Abstract
Metal nanoparticles can potentially contact human skin during their manufacture and use in commercial products. This study examined the potential of metal nanoparticles to elicit irritant contact dermatitis in a human skin equivalent model (HSEM) derived from keratinocytes. Ag (10–100 nm), TiO2 (22–214 nm) and CeO2 (15–40 nm) nanoparticles were studied. The Ag particles were either coated/shelled with silica or capped with citrate or polyvinylpyrrolidone and were in water. The TiO2 and CeO2 particles were suspended in media containing 10% fetal bovine serum. The particles (1 mg/ml) were applied to the epidermal surface of the HSEM. Positive (5% sodium dodecyl sulfate (SDS)) and negative controls (saline or media) were included. After 1 h exposure at 37 °C, the HSEM was washed with saline to remove the nanoparticles. Following a 42 h incubation (37 °C), HSEM viability was assessed using the MTT assay. A test substance is considered a dermal irritant if the HSEM viability is < 50%. The mean viability for the SDS treated HSEM was 7.8%. The viabilities of the nanoparticle treated HSEM were 91% or greater. The Ag, TiO2 and CeO2 nanoparticles examined were not dermal irritants under the conditions used in this study. The stratum corneum of the HSEM may limit penetration of metal nanoparticles to induce toxicity.
Keywords: nanoparticles, inorganic, metals, skin, irritation
Introduction
The ability to engineer materials on the nanoscale level has introduced new products that are used in medicine, cosmetics, energy and electronic fields and others. Products that contain nanomaterials, of which at least one of the dimensions of the material ranges between 1 and 100 nm, have the potential for human exposure1,2. This exposure could occur during the synthesis of the nanomaterials, manufacture and use of a product containing nanomaterials, and during their disposal. There is a need to carefully assess the toxicological properties of nanomaterials due, in part, to differences in their physical and chemical properties from bulk chemicals3.
Nanomaterials in use today have the potential to contact skin4,5. Titanium dioxide nanoparticles are used in sunscreen6 and cerium dioxide nanoparticles have been proposed to be used in this consumer product as well7. Silver nanoparticles are incorporated into textiles and have been proposed to be used as a preservative in cosmetics because of their anti-microbial properties8,9. Other occurrences of potential skin exposure to nanomaterials may happen during their manufacture or incorporation into other products. Diseases of the skin, such as irritant contact dermatitis, comprise up to 30% of all ailments related to occupational exposures10. Occupational skin diseases can impact the quality of life of the affected individual as well as the economic status of the individual and business from lost days of work and medical costs.
Irritant contact dermatitis is a non-immunologic, non-specific inflammatory response that results from direct chemical contact to the skin11,12. Clinically irritant dermatitis can range from dryness of the skin to burns13. Factors that influence the outward effects of irritant dermatitis include the properties of the chemical, concentration, acute or chronic exposure, environmental effects (e.g., temperature), skin phenotype, status of the skin (e.g., age, state of hydration) and ability of wounds to heal13. The information available on whether or not metal nanoparticles are dermal irritants is limited. Nanosized cerium dioxide (particle size of 9 nm), silica (7 and 10–20 nm), titanium dioxide (21 nm) and zinc oxide (35 nm) were not irritants in vitro to a human skin equivalent model derived from keratinocytes14, 15,16. In addition, there is no current evidence that metal nanoparticles are human dermal irritants.
The objective of this study was to assess the in vitro dermal irritation potential of cerium, silver, and titanium nanoparticles. These metal nanoparticles are found in a variety commercial products such as sunscreen (titanium dioxide, potentially cerium dioxide), fuel additives (cerium dioxide) and textiles (silver)6–9, 17. Dermal contact to these metal nanoparticles may occur from the use of these products or the release of the nanoparticles into the environment. The dermal toxicity of these nano-sized metals is not clear. For this study we used an in vitro 3-dimensional human skin equivalent model (HSEM) endorsed by the European Centre for the Validation of Alternative Methods (ECVAM) Scientific Advisory Committee as an acceptable alternative to in vivo assessment of skin irritation testing of chemicals18. This model and three similar HSEMs were used to develop in vitro skin irritation testing guidelines accepted by the Europeon Union (Organization for Economic Cooperation and Development (OECD) Test Guideline 439)19. This model is derived from normal human keratinocytes, is highly differentiated, and has a stratum corneum of approximately 10–15 cell layers. This specific skin model has been used previously to test the dermal irritancy of surfactants, personal care products and metal nanoparticles14,15,20,21. Results from studies such as presented here may aid in hazard assessments of the dermal irritancy potential of metal nanoparticles.
Materials and Methods
Test Chemicals
Various coated and sized silver nanoparticles (PVP, 50 nm; OECD PVP, 10 and 75 nm; Citrate, 10 and 50 nm; OECD Citrate, 75 nm; Silica Shelled, 50 nm; and Silica Coated, 80 and 100 nm) were purchased from nanoComposix, Inc. (San Diego, CA). Titanium dioxide nanoparticles were purchased from the following vendors: 22 nm and 25 nm anatase were purchased from Alfa Aesar (Wardfill, MA); 31 nm anatase/rutile (Aeroxide P25) was obtained from Degussa (Alpharetta, GA); 59 nm anatase/rutile was obtained from NanoAmor (Houston, TX); 142 nm anatase was purchased from Acros Organics (Morris Plains, NJ); and 214 nm rutile was obtained from Mknano (Mississauga, Ontario, Canada). Cerium dioxide nanoparticles were purchased from the following vendors: 58 nm from Alfa Aesar (Wardfill, MA); 8 nm from NanoAmor (Houston, TX); and 40 nm from Umicore (Middlesex, United Kingdom). See Kitchin et al.22 for a listing of the physical characteristics of the titanium and cerium nanoparticles used in this study.
