Abstract
Background:
The usage of antibiotics and antiseptics to washout the breast pocket, or to soak the breast implant during surgery, has come under scrutiny in recent times. Guidelines from the Centers for Disease Control and Prevention give no recommendation for or against the usage of antibiotics in this regard. They do however offer a weak recommendation for washing tissues with iodophor. This systematic review aims to investigate the efficacy and impact of such topical antibiotic or antiseptic usage in reducing infection rates.
Methods:
A systematic electronic search was performed on the PreMEDLINE, MEDLINE, EMBASE, and CENTRAL (Cochrane) databases from inception to April 2017. Reference search was performed manually through Scopus. Results of the searches were independently screened by 2 reviewers (A.F. and P.H.). Studies involving an implant or tissue expander, with appropriate controls were included. Meta-analyses were performed where possible and data summarized when not.
Results:
Three retrospective cohort studies were found to fit the review requirements. No randomized control trials were found. These studies covered a period of 1996–2010 for a total of 3,768 women undergoing augmentative surgery. The usage of antibiotics in pocket washout or implant immersion resulted in lower infection rates (RR = 0.52; P = 0.004; 95% CI = 0.34–0.81).
Conclusions:
There is a clinical benefit in using antibiotics for breast pocket irrigation and implant immersion. However, the quality of the evidence obtained in this review is low; hence, we recommend a randomized control trial for a higher level of evidence on this important issue.
INTRODUCTION
Infection rates in breast surgery are a considerable cause of morbidity and can lead to multiple secondary complications, compromising the success of surgery.1 A major category of aesthetic and reconstructive breast operations involve prostheses. These operations are at a higher risk of infections, due to the inclusion of a foreign body.2
There is a limited quantity of literature assessing the appropriate use of antibiotics and antiseptics in implant-based breast surgery. Consequently, there is controversy over the best method to administer antibiotic and antiseptic prophylaxis.
Recently, the Centers for Disease Control and Prevention (CDC) of the United States published guidelines for the prevention of surgical site infections.3 They recommended that surgeons follow established evidence-based protocols for their respective disciplines when using parenteral antibiotic prophylaxis and strongly recommend against providing additional intravenous (IV) antibiotics once incisions are closed.
There are currently no recommendations from the CDC on irrigation of tissues or soaking prosthetic devices in antibiotics.
The CDC recommends that surgeons consider the risk versus benefit trade-off when washing tissues with iodophor solution, as the net benefit is small and the evidence for that benefit is weak.4 There is no recommendation for the soaking of prosthetic devices in antiseptics.3
In Australia, the New South Wales Ministry of Health has recently issued a safety notice advising against the use of povidone-iodine for the irrigation or for soaking of prostheses. The reason for this recommendation is that this practice is “off label” and is not covered under the indications registered with the Therapeutic Goods Association of Australia.5
Two recent systematic reviews assessed the relationship of topical agents on implant-related complications. The review by Yalanis et al.6 looked at the impact of topical povidone-iodine on capsular contracture rates, whereas the review by Huang et al.7 assessed the impact of topical antibiotic prophylaxis on capsular contracture and other implant-related complications. Although Yalanis et al.6 reported infection rates with topical antibiotics, this was not a primary outcome of their study. Hence, the need remains for a systematic review to assess the existing evidence surrounding the use of topical antibiotics and antiseptic use in breast implant surgery.
Thus, this review aims to provide an evidence-based clinical tool for surgical-site infection prophylaxis in breast implant surgery and to help resolve some of the conjecture surrounding this practice.
METHODS
Search Strategy
A comprehensive search was performed in August 2016 and repeated in April 2017 on the databases of PreMEDLINE, MEDLINE, Embase, and Cochrane using the keywords: breast neoplasms, mastectomy, mammaplasty, breast implants, immersion, reconstructive surgical procedure, submerge, soak, anti-infective agent, iodine compounds, saline solution, infection, implant capsular contracture, and postoperative complication. A reference search was performed using Scopus, and all obtained articles were collated and reviewed.
The population examined was all women undergoing breast surgery, with the intervention being the usage of antibiotics or antiseptics in breast pocket irrigation or implant immersion, and the control being no agent used for the lavage or bath. The primary outcome examined was infection rates.
