Abstract
One of the primary therapeutic goals of modern cardiology is to design strategies aimed at minimizing myocardial infarct size and optimizing cardiac function following acute myocardial infarction (AMI). Patients with AMI who underwent reperfusion therapy display dysfunction of the coronary endothelium. Consequently, ischemic endothelial cells become more permeable and weaken their natural anti-thrombotic and anti-inflammatory potential. Ischemia-reperfusion injury (IRI) is associated with activation of the humoral and cellular components of the hemostatic and innate immune system, and also with excessive production of reactive oxygen species (ROS), the inhibition of nitric oxide synthase, and with inflammatory processes. Given its essential role in the regulation of vascular homeostasis, involving platelets and leukocytes among others, dysfunctional endothelium can lead to increased risk of coronary vasospasm and thrombosis. Endothelial dysfunction can be prevented by ischemic conditioning with a protective intervention based on limited intermittent periods of ischemia and reperfusion. The molecular mechanisms and signal transduction pathways underlying conditioning phenomena in the coronary endothelium have been described as involving less ROS production, reduced adhesion of neutrophils to endothelial cells and diminished inflammatory reactions. This review summarizes our current understanding of the cellular and molecular mechanisms regulating IRI-affected and -damaged coronary endothelium, and how ischemic conditioning may preserve its function.
Keywords: ischemia reperfusion injury, endothelium, inflammation, ischemic conditioning, cardioprotection
1. Introduction
The endothelium is defined as a cell monolayer covering the innermost apical surface of all blood and lymphatic vessels. Beyond its role as a physical and permeability barrier, endothelial cells (EC) can be anatomically divided into different phenotypes such as continuous, fenestrated and discontinuous endothelium, which are all functionally involved in a variety of tissue- and organ-specific functions contributing to vascular homeostasis (Kladakis and Nerem, 2004). Due to the specific expression of anti-inflammatory and anti-aggregatory molecules, quiescent EC provide a non-thrombogenic luminal surface and also actively contribute to the regulation of vascular tone, inflammation, and metabolism (de Agostini et al., 1990; Preissner, 1990; van Hinsbergh, 1997; Kladakis and Nerem, 2004; Urbich and Dimmeler, 2004). At particular sites in the vascular tree, endothelium plays a central role in innate immunity as well, allowing monocyte and neutrophil rolling and diapedesis to occur (de Agostini et al., 1990; van Hinsbergh, 1997; Urbich and Dimmeler, 2004; Rajendran et al., 2013). Stress-, damage- or metabolite-related dysfunctions of EC (Chavakis and Preissner, 2005) contribute to a variety of diseases in a wide range of organs such as heart, lung, liver or kidney (Rajendran et al., 2013). The most prominent naturally occurring endothelial dysfunction is the life-long exposure of non-laminar fluid shear stress to blood vessels at predestined sites (such as bifurcations) in the circulatory system. As a consequence, already at a very young age all individuals present early signs of atherogenesis, such as fatty streaks, that may develop into atherosclerotic plaques at a later age, depending on the person’s genetic make-up and lifestyle (Chatterjee, 2018).
Following the treatment of such atherosclerosis-related arterial occlusions in the context of myocardial infarction, the underlying process of ischemia-reperfusion (IR) induces deleterious effects not only on large vessels but also in the microcirculation of the heart. Several studies indicate that EC are more sensitive to IR than cardiomyocytes and are critical mediators of cardiac IR-injury (IRI) (Tsao et al., 1990; Lefer et al., 1991; Richard et al., 1994; Singhal et al., 2010; Hausenloy and Yellon, 2016). In this context, endothelial dysfunction implies diminished production or availability of nitric oxide (NO), an imbalance in the contribution of endothelium-derived relaxing molecules (Michiels, 2003) and a deficiency of essential vasodilators to provide control of vascular tone and blood pressure, such as prostacyclin (PGI2) (Pearson, 2000). After summarizing some of the risk and initiation factors of endothelial dysfunction, this review will focus on the protective endogenous strategy of ischemic conditioning and its potential beneficial effects on vascular homeostasis.
2. Endothelial dysfunction upon ischemia-reperfusion (IR)
Multiple mechanisms, including inflammation, increased occurrence of ROS, cellular apoptosis, increased vasoconstrictor but decreased vasodilator production and vascular remodeling are involved in the damage of EC during IR (Richard et al., 1994). Ischemia is characterized in general by the interruption of oxygen supply, either in a specific tissue or in a whole organ. Importantly, cardiovascular diseases are often initiated by ischemic episodes, contributing to the main cause of death in developed countries (Remme, 2000; Celermajer et al., 2012).
The endothelium seems to be particularly sensitive to conditions of ischemia and posterior reperfusion, because these conditions promote e.g. hypoxia-dependent changes in gene expression and permeability properties of EC, contributing to endothelial dysfunction (Harrison, 1997) and the pathogenesis of cardiovascular disorders including myocardial ischemia (Shimokawa and Yasuda, 2008). Other responses of dysfunctional EC include the chronic imbalance between ROS versus NO, the continued expression of adhesion receptors such as E-selectin, intercellular cell adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and receptor for advanced glycation endproducts (RAGE) as well as the reduction of acetylcholine production (Gaboury et al., 1993; Chavakis et al., 2003; Quagliaro et al., 2005). Consequently, the imbalance in favor of adhesive and thrombogenic properties of the endothelium renders it more prone for dysregulated leukocyte attachment, increased oxidative stress, thereby triggering inflammatory, thrombotic and necrotic processes (Heitzer et al., 2001; Galle et al., 2003). As already indicated, a major irreversible outcome and consequence of dysfunctional endothelium is associated with atherosclerosis and vascular diseases (Poredos, 2002b, a; Widmer and Lerman, 2014).
2.1. Cytotoxicity caused by Ca2+ions.
The control of cellular Ca2+ levels in arterial EC and smooth muscle cells is an integral process for the control of vascular tone, including EC-dependent vasodilation activities (Lefer et al., 1991; Sandow et al., 2012). Several studies have shown that IRI leads to EC damage in coronary arteries by disturbance of Ca2+ homeostasis (Karasawa et al., 1991; Lefer et al., 1991; Lucchesi, 1993; Vinten-Johansen et al., 1999; Kimura et al., 2000; Ladilov et al., 2000; Symons and Schaefer, 2001; Kumar et al., 2007). A combination of anoxia with extracellular acidosis (pH 6.4) results in excessive accumulation of Ca2+ in the cytosol of EC mainly due to the leak of Ca2+ from the endoplasmic reticulum. The ischemic factors (low pH and lactate) leading to cytosolic acidosis cause intracellular Ca2+ overload in EC (Ladilov et al., 2000), while anoxia and glucose deprivation play only a minor role in this context (Lefer et al., 1991). Uncontrolled depletion of Ca2+ in the ER may lead to ER-stress, followed by cleavage of ER-bound caspase-12 (Kumar et al., 2007) and caspase-3, and the activation of apoptosis through cytochrome-c (Ladilov et al., 2000; Borutaite et al., 2003; Kumar et al., 2007). By increasing intracellular Ca2+ stores, store-operated Ca2+ entry (SOCE) mainly regulates the cytosolic Ca2+ concentration of EC (Putney, 1986). Following ischemia, extracellular, but no intracellular, acidification alter EC dysfunction by suppressing SOCE (Asai et al., 2009). Additionally, unregulated influxes of Ca2+, secondary to plasma membrane damage and disruption of ion homeostasis, activate intracellular lipases, proteases and endonucleases that are important in the apoptotic signaling pathway. During reperfusion, EC increase their permeability, and an inflammatory signal transduction pathway, initiated by the oxidation of specific cellular modulators, alters the EC cytoskeleton and down-regulates natural antithrombotic and antiinflammatory processes, thus increasing membrane permeability (Higginson et al., 1982; Mullane et al., 1984; Hinshaw et al., 1989; Mertens et al., 1990; Geeraerts et al., 1991; Patel et al., 1991; Natarajan et al., 1993; Hastie et al., 1997; Ladilov et al., 2000; Kumar et al., 2007). Moreover, in in vitro studies with coronary EC, acidic preconditioning conferred protection against ischemia-apoptosis induction, associated with over-expression of the anti-apoptotic protein Bcl-XL (Kumar et al., 2008). Further investigations of Ca2+ fluxes in coronary EC may lead to the characterization of new therapeutic approaches for the treatment of chronic inflammation and coronary artery disease (Steppich et al., 2009; Stepien et al., 2012; Suades et al., 2015).
2.2. Oxidative stress.
Increased ROS levels have been generally attributed to IRI (Hinshaw et al., 1989; Lucchesi, 1993; Gaboury et al., 1994; Szocs, 2004; Therade-Matharan et al., 2005; Hernandez-Resendiz et al., 2018), whereby xanthine oxidase, NADPH oxidase, and the mitochondrial electron transport chain are the most frequently implicated sources of ROS in myocardium exposed to IR. The rapid burst of oxygen-derived free radicals during reperfusion coincides with the time course of progression in endothelial dysfunction (Karasawa et al., 1991). For example, exposure of EC for 6h to hypoxia followed by 45 min of reoxygenation increased the generation of superoxide anion (O2•−) in EC (Tsao et al., 1990; Kimura et al., 2000), whereby cytosolic xanthine oxidase and the mitochondrial electron transport chain (complexes I and III) provide the primary sources of endothelial superoxide anion. Since NADPH oxidase (NOX) 4, one of the NOX isoforms, is present in EC and co-localizes with mitochondria, it may be considered as a source of ROS as well (Szocs, 2004; Dymkowska et al., 2014).
Moreover, as a result of an excess of superoxide anion, hydroxyl (OH−) and peroxynitrite anions (ONOO−) are also produced by EC, provoking NO inactivation. As a consequence, decreased vasorelaxation and eventually vasoconstriction are observed. Peroxynitrite is a free radical that mediates lipid peroxidation and uncoupling of NO synthase (NOS). Usually, O2•− can be dismutated to H2O2, either spontaneously or by superoxide dismutase (SOD). The exposure of EC to high levels of H2O2 (<500 M) increases the intracellular Ca2+ concentration, the expression of the aforementioned adhesion receptors (Szocs, 2004; Therade-Matharan et al., 2005) as well as complement activation (Patel et al., 1991; Gaboury et al., 1994) that collectively induce neutrophil attachment to the dysfunctional vascular endothelium (Lucchesi, 1993). Needless to say, that the damage of EC by oxidative stress is reduced or prevented by ROS scavengers and antioxidants.