Silver nitrate was purchased from Sigma Chemical Co. (St. Louis, MO). A 1 mg/ml solution of silver nitrate was prepared in Milli-Q water. Silver nitrate is soluble in water. Release of metal ions (e.g., Ag+2) from metal nanoparticles is one hypothesis for toxicity of these materials23,24.
EpiDerm™ Reconstructed Human Epidermis
The three-dimensional human skin equivalent model, EpiDerm™ (EPI-200), which is derived from human keratinocytes, was obtained from MatTek Corporation (Ashland, MA).
Preparation of nanoparticle test solutions
Silver nanoparticles obtained from the manufacturer were already dispersed in Milli-Q water at a concentration of 1 mg/ml. Nanoparticles received in dry powder form (titanium dioxide and cerium dioxide) were weighed on an analytical balance, suspended in cell culture medium (Dulbecco’s Modified Eagle’s Medium (DMEM) provided by MatTek) containing 10% fetal bovine serum at a concentration of 1 mg/ml and subsequently dispersed using a probe sonicator (Misonix Microson Ultrasonic Cell Disrupter XL, Farmingdale, NY) with 4.5 watts output for 3 pulses, 2 seconds/pulse25. For each experiment, a fresh stock suspension was prepared immediately prior to dosing the tissues. The surface zeta potential of the nanoparticles was determined by dynamic light scattering (DLS) with a Malvern Zetasizer Nano (Malvern Instruments Ltd., Worcestershire, United Kingdom). DLS measurements were taken in triplicate using automated measurement times and laser attenuation settings. The measurements were checked for quality of data using the Malvern Dispersion Software (V 5.10) correlograms and error reports. The zeta potential provides an indication of the stability of the nanoparticle. Dispersions of nanoparticles with higher degrees of stability have zeta potentials greater than +25 mV or less than −25 mV26. In contrast, dispersions of nanoparticles that have a low zeta potential have a tendency to aggregate.
EpiDerm™ tissue conditioning
EpiDerm™ tissue inserts were received via overnight shipment from the supplier in 24-well plates at 4 °C, on medium-supplemented agarose gel. Upon receipt, tissues were visually inspected to check for morphological defects. The tissue inserts were removed from the agarose gel and subsequently transferred into 6-well plates containing fresh 0.9 ml culture medium. The tissues were then preconditioned by incubation for 1 h at 37 °C, 5% CO2 and 95% relative humidity (RH). The medium was then exchanged and the incubation was continued overnight for an additional 18 h to release debris and compounds associated with transport stress.
EpiDerm™ tissue exposure to test solutions
Following overnight incubation, the tissues were topically exposed to the nanoparticles for 1 h. Each tissue insert was dosed with 1 mg/ml of nanoparticle test solution; 30 μl of the 1 mg/ml solution was applied directly to the apical surface of the EpiDerm™ tissue at 1 min intervals. For 10 nm OECD PVP silver, two lots were tested (Lot 1 (L1): DAG1158; Lot 2 (L2): DAG1487). Silver nitrate (1 mg/ml Milli-Q water) was similarly applied. Nylon mesh was placed on the surface of the tissue for the homogeneous, uniform distribution of test solution. Three to four tissue inserts were used for each test substance, as well as for the negative and positive controls. For each experiment, sterile Dulbecco’s Phosphate Buffered Saline (DPBS) and 5% sodium dodecyl sulfate (SDS) solution were applied as negative and positive controls, respectively. Both controls were run in parallel and treated identically to dosed tissues. After the last tissue was dosed, tissues were placed in the incubator (37 °C, 5% CO2 and 95% RH) for 35 (±1) min. At the end of 35 min incubation, tissues were taken out of the incubator and placed in a laminar flow hood until a 1 h exposure time had elapsed. Next, each tissue insert was thoroughly rinsed (~15 times) with DPBS to remove test materials from the tissue surface. Tissues were rinsed at 1 min intervals, in the same order as they were dosed, so that the total time each tissue was exposed to a sample was approximately 1 h. After the last rinse, tissues were entirely submerged in 150 ml DPBS to remove any remaining test materials. Tissues were then gently blotted and dried with sterile cotton swabs to remove excess liquid from the surface. Tissue inserts were transferred into new 6-well plates containing fresh culture media and placed in an incubator (37 °C, 5% CO2 and 95% RH) for 24 h to allow the development of cell damage. Tissue inserts were then transferred into new 6-well plates containing fresh culture media. Tissues were further incubated for another 18 h at 37 °C, 5% CO2 and 95% RH.
Assessment of EpiDerm™ tissue viability (MTT assay)
Tissue viability was determined using the 3-(4,5 dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, where a yellow tetrazolium salt is reduced to a purple formazan dye by succinate dehydrogenase located in mitochondria of living cells. The assay was performed according to guidelines provided by the tissue supplier (MatTek Corporation, Ashland, MA). Approximately 42 h post-exposure, tissue inserts were removed from the 6-well plates, blotted on sterile absorbent pads, and transfered into 24-well plates pre-filled with 0.3 ml of MTT assay solution. For each experiment, a 1 mg/ml stock solution of MTT was prepared immediately prior to use and warmed to 37 °C in a water bath. Tissues were incubated for 3 h at 37 °C, 5% CO2 and 95% RH. Following this 3 h incubation, tissues were removed from the MTT solution, rinsed on the outer edges three times with DPBS and transferred to a new 24-well plate. Tissues were then completely submerged on both sides by adding 2 ml of isopropanol to extract the formazan salt. The plate was sealed with Parafilm to reduce solvent evaporation, protected from light using aluminum foil, and the extraction was performed by gently shaking the plate on a shaker for 2 h. Following this 2-h extraction period, tissue inserts were punctured with an injection needle (~ 20 gauge) to allow the extract to run into the well and the solution was pipetted up and down three times until completely homogenized. For each tissue, two 200 μl aliquots of extraction solution were transferred into a 96-well microtiter plate for absorbance measurement. Isopropanol was used as the blank. The plates were read at a wavelength of 570 nm and optical density (OD) was measured using a spectrophotometer.
