Abstract
Purpose
Methotrexate (MTX) chemotherapy can be associated with neurological complications during therapy and long-term neurological deficits. This study evaluated demographic and clinical factors associated with incidence of MTX neurotoxicity and described the impact of neurotoxicity on acute lymphoblastic leukemia (ALL) therapy in pediatric patients.
Experimental Design
Patients were enrolled between 2012-2017 from three pediatric cancer treatment centers in the United States. Medical records for suspected cases of MTX neurotoxicity, defined as an acute neurologic event following MTX therapy, were reviewed. Cox proportional hazards models were used to estimate the association between race/ethnicity and MTX neurotoxicity. Multivariable linear regression models compared treatment outcomes between patients with and without MTX neurotoxicity.
Results
Of the 280 newly diagnosed patients enrolled, 39 patients (13.9%) experienced MTX neurotoxicity. Compared to non-Hispanic whites, Hispanic patients experienced the greatest risk of MTX neurotoxicity (adjusted HR=2.43; 95% CI: 1.06-5.58) after accounting for sex, age at diagnosis, BMI z-score at diagnosis, and ALL risk stratification. Patients who experienced a neurotoxic event received an average of 2.25 fewer doses of IT MTX. Six of the 39 cases of neurotoxicity (15.4%) experienced relapse during the study period, compared to 13 of the 241 (2.1%) patients without neurotoxicity (p=0.0038).
Conclusion
Hispanic ethnicity was associated with increased risk of MTX neurotoxicity, which was associated with treatment modifications and relapse. Understanding the mechanism and predictors of MTX neurotoxicity is important to improving treatment outcomes in pediatric ALL.
INTRODUCTION
Methotrexate (MTX) is a key component of contemporary chemotherapy for acute lymphoblastic leukemia (ALL), the most common malignancy diagnosed among those less than 15 years of age in the United States (1). However, MTX chemotherapy can be associated with profound neurological complications during therapy, as well as long-term neurological deficits (2,3). Specifically, central nervous system-directed intrathecal (IT) and high-dose intravenous (IV) MTX have been linked to acute and subacute neurotoxicity, with a reported incidence among pediatric ALL patients ranging between 3% and 12% depending on treatment regimen (4–8). The clinical presentation of MTX-induced neurotoxicity, which typically occurs within two weeks of IT and/or IV high-dose MTX, includes altered mental status, seizures, and stroke-like symptoms (9,10). Although symptoms typically resolve with time, the incidence of neurotoxicity often results in hospitalization and modifications to leukemia therapy, which may reduce treatment efficacy and jeopardize long-term survival (9). Despite the potentially serious implications of neurotoxicity, information as to why some patients experience MTX-induced neurotoxicity, while others do not, is limited.
Several recent case series have reported a high prevalence of MTX subacute neurotoxicity among Hispanic patients with ALL suggesting sensitivity to MTX therapy may differ by race and ethnicity (11,12). Disparities and mortality in pediatric ALL have been narrowing over time (13–15). Differences in susceptibility to treatment-related toxicity may contribute to well-documented racial/ethnic disparities in ALL treatment efficacy (16–19). However, risk factors for MTX neurotoxicity and the impact of neurotoxic events on subsequent ALL treatment have not been well-described in large, diverse populations. Therefore, the objectives of this study were to: 1) evaluate demographic and clinical factors associated with incidence of acute and subacute MTX neurotoxicity, and 2) describe the impact of neurotoxicity on therapy in a large and well-characterized, multi-institutional prospective cohort of pediatric patients treated according to contemporary ALL protocols.
METHODS
Study Population
Patients undergoing therapy on a leukemia treatment protocol at three major pediatric cancer treatment centers in the United States (University of Arizona, Children’s Minnesota and Texas Children’s Cancer Center) between November 2012 and February 2017 were enrolled on a prospective study of treatment-related toxicity. Institutional review boards at each participating site approved the study, which was conducted in accordance with guidelines in the U.S. Common Rule and Belmont Report. Informed consent was obtained from parents or legal guardians of each participant, and when appropriate, assent was obtained from subjects. Subjects were eligible for the study if they were diagnosed between 2 and 18 years of age and treated on or according to one of the following Children’s Oncology Group (COG) leukemia protocols: AALL0932, AALL1231, AALL1131, AALL0434, AALL1122, AALL0031. Summary of leukemia treatment in reference to MTX therapy is provided in Supplemental Table S1. Specific treatment information for each protocol can be accessed on www.clinicaltrials.gov (20). Subjects with pre-existing medical history of developmental disability or neurologic disorders were excluded.
