Abstract
BMAL1, the nonredundant transcription factor in the core molecular clock, has been implicated in cardiometabolic diseases in mice and humans. BMAL1 controls the cyclic trafficking of Ly6chi monocytes to sites of acute inflammation. Myeloid deficiency of Bmal1 also worsens chronic inflammation in diet-induced obesity. We studied whether myeloid Bmal1 deletion promotes atherosclerosis by enhancing monocyte recruitment to atherosclerotic lesions. By generating Bmal1FloxP/FloxP;LysMCre mice on the Apoe−/− background, we showed that Bmal1 deletion in myeloid cells increased the size of atherosclerotic lesions. Bmal1 deficiency in monocytes and macrophages resulted in an increased total number of lesional macrophages in general and Ly6chi infiltrating monocyte-macrophages in particular, accompanied by skewed M2 to M1 macrophage phenotype. Ly6chi and/or Ly6clo monocyte subsets in blood, spleen, and bone marrow were not altered. Cell tracking and adoptive transfer of Ly6chi monocytes showed Bmal1 deficiency induced more trafficking of Ly6chi monocytes to atherosclerotic lesions, preferential differentiation of Ly6chi monocytes into M1 macrophages, and increased macrophage content and lesion size in the carotid arteries. We demonstrated that Bmal1 deficiency in macrophages promotes atherosclerosis by enhancing recruitment of Ly6chi monocytes to atherosclerotic lesions.—Huo, M., Huang, Y., Qu, D., Zhang, H., Wong, W. T., Chawla, A., Huang, Y., Tian, X. Y. Myeloid Bmal1 deletion increases monocyte recruitment and worsens atherosclerosis.
Keywords: molecular clock, chronic inflammation, macrophage
The molecular clock is expressed in most cell types and regulates a variety of cellular mechanisms to anticipate changes of environment (1). A significant proportion of the mammalian transcriptome has been reported to be expressed in a rhythmic pattern and is regulated by the circadian clock (2, 3). The core clock genes Bmal1 (encoded by Arntl) and Clock, when active, induce transcription of Per and Cry (4). Accumulation of PER and CRY protein is followed by their nuclear translocation to repress CLOCK:BMAL1 activity, forming a feedback regulatory loop. Meanwhile, Bmal1 expression is regulated by another feedback loop through the nuclear receptor retinoid-related orphan receptor α, which induces Bmal1 transcription, whereas nuclear receptor REV-ERBα has the opposing effect (5). Through these feedback mechanisms, molecular clock function is tightly regulated, with CLOCK:BMAL1 being the nonredundant clock genes. A genetic approach to modulate Clock or Bmal1 expression and activity has been used to study the function of molecular clock of peripheral tissues in different diseases, including diabetes, cancer, and hypertension.
Key components of the immune system, including the number of hematopoietic cells and cytokine production, follow a diurnal oscillation governed by the molecular clock (6, 7). For example, studies in mice showed that the amplitude of inflammation and mice to acute inflammation induced by pathogens peaks at early evening, accompanied by reduced survival (8, 9). This phenomenon might be an adaptation to detect and eliminate pathogens at the time when it is most likely to happen. Chronic inflammatory diseases also exhibit a circadian pattern of disease manifestation. For example, in humans, myocardial infarction incidents peak in the early morning (10). Disruption of components of the molecular clock alters immune responses. For example, deletion of Cry1 and Cry2 leads to constant elevation of proinflammatory cytokines (11). In macrophages from Bmal1−/− mice, both the basal and tumor necrosis factor (TNF)-α-induced NF-κB activation are up-regulated (12, 13). Previous study showed that Bmal1 is required to maintain diurnal oscillation of inflammatory Ly6chi monocytes in blood, spleen, and bone marrow (14). Bmal1 deletion also promoted monocyte trafficking and decreased survival in Listeria monocytogenes infection in mice (14). Higher expression of chemokines Ccl2, Ccl8, and S100a8 in monocytes and macrophages in Bmal1-deficient mice were associated with an active chromatin state (14), suggesting that Bmal1 deletion predisposes mice to acute and chronic inflammation.
Monocytes and macrophages are the key players in atherogenesis (15). Driven by hypercholesterolemia, inflammatory Ly6chi monocytes adhere to inflamed endothelium and differentiate into lesional macrophages (15). This process is critical for the initiation and exacerbation of atherosclerotic plaque formation. In addition, the functional aspects of lesional macrophages such as M1 and M2 polarization, proliferation, apoptosis, and cholesterol efflux are also important for the progression of atherogenesis. Previous studies have shown that disturbance of the molecular clock at different nodes (Clock, Bmal1, and Per) promotes atherosclerosis, whereas the Rev-Erbα agonist suppresses atherosclerosis (16–19). However, these studies have used global Clock-knockout mice rather than tissue-specific knockout mice. It remains largely unclear whether Bmal1-mediated monocyte recruitment is a major contributor for exacerbated atherosclerosis that was observed with global Bmal1 or Clock deletion. In the present study, by generating Bmal1FloxP/FloxP;LysMCre mice on Apoe−/− background, we aimed at answering whether enhanced monocyte recruitment by Bmal1 deletion in myeloid cells promotes atherosclerosis.
MATERIALS AND METHODS
Animals and treatments
Bmalf/f (ArntlfloxP), LysMCre/+ (Lyz2Cre), and Apoe−/− mice were from The Jackson Laboratory (Bar Harbor, ME, USA) and shipped to the Laboratory Animal Services Center of the Chinese University of Hong Kong. Mice were backcrossed to B6 background and later crossbred to get the Apoe−/−;Bmalf/f as control and Apoe−/−;Bmalf/f;LysMCre/+ as myeloid Bmal1-deficient mice on Apoe−/− background for experiments. To develop atherosclerosis, both genotypes were fed Western diet (WD) (0.21% cholesterol; Research Diet, New Brunswick, NJ, USA) for 12 wk starting from 6 wk of age. All mice where housed at 22°C under a 12 h/12 h light/dark cycle (light on at 6 am; light off at 6 pm). Cholesterol, non-high-density lipoprotein, and triglyceride in the plasma were measured with enzymatic methods (Stanbio, Boerne, TX, USA). All animal experiments were approved by the Hong Kong Department of Health and the Animal Experimentation Ethics Committee of the Chinese University of Hong Kong.
