Abstract
Background
Survivors of adolescent-onset cancers are at-risk for infertility, yet the majority desire children. Fertility preservation options are available for adolescents, but sperm banking remains underutilized. Patient factors which influence decisions to bank sperm are poorly understood.
Methods
Cross-sectional study of adolescent males newly diagnosed with cancer (N = 146) who completed surveys within one week of treatment initiation. Participants were 13–21 years of age (M = 16.49, SD = 2.02, 65% White) and at-risk for infertility secondary to impending gonadotoxic treatment. Participating institutions included eight leading pediatric oncology centers across the United States and Canada.
Results
Of approached patients, 80.6% participated. Parent recommendation to bank (OR=4.88; 95%CI: 1.15–20.71, p=.03), higher Tanner Stage (OR=4.25; 95%CI: 1.60–11.27, p<.01), greater perceived benefits (OR=1.41; 95%CI: 1.12–1.77, p<.01), and lower social barriers to banking (OR=0.88; 95%CI: 0.81–0.96, p<.01) was associated with adolescent collection attempts, whereas meeting with a fertility specialist (OR=3.44; 95%CI: 1.00–11.83, p=.05), parent (OR=3.02; 95%CI: 1.12–8.10, p=.03) or provider (OR=2.67; 95%CI: 1.05–6.77, p=.04) recommendation to bank, and greater adolescent self-efficacy to bank (OR=1.16; 95%CI: 1.01–1.33, p=.03) was associated with successful sperm banking.
Conclusions
Adolescents’ perceived benefits of banking, higher Tanner stage, and parent recommendation were associated with collection attempts, while perceived social barriers decreased this likelihood. Successful banking was associated with greater adolescent self-efficacy, parent and provider recommendation to bank, and consultation with a fertility specialist. Providers should consult with both adolescents and parents about fertility preservation, and interventions should be tailored to address barriers to banking, while promoting banking benefits.
Keywords: Oncology, Adolescent, Male Infertility, Cryopreservation, Psychology
INTRODUCTION
Survival rates for childhood cancer have significantly improved over the past several decades.1 Therefore, much attention has been placed on studying potential late effects of toxic cancer therapies. Cranial or pelvic radiation as well as alkylating agents are common treatments (~50%) for various types of childhood cancer2 and have been found to adversely affect the endocrine and reproductive systems.3,4 As such, survivors of childhood cancer are at increased risk for impaired fertility or sterility.
Subsequent efforts to increase fertility counselling and gamete preservation prior to the initiation of cancer treatment among newly diagnosed youth have been prioritized, but utilization of these interventions remains low.5–8 As having children is one of the top three life goals among adolescents with cancer,9 and infertility among adult survivors of childhood cancer is related to significant distress, worry, relationship difficulties, or challenges in finding a partner,10–15 it is unclear why fertility preservation remains low in this group. It may be that at the time of diagnosis, future fertility is not an immediate concern for many adolescent patients. But priorities shift over time,16 and adult survivors of childhood cancer often regret that infertility as a potential consequence of treatment was not discussed at the time of diagnosis.17 Retrospective studies have found that those adolescents who were offered fertility preservation greatly appreciated having had the option to bank sperm.18,19
The standard fertility preservation method for post-pubertal males is sperm banking/cryopreservation, but many factors contribute to decision-making and utilization of such options. The present study investigated the contribution of developmental (e.g., age, Tanner stage), communication (e.g., information and recommendation from parents, medical team providers, or fertility specialists), and psychological factors (i.e. fertility-related health beliefs, anxiety) in affecting sperm banking among at-risk adolescent males. The role that each of these factors plays in this process was tested in association with our two primary study outcomes: (1) collection attempt and (2) successful completion of banking sperm.
METHODS
Study Design and Setting
A single group, observational study design focusing on sperm banking among adolescents newly diagnosed with cancer was utilized across eight leading pediatric oncology centers in the United States and Canada from December 2010 – January 2014.
Participants
Prior to enrollment, study team members checked eligibility of new patients daily. Eligible participants were male, newly diagnosed with a first malignancy, 13–21 years of age, Tanner Stage ≥ 3, proficient in speaking and reading English or Spanish, and had the cognitive capacity to complete study questionnaires. Once initial study criteria were met, the patient’s oncologist was queried regarding the patient’s infertility risk. Patients were only considered eligible after the oncologist rated the adolescent as being at increased risk (> no risk) for infertility based on impending treatment. To control for potential bias, all patients who met eligibility were attempted for approach. After the provision of informed consent/assent, data were typically collected between one to seven days post initiation of cancer treatment. As sperm banking should occur prior to the initiation of cancer therapy,20 the timing of this data collection was chosen to increase the validity of self-reported factors that had influenced the sperm banking decision.21 Upon completion of the survey, participants received a gift card as compensation for their time and efforts. All study procedures were approved by the Institutional Review Boards of all participating centers.
Variables and Measurement
Survey content and development occurred as a function of survivor focus groups, clinical experience, literature review of previous research findings, behavioral health theory, and instrumentation, and piloting and review from the sponsoring institution’s Family Advisory Committee, all of which ultimately informed the final adolescent questionnaire.