Data Collection
MTX Neurotoxicity - Patients were prospectively monitored for the incidence of toxicity during treatment through June 2017. Electronic medical records were reviewed for suspected cases of acute or subacute MTX neurotoxicity. Suspected cases were defined as patients with a neurologic event including stroke-like symptoms, aphasia, and seizures following IT and/or IV MTX that resulted in modifications in IT and/or IV MTX therapy. Suspected cases were independently reviewed by two pediatric oncologists to confirm the diagnosis of acute or subacute MTX neurotoxicity. No discrepancies in case ascertainment were reported between reviewers.
Study Covariates - Demographic (i.e., patient sex, race, ethnicity) and clinical information (i.e., diagnosis, chemotherapy dose, treatment risk group assignment, minimal residual disease [MRD] status at day 29 of therapy, height and weight at diagnosis, date of relapse or death) were abstracted from electronic medical records. Bone marrow evaluation on COG leukemia protocols is performed at end of induction on D29 to determine risk assignment and post-induction therapy course (21, 22). Information on race and ethnicity was obtained using the standard National Institutes of Health (NIH) self-report form. Cumulative IT (number of doses) and IV (dose in g/m2) MTX were calculated through the end of the post-induction therapy. Body mass index (BMI) z-scores were calculated from diagnostic heights and weights using sex- and age-specific growth charts (23).
Statistical Methods
Statistical analyses were conducted in Stata version 14 (StataCorp, College Station, Texas) at a 5% significance threshold. Appropriate descriptive statistics for continuous (i.e., mean, standard deviation) and categorical (i.e., frequency, proportion of the total) covariates were calculated to characterize the study population. Differences in covariates by neurotoxicity status were evaluated using a t-test for continuous or Fisher’s exact test for categorical covariates. The incidence of MTX neurotoxicity by race/ethnicity groups was graphically compared using Nelson-Aalen cumulative hazard curves and statistically evaluated with the log-rank test. Cox proportional hazards models were used to estimate the crude and adjusted hazard ratio (HR) and corresponding 95% confidence interval (95% CI) for the association between race/ethnicity and MTX neurotoxicity. Adjusted models included terms for age at diagnosis, BMI z-score at diagnosis, sex, and treatment risk arm. We also evaluated the association between MTX neurotoxicity and clinical outcomes. Multivariable linear regression models were generated to compare treatment differences between patients with and without MTX neurotoxicity, adjusting for treatment risk arm, BMI z-score at diagnosis, sex, and age at diagnosis. Separate regression models were constructed for the dependent variables of cumulative cycles of IT MTX, cumulative IV MTX dose, and time from diagnosis to start of maintenance/continuation therapy. The frequency of all-cause and central nervous system (CNS) relapse was compared between patients with and without MTX neurotoxicity using the log-rank test and Cox regression models. We performed regression diagnostics to confirm that modeling assumptions were satisfied, such as the proportionality assumption of Cox regression and the multivariate normality assumption of linear regression.
