Abstract
Acute myocardial infarction (AMI) and the heart failure (HF) that often results are among the leading causes of death and disability in the world. As such, novel strategies are required to protect the heart against the detrimental effects of acute ischemia/reperfusion injury (IRI), in order to reduce myocardial infarct (MI) size and prevent the onset of HF. The endogenous cardioprotective strategy of remote ischemic conditioning (RIC), in which cycles of brief ischemia and reperfusion are applied to a tissue or organ away from the heart, has been reported in experimental studies to reduce MI size in animal models of acute IRI. In the clinical setting, RIC can be induced by simply inflating and deflating a cuff placed on the upper arm or thigh to induce brief cycles of ischemia and reperfusion, a strategy which has been shown to reduce MI size in ST-segment elevation myocardial infarction (STEMI) patients undergoing primary percutaneous coronary intervention (PPCI). The results of the ongoing CONDI2/ERIC-PPCI trial are eagerly awaited, and will provide definitive answers with regards to the cardioprotective effect and clinical outcome benefits of RIC in STEMI.
Introduction and background
Ischemic heart disease is the leading cause of morbidity and mortality in the world. This reflects the increased prevalence of cardiovascular risk factors including cigarette smoking, diabetes, hypertension and hypercholesterolemia. These conditions also predispose patients to peripheral vascular disease, cerebrovascular disease and, renal disease, adding to disease complexity of the cardiovascular patient.
Up to 25% of ST segment elevation myocardial infarcts (STEMI) are fatal (Laurie J. Lambert, James M. Brophy, Normand Racine, Stéphane Rinfret, Philippe L. L’Allier, Kevin A. Brown, Lucy J. Boothroyd, Dave Ross, Eli Segal, 2016). Survivors of the acute coronary thrombotic occlusion depend on timely revascularization with thrombolysis or primary percutaneous coronary intervention (PPCI) to abate infarction-induced lethal arrhythmias/cardiac arrest in the short term. In the long term, reduced ischemic time leads to smaller infarct size. However, despite timely PPCI or thrombolytic therapy, there remains significant morbidity and mortality following STEMI.
The ischemic insult to the myocardium is two-fold; (1) at the time of coronary occlusion; and (2) at the time of reperfusion secondary to reperfusion injury. Reperfusion injury can be responsible for up to 50% of final infarct size (Hausenloy DJ, 2013). Pump failure secondary to non-viable infarcted myocardium is one of the long-term sequelae of STEMI. The ensuing heart failure syndrome involves deleterious activation of the renin-angiotensin-aldosterone system and peripheral vasoconstriction, leading to sodium and water retention (with worsening heart failure) and left ventricular remodeling (hypertrophy, dilation and impaired cardiac function) in a patient already burdened with multiple morbidities as outlined.
To reduce the risk of short and long term complications of infarction, sophisticated and efficient systems are in place in many countries for prompt recognition and treatment of STEMI through thrombolysis or PPCI. Strategies employed have included ambulance initiation of thrombolysis and the development of designated centers providing direct PPCI services to respective catchment areas, bypassing the emergency department, with reduction in “pain-to-balloon” and “pain-to-thrombolysis” time. However, despite these measures, morbidity and mortality following PPCI or thrombolytic therapy remain significant.
Attenuating myocardial reperfusion injury, the cardiomyocyte death which occurs on reperfusing ischemic myocardium, is a potential therapeutic target for reducing infarct complications such as cardiac death and re-hospitalization for heart failure (HHF). Accessible and effective clinical interventions are required to address reperfusion injury and reduce associated complications. In this regard, remote ischemic conditioning (RIC) has been shown to reduce perioperative myocardial injury in patients undergoing coronary artery bypass graft (CABG) surgery in small studies, but the beneficial effects of RIC have not been reproduced in large clinical outcome studies. In contrast, RIC remains a promising cardioprotective strategy in STEMI patients undergoing PPCI. In this article, we review the therapeutic potential for RIC as a cardioprotective strategy for reducing MI size and improving clinical outcomes post-PPCI.
Remote ischemic conditioning – cardioprotection from a distance
Przyklenk et al. (Przyklenk K, Bauer B, Ovize M, Kloner RA, 1993) were the first to describe the cardioprotective phenomenon of remote ischemic preconditioning in 1993, where 4×5 minute cycles of occlusion and reflow to the circumflex coronary artery reduced MI size in a canine heart induced by 45 minute occlusion and 3 hrs reperfusion of the left anterior descending artery (Przyklenk K, Bauer B, Ovize M, Kloner RA, 1993). This study suggested that cardioprotection could be transferred from one coronary artery territory to another through ischemic preconditioning (Przyklenk K, Bauer B, Ovize M, Kloner RA, 1993). This concept was then extended to the remote organ, the kidney, by McClanahan et al., who showed that 10 minute occlusion and reflow in the renal artery could reduce MI size induced by 30 minute ligation and 3 hrs reperfusion of the left main coronary artery (McClanahan TB, Nao BS, Wolke LJ, Martin BJ and KP, 1993). Oxman et al. demonstrated that RIC could be applied non-invasively, using a tourniquet applied to the hindlimb (Oxman T, Arad M, Klein R, Avazov N, 1997), a key finding in the translation of RIC into the clinical setting.
Two key properties of RIC have facilitated its translation into the clinical setting (see Figure 1 for time-line of translation of RIC from experimental to clinical studies):
Figure 1.
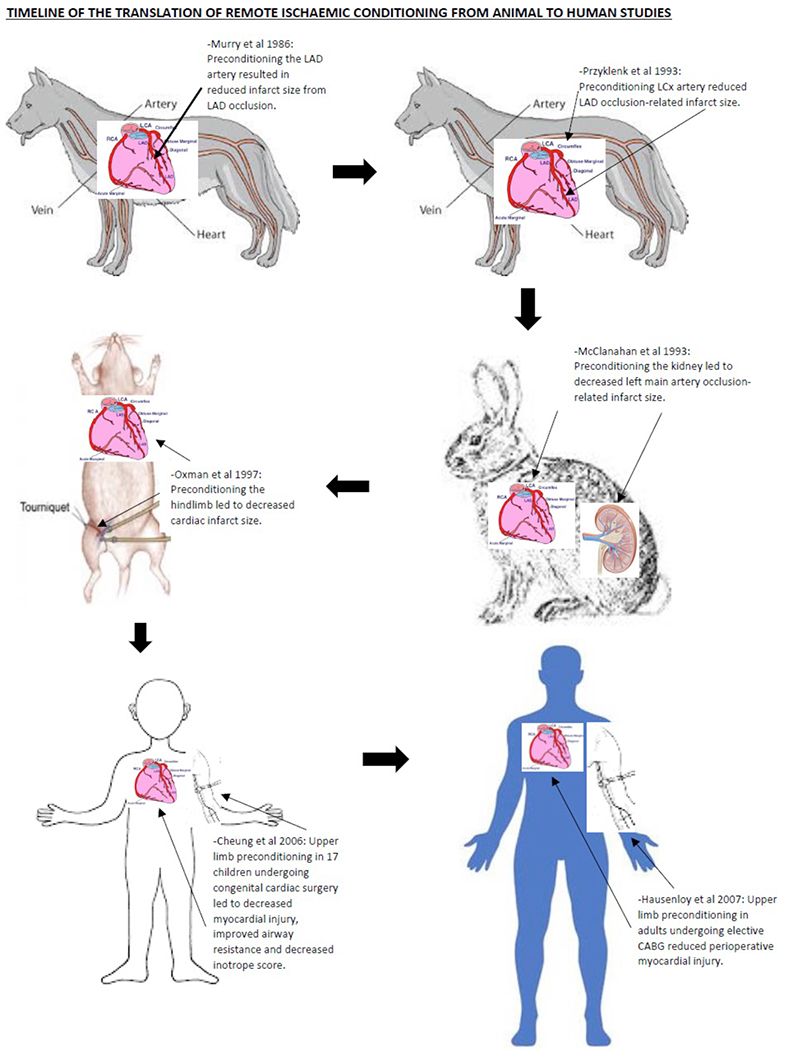
This figure shows the time-line of translation of RIC from experimental to clinical studies
(1) Feasibility: In an experimental animal MI model, the RIC stimulus could be applied to the hind limb to protect the heart against acute IRI (Oxman T, Arad M, Klein R, Avazov N, 1997) (Birnbaum Y, Hale SL, 1997). The RIC stimulus can be delivered non-invasively in human volunteers by inflating a blood pressure cuff on the upper arm to induce cycles of brief ischemia-reperfusion (Kharbanda RK, Mortensen UM, 2002). Hence, most clinical studies have applied RIC using cycles of brief ischemia-reperfusion in the upper or lower limb (limb RIC).
