Abstract
Background & Objective:
Guidelines suggest that red blood cell transfusion decisions for most hospitalized patients be based on hemoglobin (Hb) concentration and the presence of symptoms of anemia, including fatigue. However, studies differ in whether transfusion is associated with improvements in fatigue. One explanation is that the benefit of transfusion varies by baseline fatigue levels, which existing studies have not examined. The objective of this study was to determine whether the association between transfusion during hospitalization and improvements in fatigue 30 days post-discharge varies by baseline fatigue level.
Methods:
A prospective observational study of hospitalized general medicine patients with any Hb <9g/dL. Patients with sickle cell anemia and gastrointestinal bleeding were excluded since these diagnoses have alternative transfusion practices. Patients with depression were excluded because their fatigue is not primarily due to anemia. Fatigue was measured during an in-person interview and a 30 day post-discharge phone interview. Hb values and receipt of a transfusion were collected from hospital administrative data. Linear regression was used to test associations between “change in fatigue”, Hb concentration, and receipt of a transfusion.
Results:
Transfusion interacted with nadir Hb was associated with reduced fatigue post-discharge for patients with higher baseline fatigue (20% most fatigued: β=12, p=0.02; 10% most fatigued: β=17, p=0.02). Patients <50 years old with high baseline fatigue had large reductions in fatigue from transfusion (20%: β=23, p=0.02; 10%: β=29, p=0.03).
Conclusions:
Transfusion during hospitalization is associated with reduced fatigue 30 days post-discharge in patients with higher levels of baseline fatigue.
Keywords: Anemia, Fatigue, Red Blood Cell Transfusion, Symptoms of Anemia
INTRODUCTION
Restrictive red blood cell (RBC) transfusion practices have become standard of care for most hospitalized patients with anemia [1, 2]. Restrictive transfusion practices are supported by guideline recommendations which endorse as a “strong” recommendation, transfusing most hospitalized patients only when their hemoglobin (Hb) drops below restrictive transfusion thresholds (7–8g/dL) [3, 4]. Since the publication of these guidelines, most transfusion decisions in hospitalized patients have come to be based on restrictive Hb concentration thresholds alone[5]. An implication of this reliance on Hb thresholds is that differences in patients’ clinical characteristics are not routinely considered when making transfusion decisions.
It is notable, however, that guidelines from the AABB and other professional societies also suggest that transfusion decisions be influenced not only by Hb but by patient symptoms, such as fatigue [3, 6–8]. Fatigue is the primary symptom of anemia [9], and is a physiologic response to the decreased tissue oxygenation that can result from anemia. Both clinical and physiologic reasoning suggest that transfusion, which increases Hb concentration and oxygen delivery to the tissues, will improve patients’ fatigue. Yet, despite guidelines endorsing transfusion for patients’ with symptoms such as fatigue, data are limited and previous studies are mixed on whether transfusion during hospitalization improves patients’ fatigue [10–13]. The generalizability of previous studies to the broad population of hospitalized patients with anemia is limited, and confidence in the validity of the findings in the largest study (FOCUS) [12] is limited because over half of study participants were lost to follow-up. Moreover, previous studies may differ in their findings on the effect of transfusion on fatigue because patients were transfused based on Hb concentration alone, and the studies did not examine whether the association of transfusion with reduced fatigue varies by baseline (in-hospital) fatigue level.
The potential variation in the effect of transfusion on fatigue by patients’ baseline fatigue level is important because the severity of a patient’s fatigue represents the physiologic burden of their anemia, so that patients with higher fatigue levels may be more likely to benefit and experience reduced fatigue from a transfusion. Since anemia has multiple pathophysiologic mechanisms, patients with the same Hb concentration but different clinical characteristics (i.e. comorbidities, age) may experience different levels of fatigue from their anemia [14]. Consequently, at Hb concentrations within restrictive transfusion ranges, patients with higher levels of baseline fatigue may experience reduced fatigue from the increased tissue oxygenation following transfusion. Alternatively, patients with little or no fatigue are less likely to benefit from transfusion. Therefore, understanding whether the effect of transfusion on fatigue varies by patients’ baseline fatigue, may help clarify whether transfusion during hospitalization improves patients’ fatigue. Moreover, it may help clinicians understand whether measures of patients’ fatigue should be incorporated, along with Hb concentration, into transfusion decisions for hospitalized patients with anemia.
The objective of this study was to determine whether the association between transfusion during hospitalization and improvements in fatigue 30 days post-discharge varies by baseline fatigue level in hospitalized patients with anemia. We hypothesize that in hospitalized patients with anemia, transfusion in patients with high levels of fatigue will result in reduced fatigue levels 30 days after hospital discharge.
METHODS
Study Design
We performed a prospective observational study of hospitalized general medicine patients with anemia. The University of Chicago Medical Center (UCMC) institutional review board approved the study procedures and all study subjects provided informed consent.
