Abstract
Odor-guided behaviors, including homing, predator avoidance, or food and mate searching, are ubiquitous in animals. It is only recently that the neural substrate underlying olfactomotor behaviors in vertebrates was uncovered in lampreys. It consists of a neural pathway extending from the medial part of the olfactory bulb (medOB) to locomotor control centers in the brainstem via a single relay in the caudal diencephalon. This hardwired olfactomotor pathway is present throughout life and may be responsible for the olfactory-induced motor behaviors seen at all life stages. We investigated modulatory mechanisms acting on this pathway by conducting anatomical (tract tracing and immunohistochemistry) and physiological (intracellular recordings and calcium imaging) experiments on lamprey brain preparations. We show that the GABAergic circuitry of the olfactory bulb (OB) acts as a gatekeeper of this hardwired sensorimotor pathway. We also demonstrate the presence of a novel olfactomotor pathway that originates in the non-medOB and consists of a projection to the lateral pallium (LPal) that, in turn, projects to the caudal diencephalon and to the mesencephalic locomotor region (MLR). Our results indicate that olfactory inputs can induce behavioral responses by activating brain locomotor centers via two distinct pathways that are strongly modulated by GABA in the OB. The existence of segregated olfactory subsystems in lampreys suggests that the organization of the olfactory system in functional clusters may be a common ancestral trait of vertebrates.
Author summary
Olfactory-induced behaviors (homing, food or mate searching, etc.) are crucial for the survival and reproduction of most animals. A neural substrate underlying odor-induced behaviors in vertebrates was recently uncovered using a basal vertebrate model: the lamprey. It consists of a neural pathway extending from the medial olfactory bulb, a first-order relay of olfactory information in the brain, to locomotor regions. Here, we investigated modulatory mechanisms acting on this neural pathway. We show that an inhibitory circuitry that releases the neurotransmitter GABA in the olfactory bulb strongly modulates motor responses to olfactory stimulation. We also discovered and characterized a novel olfactomotor pathway that originates in the non-medial olfactory bulb and consists of a projection to the lamprey olfactory cortex that, in turn, projects to locomotor regions. This discovery of a novel pathway linking olfactory and motor centers in the brain indicates that olfactory inputs can activate locomotor centers via two distinct pathways. Both pathways are strongly modulated by the neurotransmitter GABA in the olfactory bulb. The existence of segregated olfactory subsystems in lampreys sheds light on the evolution of olfactory systems in vertebrates.
Introduction
Olfactory cues can trigger goal-directed locomotor behaviors, such as homing, predator avoidance, or food and mate searching [1–11]. It is only recently that the neural pathways and mechanisms involved in transforming olfactory inputs into locomotor behavior were characterized for the first time in a vertebrate species, the lamprey [12,13]. It consists of a specific neural pathway extending from a single glomerulus located in the medial part of the olfactory bulb (medOB) to the mesencephalic locomotor region (MLR), with a relay in the posterior tuberculum (PT) [12]. In all vertebrates, the MLR acts as a motor command center that controls locomotion via descending projections to brainstem reticulospinal (RS) neurons [14–22]. This olfactomotor pathway is present throughout the life cycle of lampreys, whether in larvae, newly transformed, parasitic, or spawning animals [12]. Yet, olfactory-induced motor behaviors can be life stage specific in lampreys. For instance, at the parasitic stage, lampreys feed on fish that they detect using olfactory cues [23]. Then, when sexually mature, the adults are attracted upstream by migratory pheromones released by larvae [24–26]. Once upstream, the females are attracted to males by sex pheromones [27,28].
The general organization of the lamprey olfactory system, from the periphery to the central nervous system (CNS), is very similar to that of other vertebrates. The peripheral olfactory organ is composed of a main olfactory epithelium and an accessory olfactory organ [29–31]. Axons from olfactory sensory neurons (OSNs) of the olfactory epithelium terminate in the olfactory bulb (OB). As in other vertebrates, the OB can be divided in two subregions, based on their inputs. The main olfactory bulb (MOB), which occupies the whole OB except its medial part (i.e., the medOB), receives inputs from the main olfactory epithelium. The medOB, on the other hand, receives inputs from OSNs located in the accessory olfactory organ [32–34]. The OB of vertebrates constitutes the primary olfactory center of the CNS and, as such, filters and actively shapes sensory inputs to secondary olfactory structures [35,36]. This processing of sensory inputs in the OB is driven by modulatory inputs coming from the numerous neurotransmitter systems present in the OB of vertebrates [37,38]. GABA is the main inhibitory neurotransmitter in the CNS, and numerous GABAergic processes are present in the OB of several vertebrate species [39–43]. GABAergic neurons of the OB are believed to play a critical role in olfactory processing by providing inhibition to the bulbar microcircuitry [44]. However, their effect on the outputs of the OB and ultimately on behavior is far less understood.
Here, we hypothesized that GABAergic neurons of the OB could play a significant role in modulating transmission in the olfactomotor pathway of lampreys. To address this, we used anatomical (tract tracing and immunohistochemistry) and physiological (intracellular recordings) techniques.
Results
GABAergic gating of olfactory inputs to RS neurons
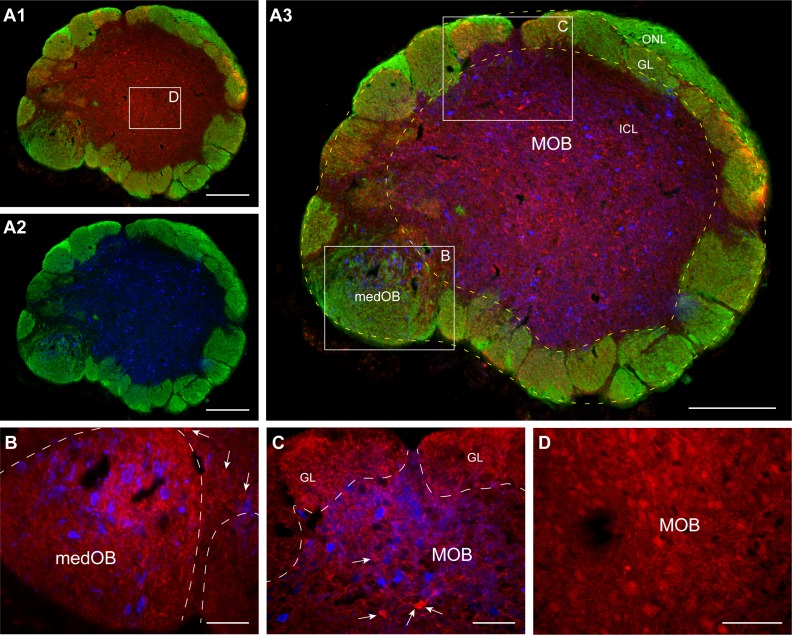
The present study showed abundant GABAergic cell bodies and processes in the OB (n = 10 adult animals, Fig 1), thus confirming the findings of Meléndez-Ferro and colleagues [43]. GABAergic neurons were mainly observed in the central region of the OB (internal cell layer [ICL], Fig 1A and 1D and S1 Fig), where the most common OB interneuron type, the granule cell, was described [45]. GABAergic processes were found all over the OB, including in and around the glomeruli of both the MOB (Fig 1A and 1C) and the medOB (Fig 1A and 1B and S2 Fig).
Fig 1. GABA-immunoreactive cell bodies and processes, as well as projection neurons in the olfactory bulb.
(A) Low-power photomicrographs from a 25-μm-thick cross section of the olfactory bulb showing multiple labelings: binding of GSIB4 to olfactory primary afferents (green), GABA immunofluorescence (red), and retrograde labeling from injections in the PT and LPal (blue). (A1) The distribution of GABAergic neurons in relation to the GL. (A2) The distribution of projection neurons in relation to the GL. (A3) Merge of A1 and A2 showing some overlap in the distribution of the GABAergic and projection neurons populations. The white frames correspond to the approximate location of photomicrographs B to D. (B) High-power photomicrograph from a section just adjacent to the one illustrated in A, showing GABA processes in the medOB glomerulus. The projection neurons (blue) were labeled from an injection in the PT and are seen inside the medOB glomerulus. Arrows point at some GABA cell bodies lying outside of the glomerulus. The green fluorescence of olfactory primary afferents was omitted for clarity, but the contours of the glomerulus are indicated with a dashed line. (C) High-power photomicrograph from the section in A, showing GABA processes in the MOB GL and in the area where projection neurons (blue) are found. Those projection neurons were labeled from an injection in the LPal. Arrows point at some GABA cell bodies. The green fluorescence of olfactory primary afferents was omitted for clarity but the contours of the glomeruli are indicated with a dashed line. (D) Photomicrograph of the central region of the olfactory bulb showing numerous GABAergic neurons and processes. This thicker section was obtained from a different experiment, in which the immunofluorescence procedure was carried out on free-floating sections. Scale bars in A = 200 μm; scale bars in B-D = 50 μm. GL, glomerular layer; GSIB4, Griffonia simplicifolia isolectin B4; ICL, internal cell layer; LPal, lateral pallium; medOB, medial part of the olfactory bulb; MOB, main olfactory bulb, ONL, olfactory nerve layer; PT, posterior tuberculum.
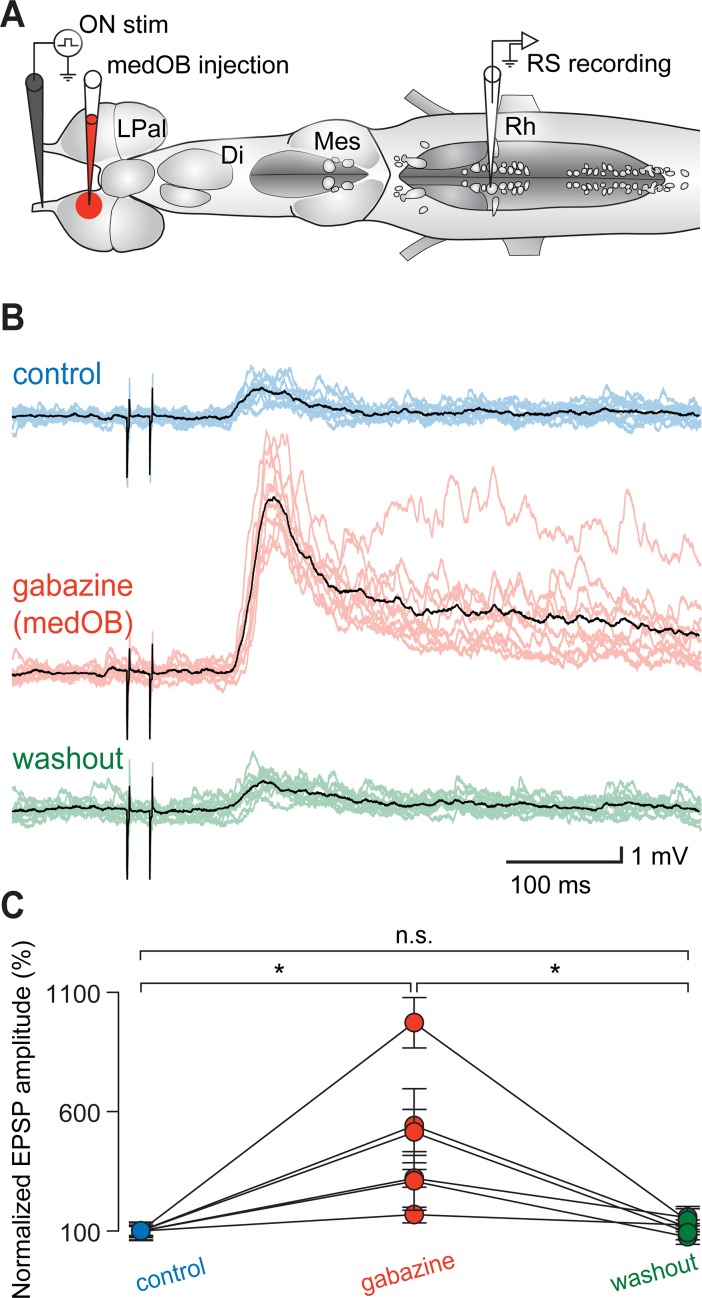
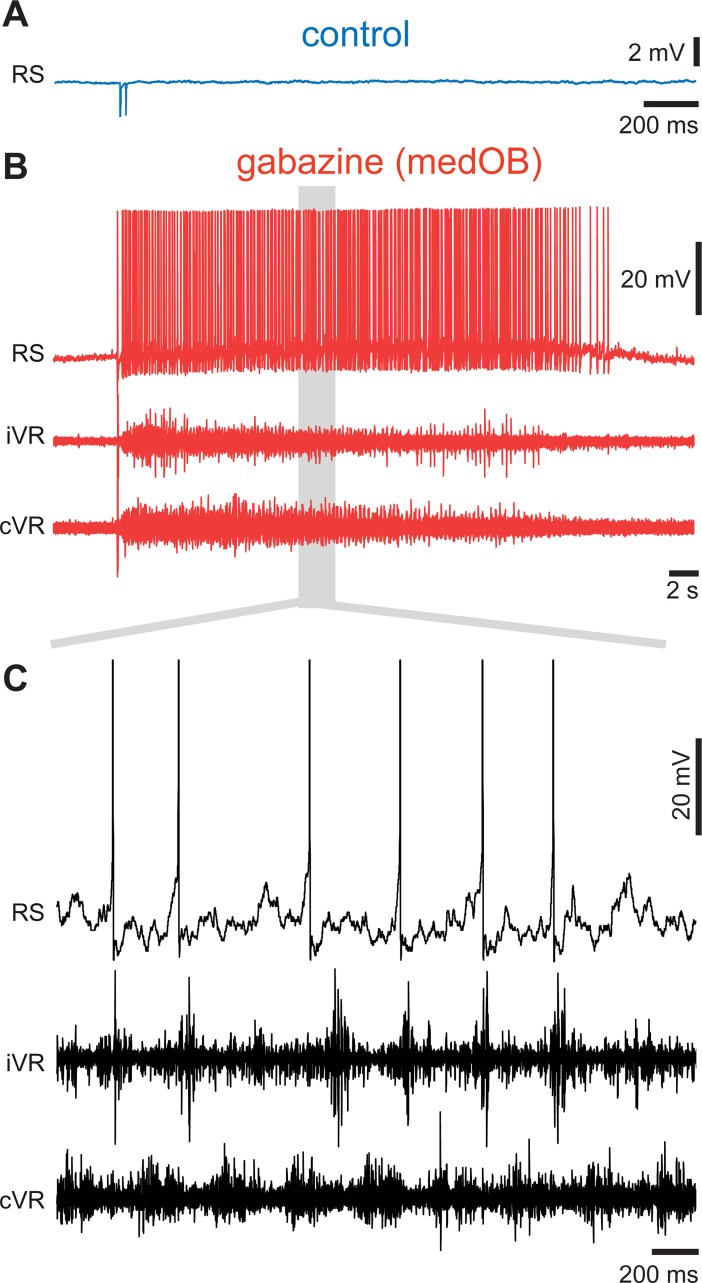
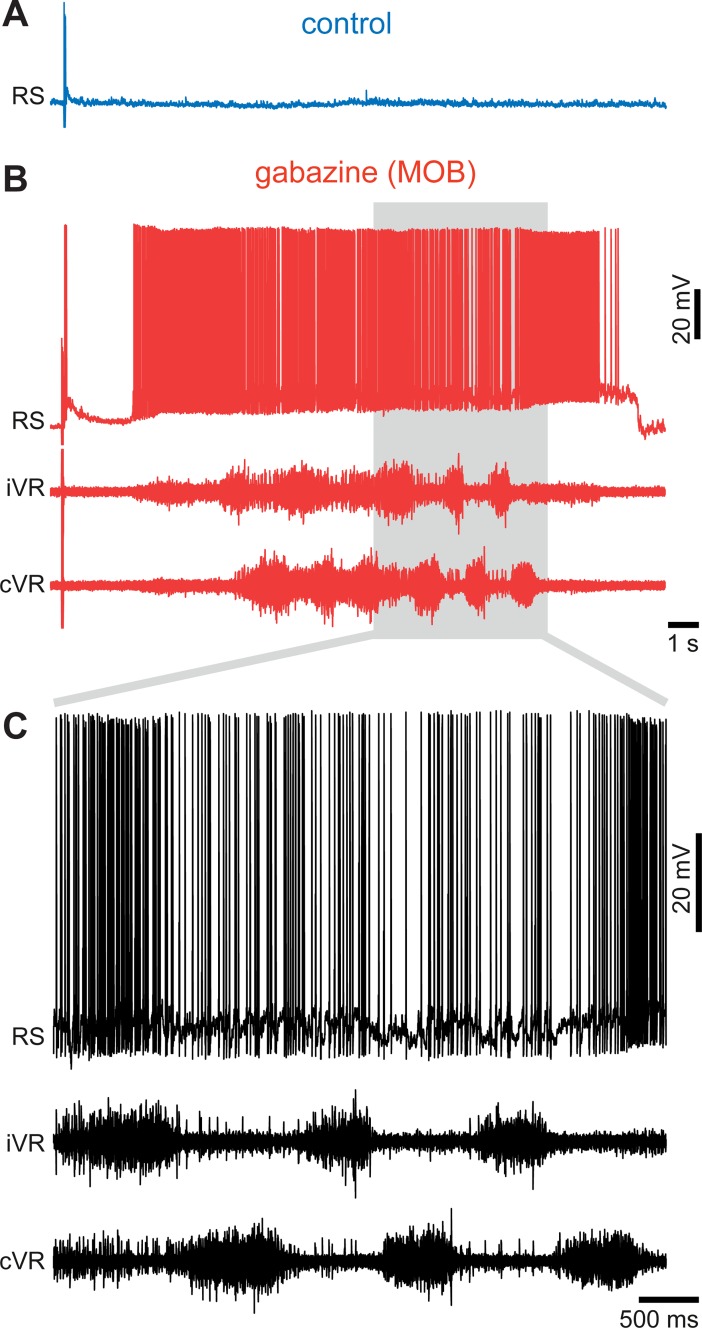
To investigate the physiological role of the GABAergic circuitry in the OB, local microinjections of the GABAA receptor antagonist, gabazine, were made into restricted areas of the OB, while stimulating the olfactory nerve (ON) and intracellularly recording from RS neurons on the same side of the brain. Gabazine injections (0.1 mM, 1.4 ± 1.6 nL) in the medOB (n = 60 synaptic responses; n = 6 neurons; n = 6 larval animals; Fig 2) were found to amplify synaptic responses of RS neurons to electrical stimulation of the ON (amplitude increase of 372.2 ± 277.5%; p < 0.05; no statistical differences between control and washout; Fig 2B and 2C). RS neurons from all four reticular nuclei (mesencephalic reticular nucleus and anterior, middle, and posterior rhombencephalic reticular nuclei) responded similarly as shown by calcium imaging experiments (n = 362 neurons; n = 6 adult animals, S3 Fig). Extracellular recordings of the OB further showed that responses of OB neurons to ON stimulation were greatly increased under gabazine (n = 60 responses; n = 6 animals, S4 Fig), thus corroborating our previous findings. In addition to increasing the responses of RS cells, stimulation of the ON after gabazine injection in the medOB even induced motor discharges in the ventral roots. The neural activity consisted of rhythmic discharges alternating on both sides, a hallmark of fictive swimming (in 58.1% of trials; n = 36 locomotor bouts out of 62 trials for gabazine versus 0 out of 78 for control; n = 9: three adult animals and six larval animals, Fig 3 and S1 Data).