Activation of endothelial cells by oxidative stress thereby promotes inflammation: Following IRI, activated EC express P-Selectin, E-Selectin and intercellular adhesion molecules (ICAMs), boosting the recruitment of neutrophils and contributing to cytokine production as major inflammatory reactions (Winn et al., 1997). Oxidative stress also induces the activation of NF-kB, playing a key role in endothelial apoptosis via the down-regulation of Bcl-2, Bax translocation and p53 upregulation (Aoki et al., 2001).
2.3. Inhibition of nitric oxide synthase (NOS).
Both, decomposition of NO during reperfusion and inhibition of NO synthesis, can increase leukocyte adherence to the venular wall. Tetrahydrobiopterine (BH4) is a cofactor, which is critical for NO production by regulating endothelial NOS (eNOS) activity. During IR, free radicals can oxidize BH4, causing a fall in the BH4/eNOS ratio, thereby uncoupling eNOS. In such a scenario, uncoupled eNOS behaves like NADPH oxidase, increasing the production of O2•−, H2O2 and OONO− to induce cytotoxicity and inflammation (Figure 1). In EC, eNOS competes with arginase for the substrate L-arginine, whereby the activity of arginase, the primary L-arginine-consuming enzyme, has been reported to become elevated following IRI (Hein et al., 2003). Since NO is a potent inhibitor of neutrophil activation and adhesion, the decreased levels of NO may lead to the development of an acute inflammatory response (Laude et al., 2001; Ong et al., 2018).
Figure 1: Overview of endothelial cell responses towards acute myocardial infarction.
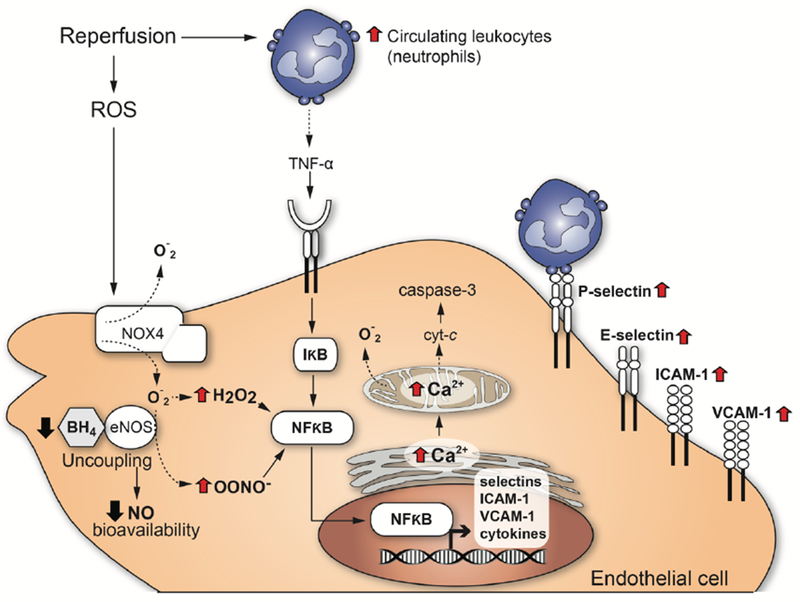
This scheme depicts the reperfusion-induced damage of endothelial cells following acute myocardial infarction.
3. Endothelial dysfunction and inflammation
The stages of acute inflammation (as part of the innate immunity response) in a blood vessel are vasodilation, increased permeability of the microvasculature, and vascular stasis (Szmitko et al., 2003). As EC undergo cytoskeletal changes that disrupt junctions in venules and capillaries, a delayed cellular response is observed in this process, starting about 6h after the initial stimulus and lasting for some days. In response to risk factors, such as IR, the endogenous protection mechanisms (anti-oxidant, anti-inflammatory, etc.) of the coronary endothelium begin to break down. The already indicated production of ROS and the reduction of NO provoke the expression of inflammatory cytokines such as interleukin-6 (IL-6) (Verma et al., 2002) or monocyte chemoattractant protein-1 (MCP-1) as well as the upregulation of VCAM-1 on EC. Upon EC activation, the first luminally expressed adhesion molecule is P-selectin (Vinten-Johansen et al., 1999), which is derived from degranulation of Weibel-Palade bodies, mediating the initial rolling of leukocytes along the vessel wall surface. After 4-6h of reperfusion, de novo expressed E-selectin, ICAM-1 and RAGE appear to induce the firm adhesion of leukocytes via there activated ß2-integrins, preparing these inflammatory cells for their transmigration across the dilated endothelium into the myocardium (Ma et al., 1992; Ampofo et al., 2017). Expression of endothelial ICAM-1, VCAM-1 and RAGE are considered as an essential process for providing firm adhesion and transmigration of neutrophils and monocytes to the subendothelial space in blood vessels, contributing to the initial steps of atherosclerotic lesion development as well (Ziche et al., 1994; Deem and Cook-Mills, 2004; Nawroth et al., 2005; Lopez-Diez et al., 2016).
The expression of the aforementioned adhesion molecules is further induced by proinflammatory cytokines such as Interleukin-1 (IL-1) or tumor necrosis factor-α (TNF-α), which have been reported to be expressed in IR within minutes (Cardozo et al., 2005; Schmidt et al., 2006). Once adhered to the inflamed endothelium, monocytes cross the arterial wall between EC and transmigrate into the neo-intimal space (Vinten-Johansen et al., 1999).
It is well known that early after reperfusion, IR induces the accumulation of platelets via P-selectin - ligand interactions on the dysfunctional (activated), but intact endothelium in the post-ischemic microvasculature (Coulter et al., 2000; Mirabet et al., 2002). The initial expression of P-selectin by the activated endothelium can be considered as an early biomarker for the IR-induced platelet-EC interactions (Massberg et al., 1998). The first attachment of platelets to the still intact but activated endothelium during IR is mediated by platelet glycoprotein Iba as a ligand for endothelial P-selectin and a receptor for von Willebrand factor, the latter being released from endothelial Weibel-Palade bodies as well (Massberg et al., 1998; Romo et al., 1999). Following adhesion, platelet activation is potentiated by the release of soluble platelet agonists at sites of EC injury, finally resulting in perpetuated granule release and the formation of platelet aggregates (Maiocchi et al., 2018). Among the platelet agonists are ROS as well as platelet granule products, including proinflammatory cytokines, chemokines, thromboxane A2, leukotrienes or proinflammatory lysolipids (Marcus, 1979; Leo et al., 1997). These events, combined with the release of vasoconstricting molecules from platelets, further exacerbate the physical obstruction of the microvasculature.
As an interventional strategy, NO was found to mediate a cardio-protective effect of a number of common clinical strategies, such as preconditioning, postconditioning and remote ischemic preconditioning, further supporting the concept that targeting of ROS, NOS and inflammatory response mechanisms may provide myocardial protection under conditions of IRI.
4. Influence of ischemic conditioning on the coronary endothelium
Brief non-lethal episodes of IR are known to protect against the deleterious effects of a sustained lethal time span of IR. This phenomenon is known as “ischemic conditioning” and has been observed in the heart and other organs (Thielmann et al., 2013; Meybohm et al., 2015; Cabrera-Fuentes et al., 2016a; Cabrera-Fuentes et al., 2016b; Hausenloy et al., 2016). Although several interrelated molecular mechanisms are operative here, ischemic conditioning protects the heart against IRI via a culminating “reperfusion injury signaling kinase” (RISK) pathway. The RISK pathway has been extensively studied in cardiomyocytes (Frohlich et al., 2013) and hepatocytes (Carini et al., 2001; Teoh et al., 2003; Hausenloy and Yellon, 2004).
4.1. Ischemic preconditioning.
One of the most promising avenues of research into potential therapeutic treatments for IRI is ischemic preconditioning (IPC). Several studies have demonstrated that IPC has direct effects on tissue cells, making them resistant to ischemic damage but also preventing the characteristic vascular dysfunction at the arteriolar, venular and capillary levels (DeFily and Chilian, 1993; Bouchard and Lamontagne, 1996; Kaeffer et al., 1996; Thourani et al., 1999; Kharbanda et al., 2001). Upon application of IPC, surgery studies in pediatric and adult patients revealed a significant reduction of tissue damage, indicated by decreased serum levels of troponin I and creatinine kinase KB (Botker et al., 2010). Likewise, other reports demonstrated a decrease of angina episodes, reduced ST deviation on the electrocardiogram and less severe arrhythmias following IPC (Jenkins et al., 1997; Sloth et al., 2014). However, specific mechanisms of IPC at the cellular and molecular level are not well understood, and most of the studies prior to or after preconditioning using a clinical approach are focused on cardiomyocyte pathophysiology.
Acute IP is operative if the time interval between a brief stimulus of preconditioning and the posterior ischemic insult lasts less than 2h (Korthuis and Gute, 1997). The second phase of protection named “delayed preconditioning” or “ischemic tolerance” becomes important if the time period between insults is longer than 24h (Korthuis and Gute, 1997). There are differences between both types of protection; the acute preconditioning is too short to be dependent on gene expression and cellular protein synthesis, while delayed preconditioning necessarily includes phenomena of de novo protein synthesis (Downey et al., 1994; Korthuis and Gute, 1997).
4.2. Cellular mechanisms in the acute preconditioning phase.
Upon the acute phase of IP, activation of PKC in cardiomyocytes induces phosphorylation of effector molecules that may contribute to cellular protection. These factors are associated with adenosine receptors via their pertussis-sensitive G proteins like ATP-sensitive potassium channels (KATP) and can contribute to protective effects of preconditioning by triggering the mKATP/ROS pathway (Heurteaux et al., 1995; Korthuis and Gute, 1997). The importance of KATP channels in this context is supported by experimental studies using respective agonists and antagonists. Although the protective effects involve KATP channels in both, plasma and mitochondrial membranes, the underlying mechanisms are still unclear (Auchampach and Gross, 1993; Jerome et al., 1995). In addition to its influence on KATP channels within the context of protective preconditioning, PKC can induce 5’ nucleotidase to increase adenosine levels during sustained ischemia. This process mobilizes cellular depots of EC metabolic energy and prevents leukocyte adherence and subsequent inflammatory reactions (Kitakaze et al., 1994).
