Abstract
IMPORTANCE
Delay in administration of the first epinephrine dose is associated with decreased survival among adults after in-hospital, nonshockable cardiac arrest. Whether this association is true in the pediatric in-hospital cardiac arrest population remains unknown.
OBJECTIVE
To determine whether time to first epinephrine dose is associated with outcomes in pediatric in-hospital cardiac arrest.
DESIGN, SETTING. AND PARTICIPANTS
We performed an analysis of data from the Get With the Guidelines–Resuscitation registry. We included US pediatric patients (age <18 years) with an in-hospital cardiac arrest and an initial nonshockable rhythm who received at least 1 dose of epinephrine. A total of 1558 patients (median age, 9 months [interquartile range [IQR], 13 days–5 years]) were included in the final cohort.
EXPOSURE
Time to epinephrine, defined as time in minutes from recognition of loss of pulse to the first dose of epinephrine.
MAIN OUTCOMES AND MEASURES
The primary outcome was survival to hospital discharge. Secondary outcomes included return of spontaneous circulation (ROSC), survival at 24 hours, and neurological outcome. A favorable neurological outcome was defined as a score of 1 to 2 on the Pediatric Cerebral Performance Category scale.
RESULTS
Among the 1558 patients, 487 (31.3%) survived to hospital discharge. The median time to first epinephrine dose was 1 minute (IQR, 0–4; range, 0–20; mean [SD], 2.6 [3.4] minutes). Longer time to epinephrine administration was associated with lower risk of survival to discharge in multivariable analysis (multivariable-adjusted risk ratio [RR] per minute delay, 0.95 [95% CI, 0.93–0.98]). Longer time to epinephrine administration was also associated with decreased risk of ROSC (multivariable-adjusted RR per minute delay, 0.97 [95% CI, 0.96–0.99]), decreased risk of survival at 24 hours (multivariable-adjusted RR per minute delay, 0.97 [95% CI, 0.95–0.99]), and decreased risk of survival with favorable neurological outcome (multivariable-adjusted RR per minute delay, 0.95 [95% CI, 0.91–0.99]). Patients with time to epinephrine administration of longer than 5 minutes (233/1558) compared with those with time to epinephrine of 5 minutes or less (1325/1558) had lower risk of in-hospital survival to discharge (21.0% [49/233] vs 33.1% [438/1325]; multivariable-adjusted RR, 0.75 [95% CI, 0.60–0.93]; P = .01).
CONCLUSIONS AND RELEVANCE
Among children with in-hospital cardiac arrest with an initial nonshockable rhythm who received epinephrine, delay in administration of epinephrine was associated with decreased chance of survival to hospital discharge, ROSC, 24-hour survival, and survival to hospital discharge with a favorable neurological outcome.
Approximately 16 000 children in the United States have a cardiac arrest each year, predominantly in a hospital setting.1,2 An initial rhythm of pulseless electrical activity or asystole (ie, a nonshockable rhythm) is most common and carries a significant mortality, with 25% to 40% of patients surviving to hospital discharge.1,3–5 Despite efforts in resuscitation research and improvement in outcomes after inhospital pediatric resuscitation during the last 30 years,4,6 there are few evidence-based interventions besides supportive care for the pediatric patient in cardiac arrest with a nonshockable rhythm.6,7
Epinephrine (or adrenaline), a potent α- and β-adrenergic agonist, is recommended by both the American Heart Association (AHA) and the European Resuscitation Council in pediatric cardiac arrest. Current guidelines recommend giving epinephrine at 0.01 mg/kg (maximum, 1 mg) as soon as vascular or intraosseous access is obtained and subsequently every 3 to 5 minutes for patients with a nonshockable rhythm.6,8 Epinephrine’s beneficial effects are thought to be mediated predominantly through α-adrenergic increase in aortic diastolic pressure and increased coronary perfusion pressure—an important determinant of return of spontaneous circulation (ROSC).9–11 Despite this, to our knowledge, no randomized trial comparing epinephrine with placebo has been conducted in this population,7 and the ethics of such a trial may currently be questionable.
Prior studies have addressed the dosage of epinephrine (standard vs high dose) in pediatric cardiac arrest.12–14 We have not identified any studies examining the association between delay in epinephrine dose and outcomes in pediatric cardiac arrest. A recent report found that delay in epinephrine administration for adult in-hospital, nonshockable cardiac arrest was associated with decreased chance of ROSC, survival to discharge, and good neurological outcome.15 We hypothesized that delay in epinephrine administration for pediatric in-hospital, nonshockable cardiac arrest would likewise be associated with decreased survival.
Methods
We used the Get With the Guidelines–Resuscitation (GWTG-R) registry, an AHA-sponsored, national, prospective, quality improvement registry of US in-hospital cardiac arrests. The details of data collection and reliability have been described previously.3,16 Cardiac arrest is defined as pulselessness, or a pulse with inadequate perfusion, requiring chest compressions, defibrillation, or both, with a hospital-wide or unit-based emergency response by acute care facility personnel. In-hospital cardiac arrest patients with prior do-not-resuscitate orders or cardiopulmonary resuscitation (CPR) events that began outside the hospital are excluded. Cases are identified and data extracted by trained personnel from cardiac arrest flow sheets, hospital paging system logs, routine checks of code carts, pharmacy drug records, and hospital billing charges for resuscitation medication.16 The registry uses Utstein-style templates for cardiac arrest, standardized reporting guidelines used to define patient variables and outcomes, to facilitate uniform reporting across hospitals.17,18 Integrity of the data is optimized through rigorous certification of data entry personnel and the use of standardized software that checks the data for completeness and accuracy.19
All participating hospitals are required to comply with local regulatory guidelines. Because data are used primarily at the local site for quality improvement, sites are granted a waiver of informed consent under the common rule.