RESULTS
A total of 280 newly diagnosed patients were eligible and enrolled in the parent study between November, 2012 and February, 2017. Individuals were followed from diagnosis of ALL to start of maintenance/continuation therapy. As of June 1, 2017, thirty-nine patients (13.9%) experienced acute or subacute MTX neurotoxicity (median follow up = 22.6 months; range: 1.3 - 55.6 months). All patients with suspected neurotoxicity presented with common manifestations (e.g., aphasia, seizure, stroke) and were independently reviewed by two pediatric oncologists (JB and ZED). Most cases of neurotoxicity presented with stroke-like symptoms (n=24), seizures (n=6), altered mental status (n=3), or both altered mental status and stroke like symptoms (n=2). There were individual cases of each of the following presentations: seizure and stroke-like symptoms, aphasia, vision changes with difficulty word finding, and syncopal episode (Supplemental Table S2). All suspected cases of neurotoxicity received at least one magnetic resonance imaging (MRI), with evidence of MTX-induced neurotoxicity detected in 72% (n=28) of cases. The demographic and clinical characteristics of the study population are presented in Table 1. The mean age at ALL diagnosis of patients included in this analysis was 8.4 years (range: 2.5 - 18.8 years). Most participants included in the analysis were male (52.1%), diagnosed with pre B-cell leukemia (85.7%), treated on high or very high-risk treatment arms (58.5%). Compared to patients without neurotoxicity, patients with acute or subacute MTX neurotoxicity were significantly older at diagnosis (12.2 years vs. 7.8 years), had greater BMI z-scores (0.80 vs. 0.14), and were more likely to receive high or very high-risk treatment (84.6% vs. 54.2%). Of the patients who experienced neurotoxicity, 74.4% (n=29) were Hispanic compared to 43.9% (n=104) of those without MTX neurotoxicity (p < 0.01). There was no statistically significant difference in type of ALL (B-cell vs. T-cell) by race/ethnicity (chi squared, p=0.362); therefore, the differences noted in neurotoxicity by race/ethnicity are not likely due to differences in ALL type.
Table 1.
Clinical and demographic characteristics of patients treated on acute lymphoblastic leukemia protocols, 2012-2017
| Overall | MTX Neurotoxicity | |||
|---|---|---|---|---|
| (n=280) | Yes (n=39) | No (n=241) | p-val. | |
|
|
||||
| Age at Diagnosis, mean(SD) | 8.40 (4.36) | 12.20 (3.27) | 7.79 (4.21) | <0.01 |
| BMI z-score, mean(SD) | 0.23 (1.31) | 0.80 (1.20) | 0.14 (1.31) | <0.01 |
| Patient Sex, n(%) | 0.99 | |||
| Male | 146 (52.1) | 20 (51.3) | 126 (52.3) | |
| Female | 134 (47.9) | 19 (48.7) | 115 (47.7) | |
| Race/Ethnicity, n(%) | <0.01 | |||
| Non-Hispanic White | 100 (36.2) | 8 (20.5) | 92 (38.8) | |
| Hispanic | 133 (48.2) | 29 (74.4) | 104 (43.9) | |
| Non-Hispanic Black | 23 (8.3) | 2 (5.1) | 21 (8.9) | |
| Non-Hispanic Other | 20 (7.3) | 0 (0.0) | 20 (8.4) | |
| Diagnosis, n(%) | 0.28 | |||
| B-ALL | 240 (85.7) | 31 (79.5) | 209 (86.7) | |
| T-ALL | 30 (10.7) | 7 (18.0) | 23 (9.5) | |
| Lymphoblastic Lymphoma | 10 (3.6) | 1 (2.5) | 9 (3.7) | |
| Treatment Arm, n(%) | <0.01 | |||
| Low/Standard Risk | 115 (41.5) | 6 (15.4) | 109 (45.8) | |
| High/Very High Risk | 162 (58.5) | 33 (84.6) | 129 (54.2) | |
| Triple IT Therapy, n(%) | 0.99 | |||
| None | 248 (89.9) | 34 (89.5) | 214 (89.9) | |
| Any | 28 (10.1) | 4 (10.5) | 24 (10.1) | |
| Day 29 MRD, n(%) | 0.11 | |||
| <0.01% | 200 (74.1) | 24 (63.2) | 176 (75.9) | |
| ≥0.01% | 70 (25.9) | 14 (36.8) | 56 (24.1) | |
SD, standard deviation; BMI, body mass index; B-ALL, B-cell acute lymphoblastic leukemia; T-ALL, T-cell acute lymphoblastic leukemia; IT, intrathecal; MRD, minimal residual disease p-value from t-test for continuous variable or Fisher’s exact test for categorical variables
The incidence of acute and subacute neurotoxicity differed significantly by race/ethnic group (Figure 1, log-rank p = 0.0049). Compared to non-Hispanic whites (Table 2), Hispanic patients experienced the greatest risk of MTX neurotoxicity (unadjusted HR=2.93; 95% CI: 1.34-6.42). Hispanic ethnicity remained robustly associated with neurotoxicity risk even after accounting for sex, age at diagnosis, BMI z-score at diagnosis, and ALL risk stratification (adjusted HR=2.43; 95% CI: 1.06-5.58). A sensitivity analysis restricted to the cases with MRI evidence of MTX neurotoxicity (n=28) did not meaningfully impact the findings.
