Supplemental Digital Content is available in the text.
Abstract
Background:
Combining liposuction with abdominoplasties was considered risky during the 1980s and 1990s due to reports of increased complications rates and the belief that liposuction posed a danger to flap circulation. However, the corresponding author’s intraoperative observations at that time, that liposuction preserved all but the smallest blood vessels, negated the prevailing opinions that liposuction increased the risk to flap circulation, and in October 1996, liposuction assisted abdominoplasty (LAA) was first performed. Thereafter, LAA was honed to become a lipoabdominoplasty technique—not merely a combination of liposuction and abdominoplasty, a technique that utilizes liposuction as a dissection tool—hydro- and lipo-dissection, to dissect free and separate the abdominal flap from the deep fascia. Enhanced flap excursion could be demonstrated intraoperatively by selectively transecting the skin retaining ligaments and limiting liposuction to the flap’s undersurface, created a vascular lining layer rich in anastomosing blood vessels that provided a rich blood supply to the flap, enabling increased flap excursion.
Methods:
Five ninety-three consecutive ambulatory LAAs with circumferential torso liposuction and other area liposuction are presented and the surgical technique is illustrated and discussed.
Results:
There were no serious adverse events, anesthesia complications, hospital transfers, no venous thromboembolism or postoperative respiratory complications in the 593 cases. Patient satisfaction was high, and the results compared favorably with abdominoplasty results published in the scientific literature.
Conclusions:
LAA is a safe and effective abdominoplasty technique. Extensive clinical experience with LAA suggests longer flap excursion and improved perfusion. It routinely incorporates circumferential torso and other areas liposuction and has commonly included buttock fat grafting.
INTRODUCTION
Abdominoplasties have grown in popularity in the United States incentivized by the growth of outpatient surgery and advances in anesthesia care that enabled safer and faster recoveries. The introduction of liposuction by Illouz1 revolutionized body contouring and the addition of liposuction of the torso to complement the results of abdominoplasties, naturally followed. Articles on combining liposuction and abdominoplasty were published during the 1990’s; however, the literature at that time was not generally supportive of this combination, advising caution.2–13 In 2001, Saldanha et al.15 described their group’s experience (since 2000) with a lipoabdominoplasty technique similar to Illouz’z Suction Abdominoplasty (1992).14 Since then lipoabdominoplasty has become synonymous with the Saldanha technique.
Liposuction assisted abdominoplasty (LAA) (formerly, liposuction abdominoplasty) was first performed in October 1996 by detaching an abdominal flap from the deep fascia with liposuction. The experience with the first 43 patients was presented in 2002 and published in 2003. It offered an increased flap excursion and enhanced perfusion.16–18 The experience subsequently gained from 337 consecutive cases (including the first 43) was published in 2009 and demonstrated in Live surgery in 2012. This clinically verified the safety, efficacy, and advantages of this approach. Two hundred fifty-six additional cases were added since 2009, and their results are included in this article.
PATIENTS AND METHODS
This prospective longitudinal ongoing study was undertaken in 1996. Five hundred ninety-three abdominoplasty candidates were divided into 2 groups. group 1, 337 patients reported on in 2009, and group 2, 256 patients added since 2009 (Table 1).
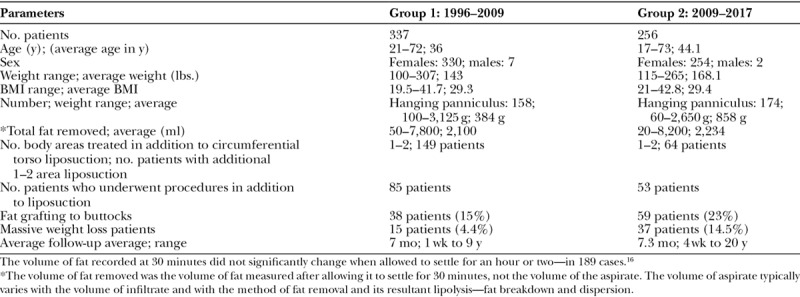
Table 1.
Data on the 593 Patients
Fifty-two of the 593 abdominoplasty patients were massive weight loss patients who lost weight with bypass surgery and/or lifestyle changes. They underwent extended abdominoplasties and body lifts. Thirty-one of the 593 patients underwent concomitant abdominal hernia repairs. All abdominoplasties since 1996 included circumferential torso liposuction and often other areas liposuction. Additional procedures, most commonly fat grafting, were also performed.
Thirty-one percentage of group 1 patients underwent liposuction of 1 additional area in addition to circumferential torso liposuction, 13%, two additional areas. This compares to 29% of group 2 patients who underwent liposuction of 1 additional area, 12.5% had 2 additional areas, 5.5% three additional areas, 1 patient had 4 areas, and another 1 had 6 additional areas liposuctioned.
Group 1 included 15 massive weight loss patients (4.4%), and group 2 included 73 (14.5%). Finally, 23% of group 1 underwent buttock fat grafting compared with 15% of group 2.
Surgery was performed in an office surgical suite under local anesthesia with monitored intravenous sedation. The usual length of surgery was 4–4.5 hours. Patients were discharged home with a responsible adult after approximately 2–3 hours in the recovery room. All patients were seen the morning after surgery, they were allowed to walk upright, showered, and returned to normal activity (Fig. 1). Strenuous abdominal exercises were avoided for 3 months when diastasis repair was performed.
Fig. 1.
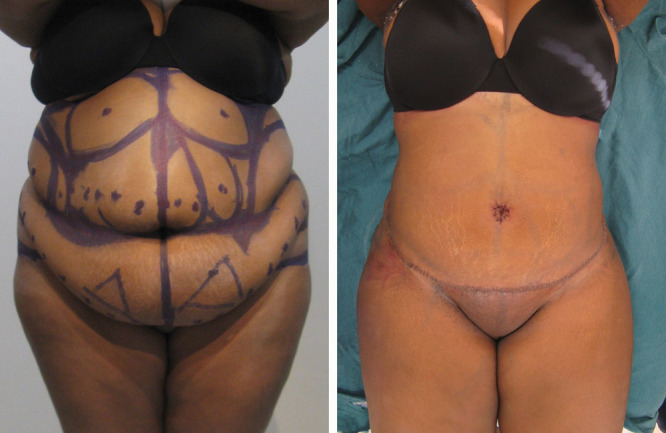
Before and next morning after LAA with circumferential torso liposuction. Note the patient is fully extended and the abdominal flap at its inferior border is healthy looking. The incision is at the pubic hairline with pubic uplift also performed.