4.3. Cellular responses upon delayed IP.
Cardiac protection can also be achieved if the ischemic episode occurs within 24h after the initial brief stimulus of ischemia or IP. This delayed response is more an adaptive response and a consequence of the induction of gene expression and de novo protein synthesis of metabolic enzymes and heat shock proteins following the first brief ischemic insult (Korthuis and Gute, 1997). In post-ischemic phases, induction of gene and protein expression in leukocytes and EC involves activation of PKC and other kinases that become translocated to the nucleus to regulate nuclear transcription factor activities (Kitakaze et al., 1994; Korthuis and Gute, 1997). Here, the nuclear factor κB (NF-κB) is a central transcription factor in delayed responses of IP at least in EC and it is considered to be the key factor for the transcription of post-ischemic inflammatory mediators. Importantly, if NF-κB translocation is blocked, the delayed phase of IP is also inhibited (Yellon and Baxter, 1995).
When EC and cardiomyocytes are exposed towards short ischemic episodes, they respond with an increased production of NO (Lu et al., 1993; Yamashita et al., 1994). NO also plays a central role during delayed responses due to a decrease in oxidative stress and cell adhesion (Korthuis and Gute, 1997). In an autocrine fashion, eNOS-produced NO is a mediator of EC protection upon delayed IP. Administration of the non-selective NOS inhibitor L-NAME prior to prolonged myocardial IR did abolish the protective effect of IPC on the coronary endothelium, indicating that NO corresponds with the EC-dependent reactions of delayed IPC (Laude et al., 2003). In addition to the influence of NO during oxidative stress, the delayed response of IP may enhance the activity of antioxidant enzymes such as SOD, catalase or glutathione peroxidase, thereby potentiating the initial protective effect of NO (Yellon and Baxter, 1995).
When arteries are subjected to IR, IPC has been shown to prevent endothelium-dependent relaxing responses to acetylcholine (Kaeffer et al., 1996). In most of such studies, the protection by IPC occurs during the reperfusion period, when arterioles are thought to be most vulnerable to dysfunction. IPC reduces the expression of EC adhesion molecules, resulting in reduced adhesion of neutrophils to the endothelium and hence, lowering the inflammatory reactions. In response to myocardial IR, circulating and heart-derived levels of TNF-α increase within minutes, mostly derived from macrophages, monocytes and mast cells. Moreover, IPC has been shown to decrease cardiac as well as circulating TNF-α levels during sustained ischemia and thereby reduces myocardial infarct size in rabbits (Meldrum et al., 1998; Belosjorow et al., 2003). In another report, IPC reduced the expression of ß2-integrins on neutrophils in preconditioned human subjects, and this observation could be linked to decreased EC injury (Kharbanda et al., 2001).
Another adaptive mechanism upon IPC is the increased biosynthesis and liberation of heat shock proteins, whereby this family of intracellular proteins may normally control cell metabolism and maintain structure and function of important proteins during stress. Nevertheless, the underlying mechanisms and signaling pathways are poorly understood (Marber and Yellon, 1996; Gray et al., 1999). The possibility to reproduce this phenomenon in human subjects may allow the identification of related (circulating) biomarkers and pathophysiological parameters by testing pharmacological approaches to prevent IRI in cardiac patients.
4.4. Ischemic Postconditioning.
Ischemic postconditioning (IPost) was first reported in dogs, and it is performed after the onset of reperfusion. In anesthetized open-chest animals, the left anterior descending artery was occluded for 1h and reperfused for 3h. In controls, there was no intervention. In pre-conditioning (precon), the LAD was occluded for 5 min and reperfused for 10 min before the prolonged occlusion. In post-conditioning (post-con), at the start of reperfusion, three cycles of 30-s reperfusion and 30-s LAD reocclusion preceded the 3h period of reperfusion. Infarct size was significantly less in the pre-con and post-con groups compared with controls (Zhao et al., 2003), however, hardly any differences were reported in the reduction of infarct sizes between animals treated with precon or post-con protocols, respectively (Donato et al., 2007). These results were confirmed in other vertebrate models such as rabbits, rats, pigs and mice (Schwartz and Lagranha, 2006; Gomez et al., 2007; Skyschally et al., 2009). Since the model algorithm is based on the delay at the first re-occlusion time point and the duration of the ischemic-reperfusion stimulus (Skyschally et al., 2009), caution should be taken for interpretation of the model algorithm used in IPost due to its high variability. Protection conferred by IPost is associated with the improvement of EC function, reduction of inflammation and necrosis (Engelman et al., 1995; Kin et al., 2004).
IPost can also protect EC from IRI via phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) activation (Zhang et al., 2007). Respective investigations of IRI in EC of human subjects confirmed that IPost inhibits the opening of the mitochondrial permeability transition pore (mPTP) by preventing KATP channel activation, leading to cellular protection from reperfusion injury (Okorie et al., 2011; Ong et al., 2015). The perturbations of coronary EC can be analyzed by brachial artery ultrasound (Lind et al., 2005), demonstrating that IPost may protect the coronary endothelium. Moreover, it has been shown that the vasodilation function of endothelium was improved in patients by IPost (Ma et al., 2006).
An important phenomenon for explaining IPost-associated protection is the increase in NO production and the reduction of the “no-reflow” phenomenon (Schwartz and Kloner, 2012). The no-reflow phenomenon is defined as the reperfusion failure at the microvascular level after primary percutaneos coronary intervention (PCI) in patients with ST-segment elevation myocardial infarction (STEMI) (Ramjane et al., 2008; Bouleti et al., 2015; Gupta and Gupta, 2016). This mechanism is a partial limitation of microcirculatory blood flow after the onset of reperfusion, despite the elimination of vascular occlusion. No-reflow is caused by vascular spasms, EC damage, sarcolemmal bubbles in the endothelium and by smooth muscle migration (Rajendran et al., 2013). Histologic analysis of the no-reflow microvasculature area shows endothelial swelling with a general loss of pinocytotic vesicles, an evidence for endothelial disruption (Kloner, 2011). Applying long periods of preconditioning, capillary no-reflow is attenuated, by a mechanism involving KATP channels (Jerome et al., 1995). Some studies showed that IPost reduces no-reflow, thereby improving the blood flow in the microcirculation. Interestingly, hypercholesterolemia may significantly reduce this beneficial effect (Reffelmann and Kloner, 2006; Zhao et al., 2007).
4.5. Remote ischemic preconditioning (RIP).
After the discovery and the medical development of the indicated conditioning procedures to directly protect the target organ, it was demonstrated that the ischemic conditioning stimulus could be applied in a non-invasive way (such as by a blood pressure cuff) and distant from the organ to be targeted (Oxman et al., 1997). Against this background, in the first human study, endothelial IRI of the forearm was induced by 20 min of upper limb ischemia followed by reperfusion. Remote preconditioning was induced by three 5 min cycles of ischemia of the contralateral limb, whereby venous occlusion plethysmography was used to assess forearm blood flow in response to acetylcholine at baseline and 15 min after reperfusion. Interestingly, the response to acetylcholine was significantly attenuated in the control group after 15 min reperfusion, but remote preconditioning prevented this reduction (Kharbanda et al., 2002). This procedure is designated as remote ischemic conditioning (RIC), whereby brief episodes of ischemia and reperfusion applied in a particular organ or vascular bed may confer protection in a remote tissue and organ exposed to IRI (Heusch et al., 2015).
According to two recent randomized trials, RIP improves long term clinical prognosis after primary percutaneous coronary intervention (PPCI) (Davies et al., 2013; Sloth et al., 2014). Nevertheless, a prospective randomized controlled clinical multi-centric trial named “Effect of Remote Ischemic Conditioning on Clinical Outcomes in STEMI Patients Undergoing PPCI” (CONDI2/ERIC-PPCI) is currently investigating whether RIC can reduce cardiac death and hospitalization for heart failure at 1 year in 5,200 patients, presenting with a ST-elevation myocardial infarction (STEMI) and treated by percutaneous coronary intervention (PPCI). The trial is planned to be completed in December 2019 (online reference https://clinicaltrials.gov/ct2/show/NCT02342522).
RIC prior to primary percutaneous coronary intervention significantly improves EC function in patients with AMI (Manchurov et al., 2014). Moreover, it has been demonstrated in animal studies that whole blood transfusion and transfer of coronary effluent from one organism to another is sufficient to transfer cardio-protection from one RIP-treated animal to a naive one. This phenomenon has been replicated in several species, supporting the role of humoral factors in RIC-mediated cardio-protection (Dickson et al., 1999). In particular, increased levels of adenosine in coronary effluent after RIC as well as neuro-humoral factors and other biochemical and electrical responses were made responsible for the RIP cardio-protective signaling system (Patel et al., 2002; Weinbrenner et al., 2004; Gross et al., 2011; Donato et al., 2013). In RIP, the activation of peripheral sensory fibers appear to activate PKCγ in a similar fashion as was observed in other ischemic conditioning techniques (Gross et al., 2013).
Besides the non-invasive procedure used for inducing RIC, it is able to confer cardio-protection when applied at different time points in relation to the induction of the main IR episode. This characteristic is also beneficial in terms of clinical outcomes (Sivaraman et al., 2015). RIC could be used immediately or 12-24h before a long ischemic episode (RIP), after ischemia but before reperfusion, at the moment of reperfusion (RIP) or 15 min following reperfusion (delayed IPC) (Sivaraman et al., 2015).
