Abstract
Bile acids (BAs) constitute an important class of steroid metabolites often displaying changes associated with disease states and other health conditions. Current analyses for these structurally similar compounds are limited by a lack of sensitivity and long separation times with often poor isomeric resolution. To overcome these challenges and provide rapid analyses for the BA isomers, we utilized cyclodextrin adducts in conjunction with novel ion mobility (IM) separation capabilities provided by structures for lossless ion manipulations (SLIM). Cyclodextrin was found to interact with both the tauro- and glyco-conjugated BA isomers studied, forming rigid noncovalent host-guest inclusion complexes. Without the use of cyclodextrin adducts, the BA isomers were found to be nearly identical in their respective mobilities and thus unable to be baseline resolved. Each separation of the cyclodextrin—bile acid host—guest inclusion complex was performed in less than 1 s, providing a much more rapid alternative to current liquid chromatography-based separations. SLIM provided capabilities for the accumulation of larger ion populations and IM peak compression that resulted in much higher resolution separations and increased signal intensities for the BA isomers studied.
Graphical Abstract

Bile acids (BAs) are a structurally similar class of steroid metabolites synthesized from cholesterol. Their structures consist of a hydroxyl group-substituted steroid backbone with a carboxyl-terminated aliphatic side chain, giving rise to the BAs amphipathic properties. In humans the side chain carboxyl group is almost always conjugated with either taurine (25%) or glycine (75%), increasing water solubility1 and enabling their roles in digestion, fat absorption, molecular signaling, and metabolic regulation.1–5
To date, BA analyses have been hampered by their chiral centers and various hydroxyl group positions on the steroid ring, creating numerous isomeric permutations, and for which only certain isomers are linked to diseases.6–9 BA analyses based upon liquid chromatography combined with mass spectrometry (LC—MS)10–12 are widely used but often ineffective for fully separating BAs.10,11 Supercritical fluid chromatography (SFC), in conjunction with acidic or basic mobile phase modifiers, has been applied to separate certain BAs that vary in polarity.12 Both LC and SFC suffer from separation times on the order of minutes to hours, as well as limited isomeric resolution,10–12 and faster and more effective separations are needed for BAs.
Ion mobility (IM) spectrometry is a rapid gas phase ion separation technique capable of distinguishing different ion structural conformations on a millisecond time scale,13 providing an attractive alternative to solution phase separations since it does not require specific mobile phases, gradient development, or stationary phase selection based on the physical properties of the analytes of interest. Furthermore, IM-MS analyses simultaneously provide both ion structural and mass information,14–16 but isomeric separations in current commercial IM-MS instrumentation suffer from poor resolution as well as sensitivity challenges.17–19 Previous IM spectrometry work has had difficulties separating the structurally similar BA isomers.20 To address these challenges, we have been developing much higher resolution IM separations with the ability to also greatly increase sensitivity by the use of large ion populations based upon the use of traveling wave (TW)-based structures for lossless ion manipulations (SLIM; see Figure 1) utilizing serpentine ultralong path with extended routing (SUPER) IM.17–19,21 Here these new capabilities were utilized for the separation of previously problematic tauro- and glyco-conjugated BA isomers, showing great promise for wide ranges of other challenging applications.
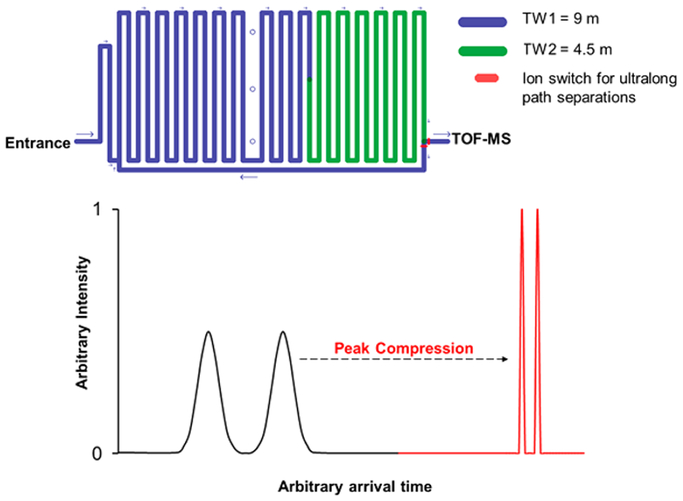
Figure 1.
