Abstract
Background
Assessing trends in breast cancer survival among young women who are largely unaffected by breast cancer screening will provide important information regarding improvements in the effectiveness of cancer care for breast cancer in the last few decades.
Methods
The study cohort consisted of women diagnosed with breast cancer between 20 and 39 years of age from the SEER Program’s 9 registry areas from 1975 to 2015. We assessed trends in breast cancer incidence rate and survival among young women.
Results
Among women 20–39 years old, breast cancer incidence increased from 24.6 per 100,000 in 1975 to 31.7 per 100,000 in 2015 (APC 0.5, 95% CI 0.4–0.6). Among female breast cancer patients, 5-year breast-cancer-specific survival increased significantly from 74.0% in 1975–1979 to 88.5% in 2010–2015 (hazard ratio for dying from breast cancer, 2010–2015 vs 1975–1979 0.37, 95% CI 0.32–0.41). The increase in cancer-specific survival reached a plateau in 2005, but in metastatic breast cancer, it continued to increase after 2005, from 45.6% in 2005–2009 to 56.5% in 2010–2015 (hazard ratio for dying from breast cancer, 2010–2015 vs 2005–2009 0.74, 95% CI 0.60–0.92). Similar patterns were also observed for 5-year overall survival and among women 20–29 and 30–39 years old.
Conclusions
There were substantial improvements in the effectiveness of breast cancer treatment on overall and cancer-specific survival from 1975–2015. However, improvements appeared to have reached a plateau after 2005, except for metastatic breast cancer in which survival continued to improve throughout the 1975–2015 period.
Keywords: Breast cancer, ductal carcinoma in situ (DCIS), incidence, survival
Introduction
Breast cancer is the most frequently diagnosed cancer, excluding skin cancers, and the second leading cause of cancer death, after lung cancer, among US women.1, 2 Advances in systemic therapy for breast cancer have greatly improved patient survival rates.3 However, quantifying the effectiveness of these advances on cancer survival rates at the population level is challenging because mammography screening also contributes to survival improvement.4, 5
Collaborative model studies of breast cancer screening strategies suggest that compared with no screening, annual screening in women 50–74 years old can reduce one third of breast cancer mortality and equals approximately 100 quality-adjusted life years (QALYs) gained per 1,000 women screened.6–8 Biennial screening results in less benefit in terms of mortality reduction, but also fewer false-positive results, compared with the annual mammography in women aged 50 to 74 years. Screened and unscreened women both experience reductions in breast cancer mortality9–11 implying that other factors (eg, cancer treatment) are also responsible for the reduced mortality. Welch et al evaluated the relative contributions of breast cancer screening and improved cancer therapy to the reduction in breast cancer mortality among women 40 years and older. They concluded that more screening tends to overdiagnose breast tumors instead of providing early detection and that most of the mortality reduction can be ascribed to improved cancer therapy.3
National guidelines for breast cancer screening with mammography usually recommend starting no earlier than 40 years of age for women with an average risk of developing breast cancer.12–16 For women at high risk of developing breast cancer, mammography screening may start at age 30, or MRI screening at age 25.17, 18 As breast cancer screening is sparse in young women <40 years of age, improvements in breast cancer survival in this population are mostly attributable to advances in breast cancer treatment. In this study, we used data from the Surveillance, Epidemiology, and End Results (SEER) Program’s 9 registry areas 1975–2015 to assess the trends in breast cancer incidence rates among young women 20–39 years old, and to evaluate the improvements in breast cancer survival among female patients diagnosed between 20 and 39 years old.
Methods
The study cohort consisted of female breast cancer patients who were diagnosed between the ages of 20 and 39 years from SEER’s 9 registry areas—including California (San Francisco and Oakland), Connecticut, Georgia (Atlanta only), Hawaii, Iowa, Michigan (Detroit only), New Mexico, Utah, and Washington (Seattle and Puget Sound region)—which cover approximately 10% of the US population.19 Details of the SEER program have been published elsewhere.20, 21 We excluded data of the first two years (SEER 1973–1974) which are generally not considered reliable, and used the most recent SEER data (1975–2015) for analyses. This study was not considered human subjects research by the Institutional Review Board at The University of Texas Medical Branch, Galveston, TX.
The SEER registry collected demographic and cancer diagnosis information. Data about breast cancer deaths were derived from information recorded in death certificates and ascertained from the US Centers for Disease Control and Prevention (CDC)'s National Center for Health Statistics (NCHS). Follow-up was through December 2015. Demographic characteristics of cancer patients were originally extracted from medical records and submitted to cancer registries. The demographic characteristics used for this analysis included sex, race/ethnicity, and age at diagnosis. Age at death was obtained from death certificates. Race was categorized as white, black, Asian/Pacific Islander, and other, and ethnicity was classified as Hispanic or non-Hispanic. Hispanic ethnicity for all cancer cases was identified by the North American Association of Central Cancer Registries (NAACCR) Hispanic/Latino Identification Algorithm (NHIA) 22.
SEER Historic Stage A was used to classify invasive breast cancer cases according to stage at diagnosis: localized (confined to breast tissue and fat including nipple and/or areola); regional (direct extension or ipsilateral regional lymph nodes involved); metastatic (distant lymph nodes involved, further contiguous extension, or metastasis); and unknown stage.23 Ductal carcinoma in situ (DCIS) was identified using ICD-O-3 codes (8201/2, 8230/2, 8401/2, 8500/2, 8501/2, 8503/2, 8504/2, 8507/2, 8521/2, 8522/2, and 8523/2).