Histology
Atherosclerotic plaque area was assessed by en face Oil Red O staining of aorta. Images were taken using a stereomicroscope connected to a camera (Leica Microsystems, Buffalo Grove, IL, USA) and analyzed using ImageJ (National Institutes of Health, Bethesda, MD, USA) for percentage of lesion coverage/total aortic area. Aortic root and left carotid artery were frozen in optimal cutting temperature compound and sectioned for Oil Red O staining to detect lipid content and in Masson’s trichrome staining to examine collagen deposition. CD68 (ab955; Abcam, Cambridge, United Kingdom), CD206 (ab64693; Abcam), and Ly6c (ab15627; Abcam) antibodies and secondary antibodies (Alexa Fluor 488 and 555; Thermo Fisher Scientific, Waltham, MA, USA) were used for immunostaining of aortic root frozen sections. Immunofluorescence images were taken with Olympus FV1000 confocal system (Olympus, Center Valley, PA, USA).
Flow cytometry
Mice were killed between zeitgeber time (ZT)4 to -6. Aortas were perfused with PBS with heparin (100 U/ml), dissected free from adventitia and perivascular adipose tissue, and dissociated with enzyme mixtures containing collagenase I (450 U/ml), collagenase XI (125 U/ml), DNase I (60 U/ml), and hyaluronidase (60 U/ml) (Worthington Biochemical, Lakewood, NJ, USA) at 37°C for 1 h with gentle shaking. Digested tissues were minced and filtered (70 μm) to create a single-cell suspension (20). Pelleted cells were resuspended in FACS buffer (PBS, 2 mM EDTA, and 5% fetal bovine serum) for immunostaining and the subsequent flow cytometric analyses. Antibodies used after Fc-block (101320) include CD45 (30-F11; 103132), F4/80 (BM8; 123121, 123110), Ly6c (HK1.4; 128016, 128012), Ly6g (1A8; 35-1276), CD11c (N418; 117327), CD115 (AFS98; 135510, 135506), CD206 (C068C2; 141707), CD8 (53-6.7; 100726) from Biolegend; CD11b (M1/70; 60-0112), CD3 (145-2C11; 50-0031), and CD4 (GK1.5; 65-0042) all from Tonbo Biosciences (San Diego, CA, USA); and CD301 (ER-MP23; MCA2392A647) from Bio-Rad (Hercules, CA, USA). Samples were fixed in FluoroFix buffer (BioLegend, San Diego, CA, USA) and stored at 4°C until analysis. For intracellular staining, cells were fixed and permeabilized using the Fixation/Permeabilization kit (BD Biosciences, San Jose, CA, USA) per the manufacturer’s instructions.
BrdU incorporation
For in vivo BrdU incorporation experiments to examine tissue macrophage proliferation, BrdU (Sigma-Aldrich, St. Louis, MO, USA) was supplemented at 0.5 mg/ml in drinking water for 1 wk. BrdU staining was performed in aortic cells with APC anti-BrdU antibody (Bu20a, catalogue no. 339808; BioLegend). The aortic cells were also stained with APC anti-Ki67 (SolA15; 50-5698-82; eBioscience, San Diego, CA, USA) for further confirmation.
In vivo monocyte labeling
To track Ly6chi monocytes in vivo, mice were first injected intraperitoneally with clodronate (1 mg) containing liposome (0.2 ml suspension) to deplete Ly6clo monocytes as previously described (21). Twenty-four hours later, mice were injected intravenously through the tail vein with 1 μm Fluoresbrite Yellow Green (YG) Microspheres (referred to hereafter as YGs) (Polysciences, Warminster, PA, USA) at ZT8 as previously described (21) and killed 48 h later. The aorta was used for flow cytometric analysis, and the left carotid artery was kept in optimal cutting temperature compound for cryosection to examine YG+ cells in the plaque.
Adoptive transfer of bone marrow monocytes
Apoe−/− mice fed WD for 9 wk were injected intraperitoneally with liposome clodronate to deplete monocytes (22). Two days later, bone marrow Ly6chi monocytes were isolated from tibia and femur from Apoe−/−;Bmalf/f and Apoe−/−;Bmalf/f;LysMCre/+ mice using a monocyte isolation kit and magnetic separation (Miltenyi Biotec, Bergisch Gladbach, Germany). Cells were stained with PKH26 (Sigma-Aldrich) per the manufacturer’s protocol. At ZT8, approximately 2 × 106 cells were injected intravenously through the tail vein into clodronate-treated Apoe−/− mice. Mice were killed 14 d later for analysis of aortic cell composition to examine percentage and cell number of PKH26+ cells. The left carotid artery was used for cryosection to examine lesion coverage and PKH26+ cell localization in the plaque.
Statistical analysis
Flow cytometry data were acquired using BD FACS Fusion and analyzed using FlowJo (Ashland, OR, USA). Bright field, phase contrast, or fluorescence images were taken with Nikon TE300 inverted microscope with CCD camera. Double immunofluorescence was taken with Olympus FV1000 confocal system. Data were analyzed with GraphPad Prism (GraphPad Software, La Jolla, CA, USA) using Student’s t test between 2 groups, or 1-way ANOVA followed by Tukey’s test for more than 2 groups. Values of P < 0.05 or P < 0.01 were considered as statistically significant.