Data Analysis
The percent viability for each individual tissue treated with a test substance (TS), and the positive (PC) and negative (NC) control was determined by the following equations:
% relative viability of TS: [ODTS/ODMeaan of NC] × 100
% relative viability of PC: [ODPC/ODMeaan of NC] × 100
% relative viability of NC: [ODNC/ODMeaan of NC] × 100
One assay is considered exposures of the test solutions to 24 individual human skin equivalents. Each assay was run on a separate day. Data in the figures (Figures 1–5, each represents an individual assay) are presented as mean ± standard deviation, N=3–4. The data were analyzed by a one-way ANOVA followed by a Dunnett’s test for post-hoc analysis. The level of significance was p < 0.05. The between assay variability was assessed by an unpaired t-test with a significance level of p < 0.05.
Figure 1.
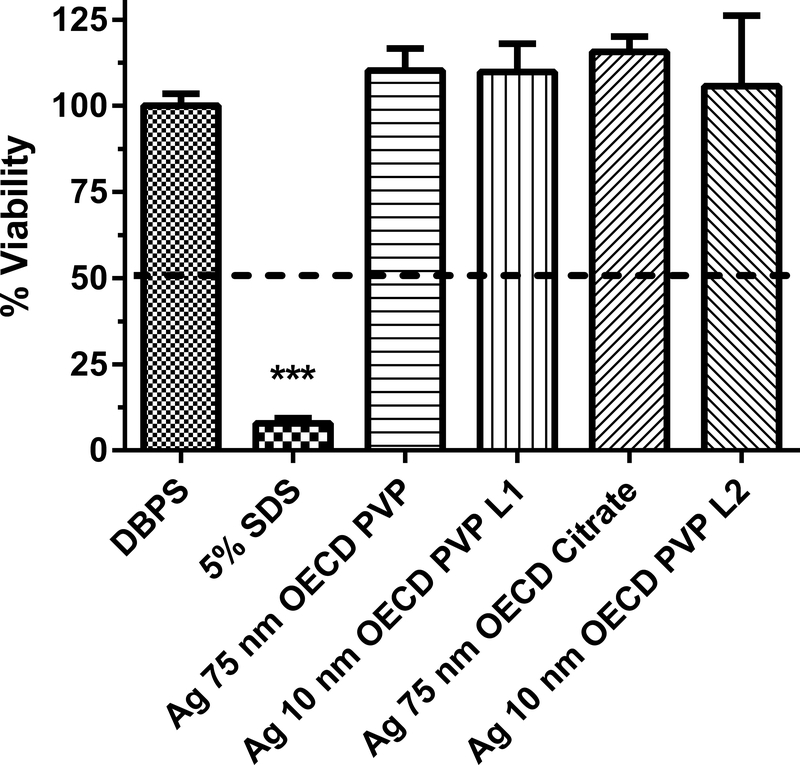
Human equivalent skin model tissue viability (%) exposed to silver nanoparticles of various sizes and coatings for 1 h. Data represents mean +/− SD, N=4. The concentration of each nanoparticle sample was 1 mg/mL. DPBS (Dulbecco’s phosphate buffered saline) was the negative control. 5% SDS (sodium dodecyl sulfate) was the positive control. PVP (polyvinyl pyrrolidine) is a coating. For Ag 10 nm PVP, two lots were used (L1 and L2). Chemicals with a mean value below the dashed line at 50% viability were considered to be dermal irritants. ***, significantly different from negative control, p < 0.001.
Figure 5.
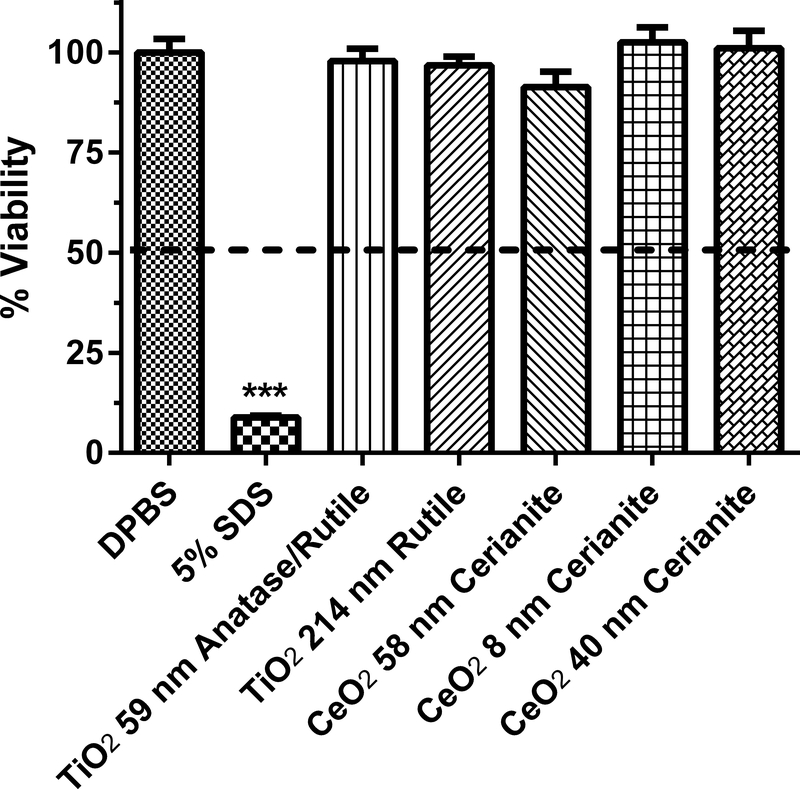
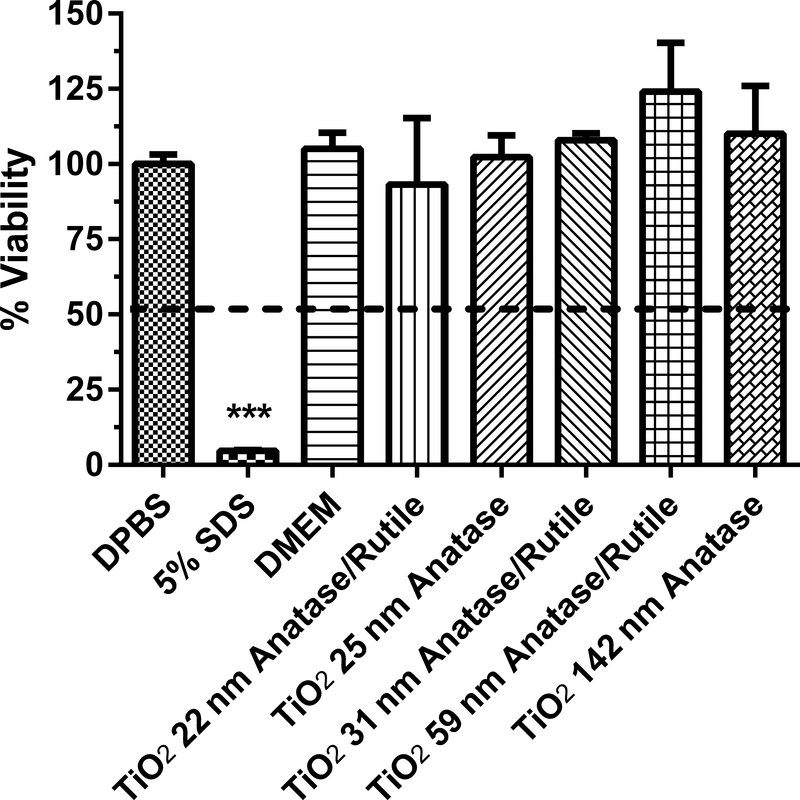
Human equivalent skin model tissue viability (%) exposed to titanium and cerium dioxide nanoparticles of various sizes and coatings for 1 h. Data represents mean ± SD, N=3. The concentration of each nanoparticle sample was 1 mg/mL. DPBS (Dulbecco’s phosphate buffered saline) was the negative control. 5% SDS (sodium dodecyl sulfate) was the positive control. DMEM (Dubelco’s minimum essential medium with 10% fetal bovine serum) was the vehicle for the TiO2 and CeO2 nanoparticles. Chemicals with a mean value below the dashed line at 50% viability were considered to be dermal irritants. ***, significantly from negative control, p < 0.001.
