Introduction
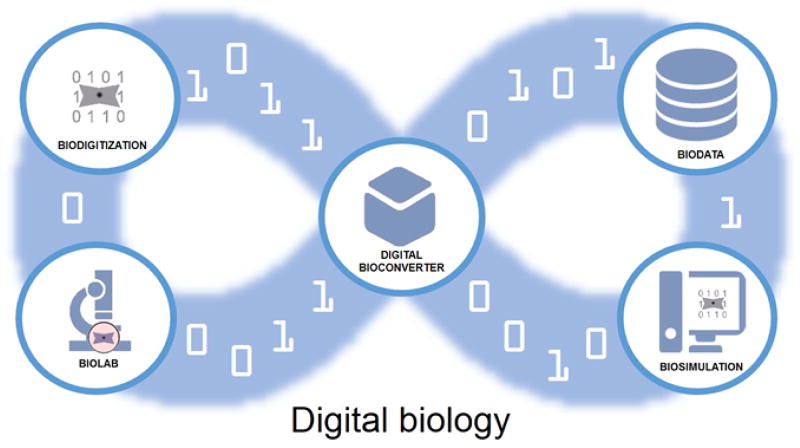
The era of Digital Biology began in 2010 with the “rebooting” of a bacterial cell using a synthetic DNA genome created from a digital template stored on a computer [1]. With this event, the creation of Mycoplasma laboratorium (nicknamed “Synthea”), came the first complete proof that DNA was the true software of life. Cells could be simulated digitally and the simulations could be tested against reality by reprograming cytoplasm with synthetic genomes generated from the digital DNA sequences driving those simulations. This in turn has created the expectation and promise that a deeper understanding of cellular function and thus life itself could be achieved on an infinite iterative loop of computer modeling and chemical synthesis (Figure 1) [2].
Figure 1.
The digital biology loop, with the digital bioconverter, a tool for instantiating data driven biosimulations into biomolecules and cells for analysis at the lab bench, digitization and further simulation and analysis.
Key components of the digital biology loop are 1) a detailed digital mapping of living systems and their biomolecular parts and the interactions of such parts-biodigitization, 2) accessible databases containing/managing this biodata, 3) computer simulation algorithms of cells driven by digital DNA sequences encoding the biomolecular parts and interactions-biosimulation, 4) laboratory technologies to deeply analyze the resulting synthetic cells-biolab-and finally and centrally 5) the digital biological converter (digital bioconverter for short). In these early days of digital biology, each of these components presents exciting bioengineering, bioscience and biomedical challenges.
Biodigitization and Biodata
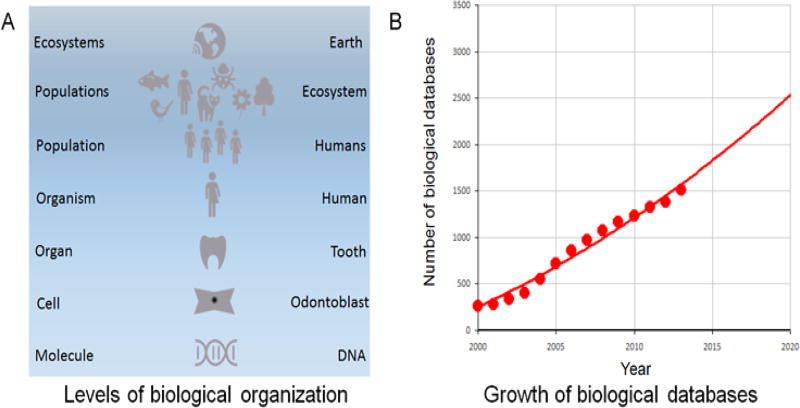
Every aspect of Earth’s biosphere is currently being digitized, from the molecular to the planetary levels and the data entered into an ever-growing collection of biologically oriented Internet databases-a mirror backup image of terrestrial life is literally being created (Figure2A)[3]. The digital acquisition of DNA sequences of phage, viruses, bacteria and human cells, the 3D structures of biomolecules as well as the detailed cellular structures and tissue and organ architecture has been underway in earnest for more than two decades now. With the advent of molecular imaging, electronic medical records, and “Big Data” [4,5], every aspect of individual organisms, populations, and ecosystems are now also being fed into the Internet based DataStream. Online biological databases are also on track for doubling every 5 years [3,6] (Figure 2B). The sheer volume of such data now threatens to overtake current data storage and search technology, and may require the development of novel technologies, including nucleic acid based data storage [7] and quantum computing [8].