Figure 1.
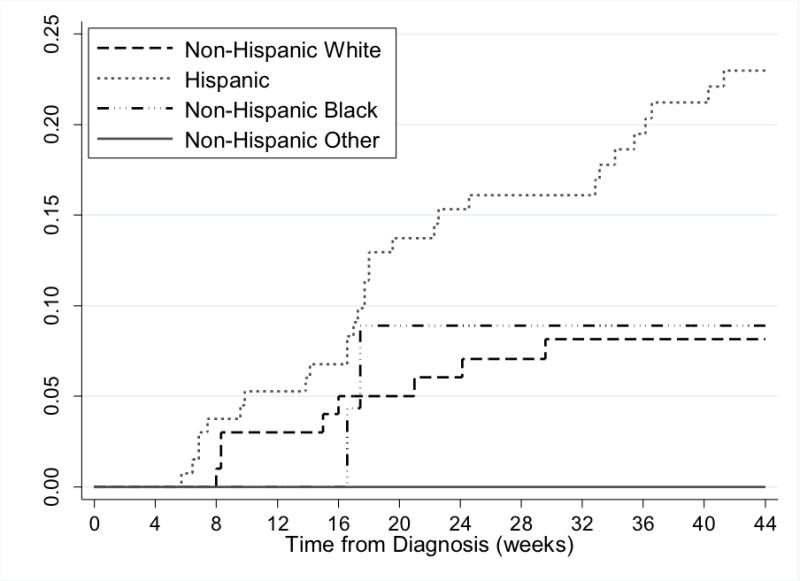
Cumulative incidence of MTX neurotoxicity by race/ethnicity
Table 2.
Association between race/ethnicity and incidence of MTX neurotoxicity among patients treated on acute lymphoblastic leukemia protocols, 2012-2017
| Patients at Baseline | Neurotoxic Events | Unadjusted Model | Adjusted Model1 | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | p-val | HR (95% CI) | p-val | |||
|
|
||||||
| Race/Ethnicity | 100 | 8 | Ref. | Ref. | ||
| Non-Hispanic White Hispanic | 133 | 29 | 2.93 (1.34-6.42) | 0.01 | 2.43 (1.06-5.58) | 0.036 |
| Non-Hispanic Black | 23 | 2 | 1.15 (0.24-5.40) | 0.86 | 1.23 (0.25-5.91) | 0.80 |
| Non-Hispanic Other | 20 | 0 | -- | -- | -- | -- |
Model adjusted for treatment risk arm, age at diagnosis, BMI z-score at diagnosis, and sex
Relative to non-Hispanic whites, Hispanic patients in the sensitivity analysis remained at increased risk of MRI-confirmed neurotoxicity in both unadjusted models (HR=2.81, 95% CI: 1.13-6.67) and models accounting for treatment risk arm, age at diagnosis, BMI z-score at diagnosis, and gender (HR=2.39, 95% CI: 0.91-6.26). A total of nine of the 39 patients with neurotoxicity (23.1%) experienced a second neurotoxic event, all of whom were Hispanic (p = 0.079). The second events were similar in nature to the symptoms in the first event: 3 patients presented with seizure; 5 with stroke-like symptoms; and 1 with altered mental status. Independent of Hispanic ethnicity, only age at diagnosis (HR=1.18, 95% CI: 1.09-1.29) remained a statistically significant predictor of neurotoxicity in multivariable models, with the risk increasing linearly with increasing age. Though not statistically significant, there was evidence of an increased risk of neurotoxicity in females (HR: 1.45, 95% CI: 0.76-2.79) and patients in high/very high treatment risk arms (HR: 1.44, 95% CI: 0.52-4.03).