Results
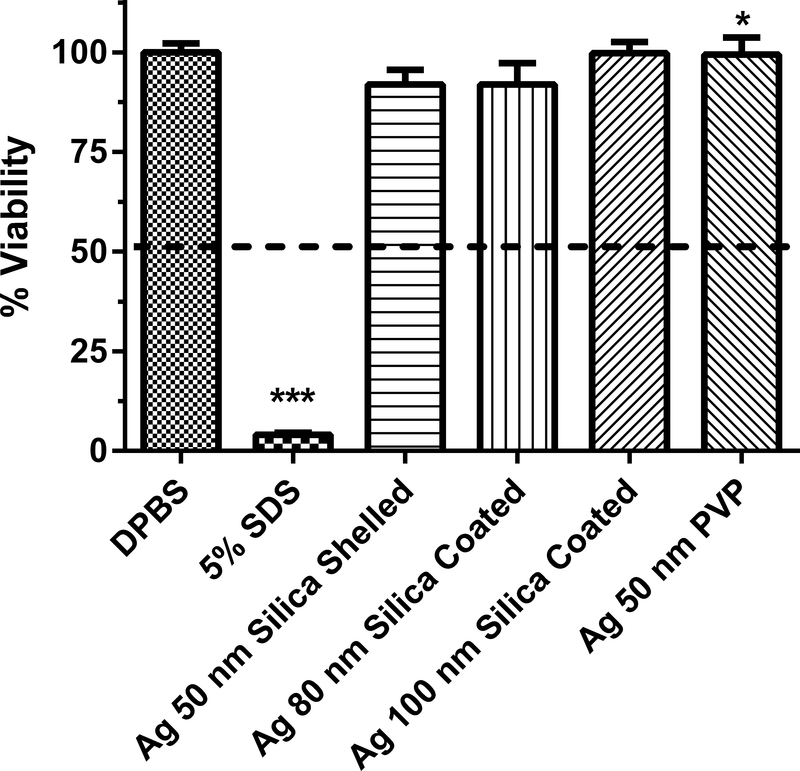
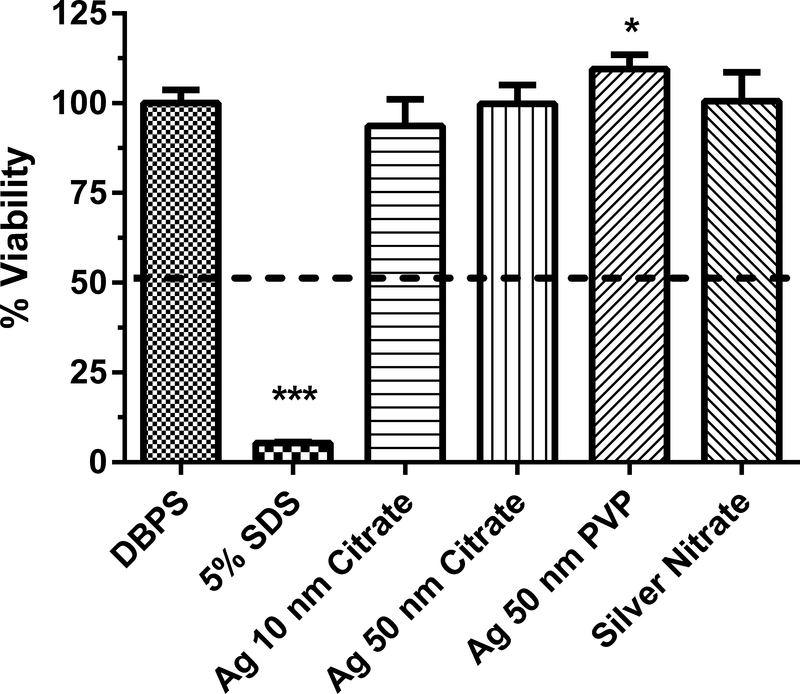
The dermal irritancy potential of Ag nanoparticles (1 mg/ml) ranging in size from 10 to 100 nm were assessed in an in vitro human skin equivalent model (Figs. 1–3). Silver nitrate, which is soluble in water, was tested because of the potential release of ionic silver from silver nanoparticles27. One potential mechanism of toxicity of silver nanoparticles is the release of silver ions28. The zeta potential of the Ag nanoparticles ranged from −47.4 to −6.4 mV (Table 1), indicating there was a wide variation in the dispersion of the silver nanoparticles in solution. All of the Ag nanoparticles and silver nitrate tested were not dermal irritants based on the conditions of the assay (i.e., no sample caused viability to be reduced to < 50%). For the Ag nanoparticles, the mean % viability of the HSEM ranged from 92 to 115% of control. Two different lots of the Ag 10 nm OECD PVP nanoparticles were tested to assess intra-lot variability (Fig. 1). The % viabilities of the HSEM between the two lots of Ag 10 nm PVP nanoparticles were 109.6 ± 8.2% (mean ± SD) and 105.7 ± 20.5% in the HSEM and were not significantly different. The between assay variability was tested with Ag 50 nm PVP (Figs. 2 and 3). The % viability of the HSEM between the two different assays was 109 ± 3.9% and 99.4 ± 4.3% for the Ag 50 nm PVP nanoparticle and were significantly different. However, the particles were not dermal irritants. In each of the silver nanoparticle assays, the positive control SDS was significantly lower (p < 0.001) than the control value. SDS is a dermal irritant in this assay.
Figure 3.
