Abstract
Estrogens influence nearly every aspect of hippocampal function, including memory formation. Although this research has traditionally focused on ovariectomized females, more recent work is providing insights into the ways in which estrogens regulate hippocampal function in both sexes. This review provides an overview of estrogenic regulation of hippocampal function in female and male rodents, with a particular emphasis on memory formation. Where applicable, we discuss the involvement of specific estrogen receptors and molecular mechanisms that mediate these effects. The review concludes by suggesting gaps in the literature that need to be filled to provide greater insights into potential sex differences in the effects of estrogens on hippocampal function.
Keywords: estrogen, hippocampus, sex differences, consolidation, ERK, estrogen receptors
Introduction
In the past three decades, increasing attention has been paid to the effects of the potent estrogen 17β-estradiol (E2) on the hippocampus. This research has provided important insights into estrogenic facilitation of hippocampal synaptic plasticity and memory formation in female rats and mice. Much less is known about how E2 regulates hippocampal memory in males, despite high levels of estrogen receptor (ER) expression and E2 levels in the male hippocampus. This review synthesizes key studies examining effects of exogenous E2 on hippocampal learning and memory in female and male rodents. Molecular mechanisms underlying the memory-enhancing effects of E2 on hippocampal memory consolidation will also be described. The review concludes by considering gaps in the literature that should be filled to better understand how E2 regulates hippocampal learning and memory in both sexes. Due to the short format of this article, we refer readers to other recent reviews (e.g., [1–8]) for more detailed information on sex differences in hippocampal memory, and effects of E2 on hippocampal morphology, physiology, and memory.
Estrogens and the hippocampus
The most well-studied ERs, ERα, ERβ, and G-protein-coupled ER (GPER), are located throughout the rodent hippocampus [9,10]. The structurally homologous ERα and ERβ are intracellular receptors that act in the nucleus as transcription factors to mediate slower classical (aka, “genomic”) effects of estrogens, and at the plasma membrane where they interact with neurotransmitter and growth factor receptors to trigger rapid non-classical (aka, “non-genomic”) effects on cell signaling and gene transcription. GPER is a membrane ER that mediates rapid G-protein-stimulated cell signaling. In both sexes, these receptors are widely distributed throughout hippocampal neurons, appearing within nuclei, dendritic spines, dendrites, axons, and axon terminals, as well as in astrocytes [11–••14]. ER localization to extranuclear sites, particularly dendritic spines [11–••14], positions them to mediate the rapid effects of estrogens on processes like cell signaling, local protein synthesis, and dendritic spinogenesis.
In females, numerous aspects of hippocampal morphology and physiology are regulated by E2, including CA1 dendritic spine density, neurogenesis, cell signaling, epigenetic processes, gene transcription, protein translation, and synaptic plasticity (see [1,3,15–19] for reviews). For example, CA1 spine density in female rats is elevated during the proestrus phase of the estrous cycle, when estrogen levels are most elevated, relative to the estrus phase, in which estrogen levels are low [20]. Bilateral ovariectomy in rats and mice reduces CA1 spine density [20], an effect that can be reversed within 30 minutes by systemic injection or dorsal hippocampal infusion of E2 [21, ••22]. These effects can be mediated by ERα or ERβ, or GPER [•23,24], and depend on rapid activation of extracellular signal-regulated kinase (ERK) and mammalian target of rapamycin (mTOR) cell signaling in the dorsal hippocampus [••22]. The mTOR pathway is activated by upstream signaling kinases including phosphoinositide 3-kinase (PI3K) and ERK, and triggers rapid local protein translation within dendrites leading to spine remodeling [25]. Both ERK and mTOR signaling are necessary for hippocampal synaptic plasticity and memory formation in male rodents [25], and for E2-induced memory consolidation in ovariectomized mice [26]. Thus, the fact that inhibitors of ERK and mTOR phosphorylation prevent E2 from increasing CA1 spine density suggests a key role for rapid local protein synthesis in E2-induced spinogenesis. However, as will be described later, E2-induced ERK phosphorylation also promotes epigenetic processes, potentially implicating gene transcription as well.
Effects of E2 on hippocampal dendritic spine density in males are similar to those in females. In vivo, bilateral gonadectomy in male rats reduces CA1 spine density, and this effect is reversed within 30 minutes of systemic testosterone or E2 injection [••27]. Likewise, ex vivo studies using hippocampal slices from gonadally-intact males, report that E2 increases CA1 spinogenesis within 2 hours of bath application. This effect depends on activation of ERα, but not ERβ [28,29]; a role for GPER has not yet been determined. In hippocampal slices, E2-induced spinogenesis depends on activation of cell-signaling kinases including protein kinase A (PKA), protein kinase C (PKC), phosphoinositide 3-kinase (PI3K), ERK, calcium calmodulin kinase II (CaMKII), LIM kinase (LIMK), and calcineurin, but not c-Jun N-terminal kinase (JNK) [30]. Inhibition of PKA, PKC, PI3K, ERK, and CaMKII also prevent E2 from enhancing long-term potentiation (LTP) in these slices [30], suggesting a role for these signaling kinases in E2-induced spinogenesis and synaptic plasticity.
Consistent with its effects on CA1 dendritic spines in females and males, E2 significantly enhances hippocampal synaptic plasticity, including NMDA-dependent LTP. In both sexes, exogenous E2 increases baseline EPSP amplitude, reduces LTP threshold, and increases LTP amplitude [19,31,32, ••101]. The LTP enhancement has been shown to depend on ERβ in adult males and females [32,33]. However, more recent work suggests important sex differences in the pre- and post-synaptic mechanisms involved in synaptic potentiation. In females, excitatory synapses are potentiated via pre-synaptic increases in glutamate release probability that are mediated by ERβ and post-synaptic increases in glutamate sensitivity that are mediated by GPER [••101]. In males, however, glutamate release probability is regulated pre-synaptically by ERα, whereas ERβ is involved post-synaptically in glutamate sensitivity [••101]. Thus, although ERβ plays a role in mediating synaptic potentiation in both sexes, the nature of its effects differs between the sexes. E2-induced LTP enhancement also involves actin polymerization. Actin polymerization, which promotes cytoskeletal shape and stabilization, is regulated by the RhoA/RhoA kinase (ROCK) signaling pathway. E2 activates this pathway in hippocampal slices from intact male rats, and reverses ovariectomy-induced reductions in RhoA levels and actin polymerization [32]. In hippocampal slices from male rats, latrunculin A, a toxin that disrupts assembly of actin filaments, blocks E2-induced LTP [32], suggesting that actin polymerization is critical for estrogenic regulation of hippocampal plasticity. Recent preliminary data from our laboratory support this assertion, as latrunculin A prevents E2 from enhancing memory consolidation in ovariectomized mice [34].
Collectively, evidence to date implicates E2 as an important modulator of hippocampal function. E2 regulates many of the morphological, biochemical, and physiological aspects of hippocampal function thought to underlie learning and memory processes, so it is perhaps not surprising that E2 also regulates memory formation. Although a thorough review of this literature is beyond the scope of this review, the sections below will provide an overview of the effects of exogenous E2 on hippocampal learning and memory in females and males, and discuss the molecular mechanisms through which E2 regulates hippocampal memory consolidation in females.