Independent of treatment risk arm, sex, BMI z-score at diagnosis, and age at diagnosis, patients who experienced a neurotoxic event received an average of 2.25 (95% CI: 1.73-2.77) fewer doses of IT MTX (Table 3). The most common treatment modification was holding lumbar punctures (LPs) and replacing IT MTX with IT cytarabine/hydrocortisone (AraC/HC) for at least one subsequent LP after the neurotoxic event (43.6%), followed by holding at least next scheduled LP and resuming IT MTX with leucovorin rescue (38.5%). LPs were generally held for 4 to 6 weeks pending symptom resolution and/or abnormality improvement on MRI. Summary of all IV and IT MTX treatment changes are presented in Supplemental Table S3. Of the 39 cases of MTX neurotoxicity, 24 (61.5%) were rechallenged with IT MTX and leucovorin rescue, 7 (17.9%) were never rechallenged, 5 (12.8%) continued to receive IT MTX with leucovorin without interruption in previously scheduled therapy. Three (7.7%) are pending final clinical decision. Six of the 39 cases of neurotoxicity (15.4%) experienced relapse during the study period, compared to 13 of the 241 (2.1%) patients without MTX neurotoxicity (Supplemental Figure S1a, log-rank p = 0.0038). Similarly, CNS relapse was significantly more frequent among patients with neurotoxicity (four of 39, 10.3%) than patients without neurotoxicity (five of 241, 2.1%) in this study (Supplemental Figure S1b, log-rank p = 0.0014). In univariate Cox regression models, MTX neurotoxicity was significantly associated with CNS relapse (unadjusted HR: 3.80, 95% CI: 1.44-10.02, p=0.007), a trend which remained after accounting for treatment risk arm (HR: 2.92, 95% CI: 1.07-7.95, p=0.036), MRD status at day 29 (HR: 3.49, 95% CI: 1.32-9.24, p=0.012), race and ethnicity (HR: 3.15, 95% CI: 1.13-8.79, p =0.028), age at diagnosis (HR: 2.56, 95% CI: 0.91-7.21, p=0.076), and gender (HR: 3.82, 95% CI: 1.44-10.10, p=0.007).
Table 3.
Treatment comparison by neurotoxicity status among patients treated on acute lymphoblastic leukemia protocols, 2012-2017
| Mean (95% CI) Treatment Comparison by Neurotoxicity | |||
|---|---|---|---|
| Neurotoxicity (n=28) |
No Neurotoxicity (n=181) |
p-val | |
|
|
|||
| IV MTX Dose, g/m2 | 10.23 (8.33-12.13) | 12.04 (11.37-12.71) | 0.084 |
| Number IT MTX Doses | 8.84 (8.36-9.33) | 11.09 (10.92-11.26) | <0.01 |
| Time to Maintenance, days | 296.9 (284.8-311.9) | 290.0 (284.8-295.3) | 0.408 |
Model adjusted for treatment risk arm, age at diagnosis, BMI z-score at diagnosis, and sex
DISCUSSION
To our knowledge, this is the first multi-institutional prospective study to evaluate demographic and clinical factors associated with incidence of MTX neurotoxicity and subsequent impact on pediatric ALL treatment. The overall incidence of MTX neurotoxicity in our patient population (13.9%) is slightly higher than previous reports, ranging from 3-12% (4–8). It is worth noting that the frequency of neurotoxicity among non-Hispanic patients in this study (6.8%) was consistent with previous studies. It appears the higher frequency of neurotoxicity observed in our study (13.9%) is a consequence of the high incidence of neurotoxicity among Hispanics (21.8%) and a high proportion of Hispanics included in the study population (47.5%), which are underrepresented in other studies of pediatric ALL neurotoxicity. Because our study eligibility limited subjects under 2 years of age at diagnosis, our study population is slightly older than the population of children with ALL in the U.S. Our gender distribution is similar to the U.S.; however, our population is over represented for Hispanics patients even though it is representative of our local populations. All reported events occurred following IT and/or IV methotrexate (approximately 14 days from last IT/IV methotrexate). This time frame is consistent with other studies of acute and subacute methotrexate neurotoxicity (4–8). We also found that Hispanic ethnicity was associated with increased risk of MTX neurotoxicity. Of the patients who experienced a neurotoxic event in our cohort, 74.4% were Hispanic, and, of the patients who experienced a second neurotoxic event, all were Hispanic. Our suggestion that Hispanics are at an increased risk of developing MTX neurotoxicity is supported by two recent case series which also reported high proportion of Hispanic patients with subacute neurotoxicity (11,12). Specifically, Afshar et al., reported on 18 cases of neurotoxicity, of which 66.7% (n=12) were Hispanic (11). Similarly, Giordano et al., described a series of five Hispanic patients who developed MTX neurotoxicity (12).
