Abstract
Daclizumab is a humanized monoclonal antibody that prevents formation of high-affinity interleukin (IL)-2 receptor (IL-2R). Because activated T cells up-regulate high-affinity IL-2R and IL-2 is used to grow activated T cells in vitro, daclizumab was envisioned to selectively inhibit activated T cells. However, the mechanism of action (MOA) of daclizumab is surprisingly broad and it includes many unanticipated effects on innate immunity. Specifically, daclizumab modulates the development of innate lymphoid cells, leading to expansion of immunoregulatory CD56bright natural killer (NK) cells. Activated CD56bright NK cells migrate to the intrathecal compartment in multiple sclerosis (MS) and regulate autoreactive T cells via cytotoxicity. Finally, daclizumab also restricts initial steps of T-cell activation by blocking trans-presentation of IL-2 by dendritic cells to antigen-specific T cells. In conclusion, daclizumab has complex immunomodulatory effects with resultant inhibition of central nervous system inflammation in MS.
Interleukin 2 (IL-2) has been called “T-cell growth factor” (Ruscetti et al. 1977; Mizel and Farrar 1979) because it is used in in vitro T-cell expansion. Once activated, T cells up-regulate IL-2 receptor (IL-2R; Fig. 1). When surface expression of IL-2R peaks (i.e., ∼ 48–72 h post-stimulation), activated naïve T cells also start producing large quantities of IL-2. This autocrine IL-2 signal was believed to be required for expansion of activated T cells. Consequently, it was presumed that blockade of IL-2 signaling would inhibit T-cell effector functions. Daclizumab was the first drug to target IL-2 signaling (Queen et al. 1989), a humanized monoclonal antibody (mAb) of an immunoglobulin (Ig)G1 subtype, which prevents interaction of CD25 with IL-2.
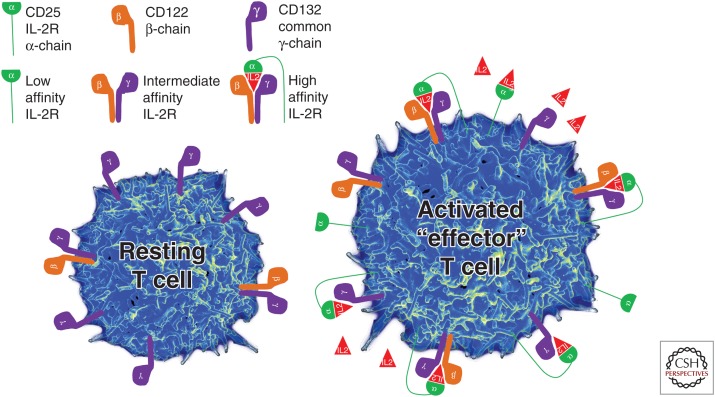
Figure 1.
Configuration of three interleukin 2 receptors (IL-2R) and their expression on the surface of resting versus activated or effector T cells.
The high-affinity IL-2R contains two signaling molecules, γ-chain (CD132) and β-chain (CD122), and the nonsignaling α-chain (CD25; Fig. 1) (Wang et al. 2005). The γ-chain is called common γ-chain (γc), because it is utilized by several cytokines (IL-2, IL-4, IL-7, IL-15, and IL-21), while the β-chain is shared by IL-2 and IL-15, which are two closely related cytokines (Waldmann et al. 1998).
Constitutive expression of γc on resting T cells underlies their responsiveness to cytokines, which mediate T-cell homeostasis and survival (e.g., IL-7). Resting human T cells also express low levels of IL-2Rβ-chain, allowing them to receive the IL-15 signal and, potentially also the IL-2 signal under conditions of IL-2 abundance. However, only a subgroup of CD4+ T cells (T regulatory cells [Tregs]) that depend on the transcriptional factor FoxP3, express constitutively high levels of CD25 in the resting state. Although expression of β- and γ-chain of IL-2R, which together form the “intermediate affinity IL-2R” is sufficient to mediate IL-2 signaling when IL-2 concentrations are high (Kd = 1 nm), T cells that express CD25 can respond to 10- to 100-fold lower concentrations of IL-2 (Fig. 1) (Kd = 10 pm; [Rickert et al. 2005]). Therefore, only resting Tregs can effectively utilize lower concentrations of IL-2 that are necessary for their in vivo survival, their homeostatic proliferation (because they have low levels of IL-7 receptor α-chain), and immunoregulatory functions (Setoguchi et al. 2005).
CD25 itself has a very limited affinity for IL-2 (Kd = 10 nm); therefore, this nonsignaling chain is called the “low-affinity IL-2R” (Fig. 1) (Rickert et al. 2005), even though in reality this receptor cannot transmit any signal. In fact, the only known role of CD25 is facilitation of IL-2 capture and, therefore, assembly of high-affinity IL-2R. This can occur “in cis” (i.e., when CD25 is expressed on the same cell that expresses intermediate affinity IL-2R), or “in trans” (when resting T cells express only intermediate affinity IL-2R), but CD25 is expressed on activated dendritic cells (DCs) (Fig. 2).
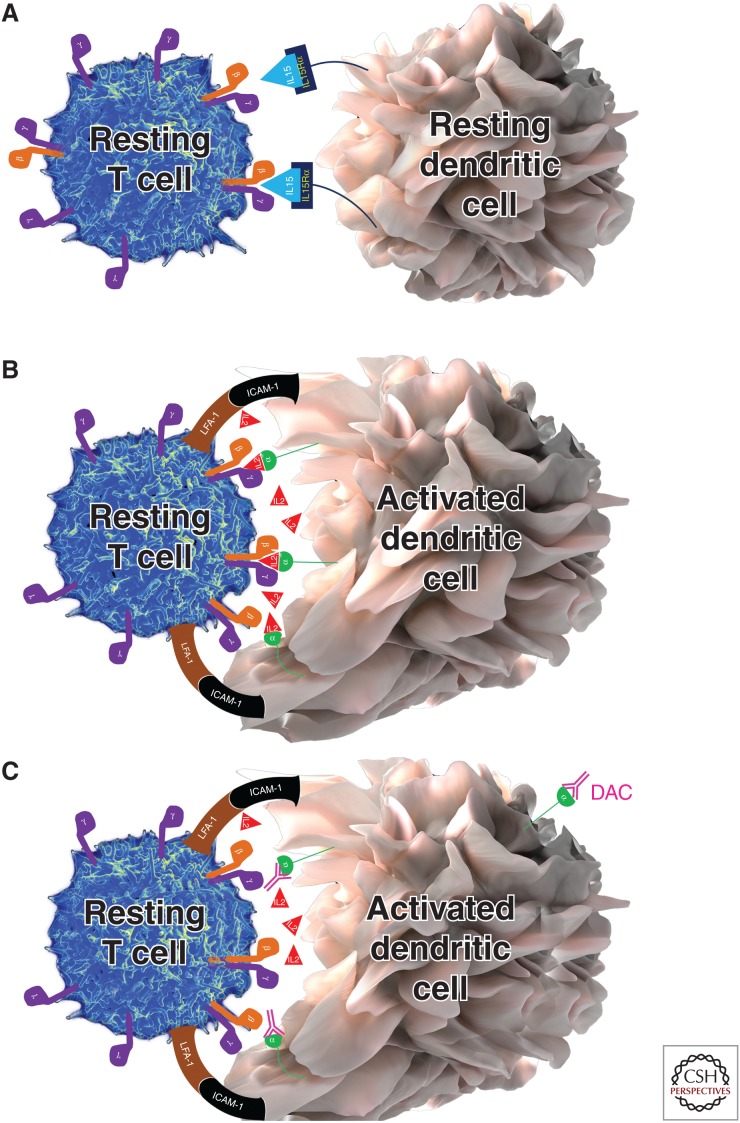
Figure 2.