Study Population
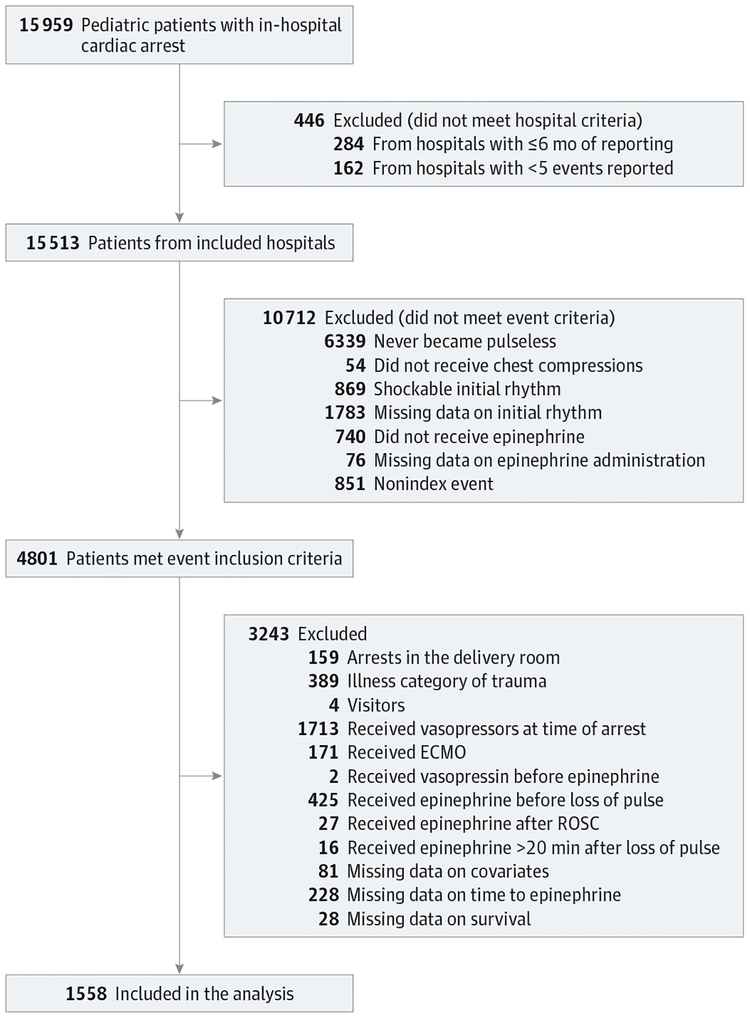
The cohort included data submitted to the GWTG-R registry between January 2000 and December 2014. We included all patients younger than 18 years who received chest compressions while pulseless with a documented nonshockable initial rhythm and who received at least 1 epinephrine bolus during resuscitation. We included index events only from hospitals with at least 6 months of reporting and at least 5 cases reported. We excluded patients with the following: (1) cardiac arrest in the delivery room, (2) an illness category of trauma or an illness category of hospital visitor, (3) vasopressor (epinephrine, norepinephrine, phenylephrine, and/or dopamine [for dopamine, at least 3 μg/kg/min]) infusion at the time of cardiac arrest, (4) treatment with extracorporeal membrane oxygenation during the event, (5) vasopressin received before epinephrine, (6) epinephrine given before loss of pulse, (7) epinephrine received after ROSC, (8) epinephrine given more than 20 minutes after loss of pulse, (9) missing data on covariates, (10) missing data on time to first epinephrine dose, and (11) missing data on in-hospital survival (Figure 1).
Figure 1. Patient Flowchart for Study of Timing of Epinephrine and Pediatric In-Hospital Nonshockable Cardiac Arrest.
The database contained data on 15 959 pediatric in-hospital cardiac arrests. Of these, 1558 met all inclusion criteria and no exclusion criteria and were included in the analysis. ECMO indicates extracorporeal membrane oxygenation; ROSC, return of spontaneous circulation.
Time to Epinephrine and Study Outcomes
Time to epinephrine was defined as the interval in minutes from recognition of loss of pulse to the first bolus dose of epinephrine. The recording of the time of pulselessness and the first dose of epinephrine was done in whole minutes. As such, a time to epinephrine of 0 minutes represents that epinephrine was given within the same whole minute that the patient lost their pulse, a time of 1 minute represents that epinephrine was given within the next whole minute, etc.
The primary outcome was survival to discharge from the hospital. Secondary outcomes were ROSC, defined as at least 20 minutes with a palpable pulse; survival at 24 hours; and favorable neurological outcome at hospital discharge. Neurological outcome was assessed with the Pediatric Cerebral Performance Category (PCPC) scale,20 in which a score of 1 indicates no neurological deficit; 2, mild cerebral disability; 3, moderate cerebral disability; 4, severe cerebral disability; 5, coma or vegetative state; and 6, brain death. A PCPC score of 1 to 2 was considered a favorable neurological outcome, and a PCPC score of 3 to 6 (death) was considered a poor neurological outcome. However, there is currently no universal definition of a favorable neurological outcome in pediatric cardiac arrest patients using the PCPC score, and multiple definitions have been used previously.4,21,22 To account for this, we did sensitivity analyses using 3 different definitions: (1) a PCPC score of 1 or 2 or no increase from baseline; (2) a PCPC score of 1, 2, or 3; and (3) a PCPC score of 1, 2, or 3 or no increase from baseline. Outcome assessors were unaware of the hypotheses of the current study. Data abstractors were not blinded to the outcomes.
Statistical Analyses
The study population was characterized using descriptive statistics. Categorical variables are presented with counts and frequencies and continuous variables in means with standard deviations or medians with interquartile ranges (IQRs) depending on the normality of the data. The χ2 test was used to compare frequencies.
