Abstract
Mammalian cell culture is foundational to biomedical research, and the reproducibility of research findings across the sciences is drawing increasing attention. While many components contribute to reproducibility, the reporting of factors that impact oxygen delivery in the general biomedical literature has the potential for both significant impact, and immediate improvement. The relationship between the oxygen consumption rate of cells and the diffusive delivery of oxygen through the overlying medium layer means parameters such as medium depth and cell type can cause significant differences in oxygenation for cultures nominally maintained under the same conditions. While oxygenation levels are widely understood to significantly impact the phenotype of cultured cells in the abstract, in practise the importance of the above parameters does not appear to be well recognized in the non-specialist research community. On analyzing two hundred articles from high-impact journals we find a large majority missing at least one key piece of information necessary to ensure consistency in replication. We propose that explicitly reporting these values should be a requirement for publication.
Introduction
Reproducibility
Reproducibility is a critical foundation of science. Shortcomings in this area are a growing concern for preclinical research in particular, given the potential for economic and human health impacts, with some studies reporting more than half of the works they investigated are incompletely reproducible [1–5] (89% [1], 78% [6], 54% [7], and 51% [8]). In the US alone, the economic impact of irreproducibility has been conservatively estimated to exceed US$28 billion annually [9]. Several initiatives have been launched to define and mitigate this problem, including “The Reproducibility Project: Cancer Biology”, which aimed to replicate 50 high impact cancer biology articles [10]. However, this approach has obvious practical and financial limitations, and the flagship project has recently been forced to scale back their objectives from 50 articles to 18 [11]. Perhaps not surprisingly, given the complexity of modern biological research, a major challenge for reproducibility is the publication of research findings with unintended, unrecognized or underreported differences in experimental method [12]. For instance, using mice of different strain, age, or sex can lead to different conclusions, even if other variables are consistent [12–14]. Similarly, passage number, cell identity and culture conditions have been shown to significantly impact reproducibility [15–17]. Other potential causes include poor experimental design, inappropriate statistical analyses [18], and of course outright scientific misconduct [19]. Here, we have chosen to focus on oxygen delivery, as the problem rests on sound and non-controversial theoretical foundations (few researchers would dispute the idea that oxygen impacts cellular phenotype, nor that aqueous solutions provide a significant diffusive barrier to oxygen delivery); and reporting–and awareness–around a few simple parameters can help reduce inconsistencies between experiments without significantly increasing the space allocated to methodology.
Oxygen levels in culture commonly differ from physiologically-relevant values
In vivo, oxygenation is finely tuned across time and length scales, including via cell-autonomous effects, vasodilation and -constriction, changes in respiratory rate, and vascular remodelling [20,21]. Nearly all of these processes are completely absent in culture, leaving oxygenation levels easily perturbed and dependant on the precise details of culture conditions–which are therefore critical to report to ensure reproducibility.
A common theme in the general literature employing mammalian cell culture is the maintenance of cells in non-physiological oxygen levels, and the use of inadequate terminology to describe these conditions. In particular, culture of cells in incubators in communication with the ambient atmosphere is often referred to as “normoxia”, while cultures in incubators with lower levels are commonly referred to as “hypoxic” [15,22,23]. In turn, “normoxic” incubators are often erroneously assumed to deliver 20.9% oxygen to the cells, without discussion of other parameters (see below), which–if true–would be substantially higher than the normal levels experienced by even well perfused tissues such as the lung parenchyma [24–27]. To avoid ambiguity, we will adopt the use of the recently coined term “physoxia” [26] to describe the oxygen levels a cell would normally encounter in vivo. We refer to higher oxygen levels as hyperoxia, and lower ones as hypoxia (or “near-anoxia” as they approach the limit of the ability of cultured cells to take up oxygen [15,26,28]).
Variations in oxygenation significantly impact cells
As a key substrate in the bioenergetics of mammalian cells, oxygen availability dictates metabolic efficiency. Aerobic metabolism allows for the theoretical production of up to thirty-six ATP molecules per glucose molecule consumed [29], in contrast to anaerobic glycolysis, which produces two. Thus, oxygenation has the potential for significant direct impacts on cellular metabolism. As cellular metabolism is able to draw oxygen out of solution even at very low concentrations, sufficiently oxygen starved cultures can even achieve near-anoxic status (2.65•10−7 mol/l) [30–32], where cellular respiration is expected to vary linearly with oxygen levels under Michaelis-Menten kinetics [30].
However, local oxygen concentration can also impact gene expression and cellular behaviour more subtly [33–36]. Under some conditions, atmospheric oxygen levels can result in a hyperoxic environment for the cultured cells. Decreases in proliferation rate, reduced plating efficiency and progressive decline in metabolic activity have all been reported as consequences of culture in the presence of excess oxygen [26,37], potentially mediated by the generation of reactive oxygen species [30,38,39]. In the case of stem cells, hyperoxia has been reported to promote differentiation, and change responses to growth factors [24]. On the other end of the spectrum, hypoxia can trigger far-reaching signaling cascades via processes such as the unfolded protein response (UPR), mTOR signaling, and hypoxia-inducible factor (HIF)-mediated gene regulation [25,26,40]. This in turn can lead to reduced metabolic rates, temporary cell cycle arrest, promote maintenance of an undifferentiated state, and upregulate production of pro-angiogenic and pro-survival signals [41–43]. It is of particular importance to note that these can be threshold-mediated responses, where a small shift in oxygen concentration can provoke a disproportionate response [40,44,45].