Inclusion and Exclusion Criteria
The results of the search were independently screened by 2 reviewers (A.F. and P.H.) for eligibility. The articles were assessed according to predetermined inclusion and exclusion criteria. Where disagreement occurred and a consensus could not be reached by discussion, it was put to a third party (S.W.) for resolution.
The inclusion criteria for studies to be incorporated were randomized control trials, prospective trials, retrospective studies, breast surgery with the use of implants or expanders, perioperative antibiotic use, irrigation of the breast pocket, and/or soaking of implant or expander in antibiotic solution, and documentation of infection rate.
Publications were excluded if they involved Case studies, lack of a comparator, experimental animal models, in vitro models, opinion pieces and letters to the editor, nonqualitative articles, revision breast surgery, or non-English text.
Assessment and Data Extraction
Selected articles were evaluated using the Newcastle-Ottawa Scale for methodological quality. This consists of 8 questions, awarding stars for meeting criteria, for a total of 9 stars. Four stars are available for questions under the selection criteria, 2 stars available for comparability, and 3 stars for questions relating to outcomes. Analysis was performed by A.F and P.H, and disagreements mediated by S.W.
Data from the included studies were extracted and compiled into a table. It encompassed key points such as author, date of published article, population, intervention (pre-, peri-, and postoperative), comparator, outcomes, type of surgical procedure, patient number, and mean age.
Data Analysis
A meta-analysis was performed on the data using RevMan (version 5.3) where applicable, otherwise the data were summarized and discussed as text. A fixed effect model was used, and the risk ratio was reported with 95% confidence intervals. A P value of < 0.05 was set to be statistically significant.
RESULTS
Search Results
The search yielded 353 records, of which 70 were removed as duplicates. Two hundred eighty-three records were screened based on titles and abstracts, and a further 254 were removed for failing to meet the criteria. The remaining 29 full-text articles were assessed for eligibility, and of these only 3 retrospective cohort studies fulfilled the review requirements (Fig. 1).8–10 No randomized control trials were found.
Fig. 1.
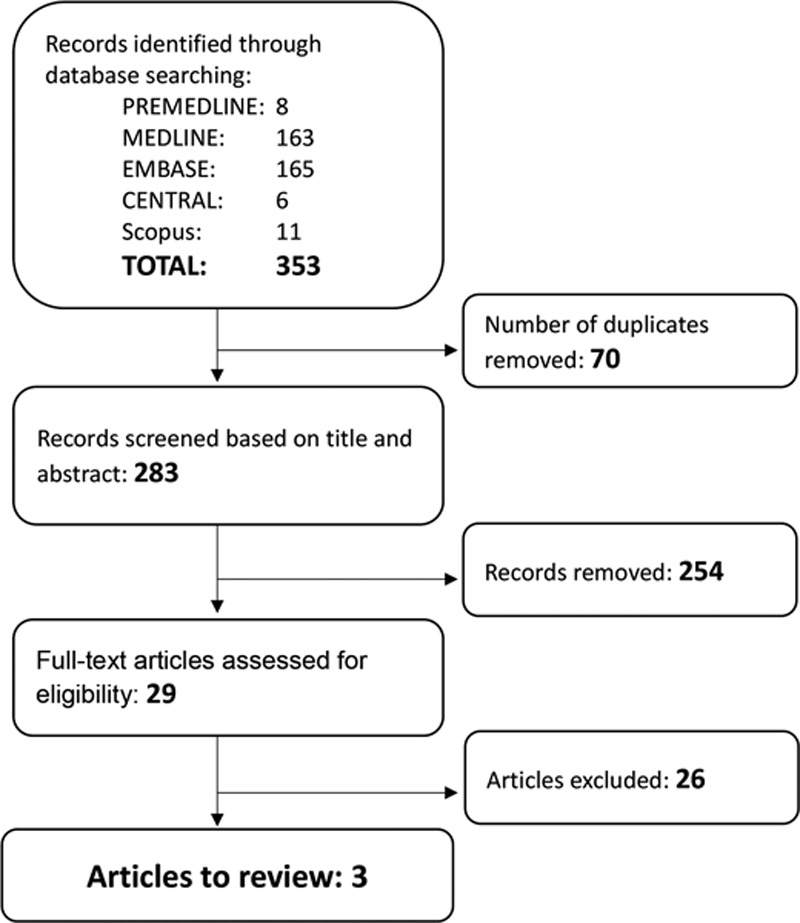
Summary of search results.
