Supplemental Digital Content is available in the text.
Abstract
Background:
Connective tissue growth factor (CTGF) is a matricellular protein that plays a key role in wound healing and scar formation. Inhibition of CTGF by a specific antisense oligonucleotide significantly reduced scarring and fibrosis in animal models. This study examined whether an antisense oligonucleotide that inhibits human CTGF expression could reduce the severity of hypertrophic scar formation in patients following surgical revision of preexisting breast scars.
Methods:
This study was a 24-week multicenter, randomized, double-blind, within-subject, placebo-controlled phase 2b study evaluating the efficacy and safety of PF-06473871 in 2 regimens of either 3 or 4 intradermal injections (postsurgery weeks 2, 5, 8, and 11) of 5 mg/cm adjacent to the new surgical incision. One hundred subjects with bilateral hypertrophic scars resulting from prior breast surgery were randomized. Efficacy was determined by the Patient and Observer Scar Assessment Scale (POSAS).
Results:
The Physician/Observer POSAS overall opinion score at (week 24) for the 4-injection regimen demonstrated a statistically significant (P = 0.022) treatment difference from placebo of 0.68, and the treatment difference for the 3-injection regimen was nonsignificant (P = 0.4). Physician evaluation of scar severity at (week 24) with the photo-guide in the 4-injection regimen had a significant reduction (point estimate of treatment difference of 0.43 favoring PF-06473871). The surgical effect was approximately 2.0 at week 24 and was nearly 3 times greater than the treatment effect. Patient evaluations using the POSAS and photo-guide were not significantly improved with either dose regimen. PF-06473871 was generally well tolerated systemically and locally.
Conclusion:
The 4-dose regimen of PF-06473871 provided statistically significant improvement, inhibiting severity of hypertrophic scar formation based on physician assessment. However, the effect of revision surgery alone is significant and may dominate the treatment effect of PF-06473871.
INTRODUCTION
There is a need for improved therapies to reduce the 40–70% incidence of hypertrophic scaring that follows surgery.1 Excessive scarring can have unpleasant physical and social consequences. Symptoms include itching, stiffness, scar contractures, tenderness, and pain. The psychosocial effects include diminished self-esteem, disruption of daily activities, anxiety, and depression.2 Current and emerging therapies have limited efficacy.1,2
Connective tissue growth factor (CTGF) is a matricellular protein known to regulate cell proliferation, migration, differentiation, angiogenesis, extracellular matrix production, and adhesion.3,4 Over-expression of CTGF has been observed in dermal injury and chronic fibrotic disorders affecting the skin.5,6 In vitro and animal studies have demonstrated that treatment with an antisense oligonucleotide7 or small molecule8 targeting CTGF led to reductions in CTGF expression and subsequent reductions in scar formation.7
The antisense oligonucleotide, PF-06473871, comprises a 20-nucleotide chain containing a phosphorothioate backbone and 2’-methoxyethyl modifications of the ribose units at the 3’ and 5’ ends, conferring resistance to nucleases and providing long residence in tissues. PF-06473871 targets CTGF expression and is delivered by intradermal injection to a surgical wound. In a small phase 2a trial (EXC-203), PF-06473871 demonstrated a statistically significant reduction of scar severity, as determined by both physicians and patients, following breast scar revision surgery of hypertrophic scars.
The current trial was a randomized, double-blind, within-subject, placebo-controlled phase 2b study evaluating efficacy and safety of PF-06473871 in patients with preexisting hypertrophic scars. The primary objective of the study was to evaluate the efficacy of PF-06473871 in reducing severity of new scar formation after undergoing elective revision of hypertrophic scars.
The study was registered with ClinicalTrial.gov (NCT01730339).
PATIENTS AND METHODS
Patient Entry Criteria Inclusion and Exclusion
Female and male patients, 18–55 years of age, who consented to enroll in this study were included. Eligible patients had scars from breast surgery, including reduction, augmentation, or mastopexy. Scar age was to be no less than 6 months and no later than 15 years from study participation. Scars met the following qualifying criteria: (1) preexisting scar from prior breast surgery with a severity equivalent to a score of 3, 4, or 5 using the photo-guide (difference in left and right scar severity must have been ≤ 1 on the photo-guide; (2) hypertrophic; both scars must have been elevated above the surrounding skin; (3) a continuous scar length of a minimum of 4–6 cm, or maximum of 12 cm (two 6-cm sections) was treated, and the 2 scars must have been separated by at least 3 cm.