Figure 2.
A. Biological data is being acquired at every level of Earth’s biosphere and B. the digitized biodata is being incorporated into Web accessible databases at a doubling rate of once every 5 years (from [6]).
Biosimulation and Biolab
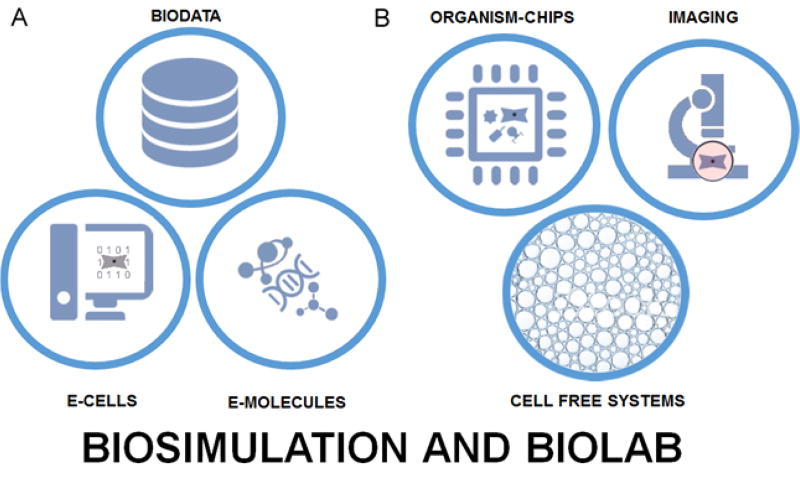
While a number of simulations of cells have been developed over the last two decades [9–11], so far only one specifically driven by a digitized DNA genome from a real cell [12], Mycoplasma genitalium, has been published. The advent of synthetic biology tools such as Tinkercell [12–14] and database driven animation [15] provide strong starts for the tools that will be needed. Detailed electronic images of biomolecules useful in simulations are already readily available from such sources as PubChem and Biosystems [6]. On the other side of the issue, biolab tools for analyzing and manipulating synthetically produced living cells are arising with breathtaking speed. Molecular imaging of structures within living cells is now possible [16–19], and even the direct physical manipulation of cellular components using optical tweezers is a routine technique [20]. Additive manufacturing tools now make the construction of artificial biofilms and organs for research a reality [21–28] (Figure 3).
Figure 3.
A. Components for biosimulation-digitized biodata, digital electronic cell models (E-CELLS), and digital electronic molecular models (E-MOLECULES). B. Biolab tools for analysis-novel cell/viruses on chip systems (ORGANISM-CHIPS), advanced microscopy (IMAGING) and advanced in vitro biochemistry (CELL FREE SYSTEMS).
Digital Bioconverter
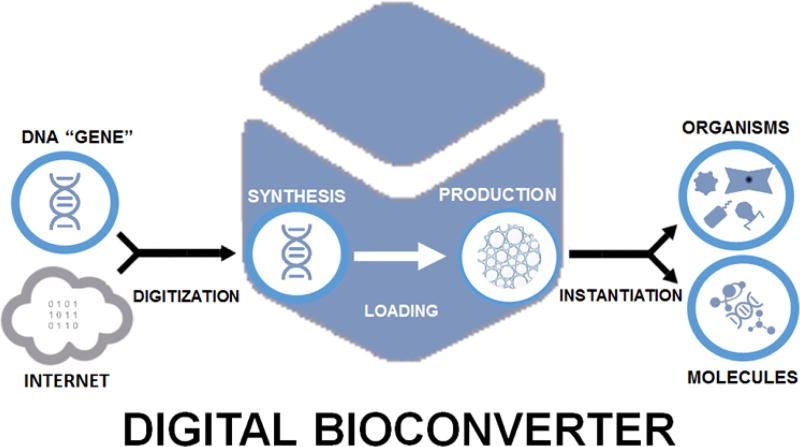
The key “gadget” in digital biology, the digital bioconverter (Figure 4), currently exists as a prototype [2] and will likely eventually evolve into a miniaturized commercially produced laboratory instrument. Such a system would allow the convenient production of cells, viruses and biological molecules directly from digitized gene encoding DNA sequences, and eventually could be as central to basic bioscience research as automated DNA sequencers are today. Significant challenges must be met before this is realized, however. While synthesis of large genomes is now possible, it remains complex and expensive. An alternative to cellular transformation could be realized via cell free systems-DNA could be loaded into such systems and then drive the production of biomolecules or organisms through a further instantiation step-possibly through reconstitution of cells and viruses (organisms) from in vitro systems [29–32] or the cell free synthesis of biomolecules.
