Abstract
Objectives:
To establish an evidence-based cutoff to differentiate between early and late recurrence and to compare clinicopathologic risk factors between the two groups.
Summary Background Data:
A clear definition of “early recurrence” after pancreatic ductal adenocarcinoma resection is currently lacking.
Methods:
Patients undergoing pancreatectomy for pancreatic ductal adenocarcinoma between 2000 and 2013 were included. Exclusion criteria were neoadjuvant therapy and incomplete follow-up. A minimum P-value approach was used to evaluate the optimal cut-off value of recurrence-free survival to divide the patients into early and late recurrence cohorts based on subsequent prognosis. Potential risk factors for early recurrencewere assessed with logistic regression models.
Results:
Of 957 included patients, 204 (21.3%) were recurrence-free at last follow-up. The optimal length of recurrence-free survival to distinguish between early (n = 388, 51.5%) and late recurrence (n = 365, 48.5%) was 12 months (P< 0.001). Patients with early recurrence had 1-, and 2-year post-recurrence survival rates of 20 and 6% compared with 45 and 22% for the late recurrence group (both P< 0.001). Preoperative risk factors for early recurrence included a Charlson age-comorbidity index ≥4 (OR 1.65), tumor size > 3.0cm on computed tomography (OR 1.53) and CA 19–9 > 210U/mL (OR 2.30). Postoperative risk factors consisted of poor tumor differentiation grade (OR 1.66), microscopic lymphovascular invasion (OR 1.70), a lymph node ratio > 0.2 (OR 2.49), and CA 19–9 > 37U/mL (OR 3.38). Adjuvant chemotherapy (OR 0.28) and chemoradiotherapy (OR 0.29) were associated with a reduced likelihood of early recurrence.
Conclusion:
A recurrence-free interval of 12 months is the optimal threshold for differentiating between early and late recurrence, based on subsequent prognosis.
Keywords: carbohydrate antigen 19–9, early recurrence, pancreatectomy, pancreatic ductal adenocarcinoma, post-recurrence survival, recurrencefree survival
Pancreatic ductal adenocarcinoma (PDAC) is a devastating disease that is projected to become the second most common cause of cancer-related death in the United States by 2030.1 Due to lack of early clinical symptoms and effective screening methods, most patients suffer from locally advanced or metastatic cancer at the time of initial presentation. Consequently, it is estimated that only 20% of newly diagnosed patients are initial candidates for resection without undergoing neoadjuvant treatement.2 Yet, even in this most favorable cohort with resectable PDAC, up to 80% of patients recur after a short recurrence-free interval.3–5 This high rate of recurrence has been attributed to the presence of occult micrometastatic disease at the time of resection and lack of effective systemic therapies.6,7
Although the term “early recurrence” is often utilized in both the academic and clinical setting, a clear definition is currently lacking, with arbitrary cut-off values varying between 6 and 12 months found in the literature.8–11 Primary surgical resection is the standard of care for localized PDAC.2 However, resectable patients susceptible to early recurrence constitute a key cohort worthy of further study, as these selected patients may benefit from a neoadjuvant-first approach.12–15 The goal of this study, therefore, was two-fold: first, to establish an evidence-based cut-off value to differentiate between early and late recurrence based on the difference in prognosis after recurrence, and second, to identify perioperative risk factors for early PDAC recurrence after resection. An evidence-based cut-off value for early recurrence has the potential to aid clinicians with prognostic stratification of post-pancreatectomy patients, while identified risk factors might help guide neoadjuvant and adjuvant treatment decisions.
METHODS
Study Population
Our institutional review board approved of this retrospective study. Patients who underwent pancreatectomy for primary resectable PDAC between 2000 and 2013 were included from a institutional database. Exclusion criteria were grossly positive resection margin (R2), synchronous distant disease at the time of resection, use of neoadjuvant therapy, and 90-day postoperative mortality. Patients with incomplete records due to follow-up done at other institutions, or with less than 24 months of follow-up in which neither recurrence nor death occurred, were also excluded. The primary outcomes of interest were recurrence-free survival (RFS), post-recurrence survival (PRS), and overall survival (OS).
Data Collection and Follow-up
Both pre- and postoperative demographics, clinicopathologic, and treatment variables were extracted from a prospectively maintained institutional database. The preoperative Charlson age-comorbidity index (CACI) was calculated from available data as a measure of frailty and patients were dichotomized using a threshold of 4 points based on recent literature.16–18 Pre- and postoperative carbohydrate antigen (CA) 19–9 values were obtained, when available. CA 19–9 values acquired at time of jaundice (total bilirubin > 5mg/dL) or later than 2 months postoperatively were excluded from analysis. Furthermore, patients who had 3 or more consecutive undetectable CA 19–9 values (<1.0U/mL) were deemed Lewis antigen negative and were also excluded from analysis. The resection margin (R) was defined as R0 when the distance of carcinoma cells to the closest resection margin was >1mm, and R1 when the distance was ≤1mm. After resection, patients were routinely referred to a medical or radiation oncologist for adjuvant treatment recommendations. Adjuvant therapy was stratified into three groups: chemotherapy, chemoradiotherapy (including patients who underwent chemotherapy followed by radiotherapy with or without radio-sensitizing chemotherapy), and no adjuvant therapy.
Our institutional follow-up strategy and definitions for diagnosis of PDAC recurrence have been described previously.5 When imaging findings were consistent with recurrence, biopsy was seldom performed. Magnetic resonance imaging and/or fluorodeoxyglucose positron emission tomography were performed if necessary to clarify ambiguous computed tomography (CT) findings. Recurrence locations were stratified into five mutually exclusive categories: “local only,” “liver only,” “lung only,” “multiple-site,” and “other.” Patients with recurrence and good performance status were generally further treated with systemic therapy or enrolled in experimental clinical trials.
Outcomes and Statistical Analysis
RFS was calculated from the date of pancreatectomy to the date of recurrence or last follow-up if recurrence did not occur. OS was defined as the time from surgery to either death or last follow-up. PRS was defined as the time from first recurrence to either death or last follow-up. Median survival outcomes were estimated with a Kaplan–Meier curve. The log-rank test was performed to compare between subgroups. A minimum P-value approach was used to evaluate the optimal threshold of RFS to divide the patients in an early and late recurrence cohort based on the length of PRS. In this approach, the log-rank test is performed for different lengths of RFS to determine the optimal cut-off point with the lowest P value.
Receiver operating characteristics (ROC) curves were constructed to estimate the optimal threshold for both pre- and postoperative CA 19–9 as a risk factor for early recurrence. The optimal cutoff value was determined to be the point of the ROC curve closest to the upper-left corner of the graph. Associations between potential risk factors and early PDAC recurrence were assessed by univariable logistic regression. Variables with a P value of < 0.10 were included as covariate in two separate multivariable logistic regression models: one for preoperative and one for postoperative risk factors. Results were presented as odds ratio (OR) with corresponding 95% confidence interval (CI). A 2-tailed P value of < 0.05 was considered statistically significant. Statistical analysis was performed with SPSS statistical software version 25.0 (SPSS Inc, Chicago, IL).