The corresponding author Daniel Brauman (D.B.) performs surgery under intravenous sedation. The coauthor Berend van der Lei (B.v.d.L.) has been performing this procedure since 2010 under general anesthesia.19,20
Surgical Technique
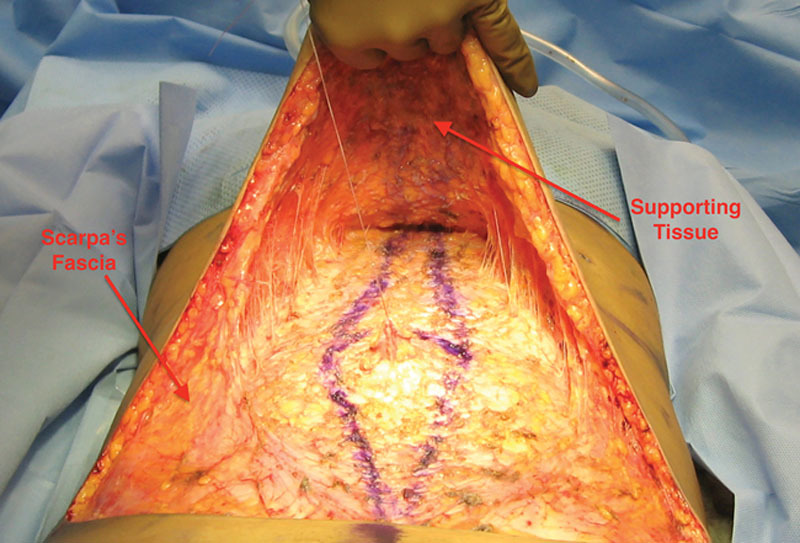
First, anesthetic fluid of the surgeon’s choosing is infiltrated between the deep fascia and the subcutaneous tissue with 3 mm infiltration cannulas attached to 60 cc Toomey syringes, to “separate” the subcutaneous tissue from the muscular abdominal wall—hydro-dissection. The point of entry is from the right or left sides, superior to the umbilicus. The entry to the surface of the muscular plane is done “by feel,” a maneuver not difficult to master. Initially infiltration/separation is performed in the epigastric area and then in the infra umbilical area. The plane of dissection remains on the deep fascia, deep to Scarpa’s fascia. The cannula slips under Scarpa’s fascia because its point of entry is superior to the umbilicus, which is where Scarpa’s fascia ends as a discernable layer (Fig. 2). Next, liposuction follows infiltration, removing the deep fat layer bordering the muscle and disrupting the stiff skin retaining ligaments while preserving the flexible blood vessels “lipo-dissection”. The skin retaining ligaments affix the skin to the fascia and form body creases such as the tendinous intersections (6 pack). “Disrupting” is done by performing liposuction while applying traction to the flap. Traction places the ligaments under tension. The ligaments are stiff and will tear, the blood vessels, however, are flexible and will move aside (see video, Supplemental Digital Content 1, which display lipo-dissection and lipo-disruption of ligaments. This video is available in the “Related Videos” section of the Full-Text article at PRSGlobalOpen.com or at http://links.lww.com/PRSGO/A856). Disruption of the skin retaining ligaments is sufficient to provide flap mobility when the patient’s skin and subcutaneous tissue is loose. Otherwise, discontinuous undermining is necessary, and some neurovascular perforators may need to be divided/sacrificed.
Fig. 2.
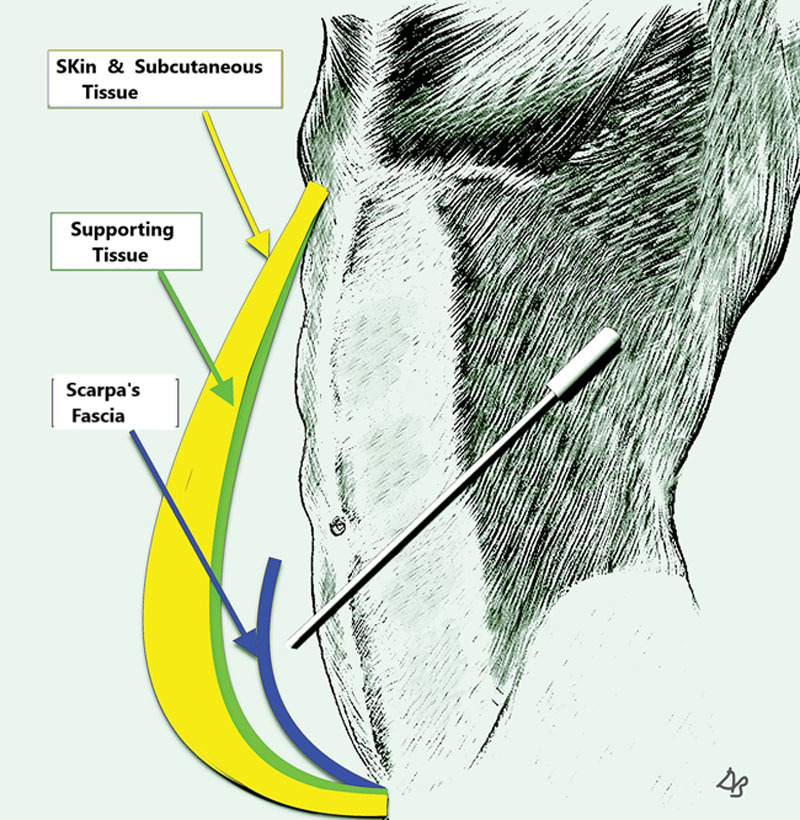
Depicting the cannula’s point of entry from the side, superior to the umbilicus. The cannula’s tip is deep to Scarpa’s fascia. Supporting tissue (in green) forms the deep layer of the abdominal flap. Supporting tissue (not shown) is also present on the deep fascia and on Scarpa’s fascia’s deep and superficial surfaces.
Video Graphic 1.