Fig 2. Injection of gabazine into the medOB increases RS neuron responses to ON stimulation.
(A) Schematic representation of the brain preparation illustrating the stimulation, injection, and recording sites. (B) Responses of RS neurons to electrical stimulation of the ON (5–15 μA). A local injection of gabazine (0.1 mM) in the medOB increased the RS neuron responses to ON stimulation. Each black trace is a mean of 10 individual responses (colored traces). (C) Univariate scatterplot showing the normalized (as a percentage of control) EPSP amplitude in control, gabazine, and washout conditions for all animals. An asterisk (*) indicates a statistically significant difference at the level p < 0.05, while n.s. indicates the absence of statistically significant difference. The numerical values underlying this figure can be found in S1 Data. Di, diencephalon; EPSP, excitatory postsynaptic potential; LPal, lateral pallium; medOB, medial part of the olfactory bulb; Mes, mesencephalon; ON, olfactory nerve; Rh, rhombencephalon; RS, reticulospinal.
Fig 3. Stimulation of the ON after a gabazine injection into the medOB can induce fictive swimming.
(A, B) Responses of a RS neuron to electrical stimulation of the ON (10 μA) before (A) and after (B, top trace) a local injection of gabazine (1 mM) in the medOB. Bottom traces in (B) show the neural activity in the iVR and cVR. (C) Details from the gray area in (B) showing fictive locomotion that is characterized by rhythmic alternating ventral root discharges on the iVR and cVR sides. The data and statistics on trials can be found in S1 Data. cVR, contralateral ventral root; iVR, ipsilateral ventral root; medOB, medial part of the olfactory bulb; ON, olfactory nerve; RS, reticulospinal.
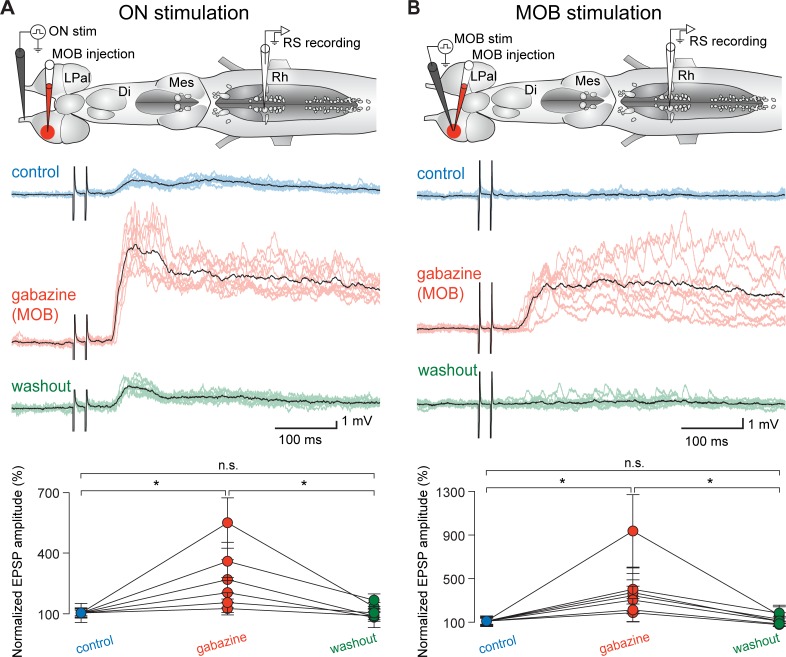
Because the density of GABAergic processes seemed relatively similar in the medOB and the MOB, we hypothesized that the neural activity in the MOB could be modulated by GABA, as observed for the medOB. To test this hypothesis, the effect of gabazine injections in the MOB on RS cell responses was examined. As shown for the medOB, gabazine injections into the MOB (0.1 mM, 1.8 ± 2.0 nL) enhanced the RS neuron responses to ON stimulations (n = 60 synaptic responses; n = 6 neurons; n = 6 larval animals; amplitude increase of 174.4 ± 167.0%, p < 0.05; no statistical differences between control and washout; Fig 4A). However, stimulation of the ON does not activate MOB neurons specifically, as it also activates medOB neurons. To rule out any involvement of the medOB in the increased RS responses after MOB gabazine injections, the effect of an electrical stimulation of the MOB with a gabazine injection (0.1 mM, 2.9 ± 1.1 nL) in the MOB was tested. Under control conditions, MOB stimulation did not induce responses in RS neurons. However, after a gabazine injection in the MOB, electrical stimulation of the MOB elicited responses in RS cells (n = 70 synaptic responses; n = 7 neurons; n = 7 larval animals; amplitude increase of 286.3 ± 296.8%; p < 0.05; no statistical differences between control and washout; Fig 4B). As a further control, electrical stimulation of the MOB under gabazine elicited significant responses in RS cells, even when the medOB had been surgically resected (n = 5 larval animals, S5 Fig). Furthermore, recordings of the ventral roots of the spinal cord showed that electrical stimulation of the MOB after a gabazine injection in the MOB can induce fictive swimming (in 65.1% of trials; 54 locomotor bouts out of 83 trials for gabazine versus 0 out of 105 for control; n = 9 larval animals, Fig 5 and S1 Data). Taken together, these findings suggest the presence of a previously unknown pathway linking the MOB to RS cells that seems to be under a strong tonic GABAergic inhibitory control.
Fig 4. Injection of gabazine into the MOB also increases RS neuron responses to olfactory stimulation.
(A) Top: schematic illustration of the brain showing stimulation, injection, and recording sites. Middle: responses of RS neurons to the electrical stimulation of the ON. A local injection of gabazine (0.1 mM) in the MOB increased the RS neuron responses to ON stimulation (5–10 μA). Each black trace is the mean of 10 individual responses (colored traces). Bottom: univariate scatterplot showing the normalized (as a percentage of control) EPSP amplitude in control, gabazine, and washout conditions for all animals. (B) Top: schematic illustration of the brain shows stimulation, injection, and recording sites. Middle: responses of RS neurons to the electrical stimulation of the MOB. Under control conditions, MOB stimulation did not induce responses in RS neurons. After a local injection of gabazine (0.1 mM) in the MOB, RS neurons responded to MOB stimulation (5–20 μA). Each black trace is the mean of 10 individual responses (colored traces). Bottom: univariate scatterplot showing the normalized (as a percentage of control) EPSP amplitude in control, gabazine, and washout conditions for all animals. An asterisk (*) indicates a statistically significant difference at the level p < 0.05, while n.s. indicates the absence of a statistically significant difference. The numerical values underlying this figure can be found in S1 Data. Di, diencephalon; EPSP, excitatory postsynaptic potential; LPal, lateral pallium; Mes, mesencephalon; MOB, main olfactory bulb; ON, olfactory nerve; Rh, rhombencephalon; RS, reticulospinal.
Fig 5. Stimulation of the MOB after an injection of gabazine into the MOB can induce fictive swimming.
(A, B) Responses of a RS neuron to electrical stimulation of the MOB (30 μA) before (A) and after (B, top trace) a gabazine (1 mM) injection into the MOB. Bottom traces in (B) show the neural activity in the iVR and cVR. (C) Detail from the gray area in (B) showing that the neural activity corresponds to fictive locomotion that is characterized by rhythmic alternating ventral root discharges on the iVR and cVR sides. The data and statistics on trials can be found in S1 Data. cVR, contralateral ventral root; iVR, ipsilateral ventral root; MOB, main olfactory bulb; RS, reticulospinal.
A lateral olfactomotor pathway
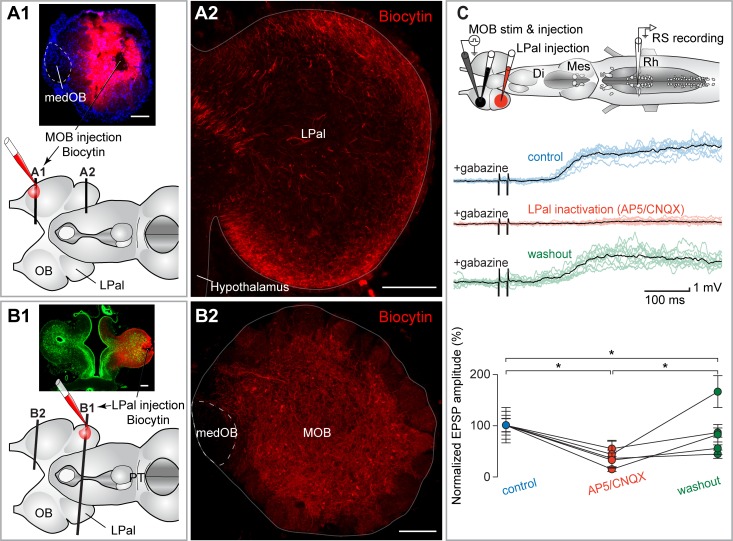
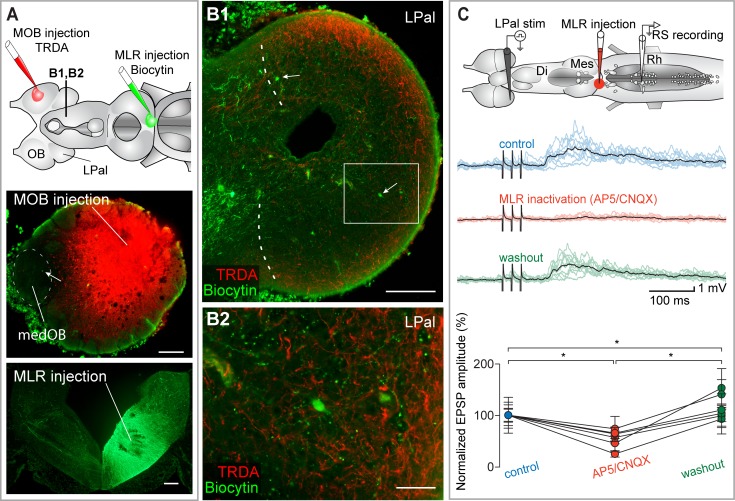
We investigated the spatial organization of projections from the MOB that would eventually reach the RS neurons. We injected the axonal tracer biocytin in the MOB (n = 13 adult animals, Fig 6A1) and found ipsilateral axonal projections to the lateral pallium (LPal), medial pallium, dorsal pallium, striatum, dorsomedial telencephalic neuropil, and habenula. Contralateral projections were found to the OB, dorsomedial telencephalic neuropil, striatum, and LPal. The MOB injections did not label any fibers in the PT. Similar olfactory projections from the OB have been reported in other species of lampreys [46,47], but the selective contribution from the medOB or the MOB was not investigated in these earlier studies. The LPal appears to be a major target of neurons in the MOB, judging by the numerous labeled fibers seen to enter this region. The fibers densely filled the outermost layer covering the entire rostro-caudal extent of the LPal (Fig 6A2). Many fibers were also seen in the more central layers of the LPal, where the neuronal cell bodies of that structure are located. Tracer injections in the LPal (n = 9 adult animals, Fig 6B1) retrogradely labeled many neurons in the MOB without ever labeling cell bodies in the medOB (Fig 6B2). The retrolabeled neurons in the MOB were found close to the glomeruli, but were almost never seen inside them.
Fig 6. The MOB inputs to RS neurons are relayed in the LPal.
(A1) Schematic representation of the brain illustrating the injection site in the MOB and the level of the cross sections shown in A1 and A2. (A2) Numerous fibers were labeled anterogradely in the LPal after a biocytin injection that included a large portion of the MOB but spared the medOB (see A1). (B1) Schematic illustration of the brain showing the injection site in the LPal and the level of the cross sections shown in B1 and B2. (B2) Projection neurons in the MOB were retrogradely labeled after an injection of biocytin in the LPal. These neurons were mainly located in the external portion of the ICL, close to the glomeruli, as opposed to the GABA-containing neurons that were more internally located in the ICL (Fig 1A and S1 Fig). The level of the injection in the LPal in B1 is similar to the level of the section illustrated in A2. Note that the medOB, which contains neurons that project directly to the PT, does not contain neurons projecting to the LPal. (C) Top: schematic illustration of the brain showing stimulation, injection, and recording sites. Middle: responses of RS neurons to the electrical stimulation (15 μA) of the MOB after injecting gabazine (0.1 mM) in the MOB. A local injection of glutamate receptor antagonists AP5/CNQX (0.5 mM/1 mM) in the LPal abolished the RS neuron responses (red traces). Each black trace is the mean of 10 individual responses (colored traces). Bottom: univariate scatterplot showing the normalized (as a percentage of control) EPSP amplitude in control, AP5/CNQX, and washout conditions for all animals. An asterisk (*) indicates a statistically significant difference at the level p < 0.05, while n.s. indicates the absence of statistically significant difference. The numerical values underlying this figure can be found in S1 Data. Scale bars in A, B = 200 μm. AP5, 2-amino-5-phosphonopentanoic acid; CNQX, 6-cyano-7-nitroquinoxaline-2,3-dione; Di, diencephalon; EPSP, excitatory postsynaptic potential; ICL, internal cell layer; LPal, lateral pallium; medOB, medial part of the olfactory bulb; Mes, mesencephalon; MOB, main olfactory bulb; PT, posterior tuberculum; Rh, rhombencephalon; RS, reticulospinal; stim, stimulation.
Physiological experiments were then carried out to characterize the effect of the pharmacological inactivation of the LPal on the responses of RS cells to the electrical stimulation of the MOB. Based on the results reported in Fig 4B, these experiments were carried out after removing the local GABAergic inhibition with a gabazine microinjection into the MOB (0.1 mM, 0.9 ± 1.0 nL, just prior each stimulation). An injection of glutamate receptor antagonists (2-amino-5-phosphonopentanoic acid [AP5]: 0.5 mM, 6-cyano-7-nitroquinoxaline-2,3-dione [CNQX]: 1 mM, 5.2 ± 0.8 nL) in the LPal strongly decreased the RS neuron responses (amplitude decrease of 64.8 ± 21.3%; p < 0.05), thus confirming the role of the LPal in relaying glutamatergic outputs from the MOB to locomotor control centers (n = 50 synaptic responses; n = 5 neurons; n = 5 larval animals, Fig 6C).