RESULTS
Bmal1 deficiency in myeloid cells increases atherosclerotic lesion coverage
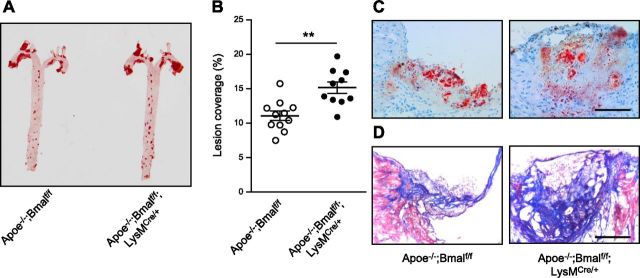
To examine the role of myeloid-specific Bmal1 on atherogenesis, we crossbred Bmalf/f and Bmalf/f;LysMCre/+ with Apoe−/− to get wild-type (Bmalf/f) mice and mice deficient in myeloid Bmal1 (Bmalf/f;LysMCre/+) on Apoe−/− background. Rhythmic expression of Bmal1 mRNA was observed in bone marrow-derived macrophages (BMDMs) from Apoe−/−;Bmalf/f mice but not from Apoe−/−;Bmalf/f;LysMCre/+ mice (Supplemental Fig. 1A). Rhythmic expressions of 2 other clock genes Cry1 and Rev-Erbα were also inhibited in BMDMs from Apoe−/−;Bmalf/f;LysMCre/+ mice (Supplemental Fig. 1B, C). It has previously been shown that BMAL1 repressed Ccl2 transcription by binding to an E-box in the Ccl2 promoter (14). Our results suggest that loss of Bmal1 resulted in attenuated suppression of Ccl2 transcription, leading to higher Ccl2 expression in BMDMs from mice deficient in myeloid Bmal1 (Supplemental Fig. 1D). Lesion coverage in aorta measured by en face Oil Red O staining demonstrated increased lesion size in Apoe−/−;Bmalf/f;LysMCre/+ mice compared with Apoe−/−;Bmalf/f fed WD for 12 wk (Fig. 1A, B). Oil Red O staining revealed increased lesion size and lipid content in the aortic root section from Apoe−/−;Bmalf/f;LysMCre/+ mice (Fig. 1C). Masson’s trichrome staining also indicated larger lesions with higher collagen content and an intact fibrous cap from Apoe−/−;Bmalf/f;LysMCre/+ mice (Fig. 1D).
Figure 1.
Myeloid Bmal1 deletion increases atherosclerotic lesion size, accompanied by more infiltrating macrophages in the lesion. Representative images (A) and summarized data (B) of lesion coverage measured by Oil Red O staining in the en face aortae from Apoe−/−; Bmalf/f; LysMCre/+ and Apoe−/−; Bmalf/f mice fed a WD for 12 wk. C, D) Representative images of Oil Red O staining (C) and Masson’s trichrome staining (from n = 6 of each group) (D) in aortic root sections from both groups. Data are means ± sem. **P < 0.01 vs. Apoe−/−; Bmalf/f.
Bmal1 deficiency leads to more macrophages and T cells in the plaque
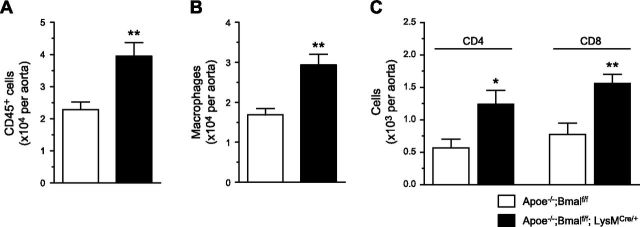
The progression of atherogenesis is highly dependent on lesional macrophage content and phenotype. Flow cytometric analysis was used to analyze the content and phenotype of cells from aortas with atherosclerotic lesions. We found that aortas from mice deficient in myeloid Bmal1 contained more CD45+ cells (Fig. 2A). This increase was accompanied by more lesional macrophages (gated as viable CD45+CD11b+F4/80int+hi) in mice deficient in myeloid Bmal1 (Fig. 2B). In addition, an increase in innate immune cell number is accompanied by an increase in adaptive immune components, reflected by more CD4+ and CD8+ T cells within the plaque with Bmal1 deficiency (Fig. 2C).
Figure 2.
Myeloid Bmal1 deletion increases macrophages and T cells in plaque. Summarized data of total CD45+ cell count (A), total macrophage numbers (B), and CD4 and CD8 T cell numbers (C) in aorta (CD45+CD3+ cells are gated on CD4 and CD8). Data are means ± sem (n = 8). *P < 0.05, **P < 0.01 vs. Apoe−/−; Bmalf/f.
Bmal1 deficiency leads to proinflammatory macrophage phenotype
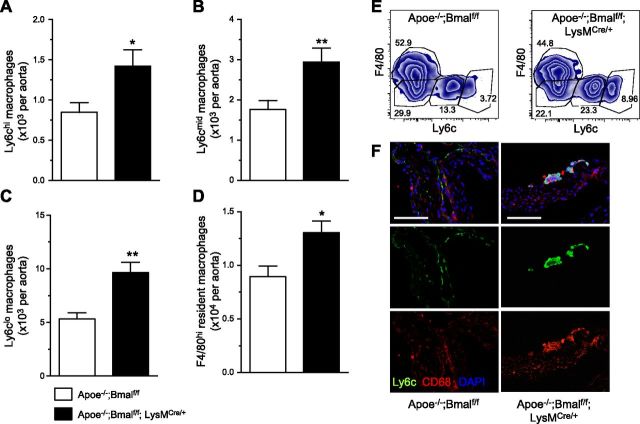
Because newly recruited monocytes gradually lose Ly6c expression and become tissue macrophages in peripheral tissues during both chronic and acute inflammation (23–26), we further analyzed the subsets of lesional macrophages from both genotypes. Macrophage subsets based on the expression of F4/80 and Ly6c (Fig. 3E) revealed increased macrophage numbers in all subsets, especially in Ly6chi, Ly6cmid, and Ly6clo cells (Fig. 3A–C), and a modest but significant increase of resident F4/80hi macrophages (Fig. 3D), indicating enhanced Ly6c+ monocyte-derived macrophage recruitment into the plaque with myeloid Bmal1 deficiency. Immunostaining of the macrophage markers CD68 and Ly6c of aortic root also showed more Ly6c expression in the plaques with myeloid Bmal1 deficiency (Fig. 3F).
Figure 3.
Myeloid Bmal1 deficiency leads to proinflammatory macrophage phenotype. A–D) Summarized data of Ly6chi monocyte-derived macrophages (A), Ly6cmid macrophages (B), Ly6clo macrophages (C), and F4/80hi macrophages (D) in atherosclerotic aorta. Data are means ± sem (n = 8). *P < 0.05, **P < 0.01 vs. Apoe−/−; Bmalf/f. E) Representative flow plots of myeloid cells (CD45+CD11b) gated on F4/80 and Ly6c for monocytes and macrophages from the aorta. F) Representative immunofluorescence staining of aortic root sections (from 4 different mice per group) showing the expression of Ly6c (green), CD68 (red), and counterstain with DAPI. Scale bars, 100 μm.