Study Design
This 24-week, multicenter, randomized, double-blind, within-subject, placebo-controlled phase 2b study evaluating the efficacy and safety of PF-06473871 was approved by institutional review boards at each investigator site. The study was conducted in accordance with legal and regulatory requirements, and the general principles of the International Ethical Guidelines for Biomedical Research Involving Human Patients Guidelines for Good clinical practice, and the Declaration of Helsinki. Randomization was implemented within 3 strata: (1) subjects with bilateral scars of equal severity (using the physician photo-guide); (2) subjects with bilateral scars of unequal severity, with the scar rated as more severe (using the physician photo-guide) being on the subject’s left side; and (3) subjects with bilateral scars of unequal severity, with the scar rated as more severe (using the physician photo-guide) being on the subject’s right side. Within each stratum, patients were randomized to 1 of 2 treatment groups (PF-06473871 and placebo: group 1: 4-dose administration and group 2: 3-dose administration) and treatments to either left or right scar.
An initial screening period of up to 21 days occurred before randomization. This was followed by a randomized, double-blind treatment period to week 11 and a further 13 week evaluation period to week 24. Pharmacokinetic assessments of plasma levels of PF-06473871 were conducted at screening, week 2 and week 8 (1 predose and one 2 hours ± 1 hour postdose). For subjects who discontinued before the end of the study, a final follow-up safety and efficacy visit was conducted.
Two weeks after scar revision surgery, PF-06473871 was administered to the preidentified 4–6 cm or 12-cm section of the revised scars via continuous intradermal injections along the perimeter of the newly formed incisions. The dose of PF-06473871 used, and dosing frequency was based on the parameters employed in the previous pilot study. Dosage was 5 mg per linear centimeter (2.5 mg per linear centimeter on each side). Each centimeter was injected with a volume of 100 µL. Dosing occurred to these predefined sections at week 2, 5, 8, and 11 after the surgical incision was closed in group 1 and at week 2, 5, and 8 in group 2.
Efficacy and Safety Assessments
The primary efficacy variable was the Physician overall opinion item of the POSAS (See figure, Supplemental Digital Content 1a, http://links.lww.com/PRSGO/A801, which displays 10-point scale as assessed at week 249). Secondary efficacy endpoints included (1) Physician scar assessment using the individual component scores in the POSAS (vascularity, pigmentation, thickness, relief, pliability, and surface area) at weeks 8, 11, 18, and 24 and overall opinion item at weeks 8, 11, and 18 (Supplemental Digital Content 1b, http://links.lww.com/PRSGO/A801); (2) Patient overall opinion of the POSAS (10-point scale at weeks 8, 11, 18, and 24 (See figure, Supplemental Digital Content 1c, http://links.lww.com/PRSGO/A801, which displays 10-point scale at weeks 8, 11, 18, and 24); (3) Physician & Patient photo-guide10,11 scar assessment (5-point scale, with scores of 3 or greater indicative of a hypertrophic scar) at weeks 8, 11, 18, and 24 (Supplemental Digital Content 2, http://links.lww.com/PRSGO/A803); (4) PR-SEQ assessed the symptoms and appearance domains at weeks 8 and 24. The PR-SEQ includes 30 items across 4 domains (appearance, symptoms, bothersomeness, and impacts (Supplemental Digital Content 3, http://links.lww.com/PRSGO/A802). Subjects completed the full 30-item assessment at baseline and an abbreviated version with only symptoms and appearance domains after the start of the study for their treated scars.12
Safety was evaluated at weeks 0, 1, 2, 5, 8, 11, 18, and 24 for (1) Incidence and severity of adverse events (AEs) that included treatment-emergent AEs (TEAEs) of special interest: maculopapular rash, pruritus, bronchospasm, dyspnea, cough, fever, diarrhea; (2) Assessment of scars for evaluation of skin reactions and electrocardiograms (at baseline and week 24); (3) vital signs and laboratory parameters.
