Abstract
Background:
It is unknown whether the new kidney transplant allocation system (KAS) has attenuated the advantages of preemptive wait-listing as a strategy to minimize pre-transplant dialysis exposure.
Methods:
We performed a retrospective study of adult US deceased donor kidney transplant (DDKT) recipients between December 4, 2011-December 3, 2014 (pre-KAS) and December 4, 2014-December 3, 2017 (post-KAS). We estimated pre-transplant dialysis durations by pre-emptive listing status in the pre- and post-KAS periods using multivariable gamma regression models.
Results:
Among 65,385 DDKT recipients, preemptively listed recipients (21%, n=13,696) were more likely to be white (59% vs 34%, p<0.001) and have private insurance (64% vs 30%, p<0.001). In the pre- and post-KAS periods, average adjusted pre-transplant dialysis durations for preemptively listed recipients were <2 years in all racial groups. Compared to recipients who were listed after starting dialysis, preemptively listed recipients experienced 3.85 (95% Confidence Interval [CI] 3.71–3.99) and 4.53 (95% CI 4.32–4.74) fewer average years of pre-transplant dialysis in the pre- and post-KAS periods, respectively (p<0.001 for all comparisons).
Conclusions:
Preemptively wait-listed DDKT recipients continue to experience substantially fewer years of pre-transplant dialysis than recipients listed after dialysis onset. Efforts are needed to improve both socioeconomic and racial disparities in preemptive wait-listing.
Keywords: kidney transplant, waiting time, Kidney, waiting list
Introduction
On December 4, 2014, the Organ Procurement and Transplantation Network implemented major changes to the deceased donor kidney allocation policy in the United States. Under the new kidney allocation system (KAS), deceased donor kidney transplant (DDKT) candidates who are waitlisted after the onset of maintenance dialysis accrue a priority point for each year of pre-listing dialysis exposure, while candidates who are preemptively listed (i.e., listed before dialysis onset) continue to receive a point for each year waiting after reaching the qualifying estimated glomerular filtration rate of 20 milliliters/minute/1.73 meters squared.1 Early studies on the effects of the KAS have indicated that more individuals with long dialysis durations are receiving DDKT, whereas fewer individuals are receiving preemptive DDKT (i.e., DDKT before the need for maintenance dialysis).2–5 Therefore, it is unknown whether DDKT candidates who were preemptively waitlisted as a strategy to minimize pre-transplant dialysis exposure will continue to receive a similar benefit under the new KAS.6,7
Prior to the KAS, DDKT candidates who were preemptively wait-listed with a qualifying eGFR and those who were wait-listed after starting maintenance dialysis accrued waiting time from their listing date onward. In this context, pre-emptively wait-listed individuals were found to have superior transplant outcomes compared to those listed after dialysis onset, a finding attributed to both socioeconomic differences and to the deleterious health impacts of prolonged dialysis exposure.6–8 As unequal access to the kidney transplant waiting list among low income and minority candidates contributed to large socioeconomic and racial disparities in DDKT,9 one of the primary objectives of the new kidney allocation system (KAS) was to improve equity in organ allocation for candidates who were wait-listed after enduring years of dialysis.10 Early studies have shown that the KAS has been successful in closing the gap in transplant rates between wait-listed whites and minorities.2 However, data are needed on other potential implications of prioritizing dialysis exposure for organ allocation, including its impacts on the known relative benefits of preemptive wait-listing.7,8,11,12
The goal of this study was to examine whether the new KAS was associated with differences in pre-transplant dialysis durations for DDKT recipients with and without preemptive waiting time. We performed a retrospective pre-post cohort study to examine whether average pre-transplant dialysis durations differed among kidney transplant recipients before and after KAS implementation based on the recipient’s preemptive listing status and by race/ethnicity.
Methods
Study Population
The study population was derived from all individuals who received DDKT in the US between December 4, 2011 and December 3, 2017, as indicated in the United Network for Organ Sharing’s (UNOS) standard transplant analytic (STAR) file. Multi-organ recipients were excluded given material differences in organ allocation protocol. To focus on DDKT recipients who did not receive pediatric priority, recipients were excluded if they were <18 years at the time of wait-listing (Appendix Figure 1). Preemptively waitlisted recipients were identified based on dialysis status at the time of wait-listing. Pre-transplant dialysis durations were calculated by subtracting recipient dialysis dates (derived from the STAR file TRR form) from transplant dates. If TRR form dialysis dates were missing, we verified that TCR form dialysis dates were also missing, or used the TCR form dialysis dates if they were non-missing. The study was approved by the Institutional Review Board at the Drexel University College of Medicine and all study procedures were in accordance with the Declaration of Helsinki.
Covariates
Variables were collected from the UNOS STAR file at the time of transplant and included recipient age at DDKT (as a continuous and squared term to account for non-linear associations), race/ethnicity (white/black/Hispanic/other), sex, diabetes history, body mass index category (categorized as <18.5 kg/m2, 18.5 to <25 kg/m2, 25 to <30 kg/m2, 30 to <40 kg/m2, and ≥40 kg/m2), human immunodeficiency virus (HIV) serostatus, hepatitis C serostatus, prior living organ donor history, prior organ transplant history, calculated panel reactive antibody (as a continuous and squared term to account for non-linear associations), blood group; waiting time from date of wait-listing to transplant, kidney donor profile index (categorized as 0 to ≤20, >20 to ≤85, >85),13 the donor’s public health service (PHS) increased risk status, zero antigen human leukocyte antigen mismatch, private versus other insurance status, educational attainment (< High School, ≥High School Graduate & < College, ≥College Graduate, Unknown), and UNOS organ procurement organization region, for which there are 11.
Statistical Analysis
All analyses were performed using STATA/MP version 14 for Mac (College Station, TX, USA). Categorical variables (e.g., sex, ethnicity) were described by their frequencies. Continuous variables (e.g., age) were described by their medians and ranges. Binary variables were compared between groups using chi-square tests. To compare continuous variables between groups, we used Wilcoxon rank-sum tests and Kruskal-Wallis tests, as appropriate.