Effects of pre-training E2 treatment on hippocampal learning and memory
The preponderance of hormones and cognition research has examined effects of exogenous E2 on hippocampal memory in young adult (2-3 months old) ovariectomized females. Most studies have administered E2 for some period prior to and/or during training, either chronically (e.g., via implanted silastic capsules or pellets) or acutely (e.g., via systemic injection or intracranial infusion). Similar studies have been conducted in gonadally-intact and castrated males, but these are far less numerous. Data from both sexes will be summarized below, including information about specific ER involvement where known. As with all pharmacological treatments, effects of E2 on memory depend on many factors, including dose, route of administration, timing and duration of administration, task difficulty, duration of handling prior to treatment, age at treatment, and duration of gonadectomy prior to treatment [1]. Nevertheless, the balance of studies in both sexes indicates that acute or chronic E2 treatment prior to training is beneficial for hippocampally-mediated spatial and non-spatial learning and memory (see Table 1 for a schematic summary of pre-training studies in both sexes).
Table 1.
Effects on memory of exogenous pre-training E2 treatment and involvement of specific estrogen receptors
| Female | Male | |||
|---|---|---|---|---|
| E2 | ERs involved | E2 | ERs involved | |
| Spatial Memory | ↑ | α, β, GPER | ↑ | ? |
| Object Recognition | ↑ | α, GPER | ? | ? |
| Social Recognition | ↑ | α, β, GPER | ? | ? |
| Fear Generalization | No effect | n/a | ↓ | ? |
In ovariectomized rats and mice, spatial memory assessed via the radial arm maze, Morris water maze, and T-maze tasks is generally enhanced by chronic E2 [35–50]. Improvements have been observed in both spatial reference memory (long-term memory for trial-independent information) and spatial working memory (short-term memory for trial-dependent information). Depending on the task and treatment, effects in females can be mediated by any ER. For example, spatial working memory in a delayed non-match-to-position (DNMTP) T-maze task is facilitated in ovariectomized rats by agonists of ERα, ERβ, or GPER administered via mini osmotic pumps for two weeks prior to training [50]. Studies using ER knockout (KO) mice report that E2 improves spatial memory and inhibitory avoidance in female ERαKO mice [51], but has no effect or impairs spatial and object recognition learning and memory in ERβKO mice [33,52], suggesting a primary role for ERβ in the memory-enhancing effects of E2. However, more recent work found that viral vector-mediated delivery of ERα to the hippocampus of female ERαKO mice improved spatial memory [53], suggesting a role for ERα in spatial memory formation as well. Although effects of E2 in GPER KO mice have not been tested, chronic systemic administration of a GPER antagonist to ovariectomized rats impairs spatial working memory in a DNMTP task and blocks the memory-enhancing effects of chronic E2, suggesting that GPER mediates effects of E2 on spatial memory in rats [54]. Consistent with the effects of E2 and ER manipulations on spatial memory, E2 also promotes the use of hippocampal-dependent spatial learning strategies in ovariectomized rats, an effect that depends on hippocampal ERα and ERβ [•55,56].
Similar memory-enhancing effects of pre-training E2 have been reported in castrated or gonadally-intact male rats and mice, as acute or chronic treatment with various forms of estradiol improve spatial reference and working memory in the radial arm maze, T-maze, and Barnes maze, and in passive avoidance [46,57–61]. In contrast to the relative wealth of ER-specific information in females, nearly nothing is known about which ERs mediate the effects of E2 on spatial memory in males. One study reported that infusion of an ERα agonist into the dorsal hippocampus of gonadally-intact male rats 30-35 minutes before training impaired spatial reference memory in the Morris water maze [62], suggesting that ERα may not be involved in the memory-enhancing effects of E2 in males. However, no studies to date have examined the role of ERα in other forms of memory or the role of other ERs in memory among castrated or intact males. Thus, this is an area ripe for future investigation.
Various tests of non-spatial hippocampal memory are also facilitated by pre-training administration of E2. In females, including object recognition, social recognition, social learning of food preferences, and trace eye-blink conditioning [63–68]. In ovariectomized mice, acute E2 administered within 45 minutes before training facilitates object recognition and social recognition, as well as the object placement test of spatial memory [63,69–•71]. All three ERs are involved in these effects, as suggested by the memory-enhancing effects of pre-training dorsal hippocampal infusion of ER agonists, although the role of any specific receptor depends on the type of memory and test difficulty. For example, in one series of studies, pre-training administration of an ERα agonist facilitated object recognition, social recognition, and object placement, whereas an ERβ agonist facilitated only object placement and a GPER agonist enhanced only social and object recognition [6, ••65, •71]. Whether pre-training E2 treatment might regulate the memory of males in these tasks remains to be investigated. However, an interesting sex difference has been reported in contextual fear generalization among gonadally-intact rats, such that fear generalization was enhanced in females relative to males [72]. This increase in females appears to be mediated by E2, as contextual fear generalization was increased in ovariectomized female rats treated with estradiol benzoate-filled silastic capsules relative to rats treated with vehicle-filled capsules [73]. Interestingly, this E2-induced fear generalization in ovariectomized females resulted from regulation of retrieval, rather than acquisition or consolidation, and depended on ERβ [73], but not membrane-associated ERs [•74]. Consistent with the sex difference observed in gonadally-intact rats, chronic pre-training exposure of gonadectomized male rats to testosterone or E2 prevented fear generalization in males, as did acute treatment 24 hours prior to retrieval [•75]. Effects of pre-retrieval E2 were mimicked by acute injection of ERα or ERβ agonists, suggesting a role for both receptors in males that differs from the singular involvement of ERβ in ovariectomized females [•75]. Together, these fear generalization-reducing effects of E2 in males directly contrast with the fear generalization-inducing effects of E2 in females, suggesting important sex differences in the role of E2 in mediating fear memory.
Together findings from pre-training studies suggest that E2 can facilitate hippocampally-mediated spatial and non-spatial learning and memory in both male and female rodents. These effects depend in part on activation of various ERs, although the specific ERs involved in each sex and type of memory may differ. Of note, however, relatively few studies have investigated effects of pre-training E2 on learning and memory in males, or have investigated the relative contributions of different ERs to learning and memory in males, so sex comparisons are difficult to draw. Nevertheless, the data thus far support a generally beneficial effect of pre-training E2 on learning and memory in both sexes.
Effects of post-training E2 treatment on hippocampal memory: Elucidating molecular mechanisms underlying estrogenic memory enhancement
Although the findings of pre-training studies suggest that E2 regulates hippocampal memory formation, the presence of E2 during both acquisition and consolidation prevents identification of the neural mechanisms through which E2 specifically influences memory formation. In recent years, numerous labs have used immediate post-training administration of acute exogenous E2 to assess effects of E2 on memory consolidation in ovariectomized rodents. In addition to pinpointing effects of E2 specifically on consolidation, these post-training treatments avoid potential confounds during acquisition resulting from E2 effects on motivation or sensorimotor functions [76]. Furthermore, because the consolidation process is confined to a limited time after training, post-training studies allow elucidation of the molecular and cellular mechanisms underlying estrogenic regulation of memory formation. This section will first describe the effects of post-training E2 on memory consolidation in females, and then summarize the receptor and intracellular mechanisms on which this consolidation depends. The section ends with a discussion of how post-training E2 treatment affects memory consolidation in males. See Table 2 for a schematic summary of post-training studies in both sexes.
Table 2.