Study Eligibility
Between April, 2014 and June, 2015, all general medicine inpatients were approached for written consent for The University of Chicago Hospitalist Project [15], a research infrastructure at UCMC. Among patients consenting to participate in the Hospitalist Project, patients were eligible if they had a Hb <9g/dL at any point during their hospitalization, which includes the range of Hb values covered by most restrictive transfusion policies [12, 16, 17, 3]. If patients were not eligible for inclusion at the time of consent for the Hospitalist Project, their Hb values were reviewed twice daily until hospital discharge to assess if their Hb was <9g/dL. Proxies were sought to answer questions for patients who failed the Short Portable Mental Status Questionnaire [18].
Patient Demographic Data Collection
Research assistants abstracted patient age and sex from the electronic health record (EHR), and asked patients to self-identify their race. Hospitalist administrative data was used to determine hospital length of stay and a Charlson Comorbidity Index score [19] for each patient using International Classification of Disease 9 codes. We also used Health Care Utilization Project (www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp) diagnosis categories to identify whether patients had sickle cell anemia (SC), gastrointestinal bleeding (GIB), or a depressive disorder (DD), because these conditions are associated with anemia (SC, GIB) and fatigue (DD) [20], and are not included as part of the Charlson Comorbidity Index.
Measuring Anemia
The first Hb <9g/dL during a patient’s hospitalization, making them eligible for study participation, was obtained through manual review of the EHR. All additional Hb values during the patient’s hospitalization were obtained from the hospital’s administrative data mart.
Determining Receipt of Red Blood Cell Transfusion While Hospitalized
Whether patients received an RBC transfusion and the number of units of RBC’s transfused during hospitalization were obtained from the hospital’s clinical data repository.
Measuring Patient Fatigue During Hospitalization and After Hospital Discharge
Baseline fatigue was measured once during the patient’s hospitalization with an in-person interview either on the first day of hospital admission for patients eligible at admission, or the day the patient became eligible for the study for those patients who were not immediately eligible at hospital admission. Fatigue was again measured with a phone call 30 days after hospital discharge. The timing for the follow-up phone call was chosen because the effects of being hospitalized from an acute illness on fatigue may have diminished, yet the effect of the increase in blood count from a transfusion is still expected be significant [21].
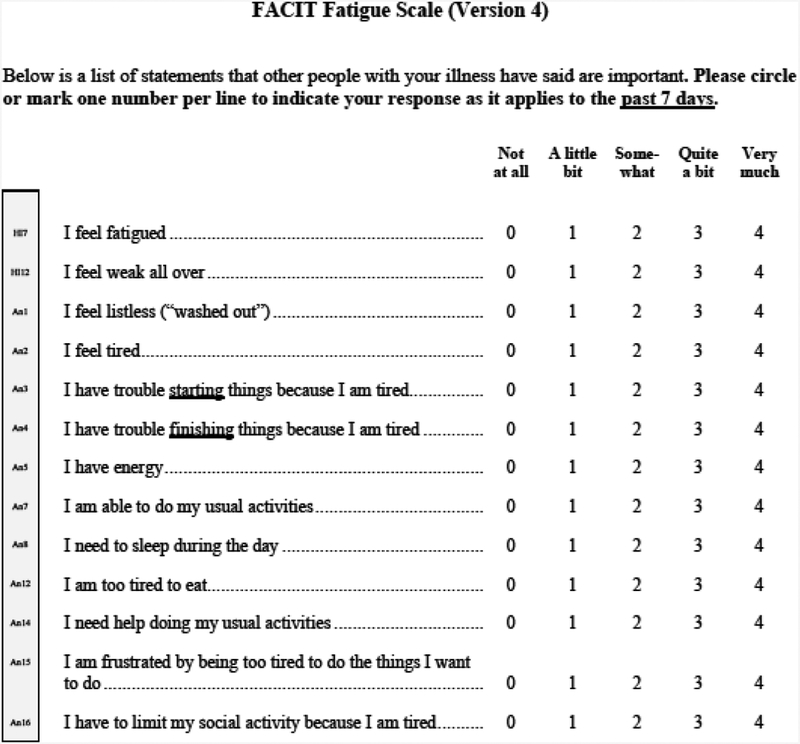
Fatigue was measured using a 13-question fatigue subscale that is part of the Functional Assessment of Chronic Illness Therapy Anemia (FACIT-An) questionnaire (appendix 1) [9, 22–25]. The fatigue subscale measures patient fatigue over the past 7 days, with scores ranging from 0–52, where lower scores reflect greater levels of fatigue. Values for any missing data in the fatigue subscale for individual subjects were filled in using a prorated score from their answered questions, in accordance with recommendations for addressing missing data in the FACIT [26].
Change in Fatigue
Our primary outcome was the change in patient fatigue level from hospitalization to 30 days post-discharge. A change in fatigue score was calculated by subtracting the FACIT fatigue score during hospitalization from the FACIT fatigue score 30 days after hospital discharge (Change in Fatigue = FACIT30 days – FACITinpt). Positive change in fatigue scores reflect decreased levels of fatigue at 30 days post-discharge compared to patients’ fatigue level during hospitalization. Negative change in fatigue scores reflect higher levels of fatigue at 30 days post discharge compared to patients’ fatigue level during hospitalization.