RESULTS
Patient Cohort
In the study period of 2000 to 2013, 1520 patients underwent upfront pancreatectomy for newly diagnosed PDAC. Excluded from this cohort were 24 patients (1.6%) who died within 90 days postsurgery and 220 patients (14.5%) with less than 24 months of followup, in which neither death, nor recurrence occurred. An additional 319 patients (21.0%) who were followed postoperatively at other institutions were also excluded. Therefore, a total of 957 patients were included in the final analysis. Demographics, clinicopathologic, and treatment characteristics of the entire study population, and dichotomized for patients with and without recurrence, are summarized in Table 1.
Table 1.
Demographics, Clinicopathologic, and Treatment Characteristics of Included Patients
| Variable | All Patients (n = 957) | No Recurrence (n = 204) | Recurrence (n = 753) | P Value |
|---|---|---|---|---|
|
| ||||
| Female, n (%) | 456 (47.6%) | 88 (43.1%) | 368 (48.9%) | 0.146 |
| Race/ethnicity, n (%) | 0.367 | |||
| Caucasian | 821 (85.8%) | 179 (87.7%) | 642 (85.3%) | |
| Other | 136 (14.2%) | 25 (12.3%) | 111 (14.7%) | |
| Age, mean years (SD) | 65.8 (10.5) | 68.2 (10.4) | 65.2 (10.5) | <0.001 |
| Charlson age-comorbidity index, n (%) | ||||
| <4 points | 657 (68.7%) | 152 (74.5%) | 505 (67.1%) | |
| ≥4 points | 300 (31.3%) | 52 (25.5%) | 248 (32.9%) | 0.042 |
| Preoperative CA 19–9 (U/mL)* | ||||
| Median (IQR) | 130 (50–398) | 72 (28–269) | 148 (56–455) | 0.006 |
| Postoperative CA 19–9 (U/mL)† | ||||
| Median (IQR) | 38 (19–113) | 28 (16–45) | 43 (22–138) | <0.001 |
| Operation procedure, n (%) | 0.100 | |||
| PPPD | 415 (43.4%) | 92 (45.1%) | 323 (42.9%) | |
| Classic PD | 383 (40.0%) | 83 (40.7%) | 300 (39.8%) | |
| Total pancreatectomy | 34 (3.6%) | 11 (5.4%) | 23 (3.1%) | |
| Distal pancreatectomy | 125 (13.1%) | 18 (8.8%) | 107 (14.2%) | |
| Complications, n (%) | 0.123 | |||
| Clavien-Dindo grade ≤II | 803 (83.9%) | 164 (80.4%) | 639 (84.9%) | |
| Clavien-Dindo grade ≥III | 154 (16.1%) | 40 (19.6%) | 114 (15.1%) | |
| Resection margin, n (%) | <0.001 | |||
| R0 (>1.0 mm) | 658 (68.8%) | 167 (81.9%) | 491 (65.2%) | |
| R1 (≤1.0 mm) | 299 (31.2%) | 37 (18.1%) | 262 (34.8%) | |
| Tumour differentiation, n (%) | <0.001 | |||
| Well-moderate | 591 (61.8%) | 148 (72.5%) | 443 (58.8%) | |
| Poor | 366 (38.2%) | 56 (27.5%) | 310 (41.2%) | |
| Tumour size, mean cm (SD) | 3.2 (1.5) | 3.0 (1.7) | 3.2 (1.4) | 0.010 |
| T-stage, n (%) | 0.028 | |||
| 1–2 | 283 (29.6%) | 73 (35.8%) | 210 (27.9%) | |
| 3–4 | 674 (70.4%) | 131 (64.2%) | 543 (72.1%) | |
| Positive lymph nodes, n (%) | 719 (75.1%) | 130 (63.7%) | 589 (78.2%) | <0.001 |
| Positive lymph node ratio, n (%) | <0.001 | |||
| ≤0.2 | 646 (67.5%) | 174 (85.3%) | 472 (62.7%) | |
| >0.2 | 311 (32.5%) | 30 (14.7%) | 281 (37.3%) | |
| Micr. perineural invasion, n (%) | 859 (89.8%) | 163 (79.9%) | 696 (92.4%) | <0.001 |
| Micr. lymphovascular invasion, n (%) | 544 (56.8%) | 81 (39.7%) | 463 (61.5%) | <0.001 |
| AJCC stage 7th edition, n (%) | 0.001 | |||
| ≤2A | 671 (28.3%) | 76 (37.3%) | 195 (25.9%) | |
| ≥2B | 686 (71.7%) | 128 (62.7%) | 558 (74.1%) | |
| Adjuvant therapy, n (%) | 0.002 | |||
| No adjuvant | 307 (32.1%) | 55 (27.0%) | 252 (33.5%) | |
| Chemotherapy | 207 (21.6%) | 62 (30.4%) | 145 (19.3%) | |
| Chemoradiotherapy | 443 (46.3%) | 87 (42.6%) | 356 (47.3%) | |
| Recurrence site, n (%) | NA | |||
| Local only | 190 (19.9%) | 0 (0%) | 190 (25.2%) | |
| Metastatic | 563 (58.8%) | 0 (0%) | 563 (74.8%) | |
| Survival (median months, 95% CI) | ||||
| Recurrence-free survival | 15.2 (14.0–16.4) | NA | 11.7 (10.8–12.6) | NA |
| Post-recurrence survival | NA | NA | 7.5 (6.8–8.2) | NA |
| Overall survival | 24.8 (23.3–26.3) | 93.0 (61.0–125.1) | 21.1 (19.2–22.9) | <0.001 |
Three hundred ninety-eight patients had preoperative CA 19–9 levels available for analysis. Excluded from analysis were 65 Lewis antigen negative patients and 494 patients with missing preoperative values.
Five hundred thirty-two patients had postoperative CA 19–9 levels available for analysis. Excluded from analysis were 65 Lewis antigen negative patients and 360 patients with missing postoperative values.
SD indicates standard deviation; CA, carbohydrate antigen; IQR, interquartile range; PPPD, pylorus-preserving pancreatoduodenectomy; PD, pancreatoduodenectomy; AJCC, American Joint Committee on Cancer; Micr, microscopic; CI, confidence interval; NA, not applicable.
Median follow-up for the entire cohort was 24.2 months (95% CI 22.7–25.8). At the time of last follow-up, 753 (78.7%) of 957 patients had recurred after a median RFS of 11.7 months (95% CI 10.8–12.6). Patients most often experienced multiple-site recurrence (n = 253, 33.6%), followed by isolated local (n = 190, 25.2%), liver only (n = 184, 24.4%), or lung only (n = 106, 14.1%) recurrence. The remaining 20 patients (2.7%) experienced first recurrence at more unusual locations such as the brain, osseous structures, or the ovaries. Median OS for all patients with recurrence was 21.1 months (95% CI 19.2–22.9). Median PRS was 7.5 months (95% CI 6.8–8.2). Median OS for the entire cohort was 24.8 months (95% CI 23.3–26.3) with 175 patients (18.3%) currently alive after a median follow-up of 71.0 months (95% CI 63.3–78.7).
