Abstract
Microtubules are a major component of the neuronal cytoskeleton. Tubulin, the subunit protein of microtubules, is an α/β heterodimer. Both α and β exist as families of isotypes, whose members are encoded by different genes and have different amino acid sequences. The βII and βIII isotypes are very prominent in the nervous system. Our previous work has suggested that βII may play a role in neuronal differentiation, but the role of βIII in neurons is not well understood. In the work reported here, we examined the roles of the different β-tubulin isotypes in response to glutamate/glycine treatment, and found that both βII and βIII bind to glutathione in the presence of ROS, especially βIII. In contrast, βI did not bind to glutathione. Our results suggest that βII and βIII, but especially βIII, may play an important role in the response of neuronal cells to stress. In view of the high levels of βII and βIII expressed in the nervous system it is conceivable that these tubulin isotypes may use their sulfhydryl groups to scavenge ROS and protect neuronal cells against oxidative stress.
Keywords: Microtubules, tubulin, glutathionylation, oxidative stress
1. INTRODUCTION
Microtubules are very prominent in the nervous system. These organelles are composed of the protein tubulin, which is a heterodimer of two subunits designated α and β (Luduena et al., 1977). Both α and β exist as families of isotypes differing in amino acid sequence and encoded by different genes (Luduena, 1998). The differences among the vertebrate β-tubulin isotypes are highly conserved in evolution, suggesting that they are functionally significant, and the nature of these functions is still being elucidated. The nervous system is well adapted to address these questions because multiple β isotypes are expressed in neurons including βIII, which is significantly different in sequence from βI and βII, which are also expressed in neurons. We have previously reported that βI was necessary for survival of the neurons, while βII was not required for survival but was necessary for neuronal differentiation, specifically neurite outgrowth. βIII was more complex since silencing it did not compromise cell survival or metabolism and had very little effect on neurite outgrowth (Guo et al., 2010).
The tubulin isotypes are rich in free thiol groups with 12 in α-tubulin and 8 in each of the β-tubulin isotypes (except for βVI, which has 10) (Britto et al., 2002), which are critical to tubulin polymerization and microtubule functions (Britto et al., 2005). Loss of these thiol groups affects tubulin polymerization in the presence of oxidative stress (Huber et al., 2008; Landino et al., 2010). Excessive ROS generation causes oxidative stress and can have potentially harmful side effects on cellular functions (Finkel, 2003). ROS include superoxide (O2−·), hydrogen peroxide (H2O2), and the highly reactive hydroxyl radical (·OH). Among other effects, these species can react with sulfhydryl groups. Superoxide can also react with NO to form peroxynitrite (ONOO−) (Beckman et al., 1994). Peroxynitrite can interact with tubulin sulfhydryl groups to inhibit microtubule assembly (Landino et al., 2002). It has long been known that microtubules are sensitive to sulfhydryl oxidizing reagents (Mellon & Rebhun, 1976) and that microtubules in neurons are harmed by oxidative stress (Malorni et al., 1991; Banan et al., 2000; Roediger & Armati, 2003). Previous work has indicated that βIII plays a unique role in protecting certain cancer cells from a variety of anti-tumor drugs, including at least one, doxorubicin, which does not bind to tubulin but does induce oxidative stress (Gan et al., 2007; Minotti et al., 1999). It is perhaps highly significant that βIII lacks a cysteine at position 239, which is present in the βI and βII isotypes and whose sulfhydryl group is highly susceptible to oxidation. In fact, oxidation of cys239 inhibits microtubule assembly (Little & Luduena, 1985; Bai et al., 1989). For this reason, it is tempting to hypothesize that βIII may protect cells from the detrimental effects of ROS. In order to address this hypothesis, we examined interactions of the βI, βII and βIII isotypes with glutathione in the presence of ROS in SK-N-SH cells, which can be a reporter of thiol oxidative stress and intracellular protein interactions with ROS. We found that the βII and βIII isotypes, particularly βIII, react with glutathione while βI does not. These results suggest that βII and βIII, and especially βIII, play roles in the response to ROS but that the roles are likely to be very complex.