Some recent studies in the context of RIC revealed the existence of an as yet uncharacterized humoral factor involved in cardio-protection (Serejo et al., 2007; Shimizu et al., 2009). This humoral factor likely has the nature of a protein with a size between 3.5 and 30 kDa, whereby the vasodilator peptide bradykinin could be excluded (Serejo et al., 2007; Shimizu et al., 2009). Some proteomic studies indicated differentially expressed proteins in plasma derived from patients exposed to RIC as compared to controls. Such putative proteins appear to be associated with inflammatory responses, hemostasis and lipid transport, supporting the hypothesis that complex interactions of molecular pathways contribute to RIC in cardio-protection (Hepponstall et al., 2012). In particular, as in other types of conditioning procedures, an increase in adenosine levels as well as the contribution of the NOS system, of stromal cell-derived factor-1 α (SMC-1a), interleukin 10, or microRNA-144 were found (Cai et al., 2012; Davidson et al., 2013; Li et al., 2014), whereby such molecules possibly play a role as local triggers of KATP channels and as NO effectors (Schmidt et al., 2007). Nevertheless, each of these factors alone is insufficient to promote the entire phenomenon of cardio-protection.
Recently, the role of the extracellular RNA (exRNA) / RNase1 system in cardiac I/R injury has achieved an increased attention (Cabrera-Fuentes et al., 2014; Cabrera-Fuentes et al., 2016a; Cabrera-Fuentes et al., 2016b). exRNA induces vascular hyper-permeability by increasing intracellular calcium ions in EC (Balint et al., 2014), and interacts with VEGF to initiate VEGF-mediated signal transduction through neuropilin-1, VEGF-R2 phosphorylation, activation of phospholipase C (PLC) and intracellular release of Ca2+ (Fischer et al., 2009). Vascular EC constitutively produce extracellular, circulating RNase1 which has different functions in EC responses (Fischer et al., 2011). In a recent clinical study, it has been shown that plasma levels of RNase1 increased after cardiac surgery subjected to RIP, while there was a significant reduction of exRNA and the pro-inflammatory mediator TNF-α, suggesting a role of vascular RNasel as a potential mediator of RIP for cardio-protection (Cabrera-Fuentes et al., 2015).
4.6. Methodology used to measure coronary EC function.
The methodologies to measure coronary EC dysfunction were designed to be non-invasive to achieve higher security margin and better acceptance from the patients. At the same time, determinations must reflect the actual state of EC function based on our knowledge of endothelial physiology and activation, described before.
Indirect assessment:
Some studies have used the assessment of vasoconstriction in response to acetylcholine that consists in flow-mediated vasodilation in brachial artery by ultrasound to detect coronary artery EC dysfunction and coronary artery disease as a surrogated measure (Anderson et al., 1995; Corretti et al., 2002). One advantage of this technique is that observer can measure EC function in response to a stimulus, however, such effect is indirect and brachial condition is assumed to be the same as coronary artery which maybe be not necessarily accurate. Another disadvantage is the operator-dependent variation and the wide range of reported normal values (Berry et al., 2000). However, brachial function is proposed as a strong predictor of coronary function in statistically significant manner, showing that a positive predictive value of abnormal brachial dilation (<3%) in predicting coronary EC dysfunction is 95% (Anderson et al., 1995). Both approaches of indirect assessment are based in endothelium-dependent relaxation as a result mainly of NO bioavailability. Nevertheless, other vascular mediators can be involved such as bradykinin.
Direct assessment:
Direct assessment is considered the “gold standard” and it determines the change in coronary artery diameter, coronary blood flow and coronary vascular resistance to an intracoronary infusion of acetylcholine (Hasdai and Lerman, 1999). In coronary arteries with a quiescent endothelium coverage, the response to intracoronary acetylcholine is epicardial and microvascular dilation results in an increase in coronary blood flow (Bonetti et al., 2003). When the EC lining is disrupted, activated or dysfunctional, intracoronary acetylcholine induces paradoxical responses, for example, vasoconstriction and a decrease in coronary blood flow. The response to acetylcholine serves as a marker for the bioavailability of NO; an abnormal response suggests a lack of NO bioavailability such as for endothelial dysfunction.
Magnetic resonance imaging (MRI) has been used for the direct assessment of the coronary endothelial dysfunction (Bulluck et al., 2017) and allows to assess coronary endothelium responses to pharmacological stimuli. Patients need to be prepared for the imaging session through the administration of vasoactive medications, selected according to the respective study and the individual patient (Hays et al., 2012). Some authors have reported that MRI is performed after an angiography to localize vascular structures more accurately (Hays et al., 2012; Bulluck et al., 2018).
5. Conclusions
A particular stimulus to mediate conditioning of organs such as the heart can act at different biological levels to engage molecular programs that produce durable (>24h) tolerance against ischemic injury. The cardio-protection conferred by ischemic conditioning is a complex system of successive events, and the mechanisms still remain to be resolved. However, we can enumerate three necessary steps to explain cellular responses after ischemic conditioning (Figure 2): (1) Generation of a cardio-protective signal in conditioned tissue with an anticipated role for EC signaling; (2) transport pathways via circulatory and nervous systems that confer the cardio-protective signal from the conditioned tissue to the heart; (3) activation of cellular signaling pathways in the heart to initiate protection. (1) and (2) involve interactions between neural and circulating factors, whereby the endothelium appears to play a crucial role in both steps (Sivaraman et al., 2015). The central role played by dysfunctional EC in promoting thrombus formation and inflammatory responses during IRI is well recognized. There is considerable progress in understanding the role of dysfunctional EC as a potential therapeutic target for myocardial protection as well. The development of approaches to control EC activation and focussing on the RISK pathway in EC may contribute to a better understanding of IRI and to provide new opportunities for its treatment or prevention.
Figure 2. Cardio-protective mediators and molecular mechanisms that are activated by ischemic conditioning in endothelial cells.
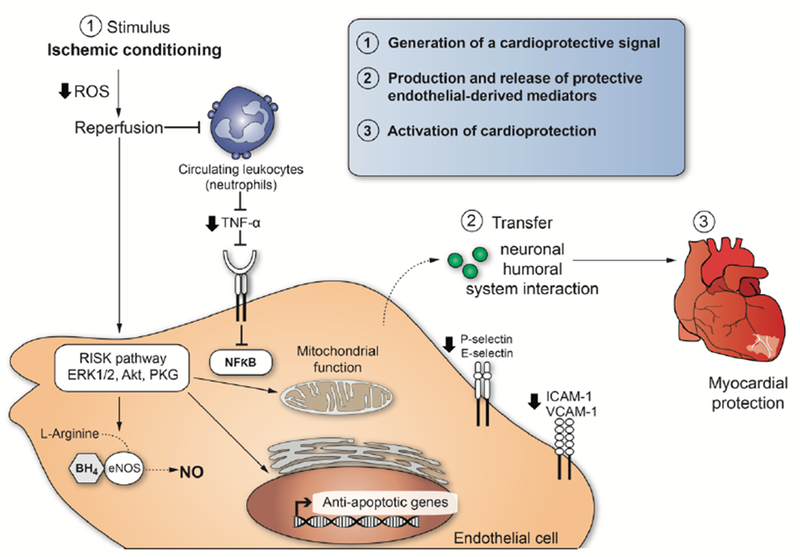
The illustration enumerates three necessary steps to explain cellular responses after ischemic conditioning induced cardioprotection.
Acknowledgments
Funding
SHR is funded by the Singapore Ministry of Health’s National Medical Research Council under its Open Fund – Young Individual Research Grant (OF-YIRG) – 0078/2018. This article is based upon work from COST Action EU-CARDIOPROTECTION CA16225 supported by COST (European Cooperation in Science and Technology). Part of the original work presented by HCF and KTP was supported by the Russian Government Program for competitive growth of Kazan Federal University, Kazan (Russian Federation), by the SHF-Foundation Research Grant (SHF/FG657P/2017) and by the von Behring-Röntgen-Foundation (Marburg, Germany). This work was supported by the European Cooperation in Science and Technology (COST Action CA16225 / EU-CARDIOPROTECTION). This project was also funded by NIH grant R01HL81863 to WAB.
Footnotes
Conflicts of interest
The authors declare that they have no conflicts of interest.