(2) Flexibility: In ischemic preconditioning (IPC), the protective stimulus has to be applied prior to ischemia and in ischemic postconditioning (IPost), at the onset of reperfusion to the heart directly. RIC can be applied at any time (before, after the onset of, or at the end of ischemia) to a remote organ or tissue.
Mechanisms underlying RIC cardioprotection
The actual mechanistic pathways underlying RIC cardioprotection is not known but it has been established that a neurohormonal pathway links the distal organ or tissue to the heart. The pathway linking the remote organ or tissue to the heart is believed to involve the release of local autacoids stimulating the sensory afferent neural pathway in the remote organ or tissue resulting in the production of a circulating transferrable blood-borne factor(s) conferring cardioprotection. Current evidence suggests the factor is thermolabile and hydrophobic, and is between 3.5 and 30 kDa. There is likely a complex interaction of signaling pathways in response to RIC, linking to the regulation of various cellular functions, including the acute phase response, immune response, hemostasis and lipid transport (V. Sivaraman and Hausenloy, 2015).
A number of candidate molecules have been suggested to be the blood-borne cardioprotective mediator of RIC including opioid (Dickson EW, Blehar DJ, Carraway RE, Heard SO and K, 2001), adenosine (Leung CH, Wang L, 2014), bradykinin (Schoemaker RG, 2000), erythropoietin, calcitonin gene related peptide, stromal derived factor 1-alpha (SDF1-α) (Davidson SM, Selvaraj P, 2013), hypoxia inducible factor 1-alpha (HIF1-α) and nanoparticles produced by cells called exosomes (Giricz Z, Varga ZV, 2014). Adenosine, bradykinin and calcitonin gene-related peptide (CGRP) may activate afferent neural pathways within the remote preconditioned organ to confer cardioprotection (Hausenloy DJ and Yellon DM, 2008). Activation of protein kinase C appears to be an important step in humoral cardioprotection in rats (Serejo FC, Rodrigues LF Jr, da Silva Tavares KC and AC, 2007).
Naloxone appears to block the cardioprotective effect of RIC in rats (Patel HH, Moore J, Hsu AK, 2002). Endogenous opioids generated by remote preconditioning may be a humoral factor conferring cardioprotection (Patel HH, Moore J, Hsu AK, 2002). It has been proposed that endocannabinoids generated by intestinal ischemia may activate CB2 endocannabinoid receptors on the myocardium in cardioprotection (Hajrasouliha AR, Tavakoli S, Ghasemi M and et al Sadeghipour H, 2008). Remote ischemic preconditioning (RIPC) appears to suppress the inflammatory response and activate an anti-inflammatory, anti-apoptotic gene transcription profile (Konstantinov IE, Arab S, Kharbanda RK, Li J, Cheung MM and Al, 2004) (Konstantinov IE, Arab S, Li J, Coles JG, Boscarino C, 2005) (Peralta C, Fernandez L, Panes J, Prats N, Sans M, 2001). Further investigation of relevance to cardioprotection is required. KATP channels of the myocardial sarcolemma and mitochondria have been implicated in IPC cardioprotection (Yellon DM, 2003). Ligand receptor binding at the cell surface activates signal transduction pathways which open mitochondrial KATP channels. The generation of mitochondrial reactive oxygen species then mediates cardioprotection by either activating pro-survival kinases (Yellon DM, 2003) or inhibiting mitochondrial permeability transition pore (mPTP) opening (Costa AD, Jakob R, Costa CL, Andrukhiv K, West IC, 2006). κ-opioid agonist induces mPTP opening (Zhang SZ, Wang NF, Xu J, Gao Q, Lin GH, 2006). Remote rat limb preconditioning can mediate cardioprotection through κ-opioid receptor blockade (Zhang SZ, Wang NF, Xu J, Gao Q, Lin GH, 2006). 8-sulphophenyltheophylline (8-SPT), a non-specific adenosine receptor antagonist, could block the cardioprotective effect of RIC performed on the rabbit kidney if administered prior to preconditioning (Pell TJ, Baxter GF, Yellon DM, 1998) and also after preconditioning (Takaoka A, Nakae I, Mitsunami K, Yabe T, Morikawa S, 1999). Elevated adenosine levels in carotid artery blood of rabbits subjected to preconditioning suggests that myocardial adenosine receptor binding is a key step in the mechanism of preconditioning (Takaoka A, Nakae I, Mitsunami K, Yabe T, Morikawa S, 1999). Free radical scavenger was able to block the cardioprotective effect of RIC (Weinbrenner C, Schulze F, Sarvary L, 2004), implicating signaling reactive oxygen species as a mediator of RIC cardioprotection. Transection of the femoral nerve before application of the RIC stimulus blocked cardioprotection (Lim SY, Yellon DM, 2010) (Steensrud T, Li J, 2010). Ding et al. noted that brief renal artery occlusion was associated with increased afferent renal nerve activity, and nerve transection also blocked RIC-induced cardioprotection (Ding YF, Zhang MM, 2001). Direct stimulation of the sensory nerve of the remote organ or tissue has been reported to reproduce the cardioprotective effect of RIC (Dong JH, Liu YX, Zhao J, Ma HJ, Guo SM, 2004) (Merlocco AC, Redington KL, 2014) (Redington KL, Disenhouse T, 2013). Stimulation of cutaneous sensory nerves, using either topical application of capsaicin (Redington KL, Disenhouse T, 2012) or surgical skin incision (Ren X, Wang Y, 2004) (Gross GJ, Baker JE, Moore J, Falck JR, 2011), has been reported to mimic RIC cardioprotection.
Clinical application of remote ischemic conditioning
Cardiac bypass surgery as a clinical setting for cardioprotection
The first clinical setting for RIC to be tested in was cardiac bypass surgery, in which the heart is subjected to a global ischemic insult when put onto cardiopulmonary bypass, followed by global reperfusion injury (acute IRI) when taken off cardiopulmonary bypass (Venugopal V, Ludman A, 2009). Direct handling of the heart, coronary embolization, and the inflammatory response to cardiopulmonary bypass can all contribute to perioperative myocardial injury (PMI)which can be quantified by measuring serum cardiac enzymes (Creatine Kinase MB isoenzyme, Troponin T and I) (Croal BL, Hillis GS, 2006) (Wang TK, Stewart RA, 2013), and can be detected as late gadolinium enhancement (LGE) on cardiovascular magnetic resonance imaging (CMR) (Selvanayagam JB, Porto I, 2005). The presence of PMI has been associated with worse clinical outcomes post-cardiac surgery (Croal BL, Hillis GS, 2006) (Wang TK, Stewart RA, 2013).
The first attempt to clinically apply RIC involved only eight patients, in a study in which remote limb preconditioning failed to affect CK-MB in elective patients undergoing cardiac surgery (Gunaydin B, Cakici I, Soncul H, Kalaycioglu S, Cevik C, 2000). This study was underpowered; CK-MB was measured 5 minutes after declamping the aorta; cuff inflation to 300 mmHg was used; and an inadequate RIPC protocol was used with two cycles of 3 minute upper limb ischemia followed by 2 minute reperfusion (Gunaydin B, Cakici I, Soncul H, Kalaycioglu S, Cevik C, 2000).
Cheung et al. were the first to successfully apply RIPC clinically (Cheung MM, Kharbanda RK, Konstantinov IE, Shimizu M, Frndova H, 2006). They reported that an RIPC protocol using four 5 minute cycles of lower limb ischemia was able to reduce myocardial injury, improve airway resistance, and decrease inotrope score in 17 children undergoing congenital cardiac surgery (Cheung MM, Kharbanda RK, Konstantinov IE, Shimizu M, Frndova H, 2006).
Hausenloy et al (Hausenloy DJ, Mwamure PK, Venugopal V, Harris J, Barnard M and Al, 2007) demonstrated that RIPC, using three-5 min cycles of upper limb ischemia, was able to reduce myocardial injury (43% reduction in serum troponin-T released over 72 hours) in adult patients undergoing elective coronary artery bypass grafting surgery. RIPC using limb ischemia has also been reported to be cardioprotective in the setting of repair of abdominal aortic aneurysm (AAA) elective surgery (Ali ZA, Callaghan CJ, Lim E, Ali AA, Nouraei SA, 2007). Ali et al demonstrated that invasive lower limb ischemia using two 10 minute episodes of iliac artery occlusion was able to reduce myocardial injury (27% reduction in serum troponin-I released over the perioperative period) and preserve renal function during elective AAA surgical repair (Ali ZA, Callaghan CJ, Lim E, Ali AA, Nouraei SA, 2007).
