Abstract
Aims
This study aimed to assess clinicopathologic features of transactive response DNA-binding protein of 43 kDa (TDP-43) pathology and its risk factors in multiple system atrophy (MSA).
Methods
Paraffin-embedded sections of the amygdala and basal forebrain from 186 autopsy-confirmed MSA cases were screened with immunohistochemistry for phospho-TDP-43. In cases having TDP-43 pathology, additional brain regions were assessed. Immunohistochemical and immunofluorescence double-staining and immunogold electron microscopy (IEM) were performed to evaluate colocalization of TDP-43 and α-synuclein. Genetic risk factors for TDP-43 pathology were also analysed.
Results
Immunohistochemistry showed various morphologies of TDP-43 pathology in 13 cases (7%), such as subpial astrocytic inclusions, neuronal inclusions, dystrophic neurites, perivascular inclusions, and glial cytoplasmic inclusions (GCIs). Multivariable logistic regression models revealed that only advanced age, but not concurrent Alzheimer's disease, argyrophilic grain disease or hippocampal sclerosis, was an independent risk factor for TDP-43 pathology in MSA (OR: 1.11, 95% CI: 1.04–1.19, P = 0.002). TDP-43 pathology was restricted to the amygdala in eight cases and extended to the hippocampus in two cases. The remaining three cases had widespread TDP-43 pathology. Immunohistochemical and immunofluorescence double-staining and IEM revealed colocalization of α-synuclein and TDP-43 in GCIs with granule-coated filaments. Pilot genetic studies failed to show associations between risk variants of TMEM106B or GRN and TDP-43 pathology.
Conclusions
TDP-43 pathology is rare in MSA and occurs mainly in the medial temporal lobe. Advanced age is a risk factor for TDP-43 pathology in MSA. Colocalization of TDP-43 and α-synuclein in GCIs suggests possible direct interaction between the two molecules.
Keywords: Multiple system atrophy, TDP-43, hippocampal sclerosis, argyrophilic grain disease, glial cytoplasmic inclusion, α-synuclein
Introduction
Multiple system atrophy (MSA) is a progressive neurodegenerative disease characterized by variable combinations of autonomic failure, parkinsonism, cerebellar ataxia, and pyramidal symptoms [1]. Some MSA patients develop cognitive impairment, mainly executive dysfunction [2-4]. In rare cases, so-called atypical MSA, patients show clinical phenotypes of frontotemporal dementia (FTD), including corticobasal syndrome or behavioral variant FTD [5]. Pathologically, MSA is classified as an α-synucleinopathy, and glial cytoplasmic inclusions (GCIs) in oligodendrocytes are the pathognomic features of the disorder [1, 6]. GCIs are found throughout the brain, with a predilection for striatonigral and olivopontocerebellar systems [7]. As with other neurodegenerative diseases, age is one of the risk factors for MSA; therefore, pathologic hallmarks of other age-related neurodegenerative diseases sometimes coexist with MSA and may modify its clinical phenotype [8, 9].
Transactive response DNA-binding protein of 43 kDa (TDP-43) aggregates in the frontotemporal cortex and motor neurons are pathologic hallmarks of frontotemporal lobar degeneration (FTLD) and amyotrophic lateral sclerosis [10-12]. In addition to these disorders, TDP-43 pathology has also been reported in other neurodegenerative diseases, including Alzheimer's disease (AD) [11-18], Lewy body disease (LBD) [12, 15, 19-21], hippocampal sclerosis [22-24], Perry syndrome [25, 26], progressive supranuclear palsy (PSP) [20, 27], corticobasal degeneration [17, 28], argyrophilic grain disease (AGD) [29, 30], and MSA [31]. TDP-43 pathology progresses in a stereotypical pattern in each disease, but the distribution is distinct among neurodegenerative diseases, suggesting that vulnerability to TDP-43 could be associated with vulnerability unique to each neurodegeneration disorder [32]. Geser et al. demonstrated that 14% (4 of 29) of MSA cases had TDP-43 aggregates in dystrophic neurites (DNs) and perivascular inclusions, but not in GCIs, predominantly in the medial temporal lobe and subcortical regions [31]. These findings suggest that TDP-43 pathology in MSA is a concomitant age-related change, rather than a primary pathologic feature of the disease.
The aims of this study were to determine the frequency, clinicopathologic features, and risk factors for TDP-43 pathology in MSA. To this end, we screened TDP-43 pathology using a large cohort of MSA cases; assessed concurrent pathologies, including AD, AGD, Lewy-related pathology, and hippocampal sclerosis; and reviewed medical records to explore possible clinicopathologic correlations. Genetic variants of TMEM106B and GRN, which are risk modifiers for TDP-43 pathology [33, 34], were also analysed.
Subjects and Methods
Case selection and ethical approval
Between 1998 and 2017, 211 cases in the Mayo Clinic brain bank have been given a neuropathologic diagnosis of MSA. Of these, 186 cases with available paraffin-embedded tissue and at least minimal medical documentation were included in this study. Brain autopsies were obtained after consent of the legal next-of-kin or individuals with legal power-of-attorney, and studies of autopsy samples are considered exempt from human subject research by the Mayo Clinic Institutional Review Board.
Neuropathologic assessment
Formalin-fixed brains underwent systematic and standardized sampling with neuropathologic evaluation by a single, experienced neuropathologist (D.W.D) [35]. Paraffin-embedded 5-μm thick sections mounted on glass slides were stained with hematoxylin and eosin (H&E) and thioflavin S stains. Braak neurofibrillary tangle (NFT) stage and Thal amyloid phase were assigned using thioflavin S fluorescent microscopy according to published criteria [36-38]. Immunohistochemistry for α-synuclein (NACP, 1:3000 rabbit polyclonal)[39] was performed on sections of the basal forebrain, striatum, midbrain, pons, medulla, and cerebellum to establish a neuropathologic diagnosis of MSA [6]. The severity of α-synuclein pathology, including GCIs, neuronal cytoplasmic inclusions (NCIs), and DNs was graded semi-quantitatively on a five-point scale (0 = absent, 1 = mild, 2 = moderate, 3 = severe, 4 = very severe) [3]. Neuropathological diagnosis of AD was based on the consensus criteria for the neuropathologic diagnosis of AD [40]. Sections of the amygdala at the level of the anterior commissure were immunostained with anti-phospho-tau antibody (CP13, 1:1000, from Dr Peter Davies, Feinstein Institute, North Shore Hospital, NY) to establish neuropathological diagnosis of AGD [41]. Select sections were processed for Gallyas silver stain and 4-repeat tau (RD4, 1:5,000; Millipore, Temecula, CA) immunohistochemistry to assist in neuropathological diagnosis of AGD. Lewy-related pathology was assessed in the cortex, amygdala, basal forebrain, and brainstem, and classified as brainstem, transitional, or diffuse Lewy body disease [42]. Hippocampal sclerosis was assessed by H&E staining as previously described [21].
TDP-43 immunohistochemistry
To characterize TDP-43 pathology in MSA, TDP-43 pathology in 186 MSA cases was screened. Sections from the amygdala, basal forebrain, putamen, globus pallidus, and anterior hypothalamus at the level of the anterior commissure were immunostained with anti-phospho-TDP43 antibody (pS409/410, mouse monoclonal, 1:5000, Cosmo Bio, Tokyo, Japan) using a DAKO Autostainer (Universal Staining System, Carpinteria, CA). For cases having TDP-43 pathology in these sections, additional sections of the motor cortex, anterior hippocampus at the level of subthalamic nucleus, thalamus, midbrain, pons, medulla, and cerebellar hemisphere with dentate nucleus were also screened. All slides were reviewed simultaneously by two observers (D.W.D., S.K.) who agreed on the presence of TDP-43 immunoreactivity, defined as NCI, GCI, DNs, fine neurites, neuronal intranuclear inclusions, spheroids, or perivascular inclusions in any region. The severity of TDP-43 pathology was graded semi-quantitatively on a four-point scale (0 = absent, 1 = mild, 2 = moderate, 3 = severe) [27].