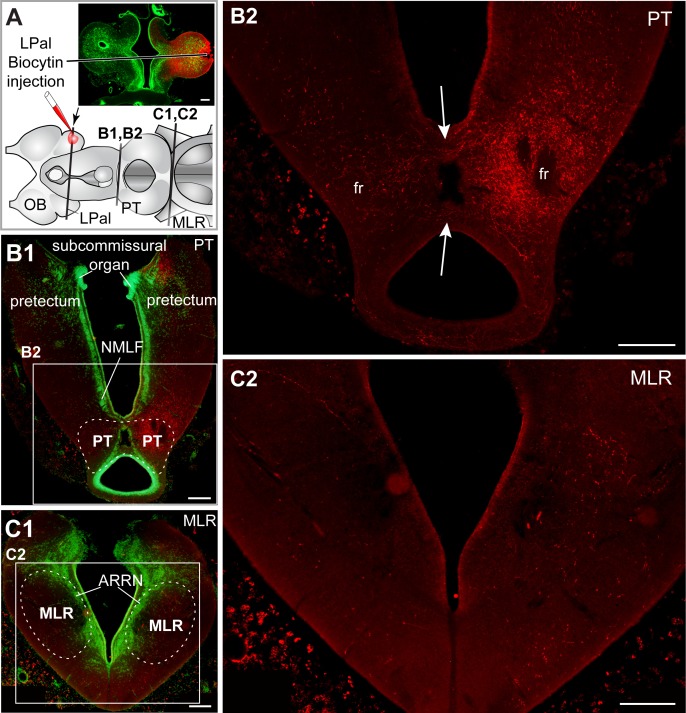
Biocytin was injected in the LPal to examine its descending projections. Emphasis was placed on regions known to be involved in the medial olfactomotor pathway, such as the PT and the MLR (n = 5 adult animals, Fig 7). Numerous fibers terminated in the PT, predominantly on the ipsilateral side (Fig 7B), with fibers crossing locally to the contralateral side (arrows in Fig 7B2). At levels immediately caudal to the PT, in the rostral mesencephalon, the number of descending fibers decreased sharply. Only a few labeled fibers continued to the level of the MLR (Fig 7C), where many appeared to terminate (Fig 7C2). More caudal levels were not investigated in the present study, but it is not excluded that some fibers continued down more caudally [48].
Fig 7. The LPal projects to the PT and MLR.
(A) Schematic illustration of the brain showing the injection site in the LPal and levels of the cross sections shown in A–C. (B) Many descending fibers from the LPal (red) reach the PT. (B1) Cross section at the level of the PT with a superimposed fluorescent Nissl stain (green). (B2) Some fibers are seen crossing to the contralateral side (arrows) at this level. Note that the Nissl stain is not illustrated here. (C) A number of descending fibers from the LPal (red) reach the MLR. (C1) Cross section at the level of the MLR with a superimposed fluorescent Nissl stain (green). (C2) Most fibers from the LPal innervate the ipsilateral MLR (right), although a few fibers were also seen contralaterally (left). Note that the Nissl stain is not illustrated here. B1 and C1 are photomontages of higher-power photomicrographs. All results come from the same animal as in Fig 6B. All scale bars = 200 μm. ARRN, anterior rhombencephalic reticular nucleus; fr, fasciculus retroflexus; LPal, lateral pallium; MLR, mesencephalic locomotor region; NMLF, nucleus of the medial longitudinal fasciculus; OB, olfactory bulb; PT, posterior tuberculum.
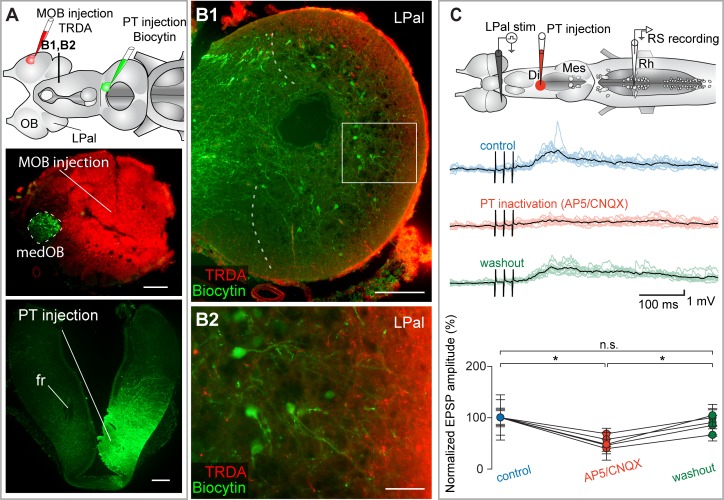
Tracing experiments were carried out to further characterize the population of LPal neurons projecting to the PT and the MLR. The organization and anatomical boundaries of the LPal in lamprey are still debated [47,49–56]. In the present study, we followed the nomenclature of Northcutt and Puzdrowski [47] and Pombal and Puelles [54]. The part of the brain that was considered to be the LPal in the present study is illustrated in S6 Fig. In this series of experiments, a solution containing Texas Red-conjugated dextran amine (TRDA) was injected in the MOB to label olfactory projections from the OB and a solution containing biocytin was injected in the PT (n = 7 adult animals and 1 larval animal, Fig 8A and S7 Fig) or the MLR (n = 4 adult animals and 1 larval animal, Fig 9A and S7 Fig) to retrogradely label neurons projecting to the PT or MLR. Typical results are shown in Figs 8 and 9 and S7 Fig. Labeled cell bodies were distributed uniformly in all regions of the LPal, dorsal, ventral, rostral and caudal, when injections were made in the PT (Fig 8A and 8B) or the MLR (Fig 9A and 9B). The dendrites of cells often extended radially towards the outermost layer of the LPal, where secondary olfactory fibers, labeled from the MOB, are located (Fig 8B and Fig 9B). These results show that fibers originating in the MOB came in proximity with LPal neurons projecting to both the PT and the MLR, suggesting that the LPal is a relay for MOB inputs to the PT and MLR.
Fig 8. The LPal inputs to RS neurons are relayed in the PT.
(A) Top: schematic representation of the brain illustrating the tracer injection sites and the level of the cross section shown in B. Bottom: the injection sites are illustrated on cross sections. Many projection neurons (green), found only in the medOB, are retrogradely labeled from the PT injection. (B1) Many neurons (in green) are retrogradely labeled in the LPal after a tracer injection in the PT. Descending fibers labeled from an MOB injection are superimposed (red). The medial border of the LPal is indicated by curved dashed lines. (B2) High magnification of the boxed area in B1 showing dendrites of neurons projecting to the PT (green) extending towards the descending fibers from the MOB (red). (C) Top: schematic illustration of the brain showing stimulation, injection, and recording sites. Middle: RS neuron responses to electrical stimulation (15–20 μA) of the LPal. A local injection of the glutamate receptor antagonists AP5/CNQX (0.5 mM/1 mM) in the PT (see schematized brain, upper panel) strongly decreased the RS neuron responses. Each black trace is a mean of 10 individual responses (colored traces). Bottom: univariate scatterplot showing the normalized (as a percentage of control) EPSP amplitude in control, AP5/CNQX, and washout conditions for all animals. An asterisk (*) indicates a statistically significant difference at the level p < 0.05, while n.s. indicates the absence of statistically significant difference. The numerical values underlying this figure can be found in S1 Data. Scale bars in A, B1 = 200 μm; scale bar in B2 = 50 μm. AP5, 2-amino-5-phosphonopentanoic acid; CNQX, 6-cyano-7-nitroquinoxaline-2,3-dione; Di, diencephalon; EPSP, excitatory postsynaptic potential; fr, fasciculus retroflexus; LPal, lateral pallium; medOB, medial part of the olfactory bulb; Mes, mesencephalon; MOB, main olfactory bulb; PT, posterior tuberculum; Rh, rhombencephalon; RS, reticulospinal; stim, stimulation; TRDA, Texas Red-conjugated dextran amine.
Fig 9. The LPal inputs to RS neurons are also relayed in the MLR.
(A) Top: schematic illustration of the brain showing the tracer injection sites and the level of the cross section shown in B. Bottom: the injection sites are illustrated on cross sections. A few medOB projection neurons are consistently retrogradely labeled by an MLR injection (one neuron here shown by an arrow in the photomicrograph of the OB). (B1) Neurons (green, two here, arrows) are retrogradely labeled in the LPal after a tracer injection in the MLR. Descending fibers labeled from a MOB injection are superimposed (red). The medial border of the LPal is indicated by curved dashed lines. (B2) High magnification of the boxed area in B1 showing neurons projecting to the MLR (green) in proximity to descending fibers from the MOB (red). (C) Top: schematic illustration of the brain showing stimulation, injection, and recording sites. Middle: RS neuron responses to the electrical stimulation (15–20 μA) of the LPal. A local injection of the glutamate receptor antagonists AP5/CNQX in the MLR (see schematized brain, upper panel) strongly decreased the RS neuron responses. Each black trace is a mean of 10 individual responses (colored traces). Bottom: univariate scatterplot showing the normalized (as a percentage of control) EPSP amplitude in control, AP5/CNQX, and washout conditions for all animals. An asterisk (*) indicates a statistically significant difference at the level p < 0.05, while n.s. indicates the absence of statistically significant difference. The numerical values underlying this figure can be found in S1 Data. Scale bars in A, B1 = 200 μm; scale bar in B2 = 50 μm. AP5, 2-amino-5-phosphonopentanoic acid; CNQX, 6-cyano-7-nitroquinoxaline-2,3-dione; Di, diencephalon; EPSP, excitatory postsynaptic potential; LPal, lateral pallium; medOB, medial part of the olfactory bulb; Mes, mesencephalon; MLR, mesencephalic locomotor region; MOB, main olfactory bulb; OB, olfactory bulb; Rh, rhombencephalon; RS, reticulospinal; stim, stimulation; TRDA, Texas Red-conjugated dextran amine.
Electrophysiological experiments were then conducted to examine the effect of deactivating the PT and the MLR on the RS neuron responses to the electrical stimulation of the LPal (Fig 8C and Fig 9C, respectively). Glutamate antagonists were locally injected in either the PT (AP5: 0.5 mM, CNQX: 1 mM, 1.1 ± 1.2 nL, Fig 8C) or the MLR (AP5: 0.5 mM, CNQX: 1 mM, 3.6 ± 2.5 nL, Fig 9C), and the RS neuron responses were markedly decreased (PT: amplitude decrease of 48.7 ± 19.7%; p < 0.05; no statistical differences between control and washout; n = 50 synaptic responses; n = 5 neurons; n = 5 larval animals; MLR: amplitude decrease of 45.3 ± 21.7%; p < 0.05; n = 60 synaptic responses; n = 6 neurons; n = 6 larval animals). Taken together with our previous findings, these results show that glutamatergic olfactory outputs from the MOB are relayed via the LPal to the PT and to the MLR before reaching RS cells.
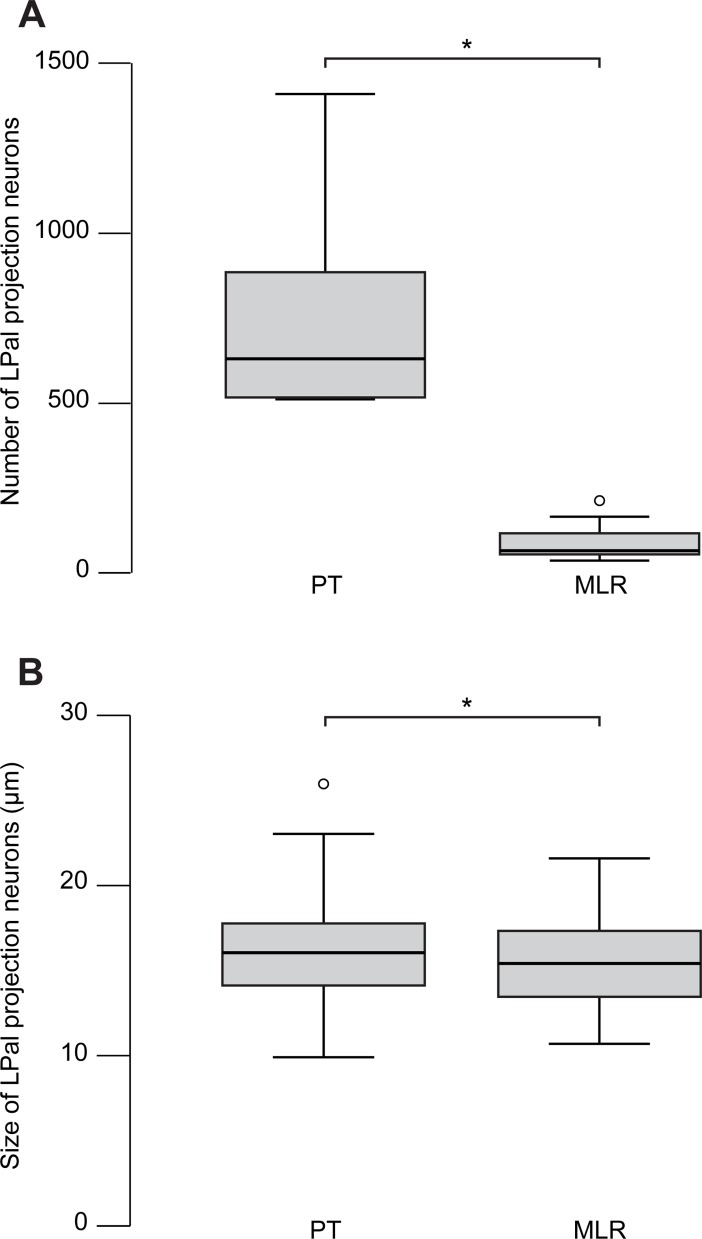
The relative importance of the projection from the LPal to the PT or to the MLR was examined by counting retrogradely labeled cells in the LPal after an injection of a fluorescent tracer in the PT or the MLR. Bilateral biocytin injections in the PT (n = 6 adult animals) followed by the analysis of 10 LPals revealed that, on average, 751 ± 283 LPal neurons (per LPal) projected to the PT (Fig 10A). Bilateral biocytin injections in the MLR (n = 5 adult animals) followed by the analysis of eight LPals revealed an average of 93 ± 62 neurons (per LPal) in these animals (Fig 10A). The size of LPal neurons projecting to the PT and MLR was measured along their long axis. LPal PT- and MLR-projecting neurons measured on average 16.3 ± 3.0 μm (n = 90 cells from a subset of three animals, Fig 10B) and 15.4 ± 2.4 μm (n = 90 cells from a subset of three animals, Fig 10B), respectively. Interestingly, a few medOB neurons were systematically labeled after an MLR tracer injection (Fig 9A), thus demonstrating a direct projection from the medOB to the MLR.
Fig 10. Number and size of LPal neurons projecting to the PT and MLR.
(A) Box plot showing the number of retrogradely labeled neurons in the LPal after injections of axonal tracer in the PT or in the MLR. (B) Box plot showing the size of retrogradely labeled neurons in the LPal after injections of axonal tracer in the PT or in the MLR. An asterisk (*) indicates a statistically significant difference at the level p < 0.05. The numerical values underlying this figure can be found in S1 Data. LPal, lateral pallium; MLR, mesencephalic locomotor region; PT, posterior tuberculum.
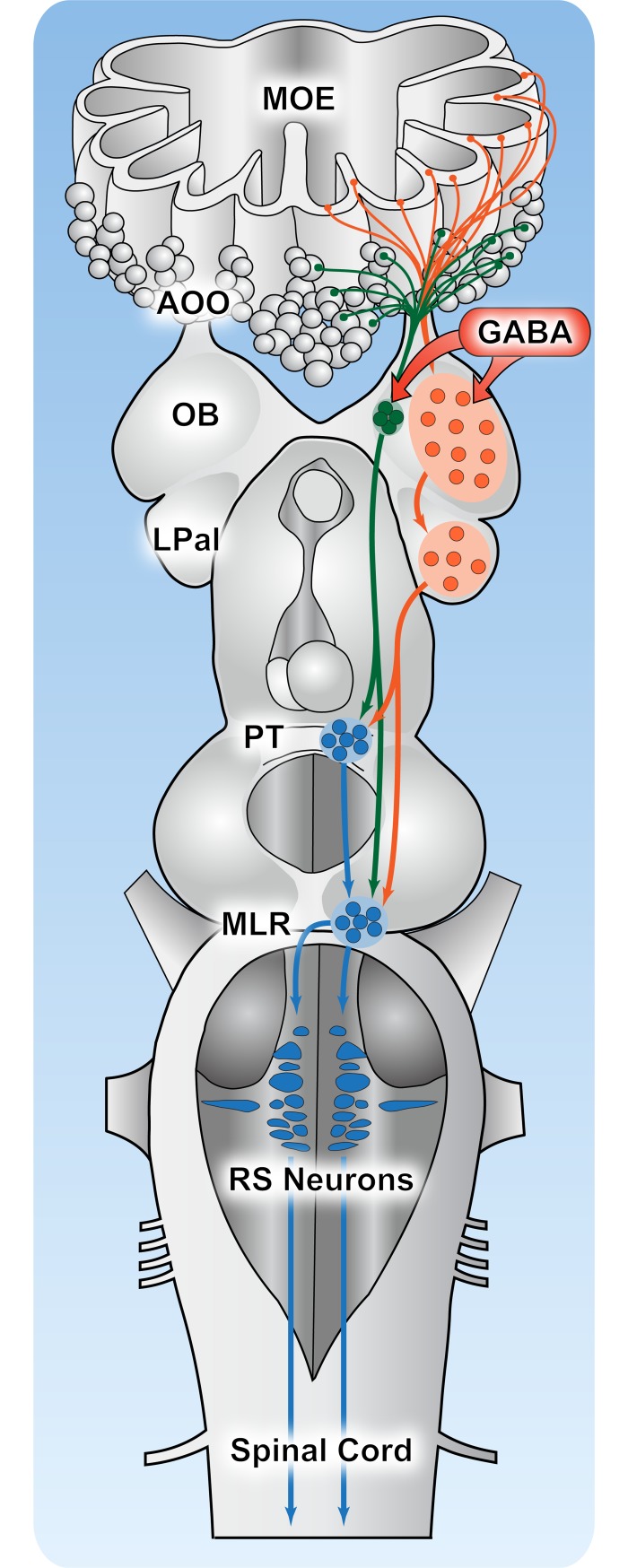
The lateral olfactomotor pathway (orange pathway in Fig 11) may contribute significantly to the motor responses of lampreys to olfactory cues in their environment, in parallel to the previously described medial olfactomotor pathway (green pathway in Fig 11).