Effects on memory of exogenous post-training E2 treatment, and involvement of specific estrogen receptors and key molecules
| Female | Male | |||||
|---|---|---|---|---|---|---|
| E2 | ERs involved | Key molecule | E2 | ERs involved | Key molecule | |
| Object Recognition | ↑ | α, β | ERK | ↑ | ? | CREB? |
| GPER | JNK | |||||
| Object Placement | ↑ | α, β | ERK | ↑ | ? | CREB? |
| GPER | JNK | |||||
Systemic injection or dorsal hippocampal infusion of E2 immediately after training in spatial memory and object recognition tasks enhances memory consolidation consistently across tasks, laboratories, and species. In the Morris water maze, systemic injection or dorsal hippocampal infusion of E2 immediately, but not two hours, post-training enhances spatial reference memory formation in ovariectomized rats and mice [77–79]. Similarly, post-training dorsal hippocampal infusion of E2 immediately after Morris water maze training enhances spatial reference memory in intact male rats [80]. It is unclear what molecular mechanisms drive these enhancements, but effects in both sexes involve activation of the cholinergic system [77, 80]. Spatial memory is also enhanced by post-training E2 in the object placement task. Using delays at which vehicle-treated subjects show no preference for the moved object, several laboratories have shown that systemic or dorsal hippocampal administration of E2 given immediately, but not 1.5 hours, after training enhances spatial memory consolidation (e.g., [69,••81–85]).
Object recognition memory consolidation, another type of memory dependent on hippocampal function [86–88], can be tested using a similar experimental approach and apparatus as the object placement task. As with object placement, systemic or dorsal hippocampal administration of E2 immediately, but not 1.5-3 hours, after training enhances object recognition memory consolidation in ovariectomized rats and mice [21,26,69,79,••81,82,84,89–92]. Interestingly, we recently showed that blocking the synthesis of E2 within the dorsal hippocampus of ovariectomized mice immediately after training prevents consolidation in both the object recognition and object placement tasks [•93], demonstrating the importance of hippocampally-synthesized E2 to memory consolidation in females. Collectively, the consolidation-enhancing effects of exogenous E2 have been so consistent across and within laboratories that we have exploited these tasks as tools to understand the molecular basis of E2-induced memory enhancement. The information gleaned from these studies thus far will be summarized below (see [15,94] for a more detailed discussion).
Given the rapid nature of estrogenic facilitation of memory consolidation, we reasoned that the memory-enhancing effects of post-training E2 were mediated by rapid non-classical effects of ERs (see Figure 1 for a diagrammatic illustration of these effects in ovariectomized mice). As such, we explored the involvement of cell-signaling cascades known to regulate memory formation and found that rapid activation of PKA, PI3K, ERK, and mTOR signaling within 5 minutes of infusion was necessary for post-training dorsal hippocampally-infused E2 to enhance object recognition memory consolidation in ovariectomized mice [26,90,95,96]. The estrogenic regulation of mTOR by ERK and PI3K was particularly intriguing because mTOR signaling triggers local protein synthesis in dendrites, which could play a role in E2’s effects on hippocampal spinogenesis. Indeed, inhibitors of ERK or mTOR phosphorylation prevent E2 from increasing CA1 dendritic spine density in ovariectomized mice [••22], linking E2-induced cell signaling to both spinogenesis and memory formation. Downstream from ERK, E2 causes rapid epigenetic changes in the dorsal hippocampus that are necessary for memory consolidation. For example, dorsal hippocampal infusion of E2 triggers ERK-dependent acetylation of the H3 histone protein in the hippocampus within 30 minutes [92]. Moreover, dorsal hippocampal infusion of a histone acetyltransferase inhibitor prevents E2 from enhancing object recognition memory consolidation [97], suggesting an essential role for H3 acetylation. Although the specific genes acetylated remain largely unknown, E2 increases H3 acetylation of specific promoters of the gene for brain derived neurotrophic factor (BDNF) [98], a memory-promoting neurotrophin.
Figure 1.
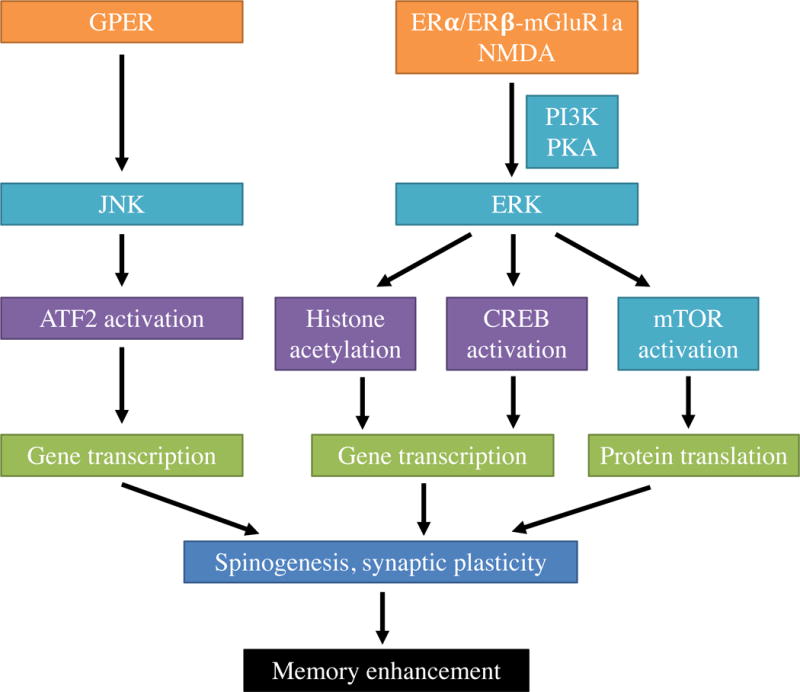
Schematic illustration of the receptors molecular mechanisms identified to date through which E2 facilitates object recognition memory consolidation in ovariectomized mice. ERα or ERβ interact with glutamate receptors (NMDA, mGluR1a) to trigger ERK signaling, which promotes H3 acetylation, CREB phosphorylation, and activation of mTOR signaling, leading to gene transcription, protein synthesis, increased CA1 dendritic spine density and synaptic plasticity, and memory enhancement. Activation of GPER also enhances memory, but via activation of JNK signaling. Preliminary work from our lab suggests that GPER activation also increases CA1 spine density. See text for references to empirical work supporting this model. Receptors are indicated in the figure in orange, cell-signaling molecules in teal, transcription factors or epigenetic events in purple, gene transcription and protein translation in green, spinogenesis and synaptic plasticity in blue, and memory enhancement in black.
Cell signaling is triggered by receptor binding, typically at ion channels and G protein-coupled receptors. Evidence that cell signaling is necessary for E2 to regulate hippocampal dendritic spine density [••22,29,30] suggests the involvement of ERs in at least some of the aforementioned effects. Systemic injection of ERα and ERβ agonists in ovariectomized rats and mice typically enhance memory consolidation in the object placement and object recognition tasks, although the effects can be dose-dependent [84,85,89]. Dorsal hippocampal infusion of an ERα agonist enhances object recognition and object placement in ovariectomized mice of both the C57BL/6 and Swiss strains [82,91], but an ERβ agonist enhances consolidation in both tasks only in C57BL/6 mice [82], suggesting potential strain differences in ER involvement. In C57BL/6 mice, the ability of E2 and agonists of ERα and ERβ to facilitate memory consolidation and ERK phosphorylation is dependent on activation of metabotropic glutamate receptor 1a (mGluR1a), and additional data showed that ERα, ERβ, and mGluR1a interact at the plasma membrane [82]. As such, these findings support non-classical actions of ERα and ERβ in which they interact with neurotransmitter receptors to rapidly trigger cell signaling. More recently, post-training infusion of ER antagonists into the dorsal hippocampus of ovariectomized C57BL/6 mice showed that ERβ antagonism blocked memory formation in both object recognition and object placement, whereas ERα antagonism blocked only object placement [•99]. Together, both agonist and antagonist data support a role for ERβ in both object recognition and spatial memory in ovariectomized C57BL/6 mice. It is curious, however, that ERα agonism enhanced memory in both tasks, whereas antagonism blocked only spatial memory. This discrepancy could be related to drug dose, or could suggest that ERα is sufficient, but not necessary to facilitate object recognition memory consolidation in females. Given the paucity of studies addressing this issue, more data will be needed to evaluate this speculative conclusion.