Primary Outcomes
The two binary primary outcomes considered were collection attempt (yes/no) and successful sperm banking (yes/no) which was obtained from the study survey via adolescent self-report. A collection attempt was considered as such if the participant endorsed one of the following options to the question of whether they had banked sperm: (a) “Yes,” (b) “No, I tried to but wasn’t able to provide a sample,” or (c) “No, I provided a sample but there was no sperm to bank in it.” Sperm banking was coded as successful only if the response was “Yes.”
Independent Outcomes
Sociodemographic Variables
Adolescent participants responded to a series of standard sociodemographic questions, including age, race/ethnicity, education, employment status, religious preference, and relationship status. For analytical purposes, race/ethnic group was collapsed into a two level White/Other variable due to a low percentage of participants endorsing any of the other race/ethnicity response options. Adolescents were also asked to report whether they had a history of masturbation, nocturnal emission (i.e., “wet dream”), and partnered sexual activity. These developmental history items were adapted from the Centers for Disease Control and Prevention’s Youth Risk Behavior Surveillance System survey.22 For analytic purposes, responses of “no” and “not sure” on these developmental were collapsed into one single “no” category. Patients’ Tanner stage and type of diagnosis were retrieved from their medical records.
Communication
Adolescents were asked a series of binary questions about whether medical team member(s), parent(s), or other family members/friends had discussed their risk of infertility with them and whether a banking recommendation was made. Adolescents were asked what their personal perception of their infertility risk was on a 0–3 scale which ranged from none (0) to high (3), and whether they met with a fertility specialist. Adolescents also completed the family communication, problem solving, affective responsiveness, and general functioning subscales from the McMaster Family Assessment Device.23 Finally, adolescents were asked whether they were familiar with fertility preservation methods prior to diagnosis.
Psychological Factors
Fertility and banking-related health beliefs were measured with subscales adapted from previous research and based on the Health Belief Model.24–28 Perceived vulnerability was assessed using a five-item scale, which included content such as “Compared to other males who have never been treated for cancer, what is your risk of developing fertility problems in the future?” Items were answered on a five-point Likert-type scale from 1–5 (much lower – much higher). Perceived severity was measured with five items, instructing adolescents to rate their agreement with statements such as “Infertility would be one of the hardest things to deal with in life” (1–5; strongly disagree – strongly agree). To measure perceived barriers, adolescents rated the relevance of 28 potential barriers from 1–4 (very unimportant – very important). Exploratory factor analysis suggested four subscales representing (a) influential authority figures (i.e., medical team and parental influences; 6 items), (b) social influences (e.g., friends, girlfriend/partner, siblings; 6 items), (c) concerns for future children (e.g., child health, genetics, desire for children; 5 items), and (d) sperm banking logistics (e.g. cost, availability; 10 items), with one item contributing to both influential authority figures and the logistics subscales. Perceived benefits were measured with six items assessing the extent adolescents agreed with statements such as “sperm banking makes an infertile man a more desirable spouse” or “sperm banking helps avoid future regret” (1–5; strongly disagree – strongly agree). Self-efficacy was assessed with four items targeting adolescent perceptions of their ability to produce a sample, arrange/schedule an appointment, arrange transportation to sperm banking facility, and pay for sperm banking (1–5; definitely no – definitely yes). Lastly, cues to action were measured by asking participants to endorse all sources of fertility-related information (choosing from seven medical, twelve social, and six media options). The number of endorsed sources were summed to a total score. Finally, anxiety during the previous week (i.e., when diagnosis and sperm banking decisions presumably occurred) was measured using the 10-item anxiety subscale of the Symptom Checklist 90-R.29
Statistical Methods
To build logistic regression models with the most appropriate variables selected as covariates, a three-step statistical strategy was employed including (a) multiple imputation (MI) for managing missing data, (b) elastic net for selection of relevant covariates, and (c) building multivariable logistic regression models. Specifically, after eliminating variables that had ≥ 20% missing data, a Markov Chain Monte Carlo method was employed to impute other missing values, based on an assumption of arbitrary missing pattern and a multivariate normal distribution of factors.30,31 This MI procedure yielded 20 imputed data sets. Second, elastic net regularization was utilized in each of the 20 imputed datasets to select final covariates. Those covariates selected 19 times or more (i.e., ≥95 % chance) based on Bayesian Information Criteria (BIC) were retained and tested in the final model.32,33 Finally, multivariate logistic models were fitted using the selected covariates in the 20 imputed datasets. Results of the final models were aggregated and presented in odds ratios (OR) and 95% confidence intervals (CIs).