To assess the independent association between time to epinephrine administration during cardiac arrest resuscitation and survival to discharge, we applied a multivariable regression model with generalized estimating equations with an exchangeable (compound symmetry) correlation matrix to account for hospital clustering. We used modified Poisson regression models with robust variance estimates to estimate risk ratios (RRs)23,24 as previously used in the adult GWTG cohort.4,25,26 For our primary analysis, we treated time to epinephrine as a linear, continuous variable.
The following variables were entered into the multivariable model: age group (neonate [<1 month], infant [1 month to <1 year], child [1–12 years], or adolescent [>12 years]), sex, year of the arrest (treated as a categorical variable with year 2000 as the reference), illness category (medical cardiac, medical noncardiac, surgical cardiac, surgical noncardiac, or newborn [ie, born this admission]), preexisting mechanical ventilation, whether the patient was monitored (presence of electrocardiography, pulse oximetry, and/or apnea monitor), whether the event was witnessed, location of arrest (intensive care unit [including postanesthesia care unit and the operating room], emergency department, floor without telemetry, floor with telemetry, or other), time of week (weekday [Monday 7 AM–Friday 11 PM] vs weekend [Friday 11 PM–Monday 7 AM]), time of day (day [7:00 AM–10:59 PM] vs night [11:00 PM–6:59 AM]), first documented pulseless rhythm (asystole vs pulseless electrical activity), and insertion or reinsertion of an airway during the event. We also included whether the hospital was primarily a pediatric hospital and hospital teaching status (major [with fellowship program], minor [with residency program], or non-teaching [no residency program]). We entered time (in minutes) to initiation of chest compressions from loss of pulse into each multivariable model to account for any delay in resuscitation. If time to CPR was negative (ie, the patient lost his or her pulse after initiation of CPR), a value of 0 minutes was imputed. All variables were chosen a priori based on prior work and clinical reasoning.22,27,28
Similar multivariable regression models were used to analyze secondary outcomes (ROSC, 24-hour survival, and survival to discharge with favorable neurological outcome), including different definitions of favorable neurological outcome. Results from the multivariable regression models are reported as RRs with 95% CIs. For both primary and secondary outcomes, the RRs represent the RR for the outcome per minute increase in time to epinephrine.
To further characterize the relationship between time to epinephrine and outcomes, we conducted a preplanned analysis in which time to epinephrine was categorized into 5 minutes or less or longer than 5 minutes, as previously used as a quality metric in the adult cardiac arrest population.29 Using this definition, we conducted similar analyses as described earlier in this section.
Outcome variables were complete for ROSC, survival at 24 hours, and survival to discharge in the included cohort. For all definitions of neurological outcome, approximately 11% of patients had missing data. For the analysis of neurological outcome, we included only patients who had these outcomes reported.
We performed a number of post hoc sensitivity analyses, including propensity score analyses, nonlinearity analyses, and multiple imputations with imputation of missing values for time to epinephrine, covariates, and the various outcomes. (Details of these analyses are provided in the eMethods in the Supplement.) We also performed post hoc tests of the following interactions with time to epinephrine in the main multivariable analysis: location of the arrest, initial rhythm, and age.
Statistical analyses were conducted with SAS version 9.4 (SAS Institute). All hypothesis tests were 2-sided, with a significance level of P < .05. No adjustments were made for multiple testing, and, as such, our secondary end points should be considered exploratory.
Results
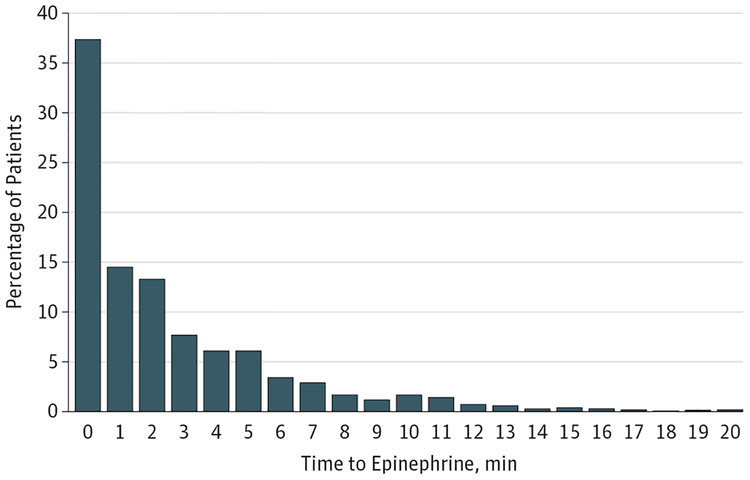
The final cohort included 1558 patients (Figure 1). Median age was 9 months (IQR, 13 days–5 years), and 46% were female. The median time to first epinephrine dose was 1 minute (IQR, 0–4; range, 0–20; mean [SD], 2.6 [3.4] minutes) (Figure 2). Median time to chest compressions was 0 minutes (IQR, 0–0). Additional patient, event, and hospital characteristics are presented in Table 1 and in eTable 1 in the Supplement.
Figure 2. Distribution of Time to Epinephrine in Pediatric In-Hospital Nonshockable Cardiac Arrest (N=1558).
The majority of the included patients received epinephrine early, with 37% receiving epinephrine within the first minute; 15% received the first dose of epinephrine more than 5 minutes after the cardiac arrest. (See Methods for definition of time to epinephrine.) No time point had zero observations.
Table 1.
