Abstract
The identification of appropriate companions and mates is essential to both speciation and the maintenance of species through prezygotic isolation. In many birds, social assortment is mediated by vocalizations learned through imitation. When imitative vocal learning occurs throughout life, emergent shared signals reflect current social associations. However, when vocal and genetic variation arises among populations, shared learned signal variants have a potential to reflect cultural or genetic origin and to limit social and reproductive intermixing, provided that signal learning occurs prior to dispersal. The red crossbill (Loxia curvirostra) is a bird species in which discrete contact call variants are associated with morphological variation, raising the possibility that learned calls play a role in limiting intermixing. I examined the process of early call learning to determine if contact call variants have a potential to limit intermixing in crossbills. I conducted a captive playback study to nestlings to evaluate potential learning predispositions. I also cross-fostered nestlings to adoptive adult pairs of either their own or a different call variant than their biological parents to assess the degree of vocal learning plasticity. Results show that young crossbills imitate the call structures of adoptive parents, generating shared family-specific calls, which could facilitate family cohesion. Learning processes that generate family-specific calls could also ensure that discrete call variants are transmitted across generations, making call variants reliable signals of crossbills’ morphological and genetic backgrounds.
Keywords: Contact call, Flight call, Red crossbill, Call learning, Family-specific call
Introduction
Population-specific signals have the potential to serve as cultural isolating mechanisms—traits that promote social isolation, limit gene flow through prezygotic processes, and facilitate other mechanisms of divergence and speciation (Irwin and Price 1999; Lachlan and Servedio 2004; Price 1998; Servedio et al. 2009). In species that learn their vocalizations through imitation, such as birds, the learning process determines the potential for shared signals to serve as cultural isolating mechanisms. In many cases, birds learn new vocalizations throughout life as they integrate into new social groups and populations, with the result that cultural signals reflect current social affiliations and do not limit social intermixing (Baker 2000; Farabaugh et al. 1994; Hultsch and Todt 2004; Mundinger 1979; Nowicki 1989; Wright and Wilkinson 2001). However, if birds learn the vocalizations of members of their natal group or population, maintain those signals after dispersal, and assort based on those vocalizations, shared signals can reliably reflect individuals’ social and genetic backgrounds and serve as cultural isolating mechanisms (Grant and Grant 1996; Price 1998; Freeberg 2000; Lachlan and Servedio 2004; MacDougall-Shackleton and MacDougall-Shackleton 2001; Servedio et al. 2009).
A number of developmental mechanisms, including learning predispositions, social experience, and constraints on production, could ensure that young birds learn the signals of their natal groups or populations and reliably link vocal signals with genetic lineages. Learning predispositions and social experience can canalize the initial memorization phase of vocal learning by influencing which tutor or model signals an animal attends to and commits to memory, thereby limiting the scope of signals that may eventually be produced (Baptista and Petrinovich 1984; Kroodsma and Pickert 1984; Marler 1997; Marler and Nelson 1993; Marler and Peters 1989; Nelson and Marler 1993; Nelson 2000; Nordby et al. 2000). Morphological constraints on production and social experience can influence the later production phase of vocal learning by biasing which subset of previously memorized signals an animal generates (Marler and Peters 1982; Nelson and Marler 1994; Podos and Nowicki 2004; West and King 1985). Together, these mechanisms may reliably link the vocal signal an animal produces with its genetic background and permit learned vocal signals to serve as cultural isolating mechanisms (Freeberg 2000; Servedio et al. 2009).
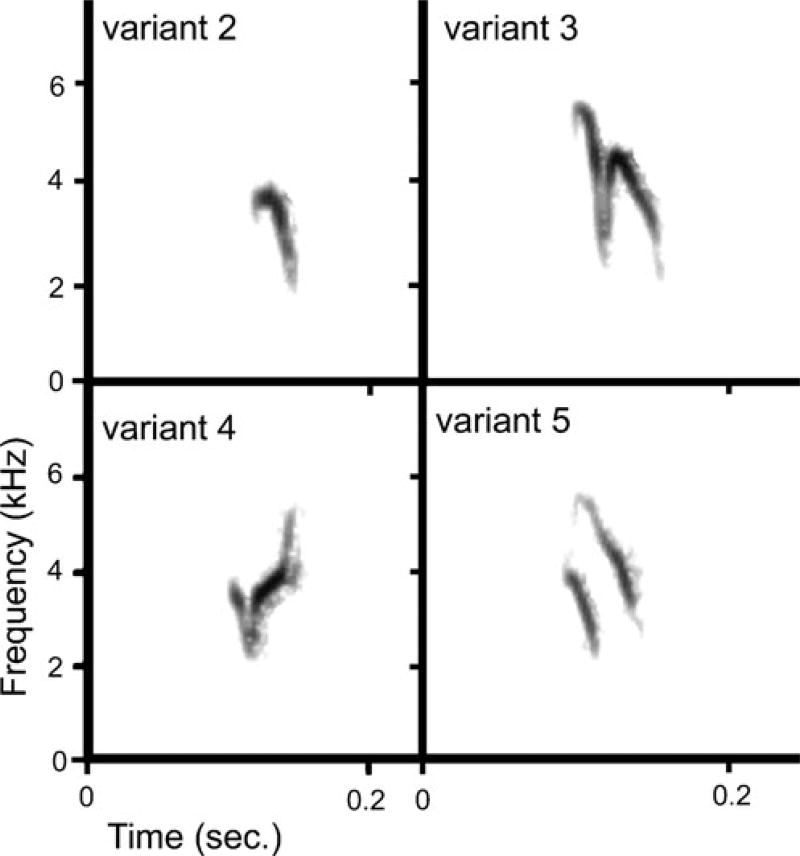
Contact calls are a class of vocalizations that have a special potential to serve as cultural isolating mechanisms because these calls mediate social assortment in many birds, positioning contact calls to drive or maintain social isolation (Marler 2004). For instance, in red crossbills (Loxia curvirostra), discrete contact call variants are associated with morphological variation (Groth 1988, 1993), suggesting that call variants do reflect genetic background and may play a role in limiting the intermixing of different lineages (Snowberg and Benkman 2007; Sewall and Hahn 2009). Crossbills in North America are composed of several sympatric ecomorphs (see “Methods” for terminology) that exhibit variation in body size and beak morphology, thought to facilitate specialization on different conifer resources (Benkman 1993; Benkman 2003; Groth 1988; Groth 1993). There are data to support the categorization of some crossbill ecomorphs as distinct species (Benkman et al. 2009; Groth 1993; see also Summers et al. 2007). However, morphological variation among other North American ecomorphs can be overlapping and genetic differences are insufficient to define some ecomorphs as separate species (Parchman et al. 2006; Questiau et al. 1999), perhaps because their divergence is too recent for neutral genetic substitutions to have accumulated (Summers et al. 2007). Thus, it has been argued that some crossbill ecomorphs, including those in the present study, are still incipient species in the process of diverging (Parchman et al. 2006). Though extremely strong selection against hybrids may achieve the divergence of crossbill ecomorphs (e.g., Benkman 2003), ecological selection could be facilitated by a cultural isolating mechanism such as vocalizations (Price 1998; Servedio et al. 2009). Discrete contact call variants are well positioned to serve as cultural isolating mechanisms in crossbills because they are the most conspicuous difference among ecomorphs (Fig. 1), they mediate the assortment of foraging flocks and breeding pairs (Adkisson 1996; Smith et al. 1999; Smith and Benkman 2007; Summers et al. 2007; Sewall and Hahn 2009), and adult birds rarely change the call variant that they produce (one of 79 wild adult crossbills changed call variant over more than one year (Keenan and Benkman 2008); none of 42 captive adult crossbills changed call variant over 2 years (Sewall 2009)). However, for discrete contact call variants to serve as cultural isolating mechanisms, young birds would have to initially learn the call variant appropriate for their lineage from members of their family or natal group.