To identify perivascular inclusions, a section including the hippocampus from a case (MSA-12) was processed for double-labelling immunohistochemistry with the combination of anti-TDP-43 antibody (MC2085, 1:1000 rabbit polyclonal, from Dr. Leonard Petrucelli, Mayo Clinic) and anti-collagen IV antibody (1:1000 mouse monoclonal, MP Biomedicals, Solon, OH) using a previously published method [43]. To screen for colocalization of α-synuclein and TDP-43 aggregates, sections including the thalamic fasciculus from three cases (MSA-11–13) and sections including the hippocampus from three cases (MSA-9, -10, and -12) were processed for double-labelling immunohistochemistry with the combination of α-synuclein (NACP, 1:3000) and anti-phospho-TDP-43 antibody (pS409/410, 1:5000).
Immunofluorescence double-staining
Immunofluorescence double-staining with the combination of α-synuclein and phospho-TDP-43 was performed to confirm colocalization of the two proteins. The deparaffinized and rehydrated sections of the thalamic fasciculus of MSA-13 were pretreated with 95% formic acid for 30 minutes and then steamed in distilled water for 30 minutes. Next, sections were blocked with Protein Block plus Serum Free (DAKO) for 1 hour and incubated with both anti-phospho-TDP43 antibody (1:500) and anti-NACP (1:2000) primary antibodies diluted in with Antibody Diluent with Background-Reducing Components (DAKO) overnight at 4°C. Sections were washed three times with 1×PBS at room temperature, and then incubated with secondary antibodies Alexa Fluor 568 (1:500, Thermo Fisher Scientific, Inc.) and Alexa Fluor 488 (1:500, Thermo Fisher Scientific, Inc.) diluted with Antibody Diluent with Background-Reducing Components (DAKO) for 1.5 hours at room temperature in a dark chamber. Sections were washed three times with 1×PBS at room temperature, incubated with 1% Sudan Black for 2 minutes, washed with distilled water and mounted with Vectashield mounting media containing DAPI (Vector Laboratories). Representative images were taken with a confocal laser-scanning fluorescent microscope (LSM 800; Carl Zeiss, Jena, Germany).
Post-embedding immunogold electron microscopy
A piece of thalamic fasciculus from formalin-fixed brain of MSA-13 was processed for immunogold electron microscopy (IEM) as described previously [44]. Rabbit polyclonal antibodies to α-synuclein [39] and TDP-43 (ProteinTech Group, Chicago, IL) were used. Goat anti-rabbit IgG conjugated with 18 nm colloidal gold particles was purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). For TDP-43 IEM, thin sections collected on Formvar-coated nickel grids were treated with citrate buffer, pH 6, for 10 minutes at 95°C before antibody incubation. EM images were obtained with a Gatan 831 Orius digital camera fitted in a Philips 208S electron microscope, and processed using Photoshop software.
Clinical Assessment
Clinical information in all MSA cases was assessed as previously described [3, 35, 45]. Antemortem clinical information for age at symptom onset, family history of dementia or parkinsonism, clinical diagnosis, and clinical symptoms and signs (autonomic dysfunctions, parkinsonism, cerebellar ataxia, cognitive impairment, etc.) were gathered from medical records and a brain bank questionnaire filled out by a close family member. Based on clinical information, each patient was assigned an MSA clinical subtype: MSA with predominant parkinsonism (MSA-P) or MSA with predominant cerebellar ataxia (MSA-C).
Genetic analysis
Genetic analyses in cases with available frozen tissue were performed: 12 TDP-43 positive cases and 153 TDP-43 negative cases. For genotyping, genomic DNA was extracted from the cerebellum of frozen brain tissue using standard procedures. Genotyping for GRN (SNP rs5848 C/T SNPs, T minor allele) and TMEM106B (rs3173615 C/G SNPs, G minor allele) was assessed with TaqMan SNP genotyping assays (Applied Biosystems, Foster City, CA) as previously reported [10, 46-48]. Genotype calls were obtained with QuantStudio™ Real-Time PCR Software (Applied Biosystems). In addition, pathogenic SNCA substitutions (A30P, A53T) were screened using the Sequenom MassArray iPLEX platform.
Statistical Analysis
All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University Saitama, Japan), which is a graphical interface for R 3.1.1 (The R Foundation for Statistical Computing, Vienna, Austria) [49]. A chi-square test or Fisher's exact test was performed for group comparisons of categorical data, as appropriate. The t-test or Mann-Whitney rank sum test was used for analyses of continuous variables, as appropriate. P-values <0.05 were considered statistically significant. Multivariable logistic regression models were built to identify independent risk factors for TDP-43 pathology using the variables with borderline significance (p < 0.15) on univariate analysis.
Results
Clinicopathologic features of MSA cases with TDP-43 pathology
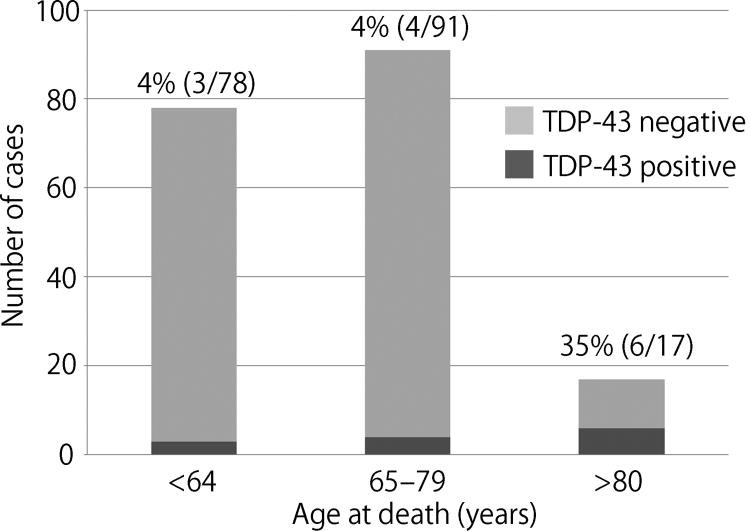
Of the 186 MSA cases, 13 (7%) had TDP-43 pathology in screening sections. Table 1 summarizes clinicopathologic features of MSA cases with TDP-43 pathology compared with cases without TDP-43 pathology. The median age at death was significantly older than that of cases without TDP-43 pathology (73 vs 65, P = 0.015). As shown in Fig. 1, when cases were divided into three age groups (i.e. 65<, 65–79, and ≥ 80 years), the frequency of TDP-43 pathology was significantly higher in the age group ≥ 80 years compared to the two younger groups (35% vs 4% vs 4%, p <0.001). Disease duration and the ratio of clinical subtype of MSA (i.e. MSA-P and MSA-C) were not different between TDP-43 positive and negative cases. It is worth noting that two of the TDP-43 positive cases were atypical MSA, which were clinically diagnosed with corticobasal syndrome (MSA-7) or progressive non-fluent aphasia (MSA-8) as previously reported [5]. Median Braak NFT stage was higher in TDP-43 positive cases than TDP-43 negative cases (II vs I, P = 0.007). Regarding concurrent pathologies, AD, AGD, hippocampal sclerosis, and Lewy-related pathology were more frequently observed in TDP-43 positive cases than in TDP-43 negative cases; of these, only hippocampal sclerosis reached statistical significance (P = 0.005). Multivariable logistic regression models using the variables with borderline significance (p < 0.15) on univariate analysis (i.e. age, Braak NFT stage, AD, AGD, and hippocampal sclerosis) revealed that only age was an independent risk factor for TDP-43 pathology in MSA (OR: 1.11, 95% CI: 1.04–1.19, P = 0.002). Clinical diagnosis, disease duration, clinical symptoms, and neurological signs of each TDP-43 positive case are given in Supplementary Table 1.
Table 1. Clinicopathologic features of MSA cases with TDP-43 pathology.
| TDP-positive N = 13 | TDP-negative N = 173 | P value | |
|---|---|---|---|
| Male, No. (%) | 6 (46%) | 101 (58%) | 0.401 |
| Age at death, years | 73 (64, 86) | 65 (60, 71) | 0.015 |
| Disease duration, years | 7 (6, 12) | 7(5, 9) | 0.307 |
| Clinical phenotype * | |||
| MSA-P | 9 (82%) | 126 (77%) | 0.265 |
| MSA-C | 2 (18%) | 38 (23%) | |
| Pathologic characteristics | |||
| Brain weight, g | 1200 (1010, 1330) | 1240 (1120, 1320) | 0.356 |
| Braak neurofibrillary tangles stage | II (II, III) | I (I, II) | 0.007 |
| Thal amyloid phase | 0 (0, 4) | 1 (0, 1) | 0.810 |
| Alzheimer's disease | 2 (15%) | 6 (3%) | 0.099 |
| Hippocampal sclerosis | 2 (15%) | 0 (0%) | 0.005 |
| Argyrophilic grain disease | 3 (23%) | 10 (6%) | 0.053 |
| Lewy-related pathology | 1 (8%) | 9 (5%) | 0.525 |
Data are displayed as median (25th, 75th range).