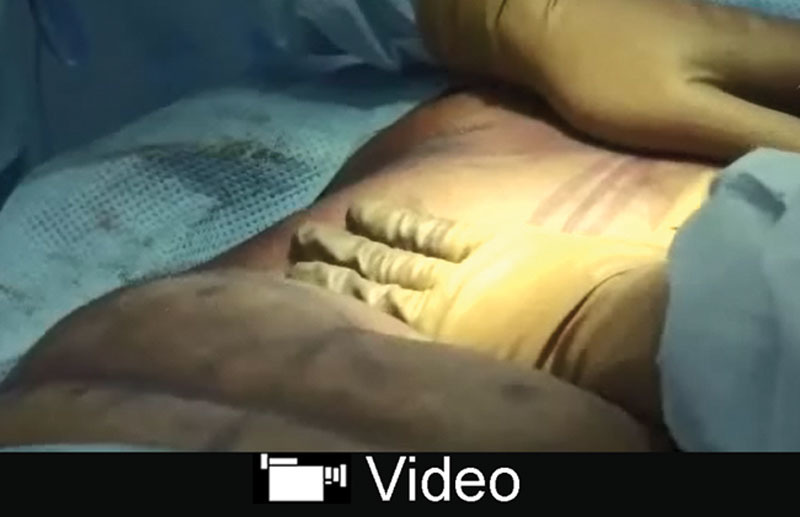
See video, Supplemental Digital Content 1, which display lipo-dissection and lipo-disruption of ligaments. This video is available in the “Related Videos” section of the Full-Text article at PRSGlobalOpen.com or at http://links.lww.com/PRSGO/A856.
Detaching the subcutaneous tissue from the muscle with liposuction and removing the fat bordering the muscle creates a “safe zone,” a space that prevents inadvertent perforations. Scarpa’s fascia’s superficial surface is next separated from the infraumbilical skin by infiltrating and liposuctioning the subcutaneous fat. This exposes Scarpa’s fascia and preserves femoral artery branches coursing on the fascia’s superficial surface (Fig. 3). Further liposuction of the flap’s undersurface is used to detach, thin and even the flap. The flap ends up being lined by a compact layer of fibrous tissue (scaffolding) containing blood vessels, nerves, and lymphatics coined - “supporting tissue” layer. The supporting tissue distributes the blood supply and forms a distinct compact deep border for the flap (Figs. 4–6).
Fig. 3.
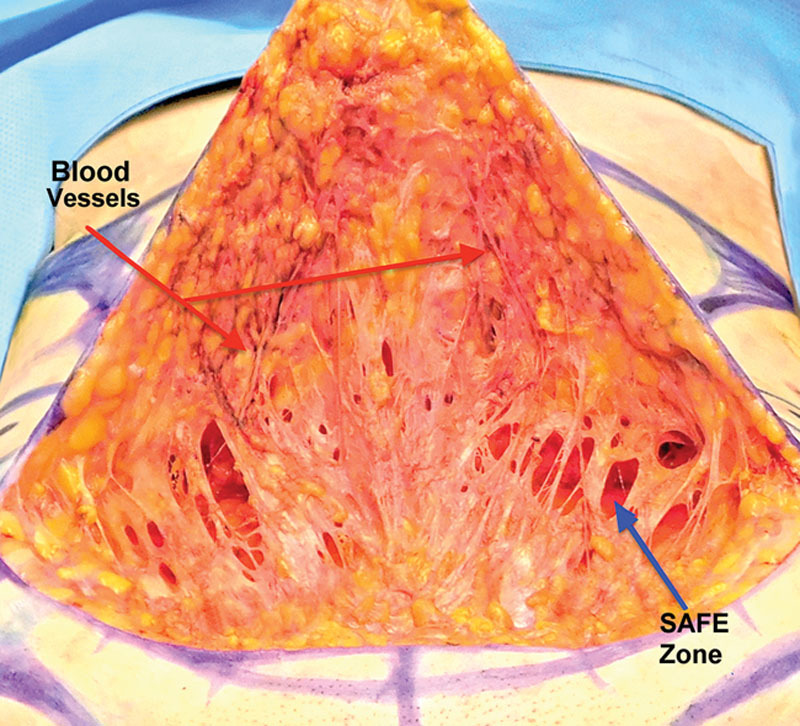
The superficial surface of Scarpa’s fascia had been separated from the skin and subcutaneous tissue with liposuction. The blue arrow points to the “safe zone,” a space created by liposuction deep to Scarpa’s that separated the deep surface of the fascia from the muscle. The red arrows point to vaso-constricted femoral artery branches coursing on the fascia toward the skin. These blood vessels can augment the abdominal flap’s blood supply.
Fig. 4.
The flap is even, lined by a supporting tissue layer that is “bluish” in color as epinephrine vaso-constriction is abating, and veins are filling up. Scarpa’s, split in the midline, its vessels still vaso-constricted, nevertheless, bleeding from the distal edge was observed. In this patient, tissues were tight, and perforators needed to be transected to obtain more mobility. The flap can withstand loss of some important perforators since it is perfused through its supporting tissue vessels.
Fig. 6.
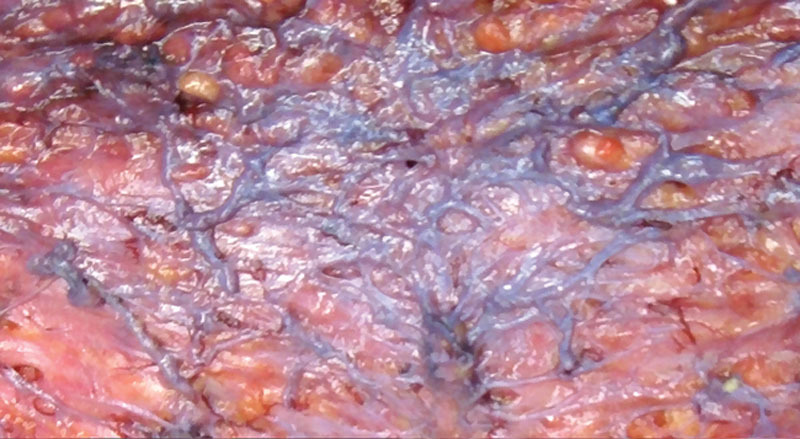
A magnified section of a supporting tissue layer. Epinephrine vaso-constriction is subsiding with veins becoming filled with blood, first.
Fig. 5.