The results of several recent meta-analyses have confirmed the cardioprotective effects of RIC cardiac bypass surgery in attenuating perioperative myocardial injury (Haji Mohd Yasin NA, Herbison P, 2014) (Healy DA, Khan WA, 2014). There have, however, been several neutral studies (Karuppasamy P, Chaubey S, 2011) (Young PJ, Dalley P, 2012) (Rahman IA, Mascaro JG, 2010) including at least one very large study (McCrindle BW, Clarizia NA, 2014).
RIPHeart study (Meybohm et al., 2015):
1403 adults undergoing elective cardiac surgery with cardiopulmonary bypass under general anesthesia with intravenous propofol were randomized to upper-limb RIPC or sham intervention. No significant differences between the RIPC group and the sham-RIPC group were seen in the level of troponin, the duration of mechanical ventilation, the length of stay in the intensive care unit, the length of hospital stay, incidence of new onset atrial fibrillation, and the incidence of postoperative delirium.
ERICCA study (Hausenloy et al., 2015a):
1612 patients undergoing elective on-pump CABG with or without valve surgery were randomly assigned to RIPC or sham conditioning. There was no standardization of anesthetic management and perioperative care. The combined primary end point was death from cardiovascular causes, nonfatal myocardial infarction, need for coronary revascularization, or stroke at 12 months from randomization. RIPC was not shown to improve clinical outcomes in patients undergoing elective on-pump CABG with or without valve surgery.
The reasons for this discrepancy may relate to: patient/clinical factors (CABG vs valve surgery, stable vs unstable patients); timing of the limb RIC protocol (before vs after surgical incision); blinding to the RIC protocol (proper vs limited blinding); the intensity of the RIC protocol (3 vs 4 cycles of limb RIC and inflation of cuff to 200mmHg vs 15mmHg above systolic blood pressure); and the presence of confounding factors.
Propofol and volatile anaesthetic agents – potential confounding factors
Attenuation of RIC has been noted when propofol anaesthesia has been used (Bautin et al., 2014), and the use of propofol, rather than volatile anaesthesia, appears to be a common factor in studies that failed to protect with RIC in CABG (Heusch, 2013; Zangrillo et al., 2015). The American College of Cardiology Foundation and the American Heart Association Task Force on Practice Guidelines have recommended the use of volatile anaesthetics in surgical patients with increased cardiovascular risk (Fleisher et al., 2008). Whether RIC provides additional cardioprotection to the use of volatile anaesthetic in patients undergoing cardiac surgery is uncertain with clinical studies showing mixed results.
Diabetes may attenuate RIC cardioprotection through neurohumoral pathways
Single-dose RIC does not appear to offer much cardioprotection in diabetic patients (Xu et al., 2014; Baranyai et al., 2015; Epps and Smart, 2016; Lejay et al., 2016) and the mechanisms of which are not well understood. About 60% to 70% of people with diabetes mellitus will eventually develop the complication of diabetic peripheral neuropathy (Boulton et al., 2005). In many of these patients, sensory C fibers mediate the cardioprotective effect of RIC, are damaged (Green et al., 2010) and this may be an important contributor to the attenuation of RIC cardioprotection (Saxena et al., 2010; Jensen et al., 2012). It may be necessary to exclude patients with diabetic neuropathy or sensory neuropathy from future clinical trials in RIC. In addition, diabetes affects the intracellular signaling pathways that are crucial for endogenous cardioprotection. These include: PI3K/Akt/glycogen synthase kinase 3 beta (PI3K/Akt/GSK3-β) signaling pathway, phosphorylation of ERK1/2 (extracellular signal-regulated protein kinases 1 and 2), generation and release of nitric oxide, ATP-sensitive potassium channels, and oxidative stress generation (Chen et al., 2012; Baumgardt et al., 2016; Wang and Zhao, 2016). This may further contribute to the attenuation of RIC cardioprotection.
PPCI as a clinical setting for RIC cardioprotection
Timely myocardial reperfusion by PPCI is the most effective therapy for limiting MI size and preserving LV systolic function in patients presenting with STEMI. Restoration of coronary blood flow in the occluded artery results in myocardial reperfusion injury which may be amenable to cardioprotection by IPost and RIC (Bulluck and Hausenloy, 2015).
In several proof-of-concept studies, limb RIC appeared to be effective when administered by paramedics in the ambulance (Botker HE, Kharbanda R, 2010a), on hospital arrival prior to PPCI (Rentoukas I, Giannopoulos G, 2010a) (White SK, Frohlich GM, 2014), and even at the onset of reperfusion with PPCI (Crimi G, Pica S, 2013). Please see Table 1 for summary of major clinical studies in STEMI patients.
Table 1.
Major clinical studies of RIC in STEMI
| Study and Year | Number of patients | Type of patients | RIC protocol | Main outcomes | Notes |
|---|---|---|---|---|---|
| Bøtker et al. (Botker HE, Kharbanda R, 2010b) CONDI (2010) |
142 | All STEMI | 4 × 5 min inflations/deflations of cuff on upper arm in the ambulance before PPCI Sham control: none |
Primary endpoint: Myocardial salvage index at 30 days post PPCI, measured by myocardial perfusion imaging as the proportion of the area at risk salvaged by treatment Increase in myocardial salvage index at 30 days No difference in MI size (SPECT or peak troponin) |
First study to test effect of RIC in patients with STEMI Reduced MI size in LAD STEMI |
| Munk et al. (Munk et al., 2010) (2010) |
242 | All STEMI | 4 × 5 min inflations/deflations of cuff on upper arm in the ambulance before PPCI Sham control: none |
Ejection fraction, LV volumes (2D and 3D echocardiography and myocardial perfusion imaging), and speckle-tracking global longitudinal strain were compared between treatment groups. Although no significant overall effect was observed, RIC seemed to result in modest improvement in LV function in high-risk patients prone to develop large myocardial infarcts |
The effect of RIC was analysed in relation to the size of the myocardial area at risk (AAR), infarct location, and target vessel patency. |
| Rentoukas et al (Rentoukas I, Giannopoulos G, 2010b) (2010) |
93 | All STEMI | 3 × 4 min inflations/deflations of cuff on upper arm at the hospital before PPCI Sham control: 3 × 5 min low-pressure inflations/deflations |
Better ST-segment resolution and lower peak troponin I Additive effects with morphine |
Combined effects of RIC with morphine |
| Crimi et al. (Crimi et al., 2013) (2013) |
100 | Anterior STEMI only | 3 × 5 min inflations/deflation of cuff on thigh at onset of reperfusion Sham control: none |
20% reduction in 72 h AUC CK–MB 21% reduction in myocardial oedema by MRI |
First study to show effect of RIC given at onset of reperfusion, and first to report effect of RIC on enzymatic MI size and myocardial oedema |
| Sloth et al. (Sloth et al., 2014) (2014) |
251 | All STEMI | 4 × 5 min inflations/deflations of cuff on upper arm in the ambulance before PPCI Sham control: none |
51% reduction in all-cause mortality, nonfatal MI, TIA or stroke, HHF at 3.8 years | First study to test effect of RIC on long-term outcomes after PPCI (secondary end point) |
| Prunier et al. (Prunier et al., 2014) (2014) |
55 | All STEMI | 3 × 5 min inflations/deflations of cuff on upper arm in before PPCI Sham control: none |
RIC immediately prior to PPCI was shown to reduce infarct size in STEMI patients, yet combining this therapy with an IPost strategy did not lead to further decrease in infarct size. | Study had arm involving PPCI combined with RIC and IPost which consisted of four cycles of 1-min inflation and 1-min deflation of the angioplasty balloon. |
| White et al. (White et al., 2015) ERIC-STEMI (2015) |
83 | All STEMI | 4 × 5 min inflations/deflations of cuff on upper arm at the hospital before PPCI Sham control: deflated cuff |
27% reduction in MI size by MRI 19% reduction in myocardial oedema by MRI |
First study to show effect of RIC given before PPCI on MI size and myocardial oedema by MRI |
| Yellon et al. (Yellon et al., 2015) ERIC-LYSIS (2015) |
519 | All STEMI | 4 × 5 min inflations/deflations of cuff on upper arm at the hospital before thrombolysis Sham control: deflated cuff |
17% reduction in enzymatic MI size (CK–MB and troponin T) | Only study to test effect of RIC in thrombolysed patients with STEMI |
| Eitel et al. (Eitel et al., 2015) LIPSIA CONDITIONING (2015) |
333 | All STEMI | 4 × 5 min inflations/deflations of cuff on upper arm at the hospital before PPCI plus IPost Sham control: none |
Increased myocardial salvage with RIC + IPost versus control (49 versus 40) No difference in MI size, MVO, or 6-month clinical end points (death, reinfarction, and heart failure at 6 months) |