2 cases are classified as atypical MSA in TDP-43 positive cases; 9 cases are unclassified because of the lack of detail clinical information.
Figure 1.
The frequency of TDP-43 pathology in each age group. Chi-square test reveals that the frequency of TDP-43 pathology in the ≥80 years age group is significantly higher compared to the other two age groups (P <0.001).
Distribution and morphology of TDP-43 pathology
A range of morphologic lesions were TDP-43 immunopositive (Fig. 2), with lesions in both neurons and glia. Table 2 summarizes the distribution, morphology, and severity of TDP-43 pathology, as well as concurrent pathologies, of all 13 TDP-43 positive cases.
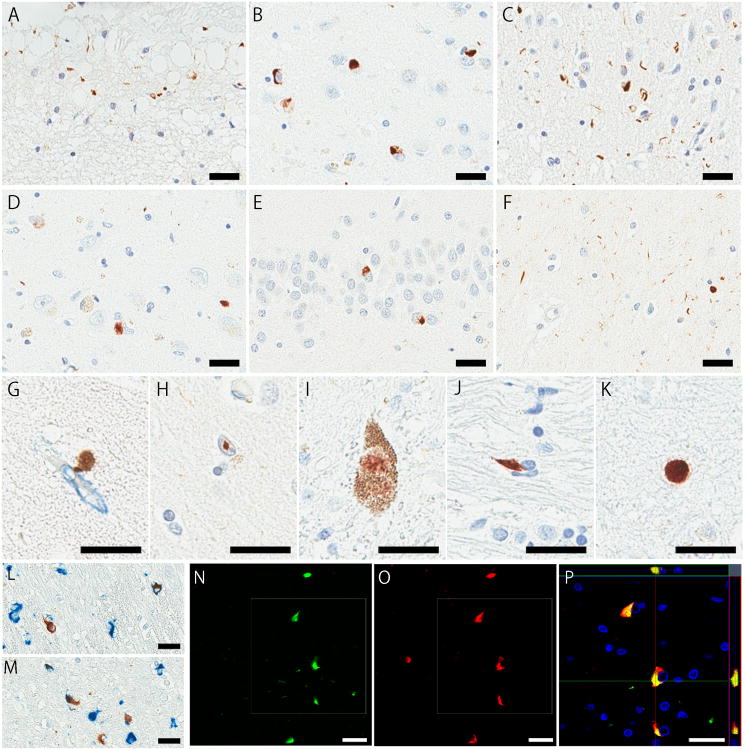
Figure 2.
Immunohistochemistry for phospho-TDP43 in the amygdala (A–C), hippocampal CA1 (D, F, H), dentate gyrus (E), substantia nigra (I), thalamic fasciculus (J), and motor cortex (K). Immunohistochemical double-staining for TDP-43 (brown) and Collagen IV (blue) in the entorhinal cortex (G), or phospho-TDP43 (brown) and phospho-tau (blue) in the mammillothalamic tract (L) and thalamic fasciculus (M). Immunofluorescence double-staining for p-TDP43 and α-synuclein in the thalamic fasciculus (N–P). Some GCIs are labelled by both phospho-TDP43 (N) and α-synuclein (O). Z-stacks confocal microscopy shows colocalization of both signals (P). Bars: 25 μm in all images.
Table 2. Pathological summary and distribution of TDP-43 pathology in MSA.
| Case | Age | Sex | AD | AGD | HS | LP | Distribution, morphology, and severity of TDP-43 pathology | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AM | HP | EC | DG | TH | BF | HT | MB | PN | ME | CW | MC | |||||||
| MSA-1 | 55 | F | - | - | - | - | + P |
- | - | - | - | - | - | - | - | - | - | - |
| MSA-2 | 57 | F | - | - | - | - | + P |
- | - | - | - | - | - | - | - | - | - | - |
| MSA-3 | 68 | F | - | - | - | - | + P |
- | - | - | - | - | - | - | - | - | - | - |
| MSA-4 | 73 | F | - | - | - | - | + P |
- | - | - | - | - | - | - | - | - | - | - |
| MSA-5 | 82 | F | - | - | - | - | + P/N |
- | - | - | - | - | - | - | - | - | - | - |
| MSA-6 | 85 | M | - | - | - | - | + P/N |
- | - | - | - | - | - | - | - | - | - | - |
| MSA-7 | 91 | M | - | - | - | - | + P/N |
- | - | - | - | - | - | - | - | - | - | - |
| MSA-8 | 88 | M | + | - | + | - | + N |
- | - | - | - | - | - | - | - | - | - | - |
| MSA-9 | 70 | F | - | - | - | + | + N/S |
+ N/D |
+ N |
+ N |
- | - | - | - | - | - | - | - |
| MSA-10 | 87 | F | + | - | - | - | +++ N/D |
++ N/D |
++ N |
++ N |
- | - | - | - | - | - | - | - |
| MSA-11 | 67 | M | - | + | - | - | - | - | - | + N |
+ N/G/D |
++ G |
- | + I/G/D |
+ G/N |
- | - | - |
| MSA-12 | 84 | M | - | + | + | - | +++ N/D/S/V |
+++ N/FN/I/V/S |
+++ N/D/V |
++ N |
+ G |
- | ++ N/G |
+ G |
- | - | - | - |
| MSA-13 | 61 | M | - | + | - | - | + N |
+++ N/FN/G/P |
++ G |
- | + G/N |
+ G |
+ N/D |
+ N/G/D |
++ G |
+++ N/G/D |
+ G |
++ G/S |
Semiquantitative assessment of severity in TDP-43 pathology: - indicates negative, + indicates mild, ++ indicates moderate, and +++ indicates severe. Abbreviations: AD, Alzheimer's disease; AGD, argyrophilic grain disease; AM, amygdala; BF, basal forebrain; CW, cerebellar white mater; D, dystrophic neurites; DD, disease duration; DG, dentate gyrus; EC, entorhinal cortex; FN; fine neurites; G, glial cytoplasmic inclusions; HP, hippocampus; HS, hippocampal sclerosis; I, neuronal intranuclear inclusions; LP, Lewy-related pathology; MB, midbrain; MC, motor cortex; ME, medulla; N, neuronal cytoplasmic inclusions; P, subpial astrocytic inclusions; PN, pontine nucleus; TH, thalamus; V, perivascular inclusions.
The amygdala was the most vulnerable region to contain TDP-43 pathology; 12 of 13 cases were positive. Subpial astrocytic lesions (Fig. 2A) were the most frequent in the amygdala, mainly in the semilunar and ambient gyri, and observed in seven cases (MSA-1–7). Of these, subpial astrocytic lesions were the only TDP-43 pathology in four cases (MSA-1–4). Six cases (MSA-5–9 and -13) had sparse NCIs in the amygdala: just one or two aggregates in the entire section. MSA-10 had numerous NCIs and a few DNs (Fig. 2B), and MSA-12 showed abundant short DNs (Fig. 2C) in the amygdala. Spheroids and perivascular inclusions characterized by small, globular, dense structures in close proximately to small vessels were rarely seen in the amygdala. The hippocampus, entorhinal cortex, and dentate gyrus were affected in four cases, respectively. NCIs were commonly observed in the hippocampal pyramidal cell layer (Fig. 2D), entorhinal cortex, and dentate gyrus (Fig. 2E). Two cases (MSA-12 and -13) had fine neurites in the hippocampal CA1 subfield (Fig. 2F), which were consistent with hippocampal sclerosis or pre-hippocampal sclerosis. A few perivascular inclusions were observed in the hippocampal CA1 and entorhinal cortex (Fig. 2G). Oligodendroglial inclusions were detected in the hippocampal alveus, fimbria, and white matter of parahippocampal gyrus. Neuronal intranuclear inclusions were rarely detected in the hippocampus (Fig. 2H). Subcortical structures, such as the thalamus, basal forebrain, and hypothalamus, and brainstem were affected in three cases. GCIs, NCIs, and DNs were commonly seen in these regions (Fig. 2I, 2J). Scattered neuronal intranuclear inclusions were detected in the substantia nigra in MSA-11. The medulla, cerebellum, and neocortices were affected in one case (MSA-13). An isolated GCI was detected in the entire section of cerebellar white matter, and moderate amounts of GCIs and spheroids (Fig. 2K) were observed in the motor cortex.