RESULTS
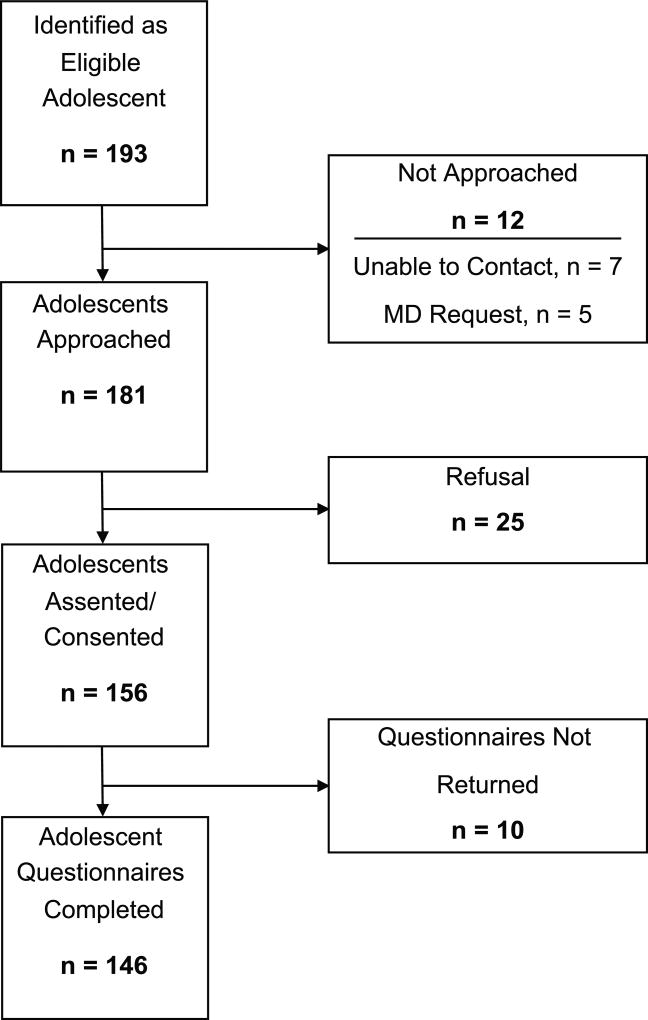
A total of 181 eligible patients were approached across the eight centers, of whom 156 agreed to participate, and 146 completed all questionnaires (see Figure 1). Patients were most often diagnosed with a leukemia or lymphoma (n=82, 56.2%), on average 16.5 years old (SD=2.0; range: 13–21 years), predominantly White (n=95, 65.1%), Christian (n=120, 92.2%), and reported dating or relationship experience (n=99, 67.8%; see Table 1, Sociodemographic Factors).
Figure 1.
Enrollment Diagram for Study Participation
Table 1.
Sociodemographic Factors among all Adolescents Newly Diagnosed with Cancer; and Separated by Sperm Banking Attempt and Successful Bankinga
| All patients |
Sperm banking attempt |
Successful sperm banking |
|||
|---|---|---|---|---|---|
| N=146 | No (n=68) |
Yes (n=78) |
No (n=82) |
Yes (n=64) |
|
|
|
|||||
| Sociodemographic Factors |
N (%) | n (%) | n (%) | n (%) | n (%) |
| Race/Ethnicity | |||||
| White | 95 (65.1) | 43 (45.3) | 52 (54.7) | 53 (55.8) | 42 (44.2) |
| Other | 51 (34.9) | 24 (47.1) | 27 (52.9) | 29 (56.9) | 22 (43.1) |
| Diagnosis | |||||
| Leukemia/Lymphoma | 82 (56.2) | 43 (63.2) | 39 (50.0) | 50 (61.0) | 32 (50.0) |
| Brain & Solid Tumors | 64 (43.8) | 25 (36.8) | 39 (50.0) | 32 (39.0) | 32 (50.0) |
| Education | |||||
| Less than H.S. Diploma | 114 (78.1) | 56 (49.1) | 58 (50.9) | 66 (57.9) | 48 (42.1) |
| H.S. Diploma/GED | 11 (07.5) | 5 (45.5) | 6 (54.5) | 5 (45.5) | 6 (54.5) |
| More than H.S. Diploma | 16 (11.0) | 4 (25.0) | 12 (75.0) | 7 (43.8) | 9 (56.3) |
| Religion | |||||
| Christian | 120 (82.2) | 57 (47.5) | 63 (52.5) | 70 (58.3) | 50 (41.7) |
| Other | 23 (15.8) | 8 (34.8) | 15 (65.2) | 10 (43.5) | 13 (56.5) |
| Relationship status | |||||
| Single, never dated | 46 (31.5) | 24 (52.2) | 22 (47.8) | 30 (65.2) | 16 (34.8) |
| Dating experience | 70 (47.9) | 32 (45.7) | 38 (54.3) | 39 (55.7) | 31 (44.3) |
| Committed relationship | 29 (19.9) | 11 (37.9) | 18 (62.1) | 13 (44.8) | 16 (55.2) |
| Tanner Stage | |||||
| Stage 3 | 13 (08.9) | 10 (76.9) | 3 (23.1) | 10 (76.9) | 3 (23.1) |
| Stage 4 | 42 (28.8) | 24 (57.1) | 18 (42.9) | 29 (69.0) | 13 (31.0) |
| Stage 5 | 84 (57.5) | 31 (36.9) | 53 (63.1) | 40 (47.6) | 44 (52.4) |
| History of nocturnal emission | |||||
| No | 84 (57.5) | 42 (50.0) | 42 (50.0) | 50 (59.5) | 34 (40.5) |
| Yes | 51 (34.9) | 18 (35.3) | 33 (64.7) | 23 (45.1) | 28 (54.9) |
| History of masturbation | |||||
| No | 26 (17.8) | 19 (73.1) | 7 (26.9) | 22 (84.6) | 4 (15.4) |
| Yes | 107 (73.3) | 40 (37.4) | 67 (62.6) | 50 (46.7) | 57 (53.3) |
| History of partnered sexual activity | |||||
| No | 81 (55.5) | 33 (48.5) | 48 (61.5) | 41 (50.0) | 40 (62.5) |
| Yes | 49 (33.6) | 25 (36.8) | 24 (30.8) | 30 (36.6) | 19 (29.7) |
| Age, M (SD) | 16.5 (2.0) | 16.2 (2.1) | 16.8 (1.9) | 16.2 (2.1) | 16.8 (1.8) |
may not equal 100% due to missing data
Of all 146 adolescent participants, approximately half made a collection attempt (53.4%, n=78). However, 14 of these males did not successfully bank, either because they were unable to provide a sample (14%, n=11) or because their sample was azoospermic (4%, n=3). Thus, 82.1% (n=64/78) of those who attempted successfully banked sperm, but overall, a minority of all at-risk patients successfully banked sperm (43.8%; n=64).