Defining Early and Late Recurrence
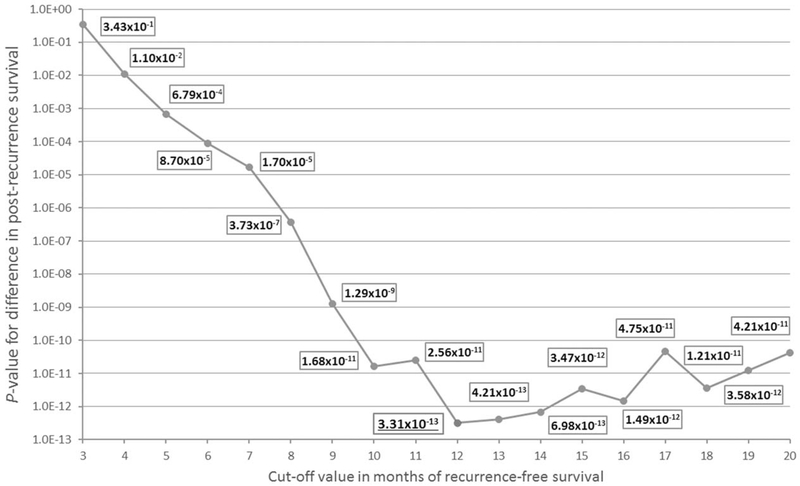
The evaluated early recurrence cut-off values and associated survival outcomes are shown in Table 2. In the current study cohort of 753 patients with recurrence, the optimal length of RFS to distinguish between early and late recurrence, based on subsequent PRS, was 12 months (P = 3.3110–13) (Fig. 1). Median RFS in the early (<12 mo) recurrence cohort (n = 388, 51.5%) was 6.5 months (95% CI 5.9–6.9), followed by a relatively limited PRS of 6.1 months (95% CI 5.5–6.8). Patients with recurrence after 12 months (n = 365, 48.5%) had a median RFS of 20.9 months (95% CI 19.4–22.4) with a median PRS of 10.8 months (95% CI 9.4–12.2). Patients with early recurrence had 1- and 2-year PRS rates of 20 and 6% compared with 45 and 22% for the late recurrence group (both P < 0.001). Median OS was significantly longer for patients with late recurrence (34.6 mo, 95% CI 31.5–37.6) when compared with patients with early recurrence (13.0 mo, 95% CI 12.2–13.8; P < 0.001).
Table 2.
Evaluated Cut-off Thresholds for Defining Early and Late Recurrence Based on the Prognosis After Recurrence
| Evaluated Cut-off | P Value | Potential Early Recurrence Cohort |
Potential Late Recurrence Cohort |
||||||
|---|---|---|---|---|---|---|---|---|---|
| N | RFS (mo) | PRS (mo) | OS (mo) | n | RFS (mo) | PRS (mo) | OS (mo) | ||
|
| |||||||||
| 3 mo | 3.43×10–1 | 85 | 1.6 | 7.9 | 9.1 | 604 | 13.7 | 7.5 | 23.7 |
| 4 mo | 1.10×10–2 | 123 | 2.1 | 6.9 | 9.8 | 630 | 14.6 | 7.7 | 24.4 |
| 5 mo | 6.79×10–4 | 157 | 2.6 | 6.5 | 9.2 | 596 | 15.1 | 7.9 | 25.1 |
| 6 mo | 8.70×10–5 | 182 | 3.3 | 6.5 | 9.9 | 571 | 15.6 | 8.0 | 25.9 |
| 7 mo | 1.70×10–5 | 222 | 3.8 | 6.5 | 10.1 | 531 | 16.6 | 8.0 | 26.6 |
| 8 mo | 3.73×10–7 | 244 | 4.0 | 6.4 | 10.2 | 509 | 17.1 | 8.3 | 28.1 |
| 9 mo | 1.29×10–9 | 277 | 4.4 | 6.1 | 10.7 | 476 | 18.1 | 8.9 | 29.6 |
| 10 mo | 1.68×10–11 | 319 | 5.3 | 6.0 | 11.2 | 434 | 19.4 | 9.7 | 31.5 |
| 11 mo | 2.56×10–11 | 353 | 5.9 | 6.2 | 12.3 | 400 | 20.2 | 10.3 | 32.5 |
| 12 mo | 3.31×10–13 | 388 | 6.5 | 6.1 | 13.0 | 365 | 20.9 | 10.8 | 34.6 |
| 13 mo | 4.21×10–13 | 410 | 6.8 | 6.2 | 13.6 | 343 | 23.0 | 10.8 | 35.6 |
| 14 mo | 6.9810–13 | 437 | 7.1 | 6.2 | 13.9 | 316 | 24.5 | 10.9 | 38.5 |
| 15 mo | 3.47×10–12 | 462 | 7.5 | 6.4 | 14.4 | 291 | 25.7 | 10.6 | 40.2 |
| 16 mo | 1.49×10–12 | 489 | 8.4 | 6.6 | 14.9 | 264 | 26.9 | 10.9 | 43.3 |
| 17 mo | 4.75×10–11 | 508 | 8.6 | 6.7 | 15.2 | 204 | 27.6 | 11.7 | 44.9 |
| 18 mo | 3.58×10–12 | 526 | 8.9 | 6.7 | 15.7 | 227 | 29.0 | 11.8 | 47.8 |
| 19 mo | 1.21×10–11 | 543 | 9.1 | 6.9 | 16.0 | 210 | 30.6 | 12.1 | 48.4 |
| 20 mo | 4.21×10–11 | 564 | 9.3 | 6.9 | 16.6 | 189 | 31.9 | 13.4 | 51.3 |
Shown in bold is the optimal cut-off threshold with the lowest P value. RFS indicates recurrence-free survival; PRS, post-recurrence survival; OS, overall survival.
FIGURE 1.
Different cut-off thresholds with corresponding P values show that the optimal threshold for defining early and late recurrence based on the difference of post-recurrence survival is 12 months.
Patients with early recurrence more often had a larger tumor, a poorly differentiated tumor, positive lymph nodes, and microscopic lymphovascular invasion (Table 3). Additionally, both pre- and postoperative CA 19–9 values were significantly higher in patients with early recurrence. On the other hand, patients with late recurrence had superior preoperative performance status and less severe postoperative complications according to the CACI and Clavien-Dindo classification, respectively. Furthermore, patients with late recurrence had more often received adjuvant chemotherapy or chemoradiotherapy. Patients with a preoperative CACI score of ≥4 (60.0 vs. 71.5%; P < 0.001) or a postoperative complication Clavien-Dindo classification ≥III (60.4 vs. 69.4%; P = 0.029) were less likely to receive any adjuvant therapy.
Table 3.