Figure 4.
The digital bioconverter, conveying electronic digitized information to the biological realm via DNA.
Grand Synthesis
Increasingly available digitized biodata coupled with advanced biosynthetic synthesis are leading to a new era of biology where electronic digital simulations can be converted to cells and biological molecules-the era of digital biology. While several major engineering and computing challenges must be tackled, these are not insurmountable and are the objects of vigorous technological innovation. In particular, the development of a standardized and commercially available bioconversion device will be critical, and with such a device in eventual widespread use a rapid cycle of model driven understanding of biological systems will get underway. Such a device and the concept of digital biology will have applications in many fields, including astrobiology [33–35], medicine [36–38], nanotechnology [39], bioinformatics [36,37,40–47], drug repurposing [48] and pharmacoengineering [49–51], while presenting the promise of placing bioengineering and biomedicine on a Moore’s Law-like curve of exponentially increasing understanding and providing exquisite control of living systems.
References
- 1.Gibson DG, Glass JI, Lartigue C, Noskov VN, Chuang RY, et al. Creation of a bacterial cell controlled by a chemically synthesized genome. Science. 2010;329:52–56. doi: 10.1126/science.1190719. [DOI] [PubMed] [Google Scholar]
- 2.Venter JC. Life at the Speed of Light: From the Double Helix to the Dawn of Digital Life. Viking Adult 2013 [Google Scholar]
- 3.Fernández-Suárez XM, Galperin MY. The 2013 Nucleic Acids Research Database Issue and the online molecular biology database collection. Nucleic Acids Res. 2013;41:D1–7. doi: 10.1093/nar/gks1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daves K. Big Data, BGI and GigaScience. Bio-IT World. 2011:1–4. [Google Scholar]
- 5.Ratner B. Statistical and Machine-Learning Data Mining: Techniques for Better Predictive Modeondling and Analysis of Big Data. CRC Press; 2011. [Google Scholar]
- 6.Abigail Acland R, Agarwala, Tanya Barrett, Jeff Beck DAB, Colleen Bollin, et al. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2013;41:D8–D20. doi: 10.1093/nar/gks1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldman N, Bertone P, Chen S, Dessimoz C, LeProust EM, et al. Towards practical, high-capacity, low-maintenance information storage in synthesized DNA. Nature. 2013;494:77–80. doi: 10.1038/nature11875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris SA, Kendon VM. Quantum-assisted biomolecular modelling. Philos Trans A Math Phys Eng Sci. 2010;368:3581–3592. doi: 10.1098/rsta.2010.0087. [DOI] [PubMed] [Google Scholar]
- 9.Ishii N, Robert M, Nakayama Y, Kanai A, Tomita M. Toward large-scale modeling of the microbial cell for computer simulation. J Biotechnol. 2004;113:281–294. doi: 10.1016/j.jbiotec.2004.04.038. [DOI] [PubMed] [Google Scholar]
- 10.Klein MT, Hou G, Quann RJ, Wei W, Liao KH, et al. BioMOL: a computer-assisted biological modeling tool for complex chemical mixtures and biological processes at the molecular level. Environ Health Perspect. 2002;110(Suppl 6):1025–1029. doi: 10.1289/ehp.02110s61025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomita M, Hashimoto K, Takahashi K, Shimizu TS, Matsuzaki Y, et al. E-CELL: software environment for whole-cell simulation. Bioinformatics. 1999;15:72–84. doi: 10.1093/bioinformatics/15.1.72. [DOI] [PubMed] [Google Scholar]
- 12.Karr JR, Sanghvi JC, Macklin DN, Gutschow MV, et al. A whole-cell computational model predicts phenotype from genotype. Elsevier. 2012;150:389–401. doi: 10.1016/j.cell.2012.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chandran D, Bergmann FT, Sauro HM. Computer-aided design of biological circuits using TinkerCell. Bioeng Bugs. 2010;1:274–281. doi: 10.4161/bbug.1.4.12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chandran D, Sauro HM. Hierarchical modeling for synthetic biology. ACS Synth Biol. 2012;1:353–364. doi: 10.1021/sb300033q. [DOI] [PubMed] [Google Scholar]