Statistical Analysis
Breast cancer incidence rates were calculated as cases per 100,000 persons and age-adjusted to the 2000 US standard population using the SEER*Stat statistical software package (version 8.3.5). Confidence intervals were calculated using the Tiwari method.24 Joinpoint regression models25 were fitted based on annual incidence data using the National Cancer Institute’s Joinpoint Regression Analysis program, version 4.6.0. This analysis program selected the best-fitting log-linear regression model to identify the conjunctures (calendar years) when annual percentage changes (APC) changed significantly, allowing for the minimum number of joinpoints necessary to fit the data. APC was calculated as (exp[β]-1)*100, where the regression coefficient (β) was estimated by fitting a least-squares regression line to the natural logarithm of the rates, using the calendar year as a regressor variable. Tests of the statistical significance of APC and differences between APCs for 2 time periods were based on methods proposed by Kleinbaum and performed in the SEER*Stat software.26
We excluded cases identified only from autopsy records or death certificates for the assessment of breast cancer survival. Only microscopically confirmed primary breast cancer cases were selected. Five-year survival probability (overall or cause-specific) was calculated using 60 monthly intervals in SEER*Stat software. Relative survival of breast cancer cases in the absence of other causes of death was calculated using survival life tables as the ratio of the proportion of observed survivors (all causes of death) in a cohort of cancer patients to the proportion of expected survivors in a comparable cohort of cancer-free individuals. SEER*Stat software used expected life tables instead of a cohort of cancer-free individuals, assuming that the cancer deaths were a negligible proportion of all deaths. Individuals who died of causes other than breast cancer were considered to be censored when we estimated breast-cancer-specific survival. Cox proportional hazard models were fitted to compare differences in 5-year survival probability across time by stage at diagnosis, controlling for age at diagnosis, and race/ethnicity. Hazard ratios (HRs) and 95% CIs were estimated from the Cox model. Kaplan-Meier curves were plotted to show differences in cumulative probability of death across time. Since high-risk women17, 18 may start breast cancer screening with mammograms at age 30, or even with MRI at age 25,18 we performed sensitivity analyses in the 20–29 age group and the 30–39 age group. Statistical significances were determined as 2-sided P values < .05.
Results
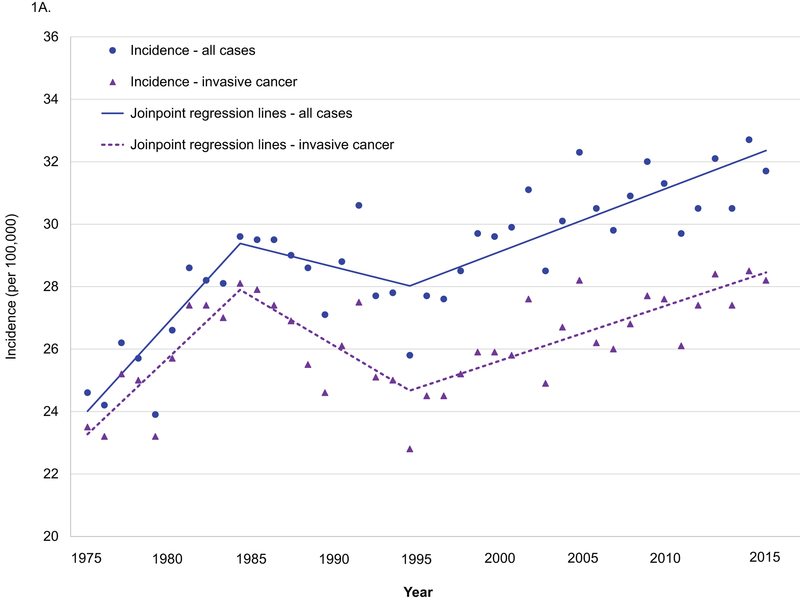
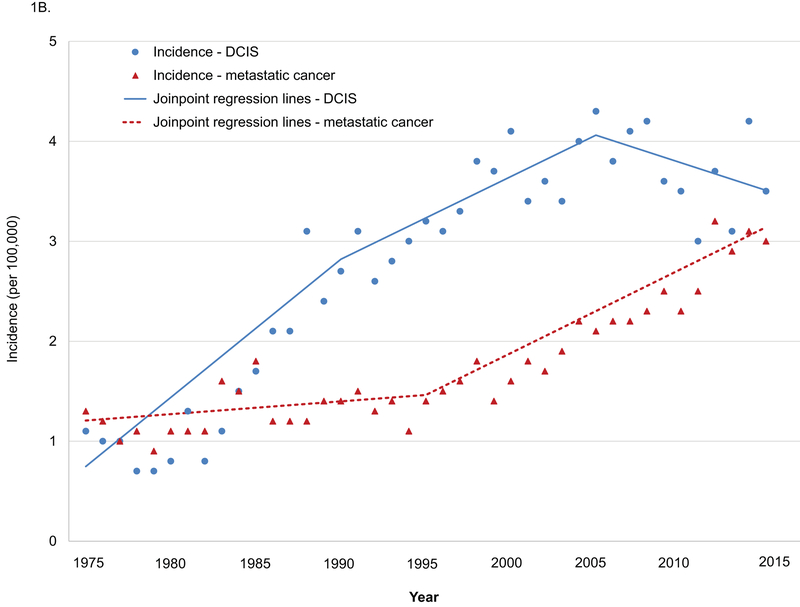
Age-adjusted incidence increased from 24.6 per 100,000 in 1975 to 31.7 per 100,000 in 2015 (APC 0.5, 95% CI 0.4–0.6) among women 20–39 years old, and joinpoint regression analyses revealed that APCs changed significantly in 1984 and 1994 (1975–1984 APC 2.3, p<0.001; 1984–1994 APC −0.5, p=0.30; 1994–2015 APC 0.7, p<0.001. 1975–1984 vs. 1984–1994 p<0.001; 1984–1994 vs. 1994–2015 p=0.02. Figure 1A). APCs for age-adjusted incidence in invasive breast cancer changed significantly in 1984 and 1994 (1975–1984 APC 2.0, p<0.001; 1984–1994 APC −1.2, p=0.008; 1994–2015 APC 0.7, p<0.001. 1975–1984 vs. 1984–1994 p<0.001; 1984–1994 vs. 1994–2015 p<0.001). Age-adjusted incidence in DCIS increased from 1.1 per 100,000 in 1975 to 3.5 per 100,000 in 2015 (APC 2.8, 95% CI 2.0–3.6, Figure 1B), and APCs changed significantly in 1990 and 2005 (1975–1990 APC 9.3, p<0.001; 1990–2005 APC 2.5, p=0.004; 2005–2015 APC −1.4, p=0.21. 1975–1990 vs. 1990–2005 p<0.001; 1990–2005 vs. 2005–2015 p=0.008). Age-adjusted incidence in metastatic cancer increased from 1.3 per 100,000 in 1975 to 3.0 per 100,000 in 2015 (APC 2.8, 95% CI 2.4–3.2), and APCs changed significantly in 1995 (1975–1985 APC 0.9, p=0.10; 1995–2015 APC 4.1, p<0.001. P value for the difference <0.001).