Fig. 1.
Spectrograms of the four crossbill contact call variants (2, 3, 4, and 5) used in these studies
Evidence that crossbills initially learn their calls comes from a cross-fostering study of four wild-caught fledglings, in which two fledglings imitated the calls of their adoptive parents of a different ecomorph (Groth 1993). In addition, field observations show that young crossbills produce calls that are more similar to those of their parents than to those of other birds living in the area (Keenan and Benkman 2008). However, it is not clear how the developmental mechanisms of vocal learning direct the acquisition of call variants by young birds. I examined the possible contributions of learning predispositions, constraints on call production, and social experience because these processes can all help canalize vocal learning and reliably link a bird’s vocal production with its genetic background. Specifically, I conducted a playback study to nestling crossbills reared by captive pairs to determine if they were predisposed to attend to particular call variants. I then cross-fostered nestlings to adults (hereafter adoptive pairs/parents) of their own call variant and ecomorph or to adoptive pairs of a different call variant and ecomorph to examine the contribution of social experience to call development. I evaluated the precision of vocal imitation by these cross-fostered nestlings as one approach to examining possible constraints on call variant production; birds cross-fostered to adoptive pairs of a different ecomorph would have genetic and morphological backgrounds mismatched with the call variant they learned (morphology has high heritability; Summers et al. 2007), which could impair the accuracy of their vocal limitations. In addition, I cross-fostered nestlings to a pair in which the male and female produced different call variants and were of different ecomorphs to determine if nestlings had a bias to memorize or produce the call variant associated with their genetic and morphological background.
Methods
Terminology
The vocal, morphological, and ecological variation within red crossbills was originally described by Groth (1988, 1993) who designated crossbill variants as “types,” numbered in the order in which they were discovered. Groth used both learned calls and morphological measurements to define “types.” Here, to distinguish the vocalizations (i.e., the behavior/cultural phenotype) from the ecological and morphological forms of crossbills (i.e., the genetic lineage/ morphological phenotype), I refer to Groth’s “types” as ecomorphs.
Subjects
The crossbill ecomorphs used as subjects in this study were morphs 2, 3, and 4 (Groth 1993). Ecomorph 3 is the smallest bodied North American crossbill form with a small bill that facilitates efficient foraging on conifers with small seeds and papery scales, such as western hemlock (Tsuga heterophylla, Benkman 1993; Groth 1993). Ecomorph 4 is a medium-sized bird that performs better on medium-sized cones such as Douglas fir (Pseudotsuga menziesii, Benkman 1993; Groth 1993). Ecomorph 2 is one of the larger crossbill forms with a robust bill that permits efficient foraging on large tough cones such as ponderosa pine (Pinus ponderosa, Benkman 1993; Groth 1993). Birds of ecomorph 4 commonly co-occur with both ecomorph 3 and ecomorph 2, but ecomorph 2 and 3 co-occur much less frequently in the wild, presumably owing to their more distinct foraging preferences and the distributions of the different conifers (T. P. Hahn, personal communication).
I captured the adults used in these studies (N=20) in Oregon and Washington, USA, over the summers of 2003 and 2004 (plus four females reared in captivity). Every adult in this study produced a call variant consistent with its ecomorph; call variants are stable in adult birds (Sewall 2009) and were stable in all adults in this study. The experimental juveniles in this study were reared during the springs and summers of 2006 and 2007. I permitted adult birds to form pairs and housed each pair in an individual flight cage (2.5×2×1 m, Corners, Limited, Kalamzoo, MI, USA) within a single room such that birds of two other call variants were adjacent to each breeding pair. This ensured that nestlings were exposed to calls of their adoptive parents’ variant prior to experimental manipulations. Six homotypic call variant and ecomorph pairs (four ecomorph 3 and two ecomorph 4 pairs) bonded and produced young. Five bonded homotypic ecomorph pairs (one ecomorph 2, two ecomorph 3 and, and two ecomorph 4 pairs) as well as one un-bonded heterotypic pair (call variant and ecomorph 3 female and call variant and ecomorph 4 male) served as adoptive pairs. Though the heterotypic pair did not engage in breeding behavior, the birds were affiliative and both parents fed and cared for the nestlings, making it unlikely that the failure of the parents to bond affected the vocal learning of the experimental subjects.
I assigned and cross-fostered nestlings to adoptive pairs of their biological call variant and ecomorph (control group, N=8) or a different call variant and ecomorph (experimental group, N=10) such that siblings were in different treatments and three or four nestlings were in each adoptive family, when the youngest nestling in a brood was 5 days old (7±2 days post-hatch). In addition, I cross-fostered five nestlings to the single heterotypic pair to explore possible biases to memorize and produce particular call variants (learning biases group, N=5). I housed each adoptive family in a separate cage, each within a separate sound attenuation chamber (IAC mini booths, Industrial Acoustics, Bronx, NY, USA).
I measured the mass, wing length, tarsus length, and bill depth of each cross-fostered subject at maturity (ca. 1 year post-hatch). I used these measures, along with measurements from 780 wild ecomorph 2 (N=117), 3 (N=361) and 4 (N= 302) red crossbills, to classify each nestling by morphology using linear discriminant analysis (LDA; Statistica Version 6.0, Statsoft, Tulsa, OK, USA). Briefly, using the database of body measurements taken from adult crossbills immediately after capture in the wild, I built a forward stepwise LDA (following StatSoft 2001) that categorized each bird by ecomorph, based on its morphological measurements. Each bird in the database had also been assigned a call variant based on aural assessment of its contact calls (researchers aurally categorized crossbill contact calls with 96% accuracy in a previous study; Sewall 2009). The LDA categorization of wild adult crossbills by their morphology was consistent with the assignment based on call variant in 84% of the cases (ecomorph 2 assignment = 78% agreement, ecomorph 3 assignment = 93% agreement, ecomorph 4 assignment = 75% agreement). The captive-raised juveniles were added to the LDA to determine their ecomorph assignment based on morphology.