SLIM SUPER IM multipass long ion path length module design used for separations (top) and a depiction of the SLIM separation compression ratio ion mobility programming (CRIMP) process (bottom).
EXPERIMENTAL SECTION
Materials and Conditions.
3-Amino 3-deoxy α−cyclodextrin was purchased from TCI Chemicals (Portland, OR). Bile acid isomers (taurodeoxycholic acid (TDCA), taurochenodeoxycholic acid (TCDCA), tauroursodeoxycholic acid (TUDCA), taurohyodeoxycholid acid (THDCA), glycodeoxycholic acid (GDCA), glycochenodeoxycholic acid (GCDCA), and glycoursodeoxycholic acid (GUDCA)) were obtained from Cayman Chemical (Ann Arbor, MI). LC-M S grade solvents were used to prepare all sam ples at total concentrations of 20 μM bile acid standards and 50 μM α− cyclodextrin in 50/50 (v/v) water/methanol with 0.5% acetic acid (v/v).
The TW SLIM serpentine ultralong path with extended routing (SUPER) IM-M S platform used for these experiments has been previously described in detail.17–19,21 Briefly, samples were infused at a flow rate of 0.3 μL/min with nanoelectrospray conditions of 3000 V nESI voltage and 110 °C for the heated ion inlet capillary. The ion funnel trap was maintained at 2.30 Torr while the SLIM SUPER IM module was at 2.36 Torr. Our traveling wave (TW) SLIM SUPER module has a total ion path length of 13.5 m (9 m for the first section and 4.5 m for the second section). For positive mode analyses the traveling wave speed was 200 m/s with an amplitude of 30 Vpp. For negative mode analyses, traveling wave speed was kept at 70 m/s with an amplitude of 20 Vpp. This difference in TW velocity and amplitude results in the differences in arrival times observed when switching between positive and negative polarities for analyses. An Agilent 6224 TOF mass spectrometer (Agilent Technologies, Santa Clara, CA) was used in all experiments. All data were acquired using home-built software. Each IM spectrum shown is a summed average of 15 individual IM separations. No new, unexpected, or significant hazards/risks were associated with this reported work.
IM Multipass Separations and Compression Ratio Ion Mobility Programming (CRIMP).
Peak compression via CRIMP has recently been described in detail.17,21 Briefly, a stuttering TW can be applied in the second (4.5 m) section of the SLIM SUPER module, which can permit ions from the first (9 m) section to be compressed while passing through the interface between the two TW regions (blue and green intersection in Figure 1). For experiments using CRIMP, the TW amplitude in both regions was kept the same as during separation (e.g., 30 V for positive mode analyses). The traveling wave velocity was kept at 200 m/s (for positive mode analyses) in the first 9 m region and stutters between 0 and 200 m/s, approximately once every 1 ms, in the second 4.5 m region during CRIMP. CRIMP was applied for ~100 ms total time in the second region to ensure the mobility range of interest is successfully compressed, and then the stuttering region reverted to normal separation conditions (constant 200 m/s velocity in positive mode and 70 m/s in negative mode) before ions exit this region. Since a stuttering (or intermittently applied/moving) traveling wave was used in the second (green) section, ions arrive slightly later at the detector compared to when no compression step is applied (since their average speed is slower in the second region when CRIMP is used). This explains the deviations in arrival times (i.e., the later arrival times when CRIMP is applied). Additionally, CRIMP is applied only once on the second pass through the compression region, not on every subsequent pass around the module. This peak compression via CRIMP resulted in the narrowing of IM peaks, thus resulting in increased signal intensities as well as increased signal-to-noise (S/N). At the end of the SLIM SUPER module, a switching region (red) enables ions to be either routed to the TOF-MS for analysis or back to the beginning of the blue section for another “pass” through the module. This switching region permits ultralong path separations by ions being able to travel around the SLIM SUPER module multiple times in a lossless fashion.20
In-SLIM Ion Accumulation.
