mol. Cell Biol. , 55–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Toyama B. H., Savas J. N., Park S. K., Harris M. S., Ingolia N. T., Yates J. R. III, Hetzer M. W. (2013) Identification of long-lived proteins reveals exceptional stability of essential cellular structures. Cell , 971–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ross C. A., Poirier M. A. (2005) Opinion: What is the role of protein aggregation in neurodegeneration? Nat. Rev. Mol. Cell Biol. , 891–898 [DOI] [PubMed] [Google Scholar]
- 48.Chen B., Retzlaff M., Roos T., Frydman J. (2011) Cellular strategies of protein quality control. Cold Spring Harb. Perspect. Biol. , a004374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tyedmers J., Mogk A., Bukau B. (2010) Cellular strategies for controlling protein aggregation. Nat. Rev. Mol. Cell Biol. , 777–788 [DOI] [PubMed] [Google Scholar]
- 50.Knowles T. P., Vendruscolo M., Dobson C. M. (2014) The amyloid state and its association with protein misfolding diseases. Nat. Rev. Mol. Cell Biol. , 384–396 [DOI] [PubMed] [Google Scholar]
- 51.Simonsen A., Cumming R. C., Brech A., Isakson P., Schubert D. R., Finley K. D. (2008) Promoting basal levels of autophagy in the nervous system enhances longevity and oxidant resistance in adult Drosophila. Autophagy , 176–184 [DOI] [PubMed] [Google Scholar]
- 52.Cuervo A. M., Dice J. F. (2000) Age-related decline in chaperone-mediated autophagy. J. Biol. Chem. , 31505–31513 [DOI] [PubMed] [Google Scholar]
- 53.Tonoki A., Kuranaga E., Tomioka T., Hamazaki J., Murata S., Tanaka K., Miura M. (2009) Genetic evidence linking age-dependent attenuation of the 26S proteasome with the aging process. Mol. Cell. Biol. , 1095–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Valera E., Dargusch R., Maher P. A., Schubert D. (2013) Modulation of 5-lipoxygenase in proteotoxicity and Alzheimer’s disease. J. Neurosci. , 10512–10525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li F., Zhang L., Craddock J., Bruce-Keller A. J., Dasuri K., Nguyen A., Keller J. N. (2008) Aging and dietary restriction effects on ubiquitination, sumoylation, and the proteasome in the heart. Mech. Ageing Dev. , 515–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bukau B., Weissman J., Horwich A. (2006) Molecular chaperones and protein quality control. Cell , 443–451 [DOI] [PubMed] [Google Scholar]
- 57.Cao S. S., Kaufman R. J. (2012) Unfolded protein response. Curr. Biol. , R622–R626 [DOI] [PubMed] [Google Scholar]
- 58.Carrara M., Prischi F., Nowak P. R., Kopp M. C., Ali M. M. (2015) Noncanonical binding of BiP ATPase domain to Ire1 and Perk is dissociated by unfolded protein CH1 to initiate ER stress signaling. eLife , e03522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sonenberg N., Hinnebusch A. G. (2009) Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell , 731–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pellegrino M. W., Nargund A. M., Haynes C. M. (2013) Signaling the mitochondrial unfolded protein response. Biochim. Biophys. Acta , 410–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schneider K., Bertolotti A. (2015) Surviving protein quality control catastrophes—from cells to organisms. J. Cell Sci. , 3861–3869 [DOI] [PubMed] [Google Scholar]
- 62.Opattova A., Cente M., Novak M., Filipcik P. (2015) The ubiquitin proteasome system as a potential therapeutic target for treatment of neurodegenerative diseases. Gen. Physiol. Biophys. , 337–352 [DOI] [PubMed] [Google Scholar]
- 63.Jung C. H., Ro S. H., Cao J., Otto N. M., Kim D. H. (2010) mTOR regulation of autophagy. FEBS Lett. , 1287–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Medzhitov R. (2008) Origin and physiological roles of inflammation. Nature , 428–435 [DOI] [PubMed] [Google Scholar]
- 65.Chovatiya R., Medzhitov R. (2014) Stress, inflammation, and defense of homeostasis. Mol. Cell , 281–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Minter M. R., Taylor J. M., Crack P. J. (2016) The contribution of neuroinflammation to amyloid toxicity in Alzheimer’s disease. J. Neurochem. , 457–474 [DOI] [PubMed] [Google Scholar]
- 67.Porhomayon J., Kolesnikov S., Nader N. D. (2014) The impact of stress hormones on post-traumatic stress disorders symptoms and memory in cardiac surgery patients. J. Cardiovasc. Thorac. Res. , 79–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fang C., Wei X., Wei Y. (2016) Mitochondrial DNA in the regulation of innate immune responses. Protein Cell , 11–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Clark I. A., Vissel B. (2015) Amyloid β: one of three danger-associated molecules that are secondary inducers of the proinflammatory cytokines that mediate Alzheimer’s disease. Br. J. Pharmacol. , 3714–3727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reed-Geaghan E. G., Savage J. C., Hise A. G., Landreth G. E. (2009) CD14 and Toll-like receptors 2 and 4 are required for fibrillar Abeta-stimulated