Demographics, Clinicopathologic, and Treatment Characteristics of All Patients With Recurrence
| Variable | Early Recurrence <12 mo (n = 388) | Late Recurrence >12 mo (n = 365) | P Value |
|---|---|---|---|
|
| |||
| Female, n (%) | 191 (49.2%) | 177 (48.5%) | 0.840 |
| Race/ethnicity, n (%) | 0.232 | ||
| Caucasian | 325 (83.8%) | 317 (86.8%) | |
| Other | 63 (16.2%) | 48 (13.2%) | |
| Age, mean years (SD) | 65.4 (11.1) | 64.9 (9.7) | 0.457 |
| Charlson age-comorbidity index, n (%) | 0.008 | ||
| <4 points | 243 (62.6%) | 262 (71.8%) | |
| ≥4 points | 145 (37.4%) | 103 (28.2%) | |
| Preoperative CA 19–9 (U/mL)* | |||
| Median (IQR) | 221 (87–685) | 91 (30–294) | <0.001 |
| Postoperative CA 19–9 (U/mL)† | |||
| Median (IQR) | 91 (30–294) | 29 (16–60) | <0.001 |
| Operation procedure, n (%) | 0.311 | ||
| PPPD | 149 (38.4%) | 151 (41.4%) | |
| Classic PD | 163 (42.0%) | 160 (43.8%) | |
| Total pancreatectomy | 15 (3.9%) | 8 (2.2%) | |
| Distal pancreatectomy | 61 (15.7%) | 46 (12.6%) | |
| Complications, n (%) | 0.013 | ||
| Clavien-Dindo grade ≤II | 317 (81.7%) | 322 (88.2%) | |
| Clavien-Dindo grade ≥III | 71 (18.3%) | 43 (11.8%) | |
| Resection margin, n (%) | 0.126 | ||
| R0 (>1.0 mm) | 243 (62.6%) | 248 (67.9%) | |
| R1 (≤1.0 mm) | 145 (37.4%) | 117 (32.1%) | |
| Tumour differentiation, n (%) | <0.001 | ||
| Well-moderate | 202 (52.1%) | 241 (66.0%) | |
| Poor | 186 (47.9%) | 124 (34.0%) | |
| Tumour size, mean cm (SD) | 3.5 (1.5) | 3.0 (1.1) | <0.001 |
| T-stage, n (%) | 0.005 | ||
| 1–2 | 91 (23.5%) | 119 (32.6%) | |
| 3–4 | 297 (76.5%) | 246 (67.4%) | |
| Positive lymph nodes, n (%) | 322 (83.0%) | 267 (73.2%) | 0.001 |
| Positive lymph node ratio, n (%) | <0.001 | ||
| ≤0.2 | 213 (54.9%) | 259 (71.0%) | |
| >0.2 | 175 (45.1%) | 106 (29.0%) | |
| Micr. perineural invasion, n (%) | 361 (93.5%) | 335 (92.0%) | 0.430 |
| Micr. lymphovascular invasion, n (%) | 257 (68.5%) | 206 (57.4%) | 0.001 |
| AJCC stage 7th edition, n (%) | 0.001 | ||
| ≤2A | 81 (20.9%) | 114 (31.2%) | |
| ≥2B | 307 (79.1%) | 251 (68.8%) | |
| Adjuvant therapy, n (%) | <0.001 | ||
| No adjuvant | 175 (45.1%) | 77 (21.1%) | |
| Chemotherapy | 65 (16.8%) | 80 (21.9%) | |
| Chemoradiotherapy | 148 (38.1%) | 208 (57.0%) | |
| Recurrence site, n (%) | |||
| Liver only | 131 (33.8%) | 53 (14.5%) | <0.001 |
| Multiple-site | 143 (36.9%) | 110 (30.1%) | 0.050 |
| Lung only | 33 (8.5%) | 73 (20.0%) | <0.001 |
| Local only | 76 (19.6%) | 114 (31.2%) | <0.001 |
| Other | 5 (1.3%) | 15 (4.1%) | 0.016 |
Three hundred fifteen patients had preoperative CA 19–9 levels available for analysis. Excluded from analysis were 50 Lewis antigen negative patients and 388 patients with missing preoperative values.
Four hundred fifty-five patients had postoperative CA 19–9 levels available for analysis. Excluded from analysis were 50 Lewis antigen negative patients and 248 patients with missing postoperative values.
SD indicates standard deviation; CA, carbohydrate antigen; IQR, interquartile range; PPPD, pylorus-preserving pancreatoduodenectomy; PD, pancreatoduodenectomy; AJCC, American Joint Committee on Cancer; Micr, microscopic.
Observed recurrence patterns were also notably different. Patients with late recurrence presented more often with local only (31.2 vs. 19.6%; P < 0.001) or lung only recurrence (20.0 vs. 8.5%; P < 0.001). On the contrary, liver only (33.8 vs. 14.5%; P < 0.001) and multiple-site recurrence (36.9 vs. 30.1%; P = 0.050) were more prevalent among the patients recurring early.
Pre- and Postoperative CA 19–9 Analysis
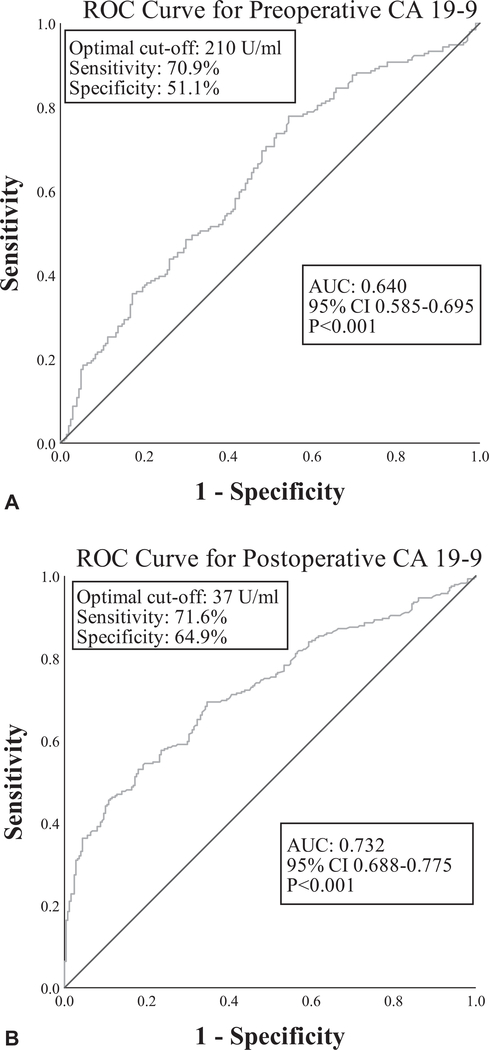
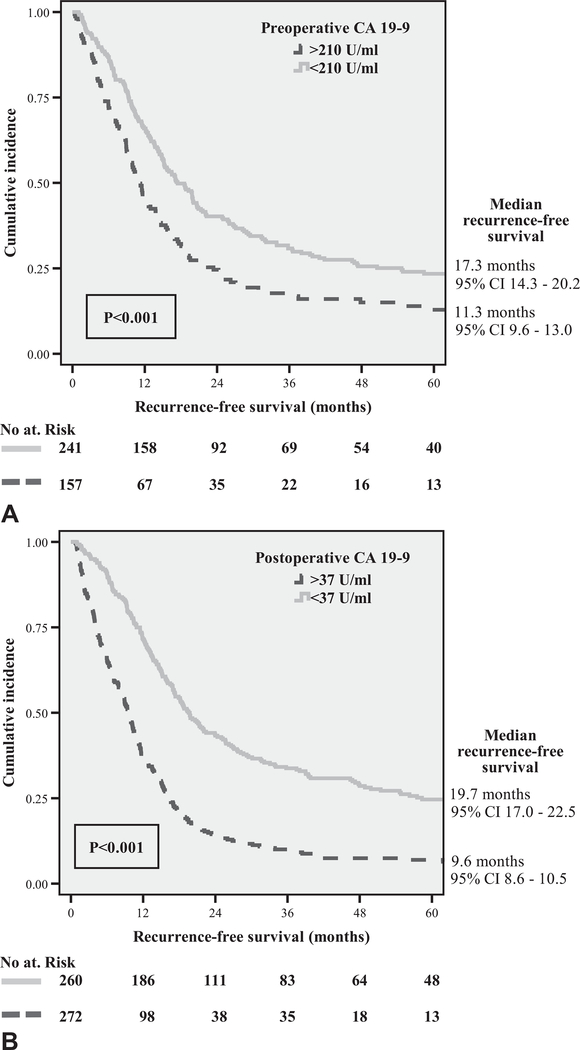
Of the entire cohort of 957 patients, 398 patients had preoperative CA 19–9 values available (median 130U/mL, IQR 50–398). Within 2 months postoperatively, 532 patients had CA 19–9 values available (median 38U/mL, IQR 19–113). Sixty-five patients (6.8%) were deemed Lewis antigen negative and were excluded from the analysis. For preoperative CA 19–9, the area under the curve (AUC) was 0.640 and the optimal threshold for predicting early recurrence was 210U/mL with a sensitivity of 70.9% and specificity of 51.1% (Fig. 2A). The best cut-off value for postoperative CA 19–9 (AUC = off value for postoperative CA 19–9 (AUC = 0.732) was 37U/mL with a sensitivity of 71.6% and specificity of 64.9% (Fig. 2B). Eighty-nine of 157 patients (56.7%) with preoperative CA 19–9 values exceeding 210U/mL recurred early, versus 86 of 241 patients (35.7%) with preoperative CA 19–9 less than 210U/mL (P < 0.001). Similarly, 175 of 272 patients (64.3%) with > 37U/mL postoperative CA 19–9 experienced early recurrence compared with 75 of 260 patients (28.8%) with < 37U/mL (P < 0.001). Elevated pre- and postoperative CA 19–9 levels were both significantly associated with decreased RFS (Fig. 3).