- 15.McGill G. Molecular movies… coming to a lecture near you. Cell. 2008;133:1127–1132. doi: 10.1016/j.cell.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 16.Grammel M, Hang HC. Chemical reporters for biological discovery. Nat Chem Biol. 2013;9:475–484. doi: 10.1038/nchembio.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung S, Takizawa PA. In vivo visualization of RNA using the U1A-based tagged RNA system. Methods Mol Biol. 2011;714:221–235. doi: 10.1007/978-1-61779-005-8_14. [DOI] [PubMed] [Google Scholar]
- 18.Courty S, Dahan M. Tracking individual intracellular proteins using quantum dots. Cold Spring Harb Protoc. 2013;2013 doi: 10.1101/pdb.prot078238. [DOI] [PubMed] [Google Scholar]
- 19.O'Rourke NA, Meyer T, Chandy G. Protein localization studies in the age of 'Omics'. Curr Opin Chem Biol. 2005;9:82–87. doi: 10.1016/j.cbpa.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Oddershede LB. Force probing of individual molecules inside the living cell is now a reality. Nat Chem Biol. 2012;8:879–886. doi: 10.1038/nchembio.1082. [DOI] [PubMed] [Google Scholar]
- 21.Lee JB, Sung JH. Organ-on-a-chip technology and microfluidic whole-body models for pharmacokinetic drug toxicity screening. Biotechnol J. 2013 doi: 10.1002/biot.201300086. [DOI] [PubMed] [Google Scholar]
- 22.Sung JH, Yu J, Luo D, Shuler ML, March JC. Microscale 3-D hydrogel scaffold for biomimetic gastrointestinal (GI) tract model. Lab Chip. 2011;11:389–392. doi: 10.1039/c0lc00273a. [DOI] [PubMed] [Google Scholar]
- 23.Neuži P, Giselbrecht S, Länge K, Huang TJ, Manz A. Revisiting lab-on-a-chip technology for drug discovery. Nat Rev Drug Discov. 2012;11:620–632. doi: 10.1038/nrd3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghaemmaghami AM, Hancock MJ, Harrington H, Kaji H, Khademhosseini A. Biomimetic tissues on a chip for drug discovery. Drug Discov Today. 2012;17:173–181. doi: 10.1016/j.drudis.2011.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sung JH, Esch MB, Prot JM, Long CJ, Smith A, et al. Microfabricated mammalian organ systems and their integration into models of whole animals and humans. Lab Chip. 2013;13:1201–1212. doi: 10.1039/c3lc41017j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atala A. Regenerative medicine strategies. J Pediatr Surg. 2012;47:17–28. doi: 10.1016/j.jpedsurg.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 27.Mironov V, Kasyanov V, Markwald RR. Organ printing: from bioprinter to organ biofabrication line. Curr Opin Biotechnol. 2011;22:667–673. doi: 10.1016/j.copbio.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Mertz L. New world of 3-d printing offers "completely new ways of thinking": q&a with author, engineer, and 3-d printing expert hod lipson. IEEE Pulse. 2013;4:12–14. doi: 10.1109/MPUL.2013.2279615. [DOI] [PubMed] [Google Scholar]
- 29.Shin J, Jardine P, Noireaux V. Genome replication, synthesis, and assembly of the bacteriophage T7 in a single cell-free reaction. ACS Synth Biol. 2012;1:408–413. doi: 10.1021/sb300049p. [DOI] [PubMed] [Google Scholar]
- 30.Abdoli A, Soleimanjahi H, Kheiri MT, Jamali A, Sohani H, et al. Reconstruction of H3N2 influenza virus based virosome in-vitro. Iran J Microbiol. 2013;5:166–171. [PMC free article] [PubMed] [Google Scholar]
- 31.Ge X, Luo D, Xu J. Cell-free protein expression under macromolecular crowding conditions. PLoS One. 2011;6:e28707. doi: 10.1371/journal.pone.0028707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sokolova E, Spruijt E, Hansen MM, Dubuc E, Groen J, et al. Enhanced transcription rates in membrane-free protocells formed by coacervation of cell lysate. Proc Natl Acad Sci U S A. 2013;110:11692–11697. doi: 10.1073/pnas.1222321110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blumberg BS. Astrobiology, space and the future age of discovery. Philos Trans A Math Phys Eng Sci. 2011;369:508–515. doi: 10.1098/rsta.2010.0239. [DOI] [PubMed] [Google Scholar]