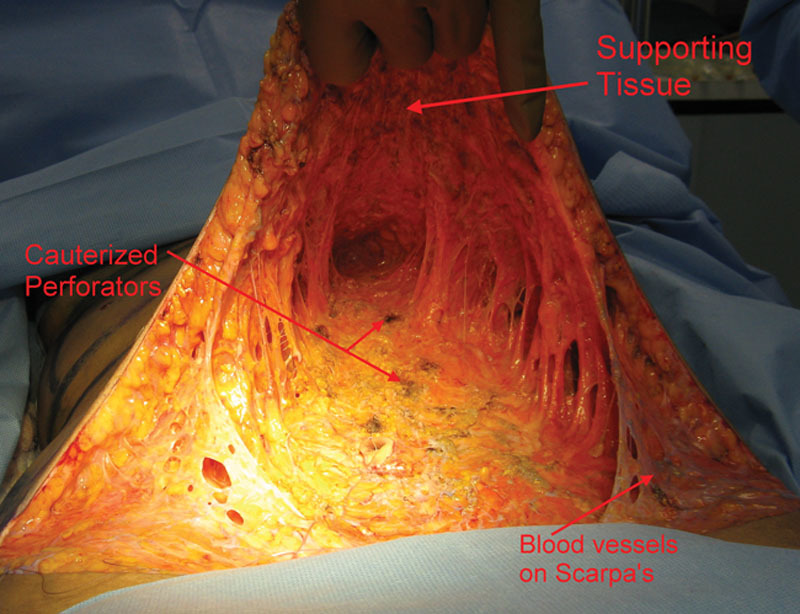
The tissue was looser and less perforators needed to be sacrificed. Note femoral artery branches on Scarpa.
Next, a curvilinear incision is made, Scarpa’s fascia is split in the midline to preserve its vessels and lymphatics, the abdominal flap is elevated sharply and bluntly from the deep fascia, preserving the supporting tissue layers on the undersurface of the flap, the abdominal wall, and the deep and superficial surfaces of Scarpa’s fascia. Traction on the flap is applied to evaluate the desired tension and neurovascular perforators preventing flap downward movement are divided. Panniculus excision is done last. The operating table is extended (not flexed), and the flap is placed under tension while the panniculus is excised. Panniculus excision is done by superficial liposuction just distal to the excision line that exposes a strip of intact supporting tissue, which is preserved distal to the flap’s edge. Bleeding from the edge is expected with the flap under tension and the patient fully extended (see video, Supplemental Digital Content 2, which displays extension of flap excursion by targeted ligament and perforator release. This video is available in the “Related Videos” section of the Full-Text article at PRSGlobalOpen.com or at http://links.lww.com/PRSGO/A857). “Segmental diastasis repair” is performed when indicated, and closure of the lower abdominal incision is done with tissue overlap to prevent linear scar contracture (see video, Supplemental Digital Content 3, which displays “supporting tissue layer” and “segmental diastasis repair”. This video is available in the “Related Videos” section of the Full-Text article at PRSGlobalOpen.com or at http://links.lww.com/PRSGO/A858).21–23 Drains may be used but weekly tapping is an alternative depending on surgeon’s choice. Tacking sutures are not recommended for fear of damage to the flap’s vascular supporting tissue layer. Patients are then positioned on their side, and circumferential torso and other areas liposuction is carried out.
Video Graphic 2.
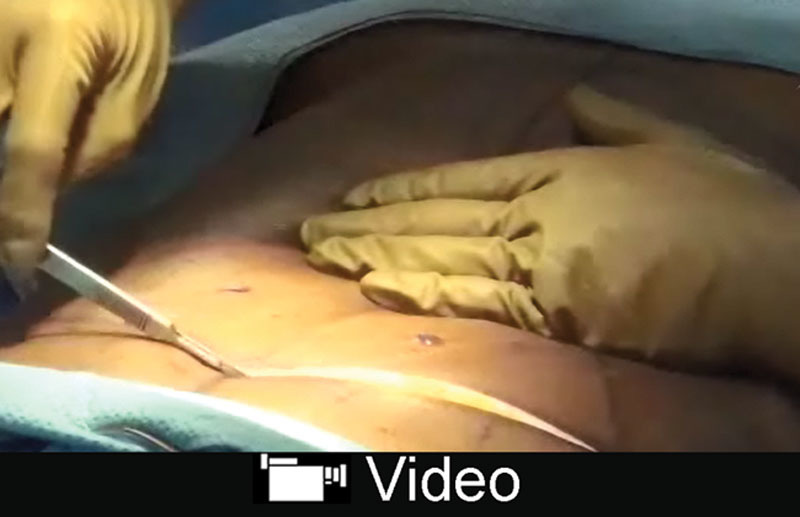
See video, Supplemental Digital Content 2, which displays extension of flap excursion by targeted ligament and perforator release. This video is available in the “Related Videos” section of the Full-Text article at PRSGlobalOpen.com or at http://links.lww.com/PRSGO/A857.
Video Graphic 3.
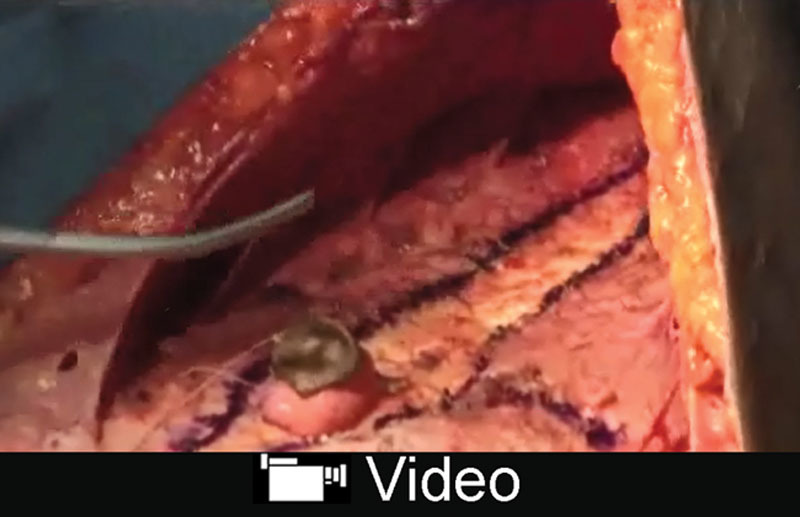
See video, Supplemental Digital Content 1, which displays supporting tissue layer and segmental diastasis repair. This video is available in the “Related Videos” section of the Full-Text article at PRSGlobalOpen.com or at http://links.lww.com/PRSGO/A858.
RESULTS
Five hundred ninety-three consecutive patients who underwent office-based LAA under local anesthesia with monitored intravenous sedation between 1996 and 2017 are included. No abdominoplasty patients were excluded unless medically unsuitable for surgery. Overall, there were no serious complications, venous thromboembolism or unplanned hospital transfers in the 593 cases. Patient satisfaction was high as evidenced by satisfaction surveys and the many referrals by satisfied patients over 21 years.