Almost a third of the 68 participants who did not attempt to bank sperm indicated they did not believe banking was necessary (27.9%, n=19), while an additional 16.2% reported not knowing what sperm banking was or not having met the developmental milestones needed to bank (n=11). Other reasons for not attempting to bank included: a lack of communication from the doctor (8.8%, n=6), not desiring biological children (5.9%, n=4), religious/moral concerns (5.9%, n=4), cost prohibitive (5.9%, n=4), concern for delaying treatment (5.9%, n=4), and fear of passing down genetic risk for cancer (1.5%, n=1). Fifteen of the 68 non-attempters (22.1%) did not report a reason for not attempting to bank.
Collection Attempt
All sociodemographic, communication, and psychological factors were tested for their association with collection attempt, and based on elastic net variable selection, the following variables were consistently identified at the univariate level: Tanner stage, several communication factors (i.e. fertility-risk communication with provider, fertility-risk communication with parent, recommendation to bank from provider, recommendation to bank from parent, familiarity with preservation methods, consultation with fertility specialist), and two of the fertility-related health belief factors (i.e. perceived benefits of banking, perceived social barriers to banking). Consequently, these variables were entered into the final logistic regression model, showing that adolescents who reported a parent recommended banking were almost five times more likely to make a collection attempt (OR=4.88; 95% CI [1.15–20.71], p=.032). In addition, adolescents with a higher Tanner stage (OR=4.25; 95% CI [1.60–11.27], p=.004) and those who more strongly endorsed the benefits of banking (OR=1.41; 95% CI [1.12–1.77], p=.004) were more likely to attempt banking. However, adolescents who reported greater barriers to banking in their social environment (e.g., friends, siblings) were less likely to make a collection attempt (OR=0.88; 95% CI [0.81–0.96], p=.005. See Table 4.
Table 4.
Multivariate Logistic Regressions on Sperm Banking Outcomes among At-risk Adolescent Males Newly Diagnosed with Cancer (N=146)
| β | SE | p | OR | 95% CI | |
|---|---|---|---|---|---|
| Sperm banking attempt | |||||
| Tanner Stage | 1.45 | 0.50 | .004 | 4.25 | [1.60 – 11.27] |
| Parent(s) recommended banking | 1.58 | 0.74 | .032 | 4.88 | [1.15 – 20.71] |
| Perceived benefits of banking | 0.34 | 0.12 | .004 | 1.41 | [1.12 – 1.77] |
| Perceived barriers: Social influences | −0.13 | 0.05 | .005 | 0.88 | [0.81 – 0.96] |
| Successful sperm banking | |||||
| Provider recommended banking | 0.98 | 0.48 | .039 | 2.67 | [1.05 – 6.77] |
| Parent(s) recommended banking | 1.10 | 0.50 | .029 | 3.02 | [1.12 – 8.10] |
| Consulted with fertility specialist | 1.24 | 0.63 | .050 | 3.44 | [1.00 – 11.83] |
| Self-efficacy | 0.15 | 0.07 | .034 | 1.16 | [1.01 – 1.33] |
Successful Completion of Sperm Banking
All sociodemographic, communication, and psychological factors were tested for their association with successful banking at the univariate level, and based on elastic net variable selection, the following variables were consistently identified: three communication factors (i.e. provider recommendation to bank, parent recommendation to bank, consultation with fertility specialist) and two fertility-related health beliefs (i.e. perceived benefits to banking, and self-efficacy). Notably, no sociodemographic factors were identified as significantly related to successful banking.
The five identified variables were entered into the final logistic regression model, showing that both adolescent-reported recommendations from a parent (OR=3.02, 95% CI [1.1–8.10], p=.029) or provider (OR=2.67, 95% CI [1.05–6.77], p=.039) were associated with an approximately three-fold greater likelihood to successfully bank. See Table 4. In addition, adolescents who reported higher self-efficacy to bank (OR=1.16; 95% CI [1.01–1.33], p=.034) were more likely to be successful. Notably, adolescents who consulted with a fertility specialist were over three times more likely to successfully bank (OR=3.44; 95% CI [1.00–11.83], p=.050). See Table 4.
DISCUSSION
Despite adolescent males’ desire for children in the future,9,34 this study found that a minority of adolescents newly diagnosed with cancer bank sperm prior to the initiation of cancer treatment. Historically, there has been a lack of clarity regarding the factors associated with adolescent sperm banking outcomes, but as this work demonstrates, adolescent factors, as well as parental and provider factors, all appear to play a role in this process.