Nestling predisposition playback
I used a playback protocol modified from Nelson (2000) to test nestling crossbills for learning predispositions, which I functionally defined as selective responsiveness to their biological contact call variant prior to the period when vocal memorization of tutor signals is reported to occur in many song birds (10 days post-hatch; reviewed in Nowicki et al. 1998). Briefly, on the day of cross-fostering (7 ± 2 days post-hatch), I placed each nestling from the control and experimental cross-fostering groups individually in a fabricated nest within a sound attenuation chamber, equipped with a video camera (Sony Handycam HDR-CX100, New York, NY, USA) and a playback speaker (Optimus PROX5, Chicago, IL, USA) attached to a Dell computer (Latitude D630, Dell, Round Rock, TX, USA) running Windows Mediaplayer (Microsoft, Redmond, WA, USA) and a playback speaker (Optimus PROX5, Chicago, IL, USA) attached to a Dell computer (Latitude D630, Dell, Round Rock, TX, USA) running Windows Mediaplayer (Microsoft, Redmond, WA, USA). I hand-fed each nestling to satiation and then permitted to acclimate for 30 min so that it was somewhat hungry at the beginning of the playback sequence. I played each nestling a unique exemplar of its biological parents’ call variant (variant 3 or 4), two familiar crossbill call variants it had been exposed to during early development (variant 2 and variant 3 or 4), a novel crossbill call variant it had not been exposed to (variant 5) and a heterospecific call (evening grosbeak, Coccothraustes vespertinus, another Cardueline finch that co-occurs with crossbills in the wild) in randomized order with 2 min of silence between stimuli. I generated playback files using a protocol described in detail elsewhere (Sewall and Hahn 2009). I video- and audio-recorded all trials and scored the response of each nestling on a scale of 1 to 3 based on whether the subject engaged in begging (score of 3), neck extensions (score of 2) or repositioning in the nest (score of 1; Nethersole-Thompson 1975). Two nestlings did not respond to any stimuli and were omitted from the statistical analysis. Further, because many of the nestlings were siblings, it could not be assumed that their responses were independent. Therefore, I averaged the response scores of siblings (N=6 families of siblings) and used a Friedman ANOVA to assess differences in response to calls of different categories (Searcy et al. 1997). This non-parametric test uses the number of dependent variables (in this case, the number of call categories, five) to determine the degrees of freedom, not the number of subjects. I then used post-hoc pair-wise comparisons of the summed scores for each call category, as described by Conover (1980), to identify differences in nestlings’ responses to different call categories.
Acoustic and statistical analysis
I recorded the vocalizations of each biological parent, adoptive adult, and juvenile (after their calls stabilized, ca. 60 days post-hatch, the calls of all birds in this study were stable 1 year later, unpublished data) by transferring them to a sound attenuation chamber equipped with a Sennheiser ME62 omnidirectional microphone attached to either an analog recorder (Sony TCM 5000EV) or a mini-disk recorder (Sony MZ-M200, non-compressing). I subsequently digitized analog recordings at a 22,050-Hz sampling rate using Syrinx software (Burt 2006) and down-sampled digital recordings made at 44,000 to 22,050 Hz using Avisoft software (Specht 2007).
I evaluated call imitation using two approaches described in detail elsewhere (Sewall 2009), but a brief synopsis follows. Spectrograms of five to nine (mode of 9) calls from each captive raised bird, biological parent, and adoptive adult were made for these analyses (FFT length 512, Hamming window, temporal resolution = 23 ms, frequency resolution = 43 Hz). In the first approach, I evaluated how similar the calls of control and experimental juveniles were to those of their adoptive and biological parents, on average, by comparing the mean values from spectrogram cross-correlation (SPCC) analyses run in Avisoft (Baker and Logue 2003; Clark et al. 1987; Nowicki and Nelson 1990). In the second approach, I used LDA of acoustic parameter measurements made in Avisoft to examine the precise nature of call imitation on a case-by-case basis (Wanker et al. 2005).
Spectrogram cross-correlation generates a similarity value, R, for two spectrograms (Baker and Logue 2003; Clark et al. 1987; Nowicki and Nelson 1990). I averaged the R values for all possible comparisons of calls from juveniles and adults recorded at the same point in time to generate one mean SPCC R value for each subject with its biological parents and one mean R value with its adoptive parents. This data set consisted of a hierarchically structured combination of fixed (e.g., cross-fostering treatment) and random (e.g., biological parents, adoptive adults) effects. Therefore, I analyzed these data using a multi-level mixed-effects linear regression (GLMM, lme package in R software, 2.7.2, Foundation for Statistical Computing, R Development Core Team 2008), which uses t-tests to test the null hypothesis that a coefficient equals 0 (Bolker et al. 2009; Crawley 2007). This model estimates parameters with restricted maximum likelihood (REML) and models random factors as an intercept. The complete GLMM aimed to determine if juveniles in the control and experimental groups produced calls that were more similar to those of their adoptive parents, compared to those of their biological parents, on average. I used additional GLMMs to compare call similarity with adoptive and biological parents for control and experimental juveniles, separately. I also ran a separate GLMM of SPCC R values to specifically compare the similarity of experimental and control juveniles’ calls with their adoptive parents only. This model was designed to determine if experimental birds imitated call variants mismatched to their ecomorph with the same accuracy that control birds imitated calls of their biological variant.
LDA of acoustic parameters permitted me to determine which adult each juvenile came to imitate most precisely. The automated parameter measurement function in Avisoft was used to measure the duration of each of the 7±2 calls from each bird, and the entropy and frequency at four equidistant points within each call to best capture differences in the temporal patterns of frequency sweeps among the call variants (Fig. 1). I then conducted forward stepwise LDA of these acoustic measures to map each of the 7±2 calls of each family member onto canonical root space, which represented the acoustic space used by the birds (Wanker and Fischer 2001). I used the classification of cases generated through LDA to determine which adult(s) and sibling(s) juveniles imitated; I defined imitation as occurring when calls produced by two individuals were not statistically discriminable by LDA. Linear discriminant analysis was the only approach I used to assess which adult the juveniles from the learning biases group imitated (i.e., the adult of their biological or of a different call variant and ecomorph).