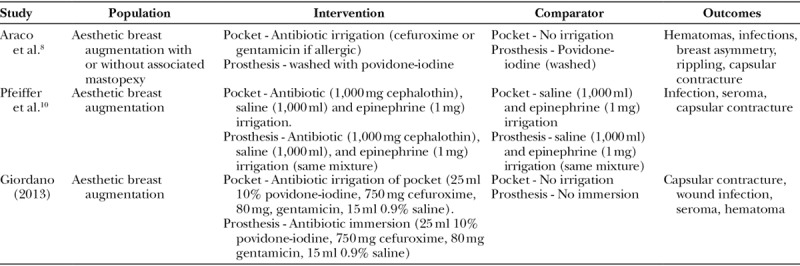
Each of these trials looked at populations of women undergoing aesthetic augmentative breast surgery. A summary of their populations, interventions, controls, and outcomes examined is provided in Table 1.
Table 1.
Summary of the Included Populations, Interventions, Controls, and Outcomes
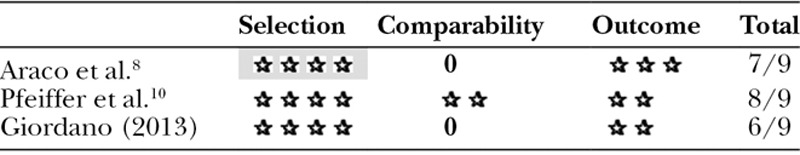
These 3 studies were appraised using the Newcastle-Ottawa Scale, obtaining an average of 7 out of 9 possible stars. This is summarized in Table 2.
Table 2.
Summary of Newcastle-Ottawa Scale Scores for Included Studies
The studies were published over the period of 2006–2013, involving 3,768 patients who had their surgery between 1996 and 2010. The primary outcome measured by Araco et al.8 was infection rates, while Pfeiffer et al.10 looked at infections and capsular contracture. Giordano et al.9 had their primary outcome as capsular contracture, but also included data on infection rates.
Breast Pocket Irrigation and Implant Immersion on Infection Rates
Araco et al.8 carried out a retrospective cohort study of women between January 1999 and December 2004. The study looked at aesthetic breast augmentation with or without associated mastopexy in 3,002 women who were patients of 2 surgeons.
There was no defined control group as it was a retrospective analysis of specific factors that could have influenced infection rates. Surgical procedure, postoperation outcomes, type of prosthesis used, and drains are examples of data extracted from the surgical records, which were then retrospectively analyzed.
Two surgical incisions techniques were used—inframammary (94.1%) for breast augmentation and periareolar (5.9%) for augmentation with associated mastopexy. Pockets and implants were created and placed with 4 different techniques: retroglandular (25%), subfascial (25%), retropectoral (33.3%), dual plane positioning (16.7%). Three different implants were used: Mentor (15.9%), Eurosilicone (58.7%), polyimplant prostheses (24.4%). Patients were given 750 mg of cefuroxime (or 1 g erythromycin for allergic patients) as an intravenous bolus 10–30 minutes before the commencement of the operation.
In this cohort, 1,902 patients had their breast pocket irrigated with cefuroxime and the prosthesis washed with povidone-iodine while 1,100 had their prosthesis bathed with povidone-iodine only. The reason for the different regimens between patients is unclear.
The exact mean follow-up period is difficult to determine, but they reported no losses to follow-up. The clinical examinations were performed at 7 and 30 days and then after 6 months. This would indicate that patients were followed up for at least 6 months.
Postoperative infections were 2 times greater in the povidone-iodine only group. The rate of infection was 15 (or 0.79%) in the antibiotic with povidone-iodine cohort, and 18 (or 1.6%) in the povidone-iodine only cohort.
The variables used to diagnose infection were swelling, cellulitis, pain, erythema, tenderness, fever, and leukocytosis. Infections were treated by implant removal, IV antibiotics, and implant replacement 6–8 weeks later.
Pfeiffer et al.10 conducted a retrospective cohort study reviewing the records of 436 female patients undergoing breast augmentation from 2000 to 2007 in Denmark.