Multivariable Modeling Strategy
First, we examined pre-transplant dialysis exposure among recipients with and without preemptive wait-listing over time using a multivariable generalized linear model with a gamma family and log link. We estimated average pre-transplant dialysis durations by race/ethnicity and preemptive listing status during each quarter (three-month period) of our study period. Next, to examine whether the KAS was associated with differences in pre-transplant dialysis durations among DDKT recipients with preemptive listing, we estimated a multivariable generalized linear model with a gamma family and log link for years of pre-transplant dialysis among DDKT recipients with preemptive listing that included an interaction term for KAS period (pre/post) and race/ethnicity. We then compared pre-transplant dialysis durations among recipients with preemptive wait-listing in the pre- and post-KAS periods, by race/ethnicity, with a difference-in-differences approach.14
To determine whether the KAS was associated with changes in the relative difference in pre-transplant dialysis durations between DDKT recipients without and without preemptive wait-listing, we estimated a multivariable generalized linear model for years of pre-transplant dialysis exposure that included a three-way interaction term for KAS period, race/ethnicity, and preemptive wait-listing status. We then compared the adjusted average difference in duration of pre-transplant dialysis among recipients with and without preemptive wait-listing, by race/ethnicity, in the pre-and post-KAS periods. We used marginal standardization (i.e., predicted probabilities summed to a weighted average of the distribution of confounders in the cohort) to calculate adjusted dialysis durations by preemptive wait-listing status and race/ethnicity group in each time frame of the study period.15 We used the ‘margins’ package in STATA, with confidence intervals estimated using the delta method.16 To account for potential clustering of waiting time by transplant center we calculated cluster-robust standard errors.17
Sensitivity Analyses
Given prior evidence of a “bolus effect” of DDKT among newly listed individuals with long dialysis durations in the immediate post-KAS period,1–3 we performed sensitivity analysis in which we estimated dialysis durations after partitioning the post-KAS period into the first 12-month period post-KAS (period 1) and the following 24 months post-KAS years (period 2). We also performed sensitivity analyses in which we compared dialysis durations in the post-KAS period to the pre-KAS period after excluding the first six months after the KAS was implemented. Finally, we considered a model in which we also excluded prior living donors, given the prioritization of these individuals for transplantation,18 to determine if this exclusion impacted our results.
Missing Data
With the exception of HIV serostatus (missing in less than 7% of the cohort), data were missing in less than 3% of the cohort on any covariates, and all primary analyses were performed on complete cases.19 In sensitivity analyses, we imputed data for missing observations of HIV serostatus using multiple imputation with 10 iterations.20 (supplementary Table 4 and 5).
Results
Study Population
Among 65,385 DDKT recipients included in the study (Appendix Figure 1), 46% (n=30,126) received DDKT in the pre-KAS period and 53% (n=35,259) received DDKT in the post-KAS period (Table 1). Compared to the pre-KAS period, DDKT recipients in the post-KAS period were younger (median 54 vs 56 years, p<0.001), fewer were white (36% vs 43%, p<0.001), and more were female (40% vs 39%, p=0.003). In the pre-KAS period, 24% of recipients (n=7,282) had been preemptively wait-listed, compared to 14.6% of recipients within the first 12 months after the KAS, and 19.8% of recipients between December 4, 2015 to December 3, 2017 (Appendix Table 1 and Appendix Figure 2). Table 2 compares characteristics between recipients with and without preemptive wait-listing in the pre- and post-KAS periods, respectively. Overall, compared to recipients without preemptive wait-listing, preemptively listed recipients were more likely to be white (59% vs 34%, p<0.001) have private insurance (64% vs 30%, p<0.001), and be college graduates (31.2% vs 20.2%, p<0.001). The proportion of organs allocated with KDPI<20 (i.e., the highest quality allografts) was similar between recipients with and without preemptive listing in the pre-KAS period (20% vs 19%, p=0.26), and was higher among individuals with preemptive listing in the post-KAS period (24% vs 19%, p<0.001).
Table 1:
Kidney Transplant Recipient Characteristics, Stratified by Transplant in Pre- or Post- Kidney Allocation System Period
| Pre-KAS December 4, 2011-December 3, 2014 |
Post-KAS December 4, 2014-December 3, 2017 |
p-value | |
|---|---|---|---|
| N=30126 | N=35259 | ||
| Race/Ethnicity | <0.001 | ||
| White | 12860 (42.7%) | 12793 (36.3%) | |
| Black | 9671 (32.1%) | 12492 (35.4%) | |
| Hispanic | 4865 (16.1%) | 6594 (18.7%) | |