While researchers who work specifically in the area of oxygenation and cellular metabolism are conscious of factors that affect it [15,46], discussions of reproducibility do not generally consider oxygenation [1–8]. However, as discussed above, if cells in one experiment are hyperoxic and in another, hypoxic, due to the relevant variables not being specified, even if all other aspects of an experimental system are adequately described, significant inconsistencies in behaviour would be quite likely. While the problem of inadequate methodological detail in the literature is well recognized, and a great many other factors also contribute to irreproducibility, we wished to quantitively determine the prevalence of this issue as related specifically to oxygenation, and to raise awareness of specific, simple steps that should be taken to avoid it. We hypothesized that a substantial proportion of articles employing mammalian tissue culture as a research tool do not report sufficient methodological detail to ensure reproducibility of the oxygenation conditions to which the cells were exposed, even in top-tier journals.
Results and discussion
Key factors affecting oxygen diffusion
Once steady state conditions are achieved following e.g. medium change, diffusive flux of oxygen through the growth medium to the cells being cultured is governed by multiple factors including cellular O2 consumption rate, cell density, O2 partial pressure, O2 solubility, temperature, media diffusion properties and the height of the media column [47–51]. Although for reasons of complexity we do not address it here, note also that the amount of oxygen delivered directly through polystyrene culture surfaces can be significant [32].
According to Fick’s first law, diffusive flux is proportional to the concentration gradient, which in turn is a function of the difference in concentration between the gas-liquid interface and the culture surface, divided by the thickness of the liquid layer. As the cells consume oxygen at a given rate, the local concentration falls, increasing the gradient (and therefore flux) until equilibrium is attained, and the local oxygen concentration stabilizes at the corresponding value. However, there is an upper limit to diffusive delivery of oxygen in a given system–oxygen levels at the culture surface cannot go below zero, at which point the diffusion gradient (and therefore the flux) cannot be further increased without increasing atmospheric oxygen levels, or reducing the thickness of the liquid layer. Beyond this point, cellular metabolism is necessarily limited (either by adaptive biological processes, or simple physics).
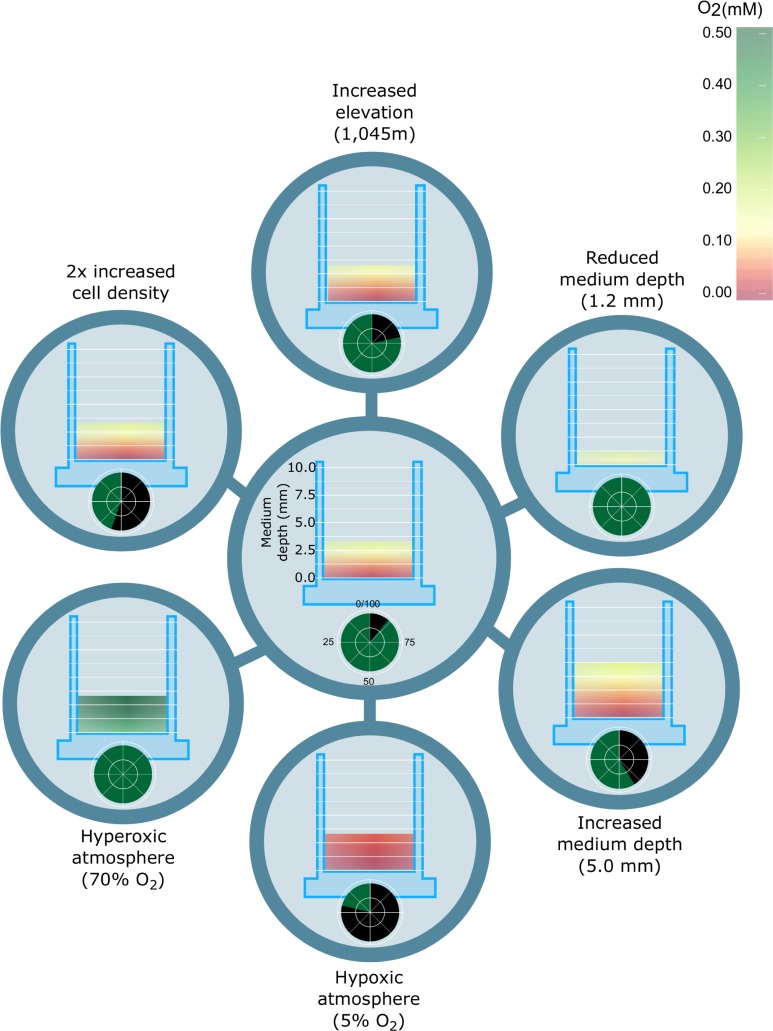
Modelling this process demonstrates that relatively minor changes to critical variables (Table 1)–within ranges that could realistically occur in routine culture–can result in significant changes to both oxygen delivery per cell and local oxygen concentration around them (Fig 1) (for detailed example calculations see Supplementary Materials).
Table 1. Critical factors determining oxygen delivery to the culture surface [32,47].
| FACTOR | DESCRIPTION |
|---|---|
| CULTURE VESSEL | In combination with medium volume, determines medium deptha |
| CELL TYPE | Oxygen consumption rates vary widely between cell typesb |
| SEEDING DENSITY | With cell type, determines oxygen consumption rate per unit areac |
| MEDIA VOLUME | With culture vessel geometry, determines medium deptha |
aMedium depth in turn determines the diffusive barrier to oxygen delivery
bAs there can be significant variation between cells nominally of the same line [30,52–57], cell type and history should be specified as precisely as possible
cWhile theoretically if cell density is reported at time of plating, a replicate experiment will duplicate the density after a fixed period of expansion, ideally cell density at time of harvest / experimentation should also be reported
Fig 1. Steady-state oxygen mass transfer in cell culture media.