In ovariectomized mice, post-training dorsal hippocampal infusion of a GPER agonist enhances, whereas a GPER antagonist impairs, consolidation in the object recognition and object placement tasks [••81], suggesting that activation of GPER may also mediate the memory-enhancing effects of E2. Our preliminary data indicates that dorsal hippocampal GPER activation also increases CA1 dendritic spine density in ovariectomized mice within 40 minutes of infusion [34]. Curiously, however, the effects of GPER activation on consolidation are not mediated by ERK [••81], as are the effects of E2 and agonists of ERα and ERβ [82,90]. Rather, the effects of GPER activation are mediated by c-Jun N-terminal kinase (JNK; Figure 1) [••81]. Moreover, GPER antagonism does not prevent E2 from facilitating memory consolidation in either task, nor does inhibition of JNK signaling [••81]. These data suggest the intriguing possibility that GPER acts independently of E2 to regulate memory consolidation in the dorsal hippocampus of ovariectomized mice. The fact that GPER antagonism prevents chronic systemic E2 from enhancing spatial working memory in a T-maze DNMTP task in ovariectomized rats [54] suggests that GPER may play a greater role in acquisition than consolidation. However, these two studies differed in many important regards, so additional study is needed to resolve these discrepancies.
In contrast to the relative wealth of data on the effects of post-training E2 treatment on memory consolidation in females, very little is known about such effect in males. One recent of castrated rats found that post-training systemic injection of E2 or testosterone enhanced memory consolidation in the object placement task [••27]. Consistent with this finding, preliminary data from our laboratory directly comparing the effects in males and females of post-training dorsal hippocampal E2 infusion on object recognition and object placement memory show that E2 enhances memory in both tasks in castrated and intact male mice in a manner similar to ovariectomized females [100]. These data suggest the absence of sex differences in the effects of exogenous E2 on memory consolidation in these tasks, and that the effects of exogenous E2 on consolidation do not depend on the presence of the testes. Interestingly, however, post-training infusion of an aromatase inhibitor into the dorsal hippocampus blocks memory consolidation in castrated, but not intact, males [100]. Thus, endogenous E2 appears essential for memory consolidation in gonadectomized male and females, but not intact males, suggesting that testicular-derived E2 or androgens may be sufficient to regulate memory in males.
Interestingly, whereas E2 in females increased ERK and Akt levels as in previous studies, no increase in ERK was detected in intact males [100]. Also, in intact and castrated males, an inhibitor of ERK phosphorylation did not prevent E2 from enhancing consolidation in the object recognition and object placement tasks, unlike previous studies in females [100]. These data suggest that ERK activation is not necessary for E2 to enhance memory as in females, and indicates a potentially important sex differences in the molecular mechanisms through which E2 regulates memory consolidation. Although it is unknown at present what cell-signaling pathways might regulate memory consolidation in males, our data indicate that E2 increases phosphorylation of the transcription factor CREB in the dorsal hippocampus of castrated males [100]. Thus, efforts are currently underway to identify the upstream signaling pathway(s) that may play a role in E2-induced memory consolidation in males.
Sex-specific molecular mechanisms have been observed previously in E2-induced facilitation of hippocampal glutamatergic neurotransmission, where glutamate release probability is mediated pre-synaptically by ERβ in females and ERα in males, and post-synaptic glutamate sensitivity is mediated by GPER in females and ERβ in males [••101]. In this study and our preliminary data [100], the end result—memory enhancement or synaptic potentiation—is similar in males and females. However, the sexes differ in the molecular mechanisms that mediate these effects. As such, more studies are needed to directly compare the molecular mechanisms underlying hippocampal function in both sexes to determine the nature and scope of potential sex differences. Such differences could have significant implications for the design of future drug treatments aimed at influencing cognitive function in neurodegenerative and psychiatric diseases.
Conclusions
In this abridged Current Opinion format, we have provided a simplified overview of the effects of E2 on hippocampal memory in females and males. Although the evidence generally suggests that E2 can benefit the formation of memories mediated by the hippocampus, a few caveats should be considered. We know far more about the effects of E2 on memory in ovariectomized females than in males, despite evidence that the male hippocampus responds to exogenous E2. For example, E2 rapidly regulates CA1 dendritic spine density in male hippocampal cultures by triggering activation of numerous cell-signaling cascades [30], including some that our laboratory has demonstrated are essential for E2 to enhance memory consolidation in ovariectomized mice. But as described above, males and females may use different molecular mechanisms to mediate the effects of E2 on memory, so these mechanisms must be examined in both males and females. Indeed, we know very little about potential sex differences in the mnemonic response to E2 because so few studies have directly compared the effects of E2 on memory in females and males. Typically, studies have been conducted using one sex, and therefore, estimations of possible sex differences require apples-to-oranges comparisons among studies in which many aspects of experimental design may differ, including behavioral protocols and E2 formulation, dose, and/or route of administration. Thus, to allow for more accurate assessment of putative sex differences in estrogenic regulation of hippocampal memory, many more studies are needed that directly compare the effects of E2 on memory in males and females within the same study using the same experimental conditions.
Even within ovariectomized females, however, we still know relatively little about how E2 facilitates memory formation. We have learned a great deal in recent years about the receptor, cell-signaling, and epigenetic mechanisms necessary for E2 to facilitate memory consolidation in ovariectomized mice, but much more remains unknown, including how these mechanisms interact within cells, in which cell populations these mechanisms are activated (e.g., pyramidal neurons, interneurons, granule cells, glia), and how E2-induced alterations in the hippocampus may affect the activity of interconnected brain regions such as the prefrontal cortex. As such, much more remains to be done.
Finally, whereas nearly all studies examining effects of estrogens on memory have been conducted using ovariectomized females, which facilitates more control over E2 levels than is possible using naturally cycling females, there is no standard convention regarding the gonadal status of males. Studies of exogenous E2 administration have been conducted in both gonadally-intact and castrated males, which complicates interpretation of results given that castrated males are deprived of endogenous circulating E2 derived from aromatization of testosterone in the testes. The studies conducted to date have generally failed to consider gonadal status in their interpretations, and to our knowledge, only our preliminary study [100] has directly compared the effects of E2 on hippocampal memory in castrated and intact males. Thus, more studies directly comparing effects of E2 on memory in castrated and intact males are necessary to determine the extent to which gonadally-derived sex steroid hormones may interact with exogenous E2 to influence memory formation.
Although the past three decades have seen a dramatic increase in research on the effects of E2 on hippocampal memory in females and males, this work is just the beginning of what is likely to be a very complicated neuroendocrinological story. With increased awareness of putative sex differences and the relative roles of gonadal vs. brain-derived E2, the field is poised to make significant breakthroughs in better understanding how this vital hormone regulates cognition.
Estradiol (E2) enhances many forms of hippocampal learning and memory
In females, rapid E2 effects are mediated by mechanisms including cell signaling
Much less is known about how E2 regulates hippocampal function in males
E2 may enhance memory in females and males via distinct molecular mechanisms
Future studies should include more direct comparisons between the sexes
Acknowledgments
The University of Wisconsin-Milwaukee supported the writing of this manuscript. Empirical work from our laboratory described herein was supported by the National Institutes of Health (R01AG022525, R01MH107886, R03MH065460), the American Federation for Aging Research, the Ellison Medical Foundation, University of Wisconsin-Milwaukee Research Growth Initiative Awards (101×240 and 101×334), the University of Wisconsin-Milwaukee, and Yale University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors have no conflicts to declare.