FIGURE 2.
The ROC curve for (A) preoperative CA 19–9 and (B) postoperative CA 19–9 for predicting early recurrence (<12 mo).
FIGURE 3.
Kaplan–Meier curves showing worse recurrencefree survival for patients with elevated (A) preoperative and (B) postoperative CA 19–9 values.
Factors Associated With Early Recurrence
Results of univariable analysis and two separate multivariable logistic regression models with pre- and postoperative risk factors are presented in Table 4. Three preoperative variables proved to be independently associated with recurrence within 12 months: CACI score of ≥4 (OR 1.65, 95% CI 1.06–2.55, P = 0.025), tumor size on the last preoperative CT scan >3.0cm (OR 1.53, 95% CI 1.11–1.95, P = 0.029) and preoperative CA 19–9 of > 210U/mL (OR 2.30, 95% CI 1.51–3.50, P < 0.001). Four postoperative risk factors were independently correlated with early recurrence, including poor tumor differentiation grade (OR 1.66, 95% CI 1.10–2.51, P = 0.016), microscopic lymphovascular invasion (OR 1.70, 95% CI 1.10–2.63, P = 0.018), positive lymph node ratio > 0.2 (OR 2.49, 95% CI 1.62– 3.84, P < 0.001), and postoperative CA 19–9 of > 37U/mL (OR 3.38, 95% CI 2.25–5.08, P < 0.001). Furthermore, both adjuvant chemotherapy (OR 0.28, 95% CI 0.16–0.51, P < 0.001) and chemoradiotherapy (OR 0.29, 95% CI 0.18–0.47, P < 0.001) were independently associated with a reduced likelihood of early recurrence.
Table 4.
Univariable and Multivariable Logistic Regression for Associations Between Pre- and Postoperative Risk Factors and Early Recurrence of Pancreatic Ductal Adenocarcinoma After Resection (<12 mo)
| Preoperative Risk Factors |
Univariable
|
Multivariable
|
||
| Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | |
|
| ||||
|---|---|---|---|---|
| Age: >70 years versus ≤70 years | 1.00 (0.77–1.30) | 0.984 | — | |
| Gender: female versus male | 1.15 (0.89–1.49) | 0.288 | — | |
| Race/ethnicity: Caucasian versus all others | 0.82 (0.57–1.17) | 0.272 | — | |
| Abdominal pain: yes versus no | 1.27 (0.96–1.67) | 0.101 | — | |
| Jaundice: yes versus no | 0.82 (0.63–1.06) | 0.130 | — | |
| Diabetes: yes versus no | 1.28 (0.94–1.75) | 0.116 | — | |
| Weight loss: yes versus no | 1.22 (0.94–1.60) | 0.138 | — | |
| Smoking: past/current versus never | 0.80 (0.59–1.09) | 0.149 | — | |
| Charlson age-comorbidity index: ≥4 versus < 4 | 1.48 (1.12–1.95) | 0.005 | 1.65 (1.06–2.55) | 0.025 |
| Tumor size * : >3.0cm versus ≤3.0 cm | 2.25 (1.69–2.99) | <0.001 | 1.53 (1.11–1.95) | 0.029 |
| Tumor location*: body/tail versus head/uncinate | 1.40 (0.99–1.97) | 0.053 | 1.45 (0.82–2.56) | 0.198 |
| Preop CA 19–9: >210 U/mL versus ≤210 U/mL | 2.36 (1.56–3.56) | <0.001 | 2.30 (1.51–3.50) | <0.001 |
|
| ||||
| Postoperative Risk Factors |
Univariable
|
Multivariable
|
||
| Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | |
|
| ||||
| Age: >70 years versus ≤70 years | 1.00 (0.77–1.30) | 0.984 | — | |
| Gender: female versus male | 1.15 (0.89–1.49) | 0.288 | — | |
| Race/ethnicity: Caucasian versus all others | 0.82 (0.57–1.17) | 0.272 | — | |
| Charlson age-comorbidity index: ≥4 versus <4 | 1.48 (1.12–1.95) | 0.005 | 1.45 (0.95–2.22) | 0.087 |
| Complications†: Clavien ≥III versus Clavien ≤II | 1.42 (1.00–2.00) | 0.047 | 1.14 (0.65–2.00) | 0.645 |
| Tumor size: > 3.0cm versus ≤3.0 cm | 1.61 (1.24–2.10) | <0.001 | 1.11 (0.74–1.68) | 0.609 |
| Tumor differentiation: poor versus well/moderate | 1.97 (1.51–2.57) | <0.001 | 1.66 (1.10–2.51) | 0.016 |
| Micr. lymphovascular invasion: yes versus no | 1.99 (1.52–2.60) | <0.001 | 1.70 (1.10–2.63) | 0.018 |
| Micr. perineural invasion: yes versus no | 2.00 (1.25–3.18) | 0.004 | 1.31 (0.57–3.02) | 0.521 |
| Positive lymph node ratio: >0.2 versus ≤0.2 | 2.71 (2.05–3.58) | <0.001 | 2.49 (1.62–3.84) | <0.001 |
| Resection margin: R1 versus R0 | 1.61 (1.23–2.13) | 0.001 | 1.24 (0.81–1.91) | 0.340 |
| Postop CA 19–9: >37 U/mL versus ≤37 U/mL | 4.45 (3.09–6.41) | <0.001 | 3.38 (2.25–5.08) | <0.001 |
| Adjuvant therapy: | ||||
| Chemotherapy versus no adjuvant | 0.34 (0.23–0.49) | <0.001 | 0.28 (0.16–0.51) | <0.001 |
| Chemoradiotherapy versus no adjuvant | 0.32 (0.24–0.43) | <0.001 | 0.29 (0.18–0.47) | <0.001 |
Shown in bold are univariable associations (P < 0.10) that were selected for multivariable analysis and significant risk factors (P < 0.05) on multivariable analysis.