Statistical Analysis
A total sample size of 100 subjects (50 per group) was planned to account for a 10% discontinuation rate. This sample size provided at least 80% power to detect a treatment difference of 1.5 points with SD of 2 using a paired test at a 1-sided 5% significance level.
Primary and secondary analyses were conducted using a repeated measure model with a covariate of the presurgery scar score, a stratification factor based on physician photo-guide, treatment, week and treatment by week interaction as fixed effect terms, and an unstructured variance–covariance matrix to account for the correlation of the data from the same patient. The stratification factor was the presurgery physician photo-guide scar equality classification described earlier. The repeated measurement analysis method assumed that all missing data were random. All efficacy endpoints and treatment differences were computed as placebo score minus PF-06473871 score and a positive value favored PF-06473871. Statistical significance was tested at the 1-sided significance level of 5%, without multiplicity adjustment. The primary analysis population for safety and efficacy is the modified intent-to-treat population (mITT), which encompasses all subjects who were randomized and received at least 1 dose of study drug.
RESULTS
Subjects
A total of 103 subjects (56 in group 1 and 47 in group 2) were randomized into the study. One hundred (97%) received at least 1 dose of study drug making the mITT population for safety and efficacy analysis (Fig. 1). Eight subjects (8.0%) (5 in group 1 and 3 in group 2) discontinued early from the study. The most common reason for discontinuation was withdrawal of consent by the subject (n = 5), lost to follow-up (n = 2), and 1 due to an AE. The majority of subjects received the planned number of dose administrations (87.6% in group 1 and 94.5% in group 2) with 2 subjects in each group receiving < 3 doses of study drug.
Fig. 1.
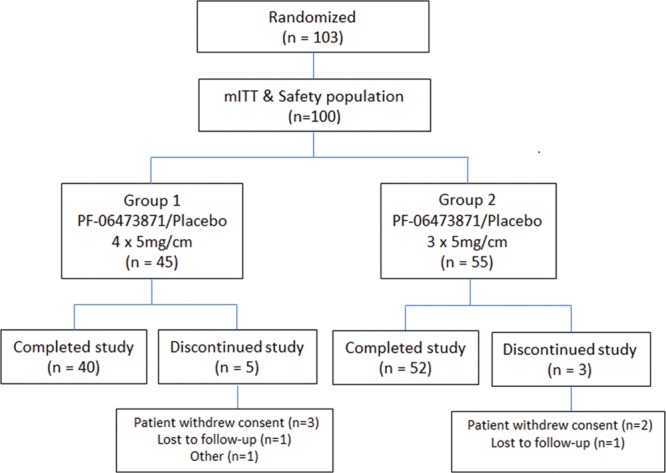
Trial design and disposition of patients in the study.
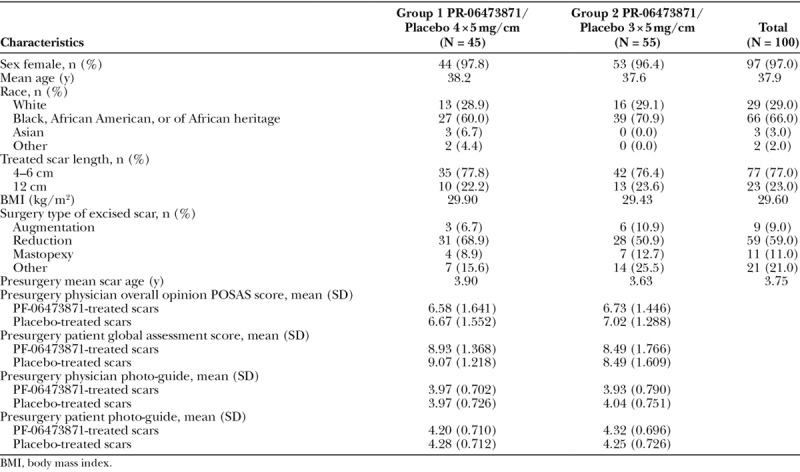
Subject demographics, baseline characteristics including presurgery scar severity assessment scores were well balanced across the 2 treatment groups (Table 1). Most subjects in the combined treatment groups were female (97.0%) and of Black, African American or of African heritage (66.0%). A majority of patients (59.0) had scars resulting from breast reduction surgery with a mean age of 3.75 years. All subjects had presurgery scar scores ≥ 3 indicative of hypertrophic scars.