| Other | 2730 (9.1%) | 3380 (9.6%) | |
| Preemptively Listed | 7282 (24.2%) | 6414 (18.2%) | <0.001 |
| Preemptively Transplanted | 2689 (8.9%) | 2368 (6.7%) | <0.001 |
| KDPI Category | <0.001 | ||
| <20 | 5805 (19.3%) | 6855 (19.4%) | |
| >20 − <85 | 21219 (70.4%) | 25346 (71.9%) | |
| >85 | 3071 (10.2%) | 3057 (8.7%) | |
| missing | 31 (0.1%) | 1 (<1%) | |
| Age (years) | 56.0 (45.0, 64.0) | 54.0 (43.0, 63.0) | <0.001 |
| Sex | 0.003 | ||
| Female | 11835 (39.3%) | 14250 (40.4%) | |
| Male | 18291 (60.7%) | 21009 (59.6%) | |
| Prior Living Donor | 0.53 | ||
| No | 30017 (99.6%) | 35136 (99.7%) | |
| Yes | 107 (0.4%) | 115 (0.3%) | |
| Missing | 2 (<1%) | 8 (<1%) | |
| Private Insurance | <0.001 | ||
| No | 17633 (58.5%) | 23242 (65.9%) | |
| Yes | 12489 (41.5%) | 12012 (34.1%) | |
| Missing | 4 (<1%) | 5 (<1%) | |
| Educational Attainment | 0.28 | ||
| Less than High School | 2197 (7.3%) | 2683 (7.6%) | |
| High School Graduate | 20208 (67.1%) | 23667 (67.1%) | |
| College Graduate or Higher | 6813 (22.6%) | 7901 (22.4%) | |
| Missing/Unknown | 908 (3.0%) | 1008 (2.9%) | |
| Diabetic | <0.001 | ||
| No | 18825 (62.5%) | 23023 (65.3%) | |
| Yes | 11223 (37.3%) | 12200 (34.6%) | |
| Missing | 78 (0.3%) | 36 (0.1%) | |
| BMI Category (kg/m2) | <0.001 | ||
| 18.5–24.9 | 8212 (27.3%) | 10279 (29.2%) | |
| <18.5 | 479 (1.6%) | 608 (1.7%) | |
| 25–29.9 | 10273 (34.1%) | 11807 (33.5%) | |
| 30–39.9 | 10591 (35.2%) | 12048 (34.2%) | |
| >40 | 571 (1.9%) | 517 (1.5%) | |
| HIV Positive | <0.001 | ||
| No | 27850 (92.4%) | 33687 (95.5%) | |
| Yes | 251 (0.8%) | 528 (1.5%) | |
| Missing | 2025 (6.7%) | 1044 (3.0%) | |
| HCV Positive | <0.001 | ||
| No | 28483 (94.5%) | 32901 (93.3%) | |
| Yes | 1502 (5.0%) | 2253 (6.4%) | |
| Missing | 141 (0.5%) | 105 (0.3%) | |
| Blood Group | 0.018 | ||
| A | 10580 (35.1%) | 12117 (34.4%) | |
| A1 | 338 (1.1%) | 349 (1.0%) | |
| A1B | 28 (0.1%) | 46 (0.1%) | |
| A2 | 57 (0.2%) | 43 (0.1%) | |
| A2B | 6 (<1%) | 13 (<1%) | |
| AB | 1570 (5.2%) | 1833 (5.2%) | |
| B | 3922 (13.0%) | 4744 (13.5%) | |
| O | 13625 (45.2%) | 16114 (45.7%) | |
| cPRA | 0.0 (0.0, 44.0) | 0.0 (0.0, 62.0) | <0.001 |
| Zero Antigen Mismatch | 2527 (8.4%) | 1726 (4.9%) | <0.001 |
| PHS Increased Risk Allograft | <0.001 | ||
| No | 25673 (85.2%) | 26884 (76.2%) | |
| Yes | 4440 (14.7%) | 8366 (23.7%) | |
| Missing | 13 (<1%) | 9 (<1%) | |
| Prior Organ Transplant | 3870 (12.8%) | 5215 (14.8%) | <0.001 |
| Days Inactive on Waiting List | 18.0 (0.0, 258.0) | 0.0 (0.0, 219.0) | <0.001 |
| Waiting Time | <0.001 | ||
| <1 Year | 6721 (22.3%) | 12047 (34.2%) | |
| 1–3 Years | 10923 (36.3%) | 10674 (30.3%) | |
| >3 Years | 12482 (41.4%) | 12538 (35.6%) |
Abbreviations: BMI—Body Mass Index; KAS—kidney allocation system; cPRA—calculated panel reactive antibody; KDPI—kidney donor profile index; kg—kilograms; m—meters; PHS—Public Health Service
Values presented as median (interquartile range) and n (%)
p-values comparing non-missing data from Chi-Square Tests and Wilcoxon Rank Sum Tests, as appropriate
Table 2.
Kidney Transplant Recipient and Allograft Characteristics, Stratified by Preemptive Listing Status and Transplant in Pre- or Post- Kidney Allocation System Period
| Pre-KAS December 4, 2011-December 3, 2014 |
Post-KAS December 4, 2014-December 3, 2017 |
|||||
|---|---|---|---|---|---|---|
| Not Preemptively Listed | Preemptively Listed | p-valuea | Not Preemptively Listed | Preemptively Listed | p-valueb | |
| 22844 | 7282 | 28845 | 6414 | |||
| Race/Ethnicity | <0.001 | <0.001 | ||||
| White | 8455 (37.0%) | 4405 (60.5%) | 9109 (31.6%) | 3684 (57.4%) | ||
| Black | 8112 (35.5%) | 1559 (21.4%) | 11094 (38.5%) | 1398 (21.8%) | ||
| Hispanic | 4212 (18.4%) | 653 (9.0%) | 5885 (20.4%) | 709 (11.1%) | ||
| Other | 2065 (9.0%) | 665 (9.1%) | 2757 (9.6%) | 623 (9.7%) | ||
| Age (years) | 55.0 (44.0, 63.0) | 58.0 (49.0, 65.0) | <0.001 | 53.0 (42.0, 62.0) | 57.0 (46.0, 65.0) | <0.001 |
| Sex | <0.001 | <0.001 | ||||
| Female | 8608 (37.7%) | 3227 (44.3%) | 11117 (38.5%) | 3133 (48.8%) | ||
| Male | 14236 (62.3%) | 4055 (55.7%) | 17728 (61.5%) | 3281 (51.2%) | ||
| Waiting Time (from Listing to Transplant) | <0.001 | <0.001 | ||||
| <1 Year | 5627 (24.6%) | 1094 (15.0%) | 10708 (37.1%) | 1339 (20.9%) | ||
| 1–2.9 Years | 8208 (35.9%) | 2715 (37.3%) | 8809 (30.5%) | 1865 (29.1%) | ||
| >3 Years | 9009 (39.4%) | 3473 (47.7%) | 9328 (32.3%) | 3210 (50.0%) | ||
| Prior Donor | 0.022 | <0.001 | ||||
| No | 22772 (99.7%) | 7245 (99.5%) | 28781 (99.8%) | 6355 (99.1%) | ||
| Yes | 71 (0.3%) | 36 (0.5%) | 58 (0.2%) | 57 (0.9%) | ||
| Missing | 1 (<1%) | 1 (<1%) | 6 (<1%) | 2 (<1%) | ||
| Private Insurance | <0.001 | <0.001 | ||||
| No | 15059 (65.9%) | 2574 (35.3%) | 20923 (72.5%) | 2319 (36.2%) | ||
| Yes | 7784 (34.1%) | 4705 (64.6%) | 7919 (27.5%) | 4093 (63.8%) | ||
| Missing | 1 (<1%) | 3 (<1%) | 3 (<1%) | 2 (<1%) | ||
| Education | <0.001 | <0.001 | ||||
| Less than High School | 1923 (8.4%) | 274 (3.8%) | 2448 (8.5%) | 235 (3.7%) | ||