Fig 11. Two olfactomotor pathways in lampreys.
Schematic representation of the brain illustrating a medial (green) and a lateral (orange) olfactomotor pathways. Both pathways act on the downstream locomotor control circuitry (blue) and thus contribute to olfactory-induced locomotor behaviors in lampreys. GABA (red) modulates both pathways at the level of the olfactory bulb. AOO, accessory olfactory organ; LPal, lateral pallium; MLR, mesencephalic locomotor region; MOE, main olfactory epithelium; OB, olfactory bulb; PT, posterior tuberculum; RS, reticulospinal.
Discussion
The transformation of sensory inputs into motor outputs is critical for the survival and reproduction of animals. As such, it represents an important part of their behavioral repertoire. While phylogenetically old nervous systems are predominantly dedicated to sensorimotor behaviors, the underlying neural pathways have been characterized in only a few cases. Recent studies in invertebrates have shed light on the neural substrate underlying some sensorimotor behaviors [57–59]. In contrast, very limited information has been collected in relation to the mechanisms of the transformation of sensory inputs into a locomotor output in vertebrates. For instance, it is well known that most animals (including humans) display odor-guided behaviors. However, the neural substrate and mechanisms associated with these behaviors have remained poorly understood.
In this study, we show that a GABAergic circuitry in the OB modulates a hardwired sensorimotor pathway. This is, to the best of our knowledge, the first report of the role of the OB GABAergic circuitry in modulating a motor behavior in any vertebrate species. Moreover, we identified a lateral olfactomotor pathway originating in the MOB and reaching locomotor control centers via a relay in the LPal. Together with our previous discovery of the medial olfactomotor pathway [12], these findings support the existence of two olfactomotor pathways in one of the most basal extant vertebrate species, the sea lamprey. As basal vertebrates, lampreys share a common brain “bauplan” with jawed vertebrates, including modern-day mammals. Therefore, knowledge gained from lamprey circuits and mechanisms provides insight into fundamental principles of vertebrate brain organization and function.
Modulation of the olfactomotor transmission
Lampreys, like many other animal species, display sex- and life stage–specific olfactory-induced motor behaviors [60–63]. The neural mechanisms accounting for the behavioral variability associated with a specific neural pathway within a species are largely unknown. However, the long-standing hypothesis that it was due to fundamental differences in brain wiring is now being challenged (reviewed in [64]). Indeed, only very subtle sex-specific differences have been found in the structure and circuitry of the brain in mammals [65–68]. Likewise, we have shown in a previous study that a hardwired olfactomotor pathway is present in both sexes at all life stages in the sea lamprey [12]. For this reason, we hypothesized in the present study that modulatory mechanisms acting on this pathway could play a role in the variability of the behavioral responses of lampreys to olfactory cues.
The OB is the first relay of the olfactomotor pathway. As such, it interfaces sensory afferents with motor control centers and it is ideally located to modulate olfactory-induced motor responses in lampreys. It has been proposed that the main function of the OB in vertebrates is the filtering and transmission of olfactory inputs [69]. Studies in mammals and turtles have shown that the sensory inputs to the OB are modulated both at presynaptic and postsynaptic levels by two classes of local GABAergic interneurons: periglomerular and granule cells [69]. Periglomerular cells inhibit glutamate release from primary olfactory axon terminals via a GABAB-mediated mechanism [70–73]. On the other hand, granule cells inhibit projection neurons via a GABAA-mediated mechanism [74–78]. Despite a rather good understanding of the cellular mechanisms responsible for the modulation of olfactory inputs, little is known about their overall effect on the OB output and, ultimately, on the resulting behavior. Using an in vitro isolated preparation of lamprey CNS, we provide the first evidence linking cellular GABAergic modulatory mechanisms in the OB to the activation of a sensorimotor pathway producing locomotor behavior.
We showed that the lamprey OB anatomical organization is very similar to that of other vertebrates, regarding its GABAergic circuitry. Our material confirms previous work showing that the lamprey OB contains numerous GABAergic neurons of different morphological types [43]. The morphology and location of the GABAergic neurons suggest that they are mainly granule cells [43,45], but not excluding possible periglomerular cells [43,51]. We also showed that both medOB and MOB glomeruli are densely innervated with GABAergic processes. These GABAergic processes are in close proximity to both primary olfactory axon terminals and dendrites or somata of OB projection neurons; this suggests possible pre- or postsynaptic contacts (S2 Fig). We have not formally identified types (i.e., axons versus dendrites) and origin (i.e., intrinsic versus extrinsic) of the GABAergic processes. The abundant GABAergic cell bodies labeled in the OB suggest that they may be of intrinsic (OB) origin (i.e., granule cells or periglomerular cells), as seen in other vertebrate species [39–43]. The lamprey granule cells are axonless [45], as in other vertebrate species. These processes are thus likely to be dendrites of granule cells or dendrites and axons of periglomerular cells, but some of these processes could be axons originating from neurons located in other parts of the brain. In mammals, most neuromodulatory inputs to the OB originate from the locus coeruleus (noradrenergic inputs), the nucleus of the diagonal band of Broca (cholinergic inputs), and the midbrain raphe (serotoninergic) (reviewed in [69,79,80]). However, some cells located in the nucleus of the diagonal band of Broca are GABAergic and project to the OB [81,82].
We now show that injection of the GABAA receptor antagonist, gabazine, in the OB potentiates RS cell responses to ON or OB stimulation, thus suggesting an enhancement of the olfactomotor transmission. In the case of OB (MOB) stimulation, however, we cannot completely exclude that electrical stimulation of the MOB might recruit not only projection neurons but also local GABAergic interneurons, and that in such a case, an injection of gabazine might block the effect of their activation. Under gabazine, the electrical stimulation of the ON or OB can induce fictive swimming—the in vitro corollary of swimming behavior. Overall, these findings suggest that the GABAA antagonist gabazine increases the output of the OB. Indeed, downstream relays of the olfactomotor pathway (PT and MLR) control locomotion in a graded fashion [12,15,83]. Consequently, the increased RS cell responses observed under gabazine are likely to result from an increased drive from the OB to the PT and/or MLR. Studies in mammals have shown that GABA acts at several locations in the OB. OSN terminals express GABAB receptors [84–86], which inhibit transmission from OSN axons to mitral cell primary dendrites upon release of GABA by periglomerular cells [70,71,87,88]. Mitral cell dendrites express both GABAA and GABAB receptors [89–93]. Pharmacological blockade or genetic alteration of GABAA receptors in mitral cells alters the OB γ oscillations and leads to increased ON-induced mitral cell discharges [78,94]. The effect of GABAB receptor activation in these cells is less clear [93]. Both periglomerular and granule cells release GABA on mitral cells; periglomerular cells contact mitral cell primary dendrites, whereas granule cells contact mitral cell secondary dendrites [69,95]. Granule and periglomerular cells also express GABAA receptors [89,91,96,97]. Genetic alteration of the GABAA receptor subtype expressed in granule cells (i.e., expressing the β3 subunit) either globally or in a cell-specific manner increases the granule cell inhibition of mitral cells and results in increased OB γ oscillations [98,99]. To the best of our knowledge, the effect of periglomerular cell GABAA receptor activation on mitral cell activity has not been investigated, but an inhibition of periglomerular cells leading to the disinhibition of mitral cells could be expected. Finally, electrophysiological evidence suggests that granule cells also possess GABAB receptors whose activation modulates granule cell inhibition of mitral cells [100]. GABA can thus depress or potentiate mitral cell activity depending on its site of action (i.e., OSNs axons, OB interneurons, or mitral cell). However, as OB interneurons act on mitral cells via GABAA receptors, the net effect of the pharmacological blockade of GABAA receptors in all OB layers is likely to be a disinhibition of mitral cells. This is consistent with our results in lampreys and those found in other vertebrate species [77,94,101–104].
The presence of both tonic and phasic inhibition in the OB has been reported in fish, amphibians, and mammals [71,77,78,94,98,99,102,105,106]. It has been suggested that tonic inhibition may modulate the strength of sensory inputs to the OB [88] or the sensitivity of second-order olfactory neurons to sensory inputs [102]. Phasic inhibition has been shown to generate neuronal synchrony (i.e., oscillations) in projection neurons [98,99]. The role of these oscillations and thus of the phasic inhibition is still debated, but several studies in insects and mammals point toward a crucial role in coding olfactory information [107–109].
Our study shows that a strong GABAergic inhibition of the OB output is present in the lamprey, one of the most basal extant vertebrate, and thus may be a common ancestral feature of the vertebrate OB.
The GABAergic modulation of the olfactomotor pathways seen in lampreys could explain some of the life stage–specific behavioral responses to olfactory cues. For instance, migratory pheromones attract only pre-spawning adult lampreys [24–26]. This is surprising because these pheromones evoke strong responses in OSNs at other life stages [110]. Somehow, the activation of OSNs only leads to locomotor responses during the pre-spawning adult life stage. Meléndez-Ferro and colleagues [43,111] have stated that the density of OB GABAergic cells declines significantly between the newly transformed and pre-spawning life stages. Whether this apparent decrease in GABAergic cell density could account for some of the life stage differences is not known at present, but it could be one plausible mechanism worth investigating.
A series of recent studies have shown that a CO2-mediated water acidification significantly impairs several olfactory-driven behaviors in fish, including prey tracking, predator avoidance, alarm response, and homing [112–116]. The mechanism at play has not been fully characterized yet, but it involves an alteration of the normal functioning of GABAA receptors, as blocking these receptors with gabazine led to a behavioral recovery [115,117]. The authors of these studies proposed that a potentiation or a reversal of the GABAA receptor function (from inhibitory to excitatory) because of changes in anionic gradients over neuronal membranes could underlie these behavioral alterations. Taken together, these studies show that GABAergic mechanisms also play a crucial role in modulating olfactomotor behaviors in fish. Further studies are needed to establish whether the neural pathways and modulatory mechanisms characterized in lampreys are also present in fish and other vertebrates.
Two distinct olfactomotor pathways
In the present study, we showed that stimulation of the MOB under gabazine led to excitatory responses in RS cells and to locomotion. This suggests the existence of a distinct pathway from the MOB to the RS cells and the presence of a strong tonic GABAergic inhibition in the MOB. We characterized the anatomy and physiology of this pathway. Anatomical data showed that the LPal receives a massive projection from the MOB and projects down to both the PT and MLR. The PT, in turn, projects to the MLR [12,118,119]. We also showed that the MLR receives a direct projection from the medOB, in addition to the already characterized projection via the PT [12]. The MLR then reaches the command cells for locomotion, the RS cells, via glutamatergic and cholinergic projections [120–123]. Physiological data confirmed that the LPal relays MOB olfactory inputs to the RS cells via the PT and MLR. This is consistent with the recent findings of Suryaranayana and colleagues [124] indicating that some LPal neurons receive monosynaptic inputs from the OB. Interestingly, Ocaña and colleagues [48] showed that a few fibers originating in the LPal could reach RS neurons directly and that some of these could be followed as far as the first spinal segments. This prompted the authors to conclude that the LPal possesses an efferent projection pattern similar to that of the amniote motor cortex [48]. It would be interesting to examine if these projections from the LPal to the RS neurons and spinal cord are also involved in olfactomotor responses. Although we cannot exclude that there may be other pathways linking olfactory centers to motor centers, our study demonstrates the existence of two distinct glutamatergic pathways linking the olfactory and motor systems in lampreys (Fig 11). Both these pathways share a common output via the PT/MLR–RS neurons system. However, they differ regarding their pathways from the OB to motor control centers (i.e., PT/MLR), as well as to their inputs from the periphery.
In lampreys, the main olfactory epithelium contains numerous tall, ciliated OSNs expressing the G-protein Golf, as in the main olfactory epithelium in other vertebrates [125–133]. The OSNs of the main olfactory epithelium project their axons to the MOB, which, in turn, projects mainly to the LPal, i.e., the putative homologue of the mammalian olfactory cortex in lampreys [134]. This pathway is strikingly similar to the main olfactory pathway of terrestrial vertebrates and thus further supports its evolutionary conservation. In addition to the main olfactory epithelium, lampreys possess an accessory olfactory organ [29–32,135–138]. The accessory olfactory organ contains short, broad, ciliated OSNs [32] that do not express the G-protein Golf and project only to the medOB [32,129]. Projection neurons of the medOB then project directly to the PT and MLR, bypassing the LPal. Taken together, these findings show that the lamprey accessory olfactory organ constitutes a discrete olfactory subsystem. It has even been suggested that the accessory olfactory organ represents a primordial vomeronasal system [29,31,138].
In other vertebrates, the presence of parallel olfactory pathways conveying the information from the periphery to high-order brain olfactory centers suggests that these systems subserve different behavioral functions [139–143]. For instance, in fish, segregated olfactory pathways, from the olfactory epithelium to the telencephalon, mediate feeding, reproductive, and alarm behaviors [139,142,144–151]. Similarly, the main and accessory (i.e., vomeronasal) systems of terrestrial vertebrates are segregated until at least the third-order neurons and their respective activation elicits different behaviors [152–155]. Physiological evidence in lampreys also supports this hypothesis, as OB local field recordings showed that the medOB and MOB have overlapping but different response profiles to feeding cues and pheromones [33,34]. Moreover, we show that the pathways from the OB to the motor control centers differ for the two olfactory subsystems. The medOB projects directly to motor control centers, whereas the MOB projects first to the LPal before reaching motor control centers. Not surprisingly, activation of both systems leads to locomotion. This could be attributed to the paucity of the behavioral repertoire of lampreys compared to mammals. However, it should be noted that reproductive, migratory, and feeding behaviors all require locomotion in lampreys. The distinction between these two subsystems thus lies in their inputs from the periphery (accessory olfactory organ versus main olfactory epithelium) as well as in the involvement of the LPal in the lateral pathway. It is tempting to propose that the medial pathway could mediate innate responses to chemical stimuli (for example, avoidance), whereas the lateral pathway could be involved in olfactomotor behaviors requiring further processing and perhaps learning (for example, olfactory navigation). A similar distinction between dual “olfactory” systems exists in invertebrates [156–158]. In mammals, it was shown that mitral cells of the MOB can develop differential responses to rewarded/unrewarded odors [159]. It has been suggested that the dichotomy between innate responses versus learned responses may be what distinguish the main and accessory systems of terrestrial vertebrates [154]. This hypothesis has, however, received little attention, and further studies are needed.
In conclusion, our study shows that olfactory inputs can activate the locomotor command system via two distinct glutamatergic pathways in lampreys. To the best of our knowledge, this is the first characterization of a dual olfactory pathway, from the periphery to the motor command system, in vertebrates. Both pathways are strongly modulated by the GABAergic circuitry of the OB that may account for some of the variability in behavioral responses to olfactory inputs in lampreys. The existence of two segregated olfactory subsystems in one of the most basal extant vertebrates sheds light on the evolution of the olfactory system and suggests that its organization in functional clusters could constitute a common ancestral trait of vertebrates.
Materials and methods
Ethics statement
For all procedures, the animals were deeply anesthetized with tricaine methanesulphonate (MS-222, 200 mg/L, Sigma-Aldrich, Oakville, ON) and then decapitated. All surgical and experimental procedures conformed to the guidelines of the Canadian Council on Animal Care and were approved by the animal care and use committee of the Université de Montréal (Protocol no. 18–018), the Université du Québec à Montréal, and the University of Windsor.