Table 1.
Patient Demographics and Baseline Characteristics (mITT)
Efficacy: POSAS
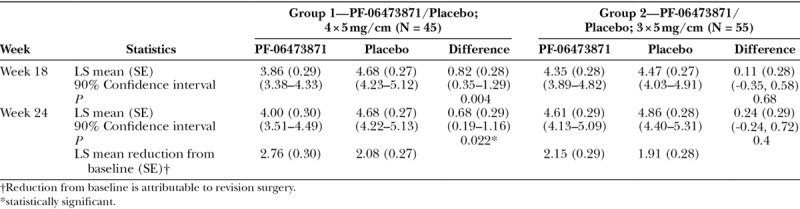
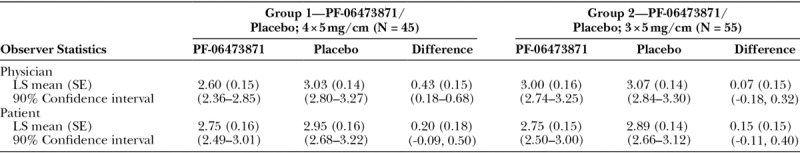
In group 1, the results from the Physician POSAS overall opinion score at week 24 (Table 2) demonstrated a statistically significant least square (LS) mean treatment difference from placebo of 0.68 (P = 0.022). In group 2, the results from the Physician POSAS overall opinion score at week 24 showed an LS mean treatment difference of 0.24 (P = 0.4). Similar results were also observed at week 18.
Table 2.
Summary of Physician POSAS Overall Opinion Scores (mITT Population)
The distribution of within-subject treatment differences in Physician POSAS overall opinion scores at week 24 are shown for the mITT population in Figure 2. A positive difference favored PF-06473871. In group 1, 61% subjects had a positive difference versus 16% negative treatment difference; in group 2, 45% positive difference versus 38% negative difference. Figure 3 shows an illustrative example of scar photographs taken at week 24 from a subject who received 4 administrations of PF-06473871. The figure illustrates the reduction in scar severity of the PF-06473871-treated scar compared with placebo (Figs. 3C, D, respectively), but also illustrates the reduction in scar severity resulting from revision surgery alone, seen when comparing the baseline scar severity with the scar severity following revision surgery on the placebo-treated side (Figs. 3B, D, respectively).
Fig. 2.
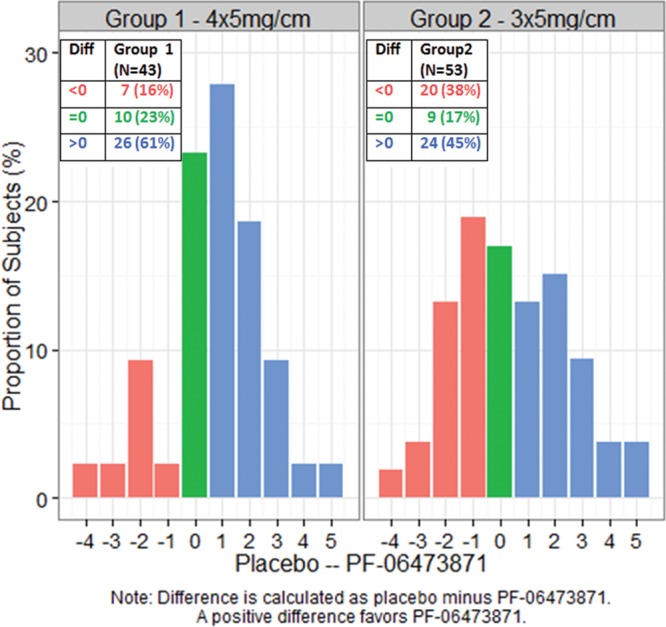
Distribution of within-subject treatment differences in physician POSAS 10-point overall opinion score at week 24 (mITT population).
Fig. 3.