Trans-presentation of interleukin (IL)-15 and IL-2. As a result of the very high affinity of the IL-15Rα chain for IL-15, the IL-15Rα chain can quickly capture and hold released IL-15 for long time periods. This IL-15Rα/IL-15 complex can then be readily trans-presented to intermediate affinity IL-2/IL-15 receptor, expressed on T cells or natural killer (NK) cells. In contrast, CD25 (IL-2Rα chain) only has low affinity for IL-2, so it is most unlikely that CD25 would be able to effectively capture small concentrations of IL-2 and create stable CD25/IL-2 complexes for trans-presentation, if IL-2 can readily diffuse to the environment. However, if IL-2 is released instead into the synaptic cleft, this is represented by an enclosed space between a peripheral supramolecular activation cluster formed by adhesion molecules such as lymphocyte function associated antigen 1 (LFA-1) and intercellular adhesion molecule 1 (ICAM-1), then diffusion is limited and high IL-2 concentrations can be achieved. Under those circumstances, when the T cell does not yet express CD25, CD25 is expressed on the surface of dendritic cells (DCs), which form stable immune synapses (IS) with antigen-specific T cells, and can effectively capture released IL-2 and trans-present it to the T cell. This cytokine signal is called signal 3. Signal 3 is delivered concomitantly with signal 1, provided by T-cell receptor (TCR) recognizing the peptide-MHC complex, and the costimulatory signal 2 (e.g., provided by interaction of CD28 with CD80 or CD86). All three signals seem necessary for efficient activation of human naïve T cells. When CD25 on the DC is blocked by daclizumab, then the primed T cell is unable to receive the IL-2-mediated signal 3, resulting in suboptimal stimulation of the T cell. Functional consequences are inhibition of antigen-specific T-cell activation, formation of antigen-specific effector, and memory T cells.
Upon activation, T cells strongly up-regulate both IL-2Rβ-chain and CD25. This allows the T cell to form a large number of high-affinity IL-2Rs on its surface and thus respond to small quantities of IL-2. Such large numbers of high-affinity IL-2R sequester most of the γ-chain, making the effector T cell relatively insensitive to IL-7 and other γ-chain-signaling cytokines. This situation is further exacerbated by down-modulation of IL-7Rα-chain upon T-cell activation. A T cell that receives a strong IL-2 signal, but lacks an IL-7 signal can proliferate and exert many effector functions, but is destined to die when the IL-2 levels become scarce (i.e., toward the end of the immune response). This phenomenon is known as activation-induced cell death (AICD). In contrast, T cells that become activated at the time when IL-2 concentrations are scarce do not undergo the same level of up-regulation of IL-2R and down-modulation of IL-7R, and they will preferentially survive at the end of the immune response as “memory” T cells.
MECHANISM OF ACTION (MOA) OF DACLIZUMAB ON THE HUMAN IMMUNE SYSTEM
Daclizumab studies in humans demonstrated that assumptions, which represented the rationale for development of daclizumab as a selective immunosuppressive agent against effector T cells, were not entirely correct.
First, the initial belief that IL-2 stimulates T-cell immunity in vivo was challenged by the observations that mice with genetic deletions of IL-2, or its signaling chains (CD25, CD122) had normal immune responses to pathogens, but instead succumbed to extreme lymphoproliferation and autoimmunity (Schorle et al. 1991; Kundig et al. 1993; Suzuki et al. 1995; Willerford et al. 1995; Wakabayashi et al. 2006). This surprising observation was later explained by a nonredundant role of IL-2 signaling in the biology of FoxP3+ Tregs (Shevach et al. 2001; Almeida et al. 2002; Malek 2003; Setoguchi et al. 2005; Turka and Walsh 2008) and an important role of high-affinity IL-2R in apoptosis of effector T cells (i.e., AICD) (Lenardo 1991; Van Parijs et al. 1999). These studies highlighted a crucial role of IL-2 in immunoregulation, which was confirmed by genetic linkage of IL-2 and/or signaling components (e.g., CD25, CD122) with several human autoimmune diseases, including MS (Hafler et al. 2007; Lowe et al. 2007; Maier et al. 2009).
However, in contrast to mice, humans with genetic deletion of CD25 have, in addition to lymphoproliferation and autoimmunity, severe immunodeficiency, which is usually the first presentation of CD25 genetic defect (Sharfe et al. 1997; Roifman 2000; Caudy et al. 2007). This indicates that the IL-2 in humans has both immune-regulatory and immune-stimulatory properties.
However, no inhibition of T-cell proliferation or their production of cytokines was noted when human T cells were polyclonally stimulated in the presence of in vivo–achievable concentrations of daclizumab (Bielekova et al. 2006). In contrast, numbers of Tregs, their in vivo proliferation, and in vitro–suppressive functions toward effector T cells were all significantly inhibited by daclizumab therapy (Oh et al. 2009; Martin et al. 2010). Furthermore, daclizumab inhibited apoptosis of effector T cells in vivo (Baan et al. 2003) and in vitro (Wuest et al. 2011), consistent with elimination of proapoptotic effects of high-affinity IL-2 signaling. Collectively, these mechanistic studies were exceedingly perplexing because they predicted that the net effect of daclizumab therapy should be an activation of T-cell immunity seen in CD25 knockout (KO) animals and humans with genetic deletion of CD25. However, clinical trials (see below) clearly demonstrated that MS disease activity is inhibited by daclizumab therapy. This apparent discrepancy suggested that daclizumab must have additional effects on the human immune system.
The first evidence supporting this hypothesis stemmed from immunophenotyping studies performed in conjunction with the first trial of daclizumb in MS (Bielekova et al. 2004), which showed remarkable expansion of lymphocytic cells that were neither T nor B cells, but rather had a high expression of CD122 (IL-2Rβ) and intermediate expression of CD8α. These turned out to be natural killer (NK) cells, representing an important component of the innate immunity against viruses and tumors (Biron and Brossay 2001; Orange 2012). However, daclizumab expanded only minor populations of blood NK cells, characterized by high expression of CD56 (Bielekova et al. 2006). These CD56bright NK cells have been labeled as “immunoregulatory” (Cooper et al. 2001; Caligiuri 2008) for several reasons: (1) They are selectively expanded in pregnancy and may participate in mediating tolerance of the mother’s immune system to the genetically foreign fetus (Nishikawa et al. 1991; Guleria and Sayegh 2007). (2) Compared to the more prevalent CD56dim NK cells, CD56bright NK cells have lower perforin levels and completely lack granzyme B (GzB). Thus, they were long considered noncytotoxic (Jacobs et al. 2001). (3) CD56bright NK cells are enriched in lymph nodes (Fehniger et al. 2003) and, via secretion of large levels of cytokines early in the immune response (Saito et al. 1993), they may alter the phenotype of newly activated T cells. However, direct evidence for their immunoregulatory role was lacking. Functional analysis of CD56bright NK cells was limited because of their low frequencies in peripheral blood (i.e., 5%–10% of NK cells, which represents ∼1% lymphocytes) and the inability to identify analogous cells in rodents, which lack a CD56 marker. Because daclizumab expanded CD56bright NK cells up to 400%–500% (Bielekova et al. 2006, 2009; Gross et al. 2016), daclizumab-treated MS patients provided a unique chance to study these cells in detail.