The study compared 2 groups of 218 patients. The intervention cohort had surgery within 2000 to 2002 and received topical antibiotic therapy, while the control (no topical antibiotic) cohort had their surgeries within 2005 to 2007.
All incisions were peri-areola except for 2 instances of inframammary incisions, and all patients had the submuscular implant pocket created via diathermy under direct vision. The implants used were textured Polytech Silimed double-lumen implants, and each patient received closed-suction drains that were removed within 24 hours postsurgery.
Both groups of women received identical perioperative prophylaxis of 1.5 g IV cefuroxime. The intervention group had their implants submerged in a solution containing 1 g/L of cephalothin and had their breast pockets washed out with the same solution. The control group did not receive any, as the antibiotic used topically on the breast implants was discontinued in Denmark.
Follow-up of these patients beyond 3 months occurred only when adverse events occurred. The mean follow-up times of the intervention and the control groups were 87 and 21.6 months, respectively.
The reported infection rates were 6.7% in the intervention group, and 12.8% in the control group (P = 0.044). Infections were diagnosed as having 1 or more of the following: fever, pain, swelling, cellulitis, elevation of leukocytes, and/or C reactive protein.
Infections in the antibiotic-exposed group were treated with antibiotics and expectant treatment, resulting in resolution of infection in all cases. Infections in the nonexposed group were treated similarly; however, 3 cases ultimately required explanation.
Giordano et al. (2013) conducted a retrospective cohort study reviewing the records of 330 consecutive female patients, in the period of 2004–2010, who underwent cosmetic breast augmentation without associated mastopexy or concurrent procedures.
Patients were divided into 2 groups, with the control group being patients treated during 2004–2009 and the intervention group treated during 2009–2010. Both groups received IV cefuroxime perioperatively; however, the intervention group also had their implants and implant pockets bathed in Betadine, gentamicin, and cefuroxime. All patients received postoperative oral antibiotics for 7 days with the intervention group receiving oral cephalexin and the control group oral levofloxacin.
All procedures were performed with an inframammary incision, with the creation of a dual-plane implant pocket through electrocautery under direct vision. Wounds were sutured in layers using absorbable sutures and covered with Micropore tape. Texturized silicone gel implants were used, together with Redon drains.
Mean follow-up time for the intervention and control groups were 22 months (± 3 months) and 24 months (±13 months), respectively. Follow-ups were planned at 4 weeks and 6 months postoperatively, and only occurred beyond 6 months in the case of adverse events.
There was no statistically significant difference in infection rates between the groups with 2 infections in the intervention group and 3 in the control (1.2% versus 1.8%; P = 0.65).
Effect of Antibiotics on Infection Rates
A summary of the infection rates when antibiotics are used versus when no antibiotics are used is shown in Table 3.
Table 3.
Summary of Results of Included Studies of Antibiotic Protocol and Infection Rates
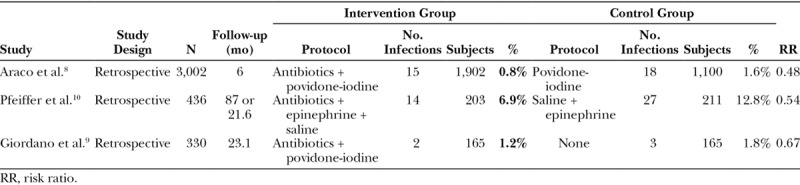
Overall, the effect of antibiotics appears to lower infection rates. A meta-analysis using the Mantel–Haenszel statistical method of the 3 studies shows a risk ratio reduction of 0.52 (95% CI = 0.34–0.81), favoring the usage of antibiotics in either breast pocket irrigation or implant immersion (Fig. 2).
Fig. 2.
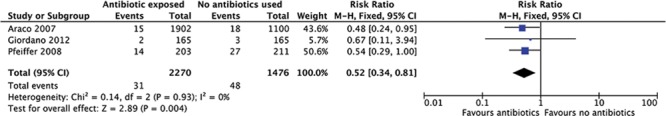
Forest plot of the effect of antibiotics on infection rates.