Bmal1 deficiency leads to enhanced M2 to M1 macrophage transformation
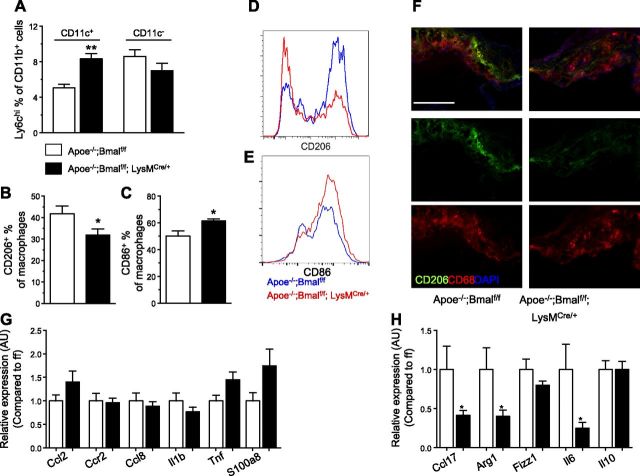
M1 macrophages are often lipid laden and express CD11c (27). We found a higher percentage of Ly6chi cells with Bmal1 deficiency in this CD11c+ macrophage subset, in addition to myeloid dendritic cells, compared with CD11c− subsets (Fig. 4A), indicating that more recruited monocytes become M1 macrophages. Furthermore, the increase of macrophage recruitment was accompanied by a decrease of the anti-inflammatory M2 macrophage marker CD206 (Fig. 4B, D) and an increase of proinflammatory M1 macrophage marker CD86 (Fig. 4C, E) in the total macrophages. Immunostaining of CD206 with CD68 together also showed similar decrease of CD206 expression in lesional macrophages from aortic root (Fig. 4F). We also examined the expression of proinflammatory M1 markers and anti-inflammatory M2 markers in aortic tissues from both genotypes. We found small but not significant increases in proinflammatory Ccl2, Tnf, and S100a8 (Fig. 4G). On the other hand, we observed a significant decrease of M2 markers, including Arg1, Fizz1, and Ccl17 in aortic tissues from myeloid-Bmal1-deleted mice (Fig. 4H), indicating Bmal1 deletion enhanced vascular inflammatory responses in the vessel wall, contributing to exacerbated atherogenesis.
Figure 4.
Myeloid Bmal1 deletion skews lesional macrophages from M2 to M1 phenotype. A) Aortic CD45+CD11b+ cells are gated on CD11c+ and CD11c− populations. CD11c+ cells contains mostly CD11c+ macrophages and some myeloid dendritic cells. CD11c+ cells from Apoe−/−; Bmalf/f; LysMCre/+ mice have more Ly6c+ cells. B) Summarized data of CD206+ % of total lesional macrophages from Apoe−/−; Bmalf/f; LysMCre/+ and Apoe−/−; Bmalf/f mice fed with WD for 12 wk. C) Summarized data of CD86+ % of total lesional macrophages. D, E) Representative histogram of CD206 (D) and CD86 (E) staining in aortic macrophages from B and C. F) Representative immunofluorescence staining of aortic root sections (from 4 different mice each group) showing the expression of CD206 (green), CD68 (red), and counterstaining with DAPI. Scale bar, 200 μm. G, H) Gene expressions of aortae from Apoe−/−; Bmalf/f and Apoe−/−; Bmalf/f; LysMCre/+ mice fed WD for 12 wk. Data are means ± sem (n = 6). *P < 0.05 and **P < 0.01 vs. Apoe−/−; Bmalf/f.
Bmal1 deficiency enhances monocyte recruitment to atherosclerotic lesion
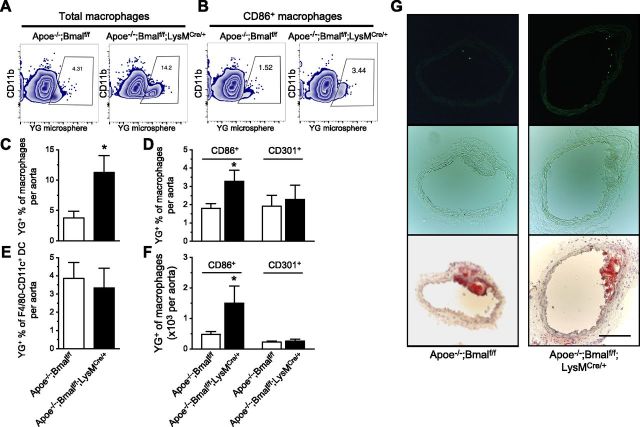
To determine whether increased lesional macrophage content is due to enhanced recruitment, we used YG latex beads to label monocytes in vivo and to examine the entry of blood monocytes into the atherosclerotic lesion. Apoe−/−;Bmalf/f and Apoe−/−;Bmalf/f;LysMCre/+ mice fed WD for 10 wk were injected intraperitoneally with 200 μl liposome clodronate, which transiently depletes blood monocytes and helps to stably label Ly6chi monocytes with YG latex beads, which enables us to monitor the recruitment of these Ly6chi cells to atherosclerotic lesions (22, 28). Twenty-four hours after the injection, these mice received tail-vein injections of YG microspheres to label Ly6chi monocytes in the blood, and were killed 48 h later (21). Flow cytometric analysis showed more lesional macrophages containing YG microspheres (YG+) in myeloid-Bmal1-deleted mice (Fig. 5A, C). In an inflammatory state, Ly6chi monocytes mostly differentiate into macrophages, and some into inflammatory TNF-α and iNOS, producing TNF-α/iNOS-producing dendritic cells (DCs) (29). The percentage of YG+ cells in the CD11b+F4/80−CD11c+ subsets (mostly myeloid DCs) (30) within the vessel wall was similar between the 2 groups (Fig. 5E), indicating that Bmal1 deficiency promotes Ly6chi monocytes to differentiate into macrophages rather than myeloid DCs. Further analysis of macrophage subsets demonstrated more YG+ M1 macrophages (CD86+) in myeloid-Bmal1-deficient mice, whereas YG+ cell absolute number and percentage in M2 macrophages (CD301+) were similar between the 2 groups (Figs. 5B, D, F), indicating that the circulating new Ly6chi monocytes labeled with YG beads preferentially differentiate into M1 but not M2 macrophages upon entering the lesions. We used CD301 as an M2 macrophage marker instead of CD206 due to limited choices of fluorescent color when YG beads were used. In line with flow cytometric analysis, fluorescent images of frozen sections from the left carotid artery also showed more YG+ cells on the surface of atherosclerotic plaque in Apoe−/−;Bmalf/f;LysMCre/+ mice (Fig. 5G).