| High School Graduate | 15676 (68.6%) | 4532 (62.2%) | 19732 (68.4%) | 3935 (61.4%) | ||
| College Graduate or Higher | 4576 (20.0%) | 2237 (30.7%) | 5854 (20.3%) | 2047 (31.9%) | ||
| Missing/Unknown | 669 (2.9%) | 239 (3.3%) | 811 (2.8%) | 197 (3.1%) | ||
| KDPI | 0.26 | <0.001 | ||||
| >20 | 18454 (80.8%) | 5836 (80.1%) | 23519 (81.5%) | 4884 (76.1%) | ||
| <20 | 4369 (19.1%) | 1436 (19.7%) | 5325 (18.5%) | 1530 (23.9%) | ||
| Missing | 21 (0.1%) | 10 (0.1%) | 1 (<1%) | 0 (0.0%) | ||
| Zero Antigen Mismatch | 1796 (7.9%) | 731 (10.0%) | <0.001 | 1177 (4.1%) | 549 (8.6%) | <0.001 |
| Prior Transplant | 2846 (12.5%) | 1024 (14.1%) | <0.001 | 4197 (14.6%) | 1018 (15.9%) | 0.007 |
| Diabetic | <0.001 | <0.001 | ||||
| No | 13832 (60.5%) | 4993 (68.6%) | 18334 (63.6%) | 4689 (73.1%) | ||
| Yes | 8962 (39.2%) | 2261 (31.0%) | 10484 (36.3%) | 1716 (26.8%) | ||
| Missing | 50 (0.2%) | 28 (0.4%) | 27 (0.1%) | 9 (0.1%) | ||
| BMI Category (kg/m2) | <0.001 | 0.003 | ||||
| 18.5–24.9 | 6193 (27.1%) | 2019 (27.7%) | 8420 (29.2%) | 1859 (29.0%) | ||
| <18.5 | 384 (1.7%) | 95 (1.3%) | 493 (1.7%) | 115 (1.8%) | ||
| 25–29.9 | 7757 (34.0%) | 2516 (34.6%) | 9589 (33.2%) | 2218 (34.6%) | ||
| 30–39.9 | 8039 (35.2%) | 2552 (35.0%) | 9889 (34.3%) | 2159 (33.7%) | ||
| >40 | 471 (2.1%) | 100 (1.4%) | 454 (1.6%) | 63 (1.0%) | ||
| HIV Positive | <0.001 | <0.001 | ||||
| No | 21062 (92.2%) | 6788 (93.2%) | 27476 (95.3%) | 6211 (96.8%) | ||
| Yes | 226 (1.0%) | 25 (0.3%) | 499 (1.7%) | 29 (0.5%) | ||
| Unknown | 1556 (6.8%) | 469 (6.4%) | 870 (3.0%) | 174 (2.7%) | ||
| HCV Positive | <0.001 | <0.001 | ||||
| No | 21475 (94.0%) | 7008 (96.2%) | 26843 (93.1%) | 6058 (94.4%) | ||
| Yes | 1263 (5.5%) | 239 (3.3%) | 1920 (6.7%) | 333 (5.2%) | ||
| Unknown | 106 (0.5%) | 35 (0.5%) | 82 (0.3%) | 23 (0.4%) | ||
| Blood Group | <0.001 | <0.001 | ||||
| A | 7849 (34.4%) | 2731 (37.5%) | 9604 (33.3%) | 2513 (39.2%) | ||
| A1 | 251 (1.1%) | 87 (1.2%) | 287 (1.0%) | 62 (1.0%) | ||
| A1B | 22 (0.1%) | 6 (0.1%) | 32 (0.1%) | 14 (0.2%) | ||
| A2 | 36 (0.2%) | 21 (0.3%) | 32 (0.1%) | 11 (0.2%) | ||
| A2B | 6 (<1%) | 0 (0.0%) | 9 (<1%) | 4 (0.1%) | ||
| AB | 1165 (5.1%) | 405 (5.6%) | 1418 (4.9%) | 415 (6.5%) | ||
| B | 3037 (13.3%) | 885 (12.2%) | 3908 (13.5%) | 836 (13.0%) | ||
| O | 10478 (45.9%) | 3147 (43.2%) | 13555 (47.0%) | 2559 (39.9%) | ||
| Calculated PRA | 0.0 (0.0, 44.0) | 0.0 (0.0, 41.0) | 0.57 | 0.0 (0.0, 59.0) | 0.0 (0.0, 70.0) | <0.001 |
|
| ||||||
Abbreviations: KAS—kidney allocation system; ECD—expanded criteria donor; DCD—donation after circulatory determination of death; cPRA—calculated panel reactive antibody
Values presented as median (interquartile range) and n (%)
p-values comparing non-missing data from Chi-Square Tests and Wilcoxon Rank Sum Tests, as appropriate among recipients in pre-KAS period
p-values comparing non-missing data from Chi-Square Tests and Wilcoxon Rank Sum Tests, as appropriate among recipients in post-KAS period
Trends in Pre-Transplant Dialysis Duration Among DDKT Recipients Who Were and Were Not Preemptively Waitlisted
Among recipients listed after dialysis, average pre-transplant dialysis durations rose steeply in the last quarter (three-month period) of 2014 and peaked in 2015, with a subsequent decline in 2016 and 2017 (Figure 1). In the fourth quarter of 2017, average pre-transplant dialysis duration among recipients without preemptive listing was 5.18 years (95% Confidence Interval [CI]: 4.85–5.51 years) among whites, 6.30 years (95% CI: 5.91–6.69 years) among blacks, 5.72 years (95% CI: 5.35–6.09 years) among Hispanics, and 5.43 years (95% CI 4.87–5.99 years) among other race/ethnicities. Average pre-transplant dialysis durations remained similar over the study period within all racial groups of preemptively listed recipients. Among preemptively listed recipients in the last quarter of 2017, average pre-transplant dialysis durations were 1.12 years (95% CI: 0.96–1.29 years) among preemptively-listed white recipients, 1.79 years (95% CI: 1.40–2.18 years) among preemptively-listed black recipients, 1.53 years among preemptively-listed Hispanic recipients (95% CI: 1.12–1.96 years), and 1.51 years among preemptively listed recipients of other races/ethnicities (95% CI 1.05–1.97 years).
Figure 1: Average Pre-Transplant Dialysis Durations of Deceased Donor Kidney Transplant Recipients in the United States by Race/Ethnicity and Preemptive Wait-Listing Status.
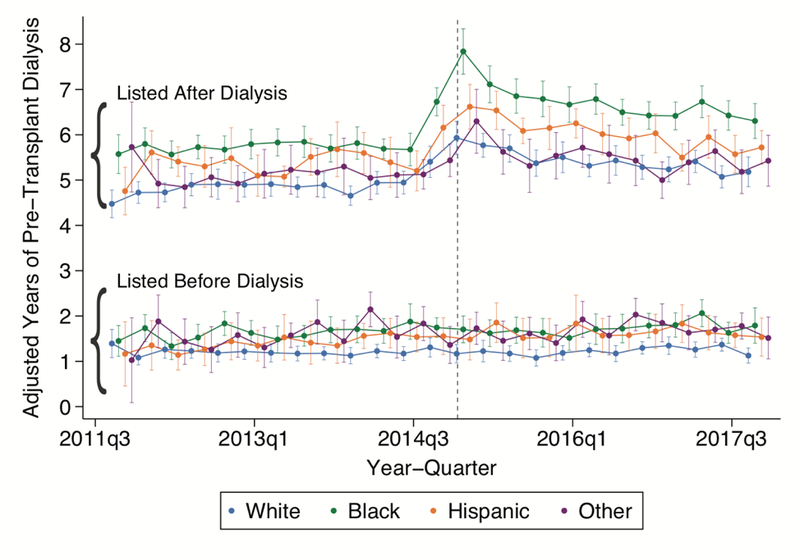
This figure displays results of multivariable generalized linear model for average pre-transplant dialysis duration, in years, by recipient race/ethnicity and preemptive wait-listing status. The dashed line indicates the onset of the new kidney allocation system on December 4, 2014. Each year/quarter point estimate (with 95% Confidence Interval) represents the predicted marginal mean dialysis duration within race/ethnicity group and preemptive listing category. Green, orange, blue, and purple circles indicate black, Hispanic, white, and other race/ethnicity recipients, respectively.