2. RESULTS AND DISCUSSION
2.1 Selective silencing of β–tubulin isotypes levels in SK-N-SH cells had little effect on the rate of ROS formation induced by glutamate/glycine treatment
Glutamate and glycine interact with neurons by binding to a complex receptor, called the NMDA (N-methyl-D-aspartic acid) receptor; the binding has multiple effects on neurons including allowing entry of calcium ions and raising intracellular levels of ROS (Reynolds & Hastings, 1995). NMDA receptors are expressed after neuronal differentiation and are not expressed in undifferentiated neurons (Pizzi et al., 2002). Treating differentiated SK-N-SH cells with glutamate and glycine caused an initial large increase in ROS generation followed by a decline to lower levels compared to the control without the treatment of glutamate/glycine (Guo et al., 2010). Since cell viability as reflected by the MTT assay was not affected by glutamate/glycine treatment over a period of 24 hours in differentiated cells (Guo et al., 2010), the results suggest that ROS may be eliminated by superoxide dismutase (SOD) or other ROS defense mechanisms such as glutathionylation. In order to determine whether specific β-tubulin isotypes have different effects on the generation of ROS by the glutamate/glycine-NMDA pathway, we selectively silenced both the βII and βIII isotypes (Figure 1A-D), and found that the levels of intracellular ROS in SK-N-SH cells respond to treatment of the cells with glutamate/glycine in a complex fashion that is not significantly affected by silencing expression of βII- or βIII-tubulin (Figure 1E), which suggests that β-tubulin isotypes may not be involved in the generation of ROS. The responses either do not occur or are minimal in undifferentiated SK-N-SH cells (data not shown). We previously reported that silencing βII caused an 18% decrease of mitochondrial dehydrogenase activity after one hour of treatment. In contrast, silencing βIII caused a 44% decrease of mitochondrial dehydrogenase. The effect of silencing βI could not be determined since βI is necessary for cell survival even in the absence of glutamate/glycine treatment, as we previously demonstrated (Guo et al., 2010).
FIGURE 1.
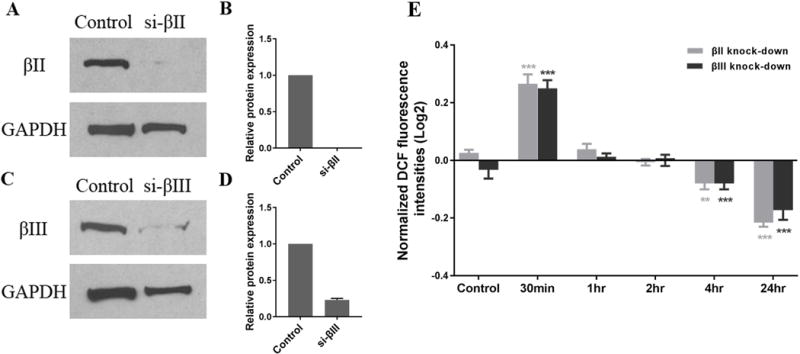
Effect of silencing β tubulin isotypes on ROS formation in differentiated SK-N-SH cells treated with glutamate and glycine. Expression of βII (A, B) and βIII (C, D) transfected with isotype-specific siRNA (siRNAs were incubated with cells for 24h) was measured after glutamate/glycine treatment for 24h in differentiated SK-N-SH cells. Negative siRNA was used as a control. GAPDH was used as a loading control. Note that each β isotype decreases significantly. (E) Cells with knockdown β tubulin isotypes were treated with 500 μM glutamate, 100 μM glycine, and 2 mM CaCl2 for the indicated times. Intracellular ROS production was determined by DCF fluorescence as described in Methods. Results are expressed as the log2 of fluorescence intensity normalized to the fluorescence intensity of undifferentiated SK-N-SH cells without any treatment, and are given as the mean ± SD. **ρ< 0.01; ***ρ<0.001 as compared with the control.