Characteristics of the Population for Study of Timing of Epinephrine and Pediatric In-Hospital Nonshockable Cardiac Arresta
| No. (%) | P Value | |||
|---|---|---|---|---|
| All Patients (N = 1558) | Survivors to Hospital Discharge (n = 487) | Nonsurvivors (n = 1071) | ||
| Sex | ||||
| Female | 709 (46) | 218 (45) | 491 (46) | .69 |
| Male | 849 (55) | 269 (55) | 580 (54) | |
| Age group | ||||
| Neonate, <1 mo | 421 (27) | 117 (24) | 304 (28) | <.001 |
| Infant, 1 mo-<1 y | 406 (26) | 147 (30) | 259 (24) | |
| Child, 1–12 y | 501 (32) | 173 (36) | 328 (31) | |
| Adolescent, >12 y | 230 (15) | 50 (10) | 180 (17) | |
| Type of admission | ||||
| Medicalcardiac | 258 (17) | 71(15) | 187 (17) | <.001 |
| Medicalnoncardiac | 777 (50) | 221 (45) | 556 (52) | |
| Surgical cardiac | 179 (11) | 80 (16) | 99 (9) | |
| Surgical noncardiac | 139 (9) | 69 (14) | 17 (7) | |
| Newbornb | 205 (13) | 46 (9) | 159 (15) | |
| Location of arrest | ||||
| Emergency department | 266 (17) | 56(12) | 210 (20) | <.001 |
| ICU/PACU/OR | 1001 (64) | 338 (69) | 663 (62) | |
| Floor with telemetry | 41 (3) | 9 (2) | 32 (3) | |
| Floor without telemetry | 144 (9) | 35 (7) | 109 (10) | |
| Other | 106 (7) | 49 (10) | 57 (5) | |
| Time of week of arrest | ||||
| Weekendc | 480 (31) | 123(25) | 357 (33) | .001 |
| Weekday | 1078 (69) | 364 (75) | 714 (67) | |
| Time of day of arrest | ||||
| Nighttimed | 457 (29) | 118 (24) | 339 (32) | .003 |
| Daytime | 1101 (71) | 369 (76) | 732 (68) | |
| Arrest witnessed | ||||
| Yes | 1401 (90) | 462 (95) | 939 (88) | <.001 |
| No | 157 (10) | 25 (5) | 132 (12) | |
| Arrest monitored | ||||
| Yes | 1313 (84) | 430 (88) | 883 (82) | .003 |
| No | 245 (16) | 57 (11) | 188 (18) | |
| Preexisting mechanical ventilation | ||||
| Yes | 768 (49) | 243 (50) | 525 (49) | .75 |
| No | 709 (51) | 244 (50) | 546 (51) | |
| Insertion of an airway | ||||
| Yes | 759 (49) | 240 (49) | 519 (48) | .06 |
| No | 799 (51) | 247 (51) | 552 (52) | |
| Initial rhythm | ||||
| Asystole | 812 (52) | 217 (45) | 595 (56) | .001 |
| Pulseless electrical activity | 746 (48) | 270 (55) | 476 (44) | |
| Time to epinephrine, min | ||||
| Median (IQR) | 1 (0–4) | 1 (0–3) | 2 (0–4) | <.001 |
| Mean (SD) | 2.6 (3.4) | 2.0 (2.8) | 2.8 (2.6) | |
| Time to chest compressions, min | ||||
| Median (IQR) | 0 (0–0) | 0 (0–0) | 0 (0–0) | .14 |
| Mean (SD) | 0.1 (1.0) | 0.1 (0.4) | 0.2 (1.1) | |
| Type of hospital | ||||
| Primarily children | 564 (36) | 202 (41) | 362 (34) | .004 |
| Primarily adult | 994 (64) | 285 (58) | 709 (66) | |
| Teaching status | ||||
| Major | 1079 (69) | 359 (74) | 420 (67) | .02 |
| Minor | 366(23) | 103 (21) | 263 (25) | |
| Nonteaching | 113 (7) | 25 (5) | 88 (8) | |
| Year of the arrest | ||||
| 2000–2002 | 109 (7) | 20 (4) | 89 (8) | .07 |
| 2003–2004 | 133 (11) | 47 (10) | 122 (11) | |
| 2005–2006 | 254 (16) | 81 (17) | 173 (16) | |
| 2007–2008 | 272 (18) | 93 (19) | 182 (17) | |
| 2009–2010 | 270 (17) | 88 (18) | 182 (17) | |
| 2011–2012 | 260 (17) | 87 (18) | 173 (16) | |
| 2013–2014 | 221 (14) | 71 (16) | 150 (14) | |
Abbreviations: ICU, intensive care unit; IQR, interquartile range; PACU, postanesthesia care unit; OR, operating room.
Continuous variables are presented as medians with interquartile ranges and categorical variables as counts (frequencies).
Defined as being born on the current admission.
Friday 11 PM to Monday 7 AM.
11:00 PM to 6:59 AM.
Primary Outcome
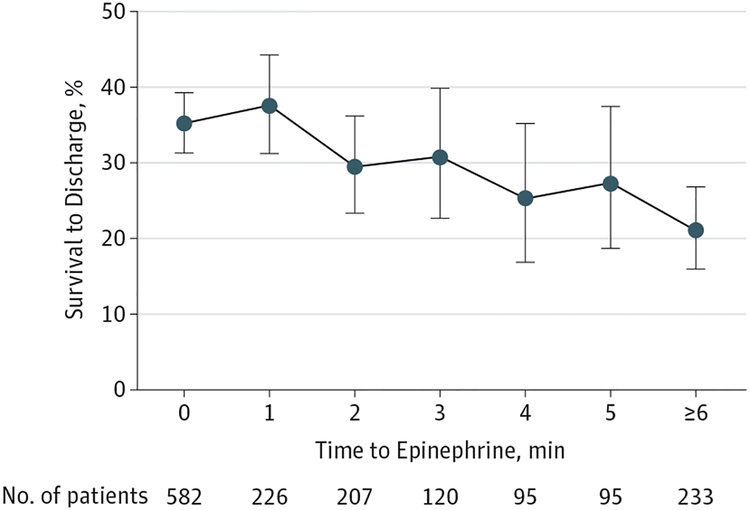
Survival to discharge was 31.3% (487/1558). Longer time to epinephrine was significantly associated with lower risk of survival to discharge in unadjusted analysis (RR per minute delay, 0.94 [95% CI, 0.91–0.97]; P < .001) (Figure 3). This association remained significant in multivariable analysis (RR per minute delay, 0.95 [95% CI, 0.93–0.98]; P < .001) (eFigure 1 in the Supplement), accounting for potentially confounding variables, displayed in Table 2.