Colocalization of α-synuclein and TDP-43 in GCIs
To examine whether TDP-43 aggregates are colocalized with α-synuclein pathology, double-staining immunohistochemistry of these two proteins were performed in five cases (MSA-9–13). Colocalization of the two proteins were not observed in the hippocampus and adjacent areas in three cases (MSA-9, -10, and -12). Sections including the thalamus were double-stained in three cases (MSA-11–13). MSA-11 did not show any colocalization, while the other two cases showed that some GCIs in the thalamic fasciculus (MSA-12 and -13) and in the mammillothalamic tract (MSA-13) were labelled by both α-synuclein and phospho-TDP-43 (Fig. 2L, 2M). In MSA-13, approximately 1% of α-synuclein-positive GCIs in the mammillothalamic tract and 13% of those in the thalamic fasciculus showed colocalization with phospho-TDP-43. An adjacent section of the thalamic fasciculus from MSA-13 was also processed for immunofluorescence double-staining. Confocal z-stack microscopy revealed colocalization of α-synuclein and phospho-TDP-43 in a subset of GCIs (Fig. 2N–P).
Immunogold electron microscopy
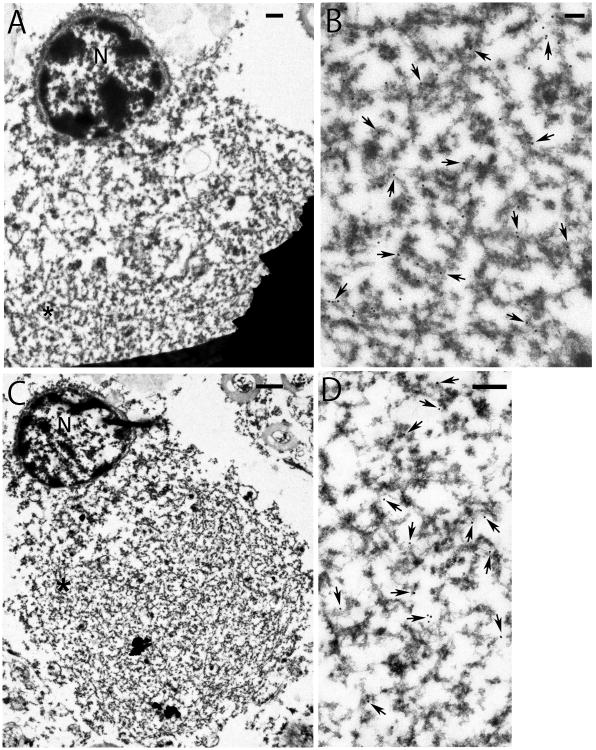
To confirm the colocalization of the two proteins, IEM using semi-serial sections of the thalamic fasciculus from MSA-13 was performed. IEM showed that GCIs were densely packed granule-associated filaments, heavily labelled with antibody to α-synuclein and moderately to TDP-43, respectively (Fig. 3). This confirms the co-localization of both proteins by immunohistochemistry and immunofluorescence double-labelling (Fig. 2L–P). Ultrastructurally, the labelled granule-coated filaments were similar to previous reports, with a diameter of ∼15 nm in uncoated segments of the filaments [12, 50]. The similar morphology makes it difficult to determine if a filament has one or both proteins.
Figure 3.
Electron micrographs of semi-serial sections of the same oligodendrocyte with cytoplasmic inclusion (GCI) immunogold labelled with anti-α-synuclein (A and B) or TDP-43 (C and D). GCI is packed with fibrillar structures. Enlargements (B, D) of asterisked areas (*) in low magnification figures (A, C) reveal robust labelling of respective antibody to granule-coated filaments in the same GCI. It appears that there are more α-synuclein than TDP-43 labelling in the inclusion. Uncoated filaments have a diameter of 15 nm. Coated filaments are heterogeneous in size, due to various amounts of associated dense granular material. Arrows point to some of the gold particles. N, nucleus. Bars: 1 μm in A and C, 0.2 μm in B and D.
Genetic analysis
To determine if reported genetic risk variants for FTLD-TDP could contribute to TDP-43 pathology in MSA, genetic analyses were performed. As shown in Table 3, minor allele frequency of TMEM106B and GRN were not significantly different between TDP-43 positive and negative cases.
Table 3. Severity of neuronal loss, α-synuclein and TDP-43 pathology in 24 regions in MSA-13.
| Neuronal loss | α-synuclein | TDP-43 | |||||
|---|---|---|---|---|---|---|---|
| GCIs | NCIs | DNs | GCIs | NCIs | DNs | ||
| Middle frontal gyrus | - | ++ | + | + | + | - | + |
| Superior temporal gyrus | - | ++ | + | + | - | - | - |
| Parietal lobe | - | ++ | - | - | + | - | + |
| Motor cortex | - | +++ | ++ | ++ | ++ | - | + |
| Visual cortex | - | + | - | - | - | - | - |
| Cingulate gyrus | - | ++ | ++ | ++ | + | - | + |
| Dentate gyrus | - | - | +++ | - | - | - | - |
| Hippocampus | - | +++ | +++ | ++ | ++ | + | +++ |
| Entorhinal cortex | - | +++ | ++ | + | ++ | - | - |
| Amygdala | - | + | +++ | ++ | + | - | - |
| Basal forebrain | + | +++ | ++ | ++ | + | - | - |
| Hypothalamus | - | +++ | ++ | ++ | - | + | + |
| Putamen | + | +++ | + | + | + | - | - |
| Globus pallidus | - | +++ | + | + | + | - | - |
| Internal capsule | - | +++ | - | - | ++ | - | - |
| Thalamus | - | ++ | ++ | ++ | + | + | - |
| Subthalamic nucleus | - | ++ | +++ | +++ | + | + | - |
| Mammillothalamic tract | - | ++++ | - | - | ++ | - | + |
| Thalamic fasciculus | - | +++ | - | - | +++ | - | + |
| Substantia nigra | +++ | +++ | ++ | ++ | + | + | + |
| Pontine tegmentum | - | ++ | + | + | + | - | - |
| Pontine nucleus | + | ++++ | ++++ | ++++ | ++ | - | - |
| Inferior olive | + | +++ | ++++ | ++++ | +++ | - | - |
| Cerebellar hemisphere | ++ | ++++ | ++ | ++ | + | - | - |
Semiquantitative assessment of severity in each pathology: - indicates negative, + indicates mild, ++ indicates moderate, +++ indicates severe, and ++++ extremely severe. Abbreviations: DNs, dystrophic neurites; GCIs, glial cytoplasmic inclusions; NCIs, neuronal cytoplasmic inclusions.
Clinicopathologic features in MSA-13
A unique widespread TDP-43 pathology of MSA-13 encouraged us to investigate clinicopathologic feature of this case; thus, clinical vignette and results of immunohistochemistry for α-synuclein, phospho-TDP-43, and phospho-tau are described here.
The patient was a 61-year-old Caucasian man who had a family history of autopsy-confirmed Lewy body disease (clinically diagnosed with Parkinson disease) in his mother. He developed erectile dysfunction at age 50 and left leg tremor at age 53. Subsequently, he developed imbalance with falls, urinary incontinence and retention, constipation, lightheadedness, difficulty swallowing, and voice hoarseness. He was diagnosed with orthostatic hypotension and began midodrine and fludrocortisone at age 56. Short-term memory loss began at that time. DaTscan detected loss of dopaminergic neuronal terminals in the striatum. He was diagnosed with probable MSA-P at age 57.