Results
Early attention to familiar call variants
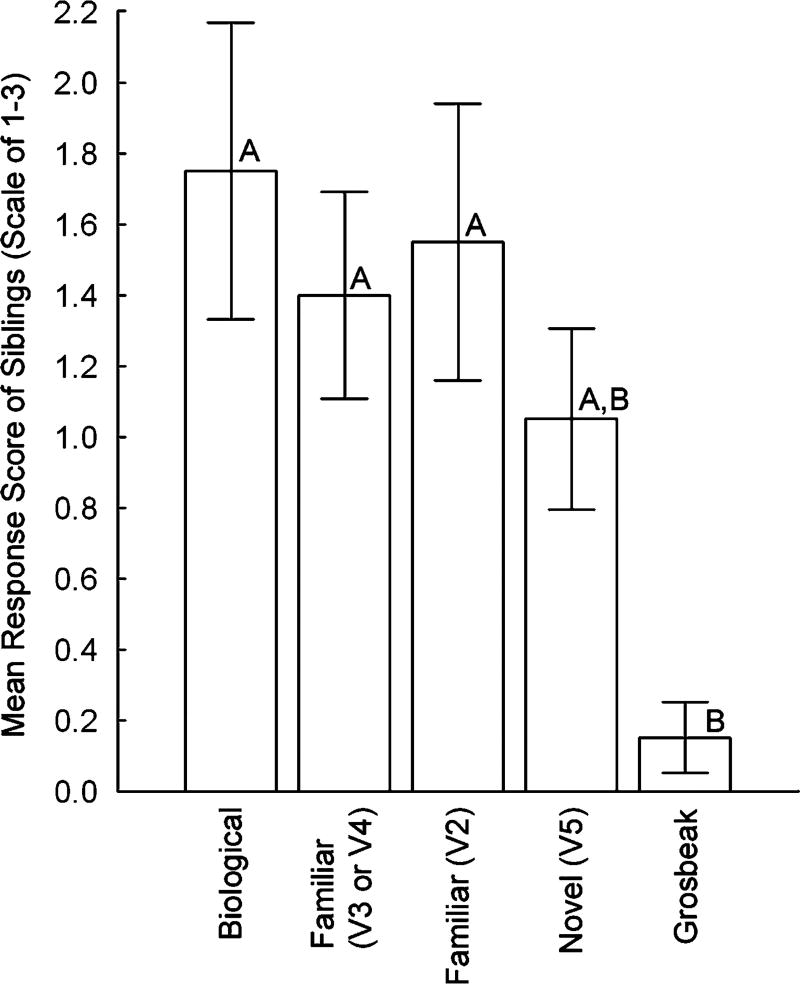
Overall, nestlings responded differently to the different call categories (Friedman ANOVA: , p<0.005). Specifically, nestling crossbills responded more strongly to crossbill call variants that they had been exposed to during early development than to heterospecific calls (post-hoc comparisons, biological variant, familiar variant 3 or 4, and familiar variant 2 vs. heterospecific, all p<0.05; Fig. 2). There were no other statistically significant differences between call categories. Thus, there was no evidence that nestlings responded selectively to calls of their biological call variant (post-hoc comparisons, biological variant vs. familiar variants 3 or 4, familiar variant 2, and novel variant, all p>0.05; Fig. 2) nor can it be concluded that nestlings responded significantly more to all crossbill call variants compared to heterospecific calls, because there was no statistically significant difference in response to novel crossbill calls and heterospecific calls (post-hoc comparison, novel vs. Grosbeak, p>0.05).
Fig. 2.
The responses of 16 nestlings aged 7±2 days to playbacks of unique exemplars of four different crossbill contact call variants and a heterospecific contact call: their biological call variant (variant 3 or 4), two crossbill call variants they had been exposed to (variant 3 or 4 and variant 2), a novel crossbill call variant they had never been exposed to (variant 5) and a heterospecific evening grosbeak call they had never been exposed to. Significant pair-wise comparisons between nestlings’ responses to different call categories are indicated with letters (different letters denote groups that were significantly different from one another)
Social learning directs early contact call production
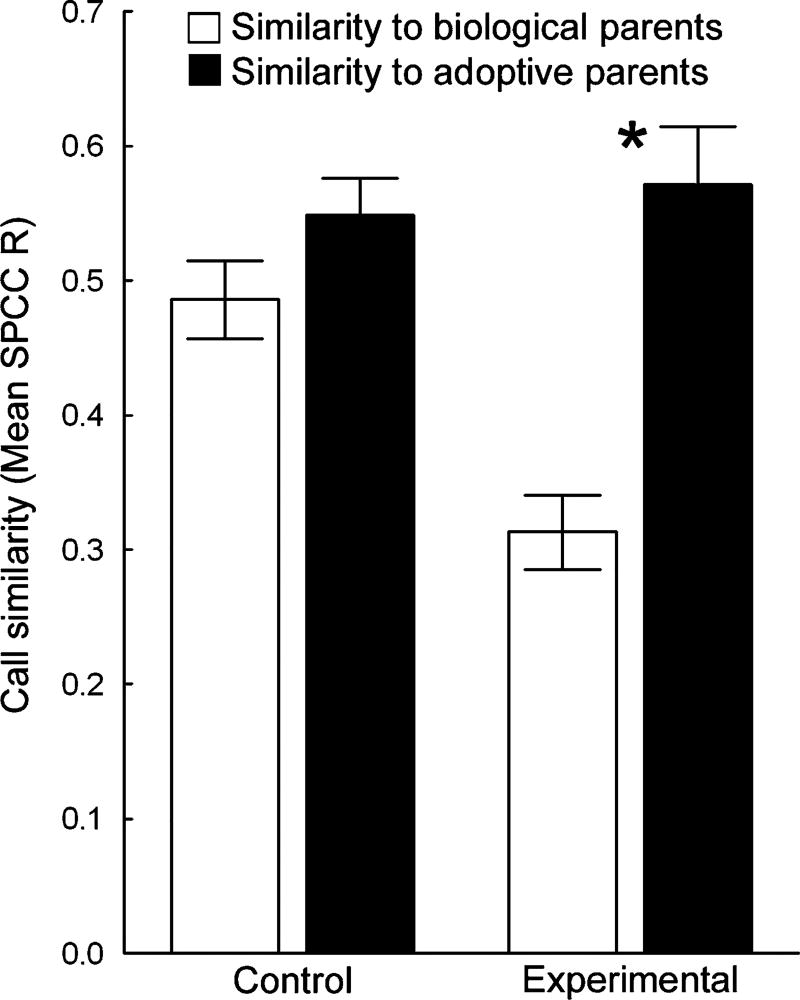
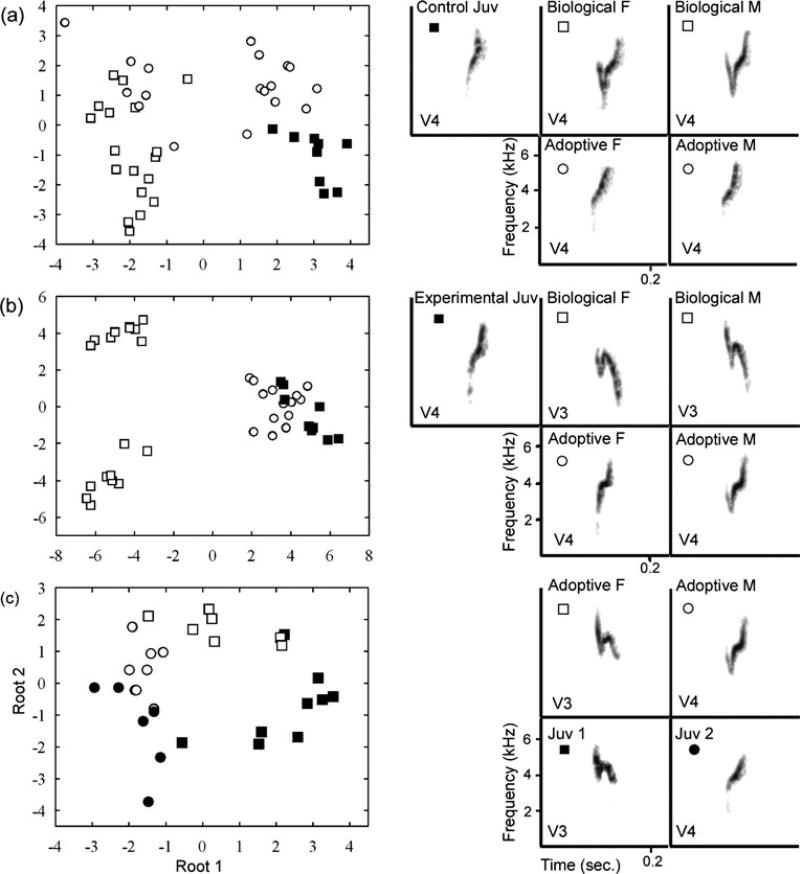
Overall, results of the GLMM of SPCC R values and examination of the LDA analysis revealed that juveniles in both the experimental and control cross-fostering groups imitated the call structure, and hence the variant, of their adoptive parents (Figs. 3 and 4). Specifically, there was a significant interaction between cross-fostering treatment and call similarity (measured as mean SPCC R value) with biological, as opposed to adoptive parents (GLMM, t25= −3.516, p=0.004; Fig. 3). There was a significant interaction in this model but there were no significant main effects (GLMM, main effect of call similarity with biological/ adoptive parents, t25=−1.364, p=0.185; GLMM, main effect of cross-fostering treatment, t3=0.359, p=0.743) because the calls of control birds were more similar to those of their biological parents than were the calls of experimental birds (additional GLMMs, no significant difference between call similarity with biological/adoptive parents for control subjects only, t11=−1.646, p=0.128; significant difference between call similarity with biological/adoptive parents for experimental subjects only, t14= −5.672, p<0.001; Fig. 3). The LDA analysis corroborates the results of the spectrographic cross-correlation: 17 of the 23 juveniles in the three cross-fostering treatments imitated the calls of at least one adoptive parent (for examples, see Fig. 4). In the six cases in which juveniles did not produce clear imitations of their adoptive parents’ calls, four birds imitated the calls of genetically unrelated “foster siblings” and one juvenile in the control group and one juvenile in the learning biases group produced calls of the same variant as (one of) their adoptive parents but their calls were distinct from those of all other family members. It is worth noting that all juveniles produced calls with sufficient variation to permit individual identification, even if many of their calls were statistically indiscriminable from those of a tutor.
Fig. 3.
The mean SPCC R values ± standard error for juveniles in the control and experimental groups when their calls were compared to those of their biological (open bars) and adoptive (closed bars) parents
Fig. 4.
Representative spectrograms and LDA plots representing the acoustic space filled by 7±2 calls from each subject and adult in three cross-fostering treatment groups: a a control juvenile (Juv, closed squares), the biological female (F) and male (M, open squares) and the adoptive female and male (open circles), (b) an experimental juvenile, its adoptive and biological parents (same symbols), and (c) two juveniles from the learning biases group (closed squares, closed circles) with the adoptive female of their biological call variant and ecomorph (open squares) and male of a different call variant and ecomorph (open circles). The call variant (V) of each subject is indicated. Note that each LD space is unique to the family analyzed and LDA axes therefore differ across the figure subdivisions
No evidence of constraint on call variant production
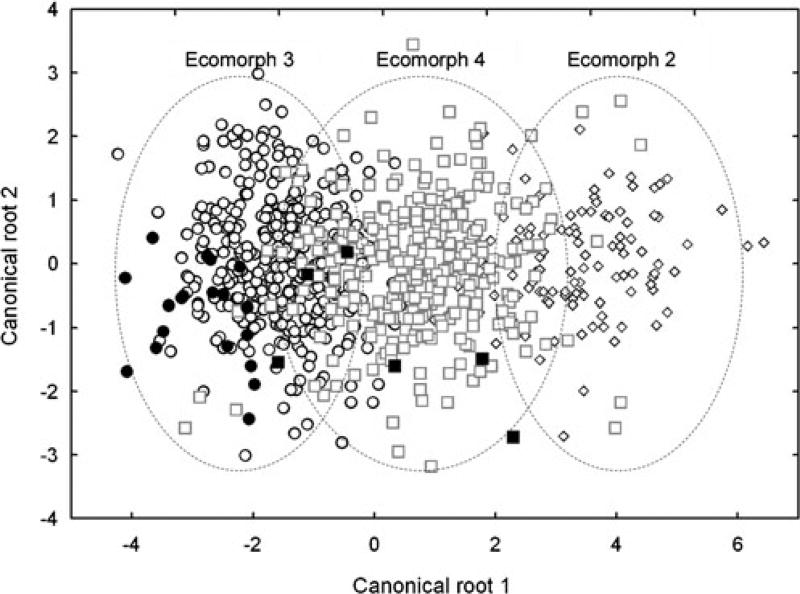
Not surprisingly, juveniles developed morphologies that were consistent with those of their biological parents’ ecomorph (Summers et al. 2007). Overall, the LDA morphological categorization of 20 of the 23 cross-fostered juveniles was consistent with the ecomorph assignment of their biological parents (87% agreement of LDA and parental ecomorph; Fig. 5). The three juveniles that were misclassified by LDA were from the control cross-fostering treatment, so their morphology could not be caused by being reared by adults of a different ecomorph.
Fig. 5.