Association of the KAS and Pre-Transplant Dialysis Duration Among Recipients with Preemptive Wait-listing by Race/Ethnicity
In a multivariable adjusted generalized linear difference-in-differences model for the outcome of pre-transplant dialysis duration, the KAS was associated with non-statistically significant differences in pre-transplant dialysis duration among preemptively listed white recipients (1.19 vs 1.24 years, p=0.29), preemptively listed black recipients (1.64 vs 1.74 years, p=0.14), and preemptively listed recipients of other races/ethnicities (1.61 vs 1.70, p=0.37). The KAS was associated with a statistically significant 0.2-year increase in dialysis exposure among preemptively listed Hispanic recipients (1.43 vs 1.63 years, p=0.01) (Table 3, Appendix Figure 3).
Table 3.
Comparison of Adjusted Pre-Transplant Dialysis Durations (in Years) among Recipients with Preemptive Wait-Listing between the Pre- and Post-KAS Periods
| Pre-Transplant Dialysis Exposure (in Years) | Difference in Years: | |||
|---|---|---|---|---|
| Pre-KAS December 4, 2011-December 3, 2014 |
Post-KAS December 4, 2014-December 3, 2017 |
Between Periods | In difference | |
| White | 1.19 (95% CI 1.10–1.28) | 1.24 (95% CI 1.14–1.34) | +0.05 (95% CI −0.04–0.14) | reference |
| Black | 1.64 (95% CI 1.51–1.78) | 1.74 (95% CI 1.59–1.89) | +0.10 (95% CI −0.03–0.23) | −0.05 (95% CI −0.18–0.09) |
| Hispanic | 1.43 (95% CI 1.25–1.61) | 1.63 (95% CI 1.48–1.79) | +0.20* (95% CI 0.05–0.36) | −0.15 (95% CI −0.32–0.02) |
| Other | 1.61 (95% CI 1.42–1.79) | 1.70 (95% CI 1.52–1.87) | +0.09 (95% CI −0.11–0.29) | −0.04 (95% CI −0.24–0.16) |
Abbreviations: KAS—Kidney Allocation System; CI—Confidence Interval
p<0.05
Estimates represent predicted marginal mean dialysis durations that are standardized to the cohort distributions of recipient age (years), sex, diabetes status, hepatitis C status, HIV status, prior living donor status, prior organ transplant status, OPTN region, calculated PRA, blood group, PHS increased risk status, kidney donor profile index category, zero HLA antigen mismatch, education level, private insurance status, and waiting time years)
Association of the KAS and the Difference in Pre-Transplant Dialysis Duration Among Recipients with and without Preemptive Wait-listing by Race/Ethnicity
Compared to recipients without preemptive wait-listing, recipients with preemptive wait-listing received DDKT with 3.85 fewer average years of pre-transplant dialysis than those listed after dialysis in the pre-KAS period (95% CI 3.71–3.99 years, p<0.001), compared to 4.53 fewer years of pre-transplant dialysis in the post-KAS period (95% CI 4.32–4.74 years, p<0.001) (difference-in-differences of 0.66 additional years, p<0.001). The post-KAS gap in pre-transplant dialysis duration between preemptively listed and non-preemptively listed recipients became wider within all race/ethnicity groups, with the widest gap in dialysis duration between black recipients with and without preemptive listing (Table 4).
Table 4.
Comparison of Adjusted Differences in Pre-Transplant Dialysis Durations (in Years) Between Transplant Recipients with and without Preemptive Wait-Listing between the Pre- and Post-KAS Periods
| Difference in Pre-Transplant Dialysis Exposure (in Years) | Difference in Years: | |||
|---|---|---|---|---|
| Pre-KAS December 1, 2012-November 30, 2014 |
Post-KAS December 1, 2014-December 31, 2016 |
Proportional Difference Between Periods | In difference | |
| White | 3.66 (95% CI 3.52–3.79) | 4.22 (95% CI 4.06–4.38) | 0.57 (95% CI 0.41–0.72)** | reference |
| Black | 4.07 (95% CI 3.87–4.27) | 5.07 (95% CI 4.78–5.35) | 0.99 (95% CI 0.74–1.24)** | −0.43 (95% CI −0.68 − −0.17) * |
| Hispanic | 3.96 (95% CI 3.69–4.24) | 4.41 (95% CI 4.08–4.76) | 0.45 (95% CI 0.28–0.93)* | 0.11 (95% CI −0.21–0.44) |
| Other | 3.47 (95% CI 3.23–3.71) | 3.86 (95% CI 3.54–4.17) | 0.39 (95% CI 0.06–0.71)* | 0.18 (95% CI −0.14 – 0.50) |
KAS—Kidney Allocation System; CI—Confidence Interval
Estimates represent predicted marginal mean dialysis durations that are standardized to the cohort distributions of recipient age, sex, waiting time, educational attainment, private insurance status, diabetes status, Human Immunodeficiency Virus serostatus, Hepatitis C serostatus, prior living donor, prior organ transplant, UNOS region, calculated PRA, blood group, PHS increased risk status, kidney donor profile index, zero HLA antigen mismatch
p<0.05
p<0.001
Sensitivity Analyses
Results were similar when comparing pre-transplant dialysis durations among preemptively listed recipients between the pre-KAS period to the early and late post-KAS periods, respectively, and after excluding the first six months of the post-KAS period (Appendix Tables 2 and 3). The post-KAS period was associated with similar gaps in pre-transplant dialysis duration between recipients with and without preemptive wait-listing following multiple imputation for missing HIV serostatus (Appendix Tables 4 and 5). Our results were also robust to the exclusion of prior living donors (Appendix Table 6).
Conclusion
This nationally representative study is the first to examine whether the KAS, in prioritizing DDKT for candidates with long dialysis durations, was associated with differences in the pre-transplant dialysis durations of recipients with and without preemptive wait-listing for DDKT. Our results demonstrate that DDKT recipients with preemptive wait-listing continue to receive DDKT with substantially fewer years of pre-transplant dialysis than those without preemptive wait-listing under the KAS, underscoring the importance of efforts to improve preemptive access to the DDKT waiting list.