Figure 3. Time to Epinephrine and Survival to Hospital Discharge After Pediatric In-Hospital Nonshockable Cardiac Arrest (N=1558).
Longer time to epinephrine administration was associated with lower risk of survival to discharge in multivariable analysis (risk ratio per minute delay, 0.95 [95% CI, 0.93–0.98]; P < .001). Error bars indicate exact binomial 95% confidence intervals.
Table 2.
Multivariable Model With Survival to Discharge as the Outcome of Pediatric In-Hospital Cardiac Arresta
| Variable | Risk Ratio (95% CI) | P Value |
|---|---|---|
| Time to epinephrine, per min | 0.95 (0.93–0.98) | <.001 |
| Time to chest compressions, per min | 0.88 (0.71–1.08) | .21 |
| Sex | ||
| Male | 1 [Reference] | |
| Female | 0.97 (0.56–1.09) | .57 |
| Age group | ||
| Child, 1–12 y | 1 [Reference] | |
| Neonate, <1 mo | 0.88 (0.71–1.09) | .24 |
| Infant, 1 mo-<1 y | 1.08 (0.92–1.27) | .35 |
| Adolescent, >12 y | 0.64 (0.49–0.84) | .001 |
| Type of admission | ||
| Medical noncardiac | 1 [Reference] | |
| Medical cardiac | 0.88 (0.71–1.08) | .22 |
| Surgical cardiac | 1.26 (1.02–1.54) | .03 |
| Surgical noncardiac | 1.55 (1.30–1.85) | <.001 |
| Newborn | 0.79 (0.58–1.08) | 1.14 |
| Location of arrest | ||
| ICU/PACU/OR | 1 [Reference] | |
| Emergency department | 0.75 (0.56–1.00) | .05 |
| Floor with telemetry | 0.64 (0.41–1.00) | .05 |
| Floor without telemetry | 0.83 (0.57–1.21) | .34 |
| Other | 1.41 (1.12–1.76) | .003 |
| Time of week of arrest | ||
| Weekday | 1 [Reference] | |
| Weekendb | 0.85 (0.74–0.98) | .02 |
| Time of day of arrest | ||
| Nighttimec | 1 [Reference] | |
| Daytime | 1.10 (0.93–1.32) | .27 |
| Arrest characteristics | ||
| Witnessed | 1.56 (1.12–2.17) | .009 |
| Monitored | 0.97 (0.72–1.32) | .86 |
| Preexisting mechanical ventilation | 0.87 (0.71–1.05) | .15 |
| Insertion of an airway | 1.20 (0.98–1.47) | .08 |
| Initial rhythm | ||
| Asystole | 1 [Reference] | |
| Pulseless electrical activity | 1.17 (1.02–1.35) | .02 |
| Hospital characteristics | ||
| Primary hospital status | ||
| Adult | 1 [Reference] | |
| Pediatric | 1.12 (0.92–1.36) | .28 |
| Teaching status | ||
| Major | 1 [Reference] | |
| Minor | 0.98 (0.80–1.21) | .88 |
| Nonteaching | 0.91 (0.66–1.27) | .59 |
Abbreviations: ICU, intensive care unit; PACU, postanesthesia care unit; OR, operating room.
We encourage the readers to interpret the results in the table carefully as the study and statistical analysis were not designed to specifically assess the association between these variables (except time to epinephrine) and survival. The model included all variables in the table as well as year of the arrest.
Friday 11 PM to Monday 7 AM.
11:00 PM to 6:59 AM.
Secondary Outcomes
Of 1558 patients, 993 (63.7%) had ROSC, and 745 (47.8%) were alive 24 hours after the arrest; 217 of 1395 patients (15.6%) had a documented favorable neurological outcome at hospital discharge (an additional 10.5% [163/1558] survived to hospital discharge but without a documented PCPC score). Increasing time to epinephrine was associated with a decreased risk of ROSC (RR per minute delay, 0.96 [95% CI, 0.94–0.97]; P < .001), lower survival at 24 hours (RR per minute delay, 0.96 [95% CI, 0.94–0.98]; P < .001), and less survival with favorable neurological outcome (RR per minute delay, 0.94 [95% CI, 0.91–0.97]; P < .001) in unadjusted analysis. These associations remained statistically significant in multivariable analysis for ROSC (RR per minute delay, 0.97 [95% CI, 0.96–0.99]; P < .001), for survival at 24 hours (RR per minute delay, 0.97 [95% CI, 0.95–0.99]; P = .003), and for survival with favorable neurological outcome (RR per minute delay, 0.95 [95% CI, 0.91–0.99]; P = .02) using our primary definition. The results of the multivariable analyses when using the 3 different sensitivity definitions of favorable neurological outcome were similar to the main definition (eTable 2 in the Supplement).
We found no sign of nonlinear (ie, quadratic or cubic) associations between time to epinephrine and survival to hospital discharge (all P > .05). None of the tested interactions as described in the Methods section were significant (all P > .05). The results of the post hoc sensitivity analyses are presented in eTable 3 in the Supplement. The association between time to epinephrine and the various outcomes remained statistically significant when using propensity score analyses and when using multiple imputation techniques for missing data.