Statistical Analysis
Statistical analysis was performed using Stata statistical software, StataCorp, College Station, TX. Descriptive statistics were used to characterize patient demographics. Mann Whitney U tests were used to compare non-normally distributed patient demographic data, baseline fatigue levels, and Hb levels for those receiving a transfusion to those not receiving a transfusion during their hospitalization. Chi Squared tests were used to compare proportions. P-values <0.05 were considered statistically significant.
Linear Regression Models to Test the Effect of Transfusion on Changes in Fatigue
Multivariable linear regression was used to test for the effect of transfusion on “change in fatigue”, our primary outcome. The dependent variable in regression analysis was change in fatigue, while the independent variables of interest were receipt of transfusion and the interaction between receipt of a transfusion and patients’ nadir Hb during hospitalization. We also used FACIT30 days as the dependent variable and controlled for baseline fatigue (FACITinpt) as an independent variable to account for potential differences in baseline fatigue levels between transfused and non-transfused patients. The results were not substantively different and therefore we report the results using patients’ change in fatigue score as the dependent variable.
Our primary analysis excluded patients with a diagnosis of SC, GIB or DD, and controls for patient age (<50, 50–64, ≥65), sex, length of hospital stay, the number of RBC units transfused, nadir Hb during hospitalization, and Charlson Comorbidity Index score. The age categories were determined by dividing the sample into near tertiles based on age. Patients with SC and/or GIB were excluded from primary analysis because transfusion guidelines exclude patients with SC from their recommendations (chronic transfusion dependent anemia)[4], and standard clinical transfusion practices in patients with SC[27, 28] and/or GIB[29, 30] do not follow restrictive transfusion practices. Additionally, transfusion practices at our institution vary significantly for patients with GIB compared to patients without a GIB, and by the location of GIB. Lastly, patients with these GIB and SC have a different association of anemia with fatigue than do other hospitalized patients with anemia [31]. Patients with DD were excluded in our primary analysis because their fatigue is a residual symptom of and primarily due to DD, rather than anemia [32–34]. In a sensitivity analysis we included patients with SC, GIB, and/or DD and control for all the same variables as in our primary model, as well as we control for whether patients had a diagnosis of SC, GIB, and/or DD.
To test the effect of transfusion at different baseline levels of fatigue, regression models were tested within stratified levels of baseline fatigue using two different approaches. First, patients were stratified into quintiles by their baseline FACIT score, with higher quintiles representing greater levels of fatigue during hospitalization. Second, fatigue was dichotomized at the median FACIT score (<28) of the sample, and at FACIT scores that represented the 40% (FACIT≤23), 30% (FACIT≤19), 20% (FACIT≤14), and 10% (FACIT≤8) most fatigued patients in the sample.
RESULTS
Patient Characteristics
9,676 patients were admitted to the general medicine service during the study period. 6,189 (64%) patients consented for participation in the Hospitalist Project, and 4,442 (72%) patients completed the Hospitalist Project inpatient interview. 1,429 (32%) of these patients had a Hb <9g/dL, and 1,135 (79%) of these patients completed the inpatient FACIT questionnaire. 763 (67%) patients were reached for the 30 day follow-up interview, 513 (67%) of these completed the 30 day follow up FACIT questionnaire, and 357 (70%) did not have a diagnosis of SC, GIB, and/or DD (Figure 1). Patients who completed the 30 day follow up interview were slightly older (56 vs 52, p<0.01) and had a slightly lower nadir Hb (7.1 g/dL vs 7.2 g/dL, p=0.05), but did not differ in their gender, race, ethnicity, Charlson comorbidity index score, length of stay, or baseline fatigue level, compared to patients who completed the inpatient interview only and did not complete the 30 day follow up interview.
Figure 1.
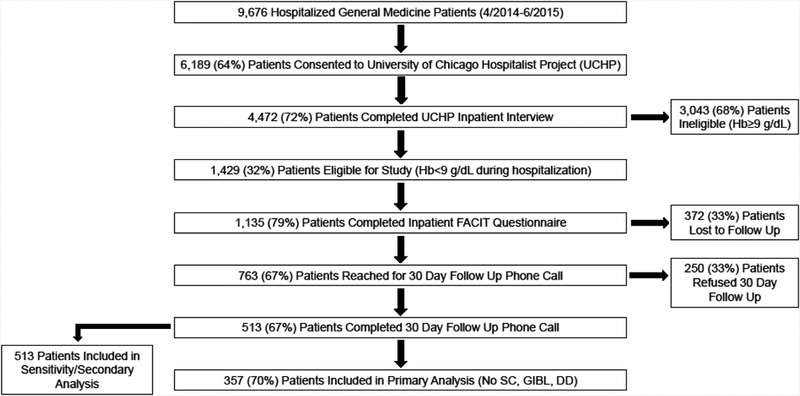
Patient Eligibility and Enrollment Diagram
Table 1 reports the demographic characteristics, comorbidities, inpatient Hb measures, and FACIT scores for patients that completed both the inpatient and follow-up interview, by whether or not they received a transfusion during their hospitalization (Table 1).