Ions were accumulated in the first of the two independently operated traveling wave module sections (blue and green sections shown in Figure 1) by stopping the TW in the second region (green) to create a potential “wall”, confining ions to the first region (blue) of the SLIM SUPER module. During in-SLIM ion accumulation, the TW speed and amplitude in the first region were kept at the same conditions as during separation (e.g., 200 m/s and 30 V for positive mode analyses). The second region was maintained at the same amplitude as the first region (30 V), but it was halted, thus having a speed of 0 m/s during in-SLIM ion accumulation. Following accumulation, separation conditions were resumed for the second region of the module. This enables the first 9 m traveling wave section of the SLIM SUPER module to function as a giant accumulation region, permitting accumulation of >109 ions (over 2–3 orders of magnitude increase in charge capacity as compared to ion introduction with an ion funnel trap).18 In the present work ions were accumulated for 1 s in-SLIM prior to the beginning of separation for all experiments. We note that considerable potential exists for further optimization of such studies, including by the use of much longer in-SLIM ion accumulation periods and the alteration and optimization of TW amplitudes in the respective module sections during the accumulation, CRIMP, and normal separation stages.
Since the first 9 m section of the SLIM SUPER module functions as a very long and extremely high capacity ion accumulation (i.e., ion trapping) region, only 4.5 m of separation path is utilized on the initial pass of ions through the module. Using the ion switch at the end of the second region;19 ions are sent through the SLIM SUPER module for another 13.5 m of IM separation. The ion switch applies either a traveling wave or a blocking voltage to a single set of electrodes to select the ion path.19 Thus, all separation path lengths in all the described experiments will be of a total distance in meters of [(4.5) + (13.5)(n)], where n is the number of passes through the SLIM SUPER module (e.g., 5 passes would equal total separation path length of 72 m).
RESULTS AND DISCUSSION
Initial Assessment of Bile Acid Isomer Separations with SLIM SUPER IM–MS.
To evaluate the utility of a rapid, high-resolution alternative to existing chromatographic approaches for BA separations, SLIM SUPER IM was initially explored for the separation of tauro- and glyco-conjugated BA isomers. Because conventional MS analyses of BAs use negative ions due to the preferred deprotonation site on the substituted aliphatic side chain,20,22,23 the [M–H]− ions for a mixture of four tauro-conjugated BA isomers (taurodeoxycholic acid (TDCA), taurochenodeoxycholic acid (TCDCA), tauroursodeoxycholic acid (TUDCA), and taurohyodeoxycholid acid (THDCA)) was assessed (Figure 2). In the analyses, TDCA and THDCA were partially resolved as their [M–H]− ions (potentially due to their well-spaced hydroxyl group substituents along the steroid ring). However, TCDCA and TUDCA were found to coarrive with portions of both the TDCA and THDCA mobility peaks, indicating that their deprotonated adducts have nearly identical mobilities (and thus collision cross sections), even after 85.5 m of separation; all four [M+K]+ isomers arrived under a single, broad IM peak so other methods were needed for their separation (Figure 2). In comparing the positive and negative mode analyses, the potassiated adducts all converge under a single mobility peak, indicating that they are unresolvable based on having identical collision cross sections (i.e., mobilities). We speculate this may be attributed to the potassium cation having multiple attachment points to OH groups, potentially resulting in multiple cation conformers present that may further challenge analyses. It is also important to mention that no metal salts were intentionally added, indicating that they are inherently present in the sample, solvent, or our nanoelectrospray emitter tip/infusion line. Surprisingly, protonated adducts, [M+H]+, and sodiated ones, [M+Na]+, were not observed for the tauro-BA isomers, but sodiated ones were observed for the glyco-BA isomers (see the Supporting Information). The sodium cation may potentially be too small to successfully form adducts with the larger tauro-BAs as compared to the smaller glyco-BAs. Some peak tailing is observed for both the deprotonated and potassiated adducts, potentially indicating the presence of conformers that are interconverting on a fast time scale, especially ones that are lower in mobility (slightly slower) than the main IM conformer distribution. These results indicate that the subtle differences in hydroxyl (OH) group positioning along the steroid backbone are indistinguishable in both positive and negative mode analyses, demonstrating the need for improved capabilities.