Group 1: 337 patients operated on between 1996 and 2009 suffered no anesthetic complications or serious surgical complications. There were 5 (1.4%) late home-acquired infections before 2005, attributable to the prolonged use of drains and 6 cases (1.7%) of minor tissue necrosis in smokers that healed without affecting the final aesthetic results. Two of the 6 cases of minor necrosis involved T junctions, and 2 were caused by drain-site pressure.16–19,21 Drains were discontinued in 2005 but reinstated several years later because of the increase in seroma incidence caused by the inclusion of many more massive weight loss patients.
In group 2, 254 patients, 2009–2017, there were also no serious complications, venous thromboembolism, anesthesia complications, or unplanned hospital transfers. One patient, placed on enoxaparin sodium prophylaxis the morning after surgery was found to have bled under the flap, a week later. She was treated with aspirations during the next few visits without any aesthetic long-term sequelae. There was 1 (0.39%) home-acquired infection of a seroma in a chronic rheumatoid arthritis, massive weight loss patient who was difficult to care for, repeatedly neglecting to close and compress her drain’s suction bulb, admitting herself to the hospital to qualify for home care and trying this again after discharge but being refused admission to another hospital. There were no other cases of infection and no marginal necrosis.
Seroma formation occurred much more commonly in massive weight loss patients. Groups-2 had 37 massive weight loss patients and seroma formation affected 7 of them (19%). In comparison, the remaining 219 nonmassive weight loss patients in that group, sustained only 3 seromas (1.3%). In Group-1, 5 of 15 massive weight loss patients had seromas (33%) and no seromas occurred in the remaining 322 patients of that group. Seromas contributed to excess fibrosis and scar contractures.24 In Group-2, there were 2 (5%) scar revisions in the 37 massive weight loss patients compared with 4 (1.3%) revisions in the remaining 219 cases. Lastly, of the 6 scar revisions in Group 2, 3 were due to seromas (50%).
Group 1 seromas were treated successfully with 4–5 mg Kenalog-10 Injections into the seromas (triamcinolone acetonide injectable suspension, USP, Bristol-Myers Squibb Company. Princeton, N.J.). In group 2, the 7 seromas were treated with dehydrated alcohol-sclerotherapy. Furthermore, the 37 massive weight loss patients who incurred 7 seromas, resulted in the reinstatement of drains. These numbers confirmed other published data that massive weight loss patients are prone to seromas and their resultant scar contractures.24 Sclerotherapy with dehydrated alcohol continues to be performed by an interventional radiologist, interested in seroma treatment at the affiliated hospital. The procedural risks in the 593 are summarized in (Table 2).
Table 2.
Procedural Risks—593 Patients: 1996–2017, Groups 1 and 2

Abdominal scar contractures can occur despite the interposition of subcutaneous tissue under the flap. The successful release of 6 contractures undertaken a year after the surgery consisted of excising contracted scars and once again interposing subcutaneous tissue under the incision. Currently, short courses of oral steroids are being evaluated in susceptible individuals to prevent contracture. Pfizer Medrol Dosepak (methylprednisolone) 4 mg. The experience gained from this large series of patients helped anticipate scar contractures and seromas. Patients who seem prone to scar contractures are offered alternative procedures such as liposuction. Liposuction can harness patients’ tendency to contract their scars and utilize it to their advantage by contracting and eliminating a hanging panniculus (Fig. 7).
Fig. 7.
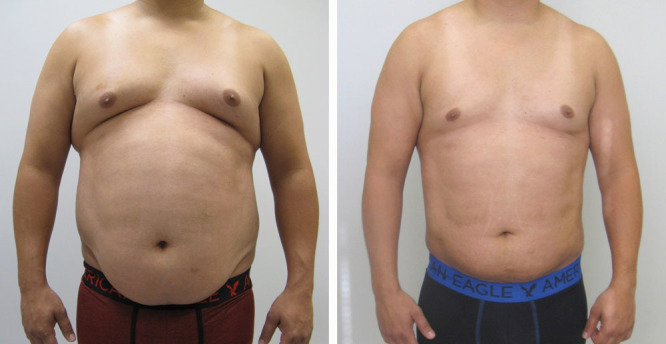
Before and 6 weeks after liposuction of abdomen chest and circumferential torso. Note the improvement in the hanging panniculus and the smooth result possible when anatomical liposuction separates the muscular abdominal wall from the panniculus enabling precise anatomic liposuction.
Concomitant abdominal hernia repair without mesh was done in 31 of the 593 abdominoplasties (groups 1 and 2). Follow-up data showed no recurrence in 18 patients who returned for follow-up (3.5 months – 6 years; average, 20.2 months).
The majority of patients reported experiencing a marked improvement in their appearance and function. Very few, mostly in the massive weight loss group, were hard to please and presented body image issues in addition to an increased frequency of seromas and scar contractures. The added experience with the technique and the newly available urine nicotine test after 2009 resulted in the absence of flap ischemia and any marginal necrosis. Patients who smoked were not treated. Overall, patient satisfaction continued to remain very high, evidenced by satisfaction surveys and referrals. Patients were pleased with aesthetic results that were enabled by the safe inclusion of routine circumferential torso liposuction, and additional area liposuction that integrated their abdominal contouring with their figure and often the ability to conceal their scars (Figs. 8, 9).
Fig. 8.
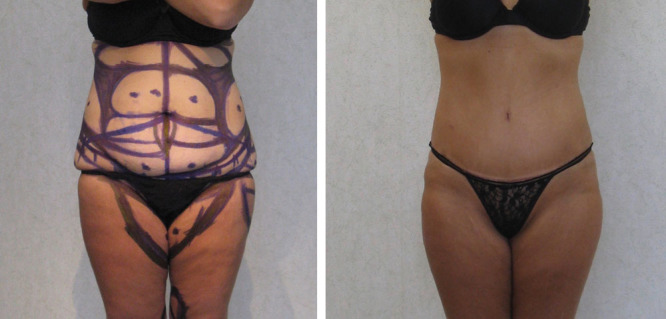
Before and after photographs of LAA with circumferential torso and thighs liposuction. This patient healed with a slightly hypertrophied scar visible just above the scant underwear but able to be hidden by it. A smooth transition to the pubic area is present. A scar contracture would have created a secondary fold negating the smooth transition, and the patient would not have been able to conceal her scar with underwear.
Fig. 9.