Shown in the centre is a plot of the calculated oxygen concentration (mmol/L) across a media column under culture conditions of media depth, atmospheric pressure at sea level, a culture density of 200,000 cells / cm2, and an oxygen consumption rate for CHO cells of [47]. Each condition shown around the perimeter represents the consequences of a change in one variable. At the base of each column the green filling within the circle indicates the fraction of the cells’ metabolic oxygen needs that can be met under each condition.
Variables impacting oxygenation are not adequately documented in the literature
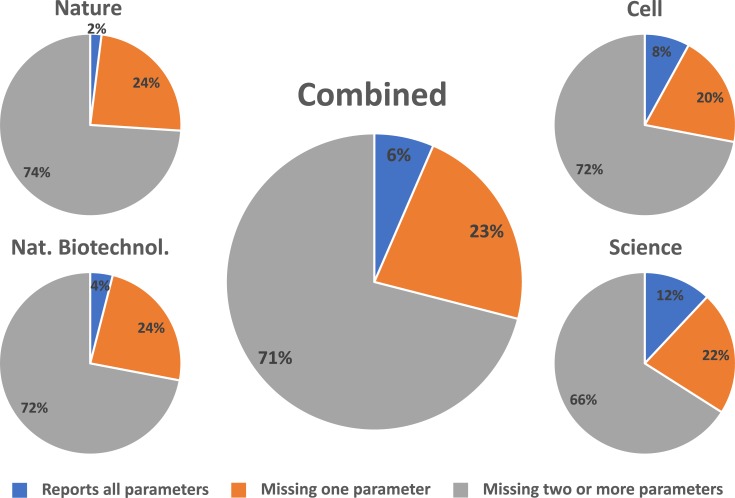
While the ideal study would include the actual oxygen levels at the culture surface, key factors to calculate this value such as the specific oxygen consumption rate per cell may not be known, while equipment to directly measure dissolved oxygen is not universally available. Therefore, we focused on parameters which would be minimally required to ensure reproducibility of oxygenation conditions, even if the absolute value was not determined (Table 1). We assessed fifty recent articles making use of mammalian tissue culture (see Materials and Methods) from each of four widely-recognized high-impact journals: Nature Biotechnology, Nature, Cell and Science. Out of the two hundred papers examined (Fig 2), 71% were missing values for more than one critical variable, 23% were missing only one and 6% had all the necessary data. None of the papers attempted to calculate the oxygen levels in the microenvironment around the cells.
Fig 2. Results from analysis of published work employing mammalian cell culture.
Manuscripts were assessed and identified as specifying all of the critical variables (blue), missing only one of the critical variables (orange), or missing multiple critical variables (grey).
While this does not necessarily mean that an attempt to reproduce the findings of a given publication would fail–the experimenters might choose conditions that result in a relatively similar oxygen environment, or alterations in gene expression or metabolism due to altered oxygenation might not dramatically affect the specific finding in question–it does draw our attention to a substantial area of risk, and provides a potential explanation that should be considered when unable to recapitulate a published finding.
Variables impacting oxygenation should be documented in all manuscripts employing mammalian cell culture
The prevalence of the problem demonstrates that a systemic solution is urgently required to ensure reproducibility of published experiments.
We propose that journal editors require all manuscripts employing mammalian cell culture to include in the materials and methods a section entitled “Oxygenation considerations”, which would at a minimum, explicitly include cell type, culture chamber specifications, media volume, and cell density both at time of seeding and of experimentation, unless those values are already included elsewhere, in which case their location should be specified in the cover letter to the editor.
Note that this information is necessary but not sufficient to ensure reproducibility. Many common cell lines are informally transferred between laboratories, potentially accumulating genetic and epigenetic alterations, and in many cases are (deliberately or otherwise) selected on the basis of phenotypes such as clonal expansion capacity. It would not be surprising for metabolism (and therefore oxygen consumption rates) to differ between two cultures of what is nominally the same cell line. Where possible, oxygen consumption rates and/or measured oxygen concentrations should be reported, as should other factors that might influence oxygen delivery, including high levels of vibration or frequent movement of culture vessels (e.g. in a crowded incubator, which would result in mixing of the medium and accelerate delivery of oxygen); obstructions to air flow below the culture vessel (which would reduce oxygen delivery through the culture surface); or the fact that a given laboratory is at a particularly high altitude (which would reduce absolute oxygen concentration in the atmosphere).
In addition to the parameters listed above, oxygen delivery to cultured cells can be influenced by other factors and reporting these would further ensure accurate replication of experiments: cell density at time of experiment, culture temperature, and partial pressure of O2 (Table 2). Theoretically, cell density should be consistent given the same starting density, culture conditions, and elapsed time, however explicitly reporting this value would remove a potential source of variability. Culture temperature is often assumed to be 37°C if not explicitly reported, and if not specified the partial pressure of O2 is generally considered to reflect exposure to atmospheric oxygen, however it is important to note that this is a function of the altitude of the location at which the culture experiment was carried out, and this value should also be reported.