Figure 1.
Age-adjusted incidence in breast cancer among women 20–39 years old, SEER 1975–2015.
A. All cases and invasive breast cancer. B. DCIS and metastatic cancer.
DCIS: ductal carcinoma in situ.
All cases: include invasive breast cancer and DCIS.
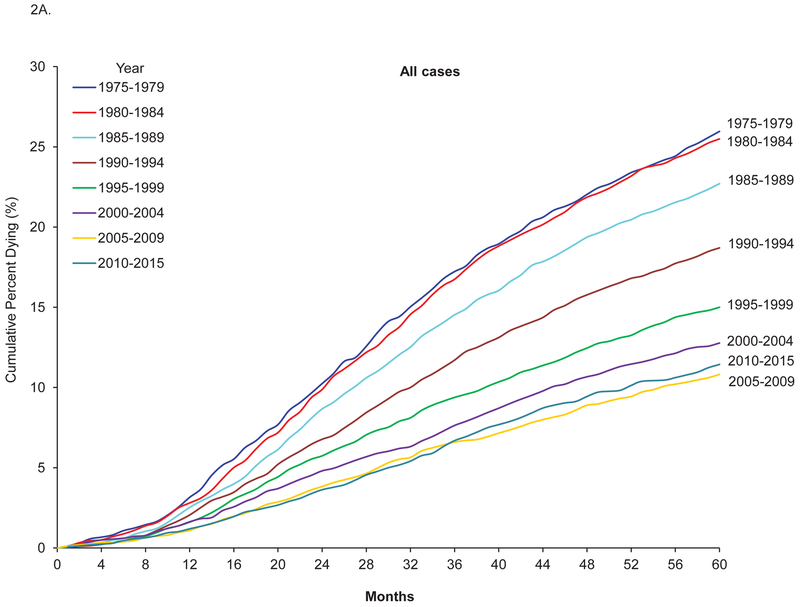
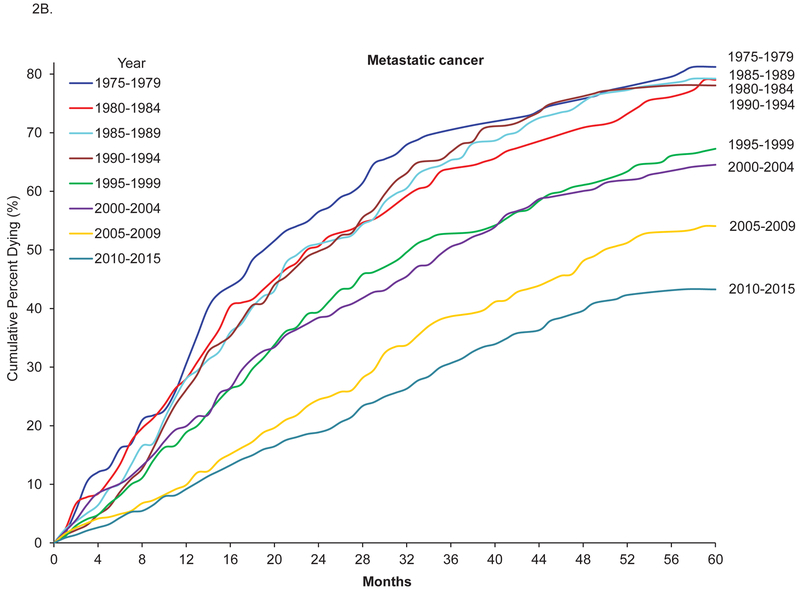
From 1975–2015, there were 39,129 cases of breast tumors with valid follow up information on mortality among women aged 20–39 years. The 5-year survival increased from 72.7% between 1975 and 1979 to 87.4% between 2010 and 2015, and the corresponding 5-year breast-cancer-specific survival and 5-year relative survival increased from 74.0% to 88.5% and from 73.1% to 87.9%, respectively (Table 1). The increase in cancer-specific survival for all breast cancer reached a plateau in 2005, but in metastatic breast cancer, it continued to increase after 2005, from 45.6% in 2005–2009 to 56.5% in 2010–2015. Figure 2 shows Kaplan-Meier curves of cumulative probability of death from breast cancer across time among breast cancer patients 20–39 years of age. The adjusted HR for breast cancer-related death among patients diagnosed between 2010 and 2015 vs patients diagnosed between 1975 and 1979 was 0.37, 95% confidence interval (CI) 0.32–0.41 (Table 2). The decrease in risk for dying from breast cancer reached a plateau between 2005 and 2009, but in metastatic breast cancer, the decrease continued after 2005–2009 (Figure 2B; HR for 2010–2015 vs 2005–2009 0.74, 95% CI 0.60–0.92). Similar patterns were also observed for 5-year overall survival (Supplemental Table 1).
Table 1.