Experimental animals and brain preparation
Experiments were performed on 57 larval and 61 adult sea lampreys (Petromyzon marinus) of both sexes. Some animals were used in more than one experiment. Larvae were collected from the Pike River stream (QC, Canada). Adults were collected from the Great Chazy River (NY, United States) and were kindly provided by agents of the U.S. Fish and Wildlife Service of Vermont. The permission to collect animals in the field was granted by the Quebec's Ministry of Natural Resources and Wildlife (permit no. 2017-03-30-2189-16-SP). All animals were kept in aerated fresh water maintained at 4–5 °C.
For all types of experiments, the animals were deeply anesthetized with tricaine methanesulphonate (MS-222, 200 mg/L, Sigma-Aldrich), decapitated caudal to the seventh branchiopore, and transferred into cold oxygenated Ringer's (8–10 °C) of the following composition (in mM): 130 NaCl, 2.1 KCl, 2.6 CaCl2, 1.8 MgCl2, 4.0 HEPES, 4.0 dextrose, and 1.0 NaHCO3, at pH 7.4. The branchial apparatus, myotomal musculature, and all soft tissues attached to the ventral side of the cranium were removed. The dorsal part of the vertebrae and cranium were removed to expose the brain and the rostral spinal cord. The peripheral olfactory organ was left intact with the ON still attached to the brain. All other nerves were cut and the choroid plexus covering the fourth and the mesencephalic ventricles was removed.
Electrophysiological recordings
The preparation was pinned down to the bottom of a recording chamber lined with Sylgard (Dow Corning, Midland, MI) and continuously perfused with cold oxygenated Ringer's (about 4 mL/min). Intracellular recordings of RS neurons were performed under visual guidance through a M3C stereomicroscope (Wild-Heerbrugg, Heerbrugg, Switzerland) using sharp glass microelectrodes (60–130 MΩ) filled with 4 M potassium acetate. The signals were amplified with an Axoclamp 2A (20 kHz sampling rate, Axon Instruments, Foster City, CA) and acquired through a Digidata 1322A interface running on pClamp 9.2 software (Axon Instruments, Foster City, CA). Only RS neurons displaying a stable membrane potential lower than −70 mV for at least 15 min were considered in this study.
Electrical stimulation (1–3 pulses, 5–30 μA, 2-ms duration, and 20-ms pulse interval) was delivered using homemade glass-coated tungsten electrodes (0.8–2 MΩ, 10–50 μm tip exposure) connected to a Grass S88 stimulator via a Grass PSIU6 photoelectric isolation unit (Astro-Med, Longueuil, QC). A delay of 50 s was allowed between each stimulation. In the figures, the synaptic responses are illustrated as the mean of 10 responses obtained with the same stimulation parameters.
In some experiments, the left and right ventral roots from one spinal segment (usually around the 10th segment) were also recorded using extracellular glass electrodes (tip diameter about 5 μm) filled with the Ringer's solution. The signals were amplified (×10,000) and filtered (100 Hz–1 kHz band-pass) using an AM systems 1800 dual channel amplifier (AM systems, Sequim, WA) and monitored for the presence of neural activity. “Fictive locomotion” (originally defined by Perret and colleagues, 1972) [160] observed in the absence of muscles and movement was defined as a neural activation of the spinal ventral (motor) roots, with a similar pattern as myotomal contractions seen during swimming.
Drug application
Drugs were purchased from Sigma-Aldrich (AP5), Tocris Bioscience (gabazine and CNQX), and Thermo Fisher Scientific (Fast Green). They were kept as frozen concentrated stock solutions and dissolved to their final concentrations in Ringer's solution prior to their use. Gabazine (SR-95531; 0.1–1 mM) and the CNQX/AP5 mixture (0.5 mM/1 mM) were pressure ejected (3–180 pulses; mean ± SD = 57 ± 46 pulses; about 4 psi, 20–40-ms pulse duration, injection volume: 0.1–6.6 nL; mean ± SD = 2.6 ± 2.1 nL) through glass micropipettes (10–20 μm tip diameter) in the brain tissue, using a Picospritzer (General Valve Corp, Fairfield, NJ). The inert dye Fast Green was added to the drug solution to monitor the extent of the injections. The spread did not exceed 300 μm in diameter for any microinjection.
Calcium imaging experiments
RS cells were retrogradely labeled by placing crystals of the calcium-sensitive indicator dye Calcium-Green dextran (3000 MW, Invitrogen, Eugene, OR) on the rostral stump of the spinal cord, transected at the level of the first spinal segments. The preparation was then kept in cold, oxygenated Ringer's solution for 24–36 h of axonal transport. Labeled cells were observed under a Nikon epifluorescence microscope equipped with a 20× (0.75 NA) objective. A filter set appropriate for fluorescein isothiocyanate (FITC) was used to visualize the neurons. The emitted light was captured with an intensified CCD video camera (Photometrics CoolSNAP HQ, Roper Scientific, Tucson, AZ) and recorded at a rate of two images per second, using Metafluor imaging software (Molecular Devices, Sunnyvale, CA). Calcium responses are expressed as relative changes in fluorescence (ΔF/F).
Anatomical experiments
Axonal tracing
Anterograde and retrograde labeling was obtained after unilateral injections of Texas Red-conjugated dextran amines (TRDA, 3000 MW, Molecular Probes, Eugene, OR) or biocytin (Sigma-Aldrich, Oakville, ON). In all cases, the site to be injected was first carefully lesioned with the fine tip of a pulled glass capillary and crystals of the tracer were immediately inserted into the lesion, all while the brain was maintained in cold, oxygenated Ringer’s. After waiting 10–15 min for the lesioned axons to pick up the dissolved tracer, the preparation was rinsed thoroughly and left in cold, oxygenated Ringer’s solution for 3–18 h to allow for the passive diffusion of the tracer in the axons. The preparation was then fixed in a solution of 4% paraformaldehyde in phosphate-buffered saline, pH 7.4, for 24 h and then transferred overnight into a solution of 20% sucrose in phosphate buffer. Transverse sections of 25-μm thickness were made with a cryostat and collected on ColorFrost Plus microscope slides (Thermo Fisher Scientific, Waltham, MA). The sections were left to dry overnight on a warming plate set at 37 °C. The following day, three changes of PBS were used to rehydrate and rinse the sections and biocytin, if present in the tissue, was revealed for 60 min with streptavidin conjugated to Alexa Fluor fluorophores of different wavelengths, depending on the needs (Streptavidin-Alexa Fluor 594, 488 or 350, dilution 1:400 in PBS, Molecular Probes, Eugene, OR). When needed, DAPI (included in the mounting medium) or Neurotrace Green (120-min incubation, diluted 1:200 in PBS at room temperature) was used as counterstain. The slides were mounted with Vectashield (H-1000 or H-1200, Vector laboratories, Burlington, ON) for observation under epifluorescence microscopy (E600 microscope equipped with a DXM1200 digital camera, Nikon Canada, Mississauga, ON).
Immunofluorescence and lectin binding
When immunofluorescence against GABA and/or lectin binding (to label primary olfactory afferents) was performed after axonal tracing, the brain preparation was immersed for 4 h in the fixative solution aforementioned, but with the addition of 2% glutaraldehyde. After thorough rinsing in PBS, the brain was sectioned on a Vibratome at 40-μm thickness and the sections were collected in PBS and processed for biocytin revelation, lectin binding, and immunofluorescence, as follows. First, the sections were incubated for 1 h in the incubation medium (PBS containing 5% normal goat serum, 0.3% Triton X-100) containing streptavidin-Alexa Fluor 350 (diluted 1:200, Invitrogen, Eugene, OR). The sections were rinsed three times for 10 min with the incubation medium, and then the primary antibody was added for an incubation of 24–36 h at 4 °C (monoclonal mouse anti-GABA, diluted 1:5,000, see below). The following day, the sections were rinsed three times for 10 min with PBS and incubated for 1 h in the incubation medium containing a goat anti-mouse-DyLight 594 secondary antibody (dilution: 1:400, Jackson ImmunoResearch Laboratories, West Grove, PA). The sections were then rinsed three times for 10 min with PBS and incubated for 1 h in PBS containing the isolectine GS-IB4-Alexa Fluor 488 (diluted 1:100, Invitrogen, Eugene, OR). The sections were rinsed once again three times for 10 min with PBS, then rinsed quickly with distilled water and mounted with Vectashield (H-1000, Vector Laboratories, Burlington, ON).
The monoclonal anti-GABA antibody, clone 3A12, was kindly donated by Dr. Peter Streit, Zürich, Switzerland, and was successfully used on the lamprey brain tissue in previous studies [118,161]. It was developed following immunization with GABA conjugated to BSA with glutaraldehyde (GABA-G-BSA) and has been well characterized [162]. Briefly, this clone was shown by enzyme-linked immunosorbent assay (ELISA) to bind strongly to GABA-G-BSA. Immunoreactivity of the same clone to β-alanine-G-BSA was 4,000 times less, and even lower for glycine-, aspartate-, glutamine-, and taurine-G-BSA. Immunoreactivity of the clone to BSA or G-BSA was almost nonexistent, and preabsorption with GABA-G-BSA completely abolished the immunohistochemical staining of rat brain sections. Double-labeling experiments using the mAb 3A12 and a commercially available rabbit anti-GABA antibody (1:100; Cat. No. AB131; Chemicon, Temecula, CA) showed immunoreactivity in the same cell populations in the lamprey brain. The AB131 antibody was developed by immunization of rabbits with GABA-G-BSA and tested for specificity on rat and human cerebellum. The immunostaining was completely abolished by preincubation of the antibody with 10–100 μg of GABA-G-BSA per mL of diluted antibody. Furthermore, spinal cord sections incubated with mAb 3A12 revealed the same pattern of immunostaining as previously shown [163,164]. No immunoreactivity was detected when the primary antibody was omitted from the immunohistochemical processing.
Data and statistics
Results are presented as mean ± SD. Statistical analyses were performed using Sigma Plot 11.0 (Systat, San Jose, CA), and statistical significance was set at p < 0.05. To test for differences in mean between groups, we performed a one-way analysis of variance for repeated measures, followed by a Holm-Sidak’s multiple-comparison post hoc test or Friedman analysis of variance for repeated measures on ranks followed by Tukey multiple-comparison post hoc test.
Supporting information
Bar graph showing the distribution of GABAergic (red) and projection (blue) neurons in the olfactory bulb. There is an overlap between the two populations, but the mean relative depth of GABAergic and projection neurons is significantly different (p < 0.001). Note that there are no GABAergic neurons in the most peripheral region of the OB and nearly no projection neurons in the most central region of the OB. The numerical values underlying this figure can be found in S1 Data. OB, olfactory bulb.
(TIF)
(A) Schematic illustration of the brain showing the tracer injection site and the level of the cross sections shown in B and C. (B) Projection neurons (blue) in medOB are labeled from an injection of biocytin in the PT. The olfactory primary afferent fibers are labeled with GSIB4 (green), and processes and cell bodies containing GABA were labeled by immunofluorescence (red). (C) High-power confocal image from an area corresponding to the white frame in B. The image was taken from an adjacent section in the same animal and is the result of a z-projection of two 1-μm optical sections from a z-stack. Scale bar in B = 100 μm; scale bar in C = 10 μm. Di, diencephalon; GSIB4, Griffonia simplicifolia isolectin B4; LPal, lateral pallium; medOB, medial part of the olfactory bulb; Mes, mesencephalon; MOB, main olfactory bulb; OB, olfactory bulb; PT, posterior tuberculum.
(TIF)
(A) Schematic illustration of the brain showing stimulation and imaging sites. (B1–4) Photomicrographs of calcium green–loaded RS neurons in the four reticular nuclei; the mesencephalic (MRN, B1), anterior rhombencephalic (ARRN, B2), middle rhombencephalic (MRRN, B3), and posterior rhombencephalic (PRRN, B4) reticular nuclei. (C1–4) Calcium responses (ΔF/F) of RS neurons from the MRN, ARRN, MRRN, and PRRN to repetitive ON stimulation (5–15 μA, 5–10 Hz, 10 s, arrows) before (C1–C4) and after a local injection of gabazine (1 mM) in the OB (D1–D4). The mean calcium response (ΔF/F) of RS neurons is significantly increased in all four reticular nuclei following the injection of gabazine (p < 0.001,). The numerical values underlying this figure can be found in S1 Data. Note that the pseudocolors here correspond to the change in fluorescence intensity (ΔF/F) according to the scale to the right, whereas the pseudocolors in B1–4 correspond to the intensity of the initial labeling in neurons. ARRN, anterior rhombencephalic reticular nucleus; Di, diencephalon; LPal, lateral pallium; medOB, medial part of the olfactory bulb; Mes, mesencephalon; MOB, main olfactory bulb; MRN, mesencephalic reticular nucleus; MRRN, middle rhombencephalic reticular nucleus; OB, olfactory bulb; ON, olfactory nerve; PRRN, posterior rhombencephalic reticular nucleus; PT, posterior tuberculum; Rh, rhombencephalon; RS, reticulospinal.
(TIF)
(A) Schematic illustration of the rostral part of the brain showing stimulation and recording sites. The meninges and the dorsalmost layer of the OB were removed with a Vibratome to gain access directly to the glomerular layer. (B) Representative examples of extracellular recordings of the OB. A bath application of gabazine (10 μM) increases the OB neuron responses (arrows) to ON electrical stimulation (5 μA). Top traces: rectified-integrated signals; bottom traces: raw signals; asterisks indicate stimulation artifacts. (C) Average OB neuron responses (normalized amplitude of the rectified-integrated signals) to ON stimulation over time in six preparations. (D) Univariate scatterplot showing the normalized (as a percentage of control) OB neuron response for all animals. The average OB neuron response is significantly increased after a bath application of 10 μM gabazine (n = 6; 10 stimulations per condition per preparation). An asterisk (*) indicates a statistically significant difference at the level p < 0.05, while n.s. indicates the absence of statistically significant difference. The numerical values underlying this figure can be found in S1 Data. Di, diencephalon; LPal, lateral pallium; Mes, mesencephalon; OB, olfactory bulb; ON, olfactory nerve.
(TIF)
(A) Schematic illustration of the brain showing stimulation, injection, and recording sites. The dashed area represents the medOB resection. (B) Responses of a RS neuron to the electrical stimulation of the MOB (15 μA) after a colocalized injection of gabazine (0.1 mM). The black trace is a mean of 10 individual responses (colored traces). Di, diencephalon; LPal, lateral pallium; Mes, mesencephalon; medOB, medial part of the olfactory bulb; MOB, main olfactory bulb; Rh, rhombencephalon; RS, reticulospinal.
(TIF)
(A-D) Photomicrographs of cross sections illustrating the general cytoarchitecture of the LPal, from rostral (A) to caudal (D). The DAPI (a blue fluorescent DNA dye) photomicrographs were inverted and converted to gray scale. The LPal was defined here as the lateral part of the evaginated telencephalon where many neurons are typically organized in many small clusters. The consistency in morphology and size of the retrogradely labeled neurons, especially those projecting to the PT, also helped in defining the extent of the LPal. This was particularly useful in the rostral part (A), where retrogradely labeled cells from the PT were only found in the ventrolateral area. Scale bar in A for all photomicrographs = 200 mm. dmtn, dorsomedial telencephalic nucleus; LPal, lateral pallium; OB, olfactory bulb; PT, posterior tuberculum.
(TIF)
(A) Schematic illustration showing the tracer injection in the PT and the level of the cross section photographed in B. The injection site in the PT is also illustrated in a photomicrograph of a cross section. (B1) Many neurons were retrogradely labeled (red) in the LPal from an injection of biocytin in the PT. The meningeal cells around the LPal show strong red autofluorescence (asterisks). (B2) The retrogradely labeled neurons from B1 superimposed over a Nissl stain (green). (C) Schematic illustration showing tracer injections and the level of the cross section photographed in D. The injection site in the MLR is also illustrated in a photomicrograph of a cross section; note that the dorsal midline at the isthmus was sectioned to gain access to the MLR. (D1) A few neurons were retrogradely labeled (arrows) in the LPal after an injection of biocytin in the MLR. The meningeal cells around the LPal show strong green autofluorescence (asterisks). (D2) The retrogradely labeled neurons from D1 superimposed over a DNA-labeling DAPI stain. All scale bars = 100 μm. LPal, lateral pallium; MLR, mesencephalic locomotor region; OB, olfactory bulb; PT, posterior tuberculum.
(TIF)
(XLSX)
Acknowledgments
We would like to thank D. Veilleux, F. Bernard, and C. Valiquette for their technical assistance.