Table 1.
Patient Characteristics
| Total N=357 | Transfusion n=133 |
No Transfusion n=224 |
p | |
|---|---|---|---|---|
| Age, Mean ± SD (years) | 59 ± 17 | 58 ± 18 | 0.57 | |
| Age, N (%) | ||||
| <50 | 36 (27) | 67 (30) | 0.66 | |
| 50–64 | 47 (35) | 69 (30) | ||
| ≥65 | 50 (38) | 88 (40) | ||
| Female, N (%) | 76 (57) | 137 (61) | 0.45 | |
| Race, N (%) | ||||
| American Indian or Alaskan Native | 1 (1) | 3 (1) | 0.7 | |
| Asian | 4 (3) | 8 (4) | ||
| Black or African American | 84 (63) | 148 (66) | ||
| Hawaiian or Other Pacific Islander | 0 (0) | 0 (0) | ||
| White | 33 (25) | 45 (20) | ||
| Multiple Reported Races | 0 (0) | 4 (2) | ||
| Don't Know/Refused | 11 (8) | 16 (7) | ||
| Ethnicity | ||||
| Hispanic or Latino | 13 (10) | 18 (8) | 0.39 | |
| Not Hispanic or Latino | 115 (86) | 202 (90) | ||
| Don’t Know/ Refused | 5 (4) | 4 (2) | ||
| Admission Comorbidities, N (%) | ||||
| Myocardial Infarction | 3 (2) | 11 (5) | 0.21 | |
| Congestive Heart Failure | 28 (21) | 62 (28) | 0.16 | |
| Peripheral Vascular Disease | 9 (7) | 17 (8) | 0.77 | |
| Cerebrovascular Disease | 3 (2) | 2 (1) | 0.29 | |
| Dementia | 0 | 0 | 0.12 | |
| Chronic Pulmonary Disease | 22 (17) | 63 (28) | 0.01 | |
| Rheumatic Disease | 12 (9) | 16 (7) | 0.52 | |
| Peptic Ulcer Disease | 9 (7) | 1 (<1) | <0.01 | |
| Liver Disease | 19 (14) | 13 (6) | <0.01 | |
| Diabetes | 42 (32) | 68 (48) | <0.01 | |
| Hemiplegia/Paraplegia | 1 (1) | 6 (3) | 0.2 | |
| Renal Disease | 23 (17) | 47 (21) | 0.4 | |
| Cancer | 18 (14) | 26 (12) | 0.59 | |
| Aids/HIV | 1 (1) | 2 (1)) | 0.89 | |
| Charlson Comorbidity Index, N (%) | ||||
| 0 | 37 (28) | 60 (27) | 0.59 | |
| 1–2 | 61 (46) | 90 (40) | ||
| 3–4 | 25 (19) | 53 (24) | ||
| ≥5 | 10 (7) | 21 (9) | ||
| Hospital Length of Stay | ||||
| Mean ± SD | 9 ± 9 | 7 ± 6 | 0.16 | |
| Median (IQR) | 6 (3–12) | 5 (3–9) | ||
| Hemoglobin Measures, g/dL | ||||
| Mean ± SD | 8.0 ± 0.9 | 8.7 ± 0.8 | <0.001 | |
| Nadir ± SD | 6.4 ± 1.0 | 7.9 ± 0.8 | <0.001 | |
| Baseline FACIT Fatigue Score, Range 0–52 | ||||
| Mean ± SD | 27 ± 14 | 28 ± 14 | 0.24 | |
| Median (IQR) | 27 (16–38) | 29 (18–39) | ||
| Baseline FACIT Fatigue Score Quintile, N (%) | ||||
| Quintile | Range | |||
| 1 (Low Fatigue) | 41–52 | 25 (19) | 58 (26) | 0.48 |
| 2 | 32–40 | 29 (22) | 37 (16) | |
| 3 | 24–31 | 25 (19) | 45 (20) | |
| 4 | 15–23 | 24 (18) | 40 (18) | |
| 5 (High Fatigue) | 0–14 | 30 (22) | 44 (20) | |
Association of Transfusion and Hb with Changes in Fatigue by Baseline Fatigue Level
Across the entire sample, when not stratifying patients by baseline fatigue level, there was no association between receipt of a transfusion or the interaction between receipt of a transfusion and nadir Hb and reduced fatigue. However, when stratifying patients by baseline fatigue quintiles, the interaction between transfusion and nadir Hb was associated with reductions in fatigue (Δ Fatigue=β=12, p=0.02) for patients with the highest level of baseline fatigue (quintile 5). This result indicates that in patients with high baseline fatigue, the effect of transfusion is dependent upon nadir Hb, and that an increase in nadir Hb of 1g/dL will on average result in an improvement of 12 points on the FACIT scale 30 days after hospital discharge. In all other quintiles, neither transfusion nor the interaction between transfusion and nadir Hb had an association with changes in fatigue (Table 2).