Figure 5.
Myeloid Bmal1 deletion promotes monocyte recruitment to lesions. A, B) Representative flow plots showing increased percentage of YG+ cells in total macrophages (A) or CD86+ macrophages (B) from Apoe−/−; Bmalf/f; LysMCre/+ mice. C–E) Summarized data on percentage of YG+ cells in total lesional macrophages (C), M1 (CD86+) and M2 (CD301+) subsets (D), and myeloid dendritic cells (E) from both groups of mice fed with WD for 10 wk. F) Summarized data of YG+ M1 and M2 subsets. Data are means ± sem (n = 6). *P < 0.05 vs. Apoe−/−; Bmalf/f. G) Representative images from 4 different mice of each group, showing YG+ cells (green fluorescence; top), phase-contrast images (middle), and Oil Red O staining (botom) of adjacent sections of left carotid arteries. Scale bar, 200 μm.
Adoptive transfer of Ly6chi monocytes from mice deficient in myeloid Bmal1 to ApoE−/− mice promotes atherogenesis
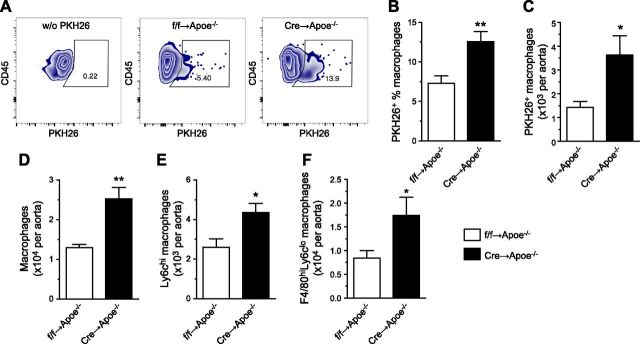
To show whether atherosclerotic progression and increased lesional macrophage content induced by Bmal1 deficiency are associated with exacerbated monocyte recruitment, adoptive transfer of Ly6chi monocytes into Apoe−/− mice with early lesions (9 wk on WD) was performed. Bone marrow Ly6chi monocytes (2 × 106 for each mouse) were labeled with red fluorescent PKH26 to track the cells after tail vein injection into the mice. Mice receiving cells from Apoe−/−;Bmalf/f;LysMCre/+ mice have more PKH26+ macrophages in the lesions 2 wk after the transfer (Fig. 6A–C). More importantly, Apoe−/− mice receiving cells from Apoe−/−;Bmalf/f;LysMCre/+ mice have an increase in total macrophage number and a similar increase in both Ly6chiF4/80int and Ly6cloF4/80hi subsets (Fig. 6D–F). These data strongly supported our hypothesis that myeloid Bmal1 deficiency promotes monocyte recruitment to exacerbate atherosclerosis.
Figure 6.
Adoptive transfer of BM monocytes with Bmal1 deletion promotes monocyte recruitment and exacerbates atherosclerosis in Apoe−/− mice. A) Representative flow plots of negative staining [without (w/o) PKH26; left panel] and PKH26 in lesional macrophages from Apoe−/− mice receiving cells from Apoe−/−; Bmalf/f mice (as ff→Apoe−/−; center panel) and Apoe−/− mice receiving cells from Apoe−/−; Bmalf/f LysMCre/+ mice (as Cre→Apoe−/−; right panel). B, C) Summarized data of flow cytometric analysis of PKH+ percentage of total lesional macrophages (B) and number of PKH+ macrophages (C) from both groups. D–F) Summarized data of flow cytometric analysis of total macrophages (D), Ly6chi newly recruited monocyte-derived macrophages (E), and resident F4/80hi macrophages (F) from both groups. Data are means ± sem (n = 6). *P < 0.05, **P < 0.01 vs. ff→Apoe−/−.
DISCUSSION
In this study, we report that myeloid-specific Bmal1 deletion promotes atherosclerosis, which is most likely due to enhanced monocyte trafficking. In myeloid Bmal1-deficient atherosclerotic mice, we observed increased lesion size with more inflammatory M1 macrophages and enhanced recruitment of Ly6chi monocytes into the lesion. Bmal1 deficiency causes preferential differentiation of Ly6chi monocytes into M1 macrophages to replenish lesional macrophages and thereby promotes atherosclerotic progression. In addition, local proliferation of lesional macrophages was unaffected by Bmal1 deficiency in either M1 or M2 macrophage subsets, indicating that increased macrophage number is not the consequence of in situ proliferation. We thereby provided evidence to explain how dysregulated monocyte trafficking induced by Bmal1 deletion promotes atherosclerosis.
The molecular clock plays an important regulatory role in cardiovascular function, immune responses, and metabolism through rhythmic expression of clock-controlled transcripts and their biologic functions (3). Previous studies have demonstrated the involvement of molecular clock in vascular inflammation and atherosclerosis (17, 19). However, the contribution of myeloid molecular clock function to chronic inflammation of the vessel wall in atherosclerosis is not thoroughly understood. For example, overexpression of Cry1 using adenoviral vector attenuates atherosclerosis in Apoe−/− mice, accompanied by the inhibition of adhesion molecules expressed by inflamed endothelium (19). An earlier study using transplantation of arterial isograft-induced arteriosclerosis model showed that global circadian clock mutation by Bmal1−/− and Per2−/−Per3−/− does not increase arteriosclerosis in transplanted arteries, whereas patients receiving grafts from Bmal1−/− have more advanced arteriosclerotic response associated with macrophage infiltration and T-cell receptor activation, suggesting the local regulatory role of tissue-intrinsic circadian clock rather than systemic alteration in this particular disease model (18). These data are different from our observation that exacerbated atherosclerosis caused by Bmal1 deletion is caused by continuous replenishment of circulating Ly6chi monocytes differentiated to lesional macrophages. Up-regulation of chemokines such as CCL2 suggests that increased recruitment of Bmal1-deficient macrophages into the lesion is possibly due to the increased chemokine production, which acts in an autocrine manner to enhance adhesion and migration and to promote inflammatory responses locally.