Based on the last preoperative computed tomography scan.
According to Clavien-Dindo classification.
CI indicates confidence interval; Preop, preoperative; CA, carbohydrate antigen; Micr, microscopic.
DISCUSSION
Although the prognostic relevance of initial recurrence is of significant clinical impact, there is presently no established and evidence-based definition for early recurrence of PDAC after pancreatectomy. Our study implied that the optimal cut-off value to differentiate between early and late recurrence, based on subsequent prognosis, is a recurrence-free interval of at least 12 months. Additionally, independent risk factors for the development of early PDAC recurrence after resection were found, including a preoperative CACI score of ≥4, tumor size on the last preoperative CT scan >3.0cm and a CA 19–9 level of > 210U/mL. Additionally, pathologic findings of poor tumor differentiation grade, microscopic lymphovascular invasion, a positive lymph node ratio > 0.2, and postoperative CA 19–9 level of > 37U/mL were independently associated with early recurrence. Lastly, adjuvant chemotherapy andchemoradiotherapy were associated with a reduced likelihood of early recurrence.
Throughout the present literature, varying cut-off values are being used to divide patients based on timing of recurrence, for instance: 6 months by Sugiura et al19 and Matsumoto et al,8 8 months by Niedergethmann et al9 and 12 months by Zhai et al,10 and Nishio et al.11 To the best of the authors’ knowledge, just one previous study has been performed with the primary goal of classifying patients into early and late recurrence groups based on the statistical assessment of the best cut-off value to differentiate in prognosis.20 In their study of 55 patients with recurrence, Yamamoto et al also established an optimal cutoff of 12 months for differentiating early and late recurrence based on OS. The 37 patients with early recurrence had a limited 5-year survival rate of 9% compared with the 42% rate found for the 18 patients with late recurrence (P < 0.001). However, no mention was made on PRS outcomes and potential differences between the two patient populations. Using OS as primary outcome when defining early and late recurrence potentially introduces bias, since OS will inevitably be better in the late recurrence cohort group as these patients already have a long recurrence-free interval. To avoid this bias, we made the conscious decision to use the difference in survival after recurrence (PRS) in our analyses to define early and late recurrence.
The current study shows that patients who recurred within 12 months had a PRS of 6.1 months compared with a PRS of 10.8 months for patients with late recurrence (P < 0.001). The fact that patients with a prolonged RFS after surgery also tended to live longer after they recurred, may suggest favorable tumor biology. Conversely, more aggressive tumor biology may lead to shorter RFS followed by a more rapid progression to death. In this way, RFS could be a clinically useful surrogate for appreciating PDAC behavior. The impact of the timing of recurrence presented in the current study could potentially aid physicians with prognostic stratification and help aid decision-making regarding the treatment of recurrence. Multiple studies focusing on the treatment of recurrence have suggested that RFS is an important factor when selecting patients for further treatment of recurrence.21 For instance, a study done at this institution on stereotactic body radiation therapy for isolated local recurrence showed that patients with a RFS > 9 months had superior survival after salvage treatment.22 In another study, Boone et al23 performed re-resections in selected patients with isolated local, liver, or pulmonary recurrence and found that survival after treatment of recurrence was significantly longer for patients with > 15 months of RFS (40.6 vs. 8.2 months; P < 0.05). Lastly, after controlling for location and treatment of recurrence, a recent Dutch study found that a RFS of > 10 months was independently associated with prolonged survival after recurrence.24
Several independent pre- and postoperative variables were identified that were associated with an increased likelihood of early recurrence after surgery for PDAC, including elevated pre- and postoperative CA 19–9. First discovered in 1979, CA 19–9 has become the most studied and well-known biomarker for PDAC.25 Multiple reports have established the association between elevated pre- and postoperative CA 19–9 levels and decreased post-pancreatectomy survival, with varying thresholds between 37 and 400U/mL being advocated.26–29 However, far fewer studies have focused on the correlation between CA 19–9 and recurrence, and there is currently no consensus regarding the CA 19–9 threshold for prediction of early recurrence.2,30 For instance, in a recent study by Nishio et al (n = 90), a preoperative CA 19–9 of > 529U/mL was recommended as the optimal cutoff for predicting recurrence within 12 months.11 Studies by Kim et al (n = 86) and Sugiura et al (n = 154) both found a preoperative threshold of > 100U/mL to have the best correlation with recurrence within 6 months.19,31 In this study, analyses of ROC curves and associated AUCs revealed optimal pre-and postoperative CA 19–9 thresholds for the prediction of early recurrence of > 210 and > 37U/mL, respectively. However, with an AUC of 0.640, sensitivity of 71% and specificity of 51%, the predictive strength of finding elevated preoperative CA 19–9 was fairly limited, highlighting the necessity of finding more accurate biomarkers in patients with PDAC.
A low performance status according to the CACI was shown to be an independent preoperative risk factor for early recurrence. High CACI scores have previously been found to correlate with worse outcomes in patients with PDAC. For instance, Dias-Santos et al32 reported that a CACI score of > 4 was predictive of death within 1 year of pancreatectomy (P < 0.001). Similarly, another recent study showed that a CACI score of ≥4 was a predictor of poor survival on multivariable analysis (P = 0.024).18 Interestingly, both a preoperative CACI score ≥4 and a postoperative complication Clavien-Dindo classification ≥III were significantly associated with early recurrence on univariable analysis. However, both lost significance in a multivariable model that included postoperative risk factors. This could in part be explained by the strong confounding effect of adjuvant therapy as a variable in the postoperative multivariable model. For instance, a study from our group reported that postoperative complications delay the time to adjuvant therapy and reduce the likelihood of adjuvant therapy.33 Likewise, Asano et al18 found that the rate of patients who received chemotherapy was significantly lower in those with a CACI score of ≥4 (69 vs. 87%, P < 0.0001). Similar correlations were observed in the current study cohort, as patients with low preoperative performance status (P < 0.001) or severe postoperative complications (P = 0.029) had a decreased likelihood of receiving any adjuvant therapy.