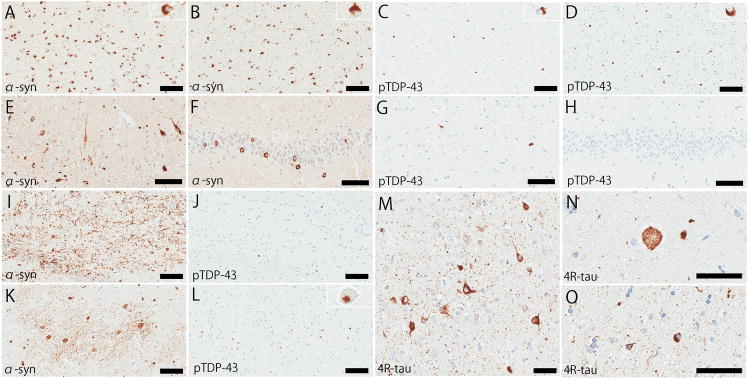
Macroscopically, the neocortex, hippocampal formation, and amygdala were free of atrophy. The basal ganglia showed no atrophy and only subtle discoloration of the lateral and posterior putamen. Horizontal sections of the midbrain and pons are remarkable for marked pontine atrophy and mild loss of pigment in the substantia nigra and locus coeruleus. There is marked atrophy of the middle cerebellar peduncle, pontine base, and cerebellar white matter. Immunohistochemistry for α-synuclein revealed widespread distribution of GCIs, consistent with a pathological diagnosis of MSA. Table 4 summarizes the severity and distribution of α-synuclein and TDP-43 pathology of this case. As shown in a section of double-staining, the mammillothalamic tract and thalamic fasciculus had numerous α-synuclein-positive GCIs (Fig. 4A, 4B) and moderate amount of TDP-43 positive GCIs (Fig. 4C, 4D). Abundant α-synuclein-positive NCIs in the hippocampal pyramidal cell layer (Fig. 4E) and dentate gyrus (Fig. 4F) resembled atypical MSA [5], but neuronal loss was minimal in these regions. The hippocampal CA1 showed some TDP-43-positive NCIs and diffuse fine neurites (fig. 4G), which were consistent with pre-hippocampal sclerosis [21], while the dentate gyrus was spared (Fig. 4H). The transverse pontine fibers had abundant α-synuclein-positive DNs (Fig. 4I), but TDP-43 immunohistochemistry showed a few GCIs in this region (Fig. 4J). TDP-43 positive NCIs were rarely detected in the locus coeruleus. The inferior olivary nucleus had numerous α-synuclein-positive NCIs, GCI, and DNs (Fig. 4K), and moderate amount of TDP-43 positive GCIs and a few NCIs (Fig. 4L). TDP-43 positive GCIs and DNs were seen in the subcortical white matter, most frequent in white matter adjacent to the motor cortex. Immunohistochemistry for phospho-tau and 4-repeat tau revealed abundant argyrophilic grains, pretangles, balloon neurons, and coiled body in the amygdala, hippocampus, and parahippocampal gyrus, which were consistent with AGD (Fig. 4M–O). Thioflavin S microscopy revealed no Alzheimer-type pathology (Braak NFT stage and Thal amyloid phase were 0).
Table 4. Genetic findings of MSA-TDP compared to MSA.
| TDP-positive N = 12 | TDP-negative N = 153 | P value | |
|---|---|---|---|
| GRN, T allele | 9/24 (38%) | 95/304 (31%) | 0.685 |
| T/T | 3/12 (25%) | 12/152 (8%) | 0.101 |
| T/C | 3/12 (25%) | 71/152 (47%) | |
| C/C | 6/12 (25%) | 69/152 (45%) | |
| TMEM106B, G allele | 7/24 (29%) | 124/306 (41%) | 0.380 |
| G/G | 0/12 (0%) | 27/153 (18%) | 0.366 |
| G/C | 7/12 (58%) | 70/153 (46%) | |
| C/C | 5/12 (42%) | 56/153 (37%) |
Genetic analysis is performed for TDP-positive (N = 12) and TDP-negative (N = 153), respectively.
Figure 4.
Immunohistochemistry for α-synuclein (A, B, E, F, I, and K) and phospho-TDP-43 (C, D, G, H, J, and L). The mammillothalamic tract (A and C), thalamic fasciculus (B and D), hippocampal CA1 (E and G), dentate gyrus (F and H), transverse fibers in pontine base (I and J), and inferior olivary nucleus (K and L). Immunohistochemistry for 4-repeat tau shows representative pathologic features of argyrophilic grain disease, including argyrophilic grains (M), pretangles (M), and balloon neurons (N) in the amygdala, and coiled bodies (O) in the white matter in the parahippocampal gyrus. Bars: 100 μm in A–L, and 50 μm in M–O.
Genetic analysis revealed that the patient did not have minor risk alleles in either TMEM106B or GRN. Since the patient had a family history of autopsy-confirmed LBD, α-synuclein-encoding gene SNCA was also analysed; however, any mutations within exons 2 and 3 of SNCA were found.
Discussion
In a cohort of 186 cases of pathologically-confirmed MSA, the present study identified 13 (7%) cases with TDP-43 immunoreactivity in sections of the amygdala and basal forebrain. Most cases had TDP-43 pathology restricted to the medial temporal regions, but three cases had widespread pathology in subcortical structures and brainstem. Colocalization of α-synuclein and TDP-43 was identified in a subset of GCIs in two cases.
As with AD and elderly healthy individuals [13, 51], the amygdala was the most vulnerable region to contain TDP-43 pathology in MSA; eight cases had this pathology restricted to the amygdala. Four cases had only subpial astrocytic lesions in the semilunar and ambient gyri, which might be an earliest change of TDP-43 pathology. In contrast, MSA-vulnerable regions, such as the putamen, substantia nigra, medulla, pons, and cerebellum, do not seem to be as vulnerable to TDP-43 pathology. More cases are necessary to conclude whether there is region-specific vulnerability to TDP-43 pathology.
IEM of serial sections is in agreement with immunohistochemistry and immunofluorescence double-staining, and further showed GCIs contain filaments associated with dense granular material. The packed and intermingled appearance in oligodendrocytes contrasts with previous reports of co-localization of α-synuclein and tau in neurons of AD with amygdala Lewy bodies [44, 52]. In the latter case, the uncoated tau filaments could be distinguished from granule-coated filaments of α-synuclein. Moreover, tau aggregates were spatially separate from aggregated α-synuclein, despite their close proximity. The mixed morphology in GCIs of MSA could be due to physical constraint of a small cytoplasm of oligodendrocytes forcing crowding of the filaments together. The nature of dense granular material is not clear. It would be interesting to see if co-existing α-synuclein and TDP-43 would mix or segregate in neurons, which have a larger cytoplasm.
In the present study, multiple regression models indicated that age was an independent risk factor for TDP-43 pathology in MSA. Not only patients with neurodegenerative diseases, but cognitively normal elderly individuals also have TDP-43 pathology, especially DNs, in the medial temporal regions [30, 51, 53]. A recent meta-analysis showed that the estimated prevalence of TDP-43 proteinopathy in cognitively normal elderly individuals (age: 80.2 ± 8.5 years) was 24% (95% CI: 13%–34%) [54]. The frequency of TDP-43 pathology in the present study was much lower than that of cognitively normal elderly individuals, probably because of younger age of death (66.7 ± 8.5 years) in the MSA cohort in the present study [31]. Indeed, when restricted to patients 80 years of age or older, the frequency of TDP-43 pathology increased to 35% in the present MSA cohort, which is within the range of other studies of TDP-43 in cognitively normal elderly individuals [54].
Besides age, several risk factors for TDP-43 pathology, such as AD, AGD, and hippocampal sclerosis were frequent in TDP-43 positive cases, although multiple regression models failed to identify them as independent risk factors. This result should be interpreted cautiously because the number of MSA cases with concomitant pathology was too small to yield statistical difference; only eight cases had AD, and two cases had hippocampal sclerosis. Interestingly, three cases with the most widespread TDP-43 pathology (MSA-11–13) had concurrent AGD. Several studies reported the high frequency of TDP-43 pathology in AGD, ranging from 55% to 60%, in the form of DNs, NCIs, and GCIs, mainly in limbic regions [29, 51]. Arnold et al. demonstrated argyrophilic grains, but not age or Alzheimer's-type pathology was correlated with frequency of TDP-43 pathology in cognitively normal elderly individuals [30]. Similarly, the previous study showed that concurrent AGD was a risk factor for TDP-43 pathology in PSP [27]. Taken together, AGD may be associated with TDP-43 pathology in MSA; however, it is still unknown whether AGD can cause widespread TDP-43 pathology beyond limbic structures affected by AGD. Additional screening of TDP-43 pathology in AGD is necessary to clarify the possible association between AGD and TDP-43 pathology.