Effect of Antibiotics on Capsular Contracture (CC)
The studies by Giordano et al.9 and Pfeiffer et al.10 also compared CC rates in their antibiotic-exposed and control cohorts, both showing a reduction in the capsular contracture rates when antibiotics are used (Fig. 3). However, the data from the studies are poor, and heterogeneity is high.
Fig. 3.
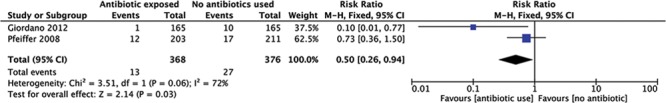
Forest plot of the effect of antibiotics on capsular contracture.
DISCUSSION
Postoperative infections are a leading cause of morbidity affecting 1.1–2.5% of patients who have had aesthetic breast augmentations.8,11–13 In the implant-based reconstructive setting, the infection rate is even higher. Tissue expander-related infection rates range from 2.5% to 24%,14–19 with a systematic review in 2013 reporting the infection rate in implant-based reconstructive surgery as 5.78%.20
There are multiple contributors that may be responsible for the higher infection rates in reconstructive patients and these include the disruption of tissue vascularity, greater exposure to endogenous bacteria from mastectomy,13 lymph node dissection,14 and adjuvant cancer therapies.21 Given this noteworthy burden of morbidity, it is important to use the correct protocol to minimize adverse outcomes.
This study was unable to find any trials looking at topical antibiotics in reconstructive cases, and it is therefore unable to present direct evidence for reconstructive breast surgery.
The 3 studies assessed in this systematic review suggest that the usage of antibiotics with or without the addition of antiseptics in breast pocket washout or implant immersion reduces infection rates, with the combined RR of the 3 studies at 0.52 (95% CI = 0.34–0.81; P = 0.004).
These findings are consistent with existing evidence for the impact of topical antibiotics on CC, which is thought to be a surrogate for subclinical infection, immunological responses, and chronic inflammation.22 CC is the most common complication of breast implant surgery,23 and in the systematic review by Huang et al.,7 the use of topical antibiotics in aesthetic implant surgery was found to be associated with a significantly diminished rated of CC. Topical antibiotics therapy reduced the incidence of CC from 6.81% to 4.86%.7
The effect of antiseptics on CC was similar in the review by Yalanis et al.6 They found that antiseptics reduced CC rates from 8.9% to 2.7%.6
We were not able to assess the independent effect of topical antiseptics on breast implant infections. Giordano et al.9 was the only study to implement different antiseptic protocols for each of their cohorts. They used topical antibiotics with antiseptics in their intervention group and no topical agents in their control group, preventing individual assessment of each topical therapy.
The most common infections after breast surgery are due to endogenous skin flora such as Staphylococcus aureus and Staphylococcus epidermidis.24 Hence, antibiotic prophylaxis should be directed toward these bacteria. This is consistent with current practice, with the IV cephalosporins being the antibiotic of choice in 79% of augmentative breast surgery cases in the United States.25
However, overuse of prophylactic antibiotics has potential hazards, including the selection of multi-drug resistant organisms.26 The routine use of antibiotics is not supported by the results of all current studies, for example, a recent study found that bacteria isolated from infections in a significant portion of implant-based breast reconstructive cases were sensitive to the topical prophylactic antibiotics administered.27
Limitations
The major limitation of this review is the lack of randomized controlled trials or prospective trials. The only reports found were of retrospective studies, which have inherent biases by nature of their design. Additionally, there were several methodological weaknesses in the trials assessed. Direct comparison of these articles was confounded by the differences in operative techniques, study design, antibiotics protocols, and their definitions of an infection.
For example, Giordano (2013) did not separate antibiotics and antiseptic usage, making it difficult to delineate which agent was primarily responsible for reducing infection rates.
Additionally, Araco et al.8 was a descriptive study without a planned control. In their analysis, they looked at a several outcomes retrospectively, including topical antibiotics, type of prosthesis, and use of drains. However, these outcomes were not controlled against each other; hence, it is difficult to determine what each individual effect was on infection. Moreover, no rationale was given for why one patient may have received antibiotics while another patient did not, thus introducing an inherent bias into the study.