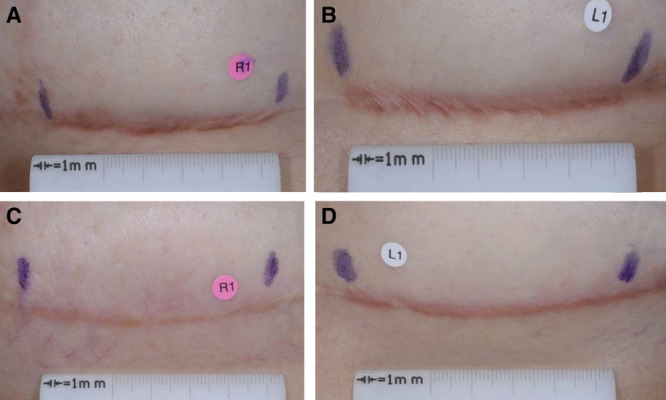
Illustrative examples of scar photographs from a subject at week 24, who received 4 administrations of PF-06473871. Photographs are right side, presurgery (baseline) (A); left side, presurgery (baseline) (B); right side, postsurgery, PF-06473871-treated (5 mg/cm, 4 treatments) (C); left-side, postsurgery, placebo-treated (D).
The results from the Physician POSAS item scores numerically favored PF-06473871 for all component scores (except pigmentation in group 2) and thickness, relief, pliability, and surface score strongly correlated strongly (r > 0.85) with the Physician POSAS overall opinion score (Supplemental Digital Content 4, http://links.lww.com/PRSGO/A804).
The treatment effect at week 8 and week 11 were not statistically different for any efficacy assessment. Generally, there was a positive trend in the observed treatment difference at these early time points, favoring PF-06473871.
Efficacy: Patient Overall Opinion
The analysis of Patient overall opinion scores showed a positive difference in favor of PF-06473871 in group 1 but a negative difference in favor of placebo in group 2. Neither of these differences was statistically significant (Supplemental Digital Content 5, http://links.lww.com/PRSGO/A805).
Efficacy: Photo-guide Assessments
The analysis of physician and patient photo-guide assessment scores showed a positive difference in favor of PF-06473871 in both groups, but none of these differences were statistically significant except physician photo-guide in group 1 (Tables 3, 4). In a dichotomous analysis, the week 24 photo-guide scores of 2 or less were classified as fine-line scars (3–5 are considered hypertrophic). In group 1, scars at the PF-06473871-treated site were classified as fine-line for 51.2% of subjects compared with 34.9% for the placebo-treated scars, with an adjusted odds ratio of treatment response of 1.91. In group 2, scars at the PF-06473871-treated site were classified as fine-line for 51.9% of subjects compared with 44.4% placebo-treated scars. These results were not statistically significant, but indicated a trend toward a fine-line scar in favor of PF-06473871.
Table 3.
Summary of Photo-guide Scar Assessment Score at Week 24 by Observer (mITT Population)
The distributions of the within-subject paired difference (placebo minus PF-06473871) in the physician photo-guide at week 24 are shown in Figure 4. The majority of group 1 subjects had a treatment difference favoring PF-06473871, 52% with ≥ 1 point improvement versus 14% worsening. In group 2, there was little treatment difference, 38% with ≥ 1 point improvement versus 31% worsening.
Fig. 4.
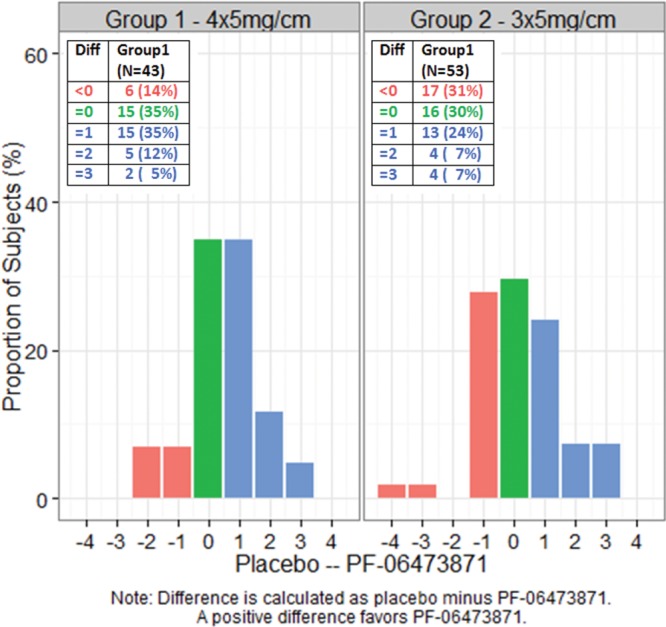
Distribution of within-subject treatment differences in 5-point physician photo-guide at week 24.