- 34.Goldman AD, Samudrala R, Baross JA. The evolution and functional repertoire of translation proteins following the origin of life. Biol Direct. 2010;5:15. doi: 10.1186/1745-6150-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldman AD, Baross JA, Samudrala R. The enzymatic and metabolic capabilities of early life. PLoS One. 2012;7:e39912. doi: 10.1371/journal.pone.0039912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horst JA, Laurenzi A, Bernard B. Computational Multitarget Drug Discovery. Polypharmacology Drug Discov. 2012:263–301. [Google Scholar]
- 37.Jenwitheesuk E, Horst JA, Rivas KL, Van Voorhis WC, Samudrala R. Novel paradigms for drug discovery: computational multitarget screening. Trends Pharmacol Sci. 2008;29:62–71. doi: 10.1016/j.tips.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jenwitheesuk E, Samudrala R. Identification of potential multitarget antimalarial drugs. JAMA. 2005;294:1490–1491. doi: 10.1001/jama.294.12.1490. [DOI] [PubMed] [Google Scholar]
- 39.Doll TA, Raman S, Dey R, Burkhard P. Nanoscale assemblies and their biomedical applications. J R Soc Interface. 2013;10:20120740. doi: 10.1098/rsif.2012.0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun X, Vilar S, Tatonetti NP. High-throughput methods for combinatorial drug discovery. Sci Transl Med. 2013;5:205rv1. doi: 10.1126/scitranslmed.3006667. [DOI] [PubMed] [Google Scholar]
- 41.Yu J, Wang J, Lin W, Li S, Li H, et al. The Genomes of Oryza sativa: a history of duplications. PLoS Biol. 2005;3:e38. doi: 10.1371/journal.pbio.0030038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hung LH, Samudrala R. PROTINFO: Secondary and tertiary protein structure prediction. Nucleic Acids Res. 2003;31:3296–3299. doi: 10.1093/nar/gkg541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Samudrala R. Modeling genome structure and function. Pure Appl Chem. 2002;74:907–914. [Google Scholar]
- 44.Hung LH, Ngan SC, Liu T, Samudrala R. PROTINFO: new algorithms for enhanced protein structure predictions. Nucleic Acids Res. 2005;33:W77–80. doi: 10.1093/nar/gki403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yuriev E, Ramsland PA. Latest developments in molecular docking: 2010–2011 in review. J Mol Recognit. 2013;26:215–239. doi: 10.1002/jmr.2266. [DOI] [PubMed] [Google Scholar]
- 46.Horst J, Samudrala R. Diversity of protein structures and difficulties in fold recognition: the curious case of protein G. F1000 Biol Rep. 2009;1:69. doi: 10.3410/B1-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ou-Yang SS, Lu JY, Kong XQ, Liang ZJ, Luo C, et al. Computational drug discovery. Acta Pharmacol Sin. 2012;33:1131–1140. doi: 10.1038/aps.2012.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xie L, Xie L, Kinnings SL, Bourne PE. Novel computational approaches to polypharmacology as a means to define responses to individual drugs. Annu Rev Pharmacol Toxicol. 2012;52:361–379. doi: 10.1146/annurev-pharmtox-010611-134630. [DOI] [PubMed] [Google Scholar]
- 49.Papworth M, Kolasinska P, Minczuk M. Designer zinc-finger proteins and their applications. Gene. 2006;366:27–38. doi: 10.1016/j.gene.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 50.Chen Y, Varani G. Engineering RNA-binding proteins for biology. FEBS J. 2013;280:3734–3754. doi: 10.1111/febs.12375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jinek M, East A, Cheng A, Lin S, Ma E, et al. RNA-programmed genome editing in human cells. Elife. 2013;2:e00471. doi: 10.7554/eLife.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]