A LDA plot representing the morphological space filled by wild red crossbills assigned as producing call variant 2 (open diamonds), 3 (open circles), and 4 (open squares; each point represents measurements from a single individual). The approximate perimeter of the LDA space representing the morphological space occupied by birds producing each call variant is included as a visual aid only. The morphological classification of cross-fostered juveniles of ecomorph 3 (closed circles) and 4 (closed squares) are also indicated
Despite the fact that juveniles in the experimental treatment had morphologies, and presumably genetic backgrounds, that were mismatched to those of their adoptive parents, they imitated the calls of their adoptive parents just as well as control juveniles did; there was no difference between experimental and control juveniles’ mean SPCC R values with adoptive parents (GLMM, call similarity with adoptive parents only, no significant main effect of treatment, t3=0.424, p=0.700; Fig. 3, closed bars). Further, there was no evidence that morphology or lineage influenced the selection of tutors or model signals; two of the five nestlings in the learning biases treatment group imitated the adult of a different ecomorph and call variant (Fig. 4c). None of the juveniles in the learning biases group were bilingual (produced both call variants) and there was no evidence that male and female juveniles were biased in imitating the male or female parent in any of the cross-fostered families.
Discussion
Understanding the potential for cultural signals to drive or maintain social and genetic isolation requires examining the process of signal learning. Here, I assessed the possible contributions of learning predispositions, social experience, and morphological constraints to the development of contact call variants associated with red crossbill ecomorphs. In a playback study assessing learning predispositions, nestlings did not respond selectively to calls of their biological variant but they did respond more strongly to calls that they were exposed to during early development, suggesting that social experience directs early attention to call variants. In subsequent cross-fostering studies, all subjects imitated the calls, and hence the call variant, of adoptive parents or siblings regardless of their morphology, supporting the role of experience in directing call production learning and indicating that morphology does not constrain call variant production. Collectively, these studies provide evidence that social experience during both the initial memorization and the latter production phases of learning directs the development of specific call variants in red crossbills. Such socially directed learning has a potential to transmit contact calls with fidelity across generations and to make call variants reliable indicators of crossbills’ ecomorphs.
The early memorization phase of learning could be influenced by learning predispositions, which were functionally defined as selective responsiveness to biological signal variants, or by social experience. Nestlings in the playback study did not respond selectively to the call variant of their biological parents; there was no evidence that nestlings discriminated between calls of their biological variant and the two other crossbill call variants that they were exposed to early in life (Fig. 2). Thus, the present playback study does not provide evidence of learning predispositions in red crossbills. Further, there was insufficient evidence to conclude that nestlings’ responded selectively to crossbill calls over heterospecific calls, as there was no statistically significant difference in nestlings’ responses to novel crossbill calls and evening grosbeak calls (Fig. 2). A future playback study in which crossbill nestlings are exposed to heterospecific calls, as well as crossbill call variants, could test for underlying predispositions to attend to species-typical calls that were overwhelmed by social experience in the present study design; stronger response to heterospecific calls heard during early development would rule out an underlying learning predisposition. Based on the present data, it seems likely that early experience alone directs attention to specific calls, as nestlings in the present study responded significantly more strongly to call variants they had been exposed to during approximately the first week of life, compared to heterospecific calls (Fig. 2).
Consistent with the evidence that experience directs crossbills’ attention to specific calls during the memorization phase of learning, the results of the cross-fostering studies support the role of social experience in directing the production of call variants. Juvenile crossbills in these studies learned to produce contact calls nearly identical to those of their adoptive parents and siblings, which is evidence that social experience directed imitative learning (Figs. 3 and 4). There was no evidence of morphological constraints on the imitation of different call variants in the crossbill ecomorphs included in this study. Juveniles developed morphologies that were consistent with those of their biological ecomorph (i.e., LDA classification of experimental birds by morphology was consistent with their genetic/parental ecomorph; Fig. 5), yet subjects in the experimental group, whose morphologies and genetic backgrounds were mismatched to those of their adoptive parents, copied the structure of their adoptive parents’ calls just as well as control juveniles (Fig. 3, closed bars). There was no evidence that young crossbills were biased in their selection of tutors or model signals associated with their genetic and morphological background either; two of the five juveniles in the learning biases group imitated the contact calls of the adoptive adult of a different ecomorph to produce a call variant that was mismatched with their lineage and morphology (Fig. 4). It is possible that the very early exposure (prior to 9 days post-hatch) that these subjects had to multiple crossbill call variants made them more plastic and better able to learn and imitate the calls of other crossbill ecomorphs. However, crossbills of the ecomorphs in this study do breed and nest sympatrically and young birds almost certainly hear multiple call variants during early development, at least in some years. Thus, the vocal plasticity described here is likely consistent with that of free-living crossbills.
Imitative learning that generates contact calls with features shared by members of families or social groups, as is documented here in red crossbills, has been observed in a number of other bird species (Bartlett and Slater 1999; Hile et al. 2000; Hile and Striedter 2000; Mammen and Nowicki 1981; Mundinger 1970, 1979; Nowicki 1989). Resulting group- and family-specific calls are thought to facilitate the identification of companions and the coordination of social behaviors (Mammen and Nowicki 1981; Farabaugh and Dooling 1996; Tyack 2008). Learning processes that generate family-specific calls may be especially beneficial in red crossbills because the beaks of young birds are initially uncrossed and only reach adult morphology at approximately 45 days of age (Adkisson 1996; unpublished data from present study), making fledglings dependent upon their parents through the period when many songbirds are reported to memorize tutors’ vocalizations (10–50 days post-hatch; reviewed in Nowicki et al. 1998). Further, in a captive cross-fostering study, Groth (1993) showed that two wild-caught fledglings that were feeding independently did not imitate the calls of adults of a different variant and ecomorph, suggesting that early imitative learning is promoted by interactions specific to families and that learning is limited once independence is reached. Crossbills’ unique foraging adaptation and resulting parental dependence may select for early imitative vocal learning that generates family-specific calls, and this learning process could also ensure that discrete call variants are transmitted with fidelity across generations.
The benefits of family-specific calls may have originally selected for early socially directed call learning in red crossbills and maintained discrete call variants when they emerged (Irwin and Price 1999). Now that crossbills exist as morphologically and culturally distinct groups, it is possible that selection also currently favors early social learning of contact calls because it reliably associates call variants with ecomorphs. Red crossbills are thought to benefit from flocking and mating with companions of the same ecological specialization and ecomorph because they pool information about foraging success (Smith et al. 1999) and hybrid offspring of birds of different ecomorphs are argued to suffer greatly reduced fitness (Benkman 1993; Benkman 2003). Early call learning from family members may facilitate family cohesion and concurrently ensure that discrete contact call variants reliably reflect ecomorph, permitting calls to mediate assortment based on ecological specializations and genetic background. The present work, in conjunction with previous studies showing that crossbills maintain and assort by discrete contact call variants in adulthood (Smith and Benkman 2007; Summers et al. 2007; Keenan and Benkman 2008; Sewall 2009; Sewall and Hahn 2009), supports the role of contact call variants as cultural isolating mechanisms in red crossbills.