Human equivalent skin model tissue viability (%) exposed to silver nanoparticles of various sizes and coatings for 1 h. Data represents mean ± SD, N=3. The concentration of each nanoparticle sample was 1 mg/mL. DPBS (Dulbecco’s phosphate buffered saline) was the negative control. 5% SDS (sodium dodecyl sulfate) was the positive control. PVP (polyvinyl pyrrolidine) is a coating. Chemicals with a mean value below the dashed line at 50% viability were considered to be dermal irritants. ***, significantly from negative control, p < 0.001. *, % viability for Ag 50 nm PVP nanoparticle significantly less than that for Ag 50 nm PVP nanoparticle in Fig 2, p < 0.05.
Table 1.
Nanoparticles analyzed for dermal irritation, size, zeta-potential and source
| Nanoparticle | Size (nm) | Zeta-potential (mV) | Source |
|---|---|---|---|
| Ag OECD PVP | 10 | −6.43 | nanoComposix |
| Ag OECD PVP | 75 | −45.2 | nanoComposix |
| Ag PVP | 50 | −36.9 | nanoComposix |
| Ag Citrate | 10 | −6.89 | nanoComposix |
| Ag Citrate | 50 | −47.3 | nanoComposix |
| Ag OECD Citrate | 75 | −37.6 | nanoComposix |
| Ag Silica Shelled | 50 | −28.2 | nanoComposix |
| Ag Silica Coated | 80 | −17.4 | nanoComposix |
| Ag Silica Coated | 100 | −7.3 | nanoComposix |
| TiO2 Anatase/Rutile | 22 | −7.06 | Alfa Aesar |
| TiO2 Anatase | 25 | −10.3 | Alfa Aesar |
| TiO2 Anatase/Rutile | 31 | −11.6 | Degussa |
| TiO2 Anatase/Rutile | 59 | −9.96 | NanoAmor |
| TiO2 Anatase | 142 | −11.4 | Acros Organics |
| TiO2 Rutile | 214 | −12.5 | Mknano |
| CeO2 Cerianite | 8 | −11.4 | NanoAmor |
| CeO2 Cerianite | 58 | −11.05 | Alfa Aesar |
| CeO2 Cerianite | 40 | −9.67 | Umicore |
Figure 2.
Human equivalent skin model tissue viability (%) exposed to silver nanoparticles of various sizes and coatings for 1 h. Data represents mean ± SD, N=3–4. The concentration of each nanoparticle sample was 1 mg/mL. DPBS (Dulbecco’s phosphate buffered saline) was the negative control. 5% SDS (sodium dodecyl sulfate) was the positive control. PVP (polyvinyl pyrrolidine) is a coating. Chemicals with a mean value below the dashed line at 50% viability were considered to be dermal irritants. ***, significantly different from negative control, p < 0.001. *, % viability for Ag 50 nm PVP nanoparticle significantly greater than that for Ag 50 nm PVP nanoparticle in Fig 3, p < 0.05.