Five-year survival among female breast cancer patients 20–39 years old by stage, SEER 1975–2015 (N=39,129).
| 5-year survival % (95% CI) | |||
|---|---|---|---|
| Overall | Cancer-specific | Relative survival | |
| All breast cancer cases (N=39,129) | |||
| 1975–1979 | 72.7(71.0–74.2) | 74.0(72.3–75.5) | 73.1(71.5–74.7) |
| 1980–1984 | 73.4(72.0–74.7) | 74.4(73.1–75.8) | 73.8(72.4–75.2) |
| 1985–1989 | 76.2(75.0–77.4) | 77.2(76.0–78.4) | 76.7(75.4–77.9) |
| 1990–1994 | 80.4(79.3–81.5) | 81.3(80.2–82.3) | 80.9(79.8–82.0) |
| 1995–1999 | 84.1(83.0–85.0) | 85.0(84.0–85.9) | 84.6(83.5–85.5) |
| 2000–2004 | 86.3(85.3–87.2) | 87.2(86.3–88.1) | 86.8(85.8–87.7) |
| 2005–2009 | 88.3(87.4–89.2) | 89.2(88.3–90.0) | 88.8(87.9–89.6) |
| 2010–2015 | 87.4(86.1–88.6) | 88.5(87.3–89.7) | 87.9(86.6–89.1) |
| Among women with localized breast cancer (n=17,718) | |||
| 1975–1979 | 84.5(82.6–86.2) | 85.4(83.5–87.1) | 85.0(83.1–86.8) |
| 1980–1984 | 85.2(83.5–86.7) | 86.2(84.6–87.7) | 85.7(84.0–87.2) |
| 1985–1989 | 88.0(86.6–89.3) | 89.1(87.7–90.3) | 88.6(87.1–89.8) |
| 1990–1994 | 90.3(89.0–91.4) | 90.8(89.6–91.9) | 90.8(89.5–91.9) |
| 1995–1999 | 92.3(91.1–93.3) | 93.2(92.1–94.1) | 92.8(91.7–93.8) |
| 2000–2004 | 93.9(92.8–94.8) | 94.8(93.8–95.6) | 94.5(93.4–95.4) |
| 2005–2009 | 95.5(94.5–96.3) | 96.1(95.2–96.8) | 96.0(95.0–96.8) |
| 2010–2015 | 94.3(92.6–95.6) | 95.4(93.8–96.6) | 94.8(93.1–96.1) |
| Among women with regional breast cancer (n=15,520) | |||
| 1975–1979 | 62.4(59.7–65.0) | 63.8(61.1–66.4) | 62.8(60.1–65.4) |
| 1980–1984 | 63.8(61.5–66.0) | 64.8(62.5–67.0) | 64.2(61.9–66.4) |
| 1985–1989 | 64.6(62.5–66.7) | 65.7(63.5–67.8) | 65.0(62.8–67.1) |
| 1990–1994 | 70.9(68.9–72.9) | 72.1(70.0–74.0) | 71.3(69.3–73.3) |
| 1995–1999 | 76.4(74.4–78.2) | 77.3(75.4–79.1) | 76.8(74.9–78.7) |
| 2000–2004 | 82.2(80.5–83.8) | 83.1(81.4–84.6) | 82.7(81.0–84.3) |
| 2005–2009 | 85.5(83.9–87.0) | 86.5(84.8–87.9) | 86.0(84.3–87.5) |
| 2010–2015 | 84.7(82.3–86.8) | 85.6(83.3–87.7) | 85.2(82.7–87.3) |
| Among women with metastatic breast cancer (n=2,320) | |||
| 1975–1979 | 17.3(11.4–24.2) | 18.0(11.8–25.3) | 17.4(11.5–24.3) |
| 1980–1984 | 19.9(14.5–26.0) | 20.3(14.7–26.6) | 20.1(14.6–26.2) |
| 1985–1989 | 19.9(14.8–25.4) | 20.1(15.0–25.7) | 20.0(14.9–25.6) |
| 1990–1994 | 21.2(16.2–26.7) | 21.2(16.1–26.8) | 21.3(16.3–26.8) |
| 1995–1999 | 31.2(25.7–36.7) | 32.2(26.7–37.9) | 31.4(25.9–37.0) |
| 2000–2004 | 33.9(28.6–39.2) | 35.0(29.6–40.5) | 34.1(28.8–39.4) |
| 2005–2009 | 43.6(38.5–48.5) | 45.6(40.4–50.6) | 43.8(38.7–48.8) |
| 2010–2015 | 54.2(47.9–60.0) | 56.5(50.1–62.4) | 54.4(48.1–60.3) |
Overall survival was defined as the probability of surviving all-causes of death.
Cancer-specific survival was defined as survival of death caused by breast cancer in the absence of other causes of death, and was calculated by specifying death caused by breast cancer and individuals who died of causes other than breast cancer were considered to be censored.
Relative survival of breast cancer cases in the absence of other causes of death was calculated using survival life tables as the ratio of the proportion of observed survivors (all causes of death) in a cohort of cancer patients to the proportion of expected survivors in a comparable cohort of cancer-free individuals.
Figure 2.
Five-year cumulative probability of death from breast cancer among breast cancer patients 20–39 years old, SEER 1975–2015.
A: All cases. B. Metastatic breast cancer.
DCIS: Ductal carcinoma in situ.
All cases: include invasive breast cancer and DCIS.
Cumulative percent dying from breast cancer = 1 - cancer-specific survival.
Table 2.