Initial communications regarding fertility risk and preservation options begin with medical providers.35,36 These messages are typically communicated during the diagnostic process, discussion of treatment plan, or during a review of potential side effects as part of the informed consent for treatment process. As such, healthcare providers can significantly influence fertility preservation decision-making,18,37 yet some oncologists prioritize disease assessment and treatment planning over fertility preservation. Adolescents who are clear in their decision to attempt/not attempt sperm banking do not typically receive a formal fertility preservation consultation, but for the majority of adolescents who are unsure or need more information, they may benefit from meeting with a fertility specialist before making a decision. Although not all pediatric oncologists believe pubertal patients should be referred to a fertility specialist,5 our results demonstrate that working with such a specialist was most influential with regard to successful sperm banking outcomes.
Parent recommendations to their sons can be important as well, and as such, they often desire involvement in sperm banking discussions as a means of developing the most informed recommendations. As parents frequently defer to physician advice regarding fertility preservation recommendations, it’s important that medical professionals provide timely, accurate, and easily digestible fertility-risk information to families, or make appropriate referrals to fertility specialists (e.g., reproductive endocrinologist, pediatric urologist, psychologist, or other practitioner). Information gained from these discussions further informs parental banking recommendations, which were found to be particularly influential with regards to adolescent collection attempts. Interventions designed to improve sperm banking outcomes in the future should target staff who interact with families and are willing to assist parents in formulating recommendations by addressing any perceived (particularly social) barrier to making a sperm banking attempt, while emphasizing/promoting adolescent understanding of the benefits associated with making a sperm banking attempt.
As adolescents must consent to all fertility preservation procedures, perhaps the most important influence on this process is the adolescent himself. Whereas previous research and clinical lore has suggested age is the most important determinant influencing fertility preservation outcomes,38,39 our data suggest Tanner stage may be more influential. Thus, Tanner stage, an indicator of physical maturity, may be a more accurate indicator of readiness and/or spermatogenesis for providers, as opposed to exclusively working from an age criterion.40 Furthermore, adolescent psychological variables, including perceived banking benefits, lack of social barriers, and self-efficacy to complete the banking process were also associated with banking outcomes. As with parents, interventions among staff who specialize in working with adolescents could aid in the targeting of these modifiable attitudes when making preservation decisions or preparing for banking.
With regard to study limitations, it should be noted that we relied on self-report for sperm banking outcomes rather than medical record verification. However, these data were collected as part of a larger study which included parent data, and we identified 100% agreement between adolescent- and parent-reports on sperm banking outcomes. In addition, the ideal study design would have included the collection of study data prior to sperm collection attempts, banking, and the initiation of treatment. Yet, variability of time from diagnosis to treatment (e.g., hours for some leukemia patients to weeks for some solid/brain tumor patients) across patients made this type of design unfeasible. As study data collection ended in 2014, interval changes in adolescent attitudes surrounding sperm banking may be possible, though unlikely. Also, participant Tanner stage and fertility risk scores were clinically assigned (as opposed to meeting standardized study-based definitions), which increases the likelihood of variability of classifications.
Future research should not only examine interventions designed to increase collection attempts and successful sperm banking, but also decisional satisfaction among adolescents to promote patients’ psychological well-being regardless of their decision and banking outcome. Study is also needed regarding the familial decision-making processes specific to experimental fertility preservation (e.g. testicular tissue cryopreservation) among prepubertal males at-risk for infertility, along with system-level factors (e.g. access to andrology labs) which could affect sperm banking opportunities among newly diagnosed adolescents.
While guidelines for practitioners regarding adolescent fertility preservation have been established by several leading oncology and pediatric healthcare organizations,41–43 this study investigated sperm banking decisions from the perspective of the adolescent. Collection attempt and sperm banking success were found to be related to adolescent developmental (e.g., Tanner stage) and psychological (e.g., benefits to banking, self-efficacy and social barriers) factors, as well as adolescents’ perception of parent recommendation, provider recommendation, and consultation with a fertility specialist. In the context of these findings, recommendations for future interventions to promote sperm banking outcomes could focus on training medical teams who can assist adolescents and their parents in navigating these factors and encourage optimal decision-making for each adolescent. Although fertility preservation is optional, accurate, digestible, and timely communications regarding fertility risk and counseling is not. By providing and participating in these discussions, healthcare providers will not only be adhering to the recommended guidelines, but also allowing patients an opportunity to maintain their option of biological fatherhood.
Table 2.