Abbreviations
- AP5
2-amino-5-phosphonopentanoic acid
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- CNS
central nervous system
- cVR
contralateral ventral root
- fr
fasciculus retroflexus
- GABA-G-BSA
GABA conjugated to BSA with glutaraldehyde
- GL
glomerular layer
- GSIB4
Griffonia simplicifolia isolectin B4
- ICL
internal cell layer
- iVR
ipsilateral ventral root
- LPal
lateral pallium
- medOB
medial part of the olfactory bulb
- MLR
mesencephalic locomotor region
- MOB
main olfactory bulb
- NMLF
nucleus of the medial longitudinal fasciculus
- OB
olfactory bulb
- ON
olfactory nerve
- ONL
olfactory nerve layer
- OSN
olfactory sensory neuron
- PT
posterior tuberculum
- Rh
rhombencephalon
- RS
reticulospinal
- TRDA
Texas Red-conjugated dextran amine
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Natural Sciences and Engineering Research Council of Canada (NSERC) http://www.nserc-crsng.gc.ca. Studentships to J-LL and J-PM. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Groupe de Recherche sur le Système Nerveux Central (GRSNC) http://www.grsnc.umontreal.ca. Fellowships to GD and DD; Studentship to EA. Fyssen Foundation http://www.fondationfyssen.fr. Fellowship to GD. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Fonds de Recherche du Québec - Santé (FRQS) http://www.frqs.gouv.qc.ca. Studentship to J-PM. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Natural Sciences and Engineering Research Council of Canada (NSERC) http://www.nserc-crsng.gc.ca/ (grant number 217435-01; RGPIN/03916-2014). Individual grants to RD and BZ, respectively. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Fonds de Recherche du Québec - Santé (FRQS) http://www.frqs.gouv.qc.ca (grant number 5249). Group grant. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Canadian Institutes of Health Research http://www.cihr-irsc.gc.ca (grant number 15129; 15176). Grants to RD. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Great Lakes Fishery Commission http://www.glfc.org (grant number 54011; 54021; 54035; 54067). Group grants to BZ and RD. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Tilden J. An account of a singular property of lamprey eels. Mem Amer Acad Sci III. 1809;XLVI: 335–336. [Google Scholar]
- 2.Pearl R. The movements and reactions of fresh-water planarians: a study in animal behaviour. London: J. & A. Churchill; 1903. [Google Scholar]
- 3.Sheldon RE. The sense of smell in selachians. J Exp Zool. 1911;10: 51–62. [Google Scholar]
- 4.Heymons RH, von Lengerken H. Biologische untersuchungen an coprophagen lamellicorniern. I. Nahrungserwerb und eortpelanzungsbiologie der gattung Scarabaeus L. Z Morphol Ökol Tiere. 1929; 14(3): 531–613. [Google Scholar]
- 5.Czeloth H. Untersuchungen über die Raumorientierung von Triton. Z Vergl Physiol. 1930;13: 74–163. [Google Scholar]
- 6.Luther W. Versuche Uber die Chemorezeption der Brachyuren. Z Vergl Phytiol. 1930;13: 177–205. [Google Scholar]
- 7.Baumann F. Experimente über den Geruchssinn und den Beuteerwerb der Viper (Vipera aspis). J Comp Physiol A. 1929;10: 36–119. [Google Scholar]
- 8.von Frisch K. Über einen Schreckstoff der Fischhaut und seine biologische Bedeutung. Z Vgl Physiol. 1941;29: 46–145. [Google Scholar]
- 9.Grimm RJ. Feeding behavior and electrical stimulation of the brain of Carassius auratus. Science. 1960;131: 162–163. [DOI] [PubMed] [Google Scholar]
- 10.Fraenkel GS, Gunn DL. The orientation of animals: kineses, taxes, and compass reactions Oxford: Clarendon Press; 1961. [Google Scholar]
- 11.Daghfous G, Green WW, Zielinski BS, Dubuc R. Chemosensory-induced motor behaviors in fish. Curr Opin Neurobiol. 2012;22(2): 223–230. 10.1016/j.conb.2011.10.009 [DOI] [PubMed] [Google Scholar]
- 12.Derjean D, Moussaddy A, Atallah E, St-Pierre M, Auclair F, Chang S, et al. A novel neural substrate for the transformation of olfactory inputs into motor output. PLoS Biol. 2010;8(12): e1000567 10.1371/journal.pbio.1000567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daghfous G, Green WW, Alford ST, Zielinski BS, Dubuc R. Sensory activation of command cells for locomotion and modulatory mechanisms: lessons from lampreys. Front Neural Circuits. 2016;10: 18 10.3389/fncir.2016.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shik ML, Severin FV, Orlovskii GN. [Control of walking and running by means of electric stimulation of the midbrain]. Biofizika. 1966;11: 659–666. [PubMed] [Google Scholar]
- 15.Sirota MG, Viana Di Prisco G, Dubuc R. Stimulation of the mesencephalic locomotor region elicits controlled swimming in semi-intact lampreys. Eur J Neurosci. 2000;12: 4081–4092. 10.1046/j.1460-9568.2000.00301.x [DOI] [PubMed] [Google Scholar]
- 16.Cabelguen JM, Bourcier-Lucas C, Dubuc R. Bimodal locomotion elicited by electrical stimulation of the midbrain in the salamander Notophthalmus viridescens. J Neurosci. 2003;23: 2434–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skinner RD, Garcia-Rill E. The mesencephalic locomotor region (MLR) in the rat. Brain Res. 1984;323: 385–389. 10.1016/0006-8993(84)90319-6 [DOI] [PubMed] [Google Scholar]
- 18.Bernau NA, Puzdrowski RL, Leonard RB. Identification of the midbrain locomotor region and its relation to descending locomotor pathways in the Atlantic stingray, Dasyatis sabina. Brain Res. 1991;557: 83–94. 10.1016/0006-8993(91)90119-G [DOI] [PubMed] [Google Scholar]
- 19.Musienko PE, Zelenin PV, Lyalka VF, Orlovsky GN, Deliagina TG. Postural performance in decerebrated rabbit. Behav Brain Res. 2008;190: 124–134. 10.1016/j.bbr.2008.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marlinsky VV, Voitenko LP. The effect of procaine injection into the medullary reticular formation on forelimb muscle activity evoked by mesencephalic locomotor region and vestibular stimulation in the decerebrated guinea-pig. Neuroscience. 1991;45: 753–759. 10.1016/0306-4522(91)90287-X [DOI] [PubMed] [Google Scholar]
- 21.Eidelberg E, Walden JG, Nguyen LH. Locomotor control in macaque monkeys. Brain. 1981;104: 647–663. 10.1093/brain/104.4.647-a [DOI] [PubMed] [Google Scholar]
- 22.Ryczko D, Dubuc R. The multifunctional mesencephalic locomotor region. Curr Pharm Des. 2013;19(24): 4448–4470. [DOI] [PubMed] [Google Scholar]
- 23.Kleerekoper H, Mogensen J. Role of olfaction in the orientation of Petromyzon marinus. I. response to a single amine in prey's body odor. Physiol Zool. 1963;36(4): 347–360. [Google Scholar]
- 24.Li W, Sorensen PW, Gallaher DG. The olfactory system of the migratory sea lamprey (Petromyzon marinus) is specifically and acutely sensitive to unique bile acids released by conspecific larvae. J Gen Physiol. 1995;105: 569–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bjerselius R, Li W, Teeter JH, Seelye JG, Johnsen PB, Maniak PJ, et al. Direct behavioural evidence that unique bile acids released by larval sea lamprey function as a migratory pheromone. Can J Fish Aquat Sci. 2000;57: 557–569. [Google Scholar]
- 26.Fine JM, Sorensen PW. Isolation and biological activity of the multi-component sea lamprey migratory pheromone. J Chem Ecol. 2008;34: 1259–1267. 10.1007/s10886-008-9535-y [DOI] [PubMed] [Google Scholar]
- 27.Li W, Scott AP, Siefkes MJ, Yan H, Liu Q, Yun SS. Bile acid secreted by male sea lamprey that acts as a sex pheromone. Science. 2002;296: 138‐141. 10.1126/science.1067797 [DOI] [PubMed] [Google Scholar]
- 28.Johnson NS, Yun SS, Thompson HT, Brant CO, Li W. A synthetized pheromone induces upstream movement in female sea lamprey and summons them into traps. Proc Natl Acad Sci USA. 2009;106: 1021‐1026. 10.1073/pnas.0808530106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scott WB. Notes on the development of Petromyzon. J Morphol. 1887;1: 253–310. 10.1002/jmor.1050010203 [DOI] [Google Scholar]
- 30.Leach WJ. The hypophysis of lampreys in relation to the nasal apparatus. J Morphol. 1951;89: 217–255. 10.1002/jmor.1050890203 [DOI] [Google Scholar]
- 31.Hagelin L, Johnels AG. On the structure and function of the accessory olfactory organ in lampreys. Acta Zool. 1955;36: 113–125. 10.1111/j.1463-6395.1955.tb00376.x [DOI] [Google Scholar]
- 32.Ren X, Chang S, Laframboise A, Green W, Dubuc R, Zielinski B. Projections from the accessory olfactory organ into the medial region of the olfactory bulb in the sea lamprey (Petromyzon marinus): a novel vertebrate sensory structure? J Comp Neurol. 2009;516(2): 105–116. 10.1002/cne.22100 [DOI] [PubMed] [Google Scholar]
- 33.Green WW, Basilious A, Dubuc R, Zielinski BS. The neuroanatomical organization of projection neurons associated with different olfactory bulb pathways in the sea lamprey, Petromyzon marinus. PLoS ONE. 2013;8(7): e69525 10.1371/journal.pone.0069525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Green WW, Boyes K, McFadden C, Daghfous G, Auclair F, Zhang H, et al. Odorant organization in the olfactory bulb of the sea lamprey. J Exp Biol. 2017; 220(7): 1350–1359. 10.1242/jeb.150466 [DOI] [PubMed] [Google Scholar]
- 35.Mori K, Nagao H, Yoshihara Y. The olfactory bulb: coding and processing of odor molecule information. Science. 1999;286(5440): 711–715. 10.1126/science.286.5440.711 [DOI] [PubMed] [Google Scholar]
- 36.Lledo PM, Gheusi G, Vincent JD. Information processing in the mammalian olfactory system. Phys Rev. 2005;85(1): 281–317. 10.1152/physrev.00008.2004 [DOI] [PubMed] [Google Scholar]
- 37.Halász N, Shepherd GM. Neurochemistry of the vertebrate olfactory bulb. Neuroscience. 1983;10: 579–619. 10.1016/0306-4522(83)90206-3 [DOI] [PubMed] [Google Scholar]
- 38.O'Connor S, Jacob TJ. Neuropharmacology of the olfactory bulb. Curr Mol Pharmacol. 2008;1(3): 181–190. 10.2174/1874467210801030181 [DOI] [PubMed] [Google Scholar]
- 39.Mugnaini E, Oertel WH, Wouterlood FF. Immunocytochemical localization of GABA neurons and dopamine neurons in the rat main and accessory olfactory bulbs. Neurosci Lett. 1984;47(3): 221–226. 10.1016/0304-3940(84)90517-2 [DOI] [PubMed] [Google Scholar]
- 40.Domenici L, Waldvogel HJ, Matute C, Streit P. Distribution of GABA-like immunoreactivity in the pigeon brain. Neuroscience. 1988;25(3): 931–950. 10.1016/0306-4522(88)90047-4 [DOI] [PubMed] [Google Scholar]
- 41.Martinoli MG, Dubourg P, Geffard M, Calas A, Kah O. Distribution of GABA-immunoreactive neurons in the forebrain of the goldfish, Carassius auratus. Cell Tissue Res. 1990;260(1): 77–84. 10.1007/BF00297492 [DOI] [PubMed] [Google Scholar]
- 42.Hamilton KA. Distribution of immunoreactivity for gamma-aminobutyric acid in the salamander olfactory bulb. J Comp Neurol. 1992;319(4): 606–614. 10.1002/cne.903190410 [DOI] [PubMed] [Google Scholar]
- 43.Meléndez-Ferro M, Pérez-Costas E, Rodríguez-Muñoz R, Gómez-López MP, Anadón R, Rodicio MC. GABA immunoreactivity in the olfactory bulbs of the adult sea lamprey Petromyzon marinus L. Brain Res. 2001;893(1–2): 253–260. 10.1016/S0006-8993(00)03316-3 [DOI] [PubMed] [Google Scholar]
- 44.Lledo PM, Saghatelyan A, Lemasson M. Inhibitory interneurons in the olfactory bulb: from development to function. Neuroscientist. 2004;10(4): 292–303. 10.1177/1073858404263460 [DOI] [PubMed] [Google Scholar]
- 45.Iwahori N, Kiyota E, Nakamura K. A Golgi study on the olfactory bulb in the lamprey, Lampetra japonica. Neurosci Res. 1987;5(2): 126–139. 10.1016/0168-0102(87)90029-0 [DOI] [PubMed] [Google Scholar]
- 46.Polenova OA, Vesselkin NP. Olfactory and nonolfactory projections in the river lamprey (Lampetra fluviatilis) telencephalon. J Hirnforsch. 1993;34(2): 261–279. [PubMed] [Google Scholar]
- 47.Northcutt RG, Puzdrowski RL. Projections of the olfactory bulb and nervus terminalis in the silver lamprey. Brain Behav Evol. 1988;32(2): 96–107. 10.1159/000116537 [DOI] [PubMed] [Google Scholar]
- 48.Ocaña FM, Suryanarayana SM, Saitoh K, Kardamakis AA, Capantini L, Robertson B, et al. The lamprey pallium provides a blueprint of the mammalian motor projections from cortex. PLoS Biol. 2015;25(4): 413–423. 10.1016/j.cub.2014.12.013 [DOI] [PubMed] [Google Scholar]
- 49.Johnston JB. The telencephalon in cyclostomes. J Comp Neurol. 1912;22: 341–404. 10.1002/cne.900220401 [DOI] [Google Scholar]
- 50.Herrick CJ, Obenchain JB. Notes on the anatomy of a cyclostome brain: Ichthyomyzon concolor. J Comp Neurol. 1913;23: 635–675. [Google Scholar]
- 51.Heier P. Fundamental principles in the structure of the brain: a study of the brain of Petromyzon fluviatilis. Acta Anat. 1948;5(Suppl 8): 1–213. 10.1159/00014069818876830 [DOI] [Google Scholar]
- 52.Schöber W. Vergleichend-anatomische Untersuchungen am Gehirn der Larven und adulten Tiere von Lampetra fluvialis (Linné, 1758) und Lampetra planeri (Bloch, 1784). J Hirnforsch. 1964;7: 107–209. [PubMed] [Google Scholar]
- 53.Nieuwenhuys R, Nicholson C. Lampreys, Petromyzontidae In: Nieuwenhuys R, ten Donkelaar HJ, Nicholson C, editors. The Central Nervous System of Vertebrates, Vol 1 Berlin: Springer-Verlag; 1998. pp. 297–495. [Google Scholar]
- 54.Pombal MA, Puelles L. Prosomeric map of the lamprey forebrain based on calretinin immunocytochemistry, Nissl stain, and ancillary markers. J Comp Neurol. 1999;414: 391–422. [DOI] [PubMed] [Google Scholar]
- 55.Pombal MA, Megías M, Bardet SM, Puelles L. New and old thoughts on the segmental organization of the forebrain in lampreys. Brain Behav. Evol. 2009;4: 7–19. 10.1159/000229009 [DOI] [PubMed] [Google Scholar]
- 56.Pombal MA, Álvarez-Otero R, Pérez-Fernández J, Solveira C, Megías M. Development and organization of the lamprey telencephalon with special reference to the GABAergic system. Front Neuroanat. 2011;5: 20 10.3389/fnana.2011.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gray JM, Hill JJ, Bargmann CI. A circuit for navigation in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2005;102(9): 3184–3191. 10.1073/pnas.0409009101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ebrahim SA, Dweck HK, Stökl J, Hofferberth JE, Trona F, Weniger K, et al. Drosophila Avoids Parasitoids by Sensing Their Semiochemicals via a Dedicated Olfactory Circuit. PLoS Biol. 2015;13(12): e1002318 10.1371/journal.pbio.1002318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Larsch J, Flavell SW, Liu Q, Gordus A, Albrecht DR, Bargmann CI. A Circuit for Gradient Climbing in C. elegans Chemotaxis. Cell Rep. 2015;12(11): 1748–1760. 10.1016/j.celrep.2015.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Faucher C, Forstreuter M, Hilker M, de Bruyne M. Behavioral responses of Drosophila to biogenic levels of carbon dioxide depend on life-stage, sex and olfactory context. J Exp Biol. 2006;209(14): 2739–2748. 10.1242/jeb.02297 [DOI] [PubMed] [Google Scholar]
- 61.Lastein S, Höglund E, Mayer I, Øverli Ø, Døving KB. Female crucian carp, Carassius carassius, lose predator avoidance behavior when getting ready to mate. J Chem Ecol. 2008;34(11): 1487–1491. 10.1007/s10886-008-9553-9 [DOI] [PubMed] [Google Scholar]