Four TiO2 nanoparticles (22–59 nm) and two TiO2 particles with sizes of 142 and 214 nm were assessed at a concentration of 1 mg/ml for in vitro dermal irritancy (Figs. 4–5). The zeta potential of the TiO2 nanoparticles and particles tested ranged from −12.5 to −7.1 mV (Table 1). The zeta potential values suggests that the TiO2 nanoparticles could have begun to aggregate. The media (DMEM with 10% fetal bovine serum (FBS)) used as a vehicle for the particles was not an irritant to the HSEM. For the TiO2 nanoparticles, the mean % viability of the HSEM ranged from 93 to 124% of control. The 59 nm TiO2 nanoparticle was tested in two different assays and the % viability was 124.0 ± 16.3% and 97.8 ± 3.1% (Figs 4 and 5). The values were not significantly different (p > 0.05). The SDS used in both assays with the TiO2 particles was significantly lower than the negative control (p < 0.001). The 31 nm TiO2 nanoparticle was tested at a dose range of 0.01 to 1 mg/ml (in DMEM with FBS) for 24 h in the HSEM and no significant irritant effects were observed (data not shown).
Figure 4.
Human equivalent skin model tissue viability (%) exposed to titanium dioxide nanoparticles of various sizes for 1 h. Data represents mean ± SD, N=3. The concentration of each nanoparticle sample was 1 mg/mL. DPBS (Dulbecco’s phosphate buffered saline) was the negative control. 5% SDS (sodium dodecyl sulfate) was the positive control. DMEM (Dubelco’s minimum essential medium with 10% fetal bovine serum) was the vehicle for the TiO2 nanoparticles. Chemicals with a mean value below the dashed line at 50% viability were considered to be dermal irritants. ***, significantly from negative control, p < 0.001.
Three CeO2 nanoparticles of sizes 8, 58 and 40 nm were assessed at a concentration of 1 mg/ml for in vitro dermal irritancy (Fig. 5). The zeta potential of the CeO2 nanoparticles tested ranged from −11.4 to −9.7 mV (Table 1). The zeta potential values suggests that the CeO2 nanoparticles had begun to aggregate. The mean % viability of the HSEM exposed to the CeO2 nanoparticles ranged from 91–103%. The CeO2 nanoparticles were not dermal irritants to the HSEM in this assay.
Discussion
The metal nanoparticles cerium, silver, and titanium tested in this study were not dermal irritants as determined by the in vitro assay using a human skin equivalent model, in that they did not reduce cell viability to less than 50% as specified by the ECVAM protocol. The positive control, 5% sodium dodecyl sulfate, was the only positive dermal irritant. Our results with cerium are consistent with Park et al.14, who tested a CeO2 nanoparticle (9 nm) used as a diesel fuel additive in the same human skin equivalent model, and did not report dermal irritation. The exposure times ranged from 16–24 h, as opposed to 1 h in the present study. Titanium dioxide (21 nm) tested in a different HSEM for 45 min was also not a skin irritant16. In addition, nanosilica (7 and 10–20 nm particle sizes), a metalloid, is not a dermal irritant following 5 and 18 h exposure periods in the same human skin equivalent model used in the present study15.
The titanium and cerium nanoparticles tested in this study are not without in vitro effect. The same TiO2 particles (22, 25, 31, 59, 142 and 214 nm) and two of the CeO2 particles (8 and 58 nm) tested in the present study have been noted to induce oxidative stress22. Although oxidative stress is a proposed mechanism of cytotoxic action of nanoparticles29–31, it should be noted that we did not test for oxidative stress in this study. Kitchin et al.22 conducted immuno-spin trapping studies and observed that the adduction of the spin trap agent 5,5-dimethyl-1-pyroline N-oxide to DNA increased with concentrations of the titanium and cerium nanoparticles starting at an exposure concentration of 100 μg/ml or greater. There were differences in potency of the nanoparticles, with the 25 and 31 nm TiO2 and 58 nm CeO2 nanoparticles being the most potent of the Ti and Ce nanoparticles tested. Sanders et al.25 and Yin et al.32 have investigated the cytotoxicity and phototoxicity of several of the TiO2 nanoparticles examined in the present study. Using a human-derived line of retinal pigment epithelial cells, Sanders et al.25 reported that the 31 nm TiO2 nanoparticle was the most potent both in the absence and presence of ultraviolet light A radiation (UVA). Yin et al.32 observed that the 31 nm TiO2 nanoparticle was the most phototoxic using UVA with a transformed epidermal human cell line (HaCaT keratinocytes). In both of the cellular studies, it was proposed that the phototoxicity was due to the generation of reactive oxygen species. AshaRani et al.33 and Mukherjee et al.34 have reported on the cytotoxicity of several Ag nanoparticles (not evaluated in the present study) in human cells and cell lines (lung fibroblasts, glioblastoma cells, keratinocytes and cervical cells). Cytotoxicity was dependent on the cell type utilized, dose and the length of exposure. In both studies, the generation of reactive oxygen species by the Ag nanoparticles was noted.