Figure 2.
Chemical structures of the tauro-BAs studied. SLIM SUPER IM–MS analyses were performed using a 85.5 m path length, and all four tauro-BA isomers were observed as [M+K]+ and [M–H]− ions. Traveling wave IM separations were optimized for best ion transmission in both polarities; however, the four isomers could not be baseline separated in either mode.
Utilization of Cyclodextrin Adducts To Increase IM Resolution of Bile Acid Isomers.
Previous IM–M S methods for increasing resolution of isomeric compounds have included the use of transition metal cation adducts,24 crown ether adducts,25 chiral gases,26,27 and noncovalent gas phase complexation or shift reagents.25,28–30 However, since these BAs only have minor differences in their hydroxyl group orientations, a suitable noncovalent complexation agent capable of creating more significant mobility differences among these BA isomers was desired. Cyclodextrins show promise for these separations as previous condensed phase studies have demonstrated their ability to form host-guest inclusion complexes with BAs.31–38 In these complexes, the aliphatic side chain of the BA can easily slip inside the hydrophobic cavity of the cyclodextrin, and the more bulky steroid portion fits between the hydrophilic exterior and hydrophobic interior.31–38 This hydrophobic cavity is created by the hydrogen atoms on H3 and H5 of the glucose moieties facing inward, with the hydrophilic rims being created from the primary and secondary hydroxyls (see the Supporting Information and refs 39–41). Based on this information, we hypothesized that the potential interactions between the cyclodextrin and tauro-BAs would result in gas phase host–guest inclusion ion complexes of varying size and shape after electrospray ionization (ESI), enabling separation with SLIM SUPER IM–MS. Furthermore, we chose 3-amino 3-deoxy α−cyclodextrin (α−CD, Figure 1) for complexation with the BAs as it has a smaller cavity size (compared to larger cavities for β or γ−cyclodextrins40) and would be more suitable for our lower molecular weight BA analytes, as well as a preferred protonation site for ionization in positive mode.
When α−CD was added to the 4 tauro-BAs mixture solution, ESI resulted in [M +α+H+K]2+ ions for each (M is the BA and α is the cyclodextrin). Extended (1 second) in-SLIM ion accumulation was used to enhance the SLIM IM–MS signal intensities, see the Experimental Section for further detail.17 In-SLIM ion accumulation permits the analysis of over a billion ions, a ~2–3 orders of magnitude increase in charge capacity compared to ion introduction with an ion funnel trap17 and was used in conjunction with CRIMP17,21 to dramatically narrow the initial broad ion distribution, resulting in greatly improved signal-to-noise (S/N) compared to without CRIMP being applied. These capabilities are demonstrated for BA separation in Figure 3A and 3B using SLIM SUPER IM separation path lengths of 18 and 72 m. In the 18 m measurements, the tauro-BAs occurred in a broader IM peak without CRIMP. This demonstrates that even with the use of CD complexation, 18 m of separation (much longer and higher resolution than conventional IM platforms, such as a 90 cm DT IM) is insufficient for complete isomeric tauro-BA resolution. As expected, the signal intensities for separations with CRIMP (Figure 3A, bottom) were much higher than without (Figure 3A, top). Separation of all four BA isomers finally occurred at 72 m when an initial CRIMP step was used (Figure 3B, bottom), but two were indistinguishable when CRIMP was not utilized (Figure 3B, top). Thus, the combination of large ion population accumulation, in the SLIM SUPER module, with a subsequent CRIMP step provides a way of increasing resolution and S/N for challenging isomeric BAs. Interestingly, the α−CD:BA complexes are potentially more rigid in structure than the BAs alone as indicated by the much narrower IM peaks evident by comparison of Figure 2 to Figure 3 (CRIMP step without CD complexation and CRIMP step with CD complexation, respectively). This demonstrates the need for the use of both the initial CRIMP step and cyclodextrin-based complexation for the high-resolution separation of BA isomers. Additionally, no other stoichiometries (e.g., 1:2 or 2:1) were observed, indicating a 1:1 ratio is preferred.
Figure 3.