Fertility-Related Communication among all Participants (N=146) and Separated by Sperm Banking Attempt and Successful Bankinga
| All patients | Sperm banking attempt | Successful sperm banking |
|||
|---|---|---|---|---|---|
| N=146 | No (n=68) |
Yes (n=78) |
No (n=82) |
Yes (n=64) |
|
|
|
|||||
| Communication Factors |
N (%) | n (%) | n (%) | n (%) | n (%) |
| Fertility risk communication by provider | |||||
| No | 33 (22.6) | 27 (81.8) | 6 (18.2) | 29 (87.9) | 4 (12.1) |
| Yes | 111 (76.0) | 38 (34.2) | 73 (65.8) | 51 (45.9) | 60 (54.1) |
| Fertility risk communication by parent | |||||
| No | 35 (24.0) | 31 (88.6) | 4 (11.4) | 31 (88.6) | 4 (11.4) |
| Yes | 108 (74.0) | 34 (31.5) | 74 (68.5) | 48 (44.4) | 60 (55.6) |
| Provider recommended sperm banking | |||||
| No | 58 (39.7) | 43 (74.1) | 15 (25.9) | 47 (81.0) | 11 (19.0) |
| Yes | 85 (58.2) | 21 (24.7) | 64 (75.3) | 32 (37.6) | 53 (62.4) |
| Parent recommended sperm banking | |||||
| No | 60 (41.1) | 48 (80.0) | 12 (20.0) | 50 (83.3) | 10 (16.7) |
| Yes | 78 (53.4) | 15 (19.2) | 63 (80.8) | 28 (35.9) | 50 (64.1) |
| Consulted with fertility specialist | |||||
| No | 114 (78.1) | 60 (52.6) | 54 (47.4) | 73 (64.0) | 41 (36.0) |
| Yes | 26 (17.8) | 4 (15.4) | 22 (84.6) | 6 (23.1) | 20 (76.9) |
| Fertility Risk, Perceived M (SD) | |||||
| 1.4 (0.7) | 1.3 (0.8) | 1.5 (0.7) | 1.3 (0.7) | 1.5 (0.7) | |
may not equal 100% due to missing data
Table 3.
Health Belief Factors among all participants; Separated by Sperm Banking Attempt and Successful Banking
| All patients | Sperm banking attempt | Successful sperm banking |
|||
|---|---|---|---|---|---|
| N=146 | No (n=68) |
Yes (n=78) |
No (n=82) |
Yes (n=64) |
|
|
| |||||
| M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | |
| Perceived vulnerability | 15.8 (5.6) | 15.2 (5.3) | 16.2 (5.8) | 15.5 (5.2) | 16.0 (6.0) |
| Perceived severity | 10.0 (3.8) | 9.1 (3.3) | 10.7 (3.9) | 9.7 (3.7) | 10.4 (3.8) |
| Barriers to banking | |||||
| Influential authority figures | 19.1 (5.8) | 19.6 (6.7) | 18.7 (5.0) | 19.1 (6.4) | 19.1 (4.9) |
| Social influences | 17.4 (7.5) | 18.9 (7.6) | 16.1 (7.2) | 18.3 (7.5) | 16.3 (7.4) |
| Concerns for future children | 16.8 (4.8) | 16.8 (5.7) | 16.7 (3.8) | 16.7 (5.5) | 16.9 (3.6) |
| Sperm banking logistics | 29.2 (10.1) | 30.4 (11.9) | 28.2 (8.1) | 29.6 (11.3) | 28.7 (8.3) |
| Benefits of banking | 22.2 (4.0) | 19.6 (3.9) | 24.0 (2.8) | 20.5 (4.0) | 24.0 (2.9) |
| Self-efficacy | 14.2 (4.6) | 11.8 (5.0) | 16.0 (3.3) | 12.2 (4.8) | 16.5 (3.0) |
| Cues to action | |||||
| Medical | 1.3 (1.1) | 1.0 (1.0) | 1.6 (1.1) | 1.1 (1.1) | 1.6 (1.1) |
| Family & friends | 1.4 (1.5) | 1.0 (1.5) | 1.7 (1.5) | 1.0 (1.4) | 1.9 (1.5) |
| Media | 0.5 (0.6) | 0.6 (0.5) | 0.4 (0.7) | 0.6 (0.5) | 0.4 (0.7) |
Acknowledgments
Funding Sources: This work was supported in part by the National Institute of Child Health and Human Development grant no. HD061296 (J. Klosky, Principal Investigator) and United States Public Health Service grant no. CA-21765 (C. Roberts, Principal Investigator) with additional support provided to St. Jude Children’s Research Hospital by the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
Conflict of Interest Disclosure: Leslie Schover has an interest in the for-profit health care company Will2Love, LLC. No other author has a conflict of interest to disclose.
Author Contributions:
James L. Klosky: funding acquisition, resources, supervision, conceptualization, methodology, investigation, project administration, data curation, writing – original draft, and writing – review and editing.
Vicky Lehmann – data curation, writing – original draft, and writing – review and editing.
Jessica S. Flynn: investigation, project administration, data curation, writing – original draft, and writing – review and editing.
Yin Su: data curation, formal analysis, writing – original draft, and writing – review and editing.
Hui Zhang: conceptualization, methodology, data curation, formal analysis, writing – original draft, and writing – review and editing.
Kathryn Russell: data curation, formal analysis, writing – original draft, and writing – review and editing.
Lauren A.-M. Schenck: writing – original draft, and writing – review and editing.
Leslie Schover: conceptualization, methodology, and writing – review and editing.