Efficacy: PR-SEQ
The analysis of PR-SEQ showed a positive treatment difference in favor of PF-06473871 in both appearance and symptom domains for both groups except the symptom domain in group 2. None of these differences were statistically significant at P ≤ 0.1 (Supplemental Digital Content 6, http://links.lww.com/PRSGO/A806).
Safety
There were no treatment-emergent Serious Adverse Events, deaths, or malignancies reported during the study (Table 4). There were 3 severe AEs of injection site pain reported. One subject in group 1 experienced a TEAE (injection site pain) that resulted in early discontinuation from study participation. Injection site pain and pruritus were the most common TEAEs in 19% and 7% of subjects, respectively.
Table 4.
Overall Summary of TEAEs by Study Phase (Safety)
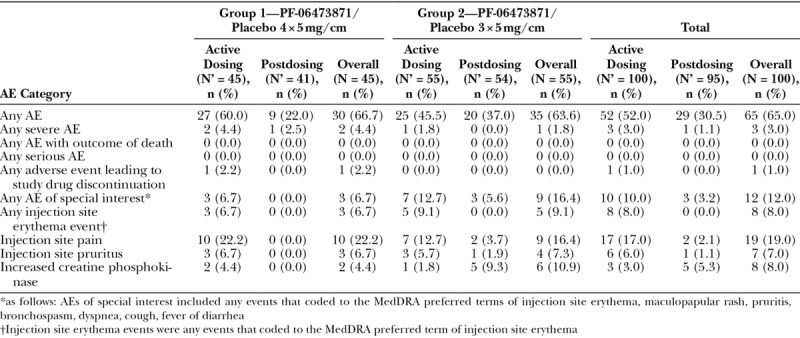
Wound dehiscence was reported as a TEAE for 4 subjects during the study. In 3 cases, the wound dehiscence occurred in first 2 weeks postsurgery (before study treatment given) and a fourth case of wound dehiscence was more than 8.5 cm from study drug treatment site. Therefore, the investigators did not consider wound dehiscence to be related to study medication.
Injection site erythema was uncommon, occurring in 7% of PF-06473871 versus 5% of placebo injection sites. The incidence was similar for both dose regimens. The injection site erythema usually occurred after the first or second dose and usually resolved within 10 days, though 1 subject reported 51 days to resolution of erythema for both the PF-06473871- and placebo-treated portions of the scars.
There were no clinically significant changes in vital signs, electrocardiograms, or laboratory abnormalities reported during the study. There was no grade 4 WHO laboratory abnormality reported during the study. Laboratory test abnormalities reported as AEs included 8 patients with reported elevation in creatine phosphokinase (2 in the 4-treatment administration group and 6 in the 3-treatment administration group) without any reported AEs of muscle weakness.
PHARMACOKINETICS
The mean (SD) of PF-06473871 concentration (ng/ml) at the week 8 postdose determination was 996.9 (722.9) for group 1 and 1206.2 (1042.6) for group 2, with median values of 830.5 and 845.0, respectively. Concentration-time data exhibited moderate variability and median concentrations were higher (< 2-fold) than the median predicted values from healthy patients.
DISCUSSION
PF-06473871 is an antisense oligonucleotide with activity against CTGF, intended to reduce the severity of newly formed scars. The current study was designed to evaluate the efficacy of PF-06473871 in reducing the severity of skin scarring in patients undergoing elective revision of preexisting hypertrophic breast scars. The goal for an effective antiscarring agent is to increase the potential of developing a fine-line scar and a more favorable assessment of the outcome by the patient and physician.
Physician-rated reductions in scar severity following the 4-dose group of PF-06473871 achieved statistical significance compared with placebo at weeks 18 and 24. Consistent with this observation, 61% of the 4-treatment regimen patients had at least a 1 point improvement in physician POSAS score compared with placebo at week 24, whereas 16% had at least a 1 point worsening. Differences in physician photo-guide scores showed significant improvement for only the 4-dose group at weeks 18 and 24. Though not statistically significant, the patient photo-guide and PR-SEQ scores showed a favorable numeric trend in the 4-treatment group. There was a modest or no improvement in POSAS overall opinion, photo-guide, and PR-SEQ scar scores in the 3-dose regimen group as reported by both physicians and patients, indicating there was no evidence of clinical effect for this regimen.