Table 2. Additional parameters that affect the amount of delivered oxygen to cultured cells.
| FACTOR | EFFECT | CITATION |
|---|---|---|
| TEMPERATURE | A) Increases in temperature cause conflicting effects of increasing the diffusion coefficient while decreasing oxygen solubility. In distilled water at 25°C that is heated to 37°C the combined effect increases the flux of oxygen by approximately 15%. | [15,58–60] |
| PARTIAL PRESSURE OF OXYGEN | A) An increase in altitude decreases the equilibrium dissolved oxygen; in our laboratory in Calgary (elev. 1045m) atmospheric pressure (and hence maximum oxygen flux) is about 13% lower than at sea level. Note that meteorological pressure reports for a given location commonly refer to “Altimeter setting” pressure (normalized to altitude), as opposed to the true barometric “Station Pressure”, which can give the false impression that local air pressure is similar to that found at sea level. B) An increase in pressure increases the solubility of oxygen, so doubling the ambient air pressure would double the oxygen flux in a system. C) The introduction of humidity and carbon dioxide effectively dilutes other atmospheric components–for dry air moving to saturation (~6% water vapour) and 5% CO2 reduces the partial pressure of oxygen by 11% (or 8% for an initial atmosphere at 50% relative humidity). |
[15,30,61,62] |
|
CONVECTIVE MIXING |
Vibration, large medium heights and temperature gradients will increase convective mixing which will increase the mass transfer of oxygen. | [59,63,64] |
| TISSUE CULTURE MEDIA COMPOSITION | A) Increases in the ionic strength of culture media of reduce the solubility of oxygen and the diffusion coefficient of oxygen; the combined effect is to reduce oxygen flux by approximately 17% compared to distilled water (*using our values in this paper, see supplementary for the range of values reported in literature). |
[15,30,59,65–67] |
| HANDLING/ REMOVAL FROM TISSUE CULTURE | A) Equilibration to a steady-state oxygen profile happens on a time scale of one to several hours, depending on medium depth but also culture chamber details. B) Opening incubator doors changes the gas mixture in the incubator, which can take on the order of an hour to equilibrate. This can cause changes in the oxygen concentration in the cell culture media, particularly during experiments in low-oxygen atmospheres, which in turn can have more extended effects. |
[15,68] |
| TISSUE CULTURE GEOMETRY | A) Oxygen diffusion through polystyrene varies between culture vessel geometries, and has been reported to be responsible for up to 30% of oxygen delivered to the tissue culture. B) A meniscus causes variation in media column height, which is particularly relevant in small wells such as in a 96 well plate. |
[15,32,69] |
Although it would be ideal to measure or calculate and report the amount of oxygen delivered per cell, reporting the recommended essential information should be sufficient to reproduce the amount of oxygen available for cultured cells. Where the necessary information is available, we recommend that investigators calculate the approximate oxygenation conditions within their culture systems as this may inform interpretation of their findings.
Oxygenation status should be considered when interpreting experiments
Methods of empirically determining oxygen concentration at the cell level in a static culture include use of electrode probes [70], florescent oxygen sensor spots [30], embedded florescent reporters [46] or biological indicators [30]. Unfortunately, these methods are expensive and generally not accessible for routine tissue culture monitoring. While continuous monitoring of true oxygen values would be ideal, in its absence mathematical modelling using a simple formulation of Fick’s law applied to static tissue culture provides an informative, if imperfect alternative [47]. To facilitate use of such a model, we have developed a spreadsheet calculator (S2 File) that allows researchers to estimate the theoretical oxygenation status of their cell cultures. The expected concentration of dissolved oxygen in an incubator under atmospheric oxygen (18.6% see [15]) at 37°C ranges from 175 μM –204 μM [30,47,59,71,72]. For our calculations we used a value of 179 μM [73] (the solubility in distilled water is 200 μM [60]. The estimated oxygen diffusion coefficient vary in literature from 0.976–3.00 x10-5 cm2/s [28,32,46,47,50,59,67,71,72]. The value used here (2.86 cm2/s [32]) is fairly conservative, and lower diffusion coefficients predict even more dramatic oxygen limitations.
Gas mixtures and atmospheric pressure directly influence the equilibrium concentration of oxygen at the air-liquid interface. The depth of the liquid and the consumption rate of oxygen by the cells allows calculation of the theoretical concentration gradient through the media. Depending on the cell density and user-specified oxygen consumption rate (either measured experimentally or obtained from the literature [30] the maximum flux of oxygen through the media may or may not be sufficient to meet the cells’ oxygen stated requirements. If the maximum flux exceeds the total consumption, the calculated culture-surface oxygen concentration at which equilibrium is reached is displayed. Otherwise, the cells will consume oxygen down to near anoxic levels [31], and the proportion of the cells’ nominal oxygen consumption rate that cannot be met under the specified culture conditions is calculated and displayed.
Conclusion
Varying O2 levels clearly have significant potential to impact culture phenotype, and while this fact is widely recognized in the community, it appears not to be taken into account on a routine basis. While any given case of irreproducibility can not necessarily be attributed to a lack of details concerning cell oxygenation status, in order to promote reproducibility in the scientific literature, journal editors should ensure that all published manuscripts employing mammalian tissue culture specify the four critical oxygenation parameters. Experimenters should routinely estimate oxygenation conditions in their culture systems and consider them in their analyses.