Table 2.
The Effect of Transfusion and Hb on Changes in Fatigue by Baseline Fatigue Quintiles
| Baseline FACIT Score | ||||||
|---|---|---|---|---|---|---|
| Unstratified | N | Mean | Range | Δ Fatigue | 95% CI | p |
| 357 | 28 | 0–52 | 2.5 | (−1.2, 6.2) | 0.19 | |
| Fatigue Quintile | N | Mean | Range | Δ Fatigue | 95% CI | p |
| 1 (Low) | 83 | 46 | 41–52 | 1.8 | (−2.8 6.4) | 0.68 |
| 2 | 66 | 36 | 32–40 | -0.84 | (−9.1, 7.4) | 0.84 |
| 3 | 70 | 28 | 24–31 | −6.9 | (−16, 1.9) | 0.12 |
| 4 | 64 | 19 | 15–23 | −4.6 | (−14, 4.7) | 0.33 |
| 5 (High) | 74 | 7.5 | 0–14 | 12 | (2.4, 23) | 0.02 |
Linear regression controlling for: age, sex, length of stay, number of units of RBC transfused, Charlson Comorbidity Index score
Fatigue=β coefficient for the interaction effect of transfusion × nadir Hb on the dependent variable change in fatigue (FACITFU – FACITinp)
When stratifying patients at 10% intervals of higher baseline fatigue, transfusion interacted with nadir Hb was again associated with reduced fatigue, and the effect was larger for patients the higher their baseline level of fatigue. The interaction between transfusion and patients’ nadir Hb resulted in change in fatigue scores of 2.3 (FACIT<28, p=0.44), 3.8 (FACIT≤23, p=0.26), 4.7 (FACIT≤19, p=0.2), 12 (FACIT≤14, p=0.02), and 17 (FACIT≤8, p=0.02) (Table 3). The direction of the interaction effect between transfusion and nadir Hb in this model was again positive, indicating that a higher nadir Hb for patients that received a transfusion resulted in improved fatigue 30 days after hospital discharge. In sensitivity analysis, including patients with SC, GIB, and/or DD, the interaction between transfusion and patients’ nadir Hb also resulted in positive changes in fatigue for patients at high levels of baseline fatigue. However, the effect size and amount of reduced fatigue was smaller both when fatigue was stratified by quintiles (supplemental Table 2) and at 10% intervals of baseline fatigue (Supplemental Table 3) compared to our primary analysis, and the effects did not quite reach statistical significance.
Table 3.
The Effect of Transfusion and Hb on Changes in Fatigue by Baseline Fatigue Level
| Baseline (In-Hospital) | |||||
|---|---|---|---|---|---|
| Fatigue Level | FACIT | N=357 | Δ Fatigue | 95% CI | p |
| Median | ≥28 | 190 | 0.34 | (−.6, 4.3) | 0.87 |
| Median | <28 | 167 | 2.3 | (−3.6, 8.3) | 0.44 |
| 60% | >23 | 219 | −0.61 | (−4.5, 3.3) | 0.76 |
| 40% | ≤23 | 138 | 3.8 | (−2.9, 10) | 0.26 |
| 70% | >19 | 248 | −0.21 | (−4.0, 3.6) | 0.91 |
| 30% | ≤19 | 109 | 4.7 | (−2.5, 12) | 0.20 |
| 80% | >14 | 283 | 0.30 | (−3.3, 3.0) | 0.87 |
| 20% | ≤14 | 74 | 12 | (2.4, 23) | 0.02 |
| 90% | >8 | 318 | 0.37 | (−3.2, 3.2) | 0.84 |
| 10% | ≤8 | 39 | 17 | (2.6, 31) | 0.02 |
Linear regression controlling for: age, sex, length of stay, number of units of RBC transfused, Charlson Comorbidity Index score
Fatigue=β coefficient for the interaction effect of transfusion × nadir Hb on the dependent variable change in fatigue (FACITFU – FACITinp)
The Effect of Transfusion and Hb on Changes in Fatigue by Baseline Fatigue Level and Age
While patient age and its effect on the relationship between transfusion and fatigue was not a pre-specified association we had anticipated, in our primary analysis age (<50) as an independent variable was consistently statistically significantly associated with reduced fatigue from transfusion. Given this, we performed exploratory analysis stratifying our models by age (<50, 50–64, ≥65).
Stratification by Baseline Fatigue and Age
When stratifying patients by baseline fatigue quintiles and age, the interaction between transfusion and nadir Hb was again positive, and associated with a large reduction in fatigue for patients with a fatigue level in the top quintile and age <50. Patients in quintile 5 and age <50 had estimated change in fatigue scores of 23 (p=0.02). This effect size is nearly double the amount of reduced fatigue seen in the model not stratified by age. For patients in the other fatigue quintiles and all other age groups, there was no association between transfusion or transfusion interacted with nadir Hb on changes in fatigue (Table 4).
Table 4.