Improved myocardial salvage when IPost combined with RIC Neither IPost alone nor RIC + IPost reduce myocardial oedema |
| Yamanaka et al. (Yamanaka et al., 2015) (2015) |
94 | All STEMI | 3 × 5 min inflations/deflations of cuff on upper arm at the hospital before PPCI Sham control: Yes |
Primary endpoint: Incidence of contrast induced-AKI after administration of contrast medium Odds ratio of CI-AKI in patients who received RIPC was 0.18 (95% confidence interval: 0.05-0.64; p=0.008) |
Lower incidence of ventricular arrhythmia was noted in the RIC group within 24 hours of RIC |
| Verouhis et al. (Verouhis et al., 2016) (2016) |
93 | Anterior STEMI only | 5-minute cycles of inflation and deflation of a blood pressure cuff around the left thigh which was continued throughout the PCI procedure Sham control: Yes |
The primary endpoint of the study was infarct size e×pressed as myocardial salvage inde× determined by CMR on days 4-7 after PCI. There was no significant difference in myocardial salvage inde× between the RIPerC and PCI group |
This study has been the only neutral study RIC STEMI study. The use of a non-standard RIC protocol comprising variable numbers of RIC cycles (as many as 7-9) were used and may have contributed to the neutral results. |
| Liu et al. (Liu et al., 2016) (2016) |
119 | All STEMI | 4 × 5 min inflations to 200 mmHg /deflations of cuff on upper arm in the ambulance before PPCI Sham control: none |
The primary study end point was early microvascular obstruction measured by CMR. There was a significant decrease in early microvascular obstruction as assessed by CMR in the RIC group |
This was the first study to assess the effect of RIC in STEMI patients through the use of CMR to detect early microvascular obstruction. |
| Gaspar et al. (Gaspar et al., 2018) (2018) |
448 | All STEMI | 3 × 5 min inflations/deflations of cuff on upper arm in before PPCI Sham control: Yes |
RIC was shown to be beneficial in a combined clinical endpoint of cardiac mortality and hospitalisation for HF. Improved EF recovery was also documented in patients with impaired LV function. In-hospital heart failure risk and need for diuretics, inotropes and/or intraaortic balloon pump were reduced in RIC group | First prospectively designed study to investigate the effect of RIC on clinical outcomes following STEMI as primary endpoint. |
| CONDI-2/ERIC-PPCI (Hausenloy et al., 2015b) | 5,400 | All STEMI | 4 × 5 min inflations/deflations of cuff on upper arm before PPCI Sham control: none or simulated |
Ongoing study Primary end point of cardiac death and HHF at 12 months |
Collaboration between Denmark, Serbia, Spain, and the UK First study to test effect of RIC on long-term clinical outcomes as primary end point |
Bøtker et al. (Botker HE, Kharbanda R, 2010b) in the CONDI trial were the first group to test the effect of RIC in patients with STEMI. The study involved 142 STEMI patients with the primary endpoint being myocardial salvage index at 30 days post PPCI, measured by myocardial perfusion imaging as the proportion of the area at risk salvaged by treatment. They found an increase in myocardial salvage index at 30 days with no difference in MI size measured by SPECT or peak troponin. Reduced MI size was found however in LAD STEMI. Crimi et al. (Crimi et al., 2013) then assessed the effect of RIC on 100 anterior STEMI patients and found a 20% reduction in 72 hour AUC CK–MB and a 21% reduction in myocardial oedema by MRI. This was the first study to show the effect of RIC given at onset of reperfusion, and the first to report the effect of RIC on enzymatic MI size and myocardial oedema.
It has been observed that the beneficial effect of RIPC can be inhibited by the opioid receptor blocker naloxone (Patel HH, Moore J, Hsu AK, 2002). Rentoukas et al (Rentoukas I, Giannopoulos G, 2010b) sought to assess the enhancement of the cardioprotective effect of RIC by opioids by having 3 arms in their study comprising of RIC only group, RIC and morphine group and control group. In paired comparisons between groups, the RIC and morphine group performed better than the control group in terms of both ST-segment reduction and peak troponin I, whereas the differences in outcomes between the RIC only group and the control group did not reach statistical significance.
Munk et al. (Munk et al., 2010) analysed the effect of RIC in relation to the size of the myocardial area at risk (AAR), infarct location, and target vessel patency in a study involving 242 STEMI patients. Ejection fraction, LV volumes (2D and 3D echocardiography and myocardial perfusion imaging), and speckle-tracking global longitudinal strain were compared between treatment groups. Although no significant overall effect was observed, RIC seemed to result in modest improvement in LV function in high-risk patients prone to develop large myocardial infarcts.
Sloth et al. (Sloth et al., 2014) performed a study involving 251 STEMI patients. The RIC intervention was 4 × 5 min inflations/deflations of cuff on upper arm in the ambulance before PPCI without sham control. This showed a 51% reduction in all-cause mortality, nonfatal MI, TIA or stroke, HHF at 3.8 years and was the first study to test effect of RIC on long-term outcomes after PPCI as a secondary end point.
Prunier et al. (Prunier et al., 2014) had a study arm involving PPCI combined with RIC and IPost which consisted of four cycles of 1-min inflation and 1-min deflation of the angioplasty balloon in their study consisting of 55 STEMI patients. RIC immediately prior to PPCI was shown to reduce infarct size in STEMI patients, yet combining this therapy with an IPost strategy did not lead to further decrease in infarct size.
In the study by White et al in 2015 (White et al., 2015), 197 patients with ST-segment elevation myocardial infarction with TIMI (Thrombolysis In Myocardial Infarction) flow grade 0 were randomly assigned to receive RIC (four 5-min cycles of upper arm cuff inflation/deflation) or control (uninflated cuff placed on upper arm for 40 min) protocols prior to PPCI. This study aimed to determine whether RIC performed (PPCI) could reduce myocardial infarct (MI) size, assessed by cardiac MRI, in patients presenting with ST-segment elevation myocardial infarction. The primary study endpoint was MI size assessed by cardiac MRI in 83 subjects on days 3 to 6 after admission. RIC was found to reduce MI size by 27%, when compared with the MI size of control subjects. At 24 hours, high-sensitivity troponin T was lower with RIC. RIC also reduced the extent of myocardial oedema measured by T2-mapping CMR and lowered mean T2 values. This precluded the use of CMR oedema imaging to accurately estimate the area at risk. When using coronary angiography jeopardy scores to estimate the area at risk, RIC was found to significantly improve the myocardial salvage index. This study demonstrated that RIC, performed in patients with STEMI treated by PPCI, reduced MI size, increased myocardial salvage, and reduced myocardial oedema when performed prior to PPCI.
Yellon et al. (Yellon et al., 2015) performed the ERIC-LYSIS study, which is the only study to test effect of RIC in thrombolysed patients with STEMI. 519 STEMI patients were randomised to 4 × 5 min inflations/deflations of cuff on upper arm at the hospital before thrombolysis or sham control with deflated cuff application. There was a 17% reduction in enzymatic MI size (CK–MB and troponin T) in the RIC group.
In the LIPSIA CONDITIONING study by Eitel et al. (Eitel et al., 2015) involving 333 STEMI patients, improved myocardial salvage was seen when IPost was combined with RIC. Neither IPost alone nor RIC + IPost reduced myocardial oedema. No difference in MI size, MVO, or 6-month clinical end points (death, re-infarction, and heart failure at 6 months) were seen.
Renal outcomes were assessed by Yamanaka et al. (Yamanaka et al., 2015) in a 94-STEMI patient study with the primary endpoint being incidence of contrast induced-AKI after administration of contrast medium. The odds ratio of CI-AKI in patients who received RIPC was 0.18 (95% confidence interval: 0.05-0.64; p=0.008). Lower incidence of ventricular arrhythmia was also noted in the RIC group within 24 hours of RIC.
The study by Verouhis et al. (Verouhis et al., 2016) has been the only neutral study of RIC in STEMI. The use of a non-standard RIC protocol comprising of variable numbers of RIC cycles (as many as 7-9) were used and may have contributed to the neutral results. The primary endpoint of the study was infarct size expressed as myocardial salvage index determined by CMR on days 4-7 after PCI. There was no significant difference in myocardial salvage index between the RIPerC and PCI group.