Mechanistic studies showed that contrary to immunological dogma, which stipulated that NK cells do not kill autologous cells that have normal expression of major histocompatibility complex (MHC) I molecules, CD56bright NK cells could kill autologous activated T cells (Bielekova et al. 2006) using granzyme K (GzK) (Jiang et al. 2011). Specifically, CD56bight NK cells kill autologous activated T cells via perforin-mediated degranulation, which induces reactive oxygen species (ROS) and loss of mitochondrial transmembrane potential in the target cells. This represents the signature of killing by two closely related granzymes (granzyme A [GzA] and GzK) (Bovenschen et al. 2009). CD56bright NK cells are the only immune cells that express GzK constitutively (Bratke et al. 2005). Inhibition of GzK expression by short interfering RNA (siRNA) technology significantly inhibited killing of syngeneic activated T cells by the NK-92 cell line, which originates from and retains functional characteristics of CD56bright NK cells (Gong et al. 1994; Jiang et al. 2011). Additionally, daclizamab therapy specifically enhanced expression of GzK, but not other granzymes (Jiang et al. 2011). A correlation between expansion of CD56bright NK cells and contraction of absolute numbers of CD4+ and CD8+ T cells induced by daclizumab therapy provided in vivo support for the NK-mediated cytotoxicity toward activated autologous T cells (Bielekova et al. 2006). Although highly controversial at the time of its publication, the idea that CD56bright NK cells in humans (and NK cells in mice) kill autologous activated T cells that express MHC I as part of a natural immunoregulatory process has now been reproduced by many investigators using different study systems (Nielsen et al. 2012; Waggoner et al. 2012; Gross et al. 2016; Laroni et al. 2016).
Daclizumab therapy makes T-cell targets also more susceptible to NK-mediated killing (Bielekova et al. 2006). There, data were again reproduced and further expanded by demonstrating involvement of CD226 (DNAM-1)/CD155 receptor–ligand interaction in the cytolysis of activated T cells by CD56bright NK cells (Gross et al. 2016). DNAM-1 is an activating receptor, which interacts with two ligands: CD112 (nectin-2 or herpesvirus entry mediator 2) and CD155 (also known as poliovirus receptor). Analogous to the CD25/IL-2 system, genetic polymorphism in CD226/DNAM-1 has been associated with susceptibility to several autoimmune diseases, including MS (Hafler et al. 2009). CD226/CD155 interaction significantly stimulated killing of autologous activated T cells by CD56bright NK cells, and the expression defect in these interacting molecules observed in MS samples was at least partially corrected by daclizumab therapy (Gross et al. 2016). Thus, CD56bright NK cells use CD226 to recognize CD155 on the surface of antigen-activated T cells, which activates NK cells to mediate GzK-dependent killing of T cells. Strong cell-permeable antioxidants can inhibit this type of cytotoxicity. Without proper evaluation in clinical trials, these data raise concern for indiscriminate use of antioxidants in subjects with inflammatory MS.
Where does the killing of activated autologous T cells happen in vivo? CD56bright NK cells are enriched in the cerebrospinal fluid (CSF) in comparison to blood and CSF levels of CD56bright NK cells further increased after daclizumab treatment (Bielekova et al. 2011; Gross et al. 2016). GzK-expressing CD56bright NK cells were also found in MS lesions, where NK cells were in contact with T cells and polarized GzK-containing granules to such contact interphase, suggesting that aforementioned cytotoxicity occurs directly in the inflamed central nervous system (CNS) tissue (Gross et al. 2016).
Intriguingly, the target of daclizumab therapy, the CD25 Tac epitope, was not only blocked completely in the blood, but also in the CSF after daclizumab treatment. When the blood–brain barrier (BBB) is intact, only 0.1% of blood concentrations of therapeutic mAb gain access to the CSF (Rubenstein et al. 2003; Komori et al. 2016). This corresponds to a peak concentration of 10 ng/mL of daclizumab in the CSF, which is ∼100-fold lower concentration in comparison to what is necessary for saturation of CD25 expression on T cells. Therefore, if T cells were reactivated in the CNS, available concentrations of daclizumab would be insufficient to saturate de novo–produced CD25. As a result, we concluded that the cells detected in the CSF-acquired blockade of CD25 Tac epitope in the blood and they were not reactivated in the CNS (Bielekova et al. 2011; Lin et al. 2015). This means that either the inflammatory process was abrogated or, possibly, during daclizumab therapy, T cells activated in CSN tissue were destroyed by CD56bright NK cells or did not migrate out of CNS tissue back to the CSF.
We also addressed the question of why daclizumab therapy expanded and activated CD56bright NK cells. CD56bright NK cells, in contrast to CD56dim NK cells express some CD25 (Cooper et al. 2001; Bielekova et al. 2006) and are selectively expanded in vivo in response to small doses of IL-2 (Caligiuri et al. 1990). This has been interpreted as evidence that CD56bright NK cells receive the IL-2 signal via high-affinity IL-2R. However, because daclizumab therapy abrogates formation of high-affinity IL-2R, if CD25 expression was the only difference between CD56bright and CD56dim NK cells, then daclizumab therapy should have inhibited, rather than expanded, CD56bright NK cells. There is another striking difference between these NK cell subsets and that is the expression of IL-2Rβ (CD122), which is at least 10-fold higher on CD56bright in comparison to CD56dim NK cells, and 100- to 1000-fold higher compared to resting T cells (Bielekova et al. 2006). We hypothesized and experimentally confirmed that the high expression of intermediate affinity IL-2R allows for CD56bright NK cells to sustain IL-2 signaling in the presence of daclizumab (Martin et al. 2010). Further, because daclizumab limits consumption of IL-2 by T cells (which are dependent on high-affinity IL-2R because of their limited CD122 expression), excess IL-2 can be utilized by CD56bright NK cells for signaling via the intermediate affinity IL-2R. Thus, paradoxically, daclizumab expands CD56bright NK cells in an IL-2-dependent manner (Martin et al. 2010).
A correlation between in vivo expansion of CD56bright NK cells and inhibition of focal brain inflammatory activity measured by CEL (Bielekova et al. 2006) suggested that immunoregulation of T cells by CD56bright NK cells represents an important MOA of daclizumab in MS. The level of expansion of CD56bright NK cells could differentiate full responders from partial responders to daclizumab treatment (Bielekova et al. 2009). These data have been independently reproduced (Wynn et al. 2010; Elkins et al. 2012). However, even patients with the smallest levels of expansion of CD56bright NK cells had discernible efficacy on magnetic resonance imaging (MRI) and clinical markers, indicating that while expansion of CD56bright NK cells is an important mechanism, it cannot be the only mechanism responsible for drug efficacy.