Time to Epinephrine as a Categorical Variable
As an additional analysis, we divided patients into 2 groups: time to epinephrine of 5 minutes or less vs longer than 5 minutes. The 5-minutes-or-less group (1325/1558 patients [85%]) had in-hospital survival to discharge of 33.1% (438/1325), compared with 21.0% (49/233) in the longer-than-5-minutes group (233/1558 patients [15%]). The crude secondary outcomes are reported in eTable 4 in the Supplement. In unadjusted analysis, the longer-than-5-minutes group had significantly lower risk of ROSC (RR, 0.73 [95% CI, 0.64–0.84]; P < .001), 24-hour survival (RR, 0.69 [95% CI, 0.58–0.83]; P < .001), survival to discharge (RR, 0.64 [95% CI, 0.49–0.83]; P = .001), and survival to hospital discharge with favorable neurological outcome (RR, 0.58 [95% CI, 0.38–0.88]; P = .01). This association remained significant in multivariable analysis for ROSC (RR, 0.85 [95% CI, 0.75–0.95]; P = .006), 24-hour survival (RR, 0.79 [95% 0.69–0.92]; P = .002), and survival to discharge (RR, 0.75 [95% CI, 0.60–0.93]; P = .01). There was no significant association with survival to hospital discharge with favorable neurological outcome (RR, 0.77 [95% CI, 0.47–1.25]; P = .29).
Discussion
In this multicenter cohort study of in-hospital pediatric cardiac arrest, delay in administration of epinephrine was associated with a decreased chance of ROSC, 24-hour survival, survival to hospital discharge, and survival to hospital discharge with a favorable neurological outcome among patients with an initial nonshockable rhythm. These associations remained when accounting for multiple predetermined potentially confounding patient, event, and hospital characteristics and in multiple different sensitivity analyses. Although the observational design precludes ascertainment of causality, the strong association with outcomes suggests that early epinephrine may be beneficial in pediatric cardiac arrest.
The physiological rationale for epinephrine is primarily through α-adrenergic increase in coronary perfusion pressure, which has been shown to be an important determinant of ROSC.9–11,30 The association between epinephrine administration and a better chance of ROSC is a consistent finding across studies.31–34 Because duration of CPR is associated with outcome21 and ROSC is a necessary first step to a meaningful recovery, the rationale for epinephrine administration as a time-sensitive intervention to improve long-term outcome becomes apparent. The lack of long-term survival data with epinephrine has previously been attributed to late drug administration in clinical trials of out-of-hospital cardiac arrest.35 Whether decreasing time to epinephrine during in-hospital and out-of-hospital cardiac arrest will improve outcomes in the pediatric or adult population remains to be clarified. Our findings do suggest, however, that there is room for improvement, with 15% of pediatric patients getting their first epinephrine dose more than 5 minutes after loss of pulse.
Epinephrine is currently recommended in pediatric cardiac arrests as the first-line pharmacological intervention despite no randomized placebo-controlled trials in this patient population.6,8 One randomized placebo-controlled study in the adult out-of-hospital cardiac arrest population found improved ROSC and short-term survival with administration of epinephrine.31 However, the study was underpowered to detect any difference in long-term outcome because of unanticipated lack of enrollment.36 Similar results were reported in a study comparing intravenous drug administration (with 79% receiving epinephrine) vs no intravenous drug administration in out-of-hospital cardiac arrest.37 In addition to these randomized studies, a number of large observational studies have been published about the adult out-of-hospital cardiac arrest population with conflicting results, even within the same data set, because of different statistical approaches.32–34 These conflicting studies have added to the complexity of clinical decision making.36,38
The aim of the current study was not to answer the question of whether or not epinephrine should be given but to clarify whether there was an association between delay in epinephrine administration and outcome when epinephrine was given during in-hospital pediatric cardiac arrest. We found that a delay in epinephrine administration was associated with a significantly decreased chance of good outcomes. There are notable differences between pediatric and adult cardiac arrest in etiology, epidemiology, and treatment, including that more children have a nonshockable rhythm.3 Despite this, the current findings in the pediatric population are in line with those previously reported for adults.15 The current study included only patients who initially had a nonshockable rhythm. We decided to analyze data only from this patient population to avoid confounding by defibrillation, which has previously been found to be a time-sensitive component of cardiac arrest resuscitation in adult patients with a shockable rhythm.39 As such, the findings should not be extrapolated to patients with a shockable rhythm; neither should they be extrapolated to out-of-hospital cardiac arrest, for which the time to initiation of therapy is often much longer.
A number of limitations should be considered when interpreting the current study. The data are observational, and the possibility of unmeasured confounding remains. We tried to account for this by multivariable regression modeling, including adjusting for time to CPR and hospital center as well as multiple patient and event characteristics. We excluded a small number of patients based on missing values for covariates, time to epinephrine, or the outcomes, which might decrease the generalizability of our results. However, the majority of patients had complete data, which allowed us to adjust for several variables, and the results did not change when using multiple imputation to account for missing data. However, the possibility remains that time to epinephrine is a marker of other aspects of the resuscitation processes and not the causal mediator.
The GWTG-R data registry is designed as a data quality improvement tool, not specifically designed to answer the current research question. The quality of data across sites may therefore vary. However, the AHA provides standardized reporting guidelines and training of all entry personnel to ensure accuracy of entered data. We furthermore included only hospitals with at least 6 months of data and at least 5 cases reported to ensure high quality of the data. Data abstractors were not blinded to the outcome of the patients, although they were unaware of the hypothesis of the current study. As such, we consider it unlikely that this limitation would bias our results. The classification of the time variables was done in whole minutes, and the actual time might therefore have been slightly misclassified. Furthermore, time variables may have been classified incorrectly on the code sheets from which data were abstracted. We believe that this potential misclassification is likely undifferentiated and that, in most cases, this would lead to bias toward the null.