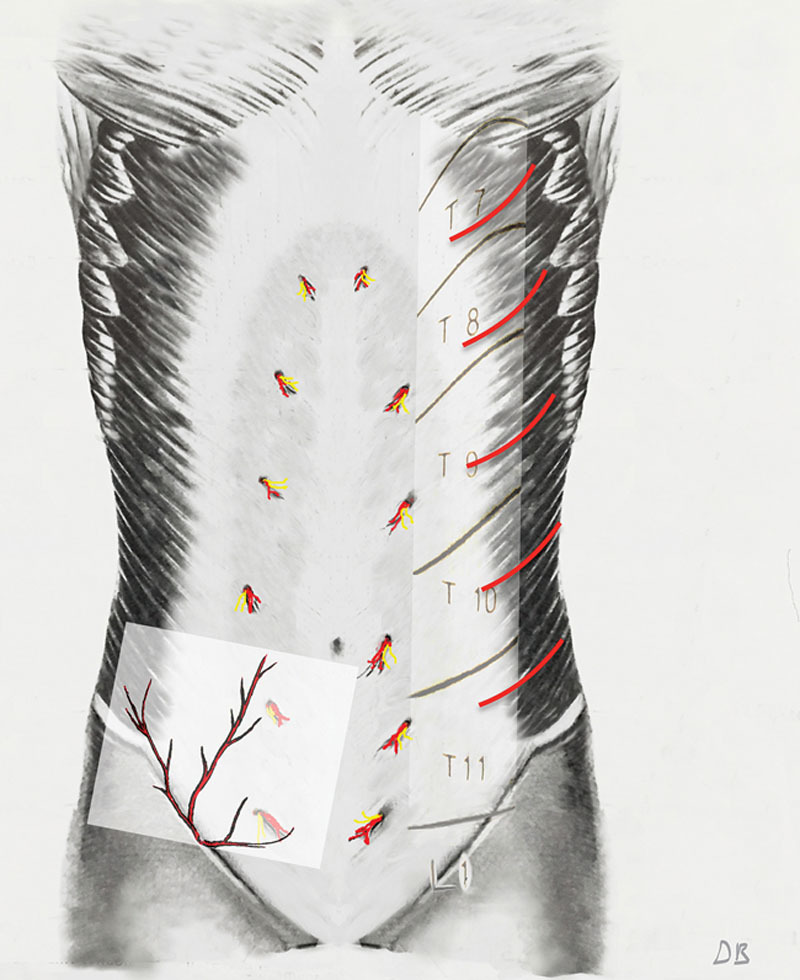
A simplified view of the abdominal wall’s blood supply. On the right, the deep anterior and lateral neurovascular perforators- T7 - L1. On the left, the 3 superficial branches of the femoral coursing on Scarpa’s fascia.
DISCUSSION
The fundamental requirement for a safe and effective abdominoplasty is a well-perfused and mobile flap. Flap mobility, in addition to umbilical release, requires the detachment of the “skin retaining ligaments” that affix the skin to the fascia (especially) at the tendinous intersections (6 pack) and along the midline (Linea Alba) and flap perfusion is maximal when it contains a well-perfused vascular network.
LAA evolved gradually. In 1996, it was named liposuction skin excision.16,25 Liposuction loosened the subcutaneous tissue, the skin was stretched, and umbilical transposition was performed. Yet, this only allowed limited flap mobility. However, intraoperative observations revealed that placing the flap under tension while performing liposuction, “disrupted - tore”, the stiff, unyielding “skin retaining ligaments” and spared the (more flexible) blood vessels resulting in greater flap mobility than was possible with liposuction alone (Supplemental Digital Content 1).
To further increase flap mobility, discontinuous dissection and selective transection of the unyielding retaining ligaments and freeing the more stretchable neurovascular perforators was performed. The flap was freed sharply and bluntly to the inframammary crease superiorly and to the flanks laterally where the surgical dissection blended with the circumferential torso liposuction that further released the flaps lateral borders. To maximize flap mobility, some periumbilical perforators could be sacrificed without a discernible effect on the flap’s profuse blood supply (Supplemental Digital Content 2).
To clinically confirm the extended downward excursion of the LAA flap compared with the Saldanha flap was done by performing liposuction only, following it with tension on the liposuctioned flap, to disrupt the ligaments and then carrying out a discontinuous dissection to further mobilize the flap (Supplemental Digital Content 2).
Further intraoperative observations revealed that a well-defined vascular layer ended up lining the flap’s undersurface. Liposuction (with blunt tip cannulas—not laser or ultrasound), limited to the flap’s deep surface, was observed to have “plucked” fat globules from the connective tissue scaffolding while leaving the loose scaffolding and its vessels and nerves largely intact. The scaffolding and its content—vessels, nerves, and lymphatics appeared to have collapsed and formed a compact connective tissue layer that was coined the “supporting tissue” layer, and when the epinephrine vasoconstriction subsided, the undersurface layer was found to consist of a rich network of arteries, nerves, and lymphatics, embedded in a fibrous-connective tissue scaffolding (Figs. 4–6).
The resultant flap ended being an island flap based on neurovascular perforators lined by a “compact vascular supporting tissue” layer rather than a friable adipose tissue undersurface that could have contained devascularized fat fragments. Furthermore, the flap could be further thinned and evened by open liposuction of its undersurface (Supplemental Digital Content 1). Not performing liposuction throughout the full thickness of the subcutaneous tissue in contrast to the Saldanha approach of liposuctioning the entire subcutaneous tissue (except for the “subcutaneous 1 cm”), seemed safer, as no intrusion into the flap was judged best.15
The flap’s blood supply is distributed through the anastomosing network of the supporting tissue undersurface supplied by deep T-7-L-1 lateral and anterior cutaneous neurovascular perforators. Additional blood supply may be harnessed by incorporating the 3 superficial branches of the femoral artery coursing on the superficial surface of Scarpa’s fascia. Abdominoplasty techniques resecting the infra-umbilical panniculus also resect Scarpa’s fascia and its vessels, LAA, can preserve some of these vessels as Scarpa’s Fascia’s superficial surface is separated with liposuction and split in the midline (Supplemental Digital Content 2). These additional vessels, when present (unless damaged by C-sections etc.), “supercharge” the flap.26–33
A recent article by Smith and Smith34 distinguishes between lipoabdominoplasty techniques such as Saldanha and Brauman and analyzes the abdominal wall’s blood supply. He concludes that if perforators of the epigastric system vessels are spared, liposuction should be able to be safely performed on the central abdominal skin, Huger zone-I.34 Smith also surmises that Brauman likely preserves these perforators.18,19,34 This may very well be so in many cases; however, when the flap is tight, epigastric central perforators are sacrificed in favor of umbilical central perforators that are usually longer, larger, and more flexible since Brauman relies heavily on the flap’s anastomosing-vascular supporting tissue lining for the distribution of blood to the central, vulnerable portion of the flap (Supplemental Digital Content 2).