Search for such alternative mechanism(s) focused on myeloid DCs, because these cells up-regulate CD25 upon their activation by microbial stimuli and are also endowed with the ability to secrete IL-2 (Granucci et al. 2001). Because mature DCs (mDCs) are the most important antigen-presenting cells (APCs) for T-cell activation, we questioned whether daclizumab affects this vital mDC function. We observed that peak in vivo achievable concentrations of daclizumab (10 µg/mL) that had no effect on proliferation of polyclonally activated T cells almost completely abolished expansion of antigen-specific T cells activated by mDCs (Wuest et al. 2011). Using selective pretreatment of mDCs or T cells with daclizumab, siRNA technology and T cells derived from a human subject with CD25 genetic deletion, we convincingly demonstrated that it is the blockade of CD25 on mDCs and not on T cells that underlies this inhibition. Further studies demonstrated that mDCs use their CD25 to present IL-2 to primed T cells “in trans”; the manner is analogous to that previously described for IL-15 (Fig. 2A) (Dubois et al. 2002). The only problem with this hypothesis was the discrepancy in affinities of IL-15Rα versus IL-2Rα (i.e., CD25) for their respective ligands; while IL-15Rα has a very strong affinity for IL-15, such that the majority (if not all) of IL-15 is bound in vivo to IL-15Rα (Fig. 2A), CD25 has a very low affinity for IL-2, which allows it to bind IL-2 only under circumstances of IL-2 abundance. Therefore, CD25 would be unable to effectively capture small quantities of IL-2 secreted to the environment. Conceptually, we remedied the problem by hypothesizing that IL-2 is trans-presented by mDCs across the immune synapse (IS) (Fig. 2B). This hypothesis turned out to be correct; instead of secreting IL-2 to the environment, mDCs release their IL-2 into the small synaptic cleft formed between an antigen-specific T cell and an mDC. Consequently, sufficiently high concentrations of IL-2 in the synaptic cleft allow effective capture of IL-2 by CD25 expressed on mDC and subsequent trans-presentation of IL-2 to the antigen-specific T cell (Wuest et al. 2011). This mDC-derived IL-2 signal was absolutely necessary for efficient expansion of antigen-specific T cells, and when it was abrogated (by blocking CD25 on mDCs) (Fig. 2C), T cells proliferated poorly, despite the fact that daclizumab-pretreated mDCs expressed high levels of MHC peptide and high levels of costimulatory molecules. Of note, we found that T cells could enter the proliferation cycle if in vitro conditions were supplemented with high doses of IL-7 as an alternative source of γc-signaling cytokine. However, T cells expanded in this manner did not express full effector functions. A similar observation was made in the animal system, where the IL-2 signal was absolutely necessary during priming of the antigen-specific CD8+ T cells for effective development of T-cell memory responses (Williams et al. 2006). Thus, the described mechanism can fully explain the strange combination of immunodeficiency with lymphoproliferation, which is characteristic of CD25 deletion in humans.
Final described MOA represents the inhibitory effect of daclizumab on innate lymphoid cells (ILCs), especially on development of their proinflammatory subtype called lymphoid tissue inducer (LTi) cells (Perry et al. 2012).
ILCs, a heterogeneous group of lymphocytes, belong to innate not adaptive immune systems. Whereas the most familiar subset of ILCs are NK cells, this category also contains cells with constitutive expression of RORγt. Depending on the tissue from which these cells have been isolated, they express slightly different phenotypes and are therefore labeled with different names (Sawa et al. 2010; Spits and Di Santo 2011), for example, LTi cells, ILC22 (i.e., IL-22-producing ILCs that also express NKp44), and ILC17 (IL-17-producing ILCs). It is clear that these different ILC categories are related developmentally because all originate from CD34+ hematopoietic precursors and all are dependent on the Id2 transcriptional regulator (Yokota et al. 1999).
Although LTi cells play a fundamental role in the formation of secondary lymphoid tissues during fetal development (Aloisi and Pujol-Borrell 2006), tertiary ectopic follicles associated with chronic inflammation can form in RORγt-deficient animals (Lochner et al. 2011), where either activated T or B cells can acquire lymphoid tissue–inducing capacity. What remains unclear is the role that adult LTi cells play. It is hypothesized that, via constitutive expression of OX40 and CD30, adult LTi cells may play a vital role in the evolution and maintenance of the CD4+ T-cell memory (Withers et al. 2012) and related B-cell/Ab responses, including formation of high-affinity class-switched IgG (Lane et al. 2012).
We observed that MS patients who were untreated have significantly greater levels of c-kit+/RORγt-expressing LTi cells in the blood and CSF (Perry et al. 2012; Lin et al. 2015) in comparison to healthy controls. These observations were reproduced in an independent cohort of MS patients, although the elevations of LTi cells in the blood did not reach formal statistical significance (Degn et al. 2015). Daclizumab therapy normalizes this CSF abnormality (Perry et al. 2012; Lin et al. 2015) by skewing development of CD34+ hematopoietic stem cells and c-kit+ undifferentiated ILC precursors away from LTi lineage toward CD56bright NK cells through enhanced intermediate affinity IL-2R-signaling (Perry et al. 2012).
Although meningeal inflammation cannot be directly visualized in living MS subjects (Magliozzi et al. 2007; Howell et al. 2011), daclizumab therapy decreased intrathecal production of chemokine CXCL13 and of IgG, measured as the IgG index (Perry et al. 2012). The affect of daclizumab on IgG production was specific for the intrathecal compartment, because earlier studies reported that daclizumab did not lower IgG, IgA, or IgM blood levels (Bielekova et al. 2004, 2006). Daclizumab does not limit migration of immune cells to the intrathecal compartment (Bielekova et al. 2011; Lin et al. 2015); therefore, decreased intrathecal CXCL13 levels that are highly expressed in tertiary lymphoid follicles (Magliozzi et al. 2004, 2007) indirectly supports the notion that inhibition of LTi cells by daclizumab may have impeded maintenance of meningeal lymphoid aggregates and associated immune responses. Because the creation of meningeal lymphoid follicles in MS is linked to greater pathology of the underlying gray matter (Howell et al. 2011), it is intriguing to note that in comparison to standard MS therapies such as interferon β (IFN-β) or glatiramer acetate, daclizumab had significantly greater inhibitory effect on brain atrophy (Kappos et al. 2015), especially of the gray matter (Borges et al. 2013).
EFFICACY AND SAFETY OF DACLIZUMAB IN MS
In the proof-of-principle, open-label, baseline-versus-treatment phase II clinical trial (Clinicaltrials.gov identifier: NCT00001934), addition of daclizumab to patients who had inadequate clinical and radiological response to IFN-β resulted in >80% inhibition of contrast-enhancing lesions (CELs) on brain MRI as well as stabilized clinical disease activity (Rose et al. 2004; Bielekova et al. 2014). In the majority of MS patients, IFN-β could be withdrawn after 6 months of combination therapy without a decline in clinical efficacy of long-term daclizumab monotherapy (Rose et al. 2007; Bielekova et al. 2009). However, IFN-β and daclizumab were also found to have synergistic effects, and optimal response in 1/3 of patients required either continued IFN-β/daclizumab combination therapy or a higher dose of daclizumab monotherapy (i.e., 2 mg/kg every 4 wk instead of the traditional 1 mg/kg every 4 wk) (Bielekova et al. 2009). This observation was puzzling because the already standard dose of 1 mg/kg led to 100% saturation of CD25 Tac epitope on peripheral blood mononuclear cells (PBMCs) (Bielekova et al. 2004), and suggested for the first time that blockade of CD25 in tissues is important for daclizumab’s effect on MS. This hypothesis was proven by demonstrating that daclizumab had to block CD25 on DCs (residing in lymphoid organs or tissues) to achieve its inhibitory effect on T-cell activation.
Whereas all open-label studies observed stabilization (Bielekova et al. 2004; Rose et al. 2004, 2007) or even improvements (Bielekova et al. 2009, 2011) of clinical outcomes, this had to be reproduced in placebo-controlled studies. Three multicenter trials were sponsored by the pharmaceutical industry: the CHOICE study (clinicaltrials.gov identifier: NCT00109161) (Wynn et al. 2010), which investigated IFN-β/daclizumab combination therapy, and two monotherapy trials: the placebo-controlled SELECT trial (clinicaltrials.gov identifier: NCT00870740) (Gold et al. 2013) and the DECIDE (clinicaltrials.gov identifier: NCT01064401) trial (Kappos et al. 2015), which tested efficacy of daclizumab against active comparator. The latter two studies used a new preparation of daclizumab called daclizumab high-yield process (DAC HYP, Zinbryta; AbbVie and Biogen), which has an identical amino-acid sequence to the original Zenapax preparation; however, changes in the production process resulted in an altered glycosylation pattern of the molecule affecting binding of daclizumab to Fc receptors. Such change can modify complement-dependent cytotoxicity (CDC) and Ab-dependent cellular cytotoxicity (ADCC), as was shown previously for different therapeutic mAb (Bielekova and Becker 2010). In addition, DAC HYP was developed for subcutaneous administration (once every 4 wk) in contrast to intravenous administration of Zenapax. The efficacy and safety of long-term DAC HYP monotherapy was examined in the extension trial called SELECTION (Giovannoni et al. 2014).