It was somewhat surprising to find that R1 resection, although associated with recurrence in general, was ultimately not a predictor of early recurrence on multivariable analysis. There might be several explanations for this finding. Firstly, patients at our institution with a close resection margin are commonly recommended to undergo adjuvant radiation therapy with either conventional or stereotactic radiotherapy for margin attenuation, possibly suppressing the impact of R1 status. Secondly, R1 margin has previously been shown to be particularly associated with local recurrence, which was more commonly seen in the late recurrence cohort.5 Lastly, recurrence following R1 margin might primarily be caused by microscopic residual disease in the remnant pancreas that has not undergone the process of hematogenous metastasis, possibly indicating a less aggressive and more favorable tumor biology resulting in a later recurrence.22 Overall, the prognostic impact of the distance of PDAC cells to the final resection margin has not been fully clarified yet, and is an intensely debated topic in recent pancreatic surgery literature.34–39
From a total of 957 patients with primary resectable patients, more than 40% (n = 388, 41.5%) recurred within 12 months. Of these patients with early recurrence, 80% had distant metastases, supporting the hypothesis that occult micrometastatic disease was present at the time of surgery. In recent years, it has been argued that a chemotherapy-first approach for resectable PDAC might help select for better tumor biology, while on the other hand sparing those patients who might have recurred early a major abdominal operation.12–14 In the near future, prospective studies (such as the PREOPANC-trial) may be able to clarify the role of neoadjuvant chemotherapy in resectable PDAC.40 Although a CACI score of ≥4, a tumor size > 3cm on preoperative CT and preoperative CA 19–9 of > 210U/mL were shown to be independently associated with early recurrence, differences in tumor biology seem to exist that cannot be accounted for by currently identified risk factors alone. Accurate preoperative identification of those patients with a high likelihood of early recurrence would greatly help clinicians and patients alike in selecting the appropriate sequence of therapies in PDAC. Recent advances in the field of “liquid biopsies” may result in a usable biomarker that reflects the presence of micrometastatic disease in patients with PDAC.41,42 A current ongoing prospective trial at our institution (NCT02974764) aims to further elucidate the usefulness of both circulating tumor cells and circulating tumor DNA as a prognostic biomarker in PDAC patients.43
Previous work from our institution, using a subset of the current cohort consisting of patients who underwent resection for a tumor in the head of the pancreas between 2000 and 2010 (n = 692), showed that specific recurrence locations have different predictive factors and possess distinct RFS curves.5 The reported findings in the present study further complement and expand on those prior results by showing that a RFS of 12 months is the best cut-off to separate early from late recurrence and by identifying risk factors that can help predict early recurrence. However, this study has several limitations worthy of consideration. First, a significant number of patients were excluded due to incomplete follow-up records, possibly limiting the generalizability of our findings to the population of PDAC patients as a whole. Second, although a prospective database from a large tertiary referral center was used for data extraction, this was a retrospective study with all the associated bias risks. Lastly, our database lacked specific information with regard to additional treatment for recurrence after pancreatectomy. Additional data on further treatments might have revealed associations not appreciated by the current study.
To summarize, there is presently no established and evidencebased definition of early recurrence following surgery for primary resectable PDAC. This study found a recurrence-free interval of 12 months to be the optimal threshold for differentiating between early and late recurrence based on subsequent prognosis. Furthermore, preoperatively (>210U/mL) and postoperatively (>37U/mL) elevated CA 19–9 were shown to be independently associated with early recurrence, albeit with relatively low sensitivity, specificity, and predictive value. Since currently acknowledged preoperative risk factors are inadequate to accurately identify patients susceptible to early PDAC recurrence, further studies are needed to identify new biomarkers for the detection of clinically occult micrometastatic disease at the time of operation.
Acknowledgments
This study was supported in the form of grants for a research fellowship by VPG by Foundation De Drie Lichten (The Netherlands), Prins Bernhard Cultuurfonds (The Netherlands), VSBfonds (The Netherlands), Prof. Michaël-van Vloten Fonds (The Netherlands), and the Living With Hope Foundation (The Netherlands).
Footnotes
The authors report no conflicts of interest.
REFERENCES
- 1.Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. [DOI] [PubMed] [Google Scholar]
- 2.Khorana AA, Mangu PB, Berlin J, et al. Potentially curable pancreatic cancer: american society of clinical oncology clinical practice guideline. J Clin Oncol. 2016;34:2541–2556. [DOI] [PubMed] [Google Scholar]
- 3.Suenaga M, Fujii T, Kanda M, et al. Pattern of first recurrent lesions in pancreatic cancer: hepatic relapse is associated with dismal prognosis and portal vein invasion. Hepatogastroenterology. 2014;61:1756–1761. [PubMed] [Google Scholar]
- 4.Parikh AA, Maiga A, Bentrem D, et al. Adjuvant therapy in pancreas cancer: does it influence patterns of recurrence? J Am Coll Surg. 2016;222: 448–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Groot VP, Rezaee N, Wu W, et al. Patterns, timing, and predictors of recurrence following pancreatectomy for pancreatic ductal adenocarcinoma. Ann Surg. 2017. Epub ahead of Print. [DOI] [PubMed] [Google Scholar]
- 6.Smeenk HG, Tran TC, Erdmann J, et al. Survival after surgical management of pancreatic adenocarcinoma: does curative and radical surgery truly exist? Langenbecks Arch Surg. 2005;390:94–103. [DOI] [PubMed] [Google Scholar]
- 7.Wolfgang CL, Herman JM, Laheru DA, et al. Recent progress in pancreatic cancer. CA Cancer J Clin. 2013;63:318–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsumoto I, Murakami Y, Shinzeki M, et al. Proposed preoperative risk factors for early recurrence in patients with resectable pancreatic ductal adenocarcinoma after surgical resection: a multi-center retrospective study. Pancreatology. 2015;15:674–680. [DOI] [PubMed] [Google Scholar]
- 9.Niedergethmann M, Hildenbrand R, Wostbrock B, et al. High expression of vascular endothelial growth factor predicts early recurrence and poor prognosis after curative resection for ductal adenocarcinoma of the pancreas. Pancreas. 2002;25:122–129. [DOI] [PubMed] [Google Scholar]