If not involved in the production of ROS, what roles do β-tubulin isotypes play in the response to oxidative stress? Evidence from Gan et al. (2007) indicates that the βIII isotype may play a role in protecting cells from ROS. Gan et al. (2007) silenced βI, βII and βIII separately in non-small cell lung cancer cells and then treated them with the anti-tumor drugs paclitaxel, vincristine and vinorelbine, which can interact with tubulin, and also with drugs that do not interact with tubulin, namely cisplatin, doxorubicin and etoposide (Gan et al., 2007). Silencing either βI or βII had no effect on cellular viability in the presence of any of these drugs. However, silencing βIII greatly increased susceptibility to all of the drugs. It is not surprising that silencing βIII would sensitize the cells to taxanes and Vinca alkaloids. First, among the β isotypes, βIII is known to interact least well with vinblastine (Khan & Luduena, 2003); second, the dynamics of microtubules made from the αβIII dimer are less sensitive to inhibition by paclitaxel than are the dynamics of microtubules made from either αβII or αβIV (Derry et al., 1997; Khan & Luduena, 2003). However, silencing βIII also enhanced susceptibility to cisplatin, doxorubicin and etoposide. If βIII exerts some kind of general protective effect on cells that express it, such an effect could help cells against ROS. In this context, it is interesting that doxorubicin, cisplatin and etoposide all promote production of free radicals and ROS in cells (Minotti et al., 1999).
A second piece of evidence that is consistent with the hypothesis that βIII protects cells from ROS is that the αβIII dimer is enriched in the outer membranes of mitochondria in certain cancer cells (Carre et al., 2002). Since mitochondria are a major cellular source of ROS, one could hypothesize that the αβIII dimer is present in the outer mitochondrial membrane to protect the cells from these ROS. If this hypothesis is correct, one might then speculate as to the mechanism by which βIII could perform this protective function. There are three pertinent observations here. First, tubulin has been found to be highly susceptible to reaction with peroxynitrite, which reacts with an as yet unidentified cysteine residue to form an intra-dimer disulfide that inhibits microtubule assembly (Landino et al., 2002). The extent of cysteine oxidation induced by peroxynitrite correlates well with inhibition of microtubule polymerization. However, the lost polymerization activity of tubulin induced by peroxynitrite can be restored by application of disulfide reducing agents, including dithiothreitol, which suggests that peroxynitrite-induced disulfide bonds are partially responsible for the inhibition of polymerization. Khan et al (1991) also confirmed that disulfide bonds in the tubulin dimers could be reduced in vitro by the thioredoxin system, which comprises thioredoxin, thioredoxin reductase and NADPH. Microtubules formed from reduced tubulin were found to be functionally and morphologically identical to those from native tubulin dimers (Khan & Luduena, 1991). The second observation is that the βI, βII and βIV isotypes of tubulin contain Cys239 whose sulfhydryl group is readily oxidized and whose oxidation inhibits microtubule assembly (Bai et al., 1989). βIII has Ser239 instead of Cys239, suggesting it would be more resistant to ROS since microtubule assembly might not be affected by ROS. Thus, one could argue that βIII forms microtubules whose assembly would be more resistant to ROS than would that of microtubules formed from βI, βII or βIV. The third observation is that βIII contains a Cys124, which is not present in the βI, βII or βIV isotypes. This cysteine is very close to the highly conserved Cys127 and Cys129, which raises the possibility that this cysteine cluster could act as a sink for ROS and reactive oxygen species by forming disulfide bonds. A similar situation occurs in Von Willebrand’s protein, which has a similar cluster of cysteines (Mayadas & Wagner, 1992; Dong et al., 1994). One could perhaps invoke the same function for Cys239 in βI and βII. The hypothesis that βIII could act as a sink is consistent with its relatively high concentration in differentiated SK-N-SH cells, where it accounts for 0.80% of the total cellular protein. Interestingly, a similar argument could be made for βII, which accounts for 1.21% of the total cellular protein (Guo et al., 2010). Protein accounts for approximately 20% of a cell’s weight. Since the average cell weight is 3.5×10−9 g, the total weight of cellular protein is 7×10−10 g (Lodish et al., 2008). Therefore, βII and βIII would have a concentration of 1.54×10−4 pmol/cell and 1.02×10−4 pmol/cell in SK-N-SH cells, respectively. Compared to these isotypes, the concentration of the enzyme SOD, which plays a protective role against free radicals, is only 4.6×10−7 pmol/cell (15 ng per 106 cells in a normal person) (Porstmann et al., 1990).