Liu et al. (Liu et al., 2016) performed the first study to assess the effect of RIC in STEMI patients through the use of CMR to detect early microvascular obstruction. The primary study end point was early microvascular obstruction measured by CMR. There was a significant decrease in early microvascular obstruction as assessed by CMR in the RIC group.
Most recently, Gaspar et al found that RIC administered prior to PPCI improved clinical outcomes following STEMI with reduced rates of HHF (Gaspar et al., 2018). This is the first prospectively designed study to investigate the effect of RIC on clinical outcomes following STEMI as primary endpoint. RIC was shown to be beneficial in a combined clinical endpoint of cardiac mortality and hospitalisation for HF. Improved EF recovery was also documented in patients with impaired LV function. In-hospital heart failure risk and need for diuretics, inotropes and/or intra-aortic balloon pump were reduced in RIC group.
In a large European multicentre study, the CONDI2/ERIC-PPCI trial (ClinicalTrials.gov Identifier: NCT02342522) is investigating, whether RIC initiated prior to PPCI can reduce the rates of cardiac death and hospitalisation for heart failure at 12 months as the primary endpoint. It is a prospective, randomized-controlled trial of 5200 STEMI patients undergoing PPCI. Patients have been randomized to either RIC or sham control. Secondary endpoints include: (i) Rates of cardiac death and heart failure hospitalization at 30 days, (ii) Rates of all-cause death, coronary revascularization, re-infarction, stroke at 30 days and at 12 months, (iii) TIMI flow post-PPCI, (iv) ST-segment resolution on ECG taken at 90 minutes, (v) Enzymatic MI size as assessed from a 48 h area-under-the-curve (AUC) high-sensitive Troponin-T (hsTrop-T) using blood samples collected at time 0, 6, 12, 24, and 48 hours in a sub-study, (vi) MI size as measured by cardiac magnetic resonance (CMR) scan performed at 6 months in a sub-study. It is well established that RIC can reduce MI size in STEMI patients who have received PPCI. It is not known whether this beneficial effect translates to improved clinical outcomes. The results of the CONDI2/ERIC-PPCI study, which will be available in Summer 2019, will establish whether limb RIC, as a non-invasive low-cost intervention, can improve long-term clinical outcomes in STEMI patients treated with PPCI.
Challenges and future directions
Several post-hoc analyses of RIC in STEMI studies have shed further insights. In the post-hoc analysis by Pryds et al. (Pryds et al., 2016a) assessing the influence of pre-infarction angina and coronary collateral blood flow (CCBF) on the effectiveness of RIC in STEMI patients, pre-infarction angina was found not to modify RIC efficacy in STEMI patients undergoing PPCI. CCBF to the infarct-related artery seemed to affect the cardioprotective efficacy of RIC, with mean myocardial salvage index (MSI) being increased in patients with CCBF versus without CCBF in the RIC with PPCI group. Pryds et al. (Pryds et al., 2016b) also found, in a separate post-hoc analysis, that RIC as adjunct to PPCI attenuated the detrimental effect of healthcare system delay on myocardial salvage in patients with STEMI. In patients with healthcare system delay >120 min, RIC with PPCI increased median MSI compared with PPCI alone, suggesting that the cardioprotective effect of RIC increases with the duration of ischemia. Sloth et al. (Sloth et al., 2015) found no significant difference in the effectiveness of RIC in subgroups of cardiovascular risk factors, lipid and glucose levels, and medication use in their post-hoc analysis. Sloth et al. (Sloth et al., 2016) also performed a post-hoc analysis addressing the issue of cost-effectiveness of RIC in STEMI patients. They found that after 4 years of follow-up, mean cumulative cardiovascular medical care costs were lower in the RIC group than in the control group, while mean major adverse cardiac and cerebrovascular event-free survival time was 0.30 years higher in the RIC than in the control group. These results suggest that RIC in STEMI appears to be a cost-effective treatment strategy in patients with STEMI.
Daily RIC following STEMI
Chronic renal failure patients undergoing haemodialysis are subjected to repeated episodes of acute myocardial ischemia resulting in myocardial stunning and chronic LV systolic impairment (Crowley LE, 2013). Limb RIC has been reported to attenuate ST-segment depression and prevent myocardial stunning in these patients (Crowley LE, 2013).
One experimental study has demonstrated that performing limb RIC daily for a period of 28 days prevented adverse post-MI LV remodeling in the rat heart (Wei M, Xin P, 2011). This approach has been tested in the clinical setting in two studies.
DREAM (Vanezis et al., 2018):
This trial assessed the role of daily RIC in improving left ventricular ejection fraction (LVEF) recovery in patients with reduced LVEF (<45%) after STEMI treated with PPCI. Patients were recruited from four UK hospitals and randomised to receive either 4 weeks of daily RIC or sham conditioning commencing on day 3 post P-PCI.
The primary endpoint was the improvement in LVEF over 4 months assessed by cardiac MRI. 73 patients (38 cases, 35 controls) completed the study. There was no difference in the improvement in LVEF over 4 months between the treatment and control groups. No differences were seen in the secondary outcome measures of changes in infarct size and left ventricular end-diastolic and systolic volumes, major adverse cardiac and cerebral events, mean Kansas City Cardiomyopathy Questionnaire score and change in N-terminal pro-brain natriuretic peptide levels. Daily RIC starting on day 3 and continued for 4 weeks following P-PCI for STEMI did not improve LVEF as assessed by CMR after 4 months when compared with a matched control group. The failure to begin RIC immediately prior to PPCI may have contributed to the neutral results of this study, and in this regard, the ongoing CRIC-RCT trial (NCT01817114) which is initiating RIC prior to PPCI and administering RIC daily for one month may provide further insights.
Summary and Conclusions
RIC provides an easily applied and very effective endogenous strategy for reducing MI size following acute IRI. Despite extensive studies, the actual mechanistic pathway underlying RIC cardioprotection remains unclear - although a neurohormonal pathway is believed to be critical, the exact interplay between the neural and hormonal pathway is yet to be determined, and the identity of the cardioprotective humoral factors remain unknown. RIC has been successfully tested in a number of clinical settings including CABG surgery, elective PCI and more recently and most promisingly in STEMI patients undergoing PPCI. The translation of RIC into patient benefit has been elusive for CABG surgery patients, and this failure may be attributed to insufficient information on the optimum RIC protocol, the effects of co-medications of RIC cardioprotection such as propofol anesthesia and use of nitrates, and the presence of age and co-morbidities such as diabetes. RIC has most promise for STEMI patients undergoing PPCI, and the results of the CONDI2/PPCI study which are due in Summer 2019 are eagerly awaited, and will provide the definitive answer as to whether RIC can improve clinical outcomes and change clinical practice.
Acknowledgments
Funding: This project was funded by NIH grant R01HL81863 to WAB. DJH is supported by the Singapore Ministry of Health’s National Medical Research Council under its Clinician Scientist-Senior Investigator scheme (NMRC/CSA-SI/0011/2017) and Collaborative Centre Grant scheme (NMRC/CGAug16C006), the Singapore Ministry of Education Academic Research Fund Tier 2 (MOE2016-T2-2-021) and the EU-CARDIOPROTECTION CA16225 Cooperation in Science and Technology (COST) Action.