- 62.Buchinger TJ, Siefkes MJ, Zielinski BS, Brant CO, Li W. Chemical cues and pheromones in the sea lamprey (Petromyzon marinus). Front Zool. 2015;12: 32 10.1186/s12983-015-0126-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walaszczyk EJ, Goheen BB, Steibel JP, Li W. Differential effects of sex pheromone compounds on adult female sea lamprey (Petromyzon marinus) locomotor patterns. J Biol Rhythm. 2016;31(3): 289–298. 10.1177/0748730416629248 [DOI] [PubMed] [Google Scholar]
- 64.Dulac C, Kimchi T. Neural mechanisms underlying sex-specific behaviors in vertebrates. Curr Opin Neurobiol. 2007;17(6): 675–683. 10.1016/j.conb.2008.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Segovia S, Guillamón A. Sexual dimorphism in the vomeronasal pathway and sex differences in reproductive behaviors. Brain Res Rev. 1993;18(1): 51–74. 10.1016/0165-0173(93)90007-M [DOI] [PubMed] [Google Scholar]
- 66.Cooke B, Hegstrom CD, Villeneuve LS, Breedlove SM. Sexual differentiation of the vertebrate brain: principles and mechanisms. Front Neuroendocrinol. 1998;19(4): 323–362. 10.1006/frne.1998.0171 [DOI] [PubMed] [Google Scholar]
- 67.Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci. 2002;25(1): 507–536. 10.1146/annurev.neuro.25.112701.142745 [DOI] [PubMed] [Google Scholar]
- 68.Shah NM, Pisapia DJ, Maniatis S, Mendelsohn MM, Nemes A, Axel R. Visualizing sexual dimorphism in the brain. Neuron. 2004;43(3): 313–319. 10.1016/j.neuron.2004.07.008 [DOI] [PubMed] [Google Scholar]
- 69.Shepherd GM, Chen WR, Greer CA. Olfactory bulb In: Shepherd GM, editor. The Synaptic Organization of the Brain. 5th ed. New York: Oxford University Press; 2003. pp 165–216. [Google Scholar]
- 70.Nickell WT, Behbehani MM, Shipley MT. Evidence for GABAB-mediated inhibition of transmission from the olfactory nerve to mitral cells in the rat olfactory bulb. Brain Res Bull. 1994;35(2): 119–123. 10.1016/0361-9230(94)90091-4 [DOI] [PubMed] [Google Scholar]
- 71.Aroniadou-Anderjaska V, Zhou FM, Priest CA, Ennis M, Shipley MT. Tonic and synaptically evoked presynaptic inhibition of sensory input to the rat olfactory bulb via GABAB heteroreceptors. J Neurophysiol. 2000;84(3): 1194–1203. 10.1152/jn.2000.84.3.1194 [DOI] [PubMed] [Google Scholar]
- 72.Murphy GJ, Darcy DP, Isaacson JS. Intraglomerular inhibition: signaling mechanisms of an olfactory microcircuit. Nat Neurosci. 2005;8(3): 354–364. 10.1038/nn1403 [DOI] [PubMed] [Google Scholar]
- 73.Karpuk N, Hayar A. Activation of postsynaptic GABAB receptors modulates the bursting pattern and synaptic activity of olfactory bulb juxtaglomerular neurons. J Neurophysiol. 2008;99(1): 308–319. 10.1152/jn.01086.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nicoll RA. Pharmacological evidence for GABA as the transmitter in granule cell inhibition in the olfactory bulb. Brain Res. 1971;35(1): 137–149. 10.1016/0006-8993(71)90600-7 [DOI] [PubMed] [Google Scholar]
- 75.Nowycky MC, Mori K, Shepherd GM. GABAergic mechanisms of dendrodendritic synapses in isolated turtle olfactory bulb. J Neurophysiol. 1981;46(3): 639–648. 10.1152/jn.1981.46.3.639 [DOI] [PubMed] [Google Scholar]
- 76.Jahr CE, Nicoll RA. An intracellular analysis of dendrodendritic inhibition in the turtle in vitro olfactory bulb. J Physiol. 1982;326: 213–234. 10.1113/jphysiol.1982.sp014187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wellis DP, Kauer JS. GABAA and glutamate receptor involvement in dendrodendritic synaptic interactions from salamander olfactory bulb. J Physiol. 1993;469: 315–339. 10.1113/jphysiol.1993.sp019816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lagier S, Panzanelli P, Russo RE, Nissant A, Bathellier B, Sassoè-Pognetto M, et al. GABAergic inhibition at dendrodendritic synapses tunes γ oscillations in the olfactory bulb. Proc Natl Acad Sci USA. 2007;104(17): 7259–7264. 10.1073/pnas.0701846104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Matsutani S, Yamamoto N. Centrifugal innervation of the mammalian olfactory bulb. Anat Sci Int. 2008;83(4): 218–227. 10.1111/j.1447-073X.2007.00223.x [DOI] [PubMed] [Google Scholar]
- 80.Ennis M, Puche AC, Holy T, Shipley MT. The olfactory system In: Paxinos G, editor. The Rat Nervous System. 4th ed. London: Elsevier; 2015. pp. 761–803. [Google Scholar]
- 81.Záborszky L, Carlsen J, Brashear HR, Heimer L. Cholinergic and GABAergic afferents to the olfactory bulb in the rat with special emphasis on the projection neurons in the nucleus of the horizontal limb of the diagonal band. J Comp Neurol. 1986;243(4): 488–509. 10.1002/cne.902430405 [DOI] [PubMed] [Google Scholar]
- 82.Gracia-Llanes FJ, Crespo C, Blasco-Ibáñez JM, Nacher J, Varea E, Rovira-Esteban L, et al. GABAergic basal forebrain afferents innervate selectively GABAergic targets in the main olfactory bulb. Neuroscience. 2010;170(3): 913–922. 10.1016/j.neuroscience.2010.07.046 [DOI] [PubMed] [Google Scholar]
- 83.Ryczko D, Grätsch S, Schläger L, Keuyalian A, Boukhatem Z, Garcia C, et al. Nigral glutamatergic neurons control the speed of locomotion. J Neurosci. 2017;37(40): 9759–9770. 10.1523/JNEUROSCI.1810-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bonino M, Cantino D, Sassoè-Pognetto M. Cellular and subcellular localization of gamma-aminobutyric acidB receptors in the rat olfactory bulb. Neurosci Lett. 1999;274(3): 195–198. 10.1016/S0304-3940(99)00697-7 [DOI] [PubMed] [Google Scholar]
- 85.Panzanelli P, López-Bendito G, Luján R, Sassoé-Pognetto M. Localization and developmental expression of GABA(B) receptors in the rat olfactory bulb. J Neurocytol. 2004;33(1): 87–99. 10.1023/B:NEUR.0000029650.28943.b2 [DOI] [PubMed] [Google Scholar]
- 86.Priest CA, Puche AC. GABAB receptor expression and function in olfactory receptor neuron axon growth. J Neurobiol. 2004;60(2): 154–165. 10.1002/neu.20011 [DOI] [PubMed] [Google Scholar]
- 87.Wachowiak M, McGann JP, Heyward PM, Shao Z, Puche AC, Shipley MT. Inhibition of olfactory receptor neuron input to olfactory bulb glomeruli mediated by suppression of presynaptic calcium influx. J Neurophysiol. 2005;94(4): 2700–2712. 10.1152/jn.00286.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pírez N, Wachowiak M. In vivo modulation of sensory input to the olfactory bulb by tonic and activity-dependent presynaptic inhibition of receptor neurons. J Neurosci. 2008;28(25): 6360–6371. 10.1523/JNEUROSCI.0793-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Laurie DJ, Seeburg PH, Wisden W. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. II. Olfactory bulb and cerebellum. J Neurosci. 1992;12(3): 1063–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fritschy JM, Mohler H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol. 1995;359(1): 154–194. 10.1002/cne.903590111 [DOI] [PubMed] [Google Scholar]
- 91.Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101(4): 815–850. 10.1016/S0306-4522(00)00442-5 [DOI] [PubMed] [Google Scholar]
- 92.Panzanelli P, Perazzini AZ, Fritschy JM, Sassoè-Pognetto M. Heterogeneity of gamma-aminobutyric acid type A receptors in mitral and tufted cells of the rat main olfactory bulb. J Comp Neurol. 2005;484(1): 121–131. 10.1002/cne.20440 [DOI] [PubMed] [Google Scholar]
- 93.Li P, Stewart R, Butler A, Gonzalez-Cota AL, Harmon S, Salkoff L. GABA-B Controls Persistent Na(+) Current and Coupled Na(+)-Activated K(+) Current. eNeuro. 2017;4(3): ENEURO.0114-17.2017. 10.1523/ENEURO.0114-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lagier S, Carleton A, Lledo PM. Interplay between local GABAergic interneurons and relay neurons generates gamma oscillations in the rat olfactory bulb. J Neurosci. 2004;24(18): 4382–4392. 10.1523/JNEUROSCI.5570-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schoppa NE, Urban NN. Dendritic processing within olfactory bulb circuits. Trends Neurosci. 2003;26(9): 501–506. 10.1016/S0166-2236(03)00228-5 [DOI] [PubMed] [Google Scholar]
- 96.Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992;12(3): 1040–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nusser Z, Ahmad Z, Tretter V, Fuchs K, Wisden W, Sieghart W, et al. Alterations in the expression of GABAA receptor subunits in cerebellar granule cells after the disruption of the alpha6 subunit gene. Eur J Neurosci. 1999;11(5): 1685–1697. 10.1046/j.1460-9568.1999.00581.x [DOI] [PubMed] [Google Scholar]
- 98.Nusser Z, Kay LM, Laurent G, Homanics GE, Mody I. Disruption of GABA(A) receptors on GABAergic interneurons leads to increased oscillatory power in the olfactory bulb network. J Neurophysiol. 2001;86(6): 2823–2833. 10.1152/jn.2001.86.6.2823 [DOI] [PubMed] [Google Scholar]
- 99.Nunes D, Kuner T. Disinhibition of olfactory bulb granule cells accelerates odour discrimination in mice. Nat Commun. 2015;6: 8950 10.1038/ncomms9950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Isaacson JS, Vitten H. GABA(B) receptors inhibit dendrodendritic transmission in the rat olfactory bulb. J Neurosci. 2003;23(6): 2032–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Duchamp-Viret P, Duchamp A. GABAergic control of odour-induced activity in the frog olfactory bulb: possible GABAergic modulation of granule cell inhibitory action. Neuroscience. 1993;56(4): 905–914. 10.1016/0306-4522(93)90136-4 [DOI] [PubMed] [Google Scholar]
- 102.Duchamp-Viret P, Duchamp A, Chaput M. GABAergic control of odor-induced activity in the frog olfactory bulb: electrophysiological study with picrotoxin and bicuculline. Neuroscience. 1993;53(1): 111–120. 10.1016/0306-4522(93)90289-R [DOI] [PubMed] [Google Scholar]
- 103.Carlson GC, Shipley MT, Keller A. Long-lasting depolarizations in mitral cells of the rat olfactory bulb. J Neurosci. 2000;20(5): 2011–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schoppa NE. Synchronization of olfactory bulb mitral cells by precisely timed inhibitory inputs. Neuron. 2006;49(2): 271–283. 10.1016/j.neuron.2005.11.038 [DOI] [PubMed] [Google Scholar]
- 105.Friedrich RW, Laurent G. Dynamic optimization of odor representations by slow temporal patterning of mitral cell activity. Science. 2001;291(5505): 889–894. 10.1126/science.291.5505.889 [DOI] [PubMed] [Google Scholar]
- 106.Labarrera C, London M, Angelo K. Tonic inhibition sets the state of excitability in olfactory bulb granule cells. J Physiol. 2013;591(Pt 7): 1841–1850. 10.1113/jphysiol.2012.241851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wehr M, Laurent G. Odour encoding by temporal sequences of firing in oscillating neural assemblies. Nature. 1996;384(6605): 162–166. 10.1038/384162a0 [DOI] [PubMed] [Google Scholar]
- 108.Stopfer M, Bhagavan S, Smith BH, Laurent G. Impaired odour discrimination on desynchronization of odour-encoding neural assemblies. Nature. 1997;390(6655): 70–74. 10.1038/36335 [DOI] [PubMed] [Google Scholar]
- 109.Kay LM. Olfactory system oscillations across phyla. Curr Opin Neurobiol. 2015;31: 141–147. 10.1016/j.conb.2014.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zielinski BS, Fredricks K, McDonald R, Zaidi AU. Morphological and electrophysiological examination of olfactory sensory neurons during the early developmental prolarval stage of the sea lamprey Petromyzon marinus L. J Neurocytol. 2005;34(3–5): 209–216. 10.1007/s11068-005-8354-0 [DOI] [PubMed] [Google Scholar]
- 111.Meléndez‐Ferro M, Pérez‐Costas E, Villar‐Cheda B, Abalo XM, Rodríguez‐Muñoz R, Rodicio MC, et al. Ontogeny of γ‐aminobutyric acid‐immunoreactive neuronal populations in the forebrain and midbrain of the sea lamprey. J Comp Neurol. 2002;446(4): 360–376. 10.1002/cne.10209 [DOI] [PubMed] [Google Scholar]
- 112.Munday PL, Dixson DL, Donelson JM, Jones GP, Pratchett MS, Devitsina GV, et al. Ocean acidification impairs olfactory discrimination and homing ability of a marine fish. Proc Natl Acad Sci USA. 2009;106(6): 1848–1852. 10.1073/pnas.0809996106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dixson DL, Munday PL, Jones GP. Ocean acidification disrupts the innate ability of fish to detect predator olfactory cues. Ecol Lett. 2010;13(1): 68–75. 10.1111/j.1461-0248.2009.01400.x [DOI] [PubMed] [Google Scholar]
- 114.Cripps IL, Munday PL, McCormick MI. Ocean acidification affects prey detection by a predatory reef fish. PLoS ONE. 2011;6(7): e22736 10.1371/journal.pone.0022736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nilsson GE, Dixson DL, Domenici P, McCormick MI, Sørensen C, Watson SA, et al. Near-future carbon dioxide levels alter fish behaviour by interfering with neurotransmitter function. Nat Clim Chang. 2012;2(3): 201–204. 10.1038/nclimate1352 [DOI] [Google Scholar]
- 116.Dixson DL, Jennings AR, Atema J, Munday PL. Odor tracking in sharks is reduced under future ocean acidification conditions. Glob Chang Biol. 2015;21(4): 1454–1462. 10.1111/gcb.12678 [DOI] [PubMed] [Google Scholar]
- 117.Chivers DP, McCormick MI, Nilsson GE, Munday PL, Watson SA, Meekan MG, et al. Impaired learning of predators and lower prey survival under elevated CO2: a consequence of neurotransmitter interference. Glob Chang Biol. 2014;20(2): 515–522. 10.1111/gcb.12291 [DOI] [PubMed] [Google Scholar]
- 118.Ménard A, Auclair F, Bourcier‐Lucas C, Grillner S, Dubuc R. Descending GABAergic projections to the mesencephalic locomotor region in the lamprey Petromyzon marinus. J Comp Neurol. 2007;501(2): 260–273. 10.1002/cne.21258 [DOI] [PubMed] [Google Scholar]
- 119.Ryczko D, Cone JJ, Alpert MH, Goetz L, Auclair F, Dubé C, et al. A descending dopamine pathway conserved from basal vertebrates to mammals. Proc Natl Acad Sci USA. 2016;113(17): E2440–E2449. 10.1073/pnas.1600684113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brocard F, Dubuc R. Differential contribution of reticulospinal cells to the control of locomotion induced by the mesencephalic locomotor region. J Neurophysiol. 2003;90(3): 1714–1727. 10.1152/jn.00202.2003 [DOI] [PubMed] [Google Scholar]
- 121.Le Ray D, Brocard F, Bourcier‐Lucas C, Auclair F, Lafaille P, Dubuc R. Nicotinic activation of reticulospinal cells involved in the control of swimming in lampreys. Eur J Neurosci. 2003;17(1): 137–148. 10.1046/j.1460-9568.2003.02417.x [DOI] [PubMed] [Google Scholar]
- 122.Grillner S, Wallén P, Saitoh K, Kozlov A, Robertson B. Neural bases of goal-directed locomotion in vertebrates—an overview. Brain Res Rev. 2008;57(1): 2–12. 10.1016/j.brainresrev.2007.06.027 [DOI] [PubMed] [Google Scholar]
- 123.Brocard F, Ryczko D, Fénelon K, Hatem R, Gonzales D, Auclair F, et al. The transformation of a unilateral locomotor command into a symmetrical bilateral activation in the brainstem. J Neurosci. 2010;30(2): 523–533. 10.1523/JNEUROSCI.3433-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Suryanarayana SM, Robertson B, Wallén P, Grillner S. The lamprey pallium provides a blueprint of the mammalian layered cortex. Curr Biol. 2017;27(21): 3264–3277.e5. 10.1016/j.cub.2017.09.034 [DOI] [PubMed] [Google Scholar]
- 125.Jones DT, Reed RR. Golf: an olfactory neuron specific-G protein involved in odorant signal transduction. Science. 1989;244(4906): 790–795. 10.1126/science.2499043 [DOI] [PubMed] [Google Scholar]
- 126.Abogadie FC, Bruch RC, Farbman AI. G-protein subunits expressed in catfish olfactory receptor neurons. Chem Senses. 1995;20(2): 199–206. 10.1093/chemse/20.2.199 [DOI] [PubMed] [Google Scholar]