Adjusted hazard ratio for dying from breast cancer in 5 years among female breast cancer patients 20–39 years old by stage, SEER 1975–2015 (N=38,776).
| Adjusted hazard ratio (95% Confidence Interval) | |||||
|---|---|---|---|---|---|
| All | Localized | Regional | Metastatic | Invasive Breast Cancer | |
| Sample Size | 38,776 | 17,586 | 15,354 | 2,281 | 35,221 |
| 1975–1979 | Reference | Reference | Reference | Reference | Reference |
| 1980–1984 | 0.98(0.90–1.08) | 0.94(0.79–1.13) | 0.98(0.86–1.10) | 0.89(0.68–1.16) | 0.99(0.90–1.09) |
| 1985–1989 | 0.86(0.79–0.95) | 0.74(0.61–0.88) | 0.94(0.83–1.06) | 0.88(0.68–1.14) | 0.91(0.83–0.99) |
| 1990–1994 | 0.69(0.63–0.76) | 0.61(0.50–0.73) | 0.72(0.64–0.82) | 0.87(0.67–1.13) | 0.74(0.67–0.81) |
| 1995–1999 | 0.54(0.49–0.60) | 0.44(0.36–0.54) | 0.57(0.50–0.65) | 0.60(0.46–0.78) | 0.59(0.53–0.65) |
| 2000–2004 | 0.45(0.40–0.49) | 0.33(0.26–0.42) | 0.40(0.35–0.46) | 0.56(0.44–0.73) | 0.49(0.44–0.54) |
| 2005–2009 | 0.37(0.33–0.41) | 0.24(0.19–0.31) | 0.31(0.27–0.36) | 0.38(0.30–0.49) | 0.40(0.36–0.45) |
| 2010–2015 | 0.37(0.32–0.41) | 0.26(0.19–0.35) | 0.31(0.26–0.37) | 0.29(0.22–0.37) | 0.39(0.35–0.44) |
Adjusted hazard ratio: hazard ratio for dying from breast cancer in 5 years, controlling for age at diagnosis and race.
Sensitivity analyses
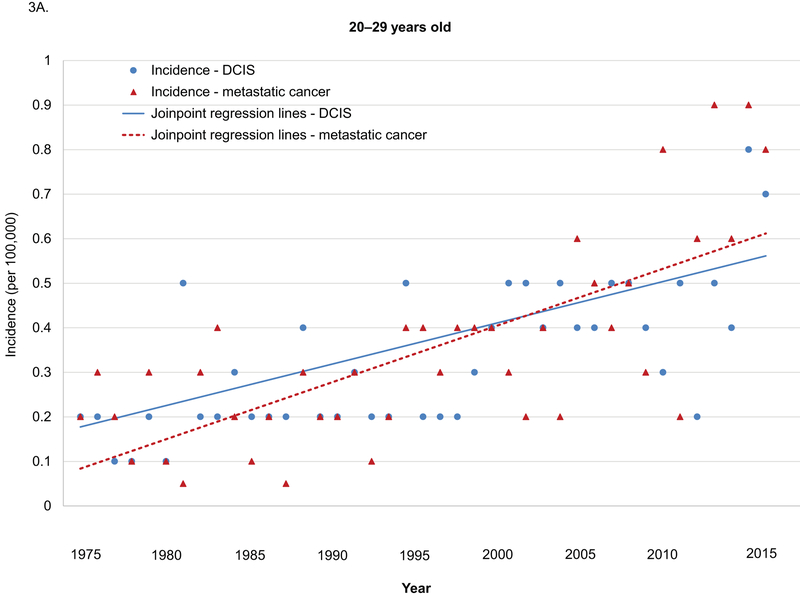
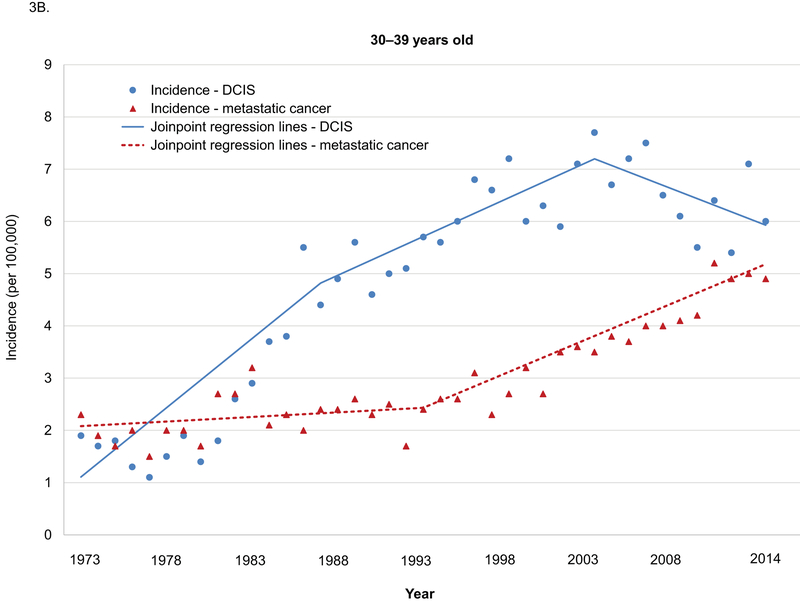
Sensitivity analyses in the age group of 20–29 years and 30–39 years yielded similar results (Supplemental Table 1–4). Among women 20–29 years old, age-adjusted incidences in metastatic breast cancer were low, but increased significantly from 1975–2015 (APC 3.8, 95% CI 2.7–4.9). No significant changes in the trends in incidence in both DCIS and metastatic cancer were observed during this period (Figure 3A). Among women 30–39 years old, the increasing trend for adjusted incidences in DCIS changed significantly in 1989 and 2005, and the APCs for age-adjusted incidences in metastatic breast cancer increased significantly in 2005 (Figure 3B).
Figure 3.
Age-adjusted incidence in DCIS and metastatic breast cancer among women 20–39 years old by age groups, SEER 1975–2015.
DCIS: ductal carcinoma in situ.