The current study was not designed to evaluate whether epinephrine should be administered. Patients who did not receive epinephrine were therefore excluded. Seven hundred forty patients did not receive epinephrine (Figure 1). Although some of these patients met other exclusion criteria, 362 patients were excluded solely on the basis of not having received epinephrine. These 362 patients had a very high rate of ROSC (94%) and a short median downtime (2 minutes [IQR, 1–5]), compared with the included cohort (64% ROSC and median downtime of 14 minutes [IQR, 6–28]). Based on this difference, we consider this patient population to be substantially different from the one included and believe that a meaningful comparison would be problematic, especially given the relatively low overall sample size.
Conclusions
Among children with in-hospital cardiac arrest with an initial nonshockable rhythm who received epinephrine, delay in administration of epinephrine was associated with decreased chance of survival to hospital discharge, ROSC, 24-hour survival, and survival to hospital discharge with a favorable neurological outcome.
Supplementary Material
Acknowledgments
Funding/Support: Dr Donnino is supported by the National Heart, Lung, and Blood Institute (NHLBI) (1K02HL107447–01A1) and American Heart Association (AHA) (14GRNT2001002). Dr K. Berg is supported by the AHA (13CRP16930000).
Role of the Funder/Sponsor: The NHLBI had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The AHA maintains the GWTG-R registry and oversees/approves data queries and manuscript submissions. However, the author group is responsible for the conception of the project, all data analyses, and manuscript writing.
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Donnino reported being a paid consultant for the American Heart Association. No other disclosures were reported.
REFERENCES
- 1.Topjian AA, Berg RA, Nadkarni VM. Pediatric cardiopulmonary resuscitation: advances in science, techniques, and outcomes. Pediatrics. 2008;122(5):1086–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berg MD, Nadkarni VM, Zuercher M, Berg RA. In-hospital pediatric cardiac arrest. Pediatr Clin North Am. 2008;55(3):589–604. [DOI] [PubMed] [Google Scholar]
- 3.Nadkarni VM, Larkin GL, Peberdy MA, et al. ; National Registry of Cardiopulmonary Resuscitation Investigators. First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. JAMA. 2006;295(1):50–57. [DOI] [PubMed] [Google Scholar]
- 4.Girotra S, Spertus JA, Li Y, Berg RA, Nadkarni VM, Chan PS; American Heart Association Get With the Guidelines–Resuscitation Investigators. Survival trends in pediatric in-hospital cardiac arrests: an analysis from Get With the Guidelines-Resuscitation. Circ Cardiovasc Qual Outcomes. 2013;6(1):42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tibballs J, Kinney S. A prospective study of outcome of in-patient paediatric cardiopulmonary arrest. Resuscitation. 2006;71(3):310–318. [DOI] [PubMed] [Google Scholar]
- 6.Kleinman ME, Chameides L, Schexnayder SM, et al. Part 14: Pediatric advanced life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122 (18)(suppl 3):S876–S908. [DOI] [PubMed] [Google Scholar]
- 7.Kleinman ME, de Caen AR, Chameides L, et al. ; Pediatric Basic and Advanced Life Support Chapter Collaborators. Part 10: Pediatric basic and advanced life support: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation. 2010;122 (16)(suppl 2):S466–S515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biarent D, Bingham R, Eich C, et al. European Resuscitation Council Guidelines for Resuscitation 2010 section 6: paediatric life support. Resuscitation. 2010;81(10):1364–1388. [DOI] [PubMed] [Google Scholar]
- 9.Sanders AB, Ewy GA, Taft TV. Prognostic and therapeutic importance of the aortic diastolic pressure in resuscitation from cardiac arrest. Crit Care Med. 1984;12(10):871–873. [DOI] [PubMed] [Google Scholar]
- 10.Niemann JT, Criley JM, Rosborough JP, Niskanen RA, Alferness C. Predictive indices of successful cardiac resuscitation after prolonged arrest and experimental cardiopulmonary resuscitation. Ann Emerg Med. 1985;14(6):521–528. [DOI] [PubMed] [Google Scholar]
- 11.Paradis NA, Martin GB, Rivers EP, et al. Coronary perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. JAMA. 1990;263(8):1106–1113. [PubMed] [Google Scholar]
- 12.Perondi MB, Reis AG, Paiva EF, Nadkarni VM, Berg RA. A comparison of high-dose and standard-dose epinephrine in children with cardiac arrest. N Engl J Med. 2004;350(17):1722–1730. [DOI] [PubMed] [Google Scholar]
- 13.Carpenter TC, Stenmark KR. High-dose epinephrine is not superior to standard-dose epinephrine in pediatric in-hospital cardiopulmonary arrest. Pediatrics. 1997;99(3): 403–408. [DOI] [PubMed] [Google Scholar]
- 14.Dieckmann RA, Vardis R. High-dose epinephrine in pediatric out-of-hospital cardiopulmonary arrest. Pediatrics. 1995;95(6): 901–913. [PubMed] [Google Scholar]
- 15.Donnino MW, Salciccioli JD, Howell MD, et al. ; American Heart Association’s Get With the Guidelines-Resuscitation Investigators. Time to administration of epinephrine and outcome after in-hospital cardiac arrest with non-shockable rhythms: retrospective analysis of large in-hospital data registry. BMJ. 2014;348:g3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peberdy MA, Kaye W, Ornato JP, et al. Cardiopulmonary resuscitation of adults in the hospital: a report of 14720 cardiac arrests from the National Registry of Cardiopulmonary Resuscitation. Resuscitation. 2003;58(3):297–308. [DOI] [PubMed] [Google Scholar]