The Effect of Transfusion and Hb on Changes in Fatigue by Baseline Fatigue Quintiles and Age
| Age<50 | 50≤Age<65 | Age≥65 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unstratified | N | Δ Fatigue | 95% CI | p | N | Δ Fatigue | 95% CI | p | N | Δ Fatigue | 95% CI | p |
| 103 | 1.2 | (−6.1, 8.5) | 0.74 | 116 | 0.49 | (−7.9, 8.9) | 0.91 | 138 | 3.4 | (−2.9, 9.7) | 0.29 | |
| Fatigue Quintile | N | Δ Fatigue | 95% CI | p | N | Δ Fatigue | 95% CI | p | N | Δ Fatigue | 95% CI | p |
| 1 (Low) | 21 | −2.3 | (−13, 8.0) | 0.64 | 28 | 1.0 | (12, 14) | 0.87 | 34 | 7.8 | (−2.5, 18) | 0.13 |
| 2 | 17 | −15 | (−36, 6.8) | 0.16 | 24 | 8.8 | (−23, 41) | 0.56 | 25 | −0.36 | (−15, 14) | 0.96 |
| 3 | 23 | −9.4 | (−26, 7.5) | 0.25 | 15 | −43 | (−138, 53) | 0.33 | 32 | 0.02 | (−14, 14) | 0.99 |
| 4 | 17 | −22 | (−56, 13) | 0.18 | 24 | −5.6 | (−29, 18) | 0.61 | 23 | 7.4 | (−6.1, 21) | 0.26 |
| 5 (High) | 25 | 23 | (4.5, 41) | 0.02 | 25 | 12 | (18, 41) | 0.34 | 24 | 0.46 | (−23, 25) | 0.97 |
Linear regression controlling for: age, sex, length of stay, number of units of RBC transfused, Charlson Comorbidity Index score
Fatigue=β coefficient for the interaction effect of transfusion × nadir Hb on the dependent variable change in fatigue (FACITFU – FACITinp)
When stratifying patients at 10% intervals of higher baseline fatigue and age, transfusion interacted with nadir Hb again resulted in a positive effect with large reductions in fatigue for patients <50 with high levels of fatigue at baseline. At each baseline level of fatigue the association with transfusion and reduced fatigue were larger than in the models not stratified by age. For patients 50–64 or ≥65, transfusion or transfusion interacted with nadir Hb was not associated with reductions in fatigue (supplemental Table 1).
Discussion
Our results support that for hospitalized patients without SC, GIB or DD, receipt of a transfusion is associated with improved fatigue 30 days after hospital discharge in patients with high levels of baseline fatigue. Additionally, among patients with high fatigue, there is a larger benefit (greater reduction in fatigue) from transfusion the higher a patient’s baseline fatigue during hospitalization. In our analysis the 20% most fatigued patients in the sample had clinically and statistically significant reductions in fatigue from transfusion 30 days after hospital discharge. Additionally, among patients with high fatigue, at each 10% interval of higher baseline fatigue, transfusion was associated with clinically larger reductions in fatigue 30 days after discharge for patients. These results are physiologically consistent with the idea that patients with more severe symptoms from their anemia are more likely to benefit from a transfusion. The variation in the effect of transfusion on reduced fatigue we observed was in a small subset of highly fatigued patients, and previous studies which did not consider patients’ baseline fatigue level may have missed the important effect of transfusion within patients with the most fatigue.
It is significant that in our study it was the interaction between transfusion and Hb that was associated with reductions in fatigue, rather than transfusion alone, because it suggests that both a measure of Hb and a measure of fatigue are necessary to identify which patients will benefit from transfusion. It also raises the possibility that a measure of fatigue could be combined with patients’ Hb in order to help clinicians and patients better balance the risk of transfusion, against the likelihood the patient will experience reduced fatigue, compared to restrictive transfusion practices based on Hb concentration alone. Therefore, our results provide empirical data to support guideline recommendations that transfusion decisions be influenced both by a patient’s Hb and whether they have symptoms from their anemia. We also believe our findings support future larger and better powered studies to identify variation in the effect of transfusion on fatigue in patients with different comorbidities, ages, admission etiologies, and hospital service types (i.e. surgical patients).
That the effect of transfusion and nadir Hb on patients’ fatigue was greater for patients in our study younger than 50 years old, raises interesting questions about fatigue as an outcome measure in patients with anemia. That older patients in our study did not benefit (experience reduced fatigue) from transfusion is consistent with a recent transfusion study in patients undergoing cardiac surgery, where a restrictive transfusion strategy benefited older patients[35]. Nonetheless, it is a surprising result since it would be expected that older patients, in whom the adverse consequences of anemia are well described, would be expected to benefit from a transfusion more than young patients who would presumably be able to better tolerate fatigue from anemia. One possible explanation for our finding is that younger patients are more active, and therefore the effects of fatigue and the benefit of a transfusion are more tangibly experienced than a patient that is more sedentary. This idea is described by the concept of fatigability, which measures and normalizes fatigue in relation to specific and defined activities[36]. While transfusion may reduce fatigue for patients at any level of activity, more active patients may experience a greater benefit because after transfusion they are able to resume a more active lifestyle. Exploring whether the reductions in fatigue from transfusion also affect patients’ activity levels is an important future direction, since it could address whether transfusion also results in improved functional outcomes in addition to or as a result of reduced fatigue levels in patients.