BA isomer SLIM SUPER IM separations with and without a CRIMP step. (A) 18 m separation of the four isomers without (top) and with compression (bottom). (B) 72 m separation of the four isomers without (top) and with compression (bottom). All species shown are the [M+α+H+K]2+ ions, where M is the BA and α is the cyclodextrin. IM peak assignments for the BA isomers from Figure 3 are based on BA species run individually (see the Supporting Information). Ions were accumulated for 1 s in-SLIM prior to separation.
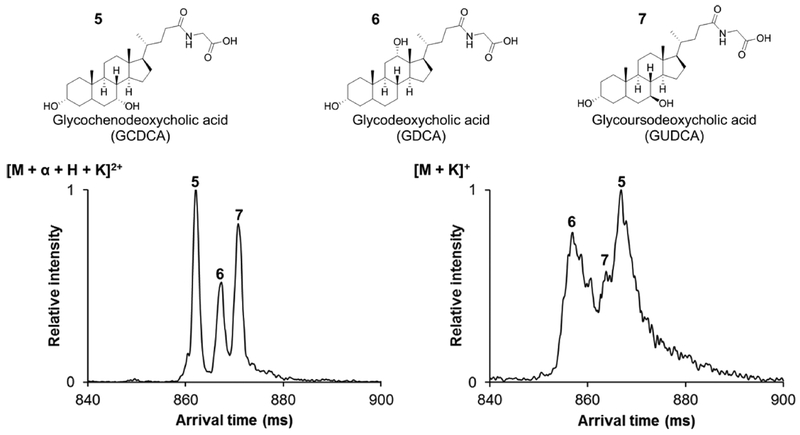
Since the tauro-BAs were separable with SLIM SUPER IM–MS when using CRIMP and upon addition of the α−CD, glycoconjugated BAs were also evaluated in this manner. Figure 4 shows SLIM SUPER IM–MS separations of three glyco-BA isomers (glycodeoxycholic acid (GDCA), glycochenodeoxycholic acid (GCDCA), and glycoursodeoxycholic acid (GUDCA)) as both their cyclodextrin complexes and as their potassiated adducts. Even with the change in functional groups (taurine to glycine), high-resolution isomer separation was again achieved for the [M+α+H+K]2+ ions using SLIM SUPER IM–M S and demonstrates that potassiated adducts alone did not enable fully resolving the glyco-BAs.
Figure 4.
Glyco-BA isomer separations. SLIM SUPER IM separation of the three glyco-BA isomers using the same parameters as the tauro-BA separations. 72 m SLIM SUPER IM separation as [M+α+H+K]2+ ions (left) and 85.5 m SLIM SUPER IM separation as [M+K]+ ions (right), where M is the BA and α is the cyclodextrin. IM peak assignments are based on BA species run individually (see the Supporting Information). Ions were accumulated for 1 s in-SLIM prior to separation.
CONCLUSION
In summary, we utilized cyclodextrin adducts in combination with extended ion accumulation and CRIMP compression in SLIM SUPER IM–MS to enable the separation and detection of structurally similar molecules that are difficult with traditional approaches. In the case of the BAs, extended ion accumulation, CRIMP compression, and cyclodextrin adduct formation were all found to be important; i.e., leaving out any of the three would either greatly reduce the signal-to-noise (S/N) or hinder separability. Evaluation of the tauro- and glyco-BA isomer groups showed that they could be resolved after a SLIM SUPER 72 m IM separation when conjugated with a cyclodextrin. Furthermore, each IM separation was possible in <1 s, providing a much faster and higher resolution alternative to existing BA separation methods. Additionally, since we were able to infuse samples at such low flow rates (300 nL/min) and separate these BA isomers in under 1 s, our total consumed was <100 pg, and much lower levels should be feasible. In comparison to previous methods for BA isomer separation, we could resolve the glyco-BAs on the order of seconds, as opposed to ~10 min via LC.10 To the best of our knowledge, this presents the first demonstration of the high-resolution IM separation of mixtures of BA isomers.20 Due to the promising results for these previously very difficult separations and the potential for significant further enhancement of both the ion accumulation step and IM resolution (with additional passes through the SLIM SUPER module), we envision that this strategy will have broad utility for the separation of other bile acid isomers, as well as other isomeric molecules such as phosphopeptides, glycans, and lipids that currently are inseparable in traditional approaches.