Footnotes
Disclosures: None
References
- Ali ZA, Callaghan CJ, Lim E, Ali AA, Nouraei SA AAM et al. (2007) Remote ischemic preconditioning reduces myocardial and renal injury after elective abdominal aortic aneurysm repair: a randomized controlled trial. Circulation 116:I98–I105. [DOI] [PubMed] [Google Scholar]
- Baranyai T, Nagy CT, Koncsos G, Onodi Z, Karolyi-Szabo M, Makkos A, Varga Z V, Ferdinandy P, Giricz Z (2015) Acute hyperglycemia abolishes cardioprotection by remote ischemic perconditioning. Cardiovasc Diabetol 14:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgardt SL, Paterson M, Leucker TM, Fang J, Zhang DX, Bosnjak ZJ, Warltier DC, Kersten JR, Ge Z-D (2016) Chronic Co-Administration of Sepiapterin and L-Citrulline Ameliorates Diabetic Cardiomyopathy and Myocardial Ischemia/Reperfusion Injury in Obese Type 2 Diabetic Mice. Circ Heart Fail 9:e002424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautin AE, Galagudza MM, Datsenko S V, Tashkhanov DM, Marichev AO, Bakanov AI, Malaia EI, Naimushin A V, Rubinchik VE, Gordeev ML (2014) [Effects of remote ischemic preconditioning on perioperative period in elective aortic valve replacement]. Anesteziol Reanimatol:11–17. [PubMed] [Google Scholar]
- Birnbaum Y, Hale SL KRA (1997) Ischemic preconditioning at a distance: reduction of myocardial infarct size by partial reduction of blood supply combined with rapid stimulation of the gastrocnemius muscle in the rabbit. . Circ 96:1641–1646. [DOI] [PubMed] [Google Scholar]
- Botker HE, Kharbanda R SM et al. (2010a) Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet 375:727–734. [DOI] [PubMed] [Google Scholar]
- Botker HE, Kharbanda R SM et al. (2010b) Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet 375:727–734. [DOI] [PubMed] [Google Scholar]
- Boulton AJM, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, Malik RA, Maser RE, Sosenko JM, Ziegler D (2005) Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care 28:956–962. [DOI] [PubMed] [Google Scholar]
- Bulluck H, Hausenloy DJ (2015) Ischaemic conditioning: are we there yet? Heart 101:1067–1077 Available at: http://heart.bmj.com/content/101/13/1067.abstract. [DOI] [PubMed] [Google Scholar]
- Chen Z-C, Cheng Y-Z, Chen L-J, Cheng K-C, Li Y-X, Cheng J-T (2012) Increase of ATP-sensitive potassium (K(ATP)) channels in the heart of type-1 diabetic rats. Cardiovasc Diabetol 11:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung MM, Kharbanda RK, Konstantinov IE, Shimizu M, Frndova H LJ et al. (2006) Randomized controlled trial of the effects of remote ischemic preconditioning on children undergoing cardiac surgery: first clinical application in humans. J Am Coll Cardiol 47:2277–2282. [DOI] [PubMed] [Google Scholar]
- Costa AD, Jakob R, Costa CL, Andrukhiv K, West IC GKD (2006) The mechanism by which the mitochondrial ATP-sensitive Kþ channel opening and H2O2 inhibit the mitochondrial permeability transition. J Biol Chem 281:20801–20808. [DOI] [PubMed] [Google Scholar]
- Crimi G, Pica S RC et al. (2013) Remote ischemic post-conditioning of the lower limb during primary percutaneous coronary intervention safely reduces enzymatic infarct size in anterior myocardial infarction: a randomized controlled trial. JACCCardiovascInterv 6:1055–1063. [DOI] [PubMed] [Google Scholar]
- Crimi G, Pica S, Raineri C, Bramucci E, De Ferrari GM, Klersy C, Ferlini M, Marinoni B, Repetto A, Romeo M, Rosti V, Massa M, Raisaro A, Leonardi S, Rubartelli P, Oltrona Visconti L, Ferrario M, Crimi G, Pica S RC et al. (2013) Remote ischemic post-conditioning of the lower limb during primary percutaneous coronary intervention safely reduces enzymatic infarct size in anterior myocardial infarction: a randomized controlled trial. JACCCardiovascInterv 6:1055–1063. [DOI] [PubMed] [Google Scholar]
- Croal BL, Hillis GS GPH et al. (2006) Relationship between postoperative cardiac troponin I levels and outcome of cardiac surgery. . Circ 114:1468–1475. [DOI] [PubMed] [Google Scholar]
- Crowley LE MC (2013) Remote ischaemic conditioning-therapeutic opportunities in renal medicine. NatRevNephrol 9:739–746. [DOI] [PubMed] [Google Scholar]
- Davidson SM, Selvaraj P HD et al. (2013) Remote ischaemic preconditioning involves signalling through the SDF-1alpha/CXCR4 signalling axis. Basic Res Cardiol 108:377. [DOI] [PubMed] [Google Scholar]
- Dickson EW, Blehar DJ, Carraway RE, Heard SO SG, K P (2001) Naloxone blocks transferred preconditioning in isolated rabbit hearts. J Mol Cell Cardiol 33:1751–1756. [DOI] [PubMed] [Google Scholar]
- Ding YF, Zhang MM HRR (2001) Role of renal nerve in cardioprotection provided by renal ischemic preconditioning in anesthetized rabbits. Sheng li xue Bao [Acta Physiol Sin 53:7–12. [PubMed] [Google Scholar]
- Dong JH, Liu YX, Zhao J, Ma HJ, Guo SM HRR (2004) High-frequency electrical stimulation of femoral nerve reduces infarct size following myocardial ischemia-reperfusion in rats. Sheng li xue Bao [Acta Physiol Sin 56:620–624. [PubMed] [Google Scholar]
- Eitel I, Stiermaier T, Rommel KP, Fuernau G, Sandri M, Mangner N, Linke A, Erbs S, Lurz P, Boudriot E, Mende M, Desch S, Schuler G, Thiele H (2015) Cardioprotection by combined intrahospital remote ischaemic perconditioning and postconditioning in ST-elevation myocardial infarction: the randomized LIPSIA CONDITIONING trial. Eur Heart J 36:3049–3057. [DOI] [PubMed] [Google Scholar]
- Epps JA, Smart NA (2016) Remote ischaemic conditioning in the context of type 2 diabetes and neuropathy: the case for repeat application as a novel therapy for lower extremity ulceration. Cardiovasc Diabetol 15:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisher LA et al. (2008) ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise. Anesth Analg 106:685–712. [DOI] [PubMed] [Google Scholar]
- Gaspar A, Lourenco AP, Pereira MA, Azevedo P, Roncon-Albuquerque RJ, Marques J, Leite-Moreira AF (2018) Randomized controlled trial of remote ischaemic conditioning in ST-elevation myocardial infarction as adjuvant to primary angioplasty (RIC-STEMI). Basic Res Cardiol 113:14. [DOI] [PubMed] [Google Scholar]
- Giricz Z, Varga ZV BT et al. (2014) Cardioprotection by remote ischemic preconditioning of the rat heart is mediated by extracellular vesicles. J Mol Cell Cardiol 68:75–78. [DOI] [PubMed] [Google Scholar]
- Green AQ, Krishnan S, Finucane FM, Rayman G (2010) Altered C-fiber function as an indicator of early peripheral neuropathy in individuals with impaired glucose tolerance. Diabetes Care 33:174–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross GJ, Baker JE, Moore J, Falck JR NK (2011) Abdominal surgical incision induces remote preconditioning of trauma (RPCT) via activation of bradykinin receptors (BK2R) and the cytochrome P450 epoxygenase pathway in canine hearts. Cardiovasc Drugs Ther 25:517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunaydin B, Cakici I, Soncul H, Kalaycioglu S, Cevik C SB et al. (2000) Does remote organ ischaemia trigger cardiac preconditioning during coronary artery surgery? Pharmacol Res 41:493–496. [DOI] [PubMed] [Google Scholar]
- Haji Mohd Yasin NA, Herbison P SP et al. (2014) The role of remote ischemic preconditioning in organ protection after cardiac surgery: a meta-analysis. JSurgRes 186:207–216. [DOI] [PubMed] [Google Scholar]
- Hajrasouliha AR, Tavakoli S, Ghasemi MJ-MP, et al. Sadeghipour HEF (2008) Endogenous cannabinoids contribute to remote ischemic preconditioning via cannabinoid CB(2) receptors in the rat heart. Eur J Pharmacol 579:246–252. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Mwamure PK, Venugopal V, Harris J, Barnard M GE, Al E (2007) Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomised controlled trial. Lancet 370:575–579. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ and Yellon DM (2008) Remote ischaemic preconditioning: underlying mechanisms and clinical application. Cardiovasc Res 79:377–386. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Candilio L, Evans R, Ariti C, Jenkins DP, Kolvekar S, Knight R, Kunst G, Laing C, Nicholas J, Pepper J, Robertson S, Xenou M, Clayton T, Yellon DM (2015a) Remote Ischemic Preconditioning and Outcomes of Cardiac Surgery. N Engl J Med 373:1408–1417 Available at: 10.1056/NEJMoa1413534. [DOI] [PubMed] [Google Scholar]