Two previous clinical studies of PF-06473871 in subjects with hypertrophic scars following breast surgery yielded divergent results. In study EXC-203 (n = 21), the physician POSAS 24 week treatment effect of 5 mg/cm for 4 doses was a 2.40 point improvement,13 whereas in a second study (NCT01038297/EXC-204, n=36), the week 24 treatment effect, again using physician POSAS, was only 0.30 (unpublished data). We hypothesized that this divergence might result from inclusion in study EXC-203 of subjects with more severe preexisting scars, resulting in greater hypertrophic scar recurrence and greater severity of placebo-treated scars than in the EXC-204 study. This lead to stricter entry criteria, 2 dose regimens, and greater sample size for the current study. The physician’s baseline assessment of a qualifying scar was confirmed by a central reviewer viewing standardized scar photographs.
To quantify the meaningfulness of change in scar appearance, a separate analysis of data collected in a validation study for the endpoints (PR-SEQ, POSAS, and photo-guide) was conducted based on internet survey data of 512 patients with linear scars.10 These analyses led to a prespecified minimal clinically important difference of at least a 1.5 point improvement (15 points on a standardized scale of 0–100) in POSAS score compared with placebo at week 24 and a positive change of at least 1 level in the 5-point photo-guide to meet the meaningfulness threshold from the patient perspective. This observation suggests that a more efficacious regimen would be needed to achieve a clinically significant improvement in scar formation as perceived by the patient, even in the presence of a detectable and statistically significant difference in the physician POSAS score.
In the repeated measure model analysis using change from baseline in Physician POSAS overall opinion score, the surgical effect, as estimated by the mean reduction from presurgery score in the placebo-treated group, was approximately 2.0 at week 24. The surgical effect was nearly 3 times greater than the treatment effect for both 4-treatment administration (0.68) and 3-treatment administration (0.24) of PF-06473871, compared with placebo. These results suggest that the effect of revision surgery alone is significant and may have dominated the treatment effect of PF-06473871. Supplementary subgroup analyses (by race, scar length, scar age, study site, and baseline scar severity) were also carried out but did not yield any compelling evidence of a greater treatment effect in any of the subpopulations studied.
The profile of AEs, vital signs, laboratory safety, and electrocardiogram findings observed demonstrated that PF-06473871, at the dose of 5 mg/linear cm, is safe and generally well tolerated following intradermal administration in the study population.
Limitations of the study were only 2 treatment regimens (3 versus 4 doses) were evaluated with the same concentration of study drug (5 mg/cm) administered 3 weeks apart. Therefore, other regimens, varying dose, frequency, or total number of doses may have been more efficacious. In addition, subjects were included with hypertrophic scars resulting from a variety of prior types of breast surgery and with different ages of scar. Both parameters may have influenced the response to PF-06473871. Being a trial of within-patient design, with active treatment given on 1 side of scar and placebo to the other side, makes it difficult to determine if systemic safety effects of PF-06473871 differ from those of placebo. As scars can continue to mature after 24 weeks, a longer evaluation would help determine how long treatment effect is sustained following the last administration.
CONCLUSIONS
The 4-treatment regimen of PF-06473871 at 5 mg/cm provided modest improvement by inhibiting severity of hypertrophic scar formation 24 weeks after scar revision surgery. The magnitude of effect was not sufficient to obtain significant improvement in scar appearance as perceived by the subject, suggesting that a more efficacious treatment regimen would be needed. The improvement observed in the Physician POSAS overall opinion score from baseline was largely due to the effect of scar revision surgery. The active drug was generally well tolerated both systemically and locally at the injection sites.
The utility of POSAS, the photo-guide, and PR-SEQ in trials of therapies to inhibit scar formation need testing with different mechanisms of action and at other anatomical sites to understand the strengths and weaknesses of these endpoints.
Supplementary Material
Footnotes
Published online 6 September 2018.
Supported by Pfizer.
This study was registered at www.clinicaltrials.gov NCT01730339.