Individuals who receive DDKT after prolonged dialysis exposure have higher risks of graft loss and death than individuals with preemptive and early DDKT.11,21,22 As expected given the prioritization of pre-listing dialysis time, preemptive transplantation rates have declined under the new KAS.3–5 However, our findings suggest that preemptive wait-listing remains advantageous to minimize pre-transplant dialysis durations under the new KAS, independent of recipients’ types of insurance coverage and their races/ethnicities. We found that DDKT recipients with preemptive wait-listing in the post-KAS period received transplant after dialysis durations of less than two years on average, compared to recipients who were listed after starting dialysis, who had average pre-transplant dialysis durations of five to six years. Numerous factors may be maintaining the wide gap in pre-transplant dialysis durations between DDKT recipients with and without preemptive listing under the new KAS. For example, individuals who begin maintenance dialysis before being wait-listed may encounter numerous additional health burdens that delay transplant referral and prolong waiting time, including increased risks of functional dependence, vascular disease, and hospitalizations.23–32 Delayed transplant referral after dialysis onset may also be a reflect variation in dialysis center transplant referral practices,33,34 a factor that is coming under increasing scrutiny as a potential quality-of-care indicator.35,36
In addition to promoting shorter pre-transplant dialysis durations, preemptive wait-listing may also increase the likelihood of receiving the highest quality allografts under the new KAS. Acknowledging the deleterious effects of long dialysis durations on health and transplant outcomes, the KAS incorporated dialysis duration into a new longevity matching paradigm, that allocates the highest quality kidneys for recipients who are expected to live longest.37 This policy change may help to explain our finding that recipients who were listed after dialysis were equally likely as preemptively listed recipients to receive the highest quality allografts before the KAS, and less likely to receive these kidneys after the KAS. In aggregate, our results suggest that efforts to further improve equitable outcomes in DDKT under the new KAS may require added focus on improving unequal access to preemptive wait-listing.9,38–40 Unequal access may be driven in part by lack of health insurance among many US individuals with non-dialysis dependent chronic kidney disease,40,41 a disparity which may be narrowing with the national gains in insurance coverage under the Affordable Care Act (ACA).42–44 In addition to providing affordable health insurance coverage options, efforts to improve access to preemptive wait-listing should also include educational interventions targeted to those most likely to lack CKD awareness and pre-dialysis health care, including low income individuals with CKD. 45–50
Historically, racial and ethnic minority DDKT candidates have been less likely to be preemptively waitlisted than white candidates.9,40,51,52 Racial disparities in DDKT rates have improved under the new KAS,6,53 but concerns have been raised that the improvements in racial disparities in DDKT may only be temporary.2–4 Experts have posited that early gains in DDKT among racial and ethnic minorities may have represented a “bolus effect” in which a relatively small proportion of individuals with very long dialysis durations receive DDKT, followed by a return to prior patterns of inequitable organ access.3,4 Consistent with this hypothesis, a recent study by Melanson and colleagues showed that dialysis durations among new DDKT recipients peaked in all racial groups immediately after implementation of the KAS, though white DDKT recipients continued to experience substantially shorter dialysis durations prior to DDKT than black and Hispanic recipients in the post-KAS period.2 Our study results suggest that this latter finding may be explained in part by racial differences in preemptive wait-listing. For example, though the absolute and relative benefits of preemptive wait-listing with respect to pre-transplant dialysis minimization were race-independent, the majority of recipients with preemptive listing were white in both the pre- and post-KAS periods. Therefore, increasing rates of preemptive listing among racial and ethnic minorities with advanced CKD is a high priority to improve equity in DDKT outcomes under the new KAS.7,11,54–56 Efforts are needed to address numerous factors that may contribute to delays in transplant referral among minorities with CKD, including geographic and provider-related variation in referral for transplant evaluation,33,57–62 perceived discrimination,63 excess financial burdens,9,51,64,65 and lack of supportive social networks.66,67
Our study has several strengths, including a large, national study sample and focus on the potential implications of policy changes on existing disparities in transplantation. However, our study must be considered with respect to its limitations, particularly concerning the possible biases associated with retrospective, observational analyses of registry data. For example, the retrospective study design may be vulnerable to selection bias due to the lack of data on CKD patients who may be eligible for preemptive wait-listing. Our analysis is also limited by a lack of granular data on recipient socioeconomic status and community-level health indicators, which may have important implications for transplant candidacy.68 Further, our study does not provide insight on other unmeasured confounders, such as social support or health literacy, that may also impact transplant referral and waiting time.69
In summary, this study found that among kidney transplant recipients of all races and ethnicities, preemptive wait-listing continues to confer a large benefit with respect to minimizing pre-transplant dialysis duration compared to listing after dialysis under the new KAS. Future studies should be directed at mitigating persistent drivers of disparate access to preemptive wait-listing.
Supplementary Material
Acknowledgements
MNH is supported by a grant (K23DK105207) from the National Institutes of Health (NIH)/National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK). MOH is supported by a grant (K99HL141678) from the NIH/National Heart, Lung, and Blood Institue (NHLBI).
This work was supported in part by Health Resources and Services Administration contract 234–2005-37011C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Abbreviations:
- BMI
Body Mass Index
- CI
Confidence Interval
- cPRA
Calculated Panel Reactive Antibody
- DDKT
Deceased Donor Kidney Transplantation
- HIV
Human Immunodeficiency Virus
- KAS
Kidney Allocation System
- KDPI
Kidney Donor Profile Index
- UNOS
United Network for Organ Sharing
- US
United States
Footnotes
Disclosures
The authors of this manuscript have no conflicts of interest to disclose.