Compared with the Saldanha’s flap, the LAA’s flap improved perfusion and excursion can be demonstrated intraoperatively as the flap is released and extended and when the panniculus is excised with the operating table extended and the flap under tension. (Supplemental Digital Content 2; see video, Supplemental Digital Content 4, which displays excision of panniculus with liposuction and bleeding from the edge with flap under tension, http://links.lww.com/PRSGO/A859). The well-perfused flap can be advanced lower, and its safe and longer excursion is attested to by the absence of marginal necroses in several hundred consecutive abdominoplasties, the surgical results obtained even in the presence of a high umbilicus and limited epigastric laxity (Fig. 10) and the ability of patients to walk upright to the recovery room after surgery. Allowing patients to walk upright, likely also helps with respiration and venous return. Additionally, flap sensibility appears to be much enhanced and infection is rare because of the flap’s abundant perfusion.
Fig. 10.
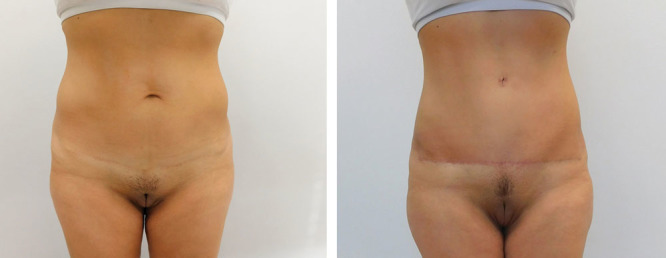
Thin patients with tight epigastric tissues/skin and high umbilicus, demonstrating the extent of the safe downward reach of the flap due to its superior mobility and rich blood supply distributed by its supporting tissue layer.
Video Graphic 4.
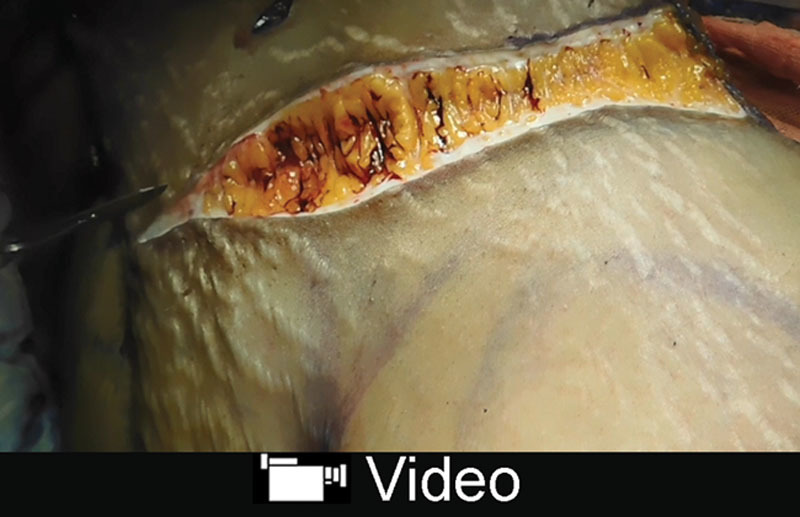
See video, Supplemental Digital Content 4, which displays excision of panniculus with liposuction and bleeding from the edge with flap under tension, http://links.lww.com/PRSGO/A859.
Seroma formation remains a persistent problem in abdominoplasty.35,36 Intraoperatively, massive weight loss patients seem to exhibit denser panniculi with an abundance of fibrous tissue and enlarged blood vessels. It seems that weight loss results in the loss of fat but the supporting tissue, containing enlarged, numerous blood vessels, nerves and lymphatics, appears to have been retained. This could explain the preponderance for seroma formation and the potential for lengthier surgeries and blood loss in massive weight loss surgery.
Preservation of the supportive tissue layer on the superficial surface of Scarpa’s fascia (that may contain lymphatics) is probably beneficial for seroma prevention. The recent finding that the lymphatic drainage from the lower abdomen courses superficial to Scarpa’s and pierces the fascia low, 2–3 cm proximal to the inguinal ligament supports this assumption.37
Seromas have been effectively treated by the corresponding author with local and systemic steroids before 2009, and Janis et al.38 have recently confirmed the inflammatory nature of seroma fluid.19 Treatment with steroids may be feasible for long-standing seromas; however, when steroids are used for prevention or treatment of fresh seromas, the benefits have to be weighed against the potential effect of steroids on the tensile strength of the healing tissues.
CONCLUSIONS
The LAA technique is based on sound surgical principals. The LAA flap’s extended downward reach is enabled by its rich blood supply. The LAA technique is uncomplicated to perform. The basic surgical maneuvers of hydro and lipo-dissection and separation of the subcutaneous tissue from the muscular fascia are straightforward and the rest follows from there. Backed by a 22-year experience gained by the authors and others, the LAA technique is suggested to surgeons as a proven alternative to other lipoabdominoplasty techniques.