The higher overall incidence of MTX neurotoxicity in our study population could be attributed to different case definitions for MTX acute or subacute neurotoxicity. For example, Bjowani et al. reported that all cases included in their study had MRI findings consistent with MTX neurotoxicity, while MRI evidence of neurotoxicity was observed in just 72% of the cases included in our study (5). Notably, restricting our analysis to cases with MRI evidence of neurotoxicity did not meaningfully impact our results. Alternatively, the high prevalence of neurotoxicity observed in this study may reflect recent advances in ALL treatment, which have improved overall survival of pediatric ALL, but may also be associated with more toxicity. A recent Children’s Oncology Group Study reported that high-risk B-ALL patients who received IV high-dose MTX versus Capizzi escalating-dose IV MTX had a significantly higher 5-year event free survival (82% compared to 75%; p=0.006) (24). These findings resulted in changes to IV MTX therapy and subsequently high dose IV MTX was recommended for pediatric patients with high risk B-ALL. This shift in the standard of care exposes more patients to high dose IV MTX, possibly increasing their risk of developing treatment-related toxicities including neurotoxicity.
Racial and ethnic minorities continue to experience inferior outcomes after being diagnosed with pediatric ALL, despite improvements in treatment regimens (16–19). Treatment-related toxicities resulting in treatment modifications or delays may contribute to racial and ethnic differences in survival. In our study, MTX neurotoxicity was significantly associated with time to any relapse as well as CNS relapse. The relapse rate of 15.4% in our patient populations is consistent with the rate reported in a small case series of 5 patients by Giordano et al. where 1 (20%) relapsed and died. Changes in IV and/or IT MTX therapy were made for patients who experienced a neurotoxic event to allow for symptom resolution and recovery from onset of neurologic changes. Accordingly, patients who experienced neurotoxicity in this study received significantly fewer doses of IT MTX and slightly lower cumulative doses of IV MTX. In addition, 29 cases (74.4%) received leucovorin rescue after IT MTX. Several publications suggest that leucovorin doses may interact with MTX to reduce efficacy and cure rate of pediatric ALL (25, 26). These findings may help us better understand what factors contribute to poorer survival among Hispanic patients with ALL.
Our findings add to the growing body of evidence indicating that minority patients, in particular Hispanics, often encounter significant disparities in terms of treatment outcomes for pediatric ALL. These findings should be further examined in larger cohort studies. Extended follow-up is also needed to further evaluate outcomes and long-term effects of MTX neurotoxicity in pediatric patients. Although we did not differentiate between acute and subacute MTX neurotoxicity, future studies with larger sample sizes should examine possible differences between acute and subacute neurotoxic events. Additionally, within each type of ALL, numerous cytogenetic subtypes exist, and the impact of these subtypes on outcomes should be examined in future studies. Future studies are also needed to evaluate biologic factors that may modify the risk of MTX neurotoxicity among vulnerable patients, including Hispanics. Understanding the mechanism and predictors of MTX neurotoxicity is critical to improving treatment outcomes. Future research from our group is investigating biomarkers for neurotoxicity which could benefit increased surveillance during therapy for these adverse treatment outcomes.
Supplementary Material
Statement of Translational Relevance.
Children from minority populations continue to experience inferior outcomes after being diagnosed with acute leukemia, despite improvements in treatment regimens. Treatment-related toxicities resulting in treatment modifications or delays may contribute to racial and ethnic differences in survival. For example, we report here that Hispanic patients experience more episodes of neurotoxicity than other groups. Accordingly, patients who experienced neurotoxicity received significantly fewer doses of IT MTX and slightly lower cumulative doses of IV MTX. These findings add to the growing body of evidence indicating that minority patients, in particular Hispanics, often encounter significant disparities in terms of treatment outcomes for pediatric ALL. Understanding the mechanisms and predictors of neurotoxicity is critical to improving treatment outcomes. Future studies are needed to evaluate biologic factors that may modify the risk of neurotoxicity among vulnerable patients, including Hispanics. Extended follow-up time is also needed to evaluate the long-term effects of neurotoxicity in pediatric patients.