The possible impact of TDP-43 pathology on clinical manifestations was a focus of the present study; however, neither the clinical subtype nor disease duration was different between TDP-43 positive and negative cases. Given the fact that TDP-43 pathology is strongly associated with cognitive deficits (i.e. memory loss and naming) in the context of AD pathology [55-57], cognitive impairment was also evaluated. Although four cases with TDP-43 pathology had documented cognitive impairment (Supplementary Table 1), in most it could be explained by other concurrent pathologies, such as AD, AGD, and hippocampal sclerosis. Intriguingly, two cases were considered to have “atypical MSA”. The previous study described these two cases were negative in TDP-43 pathology [5]; however, the present study found sparse TDP-43 pathology in the amygdala in both cases. Nevertheless, TDP-43 pathology is unlikely a key pathology of atypical MSA because the extent and severity of TDP-43 pathology was minimal. The present study also described clinical features of MSA-13, the cases with the most widespread TDP-43 pathology. Unexpectedly, however, this patient appeared to have “typical” MSA-P characterized by autonomic failure and parkinsonism. Although the patient complained of memory loss, this is sometimes observed in patients with MSA [3]. Taken together, the present study did not find convincing impact of TDP-43 pathology on clinical manifestations in MSA.
Genetic analyses in the current study were at variance with previous studies of TDP-43 in other neurodegenerative diseases. No statistically significant differences were found between MSA with and without TDP-43 pathology for TMEM106B rs3173615 and GRN. The small sample size of this cohort may contribute to this difference.
There are some limitations of the present study. First, only sections of amygdala and basal ganglia were screened for TDP-43 pathology in all cases; thus, the frequency of this pathology in MSA may be an underestimate. Given the retrospective nature of this study, clinical information was limited. To evaluate possible clinical correlates of TDP-43 pathology, it will be important to evaluate MSA patients who come to autopsy from prospective clinical studies.
A notable strength of the present study is the number of MSA cases screened for TDP-43 pathology, which is many fold greater than any previous study [31, 58]. This large number of cases allowed us to discover colocalization of TDP-43 and α-synuclein in GCIs, which were not described previously [31]. Another strength is the use of multiple methods to confirm colocalization, including immunohistochemical and immunofluorescence double-staining and IEM.
In conclusion, the present study showed that about 7% of MSA have TDP-43 pathology, mainly in the medial temporal regions. Age is a risk factor for TDP-43 pathology in MSA. Given the fact that cases with extensive TDP-43 pathology often have concurrent AGD, we hypothesize that AGD play a role in exacerbating this pathology. The present study identified colocalization of TDP-43 and α-synuclein in GCIs, which suggests possible association between the two pathologies at least in a subset of cases.
Supplementary Material
Acknowledgments
We would like to thank the patients and their families who donated brains to help further the scientific understanding of neurodegeneration. The authors would also like to acknowledge Linda Rousseau and Virginia Phillips for histologic support, and Monica Castanedes-Casey (Mayo Clinic, Jacksonville) for immunohistochemistry support. This work is supported by NIH grant P50 NS072187 and a Jaye F. and Betty F. Dyer Foundation Fellowship in progressive supranuclear palsy research.
Abbreviations
- AD
Alzheimer's disease
- AGD
argyrophilic grain disease
- DNs
dystrophic neurites
- FTD
frontotemporal dementia
- FTLD
frontotemporal lobar degeneration
- GCI
glial cytoplasmic inclusion
- IEM
immunogold electron microscopy
- MSA
multiple system atrophy
- MSA-C
MSA with predominant cerebellar ataxia
- MSA-P
MSA with predominant parkinsonism
- NCI
neuronal cytoplasmic inclusion
- NFT
neurofibrillary tangle
- PSP
progressive supranuclear palsy
- TDP-43
transactive response DNA-binding protein of 43 kDa
Footnotes
Author Contributions: Shunsuke Koga: Acquisition, analysis and interpretation of data; drafting of manuscript; execution of the statistical analysis; writing of the first draft
Wen-Lang Lin: Acquisition, analysis and interpretation of data; review and critique
Ronald L, Walton: Acquisition, analysis and interpretation of data; review and critique
Owen A. Ross: Review and critique
Dennis W. Dickson: Study concept and design; acquisition and interpretation of data; review and critique
References
- 1.Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, Wood NW, Colosimo C, Durr A, Fowler CJ, Kaufmann H, Klockgether T, Lees A, Poewe W, Quinn N, Revesz T, Robertson D, Sandroni P, Seppi K, Vidailhet M. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71:670–6. doi: 10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stankovic I, Krismer F, Jesic A, Antonini A, Benke T, Brown RG, Burn DJ, Holton JL, Kaufmann H, Kostic VS, Ling H, Meissner WG, Poewe W, Semnic M, Seppi K, Takeda A, Weintraub D, Wenning GK. Cognitive impairment in multiple system atrophy: a position statement by the Neuropsychology Task Force of the MDS Multiple System Atrophy (MODIMSA) study group. Mov Disord. 2014;29:857–67. doi: 10.1002/mds.25880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koga S, Parks A, Uitti RJ, van Gerpen JA, Cheshire WP, Wszolek ZK, Dickson DW. Profile of cognitive impairment and underlying pathology in multiple system atrophy. Mov Disord. 2017;32:405–13. doi: 10.1002/mds.26874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fiorenzato E, Antonini A, Wenning G, Biundo R. Cognitive impairment in multiple system atrophy. Mov Disord. 2017;32:1338–9. doi: 10.1002/mds.27085. [DOI] [PubMed] [Google Scholar]
- 5.Aoki N, Boyer PJ, Lund C, Lin WL, Koga S, Ross OA, Weiner M, Lipton A, Powers JM, White CL, 3rd, Dickson DW. Atypical multiple system atrophy is a new subtype of frontotemporal lobar degeneration: frontotemporal lobar degeneration associated with alpha-synuclein. Acta Neuropathol. 2015;130:93–105. doi: 10.1007/s00401-015-1442-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trojanowski JQ, Revesz T. Proposed neuropathological criteria for the post mortem diagnosis of multiple system atrophy. Neuropathol Appl Neurobiol. 2007;33:615–20. doi: 10.1111/j.1365-2990.2007.00907.x. [DOI] [PubMed] [Google Scholar]
- 7.Papp MI, Kahn JE, Lantos PL. Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy-Drager syndrome) J Neurol Sci. 1989;94:79–100. doi: 10.1016/0022-510x(89)90219-0. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed Z, Asi YT, Sailer A, Lees AJ, Houlden H, Revesz T, Holton JL. The neuropathology, pathophysiology and genetics of multiple system atrophy. Neuropathol Appl Neurobiol. 2012;38:4–24. doi: 10.1111/j.1365-2990.2011.01234.x. [DOI] [PubMed] [Google Scholar]
- 9.Koga S, Dickson DW. Recent advances in neuropathology, biomarkers and therapeutic approach of multiple system atrophy. J Neurol Neurosurg Psychiatry. 2017 doi: 10.1136/jnnp-2017-315813. [DOI] [PubMed] [Google Scholar]