- Engstrom T, Garcia Ruiz JM, Radovanovic N, Christensen EF, Sorensen HT, Ramlall M, Bulluck H, Evans R, Nicholas J, Knight R, Clayton T, Yellon DM, Botker HE (2015b) Effect of remote ischaemic conditioning on clinical outcomes in patients presenting with an ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Eur Heart J 36:1846–1848. [PubMed] [Google Scholar]
- Hausenloy DJ YD (2013) Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest 123:92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy DA, Khan WA WCS et al. (2014) Remote preconditioning and major clinical complications following adult cardiovascular surgery: systematic review and meta-analysis. IntJCardiol 176:20–31. [DOI] [PubMed] [Google Scholar]
- Heusch G (2013) Cardioprotection: chances and challenges of its translation to the clinic. Lancet (London, England) 381:166–175. [DOI] [PubMed] [Google Scholar]
- Jensen RV, Stottrup NB, Kristiansen SB, Botker HE (2012) Release of a humoral circulating cardioprotective factor by remote ischemic preconditioning is dependent on preserved neural pathways in diabetic patients. Basic Res Cardiol 107:285. [DOI] [PubMed] [Google Scholar]
- Karuppasamy P, Chaubey S DT et al. (2011) Remote intermittent ischemia before coronary artery bypass graft surgery: a strategy to reduce injury and inflammation? Basic ResCardiol 106:511–519. [DOI] [PubMed] [Google Scholar]
- Kharbanda RK, Mortensen UM WP et al. (2002) Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation 106:2881–2883. [DOI] [PubMed] [Google Scholar]
- Konstantinov IE, Arab S, Kharbanda RK, Li J, Cheung MM C V, Al E (2004) The remote ischemic preconditioning stimulus modifies inflammatory gene expression in humans. Physiol Genomics 19:143–150. [DOI] [PubMed] [Google Scholar]
- Konstantinov IE, Arab S, Li J, Coles JG, Boscarino C MA et al. (2005) The remote ischemic preconditioning stimulus modifies gene expression in mouse myocardium. J Thorac Cardiovasc Surg 130:1326–1332. [DOI] [PubMed] [Google Scholar]
- Lambert Laurie J., Brophy James M., Racine Normand, Rinfret Stéphane, Philippe LL, Kevin A. Brown, Lucy J. Boothroyd, Dave Ross, Eli Segal SK et al. (2016) Outcomes of Patients With ST-Elevation Myocardial Infarction Receiving and Not Receiving Reperfusion Therapy: The Importance of Examining All Patients. Can J Cardiol. [DOI] [PubMed] [Google Scholar]
- Lejay A, Fang F, John R, Van JAD, Barr M, Thaveau F, Chakfe N, Geny B, Scholey JW (2016) Ischemia reperfusion injury, ischemic conditioning and diabetes mellitus. J Mol Cell Cardiol 91:11–22. [DOI] [PubMed] [Google Scholar]
- Leung CH, Wang L NJ et al. (2014) Remote cardioprotection by transfer of coronary effluent from ischemic preconditioned rabbit heart preserves mitochondrial integrity and function via adenosine receptor activation. Cardiovasc Drugs Ther 28:7–17. [DOI] [PubMed] [Google Scholar]
- Lim SY, Yellon DM HDJ (2010) The neural and humoral pathways in remote limb ischemic preconditioning. Basic Res Cardiol 105:651–655. [DOI] [PubMed] [Google Scholar]
- Liu Z, Zhao L, Hong D, Gao J (2016) Remote ischaemic preconditioning reduces myocardial ischaemic reperfusion injury in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Acta Cardiol 71:596–603. [DOI] [PubMed] [Google Scholar]
- McClanahan TB, Nao BS, Wolke LJ, Martin BJ MTE, KP G (1993) Brief renal occlusion and reperfusion reduces myocardial infarct size in rabbits. FASEB J 7:A118. [Google Scholar]
- McCrindle BW, Clarizia NA KS et al. (2014) Remote ischemic preconditioning in children undergoing cardiac surgery with cardiopulmonary bypass: a single-center double-blinded randomized trial. JAmHeart Assoc 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlocco AC, Redington KL DT et al. (2014) Transcutaneous electrical nerve stimulation as a novel method of remote preconditioning: in vitro validation in an animal model and first human observations. Basic Res Cardiol 109:406. [DOI] [PubMed] [Google Scholar]
- Meybohm P et al. (2015) A Multicenter Trial of Remote Ischemic Preconditioning for Heart Surgery. N Engl J Med 373:1397–1407 Available at: 10.1056/NEJMoa1413579. [DOI] [PubMed] [Google Scholar]
- Munk K, Andersen NH, Schmidt MR, Nielsen SS, Terkelsen CJ, Sloth E, Botker HE, Nielsen TT, Poulsen SH (2010) Remote Ischemic Conditioning in Patients With Myocardial Infarction Treated With Primary Angioplasty: Impact on Left Ventricular Function Assessed by Comprehensive Echocardiography and Gated Single-Photon Emission CT. Circ Cardiovasc Imaging 3:656–662. [DOI] [PubMed] [Google Scholar]
- Oxman T, Arad M, Klein R, Avazov N RB (1997) Limb ischemia preconditions the heart against reperfusion tachyarrhythmia. Am J Physiol 273:H1707–H1712. [DOI] [PubMed] [Google Scholar]
- Patel HH, Moore J, Hsu AK GGJ (2002) Cardioprotection at a distance: mesenteric artery occlusion protects the myocardium via an opioid sensitive mechanism. J Mol Cell Cardiol 34:1317–1323. [DOI] [PubMed] [Google Scholar]
- Pell TJ, Baxter GF, Yellon DM DGM (1998) Renal ischemia preconditions myocardium: role of adenosine receptors and ATP-sensitive potassium channels. Am J Physiol 275:H1542–7. [DOI] [PubMed] [Google Scholar]
- Peralta C, Fernandez L, Panes J, Prats N, Sans M PJM et al. (2001) Preconditioning protects against systemic disorders associated with hepatic ischemia-reperfusion through blockade of tumor necrosis factor-induced P-selectin up-regulation in the rat. Hepatology 33:100–113. [DOI] [PubMed] [Google Scholar]
- Prunier F, Angoulvant D, Saint Etienne C, Vermes E, Gilard M, Piot C, Roubille F, Elbaz M, Ovize M, Biere L, Jeanneteau J, Delepine S, Benard T, Abi-Khalil W, Furber A (2014) The RIPOST-MI study, assessing remote ischemic perconditioning alone or in combination with local ischemic postconditioning in ST-segment elevation myocardial infarction. Basic Res Cardiol 109:400. [DOI] [PubMed] [Google Scholar]
- Pryds K, Bottcher M, Sloth AD, Munk K, Rahbek Schmidt M, Botker HE (2016a) Influence of preinfarction angina and coronary collateral blood flow on the efficacy of remote ischaemic conditioning in patients with ST segment elevation myocardial infarction: post hoc subgroup analysis of a randomised controlled trial. BMJ Open 6:e013314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryds K, Terkelsen CJ, Sloth AD, Munk K, Nielsen SS, Schmidt MR, Botker HE (2016b) Remote ischaemic conditioning and healthcare system delay in patients with ST-segment elevation myocardial infarction. Heart 102:1023–1028. [DOI] [PubMed] [Google Scholar]
- Przyklenk K, Bauer B, Ovize M, Kloner RA WP (1993) Regional ischemic ‘preconditioning’’ protects remote virgin myocardium from subsequent sustained coronary occlusion.’ Circulation 87:893–899. [DOI] [PubMed] [Google Scholar]
- Rahman IA, Mascaro JG SR et al. (2010) Remote ischemic preconditioning in human coronary artery bypass surgery: from promise to disappointment? Circulation 122:S53–S59. [DOI] [PubMed] [Google Scholar]
- Redington KL, Disenhouse T LJ et al. (2013) Electroacupuncture reduces myocardial infarct size and improves post-ischemic recovery by invoking release of humoral, dialyzable, cardioprotective factors. J Physiol Sci 63:219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redington KL, Disenhouse T SS et al. (2012) Remote cardioprotection by direct peripheral nerve stimulation and topical capsaicin is mediated by circulating humoral factors. Basic Res Cardiol 107:241. [DOI] [PubMed] [Google Scholar]
- Ren X, Wang Y JWK (2004) TNF-alpha is required for late ischemic preconditioning but not for remote preconditioning of trauma. J Surg Res 121:120–129. [DOI] [PubMed] [Google Scholar]