2.2 The interaction of β tubulin isotypes with glutathione
To investigate whether the thiols of cysteine residues on the β tubulin isotypes are oxidized by ROS and interact with glutathione (GSH) to form mixed disulfides, a measure of cellular (thiol) oxidative stress, we immunoprecipitated the β tubulin isotypes of differentiated SK-N-SH cells treated with glutamate and glycine; we then searched for covalently bound glutathione. The rationale is as follows: one ROS produced by glutamate/glycine treatment is superoxide (O2¯·), which is quickly transformed into H2O2 by SOD. H2O2 reacts with thiols to form sulfenic acid moieties, which rapidly react with other thiols in their vicinity, the most abundant in the cell cytosol being GSH (Cumming et al., 2004). This reaction converts protein thiols into GSS-protein mixed disulfides, a process referred to as protein S-glutathionylation. However, the catalytic mechanism of GSS-protein formation in cells in still largely unknown. De-glutathionylation is catalyzed by glutaredoxin (GRx) (Figure 2) (Gallogly & Mieyal, 2007). Cell extracts were obtained from differentiated SK-N-SH cells treated with glutamate/glycine for 30 min, 1 hr, 2 hr, 4 hr, and 24 hr, respectively. The controls consisted of extracts from cells that had not been treated with glutamate/glycine. Then βI, βII, and βIII antibodies were separately incubated with cell extracts for immunoprecipitation. Precipitates were analyzed on Western blots. Antibodies directed against protein-bound glutathione and the β tubulin antibodies were used to identify S-glutathionylated β tubulin. The results showed that βI did not detectably react with glutathione, suggesting that βI was not oxidized by ROS (Figure 3A, D). However, βII and especially βIII interacted strongly with glutathione, forming GSH-tubulin mixed disulfides (Figure 3B, C, D). In the case of βII, the extent of the reaction was strongly dependent on the length of exposure to oxidative stress, dropping at first, then rising and then dropping again (Figure 3B, D). It is possible that S-glutathionylation of βII may be regulated in such a way that it is minimal when intracellular ROS levels are abnormal (as in 30min, 4hr, and 24hr); elucidating the details of this mechanism would be a subject for future investigation. The results raise the possibility that βII and βIII may protect cells by catalyzing the reduction of excess free radicals and reactive oxygen species to water or other less harmful species.
FIGURE 2.
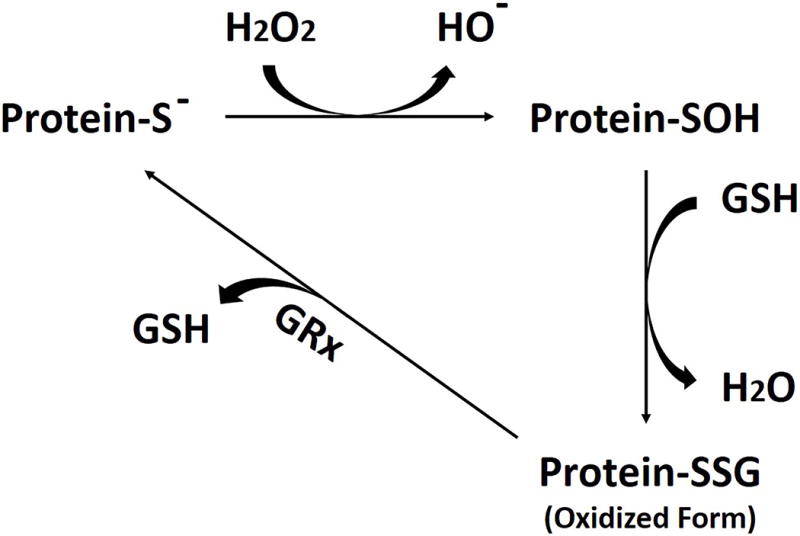
Enzymatic scavenging of reactive oxygen species (ROS). Hydrogen peroxide is converted into water; the cysteine-sulfhydryl moiety of glutathione (GSH) and protein sulfhydryls (protein-S−) are required for this reaction, in which GSH and protein-S− are converted into their oxidized form, a GSS-protein.