A. 20–29 years old. B. 30–39 years old.
Annual percentage changes (APC) and p values:
Among women 20–29 years old
DCIS: 1975–2015 APC 2.8 p<0.001
Metastatic: 1975–2015 APC 3.8 p<0.001
Among women 30–39 years old
DCIS: 1975–1989 APC 11.1, p<0.001; 1989–2005 APC 2.5, p=0.001; 2005–2015 APC −1.9, p=0.14. 1975–1989 vs. 1989–2005 p<0.001. 1989–2005 vs. 2005–2015 p=0.005)
Metastatic: 1975–1995 APC 0.8, p=0.22; 1995–2015 APC 3.9, p<0.001. P value for the difference <0.001
Discussion
Using data from SEER, we systematically compared trends in breast cancer mortality by tumor characteristics at diagnosis among young women between 20–39 years old. The main findings were the significant increase in breast cancer incidence and improved survival in cancer patients in the last 4 decades. Improvement in survival reached a plateau most recently, but among patients with metastatic cancer, survival continued to improve throughout the 1975–2015 period. The significant reduction in the probability of dying from breast cancer among young women are most likely attributed to advances in breast cancer therapy as these populations were typically not screened.3, 27
In the past few decades, treatment for breast cancer patients has undergone substantial improvement. Better-tolerated therapies have been replacing ablative surgery and aggressive chemotherapy.28 Tamoxifen or other hormonal therapies, cytotoxic,29 and targeted therapies28 shown to significantly reduce breast cancer recurrence and mortality and to improve quality of life for breast cancer patients. The introduction of taxanes in the mid-1990s greatly improved cancer survivals for patients with both early and advanced tumors.30, 31 Human epithelial growth factor receptor 2 (HER2)-directed therapies, such as the classical anti-HER2 antibody trastuzumab, have become available since late 1990s for the treatment of HER-2+ advanced breast cancer and significantly reduced cancer death in those patients.32, 33 Improved breast cancer care through multidisciplinary medical care also contributes significantly to the improvement in survival among breast cancer patients.34 In our study, we found that among young female patients breast cancer mortality has decreased by 60%−70% in the last 4 decades for each stage of breast cancer. Although our study showed improvements of cancer survival among female breast cancer patients who were diagnosed within the age range that was younger than the screening initiation age recommended by most guidelines, especially in breast cancer patients diagnosed at 20–29 years of age, we cannot definitively conclude these improvements are all attributable to improved cancer treatments. Advances in breast cancer treatments and cancer care are likely to be the major contributors. Other factors, such as dynamic changes in breast cancer risk factors (eg, breastfeeding, age at menarche, parity, and oral contraceptive use), lifestyle modifications (diet, alcohol consummation, smoking, exercise, etc.), and biological characteristics of the cancers, may also contribute to the improvements in breast cancer survival.35–39 In general, our findings demonstrate similar degree of survival improvement in women <40 years of age with invasive breast cancer to that in older women. In the screened population (women ≥40 years old), nearly all of the mortality reduction is also due to treatment advances and not screening.3 Thus, these observations may have direct implications for mammogram screening of breast cancer.
One interesting finding of this study is that cancer survival improvements reached a plateau but continued to increase among women with metastatic breast cancer in recent years. These trends may reflect no major changes in adjuvant standard of care (for adjuvant chemotherapy or hormonal therapy) in the 2010–2015 years.40 In contrast, there have been continued advances in the treatment of metastatic breast cancer (pertuzumab,41 everolimus,42, 43 and T-DM144 in recent years), which is why we found bigger changes in patients with metastatic disease, then less so with regional, then even less with localized. Stage migration may also contribute to the improved survival in patients with metastatic cancer. As lower stages usually have better survival, cancer survival will be artificially elevated in the category of metastatic tumor when stage migration occurs. Patients in lower stages (e.g., regional) could be placed in the stage of metastatic tumor because of improved diagnostic imaging, increasing use of imaging studies for staging, and changes in staging classification. Routine use of sentinel node biopsy, however, may lower the stage classification compared to radical node dissection. Johnson et al assessed incidence of metastatic breast cancer among young women in the US, and concluded that the increasing trend in cancer incidence is unlikely due to stage migration but stage migration may have some small contribution to the increasing trend after 1995.45 Therefore, the contribution of stage migration to improved survival in patients with metastatic cancer may be minimal.
Early detection followed by timely treatment can effectively improve survival for female breast cancer patients. Among young women 20–39 years old, breast cancer screening with mammography is not recommended for average-risk women, and only women at high-risk for developing breast cancer may start mammogram screening at an early age.17, 18 Those high-risk women include women with a history of chest irradiation between the ages of 10 and 30, a known genetic predisposition for breast cancer, or a strong family history of the disease. Young breast cancer patients are more likely to carry harmful mutations of BRCA1, BRCA2, or other cancer-related genes.46 However, those mutations may not negatively affect survival in breast cancer patients.47, 48 Identifying mutation carriers before they develop breast cancer offers an opportunity to start screening early or receive prophylactic treatments to prevent breast cancer. Genetic testing in young cancer patients also enables them to receive tailored treatments based on their tumor type and the molecular characteristics.49 Both the Precision Medicine Initiative and Cancer Moonshot place strong emphasis on genetic risks for aggressive breast cancer, cancer prevention, early detection, tailored interventions, and individualized cancer care with the ultimate goals to reduce cancer incidence and improve survival.50, 51
The strength of this study is that we used data from a large cohort of young breast cancer patients. Data on cancer diagnosis date, stage, and patient sociodemographic characteristics enabled us to examine changes in breast cancer incidence rates and survival across subpopulations and over time. Assessing trends in breast cancer survival among young women allowed us to focus on population largely unaffected by screening; thus, estimates from our study will provide important information regarding improvements in the effectiveness of medical care for breast cancer in the last few decades. This study also had several limitations. We used data from young women from SEER’s 9 registry areas, so that our findings may not be applicable to other areas in the US and women of other ages. Additionally, information on mammogram screening was not available in SEER; however, only women at high risk for developing breast cancer were recommended to receive mammography screening in their 30s. We also performed sensitivity analyses in younger patients diagnosed before age 30 and found similar improvements in survival.