- 10.Murray ME, Cannon A, Graff-Radford NR, Liesinger AM, Rutherford NJ, Ross OA, Duara R, Carrasquillo MM, Rademakers R, Dickson DW. Differential clinicopathologic and genetic features of late-onset amnestic dementias. Acta Neuropathol. 2014;128:411–21. doi: 10.1007/s00401-014-1302-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–3. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 12.Lin WL, Dickson DW. Ultrastructural localization of TDP-43 in filamentous neuronal inclusions in various neurodegenerative diseases. Acta Neuropathol. 2008;116:205–13. doi: 10.1007/s00401-008-0408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Josephs KA, Murray ME, Whitwell JL, Parisi JE, Petrucelli L, Jack CR, Petersen RC, Dickson DW. Staging TDP-43 pathology in Alzheimer's disease. Acta Neuropathol. 2014;127:441–50. doi: 10.1007/s00401-013-1211-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amador-Ortiz C, Lin WL, Ahmed Z, Personett D, Davies P, Duara R, Graff-Radford NR, Hutton ML, Dickson DW. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer's disease. Ann Neurol. 2007;61:435–45. doi: 10.1002/ana.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arai T, Mackenzie IR, Hasegawa M, Nonoka T, Niizato K, Tsuchiya K, Iritani S, Onaya M, Akiyama H. Phosphorylated TDP-43 in Alzheimer's disease and dementia with Lewy bodies. Acta Neuropathol. 2009;117:125–36. doi: 10.1007/s00401-008-0480-1. [DOI] [PubMed] [Google Scholar]
- 16.Davidson YS, Raby S, Foulds PG, Robinson A, Thompson JC, Sikkink S, Yusuf I, Amin H, DuPlessis D, Troakes C, Al-Sarraj S, Sloan C, Esiri MM, Prasher VP, Allsop D, Neary D, Pickering-Brown SM, Snowden JS, Mann DM. TDP-43 pathological changes in early onset familial and sporadic Alzheimer's disease, late onset Alzheimer's disease and Down's syndrome: association with age, hippocampal sclerosis and clinical phenotype. Acta Neuropathol. 2011;122:703–13. doi: 10.1007/s00401-011-0879-y. [DOI] [PubMed] [Google Scholar]
- 17.Uryu K, Nakashima-Yasuda H, Forman MS, Kwong LK, Clark CM, Grossman M, Miller BL, Kretzschmar HA, Lee VM, Trojanowski JQ, Neumann M. Concomitant TAR-DNA-binding protein 43 pathology is present in Alzheimer disease and corticobasal degeneration but not in other tauopathies. J Neuropathol Exp Neurol. 2008;67:555–64. doi: 10.1097/NEN.0b013e31817713b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McAleese KE, Walker L, Erskine D, Thomas AJ, McKeith IG, Attems J. TDP-43 pathology in Alzheimer's disease, dementia with Lewy bodies and ageing. Brain Pathol. 2017;27:472–9. doi: 10.1111/bpa.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakashima-Yasuda H, Uryu K, Robinson J, Xie SX, Hurtig H, Duda JE, Arnold SE, Siderowf A, Grossman M, Leverenz JB, Woltjer R, Lopez OL, Hamilton R, Tsuang DW, Galasko D, Masliah E, Kaye J, Clark CM, Montine TJ, Lee VM, Trojanowski JQ. Co-morbidity of TDP-43 proteinopathy in Lewy body related diseases. Acta Neuropathol. 2007;114:221–9. doi: 10.1007/s00401-007-0261-2. [DOI] [PubMed] [Google Scholar]
- 20.Yokota O, Davidson Y, Bigio EH, Ishizu H, Terada S, Arai T, Hasegawa M, Akiyama H, Sikkink S, Pickering-Brown S, Mann DM. Phosphorylated TDP-43 pathology and hippocampal sclerosis in progressive supranuclear palsy. Acta Neuropathol. 2010;120:55–66. doi: 10.1007/s00401-010-0702-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aoki N, Murray ME, Ogaki K, Fujioka S, Rutherford NJ, Rademakers R, Ross OA, Dickson DW. Hippocampal sclerosis in Lewy body disease is a TDP-43 proteinopathy similar to FTLD-TDP Type A. Acta Neuropathol. 2015;129:53–64. doi: 10.1007/s00401-014-1358-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nelson PT, Schmitt FA, Lin Y, Abner EL, Jicha GA, Patel E, Thomason PC, Neltner JH, Smith CD, Santacruz KS, Sonnen JA, Poon LW, Gearing M, Green RC, Woodard JL, Van Eldik LJ, Kryscio RJ. Hippocampal sclerosis in advanced age: clinical and pathological features. Brain. 2011;134:1506–18. doi: 10.1093/brain/awr053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nag S, Yu L, Capuano AW, Wilson RS, Leurgans SE, Bennett DA, Schneider JA. Hippocampal sclerosis and TDP-43 pathology in aging and Alzheimer disease. Ann Neurol. 2015;77:942–52. doi: 10.1002/ana.24388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cykowski MD, Takei H, Van Eldik LJ, Schmitt FA, Jicha GA, Powell SZ, Nelson PT. Hippocampal Sclerosis but Not Normal Aging or Alzheimer Disease Is Associated With TDP-43 Pathology in the Basal Forebrain of Aged Persons. J Neuropathol Exp Neurol. 2016;75:397–407. doi: 10.1093/jnen/nlw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mishima T, Koga S, Lin WL, Kasanuki K, Castanedes-Casey M, Wszolek ZK, Oh SJ, Tsuboi Y, Dickson DW. Perry Syndrome: A Distinctive Type of TDP-43 Proteinopathy. J Neuropathol Exp Neurol. 2017;76:676–82. doi: 10.1093/jnen/nlx049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wider C, Dickson DW, Stoessl AJ, Tsuboi Y, Chapon F, Gutmann L, Lechevalier B, Calne DB, Personett DA, Hulihan M, Kachergus J, Rademakers R, Baker MC, Grantier LL, Sujith OK, Brown L, Calne S, Farrer MJ, Wszolek ZK. Pallidonigral TDP-43 pathology in Perry syndrome. Parkinsonism Relat Disord. 2009;15:281–6. doi: 10.1016/j.parkreldis.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koga S, Sanchez-Contreras M, Josephs KA, Uitti RJ, Graff-Radford N, van Gerpen JA, Cheshire WP, Wszolek ZK, Rademakers R, Dickson DW. Distribution and characteristics of transactive response DNA binding protein 43 kDa pathology in progressive supranuclear palsy. Mov Disord. 2017;32:246–55. doi: 10.1002/mds.26809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kouri N, Oshima K, Takahashi M, Murray ME, Ahmed Z, Parisi JE, Yen SH, Dickson DW. Corticobasal degeneration with olivopontocerebellar atrophy and TDP-43 pathology: an unusual clinicopathologic variant of CBD. Acta Neuropathol. 2013;125:741–52. doi: 10.1007/s00401-013-1087-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujishiro H, Uchikado H, Arai T, Hasegawa M, Akiyama H, Yokota O, Tsuchiya K, Togo T, Iseki E, Hirayasu Y. Accumulation of phosphorylated TDP-43 in brains of patients with argyrophilic grain disease. Acta Neuropathol. 2009;117:151–8. doi: 10.1007/s00401-008-0463-2. [DOI] [PubMed] [Google Scholar]
- 30.Arnold SJ, Dugger BN, Beach TG. TDP-43 deposition in prospectively followed, cognitively normal elderly individuals: correlation with argyrophilic grains but not other concomitant pathologies. Acta Neuropathol. 2013;126:51–7. doi: 10.1007/s00401-013-1110-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geser F, Malunda JA, Hurtig HI, Duda JE, Wenning GK, Gilman S, Low PA, Lee VM, Trojanowski JQ. TDP-43 pathology occurs infrequently in multiple system atrophy. Neuropathol Appl Neurobiol. 2011;37:358–65. doi: 10.1111/j.1365-2990.2010.01136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan RH, Kril JJ, Fatima M, McGeachie A, McCann H, Shepherd C, Forrest SL, Affleck A, Kwok JB, Hodges JR, Kiernan MC, Halliday GM. TDP-43 proteinopathies: pathological identification of brain regions differentiating clinical phenotypes. Brain. 2015;138:3110–22. doi: 10.1093/brain/awv220. [DOI] [PubMed] [Google Scholar]