- 10.Zhai LL, Wu Y, Huang DW, et al. Increased matrix metalloproteinase-2 expression and reduced tissue factor pathway inhibitor-2 expression correlate with angiogenesis and early postoperative recurrence of pancreatic carcinoma. Am J Transl Res. 2015;7:2412–2422. [PMC free article] [PubMed] [Google Scholar]
- 11.Nishio K, Kimura K, Amano R, et al. Preoperative predictors for early recurrence of resectable pancreatic cancer. World J Surg Oncol. 2017;15:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans DB, Varadhachary GR, Crane CH, et al. Preoperative gemcitabinebased chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3496–3502. [DOI] [PubMed] [Google Scholar]
- 13.Assifi MM, Lu X, Eibl G, et al. Neoadjuvant therapy in pancreatic adenocarcinoma: a meta-analysis of phase II trials. Surgery. 2011;150:466–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Geus SW, Evans DB, Bliss LA, et al. Neoadjuvant therapy versus upfront surgical strategies in resectable pancreatic cancer: a Markov decision analysis. Eur J Surg Oncol. 2016;42:1552–1560. [DOI] [PubMed] [Google Scholar]
- 15.Dhir M, Malhotra GK, Sohal DP, et al. Neoadjuvant treatment of pancreatic adenocarcinoma: a systematic review and meta-analysis of 5520 patients. World J Surg Oncol. 2017;15:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 17.Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. [DOI] [PubMed] [Google Scholar]
- 18.Asano T, Yamada S, Fujii T, et al. The Charlson age comorbidity index predicts prognosis in patients with resected pancreatic cancer. Int J Surg. 2017;39:169–175. [DOI] [PubMed] [Google Scholar]
- 19.Sugiura T, Uesaka K, Kanemoto H, et al. Serum CA19–9 is a significant predictor among preoperative parameters for early recurrence after resection of pancreatic adenocarcinoma. J Gastrointest Surg. 2012;16:977–985. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto Y, Ikoma H, Morimura R, et al. Optimal duration of the early and late recurrence of pancreatic cancer after pancreatectomy based on the difference in the prognosis. Pancreatology. 2014;14:524–529. [DOI] [PubMed] [Google Scholar]
- 21.Groot VP, van Santvoort HC, Rombouts SJ, et al. Systematic review on the treatment of isolated local recurrence of pancreatic cancer after surgery; reresection, chemoradiotherapy and SBRT. HPB (Oxford). 2017;19:83–92. [DOI] [PubMed] [Google Scholar]
- 22.Ryan JF, Groot VP, Rosati LM, et al. Stereotactic body radiation therapy for isolated local recurrence after surgical resection of pancreatic ductal adenocarcinoma appears to be safe and effective. Ann Surg Oncol. 2018;25:280–289. [DOI] [PubMed] [Google Scholar]
- 23.Boone BA, Zeh HJ, Mock BK, et al. Resection of isolated local and metastatic recurrence in periampullary adenocarcinoma. HPB (Oxford). 2014;16:197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Groot VP, Daamen LA, Hagendoorn J, et al. Use of imaging during symptomatic follow-up after resection of pancreatic ductal adenocarcinoma. J Surg Res. 2018;221:152–160. [DOI] [PubMed] [Google Scholar]
- 25.Del Villano BC, Brennan S, Brock P, et al. Radioimmunometric assay for a monoclonal antibody-defined tumor marker, CA 19–9. Clin Chem. 1983;29:549–552. [PubMed] [Google Scholar]
- 26.Berger AC, Garcia M, Hoffman JP, et al. Postresection CA 19–9 predicts overall survival in patients with pancreatic cancer treated with adjuvant chemoradiation: a prospective validation by RTOG 9704. J Clin Oncol. 2008;26:5918–5922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reni M, Cereda S, Balzano G, et al. Carbohydrate antigen 19–9 change during chemotherapy for advanced pancreatic adenocarcinoma. Cancer. 2009;115:2630–2639. [DOI] [PubMed] [Google Scholar]
- 28.Turrini O, Schmidt CM, Moreno J, et al. Very high serum CA 19–9 levels: a contraindication to pancreaticoduodenectomy? J Gastrointest Surg. 2009;13: 1791–1797. [DOI] [PubMed] [Google Scholar]
- 29.Barton JG, Bois JP, Sarr MG, et al. Predictive and prognostic value of CA 19–9 in resected pancreatic adenocarcinoma. J Gastrointest Surg. 2009;13:2050–2058. [DOI] [PubMed] [Google Scholar]
- 30.Daamen LA, Groot VP, Heerkens HD, et al. Systematic review on the role of serum tumor markers in the detection of recurrent pancreatic cancer. HPB (Oxford). 2018. Epub ahead of Print. [DOI] [PubMed] [Google Scholar]
- 31.Kim TH, Han SS, Park SJ, et al. CA 19–9 level as indicator of early distant metastasis and therapeutic selection in resected pancreatic cancer. Int J Radiat Oncol Biol Phys. 2011;81:e743–e748. [DOI] [PubMed] [Google Scholar]
- 32.Dias-Santos D, Ferrone CR, Zheng H, et al. The Charlson age comorbidity index predicts early mortality after surgery for pancreatic cancer. Surgery. 2015;157:881–887. [DOI] [PubMed] [Google Scholar]
- 33.Wu W, He J, Cameron JL, et al. The impact of postoperative complications on the administration of adjuvant therapy following pancreaticoduodenectomy for adenocarcinoma. Ann Surg Oncol. 2014;21:2873–2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Markov P, Satoi S, Kon M. Redefining the R1 resection in patients with pancreatic ductal adenocarcinoma. J Hepatobiliary Pancreat Sci. 2016;23: 523–532. [DOI] [PubMed] [Google Scholar]
- 35.Osipov A, Nissen N, Rutgers J, et al. Redefining the positive margin in pancreatic cancer: impact on patterns of failure, long-term survival and adjuvant therapy. Ann Surg Oncol. 2017;24:3674–3682. [DOI] [PubMed] [Google Scholar]
- 36.Kim KS, Kwon J, Kim K, et al. Impact of resection margin distance on survival of pancreatic cancer: a systematic review and meta-analysis. Cancer Res Treat. 2017;49:824–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghaneh P, Kleeff J, Halloran CM, et al. The impact of positive resection margins on survival and recurrence following resection and adjuvant chemotherapy for pancreatic ductal adenocarcinoma. Ann Surg. 2017. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 38.Demir IE, Jager C, Schlitter AM, et al. R0 versus R1 resection matters after pancreaticoduodenectomy, and less after distal or total pancreatectomy for pancreatic cancer. Ann Surg. 2017. Epub ahead of Print. [DOI] [PubMed] [Google Scholar]
- 39.Strobel O, Hank T, Hinz U, et al. Pancreatic cancer surgery: the new R-status counts. Ann Surg. 2017;265:565–573. [DOI] [PubMed] [Google Scholar]
- 40.Versteijne E, van Eijck CH, Punt CJA, et al. Preoperative radiochemotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer (PREOPANC trial): study protocol for a multicentre randomized controlled trial. Trials. 2016;17:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Creemers A, Krausz S, Strijker M, et al. Clinical value of ctDNA in upper-GI cancers: a systematic review and meta-analysis. Biochim Biophys Acta. 2017;1868:394–403. [DOI] [PubMed] [Google Scholar]
- 42.Cohen JD, Javed AA, Thoburn C, et al. Combined circulating tumor DNA and protein biomarker-based liquid biopsy for the earlier detection of pancreatic cancers. Proc Natl Acad Sci U S A. 2017;114:10202–10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clinicatrials.gov (NIH). Clinical Trial NCT02974764 “ Changes in Biomarkers From Blood Over Time in Patients With Pancreatic Adenocarcinoma”. Available at: https://clinicaltrials.gov/ct2/show/NCT02974764. Published November 2016. Accessed December 18, 2017.