The formed stratum corneum in the 3-dimensional human skin equivalent model used in this study potentially retarded the diffusion of the nanoparticles into the viable regions of the HSEM. This hindrance to absorption could explain the lack of an effect observed with the tested nanoparticles. Human skin equivalent models, as used in this assay, do have barrier properties, but less so than the human epidermis35. Park et al.15 tested the dermal toxicity of nanosilica particles in cultured human keratinocytes, the same HSEM used in this study, and in vivo (Draize dermal irritation test using the rabbit as a model). The viability of the cultured keratinocytes was decreased by the nanosilica particles in a dose-dependent manner. However, the HSEM was not affected by these nanosilica particles. There was also no in vivo response of the nanosilica particles in the Draize skin irritation test. Dermal irritation of titanium dioxide and zinc oxide was not observed in vitro with a similar HSEM nor in rabbit in vivo16. Labouta and Schneider36 have reviewed the literature on the interaction of inorganic nanoparticles with skin and the studies they reviewed had shown limited (if any at all) absorption of titanium and silver nanoparticles in human skin. The dermal absorption of CeO2 nanoparticles is not known. Larese et al.37 have reported that in vitro dermal absorption of Ag nanoparticles (approx. size, 25 nm) coated with polyvinylpirrolidone in full-thickness human skin was detectable but low and that damaged skin was more permeable to the nanoparticles. Using electron microscopy, the authors found that the Ag nanoparticles were located in the stratum corneum and the upper layers of the epidermis. Pflücker et al.38 exposed human skin in vitro to one of three TiO2 nanoparticles (one of particle size 20 nm and the other two with size of 100 nm) each with different coatings. Using electron microscopy, they observed that these nanoparticles were detected only in the outer regions of the stratum corneum. Mavon et al.39 reported that the in vivo and in vitro distribution of 20 nm TiO2 nanoparticles in human skin was limited to the stratum corneum. The metal nanoparticles examined in this study may have limited ability to penetrate skin beyond the stratum corneum and thus were unable to induce dermal irritancy in vitro.
Under the conditions used in our study, the nanoparticles were applied in an aqueous vehicle. The Ag nanoparticles were obtained in water and applied directly to the tissue following sonication. The cerium and titanium nanoparticles were obtained as a powder. For this study, cerium and titanium nanoparticles were suspended in DMEM with 10% FBS and sonicated before application to the HSEM. This was done for consistency with planned toxicity studies with these nanoparticles in a two dimensional human keratinocyte model. Nanoparticles are known to react with proteins, forming a protein corona40. The potential exists that the cerium and titanium nanoparticles interacted with the FBS in the media, forming a protein corona. This interaction could have impacted the ability of the nanoparticles to interact with the skin and may also be an explanation for the lack of dermal irritancy of these nanoparticles in this in vitro model.
Conclusion
The silver, cerium and titanium nanoparticles tested can be classified as non-irritants in this human skin equivalent in vitro model. A potential explanation for this observation is that the nanoparticles have limited ability to penetrate the stratum corneum of the 3-D model, and are unable to reach sensitive cellular layers to elicit a cytotoxic effect. In addition, these nanoparticles, may have low inherent dermal irritancy potential, even though they have been noted to induce oxidative stress in vitro. Overall, under the in vitro conditions used in this study, the tested cerium, silver and titanium nanoparticles were not dermal irritants.
Acknowledgements
This article has been reviewed in accordance with the policy of the National Health and Environmental Effects Research laboratory, U.S. Environmental Protection Agency and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use. The authors thank Drs. Stephen Gavett, Jane Ellen Simmons and David Thomas for their review of a previous version of this manuscript. Funding of this project was from internal research funds of the U.S. Environmental Protection Agency.
Footnotes
Disclosure
No competing financial interests exist.
References
- 1.Seaton A, Tran L, Aitken R, et al. Nanoparticles, human health hazard and regulation. J R Soc Interface 2010:7;S119–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yokel RA, MacPhail RC. Engineered nanomaterials: exposures, hazards, and risk prevention. J Occup Med Toxicol 2011:6;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharifi S, Behzadi S, Laurent S, et al. Toxicity of nanomaterials. Chem Soc Rev 2011:41;2323–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baroli B Skin absorption and potential toxicity of nanoparticulate nanomaterials. J Biomed Nanotechnol 2010:6;485–496. [DOI] [PubMed] [Google Scholar]
- 5.Smijs TG, Bouwstra JA. Focus on skin as a possible port of entry for solid nanoparticles and the toxicological impact. J Biomed Nanotechnol 2010:6;469–484. [DOI] [PubMed] [Google Scholar]
- 6.Nohynek GJ, Lademann J, Ribaud C, et al. Grey goo on the skin? Nanotechnology, cosmetic and sunscreen safety. Crit Rev Toxicol 2007:37;251–277. [DOI] [PubMed] [Google Scholar]
- 7.Herrling T, Seivert M, Jung K. 2013. Cerium dioxide: future UV-filter in sunscreen? SOFW J 2013:5-2013;139. [Google Scholar]
- 8.Kokura S, Handa O, Takagi T, et al. Silver nanoparticles as a safe preservative for use in cosmetics. Nanomedicine: NBM 2010:6;570–574. [DOI] [PubMed] [Google Scholar]
- 9.Bartłomiejczyk T, Lankoff A, Kruszewski M, et al. Silver nanoparticles – allies or adversaries? Ann Agric Environ Med 2013:20;48–54. [PubMed] [Google Scholar]