In summary, our study reported significant improvements in survival between 1975 and 2015 among young female breast cancer patients. The survival improvement observed in these populations is likely driven by advances in breast cancer treatments, rather than screening. We also found that with the exception of metastatic breast cancer, the improvements in survival reached a plateau between 2005 and 2009.
Supplementary Material
Acknowledgement
Primary Funding Sources: Dr. Guo is currently supported by a research career development award (K12HD052023: Building Interdisciplinary Research Careers in Women’s Health Program-BIRCWH; Berenson, PI) from the Office of Research on Women’s Health (ORWH) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) at the National Institutes of Health. This study was also partly supported by a research grant from the National Cancer Institute (R01 CA207216, Shih, PI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Footnotes
Presentation: Part of the results was presented at the 2017 Building Interdisciplinary Research Careers in Women’s Health (BIRCWH) Meeting. Bethesda, MD. October 25, 2017.
Conflict of Interest Disclosures: The authors report no conflict of interest.
Author Contributions
Each author has provided substantial contributions to warrant authorship. Specific contributions are as follows: Fangjian Guo: Study planning, statistical analysis, drafting and revision of manuscript, full access to data, and responsibility for overall content of manuscript. Yong-fang Kuo: Study planning, data interpretation, and revision of manuscript. Ya Chen Tina Shih: Study planning, data interpretation, and revision of manuscript. Sharon H. Giordano: data interpretation, and revision of manuscript. Abbey B. Berenson: Study planning, data interpretation, and drafting and revision of manuscript.
References
- 1.DeSantis CE, Fedewa SA, Goding Sauer A, Kramer JL, Smith RA, Jemal A. Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. CA Cancer J Clin. 2016;66:31–42. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Breast Cancer Facts & Figures 2015–2016. Atlanta: American Cancer Society, Inc. 2015. http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-046381.pdf. Accessed on June 30, [Google Scholar]
- 3.Welch HG, Prorok PC, O'Malley AJ, Kramer BS. Breast-Cancer Tumor Size, Overdiagnosis, and Mammography Screening Effectiveness. N Engl J Med. 2016;375:1438–1447. [DOI] [PubMed] [Google Scholar]
- 4.Liu PH, Wang JD, Keating NL. Expected years of life lost for six potentially preventable cancers in the United States. Prev Med. 2013;56:309–313. [DOI] [PubMed] [Google Scholar]
- 5.U.S. Preventive Services Task Force. The Guide to Clinical Preventive Services, 2014. Available at: http://www.ahrq.gov/sites/default/files/wysiwyg/professionals/clinicians-providers/guidelines-recommendations/guide/cpsguide.pdf. Accessed August 26, 2016.
- 6.Mandelblatt JS, Stout NK, Schechter CB, et al. Collaborative Modeling of the Benefits and Harms Associated With Different U.S. Breast Cancer Screening Strategies. Ann Intern Med. 2016;164:215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mandelblatt JS, Cronin KA, Bailey S, et al. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Ann Intern Med. 2009;151:738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trentham-Dietz A, Kerlikowske K, Stout NK, et al. Tailoring Breast Cancer Screening Intervals by Breast Density and Risk for Women Aged 50 Years or Older: Collaborative Modeling of Screening Outcomes. Ann Intern Med. 2016;165:700–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson WF, Jatoi I, Devesa SS. Assessing the impact of screening mammography: Breast cancer incidence and mortality rates in Connecticut (1943–2002). Breast Cancer Res Treat. 2006;99:333–340. [DOI] [PubMed] [Google Scholar]
- 10.Tabar L, Yen MF, Vitak B, Chen HH, Smith RA, Duffy SW. Mammography service screening and mortality in breast cancer patients: 20-year follow-up before and after introduction of screening. Lancet. 2003;361:1405–1410. [DOI] [PubMed] [Google Scholar]
- 11.Autier P, Boniol M, La Vecchia C, et al. Disparities in breast cancer mortality trends between 30 European countries: retrospective trend analysis of WHO mortality database. BMJ. 2010;341:c3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.U.S. Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:716–726, W-236. [DOI] [PubMed] [Google Scholar]
- 13.Smith RA, Andrews KS, Brooks D, et al. Cancer screening in the United States, 2017: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2017;67:100–121. [DOI] [PubMed] [Google Scholar]
- 14.Bevers TB, Anderson BO, Bonaccio E, et al. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis. J Natl Compr Canc Netw. 2009;7:1060–1096. [DOI] [PubMed] [Google Scholar]
- 15.American College of Obstetricians-Gynecologists. Practice bulletin no. 122: Breast cancer screening. Obstet Gynecol. 2011;118:372–382. [DOI] [PubMed] [Google Scholar]
- 16.Mainiero MB, Lourenco A, Mahoney MC, et al. ACR Appropriateness Criteria Breast Cancer Screening . J Am Coll Radiol. 2013;10:11–14. [DOI] [PubMed] [Google Scholar]
- 17.Sung JS, Stamler S, Brooks J, et al. Breast Cancers Detected at Screening MR Imaging and Mammography in Patients at High Risk: Method of Detection Reflects Tumor Histopathologic Results. Radiology. 2016;280:716–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Breast Cancer Screening and Diagnosis. Version 1.2017. Fort Washington, PA: National Comprehensive Cancer Network, 2016. https://www.nccn.org/professionals/physician_gls/pdf/breast-screening.pdf . Accessed August 2, 2017. [Google Scholar]
- 19.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 9 Regs Research Data, Nov 2017 Sub (1973–2015) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2016 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2018, based on the November 2017 submission.