Materials and methods
Paper selection:
The following search term was used to retrieve articles, with “JOURNALNAME” replaced by each of: “Nature”, “Nature Biotechnology”, “Cell” and “Science”:
("JOURNALNAME"[Journal]) AND ((mammalian[Text Word] OR mammal[Text Word] OR human[Text Word] OR mouse[Text Word] OR rat[Text Word] OR rabbit[Text Word] OR hamster[Text Word]) AND (culture[Text Word] OR cultured[Text Word] OR cell culture[Text Word] OR tissue culture[Text Word])) AND ("2000/01/01"[Date—Publication]: "2016/06/01"[Date—Publication])
The resulting articles were then evaluated manually (S1 Fig) to restrict them to primary research articles reporting mammalian cell culture sustained for a minimum of twenty-four hours, and the most recent 50 papers from each journal were selected for scoring.
Scoring papers:
Each paper was scored by two evaluators independently, and any discrepancies were subsequently resolved by discussion to establish a consensus score. A conservative list of critical parameters was identified (Table 1), whose absence provides a challenge for efforts to reproduce the published work precisely: cell type, media volume, culture chamber specifications, and seeding density. Each paper was scored as including all parameters; missing one parameter; or missing two or more parameters.
Supporting information
(DOCX)
This spreadsheet makes initial predictions about oxygen delivery to cells cultured under a given set of conditions. As the model is fairly basic, these predictions should not be considered as definitive, but rather as a starting point for deeper consideration of culture behaviour.
(XLSX)
(XLSX)
A PubMed search was used to retrieve articles from Nature, Nature Biotechnology, Cell and Science with publication date between 2016/06/30–2000/01/01 containing the key words (“Mammalian” OR “Mammal” OR “human” OR “mouse” OR “rat” OR “rabbit” OR “hamster”) AND (“culture” OR “cultured” OR “cell culture” OR “tissue culture”). The resulting articles were then evaluated manually to restrict them to primary research articles reporting mammalian cell culture sustained for a minimum of twenty-four hours, and the most recent 50 papers from each journal were selected for scoring.
(TIF)
Acknowledgments
We thank M. Kallos and M. Doran for comments and advice, and M. Anderson for independent validation of search terms.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Funding was provided by: Natural Sciences and Engineering Research Council of Canada (http://www.nserc-crsng.gc.ca/) RGPIN-201404874 (MU), and PhD studentship (DK); Canadian Institutes of Health Research (http://cihr-irsc.gc.ca/) MOP-137095 (MU); Alberta Diabetes Institute (https://www.ualberta.ca/alberta-diabetes/) Graduate Studentship (YY); Alberta Children’s Hospital Research Institute (https://research4kids.ucalgary.ca/) Graduate Studentship (AA), and Post Doctoral Fellowship (DT). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Begley CG, Ellis LM. Drug development: Raise standards for preclinical cancer research. Nature. 2012;483: 531–533. 10.1038/483531a [DOI] [PubMed] [Google Scholar]
- 2.Begley CG, Ioannidis JPA. Reproducibility in Science: Improving the Standard for Basic and Preclinical Research. Circ Res. 2015;116: 116–126. 10.1161/CIRCRESAHA.114.303819 [DOI] [PubMed] [Google Scholar]
- 3.Baker M. 1,500 scientists lift the lid on reproducibility. Nature. 2016;533: 452–454. 10.1038/533452a [DOI] [PubMed] [Google Scholar]
- 4.Mobley A, Linder SK, Braeuer R, Ellis LM, Zwelling L. A Survey on Data Reproducibility in Cancer Research Provides Insights into Our Limited Ability to Translate Findings from the Laboratory to the Clinic. Arakawa H, editor. PLoS One. 2013;8: e63221 10.1371/journal.pone.0063221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karp NA. Reproducible preclinical research—Is embracing variability the answer? PLOS Biol. 2018;16: e2005413 10.1371/journal.pbio.2005413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prinz F, Schlange T, Asadullah K. Believe it or not: how much can we rely on published data on potential drug targets? Nat Rev Drug Discov. WORLD SCIENTIFIC; 2011;10: 712 10.1038/nrd3439-c1 [DOI] [PubMed] [Google Scholar]
- 7.Vasilevsky NA, Brush MH, Paddock H, Ponting L, Tripathy SJ, LaRocca GM, et al. On the reproducibility of science: unique identification of research resources in the biomedical literature. PeerJ. 2013;1: e148 10.7717/peerj.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartshorne JK, Schachner A. Tracking Replicability as a Method of Post-Publication Open Evaluation. Front Comput Neurosci. 2012;6: 8 10.3389/fncom.2012.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freedman LP, Cockburn IM, Simcoe TS. The Economics of Reproducibility in Preclinical Research. PLOS Biol. 2015;13: e1002165 10.1371/journal.pbio.1002165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Errington TM, Iorns E, Gunn W, Tan FE, Lomax J, Nosek BA. An open investigation of the reproducibility of cancer biology research. Elife. 2014;3: 1–9. 10.7554/eLife.04333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaiser J. Plan to replicate 50 high-impact cancer papers shrinks to just 18. Science (80-). 2018; 10.1126/science.aau9619 [DOI] [Google Scholar]