In the CHOICE study (N = 230), addition of subcutaneously administered daclizumab (low dose: 1 mg/kg every 4 wk; high dose: 2 mg/kg every 2 wk) to IFN-β decreased CEL lesions by 72% in the high-dose arm (p = 0.004) and by 25% (p = 0.51) in the low-dose arm (Wynn et al. 2010). Systemic levels of daclizumab yielded in the high-dose arm were comparable to the intravenous 1 mg/kg dose utilized in all of the previous open-label trials. In the SELECT trial (N = 600), two doses of DAC HYP monotherapy (150 mg or 300 mg every 4 wk) administered subcutaneously for 1 yr were compared to placebo. Both doses inhibited formation of CEL (by 68.8% and 79.2%; p < 0.001) and new or enlarging T2 lesions (by 70.4% and 79.0%; p < 0.001) on brain MRI (Gold et al. 2012). This efficacy on MRI parameters was paralleled by significant inhibition of annualized relapse rate (by 54.3% and 50%; p < 0.001) and by inhibition of disability progression (by 57%; p = 0.021 for 150 mg dose and by 43%; p = 0.091 for 300 mg dose).
The SELECTION extension trial examined several important issues, such as rebound phenomenon after stopping of DAC HYP treatment, efficacy after reinitiation of therapy, and long-term efficacy/safety. This trial confirmed no meaningful difference between 150 mg and 300 mg doses, lack of rebound of disease activity beyond baseline values, and continuous efficacy of daclizumab (on relapses, MRI markers, and disability) after treatment reinitiation or its continuous use (Giovannoni et al. 2014). This study also reproduced daclizumab-driven expansion of CD56bright regulatory NK cells.
Finally, the DECIDE trial demonstrated statistically significant superiority of DAC HYP (150 mg subcutaneously every 4 wk) against weekly intramuscular administration of IFN-β-1a in annualized relapse rate (45% inhibition) and the number of new or enlarging T2 lesions (54% reduction) (Kappos et al. 2015).
In general, all clinical trials in MS found daclizumab well tolerated, with little induced immunogenicity. The incidence of adverse events (AEs) and the rate of discontinuation of therapy were similar between both placebo and daclizumab arms in both CHOICE (Wynn et al. 2010) and SELECT (Gold et al. 2012) trials and between IFN-β and daclizumab in the DECIDE trial (Kappos et al. 2015). The most common AEs observed in MS trials of daclizumab belong to four categories: (1) lymphadenopathy, (2) skin rashes, (3) elevated liver function tests (LFTs), and (4) infection (Bielekova et al. 2004, 2009, 2011; Wynn et al. 2010; Gold et al. 2012; Kappos et al. 2015).
We will discuss these categories of AEs in view of the described MOA; in the CHOICE study, skin rashes appeared in 13% of daclizumab subjects and 8% of placebo subjects (Wynn et al. 2010). National Institutes of Health (NIH) trials observed frequent skin rashes of mainly mild intensity that responded to topical steroids or emollients (Cortese et al. 2016). However, they also reported few prolonged and more severe skin rashes requiring systemic steroids and/or discontinuation of therapy (Bielekova et al. 2004, 2009, 2011; Oh et al. 2014). In the SELECT trial, serious cutaneous events were observed in 1% of DAC HYP–treated subjects (Gold et al. 2012) and in 2% of DAC HYP–treated subjects in the DECIDE trial (Kappos et al. 2015). It is unclear what underlies enhanced skin reactivity in daclizumab-treated subjects; one hypothesis implicates inhibition of FoxP3 Tregs driven by daclizumab (Oh et al. 2009), while lesional skin biopsies demonstrated increased presence of CD56+ NK cells (Cortese et al. 2016), suggesting that the same cell population that mediates beneficial effects of daclizumab on MS may be responsible for cutaneous side-effects.
Often associated with daclizumab therapy is generalized mild lymphadenopathy (Bielekova et al. 2004, 2009) without pathological consequences. We have evaluated several subjects with prominent or persistent lymphadenopathy using fine needle biopsy, and found no pathological changes in flow cytometry or pathology profile (Oh et al. 2014; B Bielekova et al., unpubl.).
Elevations of LFTs have been observed in daclizumab-treated cohorts; serious elevations of LFTs (five times above upper limit of normal) were observed in 4% of DAC HYP–treated subjects in the SELECT trial and in 6% of subjects in the DECIDE trial (as compared to 3% of IFN-β-treated subjects). The mechanism behind this phenomenon is again unclear, although CD56bright NK cells represent a prominent immune cell population in the liver under physiological conditions (Moroso et al. 2010).
Although open-label NIH studies observed slight increases in the frequency of mild infectious AEs (mostly urinary tract infection [UTI] and upper respiratory tract), the CHOICE study did not demonstrate an increased incidence of infections (Wynn et al. 2010). Contrastingly, the SELECT trial reported an increase in serious infections (2%) in the DAC HYP cohort and the DECIDE trial confirmed these findings by observing a 4% rate of serious infections in the DAC HYP group (in comparison to a 2% rate in the IFN-β group). One DAC HYP–treated subject died due to complications of a psoas abscess (Gold et al. 2012).
Will daclizumab cause progressive multifocal leukoencephalopathy (PML)? Because it does not limit access of immune cells to the intrathecal compartment and expanded CD56bright NK cells may provide effective immunity against some viruses (Orange 2012), we would predict risk of PML is lower in daclizumab-treated patients compared to those treatments that directly limit access of the immune system to CNS. However, only broad postmarketing experience will determine the actual risks of long-term daclizumab administration.
Finally, none of the clinical trials demonstrated increased risk of cancer with daclizumab therapy and again, from the mechanistic standpoint, we would predict that activation of (CD56bright) NK cells may enhance NK-mediated immunosurveillance against cancer even if T-cell immunity is diminished in parallel. Our conclusion, which is supported by in vivo observations from transplantation, where, in addition to standard immunosuppressive therapy, these patients who received daclizumab had in fact lower levels of secondary cancers (Webster et al. 2004) than patients who received identical immunosuppression without daclizumab.
CONCLUSIONS
Careful in vivo observations that were supplemented by mechanistic studies revealed unexpected effects of daclizumab on cells of the innate immune system, CD56bright NK cells, mDCs, and ILCs. These effects were not previously identified in animals with genetically deleted IL-2 or its signaling components, signaling that carefully conducted mechanistic studies linked to human interventional trials have the potential to discover novel biological mechanisms (Bielekova et al. 2014). Insights such as these may not only be relevant for the MOA of the studied therapeutic, but also for the pathophysiology of the targeted disease. The MOA of daclizumab reminds us that adaptive immune responses are tightly controlled by cells of the innate immune system. Both systems must be studied via integrated design before the mechanisms that lead to the breakdown of tolerance in MS can be fully understood.
ACKNOWLEDGMENTS
The review is supported by the National Institute of Neurological Disorders and Stroke (NINDS) Intramural Research Program. The author has received patent royalty payments for her work as co-inventor on National Institutes of Health (NIH) patents related to daclizumab therapy.