The definition of infection differed between trials as well. Pfeiffer et al.10 had the highest infection rates of the 3 papers, with 12.8% in the control group and 6.9% in the intervention group. In their study, they did not use any antiseptics to irrigate the pocket or bathe the implant. However, only 3 patients required implant removal, and these were in the control group of 218 patients (1.4%). Their inclusion criteria for what constituted an infection may have been broader than Araco et al.8 and consequently falsely elevated their infection rates.
In this regard, Araco et al.8 reported infection rates of 0.8% and 2% for the intervention versus control groups, respectively, but all infected cases required implant removal. Giordano et al.9 had no cases requiring implant explanation and they were the only group to use postoperative antibiotics.
Additionally, none of the studies included in this review used the Adams’ formula, a technique of using triple antibiotic irrigation of the breast pocket (50,000 U of bacitracin, 1 g of cefazolin, and 80 mg of gentamicin, in 500 ml of saline),28 which is currently a popular choice among surgeons.
Further research is still indicated in this area in the form of a high-quality randomized controlled trial. A more appropriate trial design to delineate the relative merits of topical prophylactic measures for surgical-site infections would include 4 arms, with 1 arm each for the administration of antibiotics, antiseptics, antibiotics and antiseptics, and a control.
CONCLUSIONS
The results of this study suggest there is a clinical benefit in using antibiotics for breast pocket irrigation and implant immersion. However, given the quality of the evidence obtained in this review, no definite conclusion can be drawn with any certainty. We recommend a randomized control trial to reduce bias and to provide a higher level of evidence on this important issue. Alternatively, should this prove difficult to achieve, a study based on a national database, such as the Australian Breast Device Registry, could perhaps be undertaken to provide higher quality evidence than is currently available.
Footnotes
Published online 14 September 2018.
Presented at the third World Congress on Controversies in Breast Cancer (CoBrCa 2017) in Tokyo, Japan. Third Chris O’Brien Lifehouse Research Symposium (2017) in Sydney, Australia.
Frois and Harbour contributed equally to this work.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Townley WA, Baluch N, Bagher S, et al. A single pre-operative antibiotic dose is as effective as continued antibiotic prophylaxis in implant-based breast reconstruction: a matched cohort study. J Plast Reconstr Aesthet Surg. 2015;68:673. [DOI] [PubMed] [Google Scholar]
- 2.Ooi ASh, Song DH. Reducing infection risk in implant-based breast-reconstruction surgery: challenges and solutions. Breast Cancer (Dove Med Press). 2016;8:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berríos-Torres SI, Umscheid CA, Bratzler DW, et al. ; Healthcare Infection Control Practices Advisory Committee. Centers for Disease Control and Prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg. 2017;152:784. [DOI] [PubMed] [Google Scholar]
- 4.Andrews J, Guyatt G, Oxman AD, et al. GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol. 2013;66:719. [DOI] [PubMed] [Google Scholar]
- 5.NSW Health. In: Safety Notice 005/17: Povidone-Iodine (PVI) Use in NSW Hospitals. 2017. Sydney, NSW, Australia: NSW Government Ministry of Health; [Google Scholar]
- 6.Yalanis GC, Liu EW, Cheng HT. Efficacy and safety of povidone-iodine irrigation in reducing the risk of capsular contracture in aesthetic breast augmentation: a systematic review and meta-analysis. Plast Reconstr Surg. 2015;136:687. [DOI] [PubMed] [Google Scholar]
- 7.Huang N, Liu M, Yu P, et al. Antibiotic prophylaxis in prosthesis-based mammoplasty: a systematic review. Int J Surg. 2015;15:31. [DOI] [PubMed] [Google Scholar]
- 8.Araco A, Gravante G, Araco F, et al. Infections of breast implants in aesthetic breast augmentations: a single-center review of 3,002 patients. Aesthetic Plast Surg. 2007;31:325. [DOI] [PubMed] [Google Scholar]