- 12.Bolli R. Reflections on the Irreproducibility of Scientific Papers: Table. Circ Res. 2015;117: 665–666. 10.1161/CIRCRESAHA.115.307496 [DOI] [PubMed] [Google Scholar]
- 13.Flórez-Vargas O, Brass A, Karystianis G, Bramhall M, Stevens R, Cruickshank S, et al. Bias in the reporting of sex and age in biomedical research on mouse models. Elife. 2016;5: 1–14. 10.7554/eLife.13615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clayton JA. Studying both sexes: A guiding principle for biomedicine. FASEB J. 2016;30: 519–524. 10.1096/fj.15-279554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wenger R, Kurtcuoglu V, Scholz C, Marti H, Hoogewijs D. Frequently asked questions in hypoxia research. Hypoxia. 2015; 35 10.2147/HP.S92198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freedman LP, Gibson MC, Ethier SP, Soule HR, Neve RM, Reid YA. Reproducibility: changing the policies and culture of cell line authentication. Nat Methods. Nature Publishing Group; 2015;12: 493–497. 10.1038/nmeth.3403 [DOI] [PubMed] [Google Scholar]
- 17.Witek P, Korga A, Burdan F, Ostrowska M, Nosowska B, Iwan M, et al. The effect of a number of H9C2 rat cardiomyocytes passage on repeatability of cytotoxicity study results. Cytotechnology. Springer Netherlands; 2016;68: 2407–2415. 10.1007/s10616-016-9957-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landis SC, Amara SG, Asadullah K, Austin CP, Blumenstein R, Bradley EW, et al. A call for transparent reporting to optimize the predictive value of preclinical research. Nature. Nature Publishing Group; 2012;490: 187–191. 10.1038/nature11556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang FC, Steen RG, Casadevall A. Misconduct accounts for the majority of retracted scientific publications. Proc Natl Acad Sci. 2012;109: 17028–17033. 10.1073/pnas.1212247109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wittenberg B. Transport Of Oxygen In Muscle. Annu Rev Physiol. 1989;51: 857–878. 10.1146/annurev.ph.51.030189.004233 [DOI] [PubMed] [Google Scholar]
- 21.Muoio V, Persson PB, Sendeski MM. The neurovascular unit—concept review. Acta Physiol. 2014;210: 790–798. 10.1111/apha.12250 [DOI] [PubMed] [Google Scholar]
- 22.Saltzman DJ, Toth A, Tsai AG, Intaglietta M, Johnson PC. Oxygen tension distribution in postcapillary venules in resting skeletal muscle. Am J Physiol Heart Circ Physiol. 2003;285: H1980–H1985. 10.1152/ajpheart.00322.2002 [DOI] [PubMed] [Google Scholar]
- 23.Wild JM, Fichele S, Woodhouse N, Paley MNJ, Kasuboski L, van Beek EJR. 3D volume-localizedpO2 measurement in the human lung with3He MRI. Magn Reson Med. 2005;53: 1055–1064. 10.1002/mrm.20423 [DOI] [PubMed] [Google Scholar]
- 24.Ivanovic Z. Hypoxia or in situ normoxia: The stem cell paradigm. J Cell Physiol. 2009;219: 271–275. 10.1002/jcp.21690 [DOI] [PubMed] [Google Scholar]
- 25.Ivanovic Z., Physiological ex vivo cell oxygenation is necessary for a true insight into cytokine biology. Eur Cytokine Netw. 2009;20: 7–9. 10.1684/ecn.2009.0144 [DOI] [PubMed] [Google Scholar]
- 26.McKeown SR. Defining normoxia, physoxia and hypoxia in tumours—Implications for treatment response. Br J Radiol. 2014;87: 1–12. 10.1259/bjr.20130676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carreau A, El Hafny-Rahbi B, Matejuk A, Grillon C, Kieda C. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J Cell Mol Med. 2011;15: 1239–53. 10.1111/j.1582-4934.2011.01258.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomes A, Guillaume L, Grimes DR, Fehrenbach J, Lobjois V, Ducommun B. Oxygen Partial Pressure Is a Rate-Limiting Parameter for Cell Proliferation in 3D Spheroids Grown in Physioxic Culture Condition. PLoS One. 2016;11: e0161239 10.1371/journal.pone.0161239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heiden MG Vander Cantley LC, Thompson CB, Mammalian P, Exhibit C, Metabolism A. Understanding the Warburg Effect: Cell Proliferation. Science (80-). 2009;324: 1029 10.1126/science.1160809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wagner BA, Venkataraman S, Buettner GR. The Rate of Oxygen Utilization by Cells. Free Radic Biol Med. 2011. 10.1016/j.freeradbiomed.2011.05.024.The [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Froese G. The respiration of ascites tumour cells at low oxygen concentrations. Biochim Biophys Acta. 1962;57: 509–519. [DOI] [PubMed] [Google Scholar]
- 32.Randers-Eichhorn L, Bartlett RA, Frey DD, Rao G. Noninvasive oxygen measurements and mass transfer considerations in tissue culture flasks. Biotechnol Bioeng. 1996;51: 466–478. [DOI] [PubMed] [Google Scholar]
- 33.Wenger RH. Mammalian oxygen sensing, signalling and gene regulation. J Exp Biol. 2000;203: 1253–63. [DOI] [PubMed] [Google Scholar]