References
- 1.Wang CJ, Wetmore JB, Israni AK. Old versus new: Progress in reaching the goals of the new kidney allocation system. Hum Immunol. 2017;78(1):9–15. [DOI] [PubMed] [Google Scholar]
- 2.Melanson TA, Hockenberry JM, Plantinga L, et al. New Kidney Allocation System Associated With Increased Rates Of Transplants Among Black And Hispanic Patients. Health Aff (Millwood). 2017;36(6):1078–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Massie AB, Luo X, Lonze BE, et al. Early Changes in Kidney Distribution under the New Allocation System. Journal of the American Society of Nephrology : JASN. 2016;27(8):2495–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stewart DE, Kucheryavaya AY, Klassen DK, Turgeon NA, Formica RN, Aeder MI. Changes in Deceased Donor Kidney Transplantation One Year After KAS Implementation. Am J Transplant. 2016;16(6):1834–1847. [DOI] [PubMed] [Google Scholar]
- 5.Stewart DE, Klassen DK. Early Experience with the New Kidney Allocation System: A Perspective from UNOS. Clinical journal of the American Society of Nephrology : CJASN. 2017;12(12):2063–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schold JD, Sehgal AR, Srinivas TR, Poggio ED, Navaneethan SD, Kaplan B . Marked variation of the association of ESRD duration before and after wait listing on kidney transplant outcomes. Am J Transplant. 2010;10(9):2008–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kasiske BL, Snyder JJ, Matas AJ, Ellison MD, Gill JS, Kausz AT. Preemptive kidney transplantation: the advantage and the advantaged. Journal of the American Society of Nephrology : JASN. 2002;13(5):1358–1364. [DOI] [PubMed] [Google Scholar]
- 8.Meier-Kriesche HU, Kaplan B. Waiting time on dialysis as the strongest modifiable risk factor for renal transplant outcomes: a paired donor kidney analysis. Transplantation. 2002;74(10):1377–1381. [DOI] [PubMed] [Google Scholar]
- 9.Keith D, Ashby VB, Port FK, Leichtman AB. Insurance type and minority status associated with large disparities in prelisting dialysis among candidates for kidney transplantation. Clinical journal of the American Society of Nephrology : CJASN. 2008;3(2):463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schold JD, Reese PP. Simulating the New Kidney Allocation Policy in the United States: Modest Gains and Many Unknowns. Journal of the American Society of Nephrology : JASN. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grams ME, Chen BP, Coresh J, Segev DL. Preemptive deceased donor kidney transplantation: considerations of equity and utility. Clinical journal of the American Society of Nephrology : CJASN. 2013;8(4):575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedewald JJ, Reese PP. The kidney-first initiative: what is the current status of preemptive transplantation? Advances in chronic kidney disease. 2012;19(4):252–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee AP, Abramowicz D. Is the Kidney Donor Risk Index a step forward in the assessment of deceased donor kidney quality? Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2015;30(8):1285–1290. [DOI] [PubMed] [Google Scholar]
- 14.Dimick JB, Ryan AM. Methods for evaluating changes in health care policy: the difference-in-differences approach. Jama. 2014;312(22):2401–2402. [DOI] [PubMed] [Google Scholar]
- 15.Muller CJ, MacLehose RF. Estimating predicted probabilities from logistic regression: different methods correspond to different target populations. Int J Epidemiol. 2014;43(3):962–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitchell MN. Interpreting and Visualizing Regression Models Using Stata. Stata Press; 2012. [Google Scholar]
- 17.Wooldridge JM. Econometric Analysis of Cross Section and Panel Data. Second Edition ed. Cambridge, MA: MIT Press; 2010. [Google Scholar]
- 18.Potluri V, Harhay MN, Wilson FP, Bloom RD, Reese PP. Kidney transplant outcomes for prior living organ donors. Journal of the American Society of Nephrology : JASN. 2015;26(5):1188–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White IR, Carlin JB. Bias and efficiency of multiple imputation compared with complete-case analysis for missing covariate values. Statistics in medicine. 2010;29(28):2920–2931. [DOI] [PubMed] [Google Scholar]
- 20.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: John Wiley & Sons; 1987. [Google Scholar]
- 21.Goldfarb-Rumyantzev A, Hurdle JF, Scandling J, et al. Duration of end-stage renal disease and kidney transplant outcome. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2005;20(1):167–175. [DOI] [PubMed] [Google Scholar]
- 22.Haller MC, Kainz A, Baer H, Oberbauer R. Dialysis Vintage and Outcomes after Kidney Transplantation: A Retrospective Cohort Study. Clinical journal of the American Society of Nephrology : CJASN. 2017;12(1):122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kittiskulnam P, Sheshadri A, Johansen KL. Consequences of CKD on Functioning. Semin Nephrol. 2016;36(4):305–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiner DE, Seliger SL. Cognitive and physical function in chronic kidney disease. Current opinion in nephrology and hypertension. 2014;23(3):291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurella Tamura M, Yaffe K. Dementia and cognitive impairment in ESRD: diagnostic and therapeutic strategies. Kidney international. 2011;79(1):14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leinau L, Murphy TE, Bradley E, Fried T. Relationship between conditions addressed by hemodialysis guidelines and non-ESRD-specific conditions affecting quality of life. Clinical journal of the American Society of Nephrology : CJASN. 2009;4(3):572–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kutner NG, Zhang R. Frailty in dialysis-dependent patients with end-stage renal disease. JAMA internal medicine. 2013;173(1):78–79. [DOI] [PubMed] [Google Scholar]
- 28.Painter P, Kuskowski M. A closer look at frailty in ESRD: getting the measure right. Hemodialysis international International Symposium on Home Hemodialysis. 2013;17(1):41–49. [DOI] [PubMed] [Google Scholar]
- 29.Kim JC, Kalantar-Zadeh K, Kopple JD. Frailty and protein-energy wasting in elderly patients with end stage kidney disease. Journal of the American Society of Nephrology : JASN. 2013;24(3):337–351. [DOI] [PubMed] [Google Scholar]
- 30.Johansen KL, Chertow GM, Jin C, Kutner NG. Significance of frailty among dialysis patients. Journal of the American Society of Nephrology : JASN. 2007;18(11):2960–2967. [DOI] [PubMed] [Google Scholar]
- 31.Adams SV, Rivara M, Streja E, et al. Sex Differences in Hospitalizations with Maintenance Hemodialysis. Journal of the American Society of Nephrology : JASN. 2017;28(9):2721–2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bansal N Evolution of Cardiovascular Disease During the Transition to End-Stage Renal Disease. Semin Nephrol. 2017;37(2):120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patzer RE, Plantinga LC, Paul S, et al. Variation in Dialysis Facility Referral for Kidney Transplantation Among Patients With End-Stage Renal Disease in Georgia. Jama. 2015;314(6):582–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gander JC, Plantinga L, Zhang R, Mohan S, Pastan SO, Patzer RE. United States Dialysis Facilities With a Racial Disparity in Kidney Transplant Waitlisting. Kidney Int Rep. 2017;2(5):963–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kliger AS. Quality Measures for Dialysis: Time for a Balanced Scorecard. Clinical journal of the American Society of Nephrology : CJASN. 2016;11(2):363–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sehgal AR. Should Transplant Referral Be a Clinical Performance Measure? Journal of the American Society of Nephrology : JASN. 2017;28(3):721–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Israni AK, Salkowski N, Gustafson S, et al. New national allocation policy for deceased donor kidneys in the United States and possible effect on patient outcomes. Journal of the American Society of Nephrology : JASN. 2014;25(8):1842–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu DA, Watson CJ, Bradley JA, Johnson RJ, Forsythe JL, Oniscu GC. Global trends and challenges in deceased donor kidney allocation. Kidney international. 2017;91(6):1287–1299. [DOI] [PubMed] [Google Scholar]