References
- Ampofo E, Lachnitt N, Rudzitis-Auth J, Schmitt BM, Menger MD, Laschke MW (2017) Indole-3-carbinol is a potent inhibitor of ischemia-reperfusion-induced inflammation. J Surg Res 215:34–46. [DOI] [PubMed] [Google Scholar]
- Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D, Lieberman EH, Ganz P, Creager MA, Yeung AC, et al. (1995) Close relation of endothelial function in the human coronary and peripheral circulations. Journal of the American College of Cardiology 26:1235–1241. [DOI] [PubMed] [Google Scholar]
- Aoki M, Nata T, Morishita R, Matsushita H, Nakagami H, Yamamoto K, Yamazaki K, Nakabayashi M, Ogihara T, Kaneda Y (2001) Endothelial apoptosis induced by oxidative stress through activation of NF-kappaB: antiapoptotic effect of antioxidant agents on endothelial cells. Hypertension 38:48–55. [DOI] [PubMed] [Google Scholar]
- Asai M, Takeuchi K, Saotome M, Urushida T, Katoh H, Satoh H, Hayashi H, Watanabe H (2009) Extracellular acidosis suppresses endothelial function by inhibiting store-operated Ca2+ entry via non-selective cation channels. Cardiovasc Res 83:97–105. [DOI] [PubMed] [Google Scholar]
- Auchampach JA, Gross GJ (1993) Adenosine A1 receptors, KATP channels, and ischemic preconditioning in dogs. The American journal of physiology 264:H1327–1336. [DOI] [PubMed] [Google Scholar]
- Balint Z, Zabini D, Konya V, Nagaraj C, Vegh AG, Varo G, Wilhelm I, Fazakas C, Krizbai IA, Heinemann A, Olschewski H, Olschewski A (2014) Double-stranded RNA attenuates the barrier function of human pulmonary artery endothelial cells. PLoS One 8:e63776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belosjorow S, Bolle I, Duschin A, Heusch G, Schulz R (2003) TNF-alpha antibodies are as effective as ischemic preconditioning in reducing infarct size in rabbits. Am J Physiol Heart Circ Physiol 284:H927–930. [DOI] [PubMed] [Google Scholar]
- Berry KL, Skyrme-Jones RA, Meredith IT (2000) Occlusion cuff position is an important determinant of the time course and magnitude of human brachial artery flow-mediated dilation. Clinical science 99:261–267. [PubMed] [Google Scholar]
- Bonetti PO, Lerman LO, Lerman A (2003) Endothelial dysfunction: a marker of atherosclerotic risk. Arteriosclerosis, thrombosis, and vascular biology 23:168–175. [DOI] [PubMed] [Google Scholar]
- Borutaite V, Jekabsone A, Morkuniene R, Brown GC (2003) Inhibition of mitochondrial permeability transition prevents mitochondrial dysfunction, cytochrome c release and apoptosis induced by heart ischemia. J Mol Cell Cardiol 35:357–366. [DOI] [PubMed] [Google Scholar]
- Botker HE, Kharbanda R, Schmidt MR, Bottcher M, Kaltoft AK, Terkelsen CJ, Munk K, Andersen NH, Hansen TM, Trautner S, Lassen JF, Christiansen EH, Krusell LR, Kristensen SD, Thuesen L, Nielsen SS, Rehling M, Sorensen HT, Redington AN, Nielsen TT (2010) Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet 375:727–734. [DOI] [PubMed] [Google Scholar]
- Bouchard JF, Lamontagne D (1996) Mechanisms of protection afforded by preconditioning to endothelial function against ischemic injury. Am J Physiol 271:H1801–1806. [DOI] [PubMed] [Google Scholar]
- Bouleti C, Mewton N, Germain S (2015) The no-reflow phenomenon: State of the art. Arch Cardiovasc Dis 108:661–674. [DOI] [PubMed] [Google Scholar]
- Bulluck H, Chan MHH, Paradies V, Bryant JA, Hernandez-Resendiz S, Cabrera-Fuentes HA, Watson TJ, Chan MY, Tan JW, Hausenloy DJ (2018) Impact of Cardioprotective Therapies on the Edema-Based Area at Risk by CMR in Reperfused STEMI. J Am Coll Cardiol 71:2856–2858. [DOI] [PubMed] [Google Scholar]
- Bulluck H, Nicholas J, Crimi G, White SK, Ludman AJ, Pica S, Raineri C, Cabrera-Fuentes HA, Yellon D, Rodriguez-Palomares J, Garcia-Dorado D, Hausenloy DJ (2017) Circadian variation in acute myocardial infarct size assessed by cardiovascular magnetic resonance in reperfused STEMI patients. Int J Cardiol 230:149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera-Fuentes HA, Niemann B, Grieshaber P, Wollbrueck M, Gehron J, Preissner KT, Boning A (2015) RNase1 as a potential mediator of remote ischaemic preconditioning for cardioprotectiondagger. Eur J Cardiothorac Surg 48:732–737; discussion 737. [DOI] [PubMed] [Google Scholar]
- Cabrera-Fuentes HA et al. (2014) RNase1 prevents the damaging interplay between extracellular RNA and tumour necrosis factor-alpha in cardiac ischaemia/reperfusion injury. Thromb Haemost 112:1110–1119. [DOI] [PubMed] [Google Scholar]
- Cabrera-Fuentes HA et al. (2016a) Meeting report from the 2nd International Symposium on New Frontiers in Cardiovascular Research. Protecting the cardiovascular system from ischemia: between bench and bedside. Basic Res Cardiol 111:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera-Fuentes HA et al. (2016b) From basic mechanisms to clinical applications in heart protection, new players in cardiovascular diseases and cardiac theranostics: meeting report from the third international symposium on “New frontiers in cardiovascular research”. Basic Res Cardiol 111:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai ZP, Parajuli N, Zheng X, Becker L (2012) Remote ischemic preconditioning confers late protection against myocardial ischemia-reperfusion injury in mice by upregulating interleukin-10. Basic research in cardiology 107:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardozo AK, Ortis F, Storling J, Feng YM, Rasschaert J, Tonnesen M, Van Eylen F, Mandrup-Poulsen T, Herchuelz A, Eizirik DL (2005) Cytokines downregulate the sarcoendoplasmic reticulum pump Ca2+ ATPase 2b and deplete endoplasmic reticulum Ca2+, leading to induction of endoplasmic reticulum stress in pancreatic beta-cells. Diabetes 54:452–461. [DOI] [PubMed] [Google Scholar]
- Carini R, De Cesaris MG, Splendore R, Vay D, Domenicotti C, Nitti MP, Paola D, Pronzato MA, Albano E (2001) Signal pathway involved in the development of hypoxic preconditioning in rat hepatocytes. Hepatology 33:131–139. [DOI] [PubMed] [Google Scholar]
- Celermajer DS, Chow CK, Marijon E, Anstey NM, Woo KS (2012) Cardiovascular disease in the developing world: prevalences, patterns, and the potential of early disease detection. Journal of the American College of Cardiology 60:1207–1216. [DOI] [PubMed] [Google Scholar]
- Chatterjee S (2018) Endothelial Mechanotransduction, Redox Signaling and the Regulation of Vascular Inflammatory Pathways. Front Physiol 9:524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavakis T, Preissner KT (2005) Integrin-mediated leukocyte adhesive interactions: regulation by haemostatic factors. Hamostaseologie 25:33–38. [DOI] [PubMed] [Google Scholar]
- Chavakis T, Bierhaus A, Al-Fakhri N, Schneider D, Witte S, Linn T, Nagashima M, Morser J, Arnold B, Preissner KT, Nawroth PP (2003) The pattern recognition receptor (RAGE) is a counterreceptor for leukocyte integrins: a novel pathway for inflammatory cell recruitment. J Exp Med 198:1507–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R, International Brachial Artery Reactivity Task F (2002) Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. Journal of the American College of Cardiology 39:257–265. [DOI] [PubMed] [Google Scholar]
- Coulter SA, Cannon CP, Ault KA, Antman EM, Van de Werf F, Adgey AA, Gibson CM, Giugliano RP, Mascelli MA, Scherer J, Barnathan ES, Braunwald E, Kleiman NS (2000) High levels of platelet inhibition with abciximab despite heightened platelet activation and aggregation during thrombolysis for acute myocardial infarction: results from TIMI (thrombolysis in myocardial infarction) 14. Circulation 101:2690–2695. [DOI] [PubMed] [Google Scholar]
- Davidson SM, Selvaraj P, He D, Boi-Doku C, Yellon RL, Vicencio JM, Yellon DM (2013) Remote ischaemic preconditioning involves signalling through the SDF-1alpha/CXCR4 signalling axis. Basic research in cardiology 108:377. [DOI] [PubMed] [Google Scholar]
- Davies WR, Brown AJ, Watson W, McCormick LM, West NE, Dutka DP, Hoole SP (2013) Remote ischemic preconditioning improves outcome at 6 years after elective percutaneous coronary intervention: the CRISP stent trial long-term follow-up. Circ Cardiovasc Interv 6:246–251. [DOI] [PubMed] [Google Scholar]
- de Agostini AI, Watkins SC, Slayter HS, Youssoufian H, Rosenberg RD (1990) Localization of anticoagulantly active heparan sulfate proteoglycans in vascular endothelium: antithrombin binding on cultured endothelial cells and perfused rat aorta. The Journal of cell biology 111:1293–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deem TL, Cook-Mills JM (2004) Vascular cell adhesion molecule 1 (VCAM-1) activation of endothelial cell matrix metalloproteinases: role of reactive oxygen species. Blood 104:2385–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFily DV, Chilian WM (1993) Preconditioning protects coronary arteriolar endothelium from ischemia-reperfusion injury. Am J Physiol 265:H700–706. [DOI] [PubMed] [Google Scholar]
- Dickson EW, Reinhardt CP, Renzi FP, Becker RC, Porcaro WA, Heard SO (1999) Ischemic preconditioning may be transferable via whole blood transfusion: preliminary evidence. J Thromb Thrombolysis 8:123–129. [DOI] [PubMed] [Google Scholar]
- Donato M, Buchholz B, Rodriguez M, Perez V, Inserte J, Garcia-Dorado D, Gelpi RJ (2013) Role of the parasympathetic nervous system in cardioprotection by remote hindlimb ischaemic preconditioning. Experimental physiology 98:425–434. [DOI] [PubMed] [Google Scholar]
- Donato M, D’Annunzio V, Berg G, Gonzalez G, Schreier L, Morales C, Wikinski RL, Gelpi RJ (2007) Ischemic postconditioning reduces infarct size by activation of A1 receptors and K+(ATP) channels in both normal and hypercholesterolemic rabbits. Journal of cardiovascular pharmacology 49:287–292. [DOI] [PubMed] [Google Scholar]