Footnotes
Editors: Howard L. Weiner and Vijay K. Kuchroo
Additional Perspectives on Multiple Sclerosis available at www.perspectivesinmedicine.org
REFERENCES
- Almeida AR, Legrand N, Papiernik M, Freitas AA. 2002. Homeostasis of peripheral CD4+ T cells: IL-2Rα and IL-2 shape a population of regulatory cells that controls CD4+ T cell numbers. J Immunol 169: 4850–4860. [DOI] [PubMed] [Google Scholar]
- Aloisi F, Pujol-Borrell R. 2006. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol 6: 205–217. [DOI] [PubMed] [Google Scholar]
- Baan CC, Balk AH, van Riemsdijk IC, Vantrimpont PJ, Maat AP, Niesters HG, Zondervan PE, van Gelder T, Weimar W. 2003. Anti-CD25 monoclonal antibody therapy affects the death signals of graft-infiltrating cells after clinical heart transplantation. Transplantation 75: 1704–1710. [DOI] [PubMed] [Google Scholar]
- Bielekova B, Becker B. 2010. Monoclonal antibodies in MS: Mechanism of action. Neurology 74: S31–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielekova B, Richert N, Howard T, Blevins G, Markovic-Plese S, McCartin J, Wurfel J, Ohayon J, Waldmann TA, McFarland HF, et al. 2004. Humanized anti-CD25 (daclizumab) inhibits disease activity in multiple sclerosis patients failing to respond to interferon-β. Proc Natl Acad Sci 101: 8705–8708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielekova B, Catalfamo M, Reichert-Scrivner S, Packer A, Cerna M, Waldmann TA, McFarland H, Henkart PA, Martin R. 2006. Regulatory CD56bright natural killer cells mediate immunomodulatory effects of IL-2R-α-targeted therapy (daclizumab) in multiple sclerosis. Proc Natl Acad Sci 103: 5941–5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielekova B, Howard T, Packer AN, Richert N, Blevins G, Ohayon J, Waldmann TA, McFarland HF, Martin R. 2009. Effect of anti-CD25 antibody daclizumab in the inhibition of inflammation and stabilization of disease progression in multiple sclerosis. Arch Neurol 66: 483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielekova B, Richert N, Herman ML, Ohayon J, Waldmann TA, McFarland H, Martin R, Blevins G. 2011. Intrathecal effects of daclizumab treatment of multiple sclerosis. Neurology 77: 1877–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielekova B, Vodovotz Y, An G, Hallenbeck J. 2014. How implementation of systems biology into clinical trials accelerates understanding of diseases. Front Neurol 5: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biron CA, Brossay L. 2001. NK cells and NKT cells in innate defense against viral infections. Curr Opin Immunol 13: 458–464. [DOI] [PubMed] [Google Scholar]
- Borges IT, Shea CD, Ohayon J, Jones BC, Stone RD, Ostuni J, Shiee N, McFarland H, Bielekova B, Reich DS. 2013. The effect of daclizumab on brain atrophy in relapsing-remitting multiple sclerosis. Mult Scler Relat Disord 2: 133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovenschen N, Quadir R, van den Berg AL, Brenkman AB, Vandenberghe I, Devreese B, Joore J, Kummer JA. 2009. Granzyme K displays highly restricted substrate specificity that only partially overlaps with granzyme A. J Biol Chem 284: 3504–3512. [DOI] [PubMed] [Google Scholar]
- Bratke K, Kuepper M, Bade B, Virchow JC Jr, Luttmann W. 2005. Differential expression of human granzymes A, B, and K in natural killer cells and during CD8+ T cell differentiation in peripheral blood. Eur J Immunol 35: 2608–2616. [DOI] [PubMed] [Google Scholar]
- Caligiuri MA. 2008. Human natural killer cells. Blood 112: 461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligiuri MA, Zmuidzinas A, Manley TJ, Levine H, Smith KA, Ritz J. 1990. Functional consequences of interleukin 2 receptor expression on resting human lymphocytes. Identification of a novel natural killer cell subset with high affinity receptors. J Exp Med 171: 1509–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudy AA, Reddy ST, Chatila T, Atkinson JP, Verbsky JW. 2007. CD25 deficiency causes an immune dysregulation, polyendocrinopathy, enteropathy, X-linked-like syndrome, and defective IL-10 expression from CD4 lymphocytes. J Allergy Clin Immunol 119: 482–487. [DOI] [PubMed] [Google Scholar]
- Cooper MA, Fehniger TA, Caligiuri MA. 2001. The biology of human natural killer-cell subsets. Trends Immunol 22: 633–640. [DOI] [PubMed] [Google Scholar]
- Cortese I, Ohayon J, Fenton K, Lee CC, Raffeld M, Cowen EW, DiGiovanna JJ, Bielekova B. 2016. Cutaneous adverse events in multiple sclerosis patients treated with daclizumab. Neurology 86: 847–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degn M, Modvig S, Dyring-Andersen B, Bonefeld CM, Frederiksen JL, Geisler C, von Essen MR. 2015. Increased prevalence of lymphoid tissue inducer cells in the cerebrospinal fluid of patients with early multiple sclerosis. Mult Scler 22: 1013–1020. [DOI] [PubMed] [Google Scholar]
- Dubois S, Mariner J, Waldmann TA, Tagaya Y. 2002. IL-15Rα recycles and presents IL-15 in trans to neighboring cells. Immunity 17: 537–547. [DOI] [PubMed] [Google Scholar]
- Elkins J, Sheridan J, Armaravadi L, Riester K, O’Neil G. 2012. CD56bright natural killer cell expansion predicts response to daclizumab HYP treatment in RRMS: Results of the SELECT trial. Neurology 78 (meeting abstracts): S31.004. [Google Scholar]
- Fehniger TA, Cooper MA, Nuovo GJ, Cella M, Facchetti F, Colonna M, Caligiuri MA. 2003. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: A potential new link between adaptive and innate immunity. Blood 101: 3052–3057. [DOI] [PubMed] [Google Scholar]
- Giovannoni G, Gold R, Selmaj K, Havrdova E, Montalban X, Radue EW, Stefoski D, McNeill M, Amaravadi L, Sweetser M, et al. 2014. Daclizumab high-yield process in relapsing-remitting multiple sclerosis (SELECTION): A multicentre, randomised, double-blind extension trial. Lancet Neurol 13: 472–481. [DOI] [PubMed] [Google Scholar]
- Gold R, Giovannoni G, Selmaj K, Havrdova E, Moltalban X, Radue EW, Stefoski D, Robinson R, Riester K, Elkins J, et al. 2012. A randomized, double-blind, placebo-controlled study to evaluate the safety and efficacy of daclizumab HYP monotherapy in relapsing-remitting multiple sclerosis: Primary results of the SELECT trial. Neurology 78 (meeting abstracts): S01.005. [Google Scholar]
- Gold R, Giovannoni G, Selmaj K, Havrdova E, Montalban X, Radue EW, Stefoski D, Robinson R, Riester K, Rana J, et al. 2013. Daclizumab high-yield process in relapsing-remitting multiple sclerosis (SELECT): A randomised, double-blind, placebo-controlled trial. Lancet 381: 2167–2175. [DOI] [PubMed] [Google Scholar]
- Gong JH, Maki G, Klingemann HG. 1994. Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia 8: 652–658. [PubMed] [Google Scholar]