Acknowledgments
I thank TP Hahn, PR Marler, GL Patricelli, VV Pravosudov, EP Derryberry, KE Mabry, S Nowicki, S Peters, RC Anderson, RF Lachlan, JA Soha, IA Liu, JM Cornelius and TR Kelsey. Funding was provided by the Animal Behaviour Society, the American Ornithologist’s Union, the UC Davis Department of Neurobiology Physiology and Behavior and the UC Davis Animal Behavior Graduate Group. This research was carried out under the approval of the UC Davis IACUC and with permits from the US Fish and Wildlife Service and the states of California, Washington and Oregon.
References
- Adkisson CS. Red crossbill (Loxia curvirostra) In: Poole A, editor. The birds of North America online. no. 256. Cornell Lab of Ornithology; Ithaca, New York: 1996. [DOI] [Google Scholar]
- Baker MC. Cultural diversification in the flight call of the ringneck parrot in Western Australia. Condor. 2000;102:905–910. [Google Scholar]
- Baker MC, Logue DM. Population differentiation in a complex bird sound: a comparison of three bioacoustical analysis procedures. Ethology. 2003;109:223–242. [Google Scholar]
- Baptista LF, Petrinovich L. Social-interaction, sensitive phases and the song template hypothesis in the white-crowned sparrow. Anim Behav. 1984;32:172–181. [Google Scholar]
- Bartlett P, Slater PJB. The effect of new recruits on the flock specific call of budgerigars (Melopsittacus undulatus) Ethol Ecol Evol. 1999;11:139–147. [Google Scholar]
- Benkman CW. Adaptation to single resources and the evolution of crossbill (Loxia) diversity. Ecol Monogr. 1993;63:305–325. [Google Scholar]
- Benkman CW. Divergent selection drives the adaptive radiation of crossbills. Evolution. 2003;57:1176–1181. doi: 10.1111/j.0014-3820.2003.tb00326.x. [DOI] [PubMed] [Google Scholar]
- Benkman CW, Smith JW, Keenan PC, Parchman TL, Santisteban L. A new species of the red crossbill (Fringillidae: Loxia) from Idaho. Condor. 2009;111:169–176. [Google Scholar]
- Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White JSS. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol. 2009;24:127–135. doi: 10.1016/j.tree.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Burt JM. Syrinx. 2.6h ed. Seattle, WA: 2006. http://www.syrinxpc.com/index.html. [Google Scholar]
- Clark CW, Marler P, Beeman K. Quantitative analysis of animal vocal phonology: an application to swamp sparrow song. Ethology. 1987;76:101–115. [Google Scholar]
- Conover WJ. Practical nonparametric statistics. 2. Wiley; NY: 1980. pp. 299–310. [Google Scholar]
- Crawley MJ. The R book. Wiley; West Sussex: 2007. pp. 627–660. [Google Scholar]
- Farabaugh SM, Dooling RJ. Acoustic communication in parrots: laboratory and field studies of budgerigars, Melopsittacus undulatus. In: Kroodsma DE, Miller EH, editors. Ecology and evolution of acoustic communication in birds. Cornell University Press; Ithaca, NY: 1996. pp. 97–117. [Google Scholar]
- Farabaugh SM, Linzenbold A, Dooling RJ. Vocal plasticity in budgerigars (Melopsittacus undulatus): evidence for social factors in the learning of contact calls. J Comp Psychol. 1994;108:81–92. doi: 10.1037/0735-7036.108.1.81. [DOI] [PubMed] [Google Scholar]
- Freeberg TM. Culture and courtship in vertebrates: a review of social learning and transmission of courtship systems and mating patterns. Behav Processes. 2000;51:177–192. doi: 10.1016/s0376-6357(00)00127-3. [DOI] [PubMed] [Google Scholar]
- Grant BR, Grant PR. Cultural inheritance of song and its role in the evolution of Darwin’s finches. Evolution. 1996;50:2471–2487. doi: 10.1111/j.1558-5646.1996.tb03633.x. [DOI] [PubMed] [Google Scholar]
- Groth JG. Resolution of cryptic species in Appalachian red crossbills. Condor. 1988;90:745–760. [Google Scholar]
- Groth JG. Evolutionary differentiation in morphology, vocalizations, and allozymes among nomadic sibling species in the North American red crossbill (Loxia curvirostra) complex. University of California Press; Berkley: 1993. [Google Scholar]
- Hile AG, Striedter GF. Call convergence within groups of female budgerigars (Melopsittacus undulatus) Ethology. 2000;106:1105–1114. doi: 10.1006/anbe.1999.1438. [DOI] [PubMed] [Google Scholar]
- Hile AG, Plummer TK, Striedter GF. Male vocal imitation produces call convergence during pair bonding in budgerigars, Melopsittacus undulatus. Anim Behav. 2000;59:1209–1218. doi: 10.1006/anbe.1999.1438. [DOI] [PubMed] [Google Scholar]
- Hultsch H, Todt D. Learning to sing. In: Marler P, Slabbekoorn H, editors. Nature’s music: the science of birdsong. Elsevier Academic; San Diego, CA: 2004. pp. 80–95. [Google Scholar]
- Irwin DE, Price T. Sexual imprinting, learning and speciation. Heredity. 1999;82:347–354. doi: 10.1038/sj.hdy.6885270. [DOI] [PubMed] [Google Scholar]
- Keenan P, Benkman CW. Call imitation and call modification in red crossbills. Condor. 2008;110:93–101. [Google Scholar]
- Kroodsma DE, Pickert R. Sensitive phases for song learning—effects of social interaction and individual variation. Anim Behav. 1984;32:389–394. [Google Scholar]
- Lachlan RF, Servedio MR. Song learning accelerates allopatric speciation. Evolution. 2004;58:2049–2063. doi: 10.1111/j.0014-3820.2004.tb00489.x. [DOI] [PubMed] [Google Scholar]
- MacDougall-Shackleton EA, MacDougall-Shackleton SA. Cultural and genetic evolution in mountain white-crowned sparrows: song dialects are associated with population structure. Evolution. 2001;55:2568–2575. doi: 10.1111/j.0014-3820.2001.tb00769.x. [DOI] [PubMed] [Google Scholar]
- Mammen DL, Nowicki S. Individual differences and within-flock convergence in chickadee calls. Behav Ecol Sociobiol. 1981;9:179–186. [Google Scholar]
- Marler P. Three models of song learning: evidence from behavior. J Neurobiol. 1997;33:501–516. [PubMed] [Google Scholar]
- Marler P. Bird calls: a cornucopia for communication. In: Marler P, Slabbekoorn H, editors. Nature’s music: the science of birdsong. Elsevier Academic; San Diego, CA: 2004. pp. 132–176. [Google Scholar]