- Rentoukas I, Giannopoulos G KA et al. (2010a) Cardioprotective role of remote ischemic periconditioning in primary percutaneous coronary intervention: enhancement by opioid action. JACCCardiovascInterv 3:49–55. [DOI] [PubMed] [Google Scholar]
- Rentoukas I, Giannopoulos G KA et al. (2010b) Cardioprotective role of remote ischemic periconditioning in primary percutaneous coronary intervention: enhancement by opioid action. JACCCardiovascInterv 3:49–55. [DOI] [PubMed] [Google Scholar]
- Saxena P, Newman MAJ, Shehatha JS, Redington AN, Konstantinov IE (2010) Remote ischemic conditioning: evolution of the concept, mechanisms, and clinical application. J Card Surg 25:127–134. [DOI] [PubMed] [Google Scholar]
- Schoemaker RG van HCL (2000) Bradykinin mediates cardiac preconditioning at a distance. Am J Physiol Heart Circ Physiol 278:H1571–6. [DOI] [PubMed] [Google Scholar]
- Selvanayagam JB, Porto I CK et al. (2005) Troponin elevation after percutaneous coronary intervention directly represents the extent of irreversible myocardial injury: insights from cardiovascular magnetic resonance imaging. Circulation 111:1027–1032. [DOI] [PubMed] [Google Scholar]
- Serejo FC, Rodrigues LF Jr, da Silva Tavares KC de C, AC NJH (2007) Cardioprotective properties of humoral factors released from rat hearts subject to ischemic preconditioning. J Cardiovasc Pharmacol 49:214–220. [DOI] [PubMed] [Google Scholar]
- Sloth AD, Schmidt MR, Munk K, Kharbanda RK, Redington AN, Schmidt M, Pedersen L, Sørensen HT, Bøtker HE, Investigators C (2014) Improved long-term clinical outcomes in patients with ST-elevation myocardial infarction undergoing remote ischaemic conditioning as an adjunct to primary percutaneous coronary intervention. Eur Heart J 35:168–175 Available at: 10.1093/eurheartj/eht369. [DOI] [PubMed] [Google Scholar]
- Sloth AD, Schmidt MR, Munk K, Schmidt M, Pedersen L, Sorensen HT, Enemark U, Parner ET, Botker HE (2016) Cost-effectiveness of remote ischaemic conditioning as an adjunct to primary percutaneous coronary intervention in patients with ST-elevation myocardial infarction. Eur Hear journal Acute Cardiovasc care. [DOI] [PubMed] [Google Scholar]
- Sloth AD, Schmidt MR, Munk K, Schmidt M, Pedersen L, Toft Sørensen H, Bøtker HE, InvestigatorsBøttcherMKaltoftAKTerkelsenCJAndersenNHHansenTMTrautnerSLassen JFChristiansenEHKrusellLRKristensenSDThuesenLNielsenSSRehlingMNielsenTT C (2015) Impact of cardiovascular risk factors and medication use on the efficacy of remote ischaemic conditioning: post hoc subgroup analysis of a randomised controlled trial. BMJ Open 5:e006923 Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4390720/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensrud T, Li J DX et al. (2010) Pretreatment with the nitric oxide donor SNAP or nerve transection blocks humoral preconditioning by remote limb ischemia or intra-arterial adenosine. Am J Physiol Heart Circ Physiol 299:H1598–603. [DOI] [PubMed] [Google Scholar]
- Takaoka A, Nakae I, Mitsunami K, Yabe T, Morikawa S IT et al. (1999) Renal ischemia/reperfusion remotely improves myocardial energy metabolism during myocardial ischemia via adenosine receptors in rabbits: effects of ‘remote preconditioning’’.’ J Am Coll Cardiol 33:556–564. [DOI] [PubMed] [Google Scholar]
- Sivaraman JMJP V, Hausenloy DJ (2015) Remote ischaemic conditioning: cardiac protection from afar. Anaesthesia 70:732–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanezis AP, Arnold JR, Rodrigo G, Lai FY, Debiec R, Nazir S, Khan JN, Ng LL, Chitkara K, Coghlan JG, Hetherington SL, McCann GP, Samani NJ (2018) Daily remote ischaemic conditioning following acute myocardial infarction: a randomised controlled trial. Heart Available at: http://heart.bmj.com/content/early/2018/05/10/heartjnl-2018-313091.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopal V, Ludman A YD et al. (2009) “Conditioning” the heart during surgery. EurJCardiothoracSurg 35:977–987. [DOI] [PubMed] [Google Scholar]
- Verouhis D, Sorensson P, Gourine A, Henareh L, Persson J, Saleh N, Settergren M, Sundqvist M, Tornvall P, Witt N, Bohm F, Pernow J (2016) Effect of remote ischemic conditioning on infarct size in patients with anterior ST-elevation myocardial infarction. Am Heart J 181:66–73. [DOI] [PubMed] [Google Scholar]
- Wang TK, Stewart RA RT et al. (2013) Diagnosis of MI after CABG with high-sensitivity troponin T and new ECG or echocardiogram changes: relationship with mortality and validation of the universal definition of MI. EurHeart JAcuteCardiovascCare 2:323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhao L (2016) Calycosin ameliorates diabetes-induced cognitive impairments in rats by reducing oxidative stress via the PI3K/Akt/GSK-3beta signaling pathway. Biochem Biophys Res Commun 473:428–434. [DOI] [PubMed] [Google Scholar]
- Wei M, Xin P LS et al. (2011) Repeated remote ischemic postconditioning protects against adverse left ventricular remodeling and improves survival in a rat model of myocardial infarction. CircRes 108:1220–1225. [DOI] [PubMed] [Google Scholar]
- Weinbrenner C, Schulze F, Sarvary L SRH (2004) Remote preconditioning by infrarenal aortic occlusion is operative via delta1-opioid receptors and free radicals in vivo in the rat heart. Cardiovasc Res 61:591–599. [DOI] [PubMed] [Google Scholar]
- White SK, Frohlich GM SDM et al. (2014) Remote Ischemic Conditioning Reduces Myocardial Infarct Size and Edema in Patients With ST-Segment Elevation Myocardial Infarction. JACCCardiovascInterv. [DOI] [PubMed] [Google Scholar]
- White SK, Frohlich GM, Sado DM, Maestrini V, Fontana M, Treibel TA, Tehrani S, Flett AS, Meier P, Ariti C, Davies JR, Moon JC, Yellon DM, Hausenloy DJ (2015) Remote Ischemic Conditioning Reduces Myocardial Infarct Size and Edema in Patients With ST-Segment Elevation Myocardial Infarction. JACC Cardiovasc Interv 8:178–188 Available at: http://interventions.onlinejacc.org/content/8/1_Part_B/178.abstract. [DOI] [PubMed] [Google Scholar]
- Xu X, Zhou Y, Luo S, Zhang W, Zhao Y, Yu M, Ma Q, Gao F, Shen H, Zhang J (2014) Effect of remote ischemic preconditioning in the elderly patients with coronary artery disease with diabetes mellitus undergoing elective drug-eluting stent implantation. Angiology 65:660–666. [DOI] [PubMed] [Google Scholar]
- Yamanaka T, Kawai Y, Miyoshi T, Mima T, Takagaki K, Tsukuda S, Kazatani Y, Nakamura K, Ito H (2015) Remote ischemic preconditioning reduces contrast-induced acute kidney injury in patients with ST-elevation myocardial infarction: a randomized controlled trial. Int J Cardiol 178:136–141. [DOI] [PubMed] [Google Scholar]
- Yellon DM, Ackbarkhan AK, Balgobin V, Bulluck H, Deelchand A, Dhuny MR, Domah N, Gaoneadry D, Jagessur RK, Joonas N, Kowlessur S, Lutchoo J, Nicholas JM, Pauvaday K, Shamloll O, Walker JM, Hausenloy DJ (2015) Remote Ischemic Conditioning Reduces Myocardial Infarct Size in STEMI Patients Treated by Thrombolysis. J Am Coll Cardiol 65:2764–2765. [DOI] [PubMed] [Google Scholar]
- Yellon DM DJ (2003) Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev 83:1113–1151. [DOI] [PubMed] [Google Scholar]
- Young PJ, Dalley P GA et al. (2012) A pilot study investigating the effects of remote ischemic preconditioning in high-risk cardiac surgery using a randomised controlled double-blind protocol. Basic ResCardiol 107:1–10. [DOI] [PubMed] [Google Scholar]
- Zangrillo A, Musu M, Greco T, Di Prima AL, Matteazzi A, Testa V, Nardelli P, Febres D, Monaco F, Calabro MG, Ma J, Finco G, Landoni G (2015) Additive Effect on Survival of Anaesthetic Cardiac Protection and Remote Ischemic Preconditioning in Cardiac Surgery: A Bayesian Network Meta-Analysis of Randomized Trials. PLoS One 10:e0134264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SZ, Wang NF, Xu J, Gao Q, Lin GH BIC et al. (2006) kappa-Opioid receptors mediate cardioprotection by remote preconditioning. Anesthesiology 105:550–556. [DOI] [PubMed] [Google Scholar]