Supplementary Material
ACKNOWLEDGMENTS
Portions of this research were supported by grants from National Institute of General Medical Sciences (P41 GM103493), the National Institute of Environmental Health Sciences of the NIH (R01 ES022190), and the Laboratory Directed Research and Development Program at Pacific Northwest National Laboratory. This work was performed in the W. R. Wiley Environmental Molecular Sciences Laboratory (EMSL), a DOE national scientific user facility at the Pacific Northwest National Laboratory (PNNL). PNNL is operated by Battelle for the DOE under contract DE-AC05–76RL0 1830.
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.analchem.8b02990.
Individually run BA species and more information on the cyclodextrin molecule used in these experiments (PDF)
REFERENCES
- (1).Monte MJ; Marin JJ; Antelo A; Vazquez-Tato J World J. Gastroenterol 2009, 15 (7), 804–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Griffiths WJ; Sjovall JJ Lipid Res. 2010, 51 (1), 23–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Nagana Gowda GA; Shanaiah N; Cooper A; Maluccio M; Raftery D Lipids 2009, 44 (6), 527–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Suga T; Yamaguchi H; Sato T; Maekawa M; Goto J; Mano N PLoS One 2017, 12 (1), No. e0169719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Chen M; Gratzel M; Thomas JK J. Am. Chem. Soc 1975, 97 (8), 2052–7. [DOI] [PubMed] [Google Scholar]
- (6).Pavlidis P; Powell N; Vincent RP; Ehrlich D; Bjarnason I; Hayee B Aliment. Pharmacol. Ther 2015, 42 (7), 802–17. [DOI] [PubMed] [Google Scholar]
- (7).Ridlon JM; Kang DJ; Hylemon PB; Bajaj JS Curr. Opin. Gastroenterol 2014, 30 (3), 332–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Li T; Chiang JY Pharmacol. Rev 2014, 66 (4), 948–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Liu Y; Rong Z; Xiang D; Zhang C; Liu D Lipids Health Dis 2018, 17 (1), 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Amplatz B; Zohrer E; Haas C; Schaffer M; Stojakovic T; Jahnel J; Fauler G Clin. Chim. Acta 2017, 464, 85–92. [DOI] [PubMed] [Google Scholar]
- (11).Sarafian MH; Lewis MR; Pechlivanis A; Ralphs S; McPhail MJ; Patel VC; Dumas ME; Holmes E; Nicholson JK Anal. Chem 2015, 87 (19), 9662–70. [DOI] [PubMed] [Google Scholar]
- (12).Taguchi K; Fukusaki E; Bamba TJ Chromatogr A 2013, 1299, 103–903. [DOI] [PubMed] [Google Scholar]
- (13).Mason EA; McDaniel EW Transport properties of ions in gases; John Wiley and Sons: New York, 1988, 560 + xvi pp; AIChE J 1989, 35 (4), 701–7011. [Google Scholar]
- (14).Enders JR; McLean JA Chirality 2009, 21 (Suppl 1), E253–64. [DOI] [PubMed] [Google Scholar]
- (15).Gabelica V; Marklund E Curr. Opin. Chem. Biol 2018, 42, 51–59. [DOI] [PubMed] [Google Scholar]
- (16).Zhang X; Quinn K; Cruickshank-Quinn C; Reisdorph R; Reisdorph N Curr. Opin. Chem. Biol 2018, 42, 60–66. [DOI] [PubMed] [Google Scholar]