This study has several limitations. Although our sample size is large and includes patients with a range of comorbidities that we believe is representative of hospitalized general medicine patients, as a single center study our results may not be generalizable to other centers. Also, due to the longitudinal nature of our study not all patients were available at follow up (either lost to follow up or refused follow up phone call). While those patients did not differ significantly in their baseline demographic characteristics (including fatigue level) compared to patients who completed the follow up survey, it is possible that the 30 day follow up fatigue levels in the patients we could not contact differ from those we were able to contact, biasing our results. Additionally, although these data support a reliable association between receipt of a transfusion and improvements in fatigue 30 days after hospital discharge, the observational design of this study cannot prove that this relationship is causal. Since patients cannot be blinded to transfusion, knowledge of receipt of a transfusion could also have influenced patients’ assessment of their fatigue.
In conclusion, this study demonstrates that in hospitalized patients with anemia, red blood cell transfusion during hospitalization is associated with reduced fatigue 30 days post discharge for patients with high levels of baseline fatigue. The results of this study are consistent with physiologic reasoning in patients with anemia and the presumed effect of transfusion on symptoms when correcting anemia. This study also adds empirical support to guideline recommendations that transfusion decisions for hospitalized patients be influenced by patient symptoms, such as fatigue. Future work building on this study should focus on the differential effect of transfusion on symptoms by patient age, the effect of transfusion on fatigue and patient activity (fatigability), as well as the effect of transfusion and repeated transfusion on other quality of life measures, in order to better understand the consequences for patients receiving red blood cell transfusion during hospitalization. Additionally, while transfusion remains the primary treatment of anemia for hospitalized patients, future work should also examine whether other treatments for anemia, such as iron or erythropoiesis stimulating agents, have any effect on patient’s fatigue and/or their quality of life after hospital discharge.
Supplementary Material
Funding Source:
Dr. Prochaska is supported by a National Heart, Lung, and Blood Institute (USA) K23 Career Development Award. (NIH/NHLBI 1K23HL140132-01, Prochaska PI)
Dr. Meltzer is supported by an National Institutes of Health (USA) Clinical and Translational Science Award (NIH/NCATS UL1TR0002389-01, Solway PI).
Appendix 1.
Functional Assessment of Chronic Illness Therapy (FACIT) Anemia-Fatigue Subscale
Footnotes
Conflicts of Interest: The authors declare they have that they have no conflicts of interest relevant to the manuscript submitted.
REFERENCES:
- [1].Yazer MH, Triulzi DJ. AABB Red Blood Cell Transfusion Guidelines: Something for Almost Everyone. JAMA 2016; 316: 1984–1985. [DOI] [PubMed] [Google Scholar]
- [2].Hébert PC, Carson JL. Transfusion Threshold of 7 g per Deciliter — The New Normal. N Engl J Med 2014; 371: 1459–1461. [DOI] [PubMed] [Google Scholar]
- [3].Carson JL, Grossman BJ, Kleinman S, et al. Red Blood Cell Transfusion: A Clinical Practice Guideline From the AABB*. Ann Intern Med 2012; 157: 49–58. [DOI] [PubMed] [Google Scholar]
- [4].Carson JL, Guyatt G, Heddle NM, et al. Clinical Practice Guidelines From the AABB: Red Blood Cell Transfusion Thresholds and Storage. JAMA 2016; 316: 2025–2035. [DOI] [PubMed] [Google Scholar]
- [5].Vuille-Lessard E, Boudreault D, Girard F, et al. Red blood cell transfusion practice in elective orthopedic surgery: a multicenter cohort study. Transfusion (Paris) 2010; 50: 2117–2124. [DOI] [PubMed] [Google Scholar]
- [6].Napolitano LM, Kurek S, Luchette FA, et al. Clinical practice guideline: red blood cell transfusion in adult trauma and critical care. Crit Care Med 2009; 37: 3124–3157. [DOI] [PubMed] [Google Scholar]
- [7].Practice Guidelines for blood component therapy: A report by the American Society of Anesthesiologists Task Force on Blood Component Therapy. Anesthesiology 1996; 84: 732–747. [PubMed] [Google Scholar]
- [8].Transfusion of Blood and Blood Products: Indications and Complications - American Family Physician, http://www.aafp.org/afp/2011/0315/p719.html (accessed 7 February 2017). [PubMed]
- [9].Yellen SB, Cella DF, Webster K, et al. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage 1997; 13: 63–74. [DOI] [PubMed] [Google Scholar]
- [10].Mercadante S, Ferrera P, Villari P, et al. Effects of red blood cell transfusion on anemia-related symptoms in patients with cancer. J Palliat Med 2009; 12: 60–63. [DOI] [PubMed] [Google Scholar]