Acknowledgments
FUNDING:
This work was supported by the National Institutes of Health (R01 CA 1693398) and Reducing Ethnic Disparities in Acute Leukemia (REDIAL) Consortium, St. Baldrick’s Foundation Consortium Research Grant.
Footnotes
The authors declare no potential conflicts of interest.
References
- 1.Howlader N, Noone AM, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975-2014. National Cancer Institute; Bethesda, MD: Apr, 2017. https://seer.cancer.gov/csr/1975_2014/, based on November 2016 SEER data submission, posted to the SEER web site. [Google Scholar]
- 2.Vagace JM, Caceres-Marzal C, Jimenez M, et al. Methotrexate-induced subacute neurotoxicity in a child with acute lymphoblastic leukemia carrying genetic polymorphisms related to folate homeostasis. Am J Hematol. 2011;86(1):98–101. doi: 10.1002/ajh.21897. [DOI] [PubMed] [Google Scholar]
- 3.Reddick WE, Conklin HM. Impact of acute lymphoblastic leukemia therapy on attention and working memory in children. Expert Rev Hematol. 2010;3:655–659. doi: 10.1586/ehm.10.65. [DOI] [PubMed] [Google Scholar]
- 4.Mahoney DH, Jr, Shuster JJ, Nitschke ZR, et al. Acute neurotoxicity in children with B-precursor acute lymphoid leukemia: an association with intermediate-dose intravenous methotrexate and intrathecal triple therapy – a Pediatric Oncology Group Study. J Clin Oncol. 1998;16:1712–1722. doi: 10.1200/JCO.1998.16.5.1712. [DOI] [PubMed] [Google Scholar]
- 5.Bjowani D, Sabin ND, Pei D, et al. Methotrexate-induced neurotoxicity and leukoencephalopathy in childhood acute lymphoblastic leukemia. J Clin Oncol. 2014;32:949–959. doi: 10.1200/JCO.2013.53.0808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dufourg MN, Landman-Parker J, Auclerc MF, et al. Age and high-dose methotrexate are associated to clinical acute encephalopathy in FRALLE 93 trial for acute lymphoblastic leukemia in children. Leukemia. 2007;21:238–247. doi: 10.1038/sj.leu.2404495. [DOI] [PubMed] [Google Scholar]
- 7.Badke C, Fleming A, Iqbal A, et al. Rechallenging with intrathecal methotrexate after developing subacute neurotoxicity in children with hematologic malignancies. Pediatr Blood Cancer. 2016;63:723–726. doi: 10.1002/pbc.25850. [DOI] [PubMed] [Google Scholar]
- 8.Parasole R, Petruzziello F, Menna G, et al. Central nervous system complications during treatment of acute lymphoblastic leukemia in a single pediatric institution. Leuk Lymphoma. 2010;51(6):1063–1071. doi: 10.3109/10428191003754608. [DOI] [PubMed] [Google Scholar]
- 9.Magge RS, DeAngelis LM. The double-edged sword: Neurotoxicity of chemotherapy. Blood Rev. 2015;29:93–100. doi: 10.1016/j.blre.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bond J, Hough R, Moppett J, et al. ‘Stroke-like syndrome’ caused by intrathecal methotrexate in patients treated during the UKALL 2003 trial. Leukemia. 2013;27:954–56. doi: 10.1038/leu.2012.328. [DOI] [PubMed] [Google Scholar]
- 11.Afshar M, Birnbaum D, Golden C. Review of Dextromethorphan administration in 18 patients with subacute methotrexate central nervous system toxicity. Pediatr Neurol. 2014;50(6):625–629. doi: 10.1016/j.pediatrneurol.2014.01.048. [DOI] [PubMed] [Google Scholar]
- 12.Giordano L, Akinyede O, Bhatt N, et al. Methotrexate-induced neurotoxicity in Hispanic adolescents with high-risk acute leukemia – a case series. J Adolesc Young Adult Oncol. 2017;6(3):494–498. doi: 10.1089/jayao.2016.0094. [DOI] [PubMed] [Google Scholar]