- 39.Friedewald JJ, Samana CJ, Kasiske BL, et al. The kidney allocation system. Surg Clin North Am. 2013;93(6):1395–1406. [DOI] [PubMed] [Google Scholar]
- 40.Joshi S, Gaynor JJ, Bayers S, et al. Disparities among Blacks, Hispanics, and Whites in time from starting dialysis to kidney transplant waitlisting. Transplantation. 2013;95(2):309–318. [DOI] [PubMed] [Google Scholar]
- 41.Kurella Tamura M, Li S, Chen SC, et al. Educational programs improve the preparation for dialysis and survival of patients with chronic kidney disease. Kidney international. 2014;85(3):686–692. [DOI] [PubMed] [Google Scholar]
- 42.Trivedi AN, Sommers BD. The Affordable Care Act, Medicaid Expansion, and Disparities in Kidney Disease. Clinical journal of the American Society of Nephrology : CJASN. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frean M, Gruber J, Sommers BD. Premium subsidies, the mandate, and Medicaid expansion: Coverage effects of the Affordable Care Act. J Health Econ. 2017;53:72–86. [DOI] [PubMed] [Google Scholar]
- 44.Harhay MN, McKenna RM, Boyle SM, et al. Association between Medicaid Expansion under the Affordable Care Act and Preemptive Listings for Kidney Transplantation. Clinical journal of the American Society of Nephrology : CJASN. 2018;13(7):1069–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2016 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2017;69(3S1):A7–A8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jurkovitz CT, Li S, Norris KC, et al. Association between lack of health insurance and risk of death and ESRD: results from the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis. 2013;61(4 Suppl 2):S24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shah A, Fried LF, Chen SC, et al. Associations between access to care and awareness of CKD. Am J Kidney Dis. 2012;59(3 Suppl 2):S16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Agrawal V, Jaar BG, Frisby XY, et al. Access to health care among adults evaluated for CKD: findings from the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis. 2012;59(3 Suppl 2):S5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nee R, Yuan CM, Hurst FP, Jindal RM, Agodoa LY, Abbott KC. Impact of poverty and race on pre-end-stage renal disease care among dialysis patients in the United States. Clin Kidney J. 2017;10(1):55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tuot DS, Grubbs V. Chronic kidney disease care in the US safety net. Advances in chronic kidney disease. 2015;22(1):66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johansen KL, Zhang R, Huang Y, Patzer RE, Kutner NG. Association of race and insurance type with delayed assessment for kidney transplantation among patients initiating dialysis in the United States. Clinical journal of the American Society of Nephrology : CJASN. 2012;7(9):1490–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ifudu O, Dawood M, Iofel Y, Valcourt JS, Friedman EA. Delayed referral of black, Hispanic, and older patients with chronic renal failure. Am J Kidney Dis. 1999;33(4):728–733. [DOI] [PubMed] [Google Scholar]
- 53.United Network for Organ Sharing. OPTN/UNOS Board approves significant revisions to deceased donor kidney allocation policy. http://optn.transplant.hrsa.gov/news/newsDetail.asp?id=1600. Accessed April 16, 2014.
- 54.Gordon EJ, Ladner DP, Caicedo JC, Franklin J. Disparities in kidney transplant outcomes: a review. Semin Nephrol. 2010;30(1):81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kasiske BL, London W, Ellison MD. Race and socioeconomic factors influencing early placement on the kidney transplant waiting list. Journal of the American Society of Nephrology : JASN. 1998;9(11):2142–2147. [DOI] [PubMed] [Google Scholar]
- 56.Organ Procurement and Transplantation Network Minority Affairs Committee. Educational Guidance on Patient Referral to Kidney Transplantation. 2015; https://optn.transplant.hrsa.gov/resources/guidance/educational-guidance-on-patient-referral-to-kidney-transplantation/. Accessed January 21, 2018.
- 57.Tandon A, Wang M, Roe KC, Patel S, Ghahramani N. Nephrologists’ likelihood of referring patients for kidney transplant based on hypothetical patient scenarios. Clin Kidney J. 2016;9(4):611–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Axelrod DA, Dzebisashvili N, Schnitzler MA, et al. The interplay of socioeconomic status, distance to center, and interdonor service area travel on kidney transplant access and outcomes. Clinical journal of the American Society of Nephrology : CJASN. 2010;5(12):2276–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kutner NG, Zhang R, Huang Y, Johansen KL. Impact of race on predialysis discussions and kidney transplant preemptive wait-listing. American journal of nephrology. 2012;35(4):305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vranic GM, Ma JZ, Keith DS. The role of minority geographic distribution in waiting time for deceased donor kidney transplantation. Am J Transplant. 2014;14(11):2526–2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Epstein AM, Ayanian JZ, Keogh JH, et al. Racial disparities in access to renal transplantation--clinically appropriate or due to underuse or overuse? N Engl J Med. 2000;343(21):1537–1544, 1532 p preceding 1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ayanian JZ, Cleary PD, Keogh JH, Noonan SJ, David-Kasdan JA, Epstein AM. Physicians’ beliefs about racial differences in referral for renal transplantation. Am J Kidney Dis. 2004;43(2):350–357. [DOI] [PubMed] [Google Scholar]
- 63.Myaskovsky L, Almario Doebler D, Posluszny DM, et al. Perceived discrimination predicts longer time to be accepted for kidney transplant. Transplantation. 2012;93(4):423–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Patzer RE, Perryman JP, Schrager JD, et al. The role of race and poverty on steps to kidney transplantation in the Southeastern United States. Am J Transplant. 2012;12(2):358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Patzer RE, Amaral S, Wasse H, Volkova N, Kleinbaum D, McClellan WM. Neighborhood poverty and racial disparities in kidney transplant waitlisting. Journal of the American Society of Nephrology : JASN. 2009;20(6):1333–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Browne T The relationship between social networks and pathways to kidney transplant parity: evidence from black Americans in Chicago. Soc Sci Med. 2011;73(5):663–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gillespie A, Fink EL, Traino HM, et al. Hemodialysis Clinic Social Networks, Sex Differences, and Renal Transplantation. Am J Transplant. 2017;17(9):2400–2409. [DOI] [PubMed] [Google Scholar]
- 68.Schold JD, Buccini LD, Kattan MW, et al. The association of community health indicators with outcomes for kidney transplant recipients in the United States. Arch Surg. 2012;147(6):520–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grubbs V, Gregorich SE, Perez-Stable EJ, Hsu CY. Health literacy and access to kidney transplantation. Clinical journal of the American Society of Nephrology : CJASN. 2009;4(1):195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