Disclosure: Jeremy Gale, Jeff Jensen, Gabe Berman, William Freimuth, Gang Li, and Andreas Pleil were all employees and stockholders of Pfizer Inc., the sponsor of this clinical trial, during the conduct of this study. Malik Kutty, Andrew Rosenthal, C. B. Boswell, Magnus Noah, and Leroy Young have no potential financial conflicts of interest to declare. All the physician-authors are board-certified plastic surgeons who performed the scar revision surgeries, dosing of PF-06473871 or placebo and photo evaluations. In all cases, the physician-authors remained blinded to treatment allocation until the end of the study and the lock of the clinical database. The Article Processing Charge was paid for by the sponsor.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.Gauglitz GG, Korting HC, Pavicic T, et al. Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Mol Med. 2011;17:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monstrey S, Middelkoop E, Vranckx JJ, et al. Updated scar management practical guidelines: non-invasive and invasive measures. J Plast Reconstr Aesthet Surg. 2014;67:1017. [DOI] [PubMed] [Google Scholar]
- 3.Shi-Wen X, Leask A, Abraham D. Regulation and function of connective tissue growth factor/CCN2 in tissue repair, scarring and fibrosis. Cytokine Growth Factor Rev. 2008;19:133. [DOI] [PubMed] [Google Scholar]
- 4.Cicha I, Goppelt-Struebe M. Connective tissue growth factor: context-dependent functions and mechanisms of regulation. Biofactors. 2009;35:200. [DOI] [PubMed] [Google Scholar]
- 5.Colwell AS, Phan TT, Kong W, et al. Hypertrophic scar fibroblasts have increased connective tissue growth factor expression after transforming growth factor-beta stimulation. Plast Reconstr Surg. 2005;116:1387. [DOI] [PubMed] [Google Scholar]
- 6.Smith JC, Boone BE, Opalenik SR, et al. Gene profiling of keloid fibroblasts shows altered expression in multiple fibrosis-associated pathways. J Invest Dermatol. 2008;128:1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sisco M, Kryger ZB, O’Shaughnessy KD, et al. Antisense inhibition of connective tissue growth factor (CTGF/CCN2) mRNA limits hypertrophic scarring without affecting wound healing in vivo. Wound Repair Regen. 2008;16:661. [DOI] [PubMed] [Google Scholar]
- 8.Jurzak M, Adamczyk K, Antończak P, et al. Evaluation of genistein ability to modulate CTGF mRNA/protein expression, genes expression of TGFβ isoforms and expression of selected genes regulating cell cycle in keloid fibroblasts in vitro. Acta Pol Pharm. 2014;71:972. [PubMed] [Google Scholar]
- 9.Van de Kar AL, Corion LU, Smeulders M, et al. Reliable and feasible evaluation of linear scars by the patient and observer scar assessment scale. Plast Reconstr Surg. 2005;116:514. [DOI] [PubMed] [Google Scholar]
- 10.Pleil A, Bushmakin AG, Shields A, et al. Determining the magnitude of a detectable and a relevant treatment benefit in aesthetic medicine using a photoguide and the internet. ISPOR 16th Annual European Congress; November 2013; Dublin, Ireland. [Google Scholar]
- 11.Pleil A, Jensen J, Galipeau N, et al. Is a picture worth a thousand words? The development of a clinician and patient reported photonumeric guide to assess scar severity. Abstract #: PRM 106. Poster presented May 20, 2013 at the 18th Annual International Meeting of the International Society for Pharmacoeconomics and Outcomes Research (ISPOR); 2013; New Orleans, La. [Google Scholar]
- 12.Pleil A, Mathias A, Galipeau N, et al. Reliability of the patient-reported scar evaluation questionnaire (PR-SEQ) in a large cohort of surgical and trauma scar subjects. Abstract #: PSS30. Poster presented at the 19th Annual International Meeting of the International Society for Pharmacoeconomics and Outcomes Research (ISPOR); May 31 to June 4, 2014 Montreal, QC, Canada. [Google Scholar]
- 13.Jensen J, Gentzkow G, Berman G, et al. Anti-CTGF oligonucleotide reduces severity of postsurgical hypertrophic scars in a randomized, double-blind, within-subject, placebo-controlled study. Plast Reconstr Surg. 2018; 142:XXX. [DOI] [PubMed] [Google Scholar]