- 9.Giordano S, Peltoniemi H, Lilius P, et al. Povidone-iodine combined with antibiotic topical irrigation to reduce capsular contracture in cosmetic breast augmentation: a comparative study. Aesthet Surg J. 2013;33:675. [DOI] [PubMed] [Google Scholar]
- 10.Pfeiffer P, Jørgensen S, Kristiansen TB, et al. Protective effect of topical antibiotics in breast augmentation. Plast Reconstr Surg. 2009;124:629. [DOI] [PubMed] [Google Scholar]
- 11.Kjøller K, Hölmich LR, Jacobsen PH, et al. Epidemiological investigation of local complications after cosmetic breast implant surgery in Denmark. Ann Plast Surg. 2002;48:229. [DOI] [PubMed] [Google Scholar]
- 12.Gabriel SE, Woods JE, O’Fallon WM, et al. Complications leading to surgery after breast implantation. N Engl J Med. 1997;336:677. [DOI] [PubMed] [Google Scholar]
- 13.Pittet B, Montandon D, Pittet D. Infection in breast implants. Lancet Infect Dis. 2005;5:94. [DOI] [PubMed] [Google Scholar]
- 14.Nahabedian MY, Tsangaris T, Momen B, et al. Infectious complications following breast reconstruction with expanders and implants. Plast Reconstr Surg. 2003;112:467. [DOI] [PubMed] [Google Scholar]
- 15.Cordeiro PG, McCarthy CM. A single surgeon’s 12-year experience with tissue expander/implant breast reconstruction: part I. A prospective analysis of early complications. Plast Reconstr Surg. 2006;118:825. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong RW, Berkowitz RL, Bolding F. Infection following breast reconstruction. Ann Plast Surg. 1989;23:284. [DOI] [PubMed] [Google Scholar]
- 17.Spear SL, Majidian A. Immediate breast reconstruction in two stages using textured, integrated-valve tissue expanders and breast implants: a retrospective review of 171 consecutive breast reconstructions from 1989 to 1996. Plast Reconstr Surg. 1998;101:53. [DOI] [PubMed] [Google Scholar]
- 18.Francis SH, Ruberg RL, Stevenson KB, et al. Independent risk factors for infection in tissue expander breast reconstruction. Plast Reconstr Surg. 2009;124:1790. [DOI] [PubMed] [Google Scholar]
- 19.Handel N, Jensen JA, Black Q, et al. The fate of breast implants: a critical analysis of complications and outcomes. Plast Reconstr Surg. 1995;96:1521. [DOI] [PubMed] [Google Scholar]
- 20.Phillips BT, Bishawi M, Dagum AB, et al. A systematic review of antibiotic use and infection in breast reconstruction: what is the evidence? Plast Reconstr Surg. 2013;131:1. [DOI] [PubMed] [Google Scholar]
- 21.Selber JC, Wren JH, Garvey PB, et al. Critical evaluation of risk factors and early complications in 564 consecutive two-stage implant-based breast reconstructions using acellular dermal matrix at a single center. Plast Reconstr Surg. 2015;136:10. [DOI] [PubMed] [Google Scholar]
- 22.Steiert AE, Boyce M, Sorg H. Capsular contracture by silicone breast implants: possible causes, biocompatibility, and prophylactic strategies. Med Devices (Auckl). 2013;6:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Headon H, Kasem A, Mokbel K. Capsular contracture after breast augmentation: an update for clinical practice. Arch Plast Surg. 2015;42:532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen JB, Carroll C, Tenenbaum MM, et al. Breast implant-associated infections: the role of the National Surgical Quality Improvement Program and the local microbiome. Plast Reconstr Surg. 2015;136:921. [DOI] [PubMed] [Google Scholar]
- 25.Chopra K, Gowda AU, McNichols CHL, et al. Antimicrobial prophylaxis practice patterns in breast augmentation: a National Survey of Current Practice. Ann Plast Surg. 2017;78:629. [DOI] [PubMed] [Google Scholar]
- 26.Alanis AJ. Resistance to antibiotics: are we in the post-antibiotic era? Arch Med Res. 2005;36:697. [DOI] [PubMed] [Google Scholar]
- 27.Viola GM, Raad II, Rolston KV. Breast tissue expander-related infections: perioperative antimicrobial regimens. Infect Control Hosp Epidemiol. 2014;35:75. [DOI] [PubMed] [Google Scholar]
- 28.Adams WP, Jr, Rios JL, Smith SJ. Enhancing patient outcomes in aesthetic and reconstructive breast surgery using triple antibiotic breast irrigation: six-year prospective clinical study. Plast Reconstr Surg. 2006;117:30. [PubMed] [Google Scholar]