FIGURE 3.
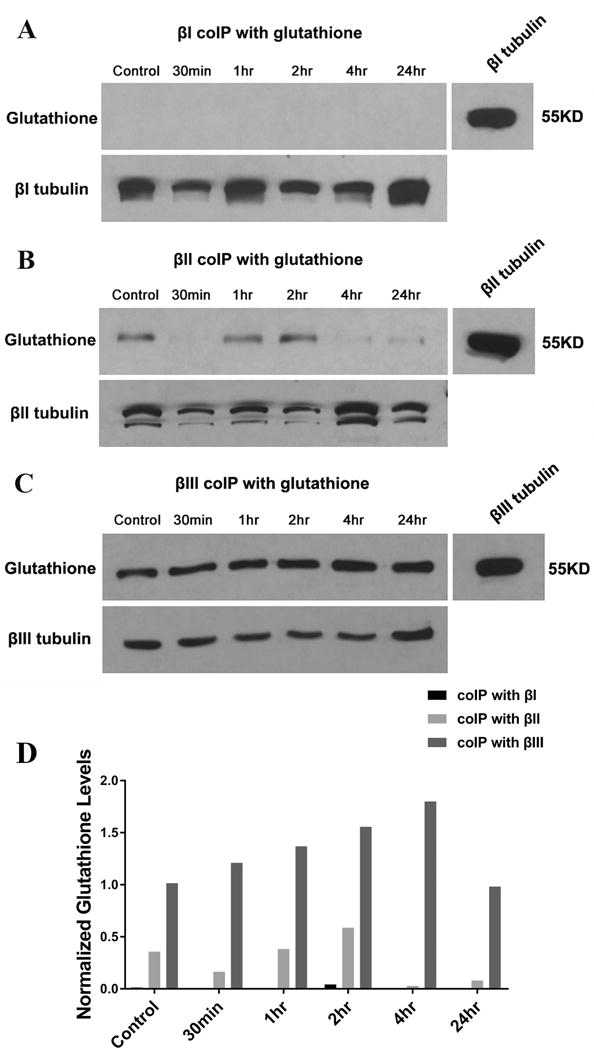
Co-Immunoprecipitation of β tubulin isotypes with glutathione in differentiated SK-N-SH cells treated with glutamate/glycine. Differentiated SK-N-SH cells were treated with 500 μM glutamate, 100 μM glycine, and 2 mM CaCl2 for the indicated times. Cell extracts were immunoprecipitated with monoclonal antibody to βI, βII, and βIII, respectively. The presence of glutathione was probed using mouse monoclonal glutathione antibody (upper row in each panel). The co-migration of glutathione with β tubulin isotypes was confirmed using the antibody to the isotypes (right image in each panel). The same nitrocellulose membrane was re-probed using β tubulin antibody to confirm the presence of β tubulin (lower row in each panel). The result showed that (A) no glutathione was detected at the position of βI, suggesting that βI did not interact with glutathione; (B) glutathione was detected at the position of βII, suggesting that βII interacted with glutathione; (C) glutathione was detected at the position of βIII, suggesting that βIII interacted with glutathione. These experiments were repeated twice. (D) Quantification of glutathione interacting with β tubulin isotypes. The figures shown in panels A, B and C were quantified using the ImageJ software available at http://rsb.info.nih.gov/ij/. Glutathione levels were normalized to β tubulin isotypes.