- Granucci F, Vizzardelli C, Pavelka N, Feau S, Persico M, Virzi E, Rescigno M, Moro G, Ricciardi-Castagnoli P. 2001. Inducible IL-2 production by dendritic cells revealed by global gene expression analysis. Nat Immunol 2: 882–888. [DOI] [PubMed] [Google Scholar]
- Gross CC, Schulte-Mecklenbeck A, Runzi A, Kuhlmann T, Posevitz-Fejfar A, Schwab N, Schneider-Hohendorf T, Herich S, Held K, Konjevic M, et al. 2016. Impaired NK-mediated regulation of T-cell activity in multiple sclerosis is reconstituted by IL-2 receptor modulation. Proc Natl Acad Sci 113: E2973–E2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guleria I, Sayegh MH. 2007. Maternal acceptance of the fetus: True human tolerance. J Immunol 178: 3345–3351. [DOI] [PubMed] [Google Scholar]
- Hafler DA, Compston A, Sawcer S, Lander ES, Daly MJ, De Jager PL, de Bakker PI, Gabriel SB, Mirel DB, Ivinson AJ, et al. 2007. Risk alleles for multiple sclerosis identified by a genomewide study. N Engl J Med 357: 851–862. [DOI] [PubMed] [Google Scholar]
- Hafler JP, Maier LM, Cooper JD, Plagnol V, Hinks A, Simmonds MJ, Stevens HE, Walker NM, Healy B, Howson JM, et al. 2009. CD226 Gly307Ser association with multiple autoimmune diseases. Genes Immunity 10: 5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell OW, Reeves CA, Nicholas R, Carassiti D, Radotra B, Gentleman SM, Serafini B, Aloisi F, Roncaroli F, Magliozzi R, et al. 2011. Meningeal inflammation is widespread and linked to cortical pathology in multiple sclerosis. Brain 134: 2755–2771. [DOI] [PubMed] [Google Scholar]
- Jacobs R, Hintzen G, Kemper A, Beul K, Kempf S, Behrens G, Sykora KW, Schmidt RE. 2001. CD56bright cells differ in their KIR repertoire and cytotoxic features from CD56dim NK cells. Eur J Immunol 31: 3121–3127. [DOI] [PubMed] [Google Scholar]
- Jiang W, Chai NR, Maric D, Bielekova B. 2011. Unexpected role for Granzyme K in CD56bright NK cell-mediated immunoregulation of multiple sclerosis. J Immunol 187: 781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappos L, Wiendl H, Selmaj K, Arnold DL, Havrdova E, Boyko A, Kaufman M, Rose J, Greenberg S, Sweetser M, et al. 2015. Daclizumab HYP versus interferon β-1a in relapsing multiple sclerosis. N Engl J Med 373: 1418–1428. [DOI] [PubMed] [Google Scholar]
- Komori M, Lin YC, Cortese I, Blake A, Ohayon J, Cherup J, Maric D, Kosa P, Wu T, Bielekova B. 2016. Insufficient disease inhibition by intrathecal rituximab in progressive multiple sclerosis. Ann Clin Transl Neurol 3: 166–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundig TM, Schorle H, Bachmann MF, Hengartner H, Zinkernagel RM, Horak I. 1993. Immune responses in interleukin-2-deficient mice. Science 262: 1059–1061. [DOI] [PubMed] [Google Scholar]
- Lane PJ, Gaspal FM, McConnell FM, Kim MY, Anderson G, Withers DR. 2012. Lymphoid tissue inducer cells: Innate cells critical for CD4+ T cell memory responses? Ann NY Acad Sci 1247: 1–15. [DOI] [PubMed] [Google Scholar]
- Laroni A, Armentani E, Kerlero de Rosbo N, Ivaldi F, Marcenaro E, Sivori S, Gandhi R, Weiner HL, Moretta A, Mancardi GL, et al. 2016. Dysregulation of regulatory CD56 NK cells/T cells interactions in multiple sclerosis. J Autoimmun 72: 8–18. [DOI] [PubMed] [Google Scholar]
- Lenardo MJ. 1991. Interleukin-2 programs mouse T lymphocytes for apoptosis. Nature 353: 858–861. [DOI] [PubMed] [Google Scholar]
- Lin YC, Winokur P, Blake A, Wu T, Romm E, Bielekova B. 2015. Daclizumab reverses intrathecal immune cell abnormalities in multiple sclerosis. Ann Clin Transl Neurol 2: 445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochner M, Ohnmacht C, Presley L, Bruhns P, Si-Tahar M, Sawa S, Eberl G. 2011. Microbiota-induced tertiary lymphoid tissues aggravate inflammatory disease in the absence of RORγt and LTi cells. J Exp Med 208: 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe CE, Cooper JD, Brusko T, Walker NM, Smyth DJ, Bailey R, Bourget K, Plagnol V, Field S, Atkinson M, et al. 2007. Large-scale genetic fine mapping and genotype-phenotype associations implicate polymorphism in the IL2RA region in type 1 diabetes. Nat Genet 39: 1074–1082. [DOI] [PubMed] [Google Scholar]
- Magliozzi R, Columba-Cabezas S, Serafini B, Aloisi F. 2004. Intracerebral expression of CXCL13 and BAFF is accompanied by formation of lymphoid follicle-like structures in the meninges of mice with relapsing experimental autoimmune encephalomyelitis. J Neuroimmunol 148: 11–23. [DOI] [PubMed] [Google Scholar]
- Magliozzi R, Howell O, Vora A, Serafini B, Nicholas R, Puopolo M, Reynolds R, Aloisi F. 2007. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain 130: 1089–1104. [DOI] [PubMed] [Google Scholar]
- Maier LM, Lowe CE, Cooper J, Downes K, Anderson DE, Severson C, Clark PM, Healy B, Walker N, Aubin C, et al. 2009. IL2RA genetic heterogeneity in multiple sclerosis and type 1 diabetes susceptibility and soluble interleukin-2 receptor production. PLoS Genet 5: e1000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek TR. 2003. The main function of IL-2 is to promote the development of T regulatory cells. J Leukoc Biol 74: 961–965. [DOI] [PubMed] [Google Scholar]
- Martin JF, Perry JS, Jakhete NR, Wang X, Bielekova B. 2010. An IL-2 paradox: Blocking CD25 on T cells induces IL-2-driven activation of CD56bright NK cells. J Immunol 185: 1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizel SB, Farrar JJ. 1979. Revised nomenclature for antigen-nonspecific T-cell proliferation and helper factors. Cell Immunol 48: 433–436. [DOI] [PubMed] [Google Scholar]
- Moroso V, Metselaar HJ, Mancham S, Tilanus HW, Eissens D, van der Meer A, van der Laan LJ, Kuipers EJ, Joosten I, Kwekkeboom J. 2010. Liver grafts contain a unique subset of natural killer cells that are transferred into the recipient after liver transplantation. Liver Transpl 16: 895–908. [DOI] [PubMed] [Google Scholar]
- Nielsen N, Odum N, Urso B, Lanier LL, Spee P. 2012. Cytotoxicity of CD56bright NK cells towards autologous activated CD4+ T cells is mediated through NKG2D, LFA-1 and TRAIL and dampened via CD94/NKG2A. PLoS ONE 7: e31959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa K, Saito S, Morii T, Hamada K, Ako H, Narita N, Ichijo M, Kurahayashi M, Sugamura K. 1991. Accumulation of CD16−CD56+ natural killer cells with high affinity interleukin 2 receptors in human early pregnancy decidua. Int Immunol 3: 743–750. [DOI] [PubMed] [Google Scholar]