Guest editors: Jill Becker, Natalie Tronson, Carrie Ferrario
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
•of special interest
••of outstanding interest
- 1.Frick KM. Sex steroid hormones matter for learning and memory: Estrogenic regulation of hippocampal function in male and female rodents. Learn Mem. 2015;22:472–493. doi: 10.1101/lm.037267.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koss WA, Frick KM. Sex differences in hippocampal function. J Neurosci Res. 2017;95:539–562. doi: 10.1002/jnr.23864. [DOI] [PubMed] [Google Scholar]
- 3.Frankfurt M, Luine V. The evolving role of dendritic spines and memory: Interaction(s) with estradiol. Horm Behav. 2015;74:28–36. doi: 10.1016/j.yhbeh.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bean LA, Ianov L, Foster TC. Estrogen receptors, the hippocampus, and memory. Neuroscientist. 2014;20:534–545. doi: 10.1177/1073858413519865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koebele SV, Bimonte-Nelson HA. Modeling menopause: The utility of rodents in translational behavioral endocrinology research. Maturitas. 2016;87:5–17. doi: 10.1016/j.maturitas.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheppard PAS, Koss WA, Frick KM, Choleris E. Rapid actions of estrogens and their receptors on memory acquisition and consolidation in females. J Neuroendocrinol. doi: 10.1111/jne.12485. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tuscher JJ, Fortress AM, Kim J, Frick KM. Regulation of object recognition and object placement by ovarian sex steroid hormones. Behav Brain Res. 2015;285:140–157. doi: 10.1016/j.bbr.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamson DK, Wainwright SR, Taylor JR, Jones BA, Watson NV, Galea LA. Androgens increase survival of adult-born neurons in the dentate gyrus by an androgen receptor-dependent mechanism in male rats. Endocrinology. 2013;154:3294–3304. doi: 10.1210/en.2013-1129. [DOI] [PubMed] [Google Scholar]
- 9.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-α and and -ß mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 10.Brailoiu E, Dun SL, Brailoiu GC, Mizuo K, Sklar LA, Oprea TI, Prossnitz ER, Dun NJ. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol. 2007:311–321. doi: 10.1677/JOE-07-0017. [DOI] [PubMed] [Google Scholar]
- 11.Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, Warrier S, Alves SE. Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. J Comp Neurol. 2005;491:81–95. doi: 10.1002/cne.20724. [DOI] [PubMed] [Google Scholar]
- 12.Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J Comp Neurol. 2001;429:355–371. [PubMed] [Google Scholar]
- 13.Mitterling KL, Spencer JL, Dziedzic N, Shenoy S, McCarthy K, Waters EM, McEwen BS, Milner TA. Cellular and subcellular localization of estrogen and progestin receptor immunoreactivities in the mouse hippocampus. J Comp Neurol. 2010;518:2729–2743. doi: 10.1002/cne.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14••.Waters EM, Thompson LI, Patel P, Gonzales AD, Ye HZ, Filardo EJ, Clegg DJ, Gorecka J, Akama KT, McEwen BS, Milner TA. G-protein-coupled estrogen receptor 1 is anatomically positioned to modulate synaptic plasticity in the mouse hippocampus. J Neurosci. 2015;35:2384–2397. doi: 10.1523/JNEUROSCI.1298-14.2015. This comprehensive study used light and electron microscopy to examine the subregional and subcellular distribution of GPER in the male and female rat hippocampus. In addition to providing invaluable quantitation of GPER immunoreactive profiles in hippocampal laminae of males and cycling females, this study also shows that GPER activation in ovariectomized rats regulates synaptic proteins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fortress AM, Frick KM. Epigenetic regulation of estrogen-dependent memory. Front Neuroendocrinol. 2014;35:530–549. doi: 10.1016/j.yfrne.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Babayan AH, Kramár EA. Rapid effects of oestrogen on synaptic plasticity: Interactions with actin and its signaling proteins. J Neuroendocrinol. 2013;25:1163–1172. doi: 10.1111/jne.12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foster TC. Interaction of rapid signal transduction cascades and gene expression in mediating estrogen effects on memory over the lifespan. Front Neuroendocrinol. 2005;26:51–64. doi: 10.1016/j.yfrne.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Mahmoud R, Wainwright SR, Galea LA. Sex hormones and adult hippocampal neurogenesis: Regulation, implications, and potential mechanisms. Front Neuroendocrinol. 2016;41:129–152. doi: 10.1016/j.yfrne.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Woolley CS. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- 20.Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inagaki T, Frankfurt M, Luine V. Estrogen-induced memory enhancements are blocked by acute bisphenol A in adult female rats: Role of dendritic spines. Endocrinology. 2012;1534:3357–3367. doi: 10.1210/en.2012-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22••.Tuscher JJ, Luine VN, Frankfurt M, Frick KM. Estradiol-mediated spine changes in the dorsal hippocampus and medial prefrontal cortex of ovariectomized female mice depend on ERK and mTOR activation in the dorsal hippocampus. J Neurosci. 2016;36:1483–1489. doi: 10.1523/JNEUROSCI.3135-15.2016. This in vivo study was the first to show that rapid activation of cell signaling pathways in the dorsal hippocampus are necessary for E2 to increase dendritic spine density not only in the hippocampus but also the medial prefrontal cortex of ovariectomized mice. These data suggest that the hippocampus and prefrontal cortex may interact to mediate the effects of hippocampal E2 on memory consolidation in females. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23••.Gabor C, Lymer J, Phan A, Choleris E. Rapid effects of the G-protein coupled oestrogen receptor (GPER) on learning and dorsal hippocampus dendritic spines in female mice. Physiol Behav. 2015;149:53–60. doi: 10.1016/j.physbeh.2015.05.017. In ovariectomized mice, systemic pre-training injection of a GPER agonist enhanced object recognition, social recognition, object placement and CA1 dendritic spine density within 40 minutes. These findings suggest rapid effects of GPER activation on dendritic morphology that may underlie its regulation of learining and memory. [DOI] [PubMed] [Google Scholar]
- 24.Waters EM, Mitterling K, Spencer JL, Mazid S, McEwen BS, Milner TA. Estrogen receptor alpha and beta specific agonists regulate expression of synaptic proteins in rat hippocampus. Brain Res. 2009;1290:1–11. doi: 10.1016/j.brainres.2009.06.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoeffer CA, Klann E. mTOR signaling: At the crossroads of plasticity, memory and disease. Trends Neurosci. 2010;33:67–75. doi: 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fortress AM, Fan L, Orr PT, Zhao Z, Frick KM. Estradiol-induced object recognition memory consolidation is dependent on activation on mTOR signaling in the dorsal hippocampus. Learn Mem. 2013;20:147–155. doi: 10.1101/lm.026732.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27••.Jacome LF, Barateli K, Buitrago D, Lema F, Frankfurt M, Luine VN. Gonadal hormones rapidly enhance spatial memory and increase hippocampal spine density in male rats. Endocrinology. 2016;157:1357–1362. doi: 10.1210/en.2015-1959. This study provided the first evidence that systemic post-training injection of E2 or testosterone enhances object placement memory consolidation in castrated male rats. In behaviorally naïve castrated males, both E2 and testosterone also increased apical CA1 dendritic spine density with 30 minutes of injection, suggesting parallel effects of these hormones on memory consolidation and rapid hippocampal spinogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mukai H, Tsurugizawa T, Murakami G, Kominami S, Ishii H, Ogiue-Ikeda M, Takata N, Tanabe N, Furukawa A, Hojo Y, Ooishi Y, et al. Rapid modulation of long-term depression and spinogenesis via synaptic estrogen receptors in hippocampal principal neurons. J Neurochem. 2007;100:950–967. doi: 10.1111/j.1471-4159.2006.04264.x. [DOI] [PubMed] [Google Scholar]