microglial activation. J. Neurosci. , 11982–11992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stewart C. R., Stuart L. M., Wilkinson K., van Gils J. M., Deng J., Halle A., Rayner K. J., Boyer L., Zhong R., Frazier W. A., Lacy-Hulbert A., El Khoury J., Golenbock D. T., Moore K. J. (2010) CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat. Immunol. , 155–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vollmar P., Kullmann J. S., Thilo B., Claussen M. C., Rothhammer V., Jacobi H., Sellner J., Nessler S., Korn T., Hemmer B. (2010) Active immunization with amyloid-beta 1-42 impairs memory performance through TLR2/4-dependent activation of the innate immune system. J. Immunol. , 6338–6347 [DOI] [PubMed] [Google Scholar]
- 73.Chiti F., Dobson C. M. (2006) Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. , 333–366 [DOI] [PubMed] [Google Scholar]
- 74.Marin I., Kipnis J. (2013) Learning and memory ... and the immune system. Learn. Mem. , 601–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Morris G. P., Clark I. A., Vissel B. (2014) Inconsistencies and controversies surrounding the amyloid hypothesis of Alzheimer’s disease. Acta Neuropathol. Commun. , 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bayer T. A., Wirths O. (2010) Intracellular accumulation of amyloid-Beta—a predictor for synaptic dysfunction and neuron loss in Alzheimer’s disease. Front. Aging Neurosci. , 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lauritzen I., Pardossi-Piquard R., Bauer C., Brigham E., Abraham J. D., Ranaldi S., Fraser P., St-George-Hyslop P., Le Thuc O., Espin V., Chami L., Dunys J., Checler F. (2012) The β-secretase-derived C-terminal fragment of βAPP, C99, but not Aβ, is a key contributor to early intraneuronal lesions in triple-transgenic mouse hippocampus. J. Neurosci. , 16243–16255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gouras G. K., Almeida C. G., Takahashi R. H. (2005) Intraneuronal Abeta accumulation and origin of plaques in Alzheimer’s disease. Neurobiol. Aging , 1235–1244 [DOI] [PubMed] [Google Scholar]
- 79.Baker-Nigh A., Vahedi S., Davis E. G., Weintraub S., Bigio E. H., Klein W. L., Geula C. (2015) Neuronal amyloid-β accumulation within cholinergic basal forebrain in ageing and Alzheimer’s disease. Brain , 1722–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chui D. H., Tanahashi H., Ozawa K., Ikeda S., Checler F., Ueda O., Suzuki H., Araki W., Inoue H., Shirotani K., Takahashi K., Gallyas F., Tabira T. (1999) Transgenic mice with Alzheimer presenilin 1 mutations show accelerated neurodegeneration without amyloid plaque formation. Nat. Med. , 560–564 [DOI] [PubMed] [Google Scholar]
- 81.Billings L. M., Oddo S., Green K. N., McGaugh J. L., LaFerla F. M. (2005) Intraneuronal Abeta causes the onset of early Alzheimer’s disease-related cognitive deficits in transgenic mice. Neuron , 675–688 [DOI] [PubMed] [Google Scholar]
- 82.Oakley H., Cole S. L., Logan S., Maus E., Shao P., Craft J., Guillozet-Bongaarts A., Ohno M., Disterhoft J., Van Eldik L., Berry R., Vassar R. (2006) Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. J. Neurosci. , 10129–10140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tampellini D., Capetillo-Zarate E., Dumont M., Huang Z., Yu F., Lin M. T., Gouras G. K. (2010) Effects of synaptic modulation on beta-amyloid, synaptophysin, and memory performance in Alzheimer’s disease transgenic mice. J. Neurosci. , 14299–14304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wirths O., Multhaup G., Czech C., Blanchard V., Moussaoui S., Tremp G., Pradier L., Beyreuther K., Bayer T. A. (2001) Intraneuronal Abeta accumulation precedes plaque formation in beta-amyloid precursor protein and presenilin-1 double-transgenic mice. Neurosci. Lett. , 116–120 [DOI] [PubMed] [Google Scholar]
- 85.Oddo S., Caccamo A., Tran L., Lambert M. P., Glabe C. G., Klein W. L., LaFerla F. M. (2006) Temporal profile of amyloid-beta (Abeta) oligomerization in an in vivo model of Alzheimer disease. A link between Abeta and tau pathology. J. Biol. Chem. , 1599–1604 [DOI] [PubMed] [Google Scholar]
- 86.Himeno E., Ohyagi Y., Ma L., Nakamura N., Miyoshi K., Sakae N., Motomura K., Soejima N., Yamasaki R., Hashimoto T., Tabira T., LaFerla F. M., Kira J. (2011) Apomorphine treatment in Alzheimer mice promoting amyloid-β degradation. Ann. Neurol. , 248–256 [DOI] [PubMed] [Google Scholar]
- 87.Chiti F., Dobson C. M. (2009) Amyloid formation by globular proteins under native conditions. Nat. Chem. Biol. , 15–22 [DOI] [PubMed] [Google Scholar]
- 88.Goldschmidt L., Teng P. K., Riek R., Eisenberg D. (2010) Identifying the amylome, proteins capable of forming amyloid-like fibrils. Proc. Natl. Acad. Sci. USA , 3487–3492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Craig-Schapiro R., Fagan A. M., Holtzman D. M. (2009) Biomarkers of Alzheimer’s disease. Neurobiol. Dis. , 128–140 [DOI] [PMC free article] [PubMed] [Google Scholar]