- 34.Wenger RH. Cellular adaptation to hypoxia: O 2 -sensing protein hydroxylases, hypoxia-inducible transcription factors, and O 2 -regulated gene expression. FASEB J. 2002;16: 1151–1162. 10.1096/fj.01-0944rev [DOI] [PubMed] [Google Scholar]
- 35.Jež M, Rožman P, Ivanović Z, Bas T. Concise Review: The Role of Oxygen in Hematopoietic Stem Cell Physiology. J Cell Physiol. 2015;230: 1999–2005. 10.1002/jcp.24953 [DOI] [PubMed] [Google Scholar]
- 36.Pahl HL, Baeuerle PA. Oxygen and the control of gene expression. BioEssays. 1994;16: 497–502. 10.1002/bies.950160709 [DOI] [PubMed] [Google Scholar]
- 37.Schoonen WGEJ Wanamarta AH, Moorsel JMVDK, Jakobs C, Joenje H. Hyperoxia-induced clonogenic killing of HeLa cells associated with respiratory failure and selective inactivation of Krebs cycle enzymes. Mutat Res. 1990;237: 173–181. [DOI] [PubMed] [Google Scholar]
- 38.Freiberger J, Coulombe K, Suliman H, Carraway M, Piantadosi C. Superoxide dismutase responds to hyperoxia in rat hippocampus. Undersea Hyperb Med. 2004;31: 227–32. [PubMed] [Google Scholar]
- 39.Lee PJ, Choi AM. Pathways of cell signaling in hyperoxia. Free Radic Biol Med. 2003;35: 341–350. 10.1016/S0891-5849(03)00279-X [DOI] [PubMed] [Google Scholar]
- 40.Majmundar AJ, Wong WJ, Simon MC. Hypoxia-Inducible Factors and the Response to Hypoxic Stress. Mol Cell. Elsevier Inc.; 2010;40: 294–309. 10.1016/j.molcel.2010.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9: 677–684. 10.1038/nm0603-677 [DOI] [PubMed] [Google Scholar]
- 42.Noman MZ, Hasmim M, Messai Y, Terry S, Kieda C, Janji B, et al. Hypoxia: a key player in antitumor immune response. A Review in the Theme: Cellular Responses to Hypoxia. Am J Physiol Physiol. 2015;309: C569–C579. 10.1152/ajpcell.00207.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chacko SM, Ahmed S, Selvendiran K, Kuppusamy ML, Khan M, Kuppusamy P. Hypoxic preconditioning induces the expression of prosurvival and proangiogenic markers in mesenchymal stem cells. Am J Physiol Physiol. 2010;299: C1562–C1570. 10.1152/ajpcell.00221.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dachs GU, Tozer GM. Hypoxia modulated gene expression: Angiogenesis, metastasis and therapeutic exploitation. Eur J Cancer. 2000;36: 1649–1660. 10.1016/S0959-8049(00)00159-3 [DOI] [PubMed] [Google Scholar]
- 45.Guitart A V., Hammoud M, Dello Sbarba P, Ivanovic Z, Praloran V. Slow-cycling/quiescence balance of hematopoietic stem cells is related to physiological gradient of oxygen. Exp Hematol. 2010;38: 847–851. 10.1016/j.exphem.2010.06.002 [DOI] [PubMed] [Google Scholar]
- 46.Kasinskas RW, Venkatasubramanian R, Forbes NS. Rapid uptake of glucose and lactate, and not hypoxia, induces apoptosis in three-dimensional tumor tissue culture. Integr Biol (United Kingdom). 2014;6: 399–410. 10.1039/c4ib00001c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McLiams WF, Blumenson LE, Tunnah K V. Kinetics of Gas Diffusion i n Mammalian Cell Culture Systems. 11. Theory. 1968;X: 741–763. [Google Scholar]
- 48.Place TL, Domann FE, Case AJ. Limitations of oxygen delivery to cells in culture: An underappreciated problem in basic and translational research. Free Radic Biol Med. Elsevier B.V.; 2017;113: 311–322. 10.1016/j.freeradbiomed.2017.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fleischaker RJ, Sinskey AJ. Oxygen demand and supply in cell culture. Eur J Appl Microbiol Biotechnol. 1981;12: 193–197. 10.1007/BF00499486 [DOI] [Google Scholar]
- 50.Van Winkle AP, Gates ID, Kallos MS. Mass Transfer Limitations in Embryoid Bodies during Human Embryonic Stem Cell Differentiation. Cells Tissues Organs. 2012;196: 34–47. 10.1159/000330691 [DOI] [PubMed] [Google Scholar]
- 51.Martin Y, Vermette P. Bioreactors for tissue mass culture: Design, characterization, and recent advances. Biomaterials. 2005;26: 7481–7503. 10.1016/j.biomaterials.2005.05.057 [DOI] [PubMed] [Google Scholar]
- 52.Baker DEC, Harrison NJ, Maltby E, Smith K, Moore HD, Shaw PJ, et al. Adaptation to culture of human embryonic stem cells and oncogenesis in vivo. Nat Biotechnol. 2007;25: 207–15. 10.1038/nbt1285 [DOI] [PubMed] [Google Scholar]
- 53.Graham S V., Tindle RW, Birnie GD. Variation in myc gene amplification and expression in sublines of HL60 cells. Leuk Res. 1985;9: 239–47. 10.1016/0145-2126(85)90086-4 [DOI] [PubMed] [Google Scholar]
- 54.Hiorns LR, Bradshaw TD, Skelton LA, Yu Q, Kelland LR, Leyland-Jones B. Variation in RNA expression and genomic DNA content acquired during cell culture. Br J Cancer. 2004;90: 476–82. 10.1038/sj.bjc.6601405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nugoli M, Chuchana P, Vendrell J, Orsetti B, Ursule L, Nguyen C, et al. Genetic variability in MCF-7 sublines: evidence of rapid genomic and RNA expression profile modifications. BMC Cancer. 2003;3: 13 10.1186/1471-2407-3-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Culliton BJ. HeLa Cells: Contaminating Cultures around the World. Science. 1974;184: 1058–9. 10.1126/science.184.4141.1058 [DOI] [PubMed] [Google Scholar]