- 29.Hojo Y, Munetomo A, Mukai H, Ikeda M, Sato R, Hatanaka Y, Murakami G, Komatsuzaki Y, Kimoto T, Kawato S. Estradiol rapidly modulates spinogenesis in hippocampal dentate gyrus: Involvement of kinase networks. Horm Behav. 2015;74:149–156. doi: 10.1016/j.yhbeh.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 30.Hasegawa Y, Hojo Y, Kojima H, Ikeda M, Hotta K, Sato R, Ooishi Y, Yoshiya M, Chung B-C, Yamazaki T, Kawato S. Estradiol rapidly modulates synaptic plasticity of hippocampal neurons: Involvement of kinase networks. Brain Res. 2015;1621:147–161. doi: 10.1016/j.brainres.2014.12.056. [DOI] [PubMed] [Google Scholar]
- 31.Smith CC, McMahon LL. Estrogen-induced increase in the magnitude of long-term potentiation occurs only when the ratio of NMDA transmission to AMPA transmission is increased. J Neurosci. 2005;26:8517–8522. doi: 10.1523/JNEUROSCI.0762-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kramár EA, Chen LY, Brandon NJ, Rex CS, Liu F, Gall CM, Lynch G. Cytoskeletal changes underlie estrogen’s acute effects on synaptic transmission and plasticity. J Neurosci. 2009;29:12982–12993. doi: 10.1523/JNEUROSCI.3059-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu F, Day M, Muniz LC, Bitran D, Arias R, Revilla-Sanchez R, Grauer S, Zhang G, Kelley C, Pulito V, Sung A, et al. Activation of estrogen receptor-beta regulates hippocampal synaptic plasticity and improves memory. Nat Neurosci. 2008;11:334–343. doi: 10.1038/nn2057. [DOI] [PubMed] [Google Scholar]
- 34.Kim J, Schalk JC, Koss WA, Frick KM. The role of actin polymerization in GPER-mediated hippocampal memory enhancement in female mice. Soc Neurosci Abstr. 2017 Poster 159.104. [Google Scholar]
- 35.Daniel JM, Dohanich GP. Acetylcholine mediates the estrogen-induced increase in NMDA receptor binding in CA1 of the hippocampus and the associated improvement in working memory. J Neurosci. 2001;21:6949–6956. doi: 10.1523/JNEUROSCI.21-17-06949.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daniel JM, Fader AJ, Spencer AL, Dohanich GP. Estrogen enhances performance of female rats during acquisition of a radial arm maze. Horm Behav. 1997;32:217–225. doi: 10.1006/hbeh.1997.1433. [DOI] [PubMed] [Google Scholar]
- 37.Fader AJ, Hendricson AW, Dohanich GP. Estrogen improves performance of reinforced T-maze alternation and prevents the amnestic effects of scopolamine administered systemically or intrahippocampally. Neurobiol Learn Mem. 1998;69:225–240. doi: 10.1006/nlme.1998.3820. [DOI] [PubMed] [Google Scholar]
- 38.Fader AJ, Johnson PEM, Dohanich GP. Estrogen improves working but not reference memory and prevents amnestic effects of scopolamine on a radial-arm maze. Pharmacol Biochem Behav. 1999;62:711–717. doi: 10.1016/s0091-3057(98)00219-6. [DOI] [PubMed] [Google Scholar]
- 39.Luine VN, Richards ST, Wu VY, Beck KD. Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters. Horm Behav. 1998;34:149–162. doi: 10.1006/hbeh.1998.1473. [DOI] [PubMed] [Google Scholar]
- 40.Bimonte HA, Denenberg VH. Estradiol facilitates performance as working memory load increases. Psychoneuroendocrinology. 1999;24:161–173. doi: 10.1016/s0306-4530(98)00068-7. [DOI] [PubMed] [Google Scholar]
- 41.Gibbs RB. Estrogen replacement enhances acquisition of a spatial memory task and reduces deficits associated with hippocampal muscarinic receptor inhibition. Horm Behav. 1999;36:222–233. doi: 10.1006/hbeh.1999.1541. [DOI] [PubMed] [Google Scholar]
- 42.O’Neal MF, Means LW, Poole MC, Hamm RJ. Estrogen affects performance of ovariectomized rats in a two-choice water-escape working memory task. Psychoneuroendocrinology. 1996;21:51–65. doi: 10.1016/0306-4530(95)00032-1. [DOI] [PubMed] [Google Scholar]
- 43.Sandstrom NJ, Williams CL. Memory retention is modulated by acute estradiol and progesterone replacement. Behav Neurosci. 2001;115:384–393. [PubMed] [Google Scholar]
- 44.Sandstrom NJ, Williams CL. Spatial memory retention is enhanced by acute and continuous estradiol replacement. Horm Behav. 2004;45:128–135. doi: 10.1016/j.yhbeh.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 45.Bowman RE, Ferguson D, Luine VN. Effects of chronic restraint stress and estradiol on open field activity, spatial memory, and monoaminergic neurotransmitters in ovariectomized rats. Neuroscience. 2002;113:401–410. doi: 10.1016/s0306-4522(02)00156-2. [DOI] [PubMed] [Google Scholar]
- 46.Heikkinen T, Puoliväli J, Liu L, Rissanen A, Tanila H. Effects of ovariectomy and estrogen treatment on learning and hippocampal neurotransmitters in mice. Horm Behav. 2002;41:22–32. doi: 10.1006/hbeh.2001.1738. [DOI] [PubMed] [Google Scholar]
- 47.Holmes MM, Wide JK, Galea LAM. Low levels of estradiol facilitate, whereas high levels of estradiol impair, working memory performance on the radial arm maze. Behav Neurosci. 2002;116:928–934. doi: 10.1037//0735-7044.116.5.928. [DOI] [PubMed] [Google Scholar]
- 48.Garza-Meilandt A, Cantu RE, Claiborne BJ. Estradiol’s effects on learning and neuronal morphology vary with route of administration. Behav Neurosci. 2006;120:905–916. doi: 10.1037/0735-7044.120.4.905. [DOI] [PubMed] [Google Scholar]
- 49.Bohacek J, Daniel JM. Increased daily handling of ovariectomized rats enhances performance on a radial-maze task and obscures effects of estradiol replacement. Horm Behav. 2007;52:237–243. doi: 10.1016/j.yhbeh.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 50.Hammond R, Mauk R, Ninaci D, Nelson D, Gibbs RB. Chronic treatment with estrogen receptor agonists restores acquisition of a spatial learning task in young ovariectomized rats. Horm Behav. 2009;56:309–314. doi: 10.1016/j.yhbeh.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fugger HN, Foster TC, Gustafsson J-A, Rissman EF. Novel effects of estradiol and estrogen receptor and on cognitive function. Brain Res. 2000;883:258–264. doi: 10.1016/s0006-8993(00)02993-0. [DOI] [PubMed] [Google Scholar]
- 52.Rissman EF, Heck AL, Leonard JE, Shupnik MA, Gustafsson JA. Disruption of estrogen receptor beta gene impairs spatial learning in female mice. Proc Natl Acad Sci USA. 2002;99:3996–4001. doi: 10.1073/pnas.012032699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Foster TC, Rani A, Kumar A, Cui L, Semple-Rowland SL. Viral vector-mediated delivery of estrogen receptor-alpha to the hippocampus improves spatial learning in estrogen receptor-alpha knockout mice. Mol Ther. 2008;16:1587–1593. doi: 10.1038/mt.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hammond R, Nelson D, Kline E, Gibbs RB. Chronic treatment with a GPR30 antagonist impairs acquisition of a spatial learning task in young female rats. Horm Behav. 2012;62:367–374. doi: 10.1016/j.yhbeh.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55•.Pisani SL, Neese SL, Katzenellenbogen JA, Schantz SL, Korol DL. Estrogen receptor-selective agonists modulate learning in female rats in a dose- and task-specific manner. Endocrinology. 2016;157:292–303. doi: 10.1210/en.2015-1616. This laboratory has demonstrated in numerous studies that systemic pre-training injection of E2 promotes the use of hippocampal-mediated spatial strategies in ovariectomized rats rather than striatial-mediated egocentric strategies to locate rewards in a plus-shaped maze. This study used ER agonists to show that hippocampal place learning can be facilitated by activation of either ERα or ERβ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Korol DL, Kolo LL. Estrogen-induced changes in place and response learning in young adult female rats. Behav Neurosci. 2002;116:411–420. doi: 10.1037//0735-7044.116.3.411. [DOI] [PubMed] [Google Scholar]