- [11].Brown E, Hurlow A, Rahman A, et al. Assessment of Fatigue after Blood Transfusion in Palliative Care Patients: A Feasibility Study. J Palliat Med 2010; 13: 1327–1330. [DOI] [PubMed] [Google Scholar]
- [12].Carson JL, Terrin ML, Noveck H, et al. Liberal or Restrictive Transfusion in High-Risk Patients after Hip Surgery. N Engl J Med 2011; 365: 2453–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chen LJ, Moeller KD, Wagman LD. Effect of red blood cell transfusions on patient-reported outcomes in an ambulatory oncology population. J Clin Oncol 2016; 34: 78–78. [Google Scholar]
- [14].Prochaska MT, Newcomb R, Block G, et al. Association Between Anemia and Fatigue in Hospitalized Patients: Does the Measure of Anemia Matter? J Hosp Med 2017; 12: 898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Meltzer D, Manning WG, Morrison J, et al. Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists. Ann Intern Med 2002; 137: 866–874. [DOI] [PubMed] [Google Scholar]
- [16].Holst LB, Haase N, Wetterslev J, et al. Transfusion requirements in septic shock (TRISS) trial - comparing the effects and safety of liberal versus restrictive red blood cell transfusion in septic shock patients in the ICU: protocol for a randomised controlled trial. Trials 2013; 14: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hébert PC, Wells G, Blajchman MA, et al. A Multicenter, Randomized, Controlled Clinical Trial of Transfusion Requirements in Critical Care. N Engl J Med 1999; 340: 409–417. [DOI] [PubMed] [Google Scholar]
- [18].Pfeiffer E A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc 1975; 23: 433–441. [DOI] [PubMed] [Google Scholar]
- [19].Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992; 45: 613–619. [DOI] [PubMed] [Google Scholar]
- [20].HCUP Clinical Classifications Software (CCS) for ICD-9-CM. Healthcare Cost and Utilization Project (HCUP). 2006–2009. Agency for Healthcare Research and Quality, Rockville, MD., https://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp (accessed 22 November 2016). [Google Scholar]
- [21].Simon TL, Snyder EL, Stowell CP, et al. (eds). Rossi’s Principles of Transfusion Medicine. 4 edition Chichester, UK; Hoboken, NJ: Wiley-Blackwell, 2009. [Google Scholar]
- [22].Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol Off J Am Soc Clin Oncol 1993; 11: 570–579. [DOI] [PubMed] [Google Scholar]
- [23].Cella D, Lai J-S, Chang C-H, et al. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer 2002; 94: 528–538. [DOI] [PubMed] [Google Scholar]
- [24].PROMIS FATIGUE AND FACIT-FATIGUE, http://www.prosettastone.org/LinkingTables/Documents/PROMIS%20Fatigue%20and%20FACIT-Fatigue%20Full%20Report.pdf (accessed 9 March 2016).
- [25].Cella D, Yount S, Rothrock N, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS). Med Care 2007; 45: S3–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Webster K, Cella D, Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: properties, applications, and interpretation. Health Qual Life Outcomes 2003; 1: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wun T, Hassell K Best practices for transfusion for patients with sickle cell disease. Hematol Rev; 1. Epub ahead of print 7 January 2010 DOI: 10.4081/hr.2009.e22. [DOI] [Google Scholar]
- [28].Telen MJ. Principles and problems of transfusion in sickle cell disease. Semin Hematol 2001; 38: 315–323. [DOI] [PubMed] [Google Scholar]
- [29].Wachter RM, Goldman L, Hollander H Hospital Medicine. Lippincott Williams & Wilkins, 2005. [Google Scholar]
- [30].Oakland K, Jairath V, Murphy MF. Advances in transfusion medicine: gastrointestinal bleeding. Transfus Med; n/a–n/a. [DOI] [PubMed] [Google Scholar]
- [31].Prochaska M, Newcomb, Block G, et al. Association Between Anemia and Fatigue in Hospitalized Patients: Does the Measure of Anemia Matter? [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Fava M, Ball S, Nelson JC, et al. Clinical relevance of fatigue as a residual symptom in major depressive disorder. Depress Anxiety 2014; 31: 250–257. [DOI] [PubMed] [Google Scholar]
- [33].Targum SD, Fava M. Fatigue as a Residual Symptom of Depression. Innov Clin Neurosci 2011; 8: 40–43. [PMC free article] [PubMed] [Google Scholar]
- [34].Ho S-Y, Rohan KJ, Parent J, et al. A longitudinal study of depression, fatigue, and sleep disturbances as a symptom cluster in women with breast cancer. J Pain Symptom Manage 2015; 49: 707–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Mazer CD, Whitlock RP, Fergusson DA, et al. Restrictive or Liberal Red-Cell Transfusion for Cardiac Surgery. N Engl J Med 2017; 377: 2133–2144. [DOI] [PubMed] [Google Scholar]
- [36].Eldadah BA. Fatigue and Fatigability in Older Adults. PM&R 2010; 2: 406–413. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