- (17).Deng L; Garimella SVB; Hamid AM; Webb IK; Attah IK; Norheim RV; Prost SA; Zheng X; Sandoval JA; Baker ES; Ibrahim YM; Smith RD Anal. Chem 2017, 89 (12), 6432–6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Deng L; Ibrahim YM; Hamid AM; Garimella SV; Webb IK; Zheng X; Prost SA; Sandoval JA; Norheim RV; Anderson GA; Tolmachev AV; Baker ES; Smith RD Anal. Chem 2016, 88 (18), 8957–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Deng L; Webb IK; Garimella SVB; Hamid AM; Zheng X; Norheim RV; Prost SA; Anderson GA; Sandoval JA; Baker ES; Ibrahim YM; Smith RD Anal. Chem 2017, 89 (8), 4628–4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Wernisch S; Afshinnia F; Rajendiran T; Pennathur S Anal. Bioanal. Chem 2018, 410 (12), 2865–2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Garimella SV; Hamid AM; Deng L; Ibrahim YM; Webb IK; Baker ES; Prost SA; Norheim RV; Anderson GA; Smith RD Anal. Chem 2016, 88 (23), 11877–11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Qiao X; Ye M; Liu CF; Yang WZ; Miao WJ; Dong J; Guo DA Steroids 2012, 77 (3), 204–11. [DOI] [PubMed] [Google Scholar]
- (23).Que AH; Konse T; Baker AG; Novotny MV Anal. Chem 2000, 72 (13), 2703–10. [DOI] [PubMed] [Google Scholar]
- (24).Zheng X; Zhang X; Schocker NS; Renslow RS; Orton DJ; Khamsi J; Ashmus RA; Almeida IC; Tang K; Costello CE; Smith RD; Michael K; Baker ES Anal. Bioanal. Chem 2017, 409 (2), 467–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Hilderbrand AE; Myung S; Clemmer DE Anal. Chem 2006, 78 (19), 6792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Dwivedi P; Wu C; Matz LM; Clowers BH; Siems WF; Hill HH Jr. Anal. Chem 2006, 78 (24), 8200–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Kulyk K; Rebrov O; Ryding M; Thomas RD; Uggerud E; Larsson MJ Am. Soc. Mass Spectrom 2017, 28 (12), 2686–2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Gaye MM; Nagy G; Clemmer DE; Pohl NL Anal. Chem 2016, 88 (4), 2335–44. [DOI] [PubMed] [Google Scholar]
- (29).Domalain V; Tognetti V; Hubert-Roux M; Lange CM; Joubert L; Baudoux J; Rouden J; Afonso CJ Am. Soc. Mass Spectrom 2013, 24 (9), 1437–45. [DOI] [PubMed] [Google Scholar]
- (30).Chouinard CD; Cruzeiro V. c. W. D.; Roitberg AE; Yost RA. J. Am. Soc. Mass Spectrom 2017, 28 (2), 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Deng J; Lu X; Constant C; Dogariu A; Fang J Chem. Commun. (Cambridge, U. K.) 2015, 51 (43), 8912–5. [DOI] [PubMed] [Google Scholar]
- (32).Hermankova E; Zak A; Polakova L; Hobzova R; Hromadka R; Sirc J Eur. J. Med. Chem 2018, 144, 300–317. [DOI] [PubMed] [Google Scholar]
- (33).Holm R; Nicolajsen HV; Hartvig RA; Westh P; Ostergaard J Electrophoresis 2007, 28 (20), 3745–52. [DOI] [PubMed] [Google Scholar]
- (34).Holm R; Schonbeck C; Askjaer S; Jensen H; Westh P; Ostergaard JJ Sep Sci 2011, 34(22), 3221–30. [DOI] [PubMed] [Google Scholar]
- (35).Liu Y; Shi J; Guo DS J. Org. Chem 2007, 72 (22), 8227–34. [DOI] [PubMed] [Google Scholar]
- (36).Momose T; Yamaguchi Y; Iida T; Goto J; Nambara T Lipids 1998, 33 (1), 101–8. [DOI] [PubMed] [Google Scholar]
- (37).Schonbeck C; Westh P; Madsen JC; Larsen KL; Stade LW; Holm R Langmuir 2011, 27 (10), 5832–41. [DOI] [PubMed] [Google Scholar]
- (38).Tidemand KD; Schonbeck C; Holm R; Westh P; Peters GH J. Phys. Chem. B 2014, 118 (37), 10889–97. [DOI] [PubMed] [Google Scholar]
- (39).Upadhyay SK; Kumar G Chem. Cent. J 2009, 3 (1), 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Del Valle EM M. Process Biochem 2004, 39 (9), 1033–1046. [Google Scholar]
- (41).Sallas F; Darcy R Eur. J. Org. Chem 2008, 2008 (6), 957–969. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.