- 57.Locklear MN, Kritzer MF. Assessment of the effects of sex and sex hormones on spatial cognition in adult rats using the Barnes maze. Horm Behav. 2014;66:298–308. doi: 10.1016/j.yhbeh.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gibbs RB, Johnson DA. Sex-specific effects of gonadectomy and hormone treatment on acquisition of a 12-arm radial maze task by Sprague Dawley rats. Endocrinology. 2008;149:3176–3183. doi: 10.1210/en.2007-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gibbs RB. Testosterone and estradiol produce different effects on cognitive performance in male rats. Horm Behav. 2005;48:268–277. doi: 10.1016/j.yhbeh.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luine V, Rodriguez M. Effects of estradiol on radial arm maze performance of young and aged rats. Behav Neural Biol. 1994;62:230–236. doi: 10.1016/s0163-1047(05)80021-4. [DOI] [PubMed] [Google Scholar]
- 61.Vázquez-Pereyra F, Rivas-Arancibia S, Loaeza-Del Castillo A, Schneider-Rivas S. Modulation of short term and long term memory by steroid sexual hormones. Life Sci. 1995;56:PL255–260. doi: 10.1016/0024-3205(95)00067-g. [DOI] [PubMed] [Google Scholar]
- 62.Talebi A, Naghdi N, Sepehri H, Rezayof A. The role of estrogen receptors on spatial learning and memory in CA1 region of adult male rat hippocampus. Iran J Pharm Res. 2010;9:183–191. [PMC free article] [PubMed] [Google Scholar]
- 63.Phan A, Gabor CS, Favaro KJ, Kaschack S, Armstrong JN, MacLusky NJ, Choleris E. Low doses of 17β-estradiol rapidly improve learning and increase hippocampal dendritic spines. Neuropsychopharmacology. 2012;37:2299–2309. doi: 10.1038/npp.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ervin KSJ, Lymer JM, Matta R, Clipperton-Allen AE, Kavaliers M, Choleris E. Estrogen involvement in social behavior in rodents: Rapid and long-term actions. Horm Behav. 2015;74:53–76. doi: 10.1016/j.yhbeh.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 65••.Phan A, Suschkov S, Molinaro L, Reynolds K, Lymer JM, Bailey CD, Kow LM, MacLusky NJ, Pfaff DW, Choleris E. Rapid increases in immature synapses parallel estrogen-induced hippocampal learning enhancements. Proc Natl Acad Sci USA. 2015;112:16018–16023. doi: 10.1073/pnas.1522150112. This exciting study used a combination of intrahippocampal infusions, electrophysiology, and biocytin staining to show that rapid E2-induced enhancements in object placement, object recognition, social recognition, and CA1 dendritic spine density were mediated by ERα in ovariectomized mice. Interestingly, the increase in spine density was associated with a decrease in excitatory glutamatergic input, suggesting a role for silent or immature synapses in the memory-enhancing effects of E2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vedder LC, Smith CC, Flannigan AE, McMahon LL. Estradiol-induced increase in novel object recognition requires hippocampal NR2B-containing NMDA receptors. Hippocampus. 2013;23:108–115. doi: 10.1002/hipo.22068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ismail N, Blaustein JD. Pubertal immune challenge blocks the ability of estradiol to enhance performance on cognitive tasks in adult female mice. Psychoneuroendocrinology. 2013;38:1170–1177. doi: 10.1016/j.psyneuen.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leuner B, Mendolia-Loffredo S, Shors TJ. High levels of estrogen enhance associative memory formation in ovariectomized females. Psychoneuroendocrinology. 2004;29:883–890. doi: 10.1016/j.psyneuen.2003.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Luine VN, Jacome LF, MacLusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- 70.Ervin KS, Mulvale E, Gallagher N, Roussel V, Choleris E. Activation of the G protein-coupled estrogen receptor, but not estrogen receptor or, rapidly enhances social learning. Psychoneuroendocrinology. 2015;58:51–66. doi: 10.1016/j.psyneuen.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 71•.Lymer J, Robinson A, Winters BD, Choleris E. Rapid effects of dorsal hippocampal G-protein coupled estrogen receptor on learning in female mice. Psychoneuroendocrinology. 2017;77:131–140. doi: 10.1016/j.psyneuen.2016.11.019. Pre-training infusion of a GPER agonist into the dorsal hippocampus of ovariectomized mice enhanced learning and memory in the object and social recognition, but not object placement, tasks. Because testing was completed within 40 minutes, these findings suggest that GPER likely regulates recognition memory via rapid non-classical effects. [DOI] [PubMed] [Google Scholar]
- 72.Lynch JF, III, Cullen PK, Jasnow AM, Riccio DC. Sex differences in the generalization of fear as a function of retention intervals. Learn Mem. 2013;20:628–632. doi: 10.1101/lm.032011.113. [DOI] [PubMed] [Google Scholar]
- 73.Lynch JF, III, Dejanovic D, Winiecki P, Mulvany J, Ortiz S, Riccio DC, Jasnow AM. Activation of ER modulates fear generalization through an effect on memory retrieval. Horm Behav. 2014;66:421–429. doi: 10.1016/j.yhbeh.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 74•.Lynch JF, III, Winiecki P, Vanderhoof T, Riccio DC, Jasnow AM. Hippocampal cytosolic estrogen receptors regulate fear generalization in females. Neurobiol Learn Mem. 2016;130:83–92. doi: 10.1016/j.nlm.2016.01.010. Previous work by this group established that E2 facilitates fear generalization to a neutral context in ovariectomized rats. This follow-up study shows that this effect depends on intracellular estrogen receptors rather than membrane-associated estrogen receptors. [DOI] [PubMed] [Google Scholar]
- 75•.Lynch JF, III, Vanderhoof T, Winiecki P, Latsko MS, Riccio DC, Jasnow AM. Aromatized testosterone attenuates contextual generalization of fear in male rats. Horm Behav. 2016;84:127–135. doi: 10.1016/j.yhbeh.2016.06.007. In their 2013 paper, Lynch et al. showed that female rats demonstrate faster fear generalization to a neutral context than males. In this follow-up study, they established that this protection from fear generalization in males is mediated by E2 rather than androgens. [DOI] [PubMed] [Google Scholar]
- 76.Frick KM, Fernandez SM, Harburger LL. A new approach to understanding the molecular mechanisms through which estrogens affect cognition. Biochim Biophys Acta Gen Subj. 2010;1800:1045–1055. doi: 10.1016/j.bbagen.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Packard MG, Teather LA. Posttraining estradiol injections enhance memory in ovariectomized rats: Cholinergic blockade and synergism. Neurobiol Learn Mem. 1997;68:172–188. doi: 10.1006/nlme.1997.3785. [DOI] [PubMed] [Google Scholar]
- 78.Packard MG, Teather LA. Intra-hippocampal estradiol infusion enhances memory in ovariectomized rats. NeuroReport. 1997;8:3009–3013. doi: 10.1097/00001756-199709290-00004. [DOI] [PubMed] [Google Scholar]