- 17.Cummins RO, Chamberlain D, Hazinski MF, et al. ; American Heart Association. Recommended guidelines for reviewing, reporting, and conducting research on in-hospital resuscitation: the in-hospital “Utstein style.” Circulation. 1997;95(8): 2213–2239. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs I, Nadkarni V, Bahr J, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update and simplification of the Utstein templates for resuscitation registries: a statement for healthcare professionals from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Councils of Southern Africa). Circulation. 2004;110(21):3385–3397. [DOI] [PubMed] [Google Scholar]
- 19.Peberdy MA, Ornato JP, Larkin GL, et al. ; National Registry of Cardiopulmonary Resuscitation Investigators. Survival from in-hospital cardiac arrest during nights and weekends. JAMA. 2008; 299(7):785–792. [DOI] [PubMed] [Google Scholar]
- 20.Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr. 1992;121(1):68–74. [DOI] [PubMed] [Google Scholar]
- 21.Matos RI, Watson RS, Nadkarni VM, et al. ; American Heart Association’s Get With the Guidelines–Resuscitation (Formerly the National Registry of Cardiopulmonary Resuscitation) Investigators. Duration of cardiopulmonary resuscitation and illness category impact survival and neurologic outcomes for in-hospital pediatric cardiac arrests. Circulation. 2013;127(4):442–451. [DOI] [PubMed] [Google Scholar]
- 22.Ortmann L, Prodhan P, Gossett J, et al. ; American Heart Association’s Get With the Guidelines–Resuscitation Investigators. Outcomes after in-hospital cardiac arrest in children with cardiac disease: a report from Get With the Guidelines-Resuscitation. Circulation. 2011;124(21): 2329–2337. [DOI] [PubMed] [Google Scholar]
- 23.Zou G A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. [DOI] [PubMed] [Google Scholar]
- 24.Zou GY, Donner A. Extension of the modified Poisson regression model to prospective studies with correlated binary data. Stat Methods Med Res. 2013;22(6):661–670. [DOI] [PubMed] [Google Scholar]
- 25.Girotra S, Chan PS. Trends in survival after in-hospital cardiac arrest. N Engl J Med. 2013;368 (7):680–681. [DOI] [PubMed] [Google Scholar]
- 26.Peng TJ, Andersen LW, Saindon BZ, et al. ; American Heart Association’s Get With the Guidelines-Resuscitation Investigators. The administration of dextrose during in-hospital cardiac arrest is associated with increased mortality and neurologic morbidity. Crit Care. 2015;19(1):160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meaney PA, Nadkarni VM, Cook EF, et al. ; American Heart Association National Registry of Cardiopulmonary Resuscitation Investigators. Higher survival rates among younger patients after pediatric intensive care unit cardiac arrests. Pediatrics. 2006;118(6):2424–2433. [DOI] [PubMed] [Google Scholar]
- 28.Donoghue AJ, Nadkarni VM, Elliott M, Durbin D; American Heart Association National Registry of Cardiopulmonary Resuscitation Investigators. Effect of hospital characteristics on outcomes from pediatric cardiopulmonary resuscitation: a report from the national registry of cardiopulmonary resuscitation. Pediatrics. 2006;118(3):995–1001. [DOI] [PubMed] [Google Scholar]
- 29.Ornato JP, Peberdy MA, Reid RD, Feeser VR, Dhindsa HS; NRCPR Investigators. Impact of resuscitation system errors on survival from in-hospital cardiac arrest. Resuscitation. 2012;83(1): 63–69. [DOI] [PubMed] [Google Scholar]
- 30.Weiner GM, Niermeyer S. Medications in neonatal resuscitation: epinephrine and the search for better alternative strategies. Clin Perinatol. 2012;39(4):843–855. [DOI] [PubMed] [Google Scholar]
- 31.Jacobs IG, Finn JC, Jelinek GA, Oxer HF, Thompson PL. Effect of adrenaline on survival in out-of-hospital cardiac arrest: a randomised double-blind placebo-controlled trial. Resuscitation. 2011;82(9):1138–1143. [DOI] [PubMed] [Google Scholar]
- 32.Hagihara A, Hasegawa M, Abe T, Nagata T, Wakata Y, Miyazaki S. Prehospital epinephrine use and survival among patients with out-of-hospital cardiac arrest. JAMA. 2012;307(11):1161–1168. [DOI] [PubMed] [Google Scholar]
- 33.Nakahara S, Tomio J, Takahashi H, et al. Evaluation of pre-hospital administration of adrenaline (epinephrine) by emergency medical services for patients with out of hospital cardiac arrest in Japan: controlled propensity matched retrospective cohort study. BMJ. 2013;347:f6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goto Y, Maeda T, Goto Y. Effects of prehospital epinephrine during out-of-hospital cardiac arrest with initial non-shockable rhythm: an observational cohort study. Crit Care. 2013;17(5):R188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reynolds JC, Rittenberger JC, Menegazzi JJ. Drug administration in animal studies of cardiac arrest does not reflect human clinical experience. Resuscitation. 2007;74(1):13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olasveengen TM. Adrenaline for out of hospital cardiac arrest? BMJ. 2013;347:f7268. [DOI] [PubMed] [Google Scholar]
- 37.Olasveengen TM, Sunde K, Brunborg C, Thowsen J, Steen PA, Wik L. Intravenous drug administration during out-of-hospital cardiac arrest: a randomized trial. JAMA. 2009;302(20):2222–2229. [DOI] [PubMed] [Google Scholar]
- 38.Callaway CW. Questioning the use of epinephrine to treat cardiac arrest. JAMA. 2012;307 (11):1198–1200. [DOI] [PubMed] [Google Scholar]
- 39.Chan PS, Krumholz HM, Nichol G, Nallamothu BK; American Heart Association National Registry of Cardiopulmonary Resuscitation Investigators. Delayed time to defibrillation after in-hospital cardiac arrest. N Engl J Med. 2008;358(1):9–17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.