- 20.Howlader N, Noone A, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2013, National Cancer Institute; Bethesda, MD, http://seer.cancer.gov/csr/1975_2013/, based on November 2015 SEER data submission, posted to the SEER web site, April 2016. [Google Scholar]
- 21.Hankey BF, Ries LA, Edwards BK. The surveillance, epidemiology, and end results program: a national resource. Cancer Epidemiol Biomarkers Prev. 1999;8:1117–1121. [PubMed] [Google Scholar]
- 22.NAACCR Race and Ethnicity Work Group. NAACCR Guideline for Enhancing Hispanic/Latino Identification: Revised NAACCR Hispanic/Latino Identification Algorithm [NHIA v2.2.1]. Springfield (IL): North American Association of Central Cancer Registries; September 2011. [Google Scholar]
- 23.Young JL Jr, Roffers SD, Ries LAG, Fritz AG, Hurlbut AA (eds). SEER Summary Staging Manual - 2000: Codes and Coding Instructions, National Cancer Institute, NIH Pub. No. 01–4969, Bethesda, MD, 2001. https://seer.cancer.gov/tools/ssm/. Accessed August 1, 2017. [Google Scholar]
- 24.Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res. 2006;15:547–569. [DOI] [PubMed] [Google Scholar]
- 25.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. [DOI] [PubMed] [Google Scholar]
- 26.Kleinbaum Kupper, and Muser. Applied Regression Analysis and Other Multivariable Methods PWS-Kent, Boston, Mass, 2nd edition, 1988. [Google Scholar]
- 27.Toriola AT, Colditz GA. Trends in breast cancer incidence and mortality in the United States: implications for prevention. Breast Cancer Res Treat. 2013;138:665–673. [DOI] [PubMed] [Google Scholar]
- 28.Sainsbury R The development of endocrine therapy for women with breast cancer. Cancer Treat Rev. 2013;39:507–517. [DOI] [PubMed] [Google Scholar]
- 29.Early Breast Cancer Trialists' Collaborative Group. Effects of adjuvant tamoxifen and of cytotoxic therapy on mortality in early breast cancer. An overview of 61 randomized trials among 28,896 women. N Engl J Med. 1988;319:1681–1692. [DOI] [PubMed] [Google Scholar]
- 30.Ghersi D, Wilcken N, Simes RJ. A systematic review of taxane-containing regimens for metastatic breast cancer. Br J Cancer. 2005;93:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nowak AK, Wilcken NR, Stockler MR, Hamilton A, Ghersi D. Systematic review of taxane-containing versus non-taxane-containing regimens for adjuvant and neoadjuvant treatment of early breast cancer. Lancet Oncol. 2004;5:372–380. [DOI] [PubMed] [Google Scholar]
- 32.Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. [DOI] [PubMed] [Google Scholar]
- 33.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. [DOI] [PubMed] [Google Scholar]
- 34.Kalager M, Haldorsen T, Bretthauer M, Hoff G, Thoresen SO, Adami HO. Improved breast cancer survival following introduction of an organized mammography screening program among both screened and unscreened women: a population-based cohort study. Breast Cancer Res. 2009;11:R44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collaborative Group on Hormonal Factors in Breast Cancer. Familial breast cancer: collaborative reanalysis of individual data from 52 epidemiological studies including 58,209 women with breast cancer and 101,986 women without the disease. Lancet. 2001;358:1389–1399. [DOI] [PubMed] [Google Scholar]
- 36.Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50302 women with breast cancer and 96973 women without the disease. Lancet. 2002;360:187–195. [DOI] [PubMed] [Google Scholar]
- 37.Chen WY, Rosner B, Hankinson SE, Colditz GA, Willett WC. Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. JAMA. 2011;306:1884–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.White E Projected changes in breast cancer incidence due to the trend toward delayed childbearing. Am J Public Health. 1987;77:495–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Partridge AH, Hughes ME, Warner ET, et al. Subtype-Dependent Relationship Between Young Age at Diagnosis and Breast Cancer Survival. J Clin Oncol. 2016;34:3308–3314. [DOI] [PubMed] [Google Scholar]
- 40.Gradishar WJ, Anderson BO, Blair SL, et al. Breast cancer version 3.2014. J Natl Compr Canc Netw. 2014;12:542–590. [DOI] [PubMed] [Google Scholar]
- 41.Swain SM, Kim SB, Cortés J, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14:461–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.André F, O'Regan R, Ozguroglu M, et al. Everolimus for women with trastuzumab-resistant, HER2-positive, advanced breast cancer (BOLERO-3): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2014;15:580–591. [DOI] [PubMed] [Google Scholar]
- 43.Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson RH, Chien FL, Bleyer A. Incidence of breast cancer with distant involvement among women in the United States, 1976 to 2009. JAMA. 2013;309:800–805. [DOI] [PubMed] [Google Scholar]
- 46.Greenup R, Buchanan A, Lorizio W, et al. Prevalence of BRCA mutations among women with triple-negative breast cancer (TNBC) in a genetic counseling cohort. Ann Surg Oncol. 2013;20:3254–3258. [DOI] [PubMed] [Google Scholar]
- 47.Wang C, Zhang J, Wang Y, et al. Prevalence of BRCA1 mutations and responses to neoadjuvant chemotherapy among BRCA1 carriers and non-carriers with triple-negative breast cancer. Ann Oncol. 2015;26:523–528. [DOI] [PubMed] [Google Scholar]
- 48.Rennert G, Bisland-Naggan S, Barnett-Griness O, et al. Clinical outcomes of breast cancer in carriers of BRCA1 and BRCA2 mutations. N Engl J Med. 2007;357:115–123. [DOI] [PubMed] [Google Scholar]
- 49.Keenan T, Moy B, Mroz EA, et al. Comparison of the Genomic Landscape Between Primary Breast Cancer in African American Versus White Women and the Association of Racial Differences With Tumor Recurrence. J Clin Oncol. 2015;33:3621–3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harris LN, Ismaila N, McShane LM, et al. Use of Biomarkers to Guide Decisions on Adjuvant Systemic Therapy for Women With Early-Stage Invasive Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34:1134–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hassett MJ, Silver SM, Hughes ME, et al. Adoption of gene expression profile testing and association with use of chemotherapy among women with breast cancer. J Clin Oncol. 2012;30:2218–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.