- 10.English JSC. Current concepts of irritant contact dermatitis. Occup Environ Med 2004:61; 722–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chew AL, Maibach HI. Occupational issues of irritant contact dermatitis. Int Arch Occup Environ Health 2003:76;339–346. [DOI] [PubMed] [Google Scholar]
- 12.Lushniak BD. Occupational contact dermatitis. Dermatol Ther 2004:17;272–277. [DOI] [PubMed] [Google Scholar]
- 13.Nosbaum A, Vocanson M, Rozieres A, et al. Allergic and irritant contact dermatitis. Eur J Dermatol 2009:119;325–332. [DOI] [PubMed] [Google Scholar]
- 14.Park B, Martin P, Harris C, et al. Initial in vitro screening approach to investigate the potential health and environmental hazards of Envirox™ – a nanoparticulate cerium oxide diesel fuel additive. Part Fibre Toxicol 2007:4;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park YH, Kim JN, Jeong SH, et al. Assessment of dermal toxicity of nanosilica using cultured keratinocytes, a human skin equivalent model and an in vivo model. Toxicology 2010:267;178–181. [DOI] [PubMed] [Google Scholar]
- 16.Choi J, Kim H, Choi J, et al. Skin corrosion and irritation test of sunscreen nanoparticles using reconstructed 3D human skin model. Environ Health Toxicol 2014:29;1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cassee FR, van Balen EC, Singh C, et al. Exposure, health and ecological effects review of engineered nanoscale cerium and cerium oxide associated with its use as a fuel additive. Crit Rev Toxicol 2011:41;213–229. [DOI] [PubMed] [Google Scholar]
- 18.ECVAM. ESAC statement on the validity of in vitro tests for skin irritation. ALTA 2007:35; 308–312. [Google Scholar]
- 19.OECD Guidelines for the Testing of Chemicals, Section 4. Test No. 439: In Vitro Skin Irritation: Reconstructed Human Epidermis Test Method. 28 July 2015. [Google Scholar]
- 20.Kubilus J, Cannon C, Neal P, et al. Response of the EpiDerm skin model to topically applied irritants and allergens. In Vitro Toxicol 1996:9;157–166. [Google Scholar]
- 21.Perkins MA, Osborne R, Rana FR, et al. Comparison of in vitro and in vivo human skin responses to consumer products and ingredients with a range of irritancy potential. Toxicol Sci 1999:48;218–229. [DOI] [PubMed] [Google Scholar]
- 22.Kitchin KT, Prasad RY, Wallace K. Oxidative stress studies of six TiO2 and two CeO2 nanomaterials: immuno-spin trapping results with DNA. Nanotoxicology 2011:5;546–556. [DOI] [PubMed] [Google Scholar]
- 23.Auffan M, Rose J Wiesner MR, et al. Chemical stability of metallic nanoparticles: a parameter controlling their potential celular toxicity in vitro. Environ Pollut 2009:157;1127–1113. [DOI] [PubMed] [Google Scholar]
- 24.Prabhu S, Poulose EK. Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications and toxicity effects. Inter Nano Lett 2012:2:32. [Google Scholar]
- 25.Sanders K, Degn LL, Mundy WR, et al. In vitro phototoxicity and hazard identification of nano-scale titanium dioxide. Toxicol Appl Pharmacol 2012:258;226–236. [DOI] [PubMed] [Google Scholar]
- 26.nanoComposix, 2012. Zeta Potential Analysis of Nanoparticles. Version 1.1, San Diego, CA. [Google Scholar]
- 27.Benn TM, Westerhoff P. Nanoparticle silver released into water from commercially available sock fabrics. Environ Sci Technol 2008:42;4133–4139. [DOI] [PubMed] [Google Scholar]
- 28.van Aerle R, Lange A, Moorhouse A, et al. Molecular mechanism of toxicity of silver nanoparticles in Zebrafish embryos. Environ Sci Technol 2013:47;8005–8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horie M, Kato H, Fujita K, et al. In vitro evaluation of cellular response induced by manufactured nanoparticles. Chem Res Toxicol 2012:24;605–619. [DOI] [PubMed] [Google Scholar]
- 30.Fard JK, Jafari S, Eghbal MA. A review of molecular mechanisms involved in toxicity of nanoparticles. Adv Pharm Bull 2014:5;447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arora S, Rajwade JM, Paknikar KM. Nanotoxicology and in vitro studies: the need of the hour. Toxicol Appl Pharmacol. 2012:258-151–165. [DOI] [PubMed] [Google Scholar]
- 32.Yin JJ, Liu J, Ehrenshaft M, et al. Phototoxicity of nano titanium dioxides in HaCaT keratinocytes – generation of reactive oxygen species and cell damage. Toxicol Appl Pharmacol 2012:263;81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.AshaRani PV, Low Kah Mun G, Hande MP, et al. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 2009;3;279–290. [DOI] [PubMed] [Google Scholar]
- 34.Mukherjee SG, O’Claonadh N, Casey A, et al. Comparative in vitro cytotoxicity study of silver nanoparticle on two mammalian cell lines. Toxicol in Vitro 2012:26;238–251. [DOI] [PubMed] [Google Scholar]
- 35.Zgoul N, Fuchs R, Lehr CM, et al. Reconstructed skin equivalents for assessing percutaneous absorption from pharmaceutical formulations. ALTEX 2001:18;103–106. [PubMed] [Google Scholar]
- 36.Labouta HI, Schneider M. 2013. Interaction of inorganic nanoparticles with the skin barrier: current status and critical review. Nanomedicine: NBM 2013:9;39–54. [DOI] [PubMed] [Google Scholar]
- 37.Larese FF, D’Agostin F, Crosera M, et al. Human skin penetration of silver nanoparticles through intact damages skin. Toxicology 2009:255;33–37. [DOI] [PubMed] [Google Scholar]
- 38.Plücker F, Wendel V, Hohenberg H, et al. The human stratum corneum layer: an effective barrier against dermal uptake of different forms of topically applied micronized titanium dioxide. Skin Pharmacol Appl Skin Physiol 2001:14 (suppl. 1);92–97. [DOI] [PubMed] [Google Scholar]
- 39.Mavon A, Miquel C, Lejeune O, et al. In vitro percutaneous absorption and in vivo stratum corneum distribution of an organic and a mineral sunscreen. Skin Pharmacol Physiol 2007:20;10–20. [DOI] [PubMed] [Google Scholar]
- 40.Docter D, Westmeir D, Markiewicz M, et al. The nanoparticle biomolecule corona – lessons learned – challenge accepted? Chem Soc Rev 2015:44;6094–6121. [DOI] [PubMed] [Google Scholar]