Another important earlier study showed that Clock- mutant (ClockΔ19/Δ19) mice on Ldlr−/− or Apoe−/− background have increased lesion size. These mice also absorb more cholesterol and have higher plasma cholesterol and triglyceride levels, indicating the inhibitory role of Clock on cholesterol level (17). In addition, macrophages from Clock-mutant mice have reduced Abca1 expression, resulting in defective cholesterol efflux (17). Unlike Clock-mutant mice, Apoe−/−;Bmalf/f;LysMCre/+ mice have a similar lipid profile compared with their wild type (Supplemental Fig. 2); thus, enhanced atherosclerosis might not be caused by hypercholesterolemia. In addition, boron-dipyrromethene fluorescence suggested that lipid content in both basal and LPS-stimulated BMDMs was similar, indicating that the ability to accumulate lipids was unlikely to be affected by Bmal1 deletion (Supplemental Fig. 3). The 2 core clock components, Clock and Bmal1, may use distinct mechanisms for the transcription of various genes to promote vascular inflammatory responses under hypercholesterolemia. However, the regulatory role of Bmal1 on cholesterol metabolism in lesional macrophages requires further characterization.
One might argue that the increased macrophage content with Bmal1 deficiency may be linked to increased myelopoiesis. In the present study, we showed that increased monocyte-macrophage recruitment is not caused by monocytosis or myelopoiesis. To determine whether myelopoiesis plays a role in this setting, major reservoirs of monocytes, including bone marrow, spleen, and blood, were analyzed to determine whether there was an increase in steady-state monocyte number. Monocytes were gated as follows: CD45+CD115+ for blood monocytes, CD45+CD11b+CD11c−F4/80−Ly6G− for splenic monocytes, and CD45+CD11b+CD11c−F4/80−Ly6G−Ly6c+ for bone marrow monocytes, as previously described (31). We found no significant changes in monocyte numbers or percentages of total CD45+ cells and no change in Ly6chi monocyte percentages in blood and spleen (Supplemental Fig. 4A–G), indicating that it is unlikely that the increased macrophage content and lesion size is due to myelopoiesis or monocytosis. In addition, the numbers of neutrophils, another myeloid cell type, gated as CD45+CD11b+Ly6G+ from blood and aorta, were comparable between 2 groups. (Supplemental Fig. 4H–K). Compared with macrophages, which are the major cell type in atherosclerotic aorta, many fewer neutrophils were detected in atherosclerotic aorta, indicating that the role of neutrophils should be minimal compared with macrophages in the current experimental setting. Macrophage phenotype may also affect lesion size and stability. Although M2 markers decreased and M1 markers increased in lesional macrophages with Bmal1 deletion, we did not find significant differences in M1 and M2 differentiation in vitro (Supplemental Fig. 5), suggesting that the in vivo phenotype switch is most likely due to the infiltration of inflammatory monocyte-derived macrophages rather than polarization within the local environment.
To verify whether local proliferation of lesional macrophages may be affected by Bmal1 deletion (32) and contribute to enhanced atherosclerosis, we analyzed proliferation of aortic macrophages using Ki67 staining and BrdU incorporation. Both methods showed that proliferation was unaltered in M1 (CD206−) or M2 (CD206+) macrophage subsets by Bmal1 deficiency (Supplemental Fig. 6). Deletion of Bmal1 did not affect proliferation of BMDMs in vitro either under basal conditions or after stimulation with LPS (Supplemental Fig. 7). These data indicate that macrophage proliferation did not contribute to the enhanced atherosclerosis induced by myeloid Bmal1 deletion.
In line with our data, a recent study using conditional Bmal1 knockout during adulthood in mice showed similar results (33). Atherosclerosis is inhibited when Bmal1 is deleted during adulthood, whereas constitutive Bmal1 deletion leads to larger plaque area (33), which is consistent with our results and with the previous study in Clock-mutant mice (17). However, these mice have global Bmal1 deletion in all tissues. Thus, it is unknown whether these conditional Bmal1-knockout mice have altered hematopoiesis and monopoiesis due to Bmal1 deletion in these cells. Moreover, whether inflammatory responses in vasculature involve the participation of other cell types (e.g., vascular smooth muscle cells, endothelial cells, and lymphocytes) might be affected by Bmal1 deletion during adulthood is uncertain. Moreover, SDF1 (or CXCL12), which is the major chemokine controlling hematopoietic stem/progenitor cell migration, follows a circadian oscillation (34). CXCL12/CXCR4 signaling protects atherosclerosis by mobilizing protective endothelial progenitor cells to promote endothelial repair (35), increases plaque stability (36), and suppresses hematopoietic stem/progenitor cell migration to the spleen, which triggers myelopoiesis (37). In the local microenvironment, binding of SDF1 CXCR4 and CXCR7 is protective against atherosclerosis by inhibiting lipid accumulation and proinflammatory chemokine secretion (38, 39). Whether SDF1/CXCR4 is involved in enhanced atherosclerosis due to disturbance of either central or peripheral clock requires further investigation.
In summary, we demonstrated that deletion of Bmal1 in monocytes and macrophages promotes atherosclerosis. Enhanced monocyte recruitment into the lesion induced by Bmal1 deletion contributed to increased macrophage content and lesion size. Our findings provide insight into the regulatory role of molecular clock in atherosclerosis and chronic inflammation in cardiovascular diseases.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This study was supported by Chinese University of Hong Kong (CUHK) Direct Grant 4054321 (to X.Y.T.), CUHK Vice-Chancellor’s Discretionary Fund (to Y.H. and X.Y.T.), the Hong Kong Research Grants Council Grant GRF464712 (to Y.H.), and Hong Kong Food and Health Bureau [Health and Medical Research Fund (HMRF) Research Fellowship Scheme 01150057] (to X.Y.T.). The authors thank Dr. Khoa Nguyen (Stanford University Blood Center, Stanford, CA, USA) for advice on flow cytometry experiments; Dr. D. Rowlands and C. Cheng (CUHK) for animal care; and Dr. X. Cui, Francis Chen, and J. Park (University of California, San Francisco) for technical assistance.