References
- 1.Howlader N, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975–2010, national cancer institute. Bethesda, MD: [Accessed May, 9, 2017]. http://seer.cancer.gov/csr/1975_2010/. Updated 2013. [Google Scholar]
- 2.Mertens A, Whitton J, Neglia JP, et al. Temporal changes in treatment exposures in the childhood cancer survivor study (CCSS) J Clin Oncol. 2015;33(15_suppl):10074. [Google Scholar]
- 3.Green DM, Kawashima T, Stovall M, et al. Fertility of male survivors of childhood cancer: A report from the childhood cancer survivor study. J Clin Oncol. 2010;28(2):332–339. doi: 10.1200/JCO.2009.24.9037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green DM, Liu W, Kutteh WH, et al. Cumulative alkylating agent exposure and semen parameters in adult survivors of childhood cancer: A report from the St. Jude lifetime cohort study. Lancet Oncol. 2014;15(11):1215–1223. doi: 10.1016/S1470-2045(14)70408-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Köhler TS, Kondapalli LA, Shah A, Chan S, Woodruff TK, Brannigan RE. Results from the survey for preservation of adolescent reproduction (SPARE) study: Gender disparity in delivery of fertility preservation message to adolescents with cancer. J Assist Reprod Genet. 2011;28(3):269–277. doi: 10.1007/s10815-010-9504-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neal MS, Nagel K, Duckworth J, et al. Effectiveness of sperm banking in adolescents and young adults with cancer: A regional experience. Cancer. 2007;110(5):1125–1129. doi: 10.1002/cncr.22889. [DOI] [PubMed] [Google Scholar]
- 7.Klosky JL, Randolph ME, Navid F, et al. Sperm cryopreservation practices among adolescent cancer patients at risk for infertility. Pediatr Hematol Oncol. 2009;26(4):252–260. doi: 10.1080/08880010902901294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diesch T, von der Weid, Nicolas Xavier, Szinnai G, Schaedelin S, De Geyter C, Rovó A. Fertility preservation in pediatric and adolescent cancer patients in Switzerland: A qualitative cross-sectional survey. Cancer Epidemiology. 2016;44:141–146. doi: 10.1016/j.canep.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 9.Klosky JL, Simmons JL, Russell KM, et al. Fertility as a priority among at-risk adolescent males newly diagnosed with cancer and their parents. Support Care Cancer. 2015;23(2):333–341. doi: 10.1007/s00520-014-2366-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frederick NN, Recklitis CJ, Blackmon JE, Bober S. Sexual dysfunction in young adult survivors of childhood cance. Peds Blood & Cancer. 2016;63(9):1622–1628. doi: 10.1002/pbc.26041. [DOI] [PubMed] [Google Scholar]
- 11.Green D, Galvin H, Horne B. The psycho-social impact of infertility on young male cancer survivors: A qualitative investigation. Psycho-oncology. 2003;12(2):141–152. doi: 10.1002/pon.622. [DOI] [PubMed] [Google Scholar]
- 12.Thompson AL, Long KA, Marsland AL. Impact of childhood cancer on emerging adult survivors' romantic relationships: A qualitative account. J Sex Med. 2012;10(S1):65–73. doi: 10.1111/j.1743-6109.2012.02950.x. [DOI] [PubMed] [Google Scholar]
- 13.Zebrack BJ, Casillas J, Nohr L, Adams H, Zeltzer LK. Fertility issues for young adult survivors of childhood cancer. Psycho-Oncology. 2004;13(10):689–699. doi: 10.1002/pon.784. [DOI] [PubMed] [Google Scholar]
- 14.Crawshaw M, Sloper P. ‘Swimming against the tide’–the influence of fertility matters on the transition to adulthood or survivorship following adolescent cancer. Eur J Cancer Care. 2010;19(5):610–620. doi: 10.1111/j.1365-2354.2009.01118.x. [DOI] [PubMed] [Google Scholar]
- 15.Lehmann V, Grönqvist H, Engvall G, et al. Negative and positive consequences of adolescent cancer 10 years after diagnosis: An interview-based longitudinal study in sweden. Psycho-Oncol. 2014;23(11):1229–1235. doi: 10.1002/pon.3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edge B, Holmes D, Makin G. Sperm banking in adolescent cancer patients. Arch Dis Child. 2006;91(2):149–152. doi: 10.1136/adc.2005.075242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stein DM, Victorson DE, Choy JT, et al. Fertility preservation preferences and perspectives among adult male survivors of pediatric cancer and their parents. J AYA Onc. 2014;3(2):75–82. doi: 10.1089/jayao.2014.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crawshaw MA, Glaser AW, Hale JP, Sloper P. Male and female experiences of having fertility matters raised alongside a cancer diagnosis during the teenage and young adult years. Eur J Cancer Care. 2009;18(4):381–390. doi: 10.1111/j.1365-2354.2008.01003.x. [DOI] [PubMed] [Google Scholar]
- 19.Saito K, Suzuki K, Iwasaki A, Yumura Y, Kubota Y. Sperm cryopreservation before cancer chemotherapy helps in the emotional battle against cancer. Cancer. 2005;104(3):521–524. doi: 10.1002/cncr.21185. [DOI] [PubMed] [Google Scholar]
- 20.Leonard M, Hammelef K, Smith GD. Fertility considerations, counseling, and semen cryopreservation for males prior to the initiation of cancer therapy. Clin J Oncol Nurs. 2004;8(2):127–31. doi: 10.1188/04.CJON.127-131. [DOI] [PubMed] [Google Scholar]
- 21.Agarwal A, Ong C, Durairajanayagam D. Contemporary and future insights into fertility preservation in male cancer patients. Trans Andrology Urology. 2014;3(1):27–40. doi: 10.3978/j.issn.2223-4683.2014.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention (CDC) [Accessed December, 6, 2016];The 2009 state and local youth risk behavior survey. ftp://ftp.cdc.gov/pub/data/yrbs/2009/2009_hs_questionnaire.pdf. Updated 2015.