- Oh U, Blevins G, Griffith C, Richert N, Maric D, Lee CR, McFarland H, Jacobson S. 2009. Regulatory T cells are reduced during anti-CD25 antibody treatment of multiple sclerosis. Arch Neurol 66: 471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J, Saidha S, Cortese I, Ohayon J, Bielekova B, Calabresi PA, Newsome SD. 2014. Daclizumab-induced adverse events in multiple organ systems in multiple sclerosis. Neurology 82: 984–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orange JS. 2012. Unraveling human natural killer cell deficiency. J Clin Invest 122: 798–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JS, Han S, Xu Q, Herman ML, Kennedy LB, Csako G, Bielekova B. 2012. Inhibition of LTi cell development by CD25 blockade is associated with decreased intrathecal inflammation in multiple sclerosis. Sci Transl Med 4: 145ra106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queen C, Schneider WP, Selick HE, Payne PW, Landolfi NF, Duncan JF, Avdalovic NM, Levitt M, Junghans RP, Waldmann TA. 1989. A humanized antibody that binds to the interleukin 2 receptor. Proc Natl Acad Sci 86: 10029–10033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickert M, Wang X, Boulanger MJ, Goriatcheva N, Garcia KC. 2005. The structure of interleukin-2 complexed with its α receptor. Science 308: 1477–1480. [DOI] [PubMed] [Google Scholar]
- Roifman CM. 2000. Human IL-2 receptor α chain deficiency. Pediatr Res 48: 6–11. [DOI] [PubMed] [Google Scholar]
- Rose JW, Watt HE, White AT, Carlson NG. 2004. Treatment of multiple sclerosis with an anti-interleukin-2 receptor monoclonal antibody. Ann Neurol 56: 864–867. [DOI] [PubMed] [Google Scholar]
- Rose JW, Burns JB, Bjorklund J, Klein J, Watt HE, Carlson NG. 2007. Daclizumab phase II trial in relapsing and remitting multiple sclerosis: MRI and clinical results. Neurology 69: 785–789. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Combs D, Rosenberg J, Levy A, McDermott M, Damon L, Ignoffo R, Aldape K, Shen A, Lee D, et al. 2003. Rituximab therapy for CNS lymphomas: Targeting the leptomeningeal compartment. Blood 101: 466–468. [DOI] [PubMed] [Google Scholar]
- Ruscetti FW, Morgan DA, Gallo RC. 1977. Functional and morphologic characterization of human T cells continuously grown in vitro. J Immunol 119: 131–138. [PubMed] [Google Scholar]
- Saito S, Nishikawa K, Morii T, Enomoto M, Narita N, Motoyoshi K, Ichijo M. 1993. Cytokine production by CD16-CD56bright natural killer cells in the human early pregnancy decidua. Int Immunol 5: 559–563. [DOI] [PubMed] [Google Scholar]
- Sawa S, Cherrier M, Lochner M, Satoh-Takayama N, Fehling HJ, Langa F, Di Santo JP, Eberl G. 2010. Lineage relationship analysis of RORγt+ innate lymphoid cells. Science 330: 665–669. [DOI] [PubMed] [Google Scholar]
- Schorle H, Holtschke T, Hunig T, Schimpl A, Horak I. 1991. Development and function of T cells in mice rendered interleukin-2 deficient by gene targeting. Nature 352: 621–624. [DOI] [PubMed] [Google Scholar]
- Setoguchi R, Hori S, Takahashi T, Sakaguchi S. 2005. Homeostatic maintenance of natural Foxp3+ CD25+ CD4+ regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med 201: 723–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharfe N, Dadi HK, Shahar M, Roifman CM. 1997. Human immune disorder arising from mutation of the α chain of the interleukin-2 receptor. Proc Natl Acad Sci 94: 3168–3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevach EM, McHugh RS, Piccirillo CA, Thornton AM. 2001. Control of T-cell activation by CD4+ CD25+ suppressor T cells. Immunol Rev 182: 58–67. [DOI] [PubMed] [Google Scholar]
- Spits H, Di Santo JP. 2011. The expanding family of innate lymphoid cells: Regulators and effectors of immunity and tissue remodeling. Nat Immunol 12: 21–27. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Kundig TM, Furlonger C, Wakeham A, Timms E, Matsuyama T, Schmits R, Simard JJ, Ohashi PS, Griesser H, et al. 1995. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor β. Science 268: 1472–1476. [DOI] [PubMed] [Google Scholar]
- Turka LA, Walsh PT. 2008. IL-2 signaling and CD4+ CD25+ Foxp3+ regulatory T cells. Front Biosci 13: 1440–1446. [DOI] [PubMed] [Google Scholar]
- Van Parijs L, Refaeli Y, Lord JD, Nelson BH, Abbas AK, Baltimore D. 1999. Uncoupling IL-2 signals that regulate T cell proliferation, survival, and Fas-mediated activation-induced cell death. Immunity 11: 281–288. [DOI] [PubMed] [Google Scholar]
- Waggoner SN, Cornberg M, Selin LK, Welsh RM. 2012. Natural killer cells act as rheostats modulating antiviral T cells. Nature 481: 394–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi K, Lian ZX, Moritoki Y, Lan RY, Tsuneyama K, Chuang YH, Yang GX, Ridgway W, Ueno Y, Ansari AA, et al. 2006. IL-2 receptor α–/– mice and the development of primary biliary cirrhosis. Hepatology 44: 1240–1249. [DOI] [PubMed] [Google Scholar]
- Waldmann T, Tagaya Y, Bamford R. 1998. Interleukin-2, interleukin-15, and their receptors. Int Rev Immunol 16: 205–226. [DOI] [PubMed] [Google Scholar]
- Wang X, Rickert M, Garcia KC. 2005. Structure of the quaternary complex of interleukin-2 with its α, β, and γc receptors. Science 310: 1159–1163. [DOI] [PubMed] [Google Scholar]
- Webster AC, Playford EG, Higgins G, Chapman JR, Craig JC. 2004. Interleukin 2 receptor antagonists for renal transplant recipients: A meta-analysis of randomized trials. Transplantation 77: 166–176. [DOI] [PubMed] [Google Scholar]
- Willerford DM, Chen J, Ferry JA, Davidson L, Ma A, Alt FW. 1995. Interleukin-2 receptor α chain regulates the size and content of the peripheral lymphoid compartment. Immunity 3: 521–530. [DOI] [PubMed] [Google Scholar]
- Williams MA, Tyznik AJ, Bevan MJ. 2006. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature 441: 890–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers DR, Gaspal FM, Mackley EC, Marriott CL, Ross EA, Desanti GE, Roberts NA, White AJ, Flores-Langarica A, McConnell FM, et al. 2012. Cutting edge: Lymphoid tissue inducer cells maintain memory CD4 T cells within secondary lymphoid tissue. J Immunol 189: 2094–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuest SC, Edwan JH, Martin JF, Han S, Perry JS, Cartagena CM, Matsuura E, Maric D, Waldmann TA, Bielekova B. 2011. A role for interleukin-2 trans-presentation in dendritic cell-mediated T cell activation in humans, as revealed by daclizumab therapy. Nat Med 17: 604–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn D, Kaufman M, Montalban X, Vollmer T, Simon J, Elkins J, O’Neill G, Neyer L, Sheridan J, Wang C, et al. 2010. Daclizumab in active relapsing multiple sclerosis (CHOICE study): A phase 2, randomised, double-blind, placebo-controlled, add-on trial with interferon β. Lancet Neurol 9: 381–390. [DOI] [PubMed] [Google Scholar]
- Yokota Y, Mansouri A, Mori S, Sugawara S, Adachi S, Nishikawa S, Gruss P. 1999. Development of peripheral lymphoid organs and natural killer cells depends on the helix–loop–helix inhibitor Id2. Nature 397: 702–706. [DOI] [PubMed] [Google Scholar]