- Downey JM, Cohen MV, Ytrehus K, Liu Y (1994) Cellular mechanisms in ischemic preconditioning: the role of adenosine and protein kinase C. Annals of the New York Academy of Sciences 723:82–98. [PubMed] [Google Scholar]
- Dymkowska D, Drabarek B, Podszywalow-Bartnicka P, Szczepanowska J, Zablocki K (2014) Hyperglycaemia modifies energy metabolism and reactive oxygen species formation in endothelial cells in vitro. Arch Biochem Biophys 542:7–13. [DOI] [PubMed] [Google Scholar]
- Engelman DT, Watanabe M, Engelman RM, Rousou JA, Flack JE 3rd, Deaton DW, Das DK (1995) Constitutive nitric oxide release is impaired after ischemia and reperfusion. The Journal of thoracic and cardiovascular surgery 110:1047–1053. [DOI] [PubMed] [Google Scholar]
- Fischer S, Nishio M, Dadkhahi S, Gansler J, Saffarzadeh M, Shibamiyama A, Kral N, Baal N, Koyama T, Deindl E, Preissner KT (2011) Expression and localisation of vascular ribonucleases in endothelial cells. Thromb Haemost 105:345–355. [DOI] [PubMed] [Google Scholar]
- Fischer S, Nishio M, Peters SC, Tschernatsch M, Walberer M, Weidemann S, Heidenreich R, Couraud PO, Weksler BB, Romero IA, Gerriets T, Preissner KT (2009) Signaling mechanism of extracellular RNA in endothelial cells. FASEB J 23:2100–2109. [DOI] [PubMed] [Google Scholar]
- Frohlich GM, Meier P, White SK, Yellon DM, Hausenloy DJ (2013) Myocardial reperfusion injury: looking beyond primary PCI. Eur Heart J 34:1714–1722. [DOI] [PubMed] [Google Scholar]
- Gaboury J, Woodman RC, Granger DN, Reinhardt P, Kubes P (1993) Nitric oxide prevents leukocyte adherence: role of superoxide. The American journal of physiology 265:H862–867. [DOI] [PubMed] [Google Scholar]
- Gaboury JP, Anderson DC, Kubes P (1994) Molecular mechanisms involved in superoxide-induced leukocyte-endothelial cell interactions in vivo. Am J Physiol 266:H637–642. [DOI] [PubMed] [Google Scholar]
- Galle J, Quaschning T, Seibold S, Wanner C (2003) Endothelial dysfunction and inflammation: what is the link? Kidney international Supplement:S 45–49. [DOI] [PubMed] [Google Scholar]
- Geeraerts MD, Ronveaux-Dupal MF, Lemasters JJ, Herman B (1991) Cytosolic free Ca2+ and proteolysis in lethal oxidative injury in endothelial cells. Am J Physiol 261:C889–896. [DOI] [PubMed] [Google Scholar]
- Gomez L, Thibault H, Gharib A, Dumont JM, Vuagniaux G, Scalfaro P, Derumeaux G, Ovize M (2007) Inhibition of mitochondrial permeability transition improves functional recovery and reduces mortality following acute myocardial infarction in mice. American journal of physiology Heart and circulatory physiology 293:H1654–1661. [DOI] [PubMed] [Google Scholar]
- Gray CC, Amrani M, Yacoub MH (1999) Heat stress proteins and myocardial protection: experimental model or potential clinical tool? The international journal of biochemistry & cell biology 31:559–573. [DOI] [PubMed] [Google Scholar]
- Gross ER, Hsu AK, Urban TJ, Mochly-Rosen D, Gross GJ (2013) Nociceptive-induced myocardial remote conditioning is mediated by neuronal gamma protein kinase C. Basic research in cardiology 108:381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross GJ, Baker JE, Moore J, Falck JR, Nithipatikom K (2011) Abdominal surgical incision induces remote preconditioning of trauma (RPCT) via activation of bradykinin receptors (BK2R) and the cytochrome P450 epoxygenase pathway in canine hearts. Cardiovascular drugs and therapy 25:517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Gupta MM (2016) No reflow phenomenon in percutaneous coronary interventions in ST-segment elevation myocardial infarction. Indian Heart J 68:539–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DG (1997) Cellular and molecular mechanisms of endothelial cell dysfunction. The Journal of clinical investigation 100:2153–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasdai D, Lerman A (1999) The assessment of endothelial function in the cardiac catheterization laboratory in patients with risk factors for atherosclerotic coronary artery disease. Herz 24:544–547. [DOI] [PubMed] [Google Scholar]
- Hastie LE, Patton WF, Hechtman HB, Shepro D (1997) Filamin redistribution in an endothelial cell reoxygenation injury model. Free Radic Biol Med 22:955–966. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Yellon DM (2004) New directions for protecting the heart against ischaemia-reperfusion injury: targeting the Reperfusion Injury Salvage Kinase (RISK)-pathway. Cardiovasc Res 61:448–460. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Yellon DM (2016) Ischaemic conditioning and reperfusion injury. Nat Rev Cardiol 13:193–209. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ et al. (2016) Ischaemic conditioning and targeting reperfusion injury: a 30 year voyage of discovery. Basic Res Cardiol 111:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays AG, Kelle S, Hirsch GA, Soleimanifard S, Yu J, Agarwal HK, Gerstenblith G, Schar M, Stuber M, Weiss RG (2012) Regional coronary endothelial function is closely related to local early coronary atherosclerosis in patients with mild coronary artery disease: pilot study. Circulation Cardiovascular imaging 5:341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein TW, Zhang C, Wang W, Chang CI, Thengchaisri N, Kuo L (2003) Ischemia-reperfusion selectively impairs nitric oxide-mediated dilation in coronary arterioles: counteracting role of arginase. FASEB J 17:2328–2330. [DOI] [PubMed] [Google Scholar]
- Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T (2001) Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation 104:2673–2678. [DOI] [PubMed] [Google Scholar]
- Hepponstall M, Ignjatovic V, Binos S, Monagle P, Jones B, Cheung MH, d’Udekem Y, Konstantinov IE (2012) Remote ischemic preconditioning (RIPC) modifies plasma proteome in humans. PloS one 7:e48284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Resendiz S, Chinda K, Ong SB, Cabrera-Fuentes H, Zazueta C, Hausenloy DJ (2018) The Role of Redox Dysregulation in the Inflammatory Response to Acute Myocardial Ischaemia-reperfusion Injury - Adding Fuel to the Fire. Curr Med Chem 25:1275–1293. [DOI] [PubMed] [Google Scholar]
- Heurteaux C, Lauritzen I, Widmann C, Lazdunski M (1995) Essential role of adenosine, adenosine A1 receptors, and ATP-sensitive K+ channels in cerebral ischemic preconditioning. Proceedings of the National Academy of Sciences of the United States of America 92:4666–4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heusch G, Botker HE, Przyklenk K, Redington A, Yellon D (2015) Remote ischemic conditioning. J Am Coll Cardiol 65:177–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higginson LA, White F, Heggtveit HA, Sanders TM, Bloor CM, Covell JW (1982) Determinants of myocardial hemorrhage after coronary reperfusion in the anesthetized dog. Circulation 65:62–69. [DOI] [PubMed] [Google Scholar]
- Hinshaw DB, Burger JM, Armstrong BC, Hyslop PA (1989) Mechanism of endothelial cell shape change in oxidant injury. J Surg Res 46:339–349. [DOI] [PubMed] [Google Scholar]
- Jenkins DP, Pugsley WB, Alkhulaifi AM, Kemp M, Hooper J, Yellon DM (1997) Ischaemic preconditioning reduces troponin T release in patients undergoing coronary artery bypass surgery. Heart 77:314–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerome SN, Akimitsu T, Gute DC, Korthuis RJ (1995) Ischemic preconditioning attenuates capillary no-reflow induced by prolonged ischemia and reperfusion. Am J Physiol 268:H2063–2067. [DOI] [PubMed] [Google Scholar]
- Kaeffer N, Richard V, Francois A, Lallemand F, Henry JP, Thuillez C (1996) Preconditioning prevents chronic reperfusion-induced coronary endothelial dysfunction in rats. Am J Physiol 271:H842–849. [DOI] [PubMed] [Google Scholar]
- Karasawa A, Rochester JA, Ma XL, Lefer AM (1991) Protection of endothelial damage and systemic shock by benidipine, a calcium antagonist, in rats subjected to splanchnic ischemia and reperfusion. Circ Shock 33:135–141. [PubMed] [Google Scholar]
- Kharbanda RK, Peters M, Walton B, Kattenhorn M, Mullen M, Klein N, Vallance P, Deanfield J, MacAllister R (2001) Ischemic preconditioning prevents endothelial injury and systemic neutrophil activation during ischemia-reperfusion in humans in vivo. Circulation 103:1624–1630. [DOI] [PubMed] [Google Scholar]
- Kharbanda RK, Mortensen UM, White PA, Kristiansen SB, Schmidt MR, Hoschtitzky JA, Vogel M, Sorensen K, Redington AN, MacAllister R (2002) Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation 106:2881–2883. [DOI] [PubMed] [Google Scholar]
- Kimura C, Oike M, Ito Y (2000) Hypoxia-induced alterations in Ca(2+) mobilization in brain microvascular endothelial cells. Am J Physiol Heart Circ Physiol 279:H2310–2318. [DOI] [PubMed] [Google Scholar]
- Kin H, Zhao ZQ, Sun HY, Wang NP, Corvera JS, Halkos ME, Kerendi F, Guyton RA, Vinten-Johansen J (2004) Postconditioning attenuates myocardial ischemia-reperfusion injury by inhibiting events in the early minutes of reperfusion. Cardiovascular research 62:74–85. [DOI] [PubMed] [Google Scholar]
- Kitakaze M, Hori M, Morioka T, Minamino T, Takashima S, Sato H, Shinozaki Y, Chujo M, Mori H, Inoue M, et al. (1994) Infarct size-limiting effect of ischemic preconditioning is blunted by inhibition of 5’-nucleotidase activity and attenuation of adenosine release. Circulation 89:1237–1246. [DOI] [PubMed] [Google Scholar]
- Kladakis SM, Nerem RM (2004) Endothelial cell monolayer formation: effect of substrate and fluid shear stress. Endothelium : journal of endothelial cell research 11:29–44. [DOI] [PubMed] [Google Scholar]
- Kloner RA (2011) No-reflow phenomenon: maintaining vascular integrity. J Cardiovasc Pharmacol Ther 16:244–250. [DOI] [PubMed] [Google Scholar]