Glossary
- BMDM
bone marrow-derived macrophage
- DC
dendritic cell
- TNF
tumor necrosis factor
- WD
Western diet
- YG
yellow green
- ZT
zeitgeber time
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
X. Y. Tian designed the study, analyzed the data, and wrote the manuscript; X. Y. Tian, M. Huo, Y. Huang, H. Zhang, and D. Qu researched the data and contributed to discussion; W. T. Wong, A. Chawla, and Y. Huang provided critical reagents, contributed to the discussion, and reviewed and edited the manuscript; and X. Y. Tian was the guarantor of this study, had full access to, and is responsible for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.McAlpine C. S., Swirski F. K. (2016) Circadian influence on metabolism and inflammation in atherosclerosis. Circ. Res. , 131–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu B., Gates L. A., Stashi E., Dasgupta S., Gonzales N., Dean A., Dacso C. C., York B., O’Malley B. W. (2015) Coactivator-dependent oscillation of chromatin accessibility dictates circadian gene amplitude via REV-ERB loading. Mol. Cell , 769–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takahashi J. S. (2015) Molecular components of the circadian clock in mammals. Diabetes Obes. Metab. (Suppl 1), 6–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asher G., Sassone-Corsi P. (2015) Time for food: the intimate interplay between nutrition, metabolism, and the circadian clock. Cell , 84–92 [DOI] [PubMed] [Google Scholar]
- 5.Yang G., Paschos G., Curtis A. M., Musiek E. S., McLoughlin S. C., FitzGerald G. A. (2013) Knitting up the raveled sleave of care. Sci. Transl. Med. , 212rv3 [DOI] [PubMed] [Google Scholar]
- 6.Carter S. J., Durrington H. J., Gibbs J. E., Blaikley J., Loudon A. S., Ray D. W., Sabroe I. (2016) A matter of time: study of circadian clocks and their role in inflammation. J. Leukoc. Biol. , 549–560 [DOI] [PubMed] [Google Scholar]
- 7.Scheiermann C., Kunisaki Y., Frenette P. S. (2013) Circadian control of the immune system. Nat. Rev. Immunol. , 190–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibbs J. E., Blaikley J., Beesley S., Matthews L., Simpson K. D., Boyce S. H., Farrow S. N., Else K. J., Singh D., Ray D. W., Loudon A. S. (2012) The nuclear receptor REV-ERBα mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc. Natl. Acad. Sci. USA , 582–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellet M. M., Deriu E., Liu J. Z., Grimaldi B., Blaschitz C., Zeller M., Edwards R. A., Sahar S., Dandekar S., Baldi P., George M. D., Raffatellu M., Sassone-Corsi P. (2013) Circadian clock regulates the host response to Salmonella. Proc. Natl. Acad. Sci. USA , 9897–9902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ammirati E., Cristell N., Cianflone D., Vermi A. C., Marenzi G., De Metrio M., Uren N. G., Hu D., Ravasi T., Maseri A., Cannistraci C. V. (2013) Questing for circadian dependence in ST-segment-elevation acute myocardial infarction: a multicentric and multiethnic study. Circ. Res. , e110–e114 [DOI] [PubMed] [Google Scholar]
- 11.Narasimamurthy R., Hatori M., Nayak S. K., Liu F., Panda S., Verma I. M. (2012) Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proc. Natl. Acad. Sci. USA , 12662–12667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curtis A. M., Fagundes C. T., Yang G., Palsson-McDermott E. M., Wochal P., McGettrick A. F., Foley N. H., Early J. O., Chen L., Zhang H., Xue C., Geiger S. S., Hokamp K., Reilly M. P., Coogan A. N., Vigorito E., FitzGerald G. A., O’Neill L. A. (2015) Circadian control of innate immunity in macrophages by miR-155 targeting Bmal1. Proc. Natl. Acad. Sci. USA , 7231–7236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spengler M. L., Kuropatwinski K. K., Comas M., Gasparian A. V., Fedtsova N., Gleiberman A. S., Gitlin I. I., Artemicheva N. M., Deluca K. A., Gudkov A. V., Antoch M. P. (2012) Core circadian protein CLOCK is a positive regulator of NF-κB-mediated transcription. Proc. Natl. Acad. Sci. USA , E2457–E2465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen K. D., Fentress S. J., Qiu Y., Yun K., Cox J. S., Chawla A. (2013) Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science , 1483–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tabas I., Bornfeldt K. E. (2016) Macrophage phenotype and function in different stages of atherosclerosis. Circ. Res. , 653–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sitaula S., Billon C., Kamenecka T. M., Solt L. A., Burris T. P. (2015) Suppression of atherosclerosis by synthetic REV-ERB agonist. Biochem. Biophys. Res. Commun. , 566–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan X., Jiang X. C., Hussain M. M. (2013) Impaired cholesterol metabolism and enhanced atherosclerosis in clock mutant mice. Circulation , 1758–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng B., Anea C. B., Yao L., Chen F., Patel V., Merloiu A., Pati P., Caldwell R. W., Fulton D. J., Rudic R. D. (2011) Tissue-intrinsic dysfunction of circadian clock confers transplant arteriosclerosis. Proc. Natl. Acad. Sci. USA , 17147–17152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang L., Chu Y., Wang L., Wang Y., Zhao X., He W., Zhang P., Yang X., Liu X., Tian L., Li B., Dong S., Gao C. (2015) Overexpression of CRY1 protects against the development of atherosclerosis via the TLR/NF-κB pathway. Int. Immunopharmacol. , 525–530 [DOI] [PubMed] [Google Scholar]
- 20.Butcher M. J., Herre M., Ley K., Galkina E. (2011) Flow cytometry analysis of immune cells within murine aortas. J. Vis. Exp. (53):2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tacke F., Alvarez D., Kaplan T. J., Jakubzick C., Spanbroek R., Llodra J., Garin A., Liu J., Mack M., van Rooijen N., Lira S. A., Habenicht A. J., Randolph G. J. (2007) Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J. Clin. Invest. , 185–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Rooijen N., Sanders A. (1994) Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J. Immunol. Methods , 83–93 [DOI] [PubMed] [Google Scholar]