- 127.DellaCorte C, Restrepo D, Menco BPM, Andreini I, Kalinoski DL. Gα q/Gα 11: immunolocalization in the olfactory epithelium of the rat (Rattus rattus) and the channel catfish (Ictalurus punctatus). Neuroscience. 1996;74(1): 261–273. 10.1016/0306-4522(96)00115-7 [DOI] [PubMed] [Google Scholar]
- 128.Morita Y, Finger TE. Differential projections of ciliated and microvillous olfactory receptor cells in the catfish, Ictalurus punctatus. J Comp Neurol. 1998;398(4): 539–550. [DOI] [PubMed] [Google Scholar]
- 129.Frontini A, Zaidi AU, Hua H, Wolak TP, Greer CA, Kafitz KW, et al. Glomerular territories in the olfactory bulb from the larval stage of the sea lamprey Petromyzon marinus. J Comp Neurol. 2003;465(1): 27–37. 10.1002/cne.10811 [DOI] [PubMed] [Google Scholar]
- 130.Hansen A, Zielinski BS. Diversity in the olfactory epithelium of bony fishes: development, lamellar arrangement, sensory neuron cell types and transduction components. J Neurocytol. 2005;34(3–5): 183–208. 10.1007/s11068-005-8353-1 [DOI] [PubMed] [Google Scholar]
- 131.Hansen A. Olfactory and solitary chemosensory cells: two different chemosensory systems in the nasal cavity of the American alligator, Alligator mississippiensis. BMC Neurosci. 2007;8: 64 10.1186/1471-2202-8-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Laframboise AJ, Ren X, Chang S, Dubuc R, Zielinski BS. Olfactory sensory neurons in the sea lamprey display polymorphisms. Neurosci Lett. 2007;414(3): 277–281. 10.1016/j.neulet.2006.12.037 [DOI] [PubMed] [Google Scholar]
- 133.Oka Y, Korsching SI. Shared and unique G alpha proteins in the zebrafish versus mammalian senses of taste and smell. Chem Senses. 2011;36(4): 357–365. 10.1093/chemse/bjq138 [DOI] [PubMed] [Google Scholar]
- 134.Villar‐Cerviño V, Barreiro‐Iglesias A, Anadon R, Rodicio MC. Distribution of glycine immunoreactivity in the brain of adult sea lamprey (Petromyzon marinus). Comparison with γ‐aminobutyric acid. J Comp Neurol. 2008;507(3): 1441–1463. 10.1002/cne.21634 [DOI] [PubMed] [Google Scholar]
- 135.Vogt KC, Yung É. Lehrbuch der praktischen vergleichenden Anatomie, Vol 2 Braunschweig: Vieweg; 1894. 10.5962/bhl.title.61779 [DOI] [Google Scholar]
- 136.Lubosch W. Die Entwicklung und Metamorphose der Geruchsorganes von Petromyzon und seine Bedeutung fur die vergleichende Anatomie des Geruchsorganes. Jen Zschr Natw. 1905;40: 90–143. [Google Scholar]
- 137.De Beer GR. On a problematical organ of the lamprey. J Anat. 1924;59(Pt 1): 97–107. [PMC free article] [PubMed] [Google Scholar]
- 138.Chang S, Chung-Davidson Y-W, Libants SV, Nanlohy KG, Kiupel M, Brown CT, et al. The sea lamprey has a primordial accessory olfactory system. BMC Evol Biol. 2013;13: 172 10.1186/1471-2148-13-172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Døving KB, Selset R. Behavior patterns in cod released by electrical stimulation of olfactory tract bundlets. Science. 1980;207(4430): 559–560. 10.1126/science.7352272 [DOI] [PubMed] [Google Scholar]
- 140.Eisthen HL. Evolution of vertebrate olfactory systems. Brain Behav Evol. 1997;50(4): 222–233. 10.1159/000113336 [DOI] [PubMed] [Google Scholar]
- 141.Breer H, Hoppe R, Kaluza J, Levai O, Strotmann J. Olfactory subsystems in mammals: specific roles in recognizing chemical signals? Chemi Senses. 2005;30(Suppl 1): i144–i145. 10.1093/chemse/bjh155 [DOI] [PubMed] [Google Scholar]
- 142.Hamdani EH, Døving KB. The functional organization of the fish olfactory system. Prog Neurobiol. 2007;82(2): 80–86. 10.1016/j.pneurobio.2007.02.007 [DOI] [PubMed] [Google Scholar]
- 143.Munger SD, Leinders-Zufall T, Zufall F. Subsystem organization of the mammalian sense of smell. Annu Rev Physiol. 2009;71: 115–140. 10.1146/annurev.physiol.70.113006.100608 [DOI] [PubMed] [Google Scholar]
- 144.Hamdani EH, Stabell OB, Alexander G, Døving KB. Alarm reaction in the crucian carp is mediated by the medial bundle of the medial olfactory tract. Chem Senses. 2000;25(1): 103–109. 10.1093/chemse/25.1.103 [DOI] [PubMed] [Google Scholar]
- 145.Hamdani EH, Kasumyan A, Døving KB. Is feeding behaviour in crucian carp mediated by the lateral olfactory tract? Chem Senses. 2001a;26(9): 1133–1138. 10.1093/chemse/26.9.1133 [DOI] [PubMed] [Google Scholar]
- 146.Hamdani EH, Alexander G, Døving KB. Projection of sensory neurons with microvilli to the lateral olfactory tract indicates their participation in feeding behaviour in crucian carp. Chem Senses. 2001b;26(9): 1139–1144. 10.1093/chemse/26.9.1139 [DOI] [PubMed] [Google Scholar]
- 147.Hamdani EH, Døving KB. The alarm reaction in crucian carp is mediated by olfactory neurons with long dendrites. Chem Senses. 2002;27(4): 395–398. 10.1093/chemse/27.4.395 [DOI] [PubMed] [Google Scholar]
- 148.Hamdani EH, Døving KB. Sensitivity and selectivity of neurons in the medial region of the olfactory bulb to skin extract from conspecifics in crucian carp, Carassius carassius. Chem Senses. 2003;28(3): 181–189. 10.1093/chemse/28.3.181 [DOI] [PubMed] [Google Scholar]
- 149.Hamdani EH, Døving KB. Specific projection of the sensory crypt cells in the olfactory system in crucian carp, Carassius carassius. Chem Senses. 2006;31(1): 63–67. 10.1093/chemse/bjj006 [DOI] [PubMed] [Google Scholar]
- 150.Nikonov AA, Caprio J. Electrophysiological evidence for a chemotopy of biologically relevant odors in the olfactory bulb of the channel catfish. J Neurophysiol. 2001;86(4): 1869–1876. 10.1152/jn.2001.86.4.1869 [DOI] [PubMed] [Google Scholar]
- 151.Nikonov AA, Finger TE, Caprio J. Beyond the olfactory bulb: An odotopic map in the forebrain. Proc Natl Acad Sci USA. 2005;102(51): 18688–18693. 10.1073/pnas.0505241102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Scalia F, Winans SS. The differential projections of the olfactory bulb and accessory olfactory bulb in mammals. J Comp Neurol. 1975;161(1): 31–55. 10.1002/cne.901610105 [DOI] [PubMed] [Google Scholar]
- 153.Halpern M, Martınez-Marcos A. Structure and function of the vomeronasal system: an update. Prog Neurobiol. 2003;70(3): 245–318. 10.1016/S0301-0082(03)00103-5 [DOI] [PubMed] [Google Scholar]
- 154.Baxi KN, Dorries KM, Eisthen HL. Is the vomeronasal system really specialized for detecting pheromones? Trends Neurosci. 2006;29(1): 1–7. 10.1016/j.tins.2005.10.002 [DOI] [PubMed] [Google Scholar]
- 155.Eisthen HL, Polese G. Evolution of vertebrate olfactory subsystems In: Kaas JH, editor. Evolution of nervous systems, Vol 2 Academic Press; 2007. pp. 355–406. 10.1016/B0-12-370878-8/00142-7 [DOI] [Google Scholar]
- 156.Suh GS, Wong AM, Hergarden AC, Wang JW, Simon AF, Benzer S, et al. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature. 2004;431(7010): 854–859. 10.1038/nature02980 [DOI] [PubMed] [Google Scholar]
- 157.Galizia CG, Rössler W. Parallel olfactory systems in insects: anatomy and function. Annu Rev Entomol. 2010;55: 399–420. 10.1146/annurev-ento-112408-085442 [DOI] [PubMed] [Google Scholar]
- 158.Riffell JA, Lei H, Abrell L, Hildebrand JG. Neural basis of a pollinator’s buffet: olfactory specialization and learning in Manduca sexta. Science. 2013;339(6116): 200–204. 10.1126/science.1225483 [DOI] [PubMed] [Google Scholar]
- 159.Doucette W, Restrepo D. Profound context-dependent plasticity of mitral cell responses in olfactory bulb. PLoS Biol. 2008;6(10): e258 10.1371/journal.pbio.0060258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Perret C, Millanvoye M, Cabelguen JM. [Ascending spinal messages during fictitious locomotion in curarized cats]. J Physiol (Paris). 1972;65: 153A. [PubMed] [Google Scholar]
- 161.Robertson B, Auclair F, Ménard A, Grillner S, Dubuc R. GABA distribution in lamprey is phylogenetically conserved. J Comp Neurol. 2007;503(1): 47–63. 10.1002/cne.21348 [DOI] [PubMed] [Google Scholar]
- 162.Matute C, Streit P. Monoclonal antibodies demonstrating GABA-like immunoreactivity. Histochemistry. 1986;86(2): 147–157. 10.1007/BF00493380 [DOI] [PubMed] [Google Scholar]
- 163.Christenson J, Shupliakov O, Cullheim S, Grillner S. Possible morphological substrates for GABA-mediated presynaptic inhibition in the lamprey spinal cord. J Comp Neurol. 1993;328(4): 463–72. 10.1002/cne.903280402 [DOI] [PubMed] [Google Scholar]
- 164.Ruiz Y, Pombal MA, Megías M. Development of GABA-immunoreactive cells in the spinal cord of the sea lamprey, P. marinus. J Comp Neurol. 2004;470(2): 151–163. 10.1002/cne.11032 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Bar graph showing the distribution of GABAergic (red) and projection (blue) neurons in the olfactory bulb. There is an overlap between the two populations, but the mean relative depth of GABAergic and projection neurons is significantly different (p < 0.001). Note that there are no GABAergic neurons in the most peripheral region of the OB and nearly no projection neurons in the most central region of the OB. The numerical values underlying this figure can be found in S1 Data. OB, olfactory bulb.
(TIF)
(A) Schematic illustration of the brain showing the tracer injection site and the level of the cross sections shown in B and C. (B) Projection neurons (blue) in medOB are labeled from an injection of biocytin in the PT. The olfactory primary afferent fibers are labeled with GSIB4 (green), and processes and cell bodies containing GABA were labeled by immunofluorescence (red). (C) High-power confocal image from an area corresponding to the white frame in B. The image was taken from an adjacent section in the same animal and is the result of a z-projection of two 1-μm optical sections from a z-stack. Scale bar in B = 100 μm; scale bar in C = 10 μm. Di, diencephalon; GSIB4, Griffonia simplicifolia isolectin B4; LPal, lateral pallium; medOB, medial part of the olfactory bulb; Mes, mesencephalon; MOB, main olfactory bulb; OB, olfactory bulb; PT, posterior tuberculum.
(TIF)
(A) Schematic illustration of the brain showing stimulation and imaging sites. (B1–4) Photomicrographs of calcium green–loaded RS neurons in the four reticular nuclei; the mesencephalic (MRN, B1), anterior rhombencephalic (ARRN, B2), middle rhombencephalic (MRRN, B3), and posterior rhombencephalic (PRRN, B4) reticular nuclei. (C1–4) Calcium responses (ΔF/F) of RS neurons from the MRN, ARRN, MRRN, and PRRN to repetitive ON stimulation (5–15 μA, 5–10 Hz, 10 s, arrows) before (C1–C4) and after a local injection of gabazine (1 mM) in the OB (D1–D4). The mean calcium response (ΔF/F) of RS neurons is significantly increased in all four reticular nuclei following the injection of gabazine (p < 0.001,). The numerical values underlying this figure can be found in S1 Data. Note that the pseudocolors here correspond to the change in fluorescence intensity (ΔF/F) according to the scale to the right, whereas the pseudocolors in B1–4 correspond to the intensity of the initial labeling in neurons. ARRN, anterior rhombencephalic reticular nucleus; Di, diencephalon; LPal, lateral pallium; medOB, medial part of the olfactory bulb; Mes, mesencephalon; MOB, main olfactory bulb; MRN, mesencephalic reticular nucleus; MRRN, middle rhombencephalic reticular nucleus; OB, olfactory bulb; ON, olfactory nerve; PRRN, posterior rhombencephalic reticular nucleus; PT, posterior tuberculum; Rh, rhombencephalon; RS, reticulospinal.
(TIF)
(A) Schematic illustration of the rostral part of the brain showing stimulation and recording sites. The meninges and the dorsalmost layer of the OB were removed with a Vibratome to gain access directly to the glomerular layer. (B) Representative examples of extracellular recordings of the OB. A bath application of gabazine (10 μM) increases the OB neuron responses (arrows) to ON electrical stimulation (5 μA). Top traces: rectified-integrated signals; bottom traces: raw signals; asterisks indicate stimulation artifacts. (C) Average OB neuron responses (normalized amplitude of the rectified-integrated signals) to ON stimulation over time in six preparations. (D) Univariate scatterplot showing the normalized (as a percentage of control) OB neuron response for all animals. The average OB neuron response is significantly increased after a bath application of 10 μM gabazine (n = 6; 10 stimulations per condition per preparation). An asterisk (*) indicates a statistically significant difference at the level p < 0.05, while n.s. indicates the absence of statistically significant difference. The numerical values underlying this figure can be found in S1 Data. Di, diencephalon; LPal, lateral pallium; Mes, mesencephalon; OB, olfactory bulb; ON, olfactory nerve.
(TIF)
(A) Schematic illustration of the brain showing stimulation, injection, and recording sites. The dashed area represents the medOB resection. (B) Responses of a RS neuron to the electrical stimulation of the MOB (15 μA) after a colocalized injection of gabazine (0.1 mM). The black trace is a mean of 10 individual responses (colored traces). Di, diencephalon; LPal, lateral pallium; Mes, mesencephalon; medOB, medial part of the olfactory bulb; MOB, main olfactory bulb; Rh, rhombencephalon; RS, reticulospinal.
(TIF)
(A-D) Photomicrographs of cross sections illustrating the general cytoarchitecture of the LPal, from rostral (A) to caudal (D). The DAPI (a blue fluorescent DNA dye) photomicrographs were inverted and converted to gray scale. The LPal was defined here as the lateral part of the evaginated telencephalon where many neurons are typically organized in many small clusters. The consistency in morphology and size of the retrogradely labeled neurons, especially those projecting to the PT, also helped in defining the extent of the LPal. This was particularly useful in the rostral part (A), where retrogradely labeled cells from the PT were only found in the ventrolateral area. Scale bar in A for all photomicrographs = 200 mm. dmtn, dorsomedial telencephalic nucleus; LPal, lateral pallium; OB, olfactory bulb; PT, posterior tuberculum.
(TIF)
(A) Schematic illustration showing the tracer injection in the PT and the level of the cross section photographed in B. The injection site in the PT is also illustrated in a photomicrograph of a cross section. (B1) Many neurons were retrogradely labeled (red) in the LPal from an injection of biocytin in the PT. The meningeal cells around the LPal show strong red autofluorescence (asterisks). (B2) The retrogradely labeled neurons from B1 superimposed over a Nissl stain (green). (C) Schematic illustration showing tracer injections and the level of the cross section photographed in D. The injection site in the MLR is also illustrated in a photomicrograph of a cross section; note that the dorsal midline at the isthmus was sectioned to gain access to the MLR. (D1) A few neurons were retrogradely labeled (arrows) in the LPal after an injection of biocytin in the MLR. The meningeal cells around the LPal show strong green autofluorescence (asterisks). (D2) The retrogradely labeled neurons from D1 superimposed over a DNA-labeling DAPI stain. All scale bars = 100 μm. LPal, lateral pallium; MLR, mesencephalic locomotor region; OB, olfactory bulb; PT, posterior tuberculum.
(TIF)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.