Supplementary Material
Footnotes
Published online 14 September 2018.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.Illouz YG. Body contouring by lipolysis: a 5-year experience with over 3000 cases. Plast Reconstr Surg. 1983;72:591. [DOI] [PubMed] [Google Scholar]
- 2.Christman KD. Death following suction lipectomy and abdominoplasty. Plast Reconstr Surg. 1986;78:428. [DOI] [PubMed] [Google Scholar]
- 3.Abbes M, Bourgeon Y. Fat embolism after dermolipectomy and liposuction. Plast Reconstr Surg. 1989;84:546. [DOI] [PubMed] [Google Scholar]
- 4.Ersek RA. Serial suction lipectomy. Clin Plast Surg. 1989;16:313. [PubMed] [Google Scholar]
- 5.Dellerud E. Abdominoplasty combined with suction lipoplasty: a study of complication, revisions, and risk factors in 487 cases. Ann Plast Surg. 1990;25:333. [DOI] [PubMed] [Google Scholar]
- 6.Lockwood T. High-lateral-tension abdominoplasty with superficial fascial system suspension. Plast Reconstr Surg. 1995;96:603. [DOI] [PubMed] [Google Scholar]
- 7.Matarasso A. Liposuction as an adjunct to a full abdominoplasty revisited. Plast Reconstr Surg. 2000;106:1197. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen TT, Kim KA, Young RB. Tumescent mini abdominoplasty. Ann Plast Surg. 1997;38:209. [DOI] [PubMed] [Google Scholar]
- 9.Cárdenas-Camarena L, González LE. Large-volume liposuction and extensive abdominoplasty: a feasible alternative for improving body shape. Plast Reconstr Surg. 1998;102:1698. [DOI] [PubMed] [Google Scholar]
- 10.Baroudi R. Liposuction as an adjunct to a full abdominoplasty revisited (Discussion). Plast Reconstr Surg. 2000;106:1203. [DOI] [PubMed] [Google Scholar]
- 11.Shestak KC. Liposuction as an adjunct to a full abdominoplasty revisited (Discussion). Plast Reconstr Surg. 2000;106:1205. [DOI] [PubMed] [Google Scholar]
- 12.Heppe HP. Combined liposuction with abdominoplasty. Plast Reconstr Surg. 2001;108:577. [DOI] [PubMed] [Google Scholar]
- 13.Avelar JM. Abdominoplasty without panniculus undermining and resection: analysis and 3-year follow-up of 97 consecutive cases. Aesthet Surg J. 2002;22:16. [DOI] [PubMed] [Google Scholar]
- 14.Illouz YG. A new safe and aesthetic approach to suction abdominoplasty. Aesthetic Plast Surg. 1992;16:3. [DOI] [PubMed] [Google Scholar]
- 15.Saldanha OR, Pinto EB, Matos WN, Jr, et al. Lipoabdominoplasty without undermining. Aesthet Surg J. 2001;21:518. [DOI] [PubMed] [Google Scholar]
- 16.Brauman D. Lipoplasty: a case for a low-volume procedure. Aesth Surg J. 2000;20:373. [Google Scholar]
- 17.Brauman D. Liposuction abdomminoplasty: an evolving concept. Presented at the ASPS/PSEF/ASMS 71st annual scientific meeting November 2–6, 2002 San Antonio, Tex. [Google Scholar]
- 18.Brauman D. Liposuction abdominoplasty: an evolving concept. Plast Reconstr Surg. 2003;112:288. [DOI] [PubMed] [Google Scholar]
- 19.Brauman D, Capocci J. Liposuction abdominoplasty: an advanced body contouring technique. Plast Reconstr Surg. 2009;124:1685. Brauman D. Anatomical Liposuction & Liposuction Abdominoplasty. The second Huffstadt International Conference in Groningen. The Netherlands. University Medical Centre of Groningen. November 12–13, 2010. [DOI] [PubMed] [Google Scholar]
- 20.Brauman D. Anatomical Liposuction & Liposuction Abdominoplasty: Live Surgery. Amsterdam, The Netherlands: Sint Lucas Andreas Hospital; NVEPC 6th International Live Surgery and Congress on Aesthetic Plastic Surgery Body contouring, Amsterdam, The Netherlands. December 7 & 8, 2012. [Google Scholar]
- 21.Brauman D. Diastasis recti: clinical anatomy. Plast Reconstr Surg. 2008;122:1564. [DOI] [PubMed] [Google Scholar]
- 22.Brauman D. Reply, Letter to the Editor. Diastasis recti: clinical anatomy. Plast Reconstr Surg. 2009;123:1885.19483598 [Google Scholar]
- 23.Brauman D. Reply, Letter to the Editor. Factors that may affect. Plast Reconstr Surg. 2009;124:334.19568121 [Google Scholar]
- 24.di Summa PG, Wettstein R, Erba P, et al. Scar asymmetry after abdominoplasty: the unexpected role of seroma. Ann Plast Surg. 2013;71:461. [DOI] [PubMed] [Google Scholar]
- 25.Pitman GH. Commentary. Aesthet Surg J. 2000;20:380. [Google Scholar]
- 26.Huger WE., Jr The anatomic rationale for abdominal lipectomy. Am Surg. 1979;45:612. [PubMed] [Google Scholar]
- 27.Jamieson EBA. Illustrations of Regional Anatomy. Section III, Abdomen and Section VII, Lower limb. 1936Edinburgh, Scotland: E & S Livingston Ltd; [Google Scholar]
- 28.Ellis H. Clinical Anatomy. Blackwell Scientific Publications; 1966:5th ed Oxford, London, Edinburgh, Melbourne: 53. [Google Scholar]
- 29.Romanes GJ. Cunnigham’s Manual of Practical Anatomy. Thorax and Abdomen. 1968:Volume 2 13th ed London, United Kingdom: Oxford University Press; 93. [Google Scholar]
- 30.Jamieson EBA. Companion to Manuals of Anatomy. 1965:7th ed London, United Kingdom: Oxford University Press; 359. [Google Scholar]
- 31.Warwick, Williams Gray’s Anatomy. 1973:35th British Edition Philadelphia, Pa.: W.B. Saunders; 519. [Google Scholar]
- 32.Grant JCB. Grant’s Atlas of Anatomy. 1972:6th ed Baltimore, Md.: Williams & Wilkins; 105. [Google Scholar]
- 33.Clemente CD. Anatomy, A Regional Atlas of the Human Body. 1996:4th ed Williams and Wilkins; 246. [Google Scholar]
- 34.Smith LF, Smith LF., Jr Safely combining abdominoplasty with aggressive abdominal liposuction based on perforator vessels: technique and a review of 300 consecutive cases. Plast Reconstr Surg. 2015;135:1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shermak MA, Rotellini-Coltvet LA, Chang D. Seroma development following body contouring surgery for massive weight loss: patient risk factors and treatment strategies. Plast Reconstr Surg. 2008;122:280. [DOI] [PubMed] [Google Scholar]
- 36.Gusenoff JA. Prevention and management of complications in body contouring surgery. Clin Plast Surg. 2014;41:805. [DOI] [PubMed] [Google Scholar]
- 37.Saam S, Tourani SS, Taylor I, et al. Scarpa fascia preservation in abdominoplasty: does it preserve the lymphatics? Plast Reconstr Surg. 2015;136:258. [DOI] [PubMed] [Google Scholar]
- 38.Janis JE, Khansa L, Khansa I. Strategies for postoperative seroma prevention: a systematic review. Plast Reconstr Surg. 2016;138:240. [DOI] [PubMed] [Google Scholar]