- 57.Macville M, Schröck E, Padilla-Nash H, Keck C, Ghadimi BM, Zimonjic D, et al. Comprehensive and definitive molecular cytogenetic characterization of HeLa cells by spectral karyotyping. Cancer Res. 1999;59: 141–50. [PubMed] [Google Scholar]
- 58.Han P, Bartels DM. Temperature Dependence of Oxygen Diffusion in H 2 O and D 2 O †. J Phys Chem. 1996;100: 5597–5602. 10.1021/jp952903y [DOI] [Google Scholar]
- 59.Langø T, Mørland T, Brubakk AO. Diffusion coefficients and solubility coefficients for gases in biological fluids and tissues: a review. Undersea Hyperb Med. 1996;23: 247–72. [PubMed] [Google Scholar]
- 60.Temperature Tromans D. and pressure dependent solubility of oxygen in water: a thermodynamic analysis. Hydrometallurgy. 1998;48: 327–342. 10.1016/S0304-386X(98)00007-3 [DOI] [Google Scholar]
- 61.Yang J ‐D, Wang NS. Oxygen Mass Transfer Enhancement via Fermentor Headspace Pressurization. Biotechnol Prog. 1992;8: 244–251. 10.1021/bp00015a010 [DOI] [PubMed] [Google Scholar]
- 62.Sangster WE. An Improved Technique for Computing the Horizontal Pressure-Gradient Force at the Earth’s Surface. Mon Weather Rev. 1987;115: 1358–1369. [DOI] [Google Scholar]
- 63.Balin AK, Goodman DBP, Rasmussen H, Cristofalo VJ. The effect of oxygen tension on the growth and metabolism of WI‐38 cells. J Cell Physiol. 1976;89: 235–249. 10.1002/jcp.1040890207 [DOI] [PubMed] [Google Scholar]
- 64.Berry JD, Godara P, Liovic P, Haylock DN. Predictions for optimal mitigation of paracrine inhibitory signalling in haemopoietic stem cell cultures. Stem Cell Res Ther. 2015;6: 58 10.1186/s13287-015-0048-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ju L-K, Ho CS. Measuring oxygen diffusion coefficients with polarographic oxygen electrodes: I. Electrolyte solutions. Biotechnol Bioeng. 1985;27: 1495–1499. 10.1002/bit.260271015 [DOI] [PubMed] [Google Scholar]
- 66.Ho CS, Ju L-K, Ho C-T. Measuring oxygen diffusion coefficients with polarographic oxygen electrodes. II. Fermentation Media. Biotechnol Bioeng. 1986;28: 1086–1092. 10.1002/bit.260280720 [DOI] [PubMed] [Google Scholar]
- 67.Jamnongwong M, Loubiere K, Dietrich N, Hébrard G. Experimental study of oxygen diffusion coefficients in clean water containing salt, glucose or surfactant: Consequences on the liquid-side mass transfer coefficients. Chem Eng J. 2010;165: 758–768. 10.1016/j.cej.2010.09.040 [DOI] [Google Scholar]
- 68.Allen CB, Schneider BK, White CW. Limitations to oxygen diffusion and equilibration in in vitro cell exposure systems in hyperoxia and hypoxia. Am J Physiol Lung Cell Mol Physiol. 2001;281: L1021–7. 10.1152/ajplung.2001.281.4.L1021 [DOI] [PubMed] [Google Scholar]
- 69.Cassileth B, Brown C, Liberatore C, Lovejoy J, Parry S, Streeto C, et al. Limitations to oxygen diffusion and equilibration in in vitro cell exposure systems in hyperoxia and hypoxia. Am J Physiol Lung Cell Mol Physiol. 2001;4: 261–263. 10.1080/08858198909528020 [DOI] [PubMed] [Google Scholar]
- 70.Allen CB, Schneider BK, White CW. Limitations to oxygen diffusion and equilibration in in vitro cell exposure systems in hyperoxia and hypoxia. Am J Physiol Cell Mol Physiol. 2001;281: L1021–L1027. 10.1152/ajplung.2001.281.4.L1021 [DOI] [PubMed] [Google Scholar]
- 71.Kim MC, Lam RHW, Thorsen T, Asada HH. Mathematical analysis of oxygen transfer through polydimethylsiloxane membrane between double layers of cell culture channel and gas chamber in microfluidic oxygenator. Microfluid Nanofluidics. 2013;15: 285–296. 10.1007/s10404-013-1142-8 [DOI] [Google Scholar]
- 72.Buchwald P. FEM-based oxygen consumption and cell viability models for avascular pancreatic islets. Theor Biol Med Model. 2009;6 10.1186/1742-4682-6-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vendruscolo F, Rossi MJ, Schmidell W, Ninow JL. Determination of Oxygen Solubility in Liquid Media. ISRN Chem Eng. 2012;2012: 1–5. 10.5402/2012/601458 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
This spreadsheet makes initial predictions about oxygen delivery to cells cultured under a given set of conditions. As the model is fairly basic, these predictions should not be considered as definitive, but rather as a starting point for deeper consideration of culture behaviour.
(XLSX)
(XLSX)
A PubMed search was used to retrieve articles from Nature, Nature Biotechnology, Cell and Science with publication date between 2016/06/30–2000/01/01 containing the key words (“Mammalian” OR “Mammal” OR “human” OR “mouse” OR “rat” OR “rabbit” OR “hamster”) AND (“culture” OR “cultured” OR “cell culture” OR “tissue culture”). The resulting articles were then evaluated manually to restrict them to primary research articles reporting mammalian cell culture sustained for a minimum of twenty-four hours, and the most recent 50 papers from each journal were selected for scoring.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.