- 33.Dickson DW, Baker M, Rademakers R. Common variant in GRN is a genetic risk factor for hippocampal sclerosis in the elderly. Neurodegener Dis. 2010;7:170–4. doi: 10.1159/000289231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu L, De Jager PL, Yang J, Trojanowski JQ, Bennett DA, Schneider JA. The TMEM106B locus and TDP-43 pathology in older persons without FTLD. Neurology. 2015;84:927–34. doi: 10.1212/WNL.0000000000001313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koga S, Dickson DW, Bieniek KF. Chronic Traumatic Encephalopathy Pathology in Multiple System Atrophy. J Neuropathol Exp Neurol. 2016;75:963–70. doi: 10.1093/jnen/nlw073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thal DR, Rub U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 37.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 38.Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Trojanowski JQ, Vinters HV, Hyman BT. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol. 2012;123:1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gwinn-Hardy K, Mehta ND, Farrer M, Maraganore D, Muenter M, Yen SH, Hardy J, Dickson DW. Distinctive neuropathology revealed by alpha-synuclein antibodies in hereditary parkinsonism and dementia linked to chromosome 4p. Acta Neuropathol. 2000;99:663–72. doi: 10.1007/s004010051177. [DOI] [PubMed] [Google Scholar]
- 40.Hyman BT, Trojanowski JQ. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol. 1997;56:1095–7. doi: 10.1097/00005072-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 41.Ferrer I, Santpere G, van Leeuwen FW. Argyrophilic grain disease. Brain. 2008;131:1416–32. doi: 10.1093/brain/awm305. [DOI] [PubMed] [Google Scholar]
- 42.Kosaka K, Yoshimura M, Ikeda K, Budka H. Diffuse type of Lewy body disease: progressive dementia with abundant cortical Lewy bodies and senile changes of varying degree--a new disease? Clin Neuropathol. 1984;3:185–92. [PubMed] [Google Scholar]
- 43.Lin WL, Castanedes-Casey M, Dickson DW. Transactivation response DNA-binding protein 43 microvasculopathy in frontotemporal degeneration and familial Lewy body disease. J Neuropathol Exp Neurol. 2009;68:1167–76. doi: 10.1097/NEN.0b013e3181baacec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uchikado H, Lin WL, DeLucia MW, Dickson DW. Alzheimer disease with amygdala Lewy bodies: a distinct form of alpha-synucleinopathy. J Neuropathol Exp Neurol. 2006;65:685–97. doi: 10.1097/01.jnen.0000225908.90052.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koga S, Aoki N, Uitti RJ, van Gerpen JA, Cheshire WP, Josephs KA, Wszolek ZK, Langston JW, Dickson DW. When DLB, PD, and PSP masquerade as MSA: an autopsy study of 134 patients. Neurology. 2015;85:404–12. doi: 10.1212/WNL.0000000000001807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Deerlin VM, Sleiman PM, Martinez-Lage M, Chen-Plotkin A, Wang LS, Graff-Radford NR, Dickson DW, Rademakers R, Boeve BF, Grossman M, Arnold SE, Mann DM, Pickering-Brown SM, Seelaar H, Heutink P, van Swieten JC, Murrell JR, Ghetti B, Spina S, Grafman J, Hodges J, Spillantini MG, Gilman S, Lieberman AP, Kaye JA, Woltjer RL, Bigio EH, Mesulam M, Al-Sarraj S, Troakes C, Rosenberg RN, White CL, 3rd, Ferrer I, Llado A, Neumann M, Kretzschmar HA, Hulette CM, Welsh-Bohmer KA, Miller BL, Alzualde A, Lopez de Munain A, McKee AC, Gearing M, Levey AI, Lah JJ, Hardy J, Rohrer JD, Lashley T, Mackenzie IR, Feldman HH, Hamilton RL, Dekosky ST, van der Zee J, Kumar-Singh S, Van Broeckhoven C, Mayeux R, Vonsattel JP, Troncoso JC, Kril JJ, Kwok JB, Halliday GM, Bird TD, Ince PG, Shaw PJ, Cairns NJ, Morris JC, McLean CA, DeCarli C, Ellis WG, Freeman SH, Frosch MP, Growdon JH, Perl DP, Sano M, Bennett DA, Schneider JA, Beach TG, Reiman EM, Woodruff BK, Cummings J, Vinters HV, Miller CA, Chui HC, Alafuzoff I, Hartikainen P, Seilhean D, Galasko D, Masliah E, Cotman CW, Tunon MT, Martinez MC, Munoz DG, Carroll SL, Marson D, Riederer PF, Bogdanovic N, Schellenberg GD, Hakonarson H, Trojanowski JQ, Lee VM. Common variants at 7p21 are associated with frontotemporal lobar degeneration with TDP-43 inclusions. Nat Genet. 2010;42:234–9. doi: 10.1038/ng.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rademakers R, Eriksen JL, Baker M, Robinson T, Ahmed Z, Lincoln SJ, Finch N, Rutherford NJ, Crook RJ, Josephs KA, Boeve BF, Knopman DS, Petersen RC, Parisi JE, Caselli RJ, Wszolek ZK, Uitti RJ, Feldman H, Hutton ML, Mackenzie IR, Graff-Radford NR, Dickson DW. Common variation in the miR-659 binding-site of GRN is a major risk factor for TDP43-positive frontotemporal dementia. Hum Mol Genet. 2008;17:3631–42. doi: 10.1093/hmg/ddn257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rutherford NJ, Carrasquillo MM, Li M, Bisceglio G, Menke J, Josephs KA, Parisi JE, Petersen RC, Graff-Radford NR, Younkin SG, Dickson DW, Rademakers R. TMEM106B risk variant is implicated in the pathologic presentation of Alzheimer disease. Neurology. 2012;79:717–8. doi: 10.1212/WNL.0b013e318264e3ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–8. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Forno LS. Neuropathology of Parkinson's disease. J Neuropathol Exp Neurol. 1996;55:259–72. doi: 10.1097/00005072-199603000-00001. [DOI] [PubMed] [Google Scholar]
- 51.Uchino A, Takao M, Hatsuta H, Sumikura H, Nakano Y, Nogami A, Saito Y, Arai T, Nishiyama K, Murayama S. Incidence and extent of TDP-43 accumulation in aging human brain. Acta Neuropathol Commun. 2015;3:35. doi: 10.1186/s40478-015-0215-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fujishiro H, Tsuboi Y, Lin WL, Uchikado H, Dickson DW. Co-localization of tau and alpha-synuclein in the olfactory bulb in Alzheimer's disease with amygdala Lewy bodies. Acta Neuropathol. 2008;116:17–24. doi: 10.1007/s00401-008-0383-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kovacs GG, Milenkovic I, Wohrer A, Hoftberger R, Gelpi E, Haberler C, Honigschnabl S, Reiner-Concin A, Heinzl H, Jungwirth S, Krampla W, Fischer P, Budka H. Non-Alzheimer neurodegenerative pathologies and their combinations are more frequent than commonly believed in the elderly brain: a community-based autopsy series. Acta Neuropathol. 2013;126:365–84. doi: 10.1007/s00401-013-1157-y. [DOI] [PubMed] [Google Scholar]
- 54.Nascimento C, Di Lorenzo Alho AT, Bazan Conceicao Amaral C, Leite REP. Leite REP, Nitrini R, Jacob-Filho W, Pasqualucci CA, Hokkanen SRK, Hunter S, Keage H, Kovacs GG, Grinberg LT, Suemoto CK. Prevalence of transactive response DNA-binding protein 43 (TDP-43) proteinopathy in cognitively normal older adults: systematic review and meta-analysis. Neuropathol Appl Neurobiol. 2017 doi: 10.1111/nan.12430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Josephs KA, Whitwell JL, Knopman DS, Hu WT, Stroh DA, Baker M, Rademakers R, Boeve BF, Parisi JE, Smith GE, Ivnik RJ, Petersen RC, Jack CR, Jr, Dickson DW. Abnormal TDP-43 immunoreactivity in AD modifies clinicopathologic and radiologic phenotype. Neurology. 2008;70:1850–7. doi: 10.1212/01.wnl.0000304041.09418.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Josephs KA, Whitwell JL, Tosakulwong N, Weigand SD, Murray ME, Liesinger AM, Petrucelli L, Senjem ML, Ivnik RJ, Parisi JE, Petersen RC, Dickson DW. TAR DNA-binding protein 43 and pathological subtype of Alzheimer's disease impact clinical features. Ann Neurol. 2015;78:697–709. doi: 10.1002/ana.24493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.James BD, Wilson RS, Boyle PA, Trojanowski JQ, Bennett DA, Schneider JA. TDP-43 stage, mixed pathologies, and clinical Alzheimer's-type dementia. Brain. 2016;139:2983–93. doi: 10.1093/brain/aww224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cairns NJ, Neumann M, Bigio EH, Holm IE, Troost D, Hatanpaa KJ, Foong C, White CL, 3rd, Schneider JA, Kretzschmar HA, Carter D, Taylor-Reinwald L, Paulsmeyer K, Strider J, Gitcho M, Goate AM, Morris JC, Mishra M, Kwong LK, Stieber A, Xu Y, Forman MS, Trojanowski JQ, Lee VM, Mackenzie IR. TDP-43 in familial and sporadic frontotemporal lobar degeneration with ubiquitin inclusions. Am J Pathol. 2007;171:227–40. doi: 10.2353/ajpath.2007.070182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.