- 13.Kahn JM, Keegan T, Tao L, et al. Racial disparities in the survival of American children, adolescents and young adults with acute lymphoblastic leukemia, acute myelogenous leukemia and Hodgkin lymphoma. Cancer. 2016;122(17):2723–2730. doi: 10.1002/cncr.30089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang L, Bhatia S, Gomez SL, et al. Differential inequality trends over time in survival among U.S. children with acute lymphoblastic leukemia by race/ethnicity, age at diagnosis and sex. Cancer Epidemiol Biomarkers Prev. 2015;24(11):1781–1788. doi: 10.1158/1055-9965.EPI-15-0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kadan-Lottick NS, Ness KK, Bhatia S, et al. Survival variability by race and ethnicity in childhood acute lymphoblastic leukemia. JAMA. 2003;290(15):2008–2014. doi: 10.1001/jama.290.15.2008. [DOI] [PubMed] [Google Scholar]
- 16.Walsh A, Chewning J, Li X, et al. Inferior outcomes for black children with high risk acute lymphoblastic leukemia and the impact of socioeconomic variables. Pediatr Blood Cancer. 2017;64:267–274. doi: 10.1002/pbc.26222. [DOI] [PubMed] [Google Scholar]
- 17.Abrahao R, Lichtensztajn DY, Ribeiro RC, et al. Racial/ethnic and socioeconomic disparities in survival among children with acute lymphoblastic leukemia in California, 1988-2011: A population-based observational study. Pediatr Blood Cancer. 2015;62:1819–1825. doi: 10.1002/pbc.25544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pulte D, Redaniel MT, Jansen L, et al. Recent trends in survival of adult patients with acute leukemia: overall improvements, but persistent and partly increasing disparity in survival of patients from minority groups. Haematologica. 2013;98(2):222–229. doi: 10.3324/haematol.2012.063602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhatia S, Sather HN, Heerema NA, et al. Racial and ethnic differences in survival of children with acute lymphoblastic leukemia. Blood. 2002;100:1957–1964. doi: 10.1182/blood-2002-02-0395. [DOI] [PubMed] [Google Scholar]
- 20.Clinicaltrials.gov National Library of Medicine (US) 2017 Identification Nos. NCT00022737, NCT00408005, NCT01190930, NCT01460160, NCT02883049, NCT02112916. Retrieved from https://clinicaltrials.gov.
- 21.Cazzaniga G, Valsecchi MG, Gaipa G, et al. Defining the correct role of minimal residual disease tests in the management of acute lymphoblastic leukaemia. Br J Haematol. 2011;155:45–52. doi: 10.1111/j.1365-2141.2011.08795.x. [DOI] [PubMed] [Google Scholar]
- 22.Schultz KR, Pullen DJ, Sather HN, et al. Risk- and response-based classification of childhood B-precursor acute lymphoblastic leukemia: a combined analysis of prognostic markers from the Pediatric Oncology Group (POG) and Children’s Cancer Group (CCG) Blood. 2007;109(3):926–935. doi: 10.1182/blood-2006-01-024729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. 2000 CDC Growth Charts for the United States: Methods and Development. Available at: http://www.cdc.gov/nchs/data/series/sr_11/sr11_246.pdf. Accessed 1 July, 2017. [PubMed]
- 24.Larsen EC, Devidas M, Chen S, et al. Dexamethasone and high-dose methotrexate improve outcome for children and young adults with high-risk B-acute lymphoblastic leukemia: A report from Children’s Oncology Group Study AALL0232. J Clin Oncol. 2016;34:2380–2388. doi: 10.1200/JCO.2015.62.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skarby TV, Anderson H, Heldrup J, et al. High leucovorin doses during high-dose methotrexate treatment may reduce the cure rate in childhood acute lymphoblastic leukemia. Leukemia. 2006;20:1955–1962. doi: 10.1038/sj.leu.2404404. [DOI] [PubMed] [Google Scholar]
- 26.Sterba J, Dusek L, Demlova R, et al. Pretreatment plasma folate modulates the pharmacodynamics effect of high-dose methotrexate in children with acute lymphoblastic leukemia and non-Hodgkin lymphoma: “Folate Overrescue” concept revisited. Clin Chem. 2006;52:692–700. doi: 10.1373/clinchem.2005.061150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