- 23.Epstein NB, Baldwin LM, Bishop DS. The McMaster family assessment device. J Marital Fam Ther. 1983;9(2):171–180. [Google Scholar]
- 24.Schover LR, Brey K, Lichtin A, Lipshultz LI, Jeha S. Knowledge and experience regarding cancer, infertility, and sperm banking in younger male survivors. J Clin Oncol. 2002;20(7):1880–1889. doi: 10.1200/JCO.2002.07.175. [DOI] [PubMed] [Google Scholar]
- 25.Watt LD. Pregnancy prevention in primary care for adolescent males. J Peds Health Care. 2001;15(5):223–228. doi: 10.1067/mph.2001.113088. [DOI] [PubMed] [Google Scholar]
- 26.Booth RE, Zhang Y, Kwiatkowski CF. The challenge of changing drug and sex risk behaviors of runaway and homeless adolescents. Child Abuse Negl. 1999;23(12):1295–1306. doi: 10.1016/s0145-2134(99)00090-3. [DOI] [PubMed] [Google Scholar]
- 27.Lollis CM, Johnson EH, Antoni MH. The efficacy of the health belief model for predicting condom usage and risky sexual practices in university students. AIDS Educ Prev. 1997;9(6):551–563. [PubMed] [Google Scholar]
- 28.Janz NK, Becker MH. The health belief model: A decade later. Health Education & Behavior. 1984;11(1):1–47. doi: 10.1177/109019818401100101. [DOI] [PubMed] [Google Scholar]
- 29.Derogatis LR, Unger R. Symptom checklist-90-revised. In: Weiner IB, editor. The corsini encyclopedia of psychology. Hoboken, NJ: John Wiley & Sons Inc; 2010. pp. 2447–2450. [Google Scholar]
- 30.Van Buuren S. Flexible imputation of missing data. Boca Raton, FL: Chapman & Hall/CRC Press; 2012. [Google Scholar]
- 31.Schafer JL. Analysis of incomplete multivariate data. New York: Chapman & Hall/CRC press; 1997. [Google Scholar]
- 32.Zou H, Hastie T. Regularization and variable selection via the elastic net. J R Stats Soc Ser. 2005;67(2):301–320. [Google Scholar]
- 33.Hastie T, Tibshirani R, Wainwright M. Statistical learning with sparsity. Boca Raton, FL: Chapman & Hall/CRC Press; 2015. [Google Scholar]
- 34.Geue K, Richter D, Schmidt R, et al. The desire for children and fertility issues among young German cancer survivors. J Adolesc Health. 2014;54(5):527–535. doi: 10.1016/j.jadohealth.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 35.Quinn GP, Murphy D, Knapp C, et al. Who decides? decision making and fertility preservation in teens with cancer: A review of the literature. Journal of Adolescent Health. 2011;49(4):337–346. doi: 10.1016/j.jadohealth.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bastings L, Baysal Ö, Beerendonk C, et al. Deciding about fertility preservation after specialist counselling. Hum Reprod. 2014;29(8):1721–1729. doi: 10.1093/humrep/deu136. [DOI] [PubMed] [Google Scholar]
- 37.Peddie V, Porter MA, Barbour R, et al. Factors affecting decision making about fertility preservation after cancer diagnosis: A qualitative study. BJOG. 2012;119(9):1049–1057. doi: 10.1111/j.1471-0528.2012.03368.x. [DOI] [PubMed] [Google Scholar]
- 38.Anderson RA, Weddell A, Spoudeas HA, et al. Do doctors discuss fertility issues before they treat young patients with cancer? Hum Reprod. 2008;23(10):2246–2251. doi: 10.1093/humrep/den252. [DOI] [PubMed] [Google Scholar]
- 39.Fuchs AB, Brannigan RE. Disparities in adolescent patient–provider communication regarding fertility preservation care. In: Woodruff TK, Clayman ML, Waimey KE, editors. Oncofertility communication. New York: Springer; 2014. pp. 111–120. [Google Scholar]
- 40.Nielsen CT, Skakkebaek NE, Richardson DW, et al. Onset of the release of spermatozoa (spermarche) in boys in relation to age, testicular growth, pubic hair and height. J Clin Endocrinol Metab. 1986;62(3):532–535. doi: 10.1210/jcem-62-3-532. [DOI] [PubMed] [Google Scholar]
- 41.Loren AW, Mangu PB, Beck LN, et al. Fertility preservation for patients with cancer: American society of clinical oncology clinical practice guideline update. J Clin Oncol. 2013;31(19):2500–2510. doi: 10.1200/JCO.2013.49.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.National Comprehensive Cancer Network. [Accessed May 9, 2017];Clinical practice guidelines in oncology – adolescent and young adult (AYA) oncology, version 2. www.aphon.org/education/files/guideline_aya.pdf. Updated 2014.
- 43.Fallat ME, Hutter J American Academy of Pediatrics Committee on Bioethics, American Academy of Pediatrics Section on Hematology/Oncology, American Academy of Pediatrics Section on Surgery. Preservation of fertility in pediatric and adolescent patients with cancer. Pediatrics. 2008;121(5):e1461–9. doi: 10.1542/peds.2008-0593. [DOI] [PubMed] [Google Scholar]