- 79.Gresack JE, Frick KM. Post-training estrogen enhances spatial and object memory consolidation in female mice. Pharmacol Biochem Behav. 2006;84:112–119. doi: 10.1016/j.pbb.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 80.Packard MG, Kohlmaier JR, Alexander GM. Posttraining intrahippocampal estradiol injections enhance spatial memory in male rats: Interaction with cholinergic systems. Behav Neurosci. 1996;110:626–632. doi: 10.1037//0735-7044.110.3.626. Studies using post-training infusion of E2, a GPER agonist, and a GPER antagonist demonstrate that activation of GPER facilitates object recognition and object placement memory consolidation in ovariectomized mice in a manner similar to E2. However, additional experiments showed that E2 and GPER regulate memory consoliation via different cell-signaling mechanisms, suggesting that GPER influences memory independently of E2. [DOI] [PubMed] [Google Scholar]
- 81••.Kim J, Szinte JS, Boulware MI, Frick KM. 17β-estradiol and agonism of G-protein Coupled Estrogen Receptor (GPER) enhance hippocampal memory via different cell-signaling mechanisms. J Neurosci. 2016;36:3309–3321. doi: 10.1523/JNEUROSCI.0257-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boulware MI, Heisler JD, Frick KM. The memory-enhancing effects of hippocampal estrogen receptor activation involve metabotropic glutamate receptor signaling. J Neurosci. 2013;33:15184–15194. doi: 10.1523/JNEUROSCI.1716-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Inagaki T, Gautreaux C, Luine V. Acute estrogen treatment facilitates recognition memory consolidation and alters monoamine levels in memory-related brain areas. Horm Behav. 2010;58:415–426. doi: 10.1016/j.yhbeh.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Walf AA, Koonce CJ, Frye CA. Estradiol or diarylpropionitrile administration to wild type, but not estrogen receptor beta knockout, mice enhances performance in the object recognition and object placement tasks. Neurobiol Learn Mem. 2008;89:513–521. doi: 10.1016/j.nlm.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Frye CA, Duffy CK, Walf AA. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol Learn Mem. 2007;88:208–216. doi: 10.1016/j.nlm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cohen SJ, Munchow AH, Rios LM, Zhang G, Asgeirsdottir HN, Stackman RW., Jr The rodent hippocampus is essential for nonspatial object memory. Curr Biol. 2013;23:1685–1690. doi: 10.1016/j.cub.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cohen SJ, Stackman RW., Jr Assessing rodent hippocampal involvement in the novel object recognition task. A review. Behav Brain Res. 2015;285:105–117. doi: 10.1016/j.bbr.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Baker KB, Kim JJ. Effects of stress and hippocampal NMDA receptor antagonism on recognition memory in rats. Learn Mem. 2002;9:58–65. doi: 10.1101/lm.46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Walf AA, Rhodes ME, Frye CA. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiol Learn Mem. 2006;86:35–46. doi: 10.1016/j.nlm.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM. Estradiol-induced enhancement of object memory consolidation involves hippocampal ERK activation and membrane-bound estrogen receptors. J Neurosci. 2008;28:8660–8667. doi: 10.1523/JNEUROSCI.1968-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pereira LM, Bastos CP, de Souza JM, Ribeiro FM, Pereira GS. Estradiol enhances object recognition memory in Swiss female mice by activating hippocampal estrogen receptor. Neurobiol Learn Mem. 2014;114:1–9. doi: 10.1016/j.nlm.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 92.Zhao Z, Fan L, Frick KM. Epigenetic alterations regulate the estradiol-induced enhancement of memory consolidation. Proc Natl Acad Sci USA. 2010;107:5605–5610. doi: 10.1073/pnas.0910578107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93•.Tuscher JJ, Szinte JS, Starrett JR, Krentzel AA, Fortress AM, Remage-Healey L, Frick KM. Inhibition of local estrogen synthesis in the hippocampus impairs hippocampal memory consolidation in ovariectomized female mice. Horm Behav. 2016;83:60–67. doi: 10.1016/j.yhbeh.2016.05.001. The hippocampus synthesizes its own E2 via local aromatization of testosterone. In this study, post-training infusion of an aromatase inhibitor into the dorsal hippocampus of ovariectomized mice prevented memory consolidation in the object recognition and object placement tasks. This finding was the first to demonstrate that hippocampally-synthesized E2 regulates memory formation in mammals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Frick KM. Molecular mechanisms underlying the memory-enhancing effects of estradiol. Horm Behav. 2015;74:4–18. doi: 10.1016/j.yhbeh.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fan L, Zhao Z, Orr PT, Chambers CH, Lewis MC, Frick KM. Estradiol-induced object memory consolidation in middle-aged female mice requires dorsal hippocampal extracellular signal-regulated kinase and phosphatidylinositol 3-kinase activation. J Neurosci. 2010;30:4390–4400. doi: 10.1523/JNEUROSCI.4333-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lewis MC, Kerr KM, Orr PT, Frick KM. Estradiol-induced enhancement of object memory consolidation involves NMDA receptors and protein kinase A in the dorsal hippocampus of female C57BL/6 mice. Behav Neurosci. 2008;122:716–721. doi: 10.1037/0735-7044.122.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhao Z, Fan L, Fortress AM, Boulware MI, Frick KM. Hippocampal histone acetylation regulates object recognition and the estradiol-induced enhancement of object recognition. J Neurosci. 2012;32:2344–2351. doi: 10.1523/JNEUROSCI.5819-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fortress AM, Kim J, Poole RL, Gould TJ, Frick KM. 17β-Estradiol regulates histone alterations associated with memory consolidation and increases Bdnf promoter acetylation in middle-aged female mice. Learn Mem. 2014;21:457–467. doi: 10.1101/lm.034033.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99•.Kim J, Frick KM. Distinct effects of estrogen receptor antagonism on object recognition and spatial memory consolidation in ovariectomized mice. Psychoneuroendocrinology. 2017;85:110–114. doi: 10.1016/j.psyneuen.2017.08.013. Post-training infusion of an ERα antagonist into the dorsal hippocampus of ovariectomized mice enhanced memory consolidation in object placement, but not object recognition, whereas an ERβ antagonist impaired memory in both tasks. These findings suggest a role for both ERs in memory consolidation among females that is memory- or task-specific. [DOI] [PubMed] [Google Scholar]
- 100.Koss WA, Gremminger RL, Philippi SM, Frick KM. Effects of dorsal hippocampal estradiol treatment and aromatase inhibition on memory consolidation in male mice. Soc Neurosci Abstr. 2017 Poster 159.109. [Google Scholar]
- 101••.Oberlander JG, Woolley CS. 17β-estradiol acutely potentiates glutamatergic synaptic transmission in the hippocampus through distinct mechanisms in males and females. J Neurosci. 2017;37:12314–12327. doi: 10.1523/JNEUROSCI.4437-15.2016. This innovative study used whole-cell voltage-clamp recordings and two-photon glutamate uncaging to demonstrate that E2 acutely potentiates glutamatergic synaptic transmission in hippocampal slices from gonadectomized male and female rats via different estrogen receptor mechanisms. In females, ERβ increases presynaptic glutamate release, whereas GPER increase postsynaptic glutamate sensitivity. In males, ERα increases presynaptic glutamate release, and ERβ increase postsynaptic glutamate sensitivity. Note that an earlier version of this paper containing an error was published in 2016; this reference is for the corrected version. [DOI] [PMC free article] [PubMed] [Google Scholar]