- Marler P, Nelson DA. Action-based learning—a new form of developmental plasticity in bird song. Neth J Zool. 1993;43:91–103. [Google Scholar]
- Marler P, Peters S. Developmental overproduction and selective attrition: new processes in the epigenesis of birdsong. Dev Psychobiol. 1982;15:369–378. doi: 10.1002/dev.420150409. [DOI] [PubMed] [Google Scholar]
- Marler P, Peters S. Species differences in auditory responsiveness in early vocal learning. In: Dooling RJ, Hulse SH, editors. The comparative psychology of audition: perceiving complex sounds. Erlbaum; Hillsdale, NJ: 1989. pp. 243–273. [Google Scholar]
- Mundinger PC. Vocal imitation and individual recognition of finch calls. Science. 1970;168:480–482. doi: 10.1126/science.168.3930.480. [DOI] [PubMed] [Google Scholar]
- Mundinger PC. Call learning in the Carduelinae: ethological and systematic considerations. Syst Zool. 1979;28:270–283. [Google Scholar]
- Nelson DA. A preference for own-subspecies song guides vocal learning in a song bird. Proc Natl Acad Sci USA. 2000;97:13348–13353. doi: 10.1073/pnas.240457797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DA, Marler P. Innate recognition of song in white-crowned sparrows—a role in selective vocal learning. Anim Behav. 1993;46:806–808. [Google Scholar]
- Nelson DA, Marler P. Selection-based learning in bird song development. Proc Natl Acad Sci USA. 1994;91:10498–10501. doi: 10.1073/pnas.91.22.10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nethersole-Thompson D. Pine crossbills: a Scottish contribution. T and AD Poyser; Berkhamsted, UK: 1975. [Google Scholar]
- Nordby JC, Campbell SE, Burt JM, Beecher MD. Social influences during song development in the song sparrow: a laboratory experiment simulating field conditions. Anim Behav. 2000;59:1187–1197. doi: 10.1006/anbe.1999.1412. [DOI] [PubMed] [Google Scholar]
- Nowicki S. Vocal plasticity in captive black-capped chickadees: the acoustic basis and rate of call convergence. Anim Behav. 1989;37:64–73. [Google Scholar]
- Nowicki S, Nelson DA. Defining natural categories in acoustic signals: comparison of three methods applied to ‘chick-a-dee’ call notes. Ethology. 1990;86:89–101. [Google Scholar]
- Nowicki S, Peters S, Podos J. Song learning, early nutrition and sexual selection in songbirds. Amer Zool. 1998;38:179–190. [Google Scholar]
- Parchman TL, Benkman CW, Britch SC. Patterns of genetic variation in the adaptive radiation of New World crossbills (Aves: Loxia) Mol Ecol. 2006;15:1873–1887. doi: 10.1111/j.1365-294X.2006.02895.x. [DOI] [PubMed] [Google Scholar]
- Podos J, Nowicki S. Performance limits on birdsong. In: Marler P, Slabbekoorn H, editors. Nature’s music: the science of birdsong. Elsevier Academic; San Diego, CA: 2004. pp. 318–342. [Google Scholar]
- Price T. Sexual selection and natural selection in bird speciation. Philoso Trans Biol Sci. 1998;353:251–260. [Google Scholar]
- Questiau S, Gielly L, Clouet M, Taberlet P. Phylogeographical evidence of gene flow among common crossbill (Loxia curvirostra, Aves, Fringillidae) populations at the continental level. Heredity. 1999;83:196–205. doi: 10.1046/j.1365-2540.1999.00551.x. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing 2008 [Google Scholar]
- R Foundation for Statistical Computing; Vienna, Austria: ( http://www.R-project.org) [Google Scholar]
- Searcy WA, Nowicki S, Hughes M. The response of male and female song sparrows to geographic variation in song. Condor. 1997;99:651–657. [Google Scholar]
- Servedio MR, Saether SA, Saetre GP. Reinforcement and learning. Evol Ecol. 2009;23:109–123. [Google Scholar]
- Sewall KB. Limited adult vocal learning maintains call dialects but permits pair-distinctive calls in red crossbills. Anim Behav. 2009;77:1303–1311. [Google Scholar]
- Sewall KB, Hahn TP. Social experience modifies behavioural responsiveness to a preferred vocal signal in red crossbills, Loxia curvirostra. Anim Behav. 2009;77:123–128. [Google Scholar]
- Smith JW, Benkman CW. A coevolutionary arms race causes ecological speciation in crossbills. Am Nat. 2007;169:455–465. doi: 10.1086/511961. [DOI] [PubMed] [Google Scholar]
- Smith JW, Benkman CW, Coffey K. The use and misuse of public information by foraging red crossbills. Behav Ecol. 1999;10:54–62. [Google Scholar]
- Snowberg LK, Benkman CW. The role of marker traits in the assortative mating within red crossbills, Loxia curvirostra complex. J Evol Biol. 2007;20:1924–1932. doi: 10.1111/j.1420-9101.2007.01372.x. [DOI] [PubMed] [Google Scholar]
- Specht R. Avisoft-SAS Lab Pro. 4.40 ed. Sound Analysis and Synthesis Lab; Berlin, Germany: 2007. [Google Scholar]
- StatSoft I. Statistica (data analysis software system) (6) 2001 ( www.statsoft.com)
- Summers RW, Dawson RJ, Phillips RE. Assortative mating and patterns of inheritance indicate that the three crossbill taxa in Scotland are species. J Avian Biol. 2007;38:153–162. [Google Scholar]
- Tyack PL. Convergence of calls as animals form social bonds, active compensation for noisy communication channels, and the evolution of vocal learning in mammals. J Comp Psychol. 2008;122:319–331. doi: 10.1037/a0013087. [DOI] [PubMed] [Google Scholar]
- Wanker R, Fischer J. Intra- and interindividual variation in the contact calls of spectacled parrotlets (Forpus conspicillatus) Behaviour. 2001;138:709–726. [Google Scholar]
- Wanker R, Sugama Y, Prinage S. Vocal labelling of family members in spectacled parrotlets, Forpus conspicillatus. Anim Behav. 2005;70:111–118. [Google Scholar]
- West MJ, King AP. Social guidance of vocal learning by female cowbirds—validating its functional significance. J Comp Ethol. 1985;70:225–235. [Google Scholar]
- Wright TF, Wilkinson GS. Population genetic structure and vocal dialects in an Amazon parrot. Proc R Soc Lond B Biol Sci. 2001;268:609–616. doi: 10.1098/rspb.2000.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]