- Korthuis RJ, Gute DC (1997) Postischemic leukocyte/endothelial cell interactions and microvascular barrier dysfunction in skeletal muscle: cellular mechanisms and effect of Daflon 500 mg. International journal of microcirculation, clinical and experimental 17 Suppl 1:11–17. [DOI] [PubMed] [Google Scholar]
- Kumar S, Reusch HP, Ladilov Y (2008) Acidic pre-conditioning suppresses apoptosis and increases expression of Bcl-xL in coronary endothelial cells under simulated ischaemia. J Cell Mol Med 12:1584–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Kasseckert S, Kostin S, Abdallah Y, Schafer C, Kaminski A, Reusch HP, Piper HM, Steinhoff G, Ladilov Y (2007) Ischemic acidosis causes apoptosis in coronary endothelial cells through activation of caspase-12. Cardiovasc Res 73:172–180. [DOI] [PubMed] [Google Scholar]
- Ladilov Y, Schafer C, Held A, Schafer M, Noll T, Piper HM (2000) Mechanism of Ca(2+) overload in endothelial cells exposed to simulated ischemia. Cardiovasc Res 47:394–403. [DOI] [PubMed] [Google Scholar]
- Laude K, Thuillez C, Richard V (2001) Coronary endothelial dysfunction after ischemia and reperfusion: a new therapeutic target? Braz J Med Biol Res 34:1–7. [DOI] [PubMed] [Google Scholar]
- Laude K, Favre J, Thuillez C, Richard V (2003) NO produced by endothelial NO synthase is a mediator of delayed preconditioning-induced endothelial protection. Am J Physiol Heart Circ Physiol 284:H2053–2060. [DOI] [PubMed] [Google Scholar]
- Lefer AM, Tsao PS, Lefer DJ, Ma XL (1991) Role of endothelial dysfunction in the pathogenesis of reperfusion injury after myocardial ischemia. FASEB J 5:2029–2034. [DOI] [PubMed] [Google Scholar]
- Leo R, Pratico D, Iuliano L, Pulcinelli FM, Ghiselli A, Pignatelli P, Colavita AR, FitzGerald GA, Violi F (1997) Platelet activation by superoxide anion and hydroxyl radicals intrinsically generated by platelets that had undergone anoxia and then reoxygenated. Circulation 95:885–891. [DOI] [PubMed] [Google Scholar]
- Li J, Rohailla S, Gelber N, Rutka J, Sabah N, Gladstone RA, Wei C, Hu P, Kharbanda RK, Redington AN (2014) MicroRNA-144 is a circulating effector of remote ischemic preconditioning. Basic research in cardiology 109:423. [DOI] [PubMed] [Google Scholar]
- Lind L, Fors N, Hall J, Marttala K, Stenborg A (2005) A comparison of three different methods to evaluate endothelium-dependent vasodilation in the elderly: the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. Arterioscler Thromb Vasc Biol 25:2368–2375. [DOI] [PubMed] [Google Scholar]
- Lopez-Diez R, Shekhtman A, Ramasamy R, Schmidt AM (2016) Cellular mechanisms and consequences of glycation in atherosclerosis and obesity. Biochim Biophys Acta 1862:2244–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D, Maulik N, Moraru II, Kreutzer DL, Das DK (1993) Molecular adaptation of vascular endothelial cells to oxidative stress. The American journal of physiology 264:C715–722. [DOI] [PubMed] [Google Scholar]
- Lucchesi BR (1993) Complement activation, neutrophils, and oxygen radicals in reperfusion injury. Stroke 24:I41–47; discussion 138-40. [DOI] [PubMed] [Google Scholar]
- Ma X, Zhang X, Li C, Luo M (2006) Effect of postconditioning on coronary blood flow velocity and endothelial function and LV recovery after myocardial infarction. J Interv Cardiol 19:367–375. [DOI] [PubMed] [Google Scholar]
- Ma XL, Lefer DJ, Lefer AM, Rothlein R (1992) Coronary endothelial and cardiac protective effects of a monoclonal antibody to intercellular adhesion molecule-1 in myocardial ischemia and reperfusion. Circulation 86:937–946. [DOI] [PubMed] [Google Scholar]
- Maiocchi S, Alwis I, Wu MCL, Yuan Y, Jackson SP (2018) Thromboinflammatory Functions of Platelets in Ischemia-Reperfusion Injury and Its Dysregulation in Diabetes. Semin Thromb Hemost 44:102–113. [DOI] [PubMed] [Google Scholar]
- Manchurov V, Ryazankina N, Khmara T, Skrypnik D, Reztsov R, Vasilieva E, Shpektor A (2014) Remote ischemic preconditioning and endothelial function in patients with acute myocardial infarction and primary PCI. Am J Med 127:670–673. [DOI] [PubMed] [Google Scholar]
- Marber MS, Yellon DM (1996) Myocardial adaptation, stress proteins, and the second window of protection. Annals of the New York Academy of Sciences 793:123–141. [DOI] [PubMed] [Google Scholar]
- Marcus AJ (1979) Pathways of oxygen utilization by stimulated platelets and leukocytes. Semin Hematol 16:188–195. [PubMed] [Google Scholar]
- Massberg S, Enders G, Leiderer R, Eisenmenger S, Vestweber D, Krombach F, Messmer K (1998) Platelet-endothelial cell interactions during ischemia/reperfusion: the role of P-selectin. Blood 92:507–515. [PubMed] [Google Scholar]
- Meldrum DR, Dinarello CA, Shames BD, Cleveland JC Jr., Cain BS, Banerjee A, Meng X, Harken AH (1998) Ischemic preconditioning decreases postischemic myocardial tumor necrosis factor-alpha production. Potential ultimate effector mechanism of preconditioning. Circulation 98:11214–218; discussion 11218-219. [PubMed] [Google Scholar]
- Mertens S, Noll T, Spahr R, Krutzfeldt A, Piper HM (1990) Energetic response of coronary endothelial cells to hypoxia. Am J Physiol 258:H689–694. [DOI] [PubMed] [Google Scholar]
- Meybohm P et al. (2015) A Multicenter Trial of Remote Ischemic Preconditioning for Heart Surgery. N Engl J Med 373:1397–1407. [DOI] [PubMed] [Google Scholar]
- Michiels C (2003) Endothelial cell functions. J Cell Physiol 196:430–443. [DOI] [PubMed] [Google Scholar]
- Mirabet M, Garcia-Dorado D, Inserte J, Barrabes JA, Lidon RM, Soriano B, Azevedo M, Padilla F, Agullo L, Ruiz-Meana M, Massaguer A, Pizcueta P, Soler-Soler J (2002) Platelets activated by transient coronary occlusion exacerbate ischemia-reperfusion injury in rat hearts. Am J Physiol Heart Circ Physiol 283:H1134–1141. [DOI] [PubMed] [Google Scholar]
- Mullane KM, Read N, Salmon JA, Moncada S (1984) Role of leukocytes in acute myocardial infarction in anesthetized dogs: relationship to myocardial salvage by anti-inflammatory drugs. J Pharmacol Exp Ther 228:510–522. [PubMed] [Google Scholar]
- Natarajan V, Taher MM, Roehm B, Parinandi NL, Schmid HH, Kiss Z, Garcia JG (1993) Activation of endothelial cell phospholipase D by hydrogen peroxide and fatty acid hydroperoxide. J Biol Chem 268:930–937. [PubMed] [Google Scholar]
- Nawroth P, Bierhaus A, Marrero M, Yamamoto H, Stern DM (2005) Atherosclerosis and restenosis: is there a role for RAGE? Curr Diab Rep 5:11–16. [DOI] [PubMed] [Google Scholar]
- Okorie MI, Bhavsar DD, Ridout D, Charakida M, Deanfield JE, Loukogeorgakis SP, MacAllister RJ (2011) Postconditioning protects against human endothelial ischaemia-reperfusion injury via subtype-specific KATP channel activation and is mimicked by inhibition of the mitochondrial permeability transition pore. Eur Heart J 32:1266–1274. [DOI] [PubMed] [Google Scholar]
- Ong SB, Dongworth RK, Cabrera-Fuentes HA, Hausenloy DJ (2015) Role of the MPTP in conditioning the heart - translatability and mechanism. Br J Pharmacol 172:2074–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong SB, Hernandez-Resendiz S, Crespo-Avilan GE, Mukhametshina RT, Kwek XY, Cabrera-Fuentes HA, Hausenloy DJ (2018) Inflammation following acute myocardial infarction: Multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol Ther 186:73–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxman T, Arad M, Klein R, Avazov N, Rabinowitz B (1997) Limb ischemia preconditions the heart against reperfusion tachyarrhythmia. Am J Physiol 273:H1707–1712. [DOI] [PubMed] [Google Scholar]
- Patel HH, Moore J, Hsu AK, Gross GJ (2002) Cardioprotection at a distance: mesenteric artery occlusion protects the myocardium via an opioid sensitive mechanism. Journal of molecular and cellular cardiology 34:1317–1323. [DOI] [PubMed] [Google Scholar]
- Patel KD, Zimmerman GA, Prescott SM, McEver RP, McIntyre TM (1991) Oxygen radicals induce human endothelial cells to express GMP-140 and bind neutrophils. J Cell Biol 112:749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson JD (2000) Normal endothelial cell function. Lupus 9:183–188. [DOI] [PubMed] [Google Scholar]
- Poredos P (2002a) Endothelial dysfunction in the pathogenesis of atherosclerosis. International angiology : a journal of the International Union of Angiology 21:109–116. [PubMed] [Google Scholar]
- Poredos P (2002b) Endothelial dysfunction and cardiovascular disease. Pathophysiology of haemostasis and thrombosis 32:274–277. [DOI] [PubMed] [Google Scholar]
- Preissner KT (1990) Physiological role of vessel wall related antithrombotic mechanisms: contribution of endogenous and exogenous heparin-like components to the anticoagulant potential of the endothelium. Haemostasis 20 Suppl 1:30–49. [DOI] [PubMed] [Google Scholar]
- Putney JW Jr. (1986) A model for receptor-regulated calcium entry. Cell Calcium 7:1–12. [DOI] [PubMed] [Google Scholar]
- Quagliaro L, Piconi L, Assaloni R, Da Ros R, Maier A, Zuodar G, Ceriello A (2005) Intermittent high glucose enhances ICAM-1, VCAM-1 and E-selectin expression in human umbilical vein endothelial cells in culture: the distinct role of protein kinase C and mitochondrial superoxide production. Atherosclerosis 183:259–267. [DOI] [PubMed] [Google Scholar]
- Rajendran P, Rengarajan T, Thangavel J, Nishigaki Y, Sakthisekaran D, Sethi G, Nishigaki I (2013) The vascular endothelium and human diseases. Int J Biol Sci 9:1057–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramjane K, Han L, Jin C (2008) The diagnosis and treatment of the no-reflow phenomenon in patients with myocardial infarction undergoing percutaneous coronary intervention. Exp Clin Cardiol 13:121–128. [PMC free article] [PubMed] [Google Scholar]
- Reffelmann T, Kloner RA (2006) The no-reflow phenomenon: A basic mechanism of myocardial ischemia and reperfusion. Basic research in cardiology 101:359–372. [DOI] [PubMed] [Google Scholar]