- 23.Belliere J., Casemayou A., Ducasse L., Zakaroff-Girard A., Martins F., Iacovoni J. S., Guilbeau-Frugier C., Buffin-Meyer B., Pipy B., Chauveau D., Schanstra J. P., Bascands J. L. (2015) Specific macrophage subtypes influence the progression of rhabdomyolysis-induced kidney injury. J. Am. Soc. Nephrol. , 1363–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fink L. N., Costford S. R., Lee Y. S., Jensen T. E., Bilan P. J., Oberbach A., Blüher M., Olefsky J. M., Sams A., Klip A. (2014) Pro-inflammatory macrophages increase in skeletal muscle of high fat-fed mice and correlate with metabolic risk markers in humans. Obesity (Silver Spring) , 747–757 [DOI] [PubMed] [Google Scholar]
- 25.Pérez-Rial S., del Puerto-Nevado L., Terrón-Expósito R., Girón-Martínez Á., González-Mangado N., Peces-Barba G. (2013) Role of recently migrated monocytes in cigarette smoke-induced lung inflammation in different strain of mice. PLoS One , e72975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davies L. C., Rosas M., Jenkins S. J., Liao C. T., Scurr M. J., Brombacher F., Fraser D. J., Allen J. E., Jones S. A., Taylor P. R. (2013) Distinct bone marrow-derived and tissue-resident macrophage lineages proliferate at key stages during inflammation. Nat. Commun. , 1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gordon S. (2007) Macrophage heterogeneity and tissue lipids. J. Clin. Invest. , 89–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tacke F., Ginhoux F., Jakubzick C., van Rooijen N., Merad M., Randolph G. J. (2006) Immature monocytes acquire antigens from other cells in the bone marrow and present them to T cells after maturing in the periphery. J. Exp. Med. , 583–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aldridge J. R. Jr., Moseley C. E., Boltz D. A., Negovetich N. J., Reynolds C., Franks J., Brown S. A., Doherty P. C., Webster R. G., Thomas P. G. (2009) TNF/iNOS-producing dendritic cells are the necessary evil of lethal influenza virus infection. Proc. Natl. Acad. Sci. USA , 5306–5311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Subramanian M., Thorp E., Hansson G. K., Tabas I. (2013) Treg-mediated suppression of atherosclerosis requires MYD88 signaling in DCs. J. Clin. Invest. , 179–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tian X. Y., Ganeshan K., Hong C., Nguyen K. D., Qiu Y., Kim J., Tangirala R. K., Tontonoz P., Chawla A. (2016) Thermoneutral housing accelerates metabolic inflammation to potentiate atherosclerosis but not insulin resistance. Cell Metab. , 165–178 [Erratum]https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26549485&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robbins C. S., Hilgendorf I., Weber G. F., Theurl I., Iwamoto Y., Figueiredo J. L., Gorbatov R., Sukhova G. K., Gerhardt L. M., Smyth D., Zavitz C. C., Shikatani E. A., Parsons M., van Rooijen N., Lin H. Y., Husain M., Libby P., Nahrendorf M., Weissleder R., Swirski F. K. (2013) Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat. Med. , 1166–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang G., Chen L., Grant G. R., Paschos G., Song W. L., Musiek E. S., Lee V., McLoughlin S. C., Grosser T., Cotsarelis G., FitzGerald G. A. (2016) Timing of expression of the core clock gene Bmal1 influences its effects on aging and survival. Sci. Transl. Med. , 324ra16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Méndez-Ferrer S., Lucas D., Battista M., Frenette P. S. (2008) Haematopoietic stem cell release is regulated by circadian oscillations. Nature , 442–447 [DOI] [PubMed] [Google Scholar]
- 35.Zernecke A., Bidzhekov K., Noels H., Shagdarsuren E., Gan L., Denecke B., Hristov M., Köppel T., Jahantigh M. N., Lutgens E., Wang S., Olson E. N., Schober A., Weber C. (2009) Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci. Signal. , ra81 [DOI] [PubMed] [Google Scholar]
- 36.Akhtar S., Gremse F., Kiessling F., Weber C., Schober A. (2013) CXCL12 promotes the stabilization of atherosclerotic lesions mediated by smooth muscle progenitor cells in Apoe-deficient mice. Arterioscler. Thromb. Vasc. Biol. , 679–686 [DOI] [PubMed] [Google Scholar]
- 37.Dutta P., Courties G., Wei Y., Leuschner F., Gorbatov R., Robbins C. S., Iwamoto Y., Thompson B., Carlson A. L., Heidt T., Majmudar M. D., Lasitschka F., Etzrodt M., Waterman P., Waring M. T., Chicoine A. T., van der Laan A. M., Niessen H. W. M., Piek J. J., Rubin B. B., Butany J., Stone J. R., Katus H. A., Murphy S. A., Morrow D. A., Sabatine M. S., Vinegoni C., Moskowitz M. A., Pittet M. J., Libby P., Lin C. P., Swirski F. K., Weissleder R., Nahrendorf M. (2012) Myocardial infarction accelerates atherosclerosis. Nature , 325–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Irvine K. M., Andrews M. R., Fernandez-Rojo M. A., Schroder K., Burns C. J., Su S., Wilks A. F., Parton R. G., Hume D. A., Sweet M. J. (2009) Colony-stimulating factor-1 (CSF-1) delivers a proatherogenic signal to human macrophages. J. Leukoc. Biol. , 278–288 [DOI] [PubMed] [Google Scholar]
- 39.Li X., Zhu M., Penfold M. E., Koenen R. R., Thiemann A., Heyll K., Akhtar S., Koyadan S., Wu Z., Gremse F., Kiessling F., van Zandvoort M., Schall T. J., Weber C., Schober A. (2014) Activation of CXCR7 limits atherosclerosis and improves hyperlipidemia by increasing cholesterol uptake in adipose tissue. Circulation , 1244–1253 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.