- Remme WJ (2000) Overview of the relationship between ischemia and congestive heart failure. Clinical cardiology 23:IV4–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard V, Kaeffer N, Tron C, Thuillez C (1994) Ischemic preconditioning protects against coronary endothelial dysfunction induced by ischemia and reperfusion. Circulation 89:1254–1261. [DOI] [PubMed] [Google Scholar]
- Romo GM, Dong JF, Schade AJ, Gardiner EE, Kansas GS, Li CQ, McIntire LV, Berndt MC, Lopez JA (1999) The glycoprotein Ib-IX-V complex is a platelet counterreceptor for P-selectin. J Exp Med 190:803–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandow SL, Senadheera S, Grayson TH, Welsh DG, Murphy TV (2012) Calcium and endothelium-mediated vasodilator signaling. Adv Exp Med Biol 740:811–831. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Geigenmuller S, Volker W, Buddecke E (2006) The antiatherogenic and antiinflammatory effect of HDL-associated lysosphingolipids operates via Akt -->NF-kappaB signalling pathways in human vascular endothelial cells. Basic Res Cardiol 101:109–116. [DOI] [PubMed] [Google Scholar]
- Schmidt MR, Smerup M, Konstantinov IE, Shimizu M, Li J, Cheung M, White PA, Kristiansen SB, Sorensen K, Dzavik V, Redington AN, Kharbanda RK (2007) Intermittent peripheral tissue ischemia during coronary ischemia reduces myocardial infarction through a KATP-dependent mechanism: first demonstration of remote ischemic perconditioning. American journal of physiology Heart and circulatory physiology 292:H1883–1890. [DOI] [PubMed] [Google Scholar]
- Schwartz BG, Kloner RA (2012) Coronary no reflow. Journal of molecular and cellular cardiology 52:873–882. [DOI] [PubMed] [Google Scholar]
- Schwartz LM, Lagranha CJ (2006) Ischemic postconditioning during reperfusion activates Akt and ERK without protecting against lethal myocardial ischemia-reperfusion injury in pigs. American journal of physiology Heart and circulatory physiology 290:H1011–1018. [DOI] [PubMed] [Google Scholar]
- Serejo FC, Rodrigues LF Jr., da Silva Tavares KC, de Carvalho AC, Nascimento JH (2007) Cardioprotective properties of humoral factors released from rat hearts subject to ischemic preconditioning. Journal of cardiovascular pharmacology 49:214–220. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Tropak M, Diaz RJ, Suto F, Surendra H, Kuzmin E, Li J, Gross G, Wilson GJ, Callahan J, Redington AN (2009) Transient limb ischaemia remotely preconditions through a humoral mechanism acting directly on the myocardium: evidence suggesting cross-species protection. Clinical science 117:191–200. [DOI] [PubMed] [Google Scholar]
- Shimokawa H, Yasuda S (2008) Myocardial ischemia: current concepts and future perspectives. J Cardiol 52:67–78. [DOI] [PubMed] [Google Scholar]
- Singhal AK, Symons JD, Boudina S, Jaishy B, Shiu YT (2010) Role of Endothelial Cells in Myocardial Ischemia-Reperfusion Injury. Vasc Dis Prev 7:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaraman V, Pickard JM, Hausenloy DJ (2015) Remote ischaemic conditioning: cardiac protection from afar. Anaesthesia 70:732–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skyschally A, van Caster P, Iliodromitis EK, Schulz R, Kremastinos DT, Heusch G (2009) Ischemic postconditioning: experimental models and protocol algorithms. Basic research in cardiology 104:469–483. [DOI] [PubMed] [Google Scholar]
- Sloth AD, Schmidt MR, Munk K, Kharbanda RK, Redington AN, Schmidt M, Pedersen L, Sorensen HT, Botker HE, Investigators C (2014) Improved long-term clinical outcomes in patients with ST-elevation myocardial infarction undergoing remote ischaemic conditioning as an adjunct to primary percutaneous coronary intervention. Eur Heart J 35:168–175. [DOI] [PubMed] [Google Scholar]
- Stepien E, Stankiewicz E, Zalewski J, Godlewski J, Zmudka K, Wybranska I (2012) Number of microparticles generated during acute myocardial infarction and stable angina correlates with platelet activation. Arch Med Res 43:31–35. [DOI] [PubMed] [Google Scholar]
- Steppich BA, Braun SL, Stein A, Demetz G, Groha P, Schomig A, von Beckerath N, Kastrati A, Ott I (2009) Plasma TF activity predicts cardiovascular mortality in patients with acute myocardial infarction. Thromb J 7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suades R, Padro T, Vilahur G, Martin-Yuste V, Sabate M, Sans-Rosello J, Sionis A, Badimon L (2015) Growing thrombi release increased levels of CD235a(+) microparticles and decreased levels of activated platelet-derived microparticles. Validation in ST-elevation myocardial infarction patients. J Thromb Haemost 13:1776–1786. [DOI] [PubMed] [Google Scholar]
- Symons JD, Schaefer S (2001) Na(+)/H(+) exchange subtype 1 inhibition reduces endothelial dysfunction in vessels from stunned myocardium. Am J Physiol Heart Circ Physiol 281:H1575–1582. [DOI] [PubMed] [Google Scholar]
- Szmitko PE, Wang CH, Weisel RD, de Almeida JR, Anderson TJ, Verma S (2003) New markers of inflammation and endothelial cell activation: Part I. Circulation 108:1917–1923. [DOI] [PubMed] [Google Scholar]
- Szocs K (2004) Endothelial dysfunction and reactive oxygen species production in ischemia/reperfusion and nitrate tolerance. Gen Physiol Biophys 23:265–295. [PubMed] [Google Scholar]
- Teoh N, Leclercq I, Pena AD, Farrell G (2003) Low-dose TNF-alpha protects against hepatic ischemia-reperfusion injury in mice: implications for preconditioning. Hepatology 37:118–128. [DOI] [PubMed] [Google Scholar]
- Therade-Matharan S, Laemmel E, Carpentier S, Obata Y, Levade T, Duranteau J, Vicaut E (2005) Reactive oxygen species production by mitochondria in endothelial cells exposed to reoxygenation after hypoxia and glucose depletion is mediated by ceramide. Am J Physiol Regul Integr Comp Physiol 289:R1756–1762. [DOI] [PubMed] [Google Scholar]
- Thielmann M, Kottenberg E, Kleinbongard P, Wendt D, Gedik N, Pasa S, Price V, Tsagakis K, Neuhauser M, Peters J, Jakob H, Heusch G (2013) Cardioprotective and prognostic effects of remote ischaemic preconditioning in patients undergoing coronary artery bypass surgery: a single-centre randomised, double-blind, controlled trial. Lancet 382:597–604. [DOI] [PubMed] [Google Scholar]
- Thourani VH, Nakamura M, Duarte IG, Bufkin BL, Zhao ZQ, Jordan JE, Shearer ST, Guyton RA, Vinten-Johansen J (1999) Ischemic preconditioning attenuates postischemic coronary artery endothelial dysfunction in a model of minimally invasive direct coronary artery bypass grafting. J Thorac Cardiovasc Surg 117:383–389. [DOI] [PubMed] [Google Scholar]
- Tsao PS, Aoki N, Lefer DJ, Johnson G 3rd, Lefer AM (1990) Time course of endothelial dysfunction and myocardial injury during myocardial ischemia and reperfusion in the cat. Circulation 82:1402–1412. [DOI] [PubMed] [Google Scholar]
- Urbich C, Dimmeler S (2004) Endothelial progenitor cells: characterization and role in vascular biology. Circulation research 95:343–353. [DOI] [PubMed] [Google Scholar]
- van Hinsbergh VW (1997) Endothelial permeability for macromolecules. Mechanistic aspects of pathophysiological modulation. Arteriosclerosis, thrombosis, and vascular biology 17:1018–1023. [DOI] [PubMed] [Google Scholar]
- Verma S, Li SH, Badiwala MV, Weisel RD, Fedak PW, Li RK, Dhillon B, Mickle DA (2002) Endothelin antagonism and interleukin-6 inhibition attenuate the proatherogenic effects of C-reactive protein. Circulation 105:1890–1896. [DOI] [PubMed] [Google Scholar]
- Vinten-Johansen J, Zhao ZQ, Nakamura M, Jordan JE, Ronson RS, Thourani VH, Guyton RA (1999) Nitric oxide and the vascular endothelium in myocardial ischemia-reperfusion injury. Ann N Y Acad Sci 874:354–370. [DOI] [PubMed] [Google Scholar]
- Weinbrenner C, Schulze F, Sarvary L, Strasser RH (2004) Remote preconditioning by infrarenal aortic occlusion is operative via delta1-opioid receptors and free radicals in vivo in the rat heart. Cardiovascular research 61:591–599. [DOI] [PubMed] [Google Scholar]
- Widmer RJ, Lerman A (2014) Endothelial dysfunction and cardiovascular disease. Global cardiology science practice 2014:291–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn RK, Ramamoorthy C, Vedder NB, Sharar SR, Harlan JM (1997) Leukocyte-endothelial cell interactions in ischemia-reperfusion injury. Ann N Y Acad Sci 832:311–321. [DOI] [PubMed] [Google Scholar]
- Yamashita N, Nishida M, Hoshida S, Kuzuya T, Hori M, Taniguchi N, Kamada T, Tada M (1994) Induction of manganese superoxide dismutase in rat cardiac myocytes increases tolerance to hypoxia 24 hours after preconditioning. The Journal of clinical investigation 94:2193–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellon DM, Baxter GF (1995) A “second window of protection” or delayed preconditioning phenomenon: future horizons for myocardial protection? J Mol Cell Cardiol 27:1023–1034. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Park TS, Gidday JM (2007) Hypoxic preconditioning protects human brain endothelium from ischemic apoptosis by Akt-dependent survivin activation. Am J Physiol Heart Circ Physiol 292:H2573–2581. [DOI] [PubMed] [Google Scholar]
- Zhao JL, Yang YJ, You SJ, Cui CJ, Gao RL (2007) Different effects of postconditioning on myocardial no-reflow in the normal and hypercholesterolemic mini-swines. Microvascular research 73:137–142. [DOI] [PubMed] [Google Scholar]
- Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J (2003) Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. American journal of physiology Heart and circulatory physiology 285:H579–588. [DOI] [PubMed] [Google Scholar]
- Ziche M, Morbidelli L, Masini E, Amerini S, Granger HJ, Maggi CA, Geppetti P, Ledda F (1994) Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J Clin Invest 94:2036–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]