That glutathione can interact with tubulin has long been known. Banerjee et al. (Banerjee et al., 1985) showed that 5 mM glutathione could inhibit colchicine binding to tubulin in vitro as well as induce an abnormal aggregation of tubulin into amorphous structures. Both of these effects were inhibited by 5-10 mM oxidized glutathione (Banerjee et al., 1985). Glutathione concentrations in cells range from 2 mM to 10 mM depending on the cell type (Cotgreave, 2003). In mammalian cells, almost 90% of the glutathione is in the cytosol and up to 10% in the mitochondria. All the glutathione is synthesized in the cytoplasm. Most of the glutathione (>98%) exists in the form of GSH, while <1% of total glutathione is in the form of GSSG (Hansen et al., 2006). These observed in vivo concentrations of glutathione as well as the results of Banerjee et al. (Banerjee et al., 1985) are consistent with our observation of binding of glutathione to tubulin. In addition to affecting tubulin polymerization, glutathione has previously been shown to bind covalently to tubulin in vivo (Luduena, 2008). Here, however, we examine for the first time binding to individual isotypes. Because of the distribution of thiols in the sequences of the isotypes, it is quite unexpected that βII and βIII bind to glutathione while βI does not. βI and βII have the exact same distribution of thiols, including C239, while βIII lacks C239 but has C124, which βI and βII do not. If C239 were the residue that reacted with glutathione, one would expect to see glutathionylaton in βI and βII but not in βIII. Conversely, if C124 were the reactive residue then one would expect that βIII would be glutathionylated and not the other two. The fact that only βII and βIII are glutathionylated suggests that the interaction is very complex, perhaps involving a thiol other than the ones of C124 or C239 and conceivably being mediated by a conformational difference whereby the reactive thiol or thiols are only available in βII and βIII and not in βI. Alternatively, perhaps the specific environment mandates that only C239 reacts in βII and only C124 in βIII. Pursuing this same line of reasoning, such conformational changes could account for the apparent detachment of glutathione from βII during the reaction. Since βII has a much less rigid conformation than βIII (Schwarz et al., 1998), one could envision a conformational change in βII, provoked by further reaction of βII with ROS, which brings a cysteine close enough to the covalently bound glutathione to permit a sulfhydryl-disulfide interchange to detach the glutathione from the tubulin molecule. βIII, with its much more rigid conformation, may not allow for such a reaction to happen so rapidly. The fact that the 24 hr time-point for βIII appears to be relatively lower than for earlier time points (Figure 3D) raises the possibility that if allowed to react long enough, βIII also would undergo the same conformational change that would allow the glutathione to detach.
It should be noted that there is considerable binding of glutathione to both βII and βIII even in cells not treated with glutamate/glycine (Figures 3B, C) and although there is some variation in glutathione binding noted with βII following glutamate/glycine treatment, it appears that covalent binding of glutathione to the βII and βIII isotypes is likely to be significant even under normal conditions. S-Glutathionylation of these isotypes may even count as a novel post-translational modification of tubulin, which certainly has a large number of these already (Landino et al., 2004). Glutathionylation of cellular proteins is controlled by the glutaredoxin system (Holmgren, 1989). There is often a basal level of protein glutathionylation even when cells are not stressed (Ghezzi et al., 2002), as we have observed here. In addition to protecting proteins against oxidative stress, reversible glutathionylation is also important in regulating a variety of cellular processes by modulating protein activity, guiding correct protein folding, and regulating proteins in redox signaling pathways as a specific post-translational modification in unstressed cells (Bibert et al., 2011; Chen et al., 2007; Dalle-Donne et al., 2003; Demasi et al., 2003; Kemmerling et al., 2007; Pan & Berk, 2007; Rinna et al., 2006).
In summary, our investigation suggests that β tubulin, especially βII and βIII, may play an important role in protecting neurons from oxidative stress; such stress could cause deleterious alterations in microtubule structure and function. Furthermore, the interaction of specific tubulin isotypes with glutathione raises the possibility of the existence of a novel, as yet poorly understood, pathway that may regulate microtubule assembly and/or function. Also, our findings might contribute to understanding the pathogenic events involved in neuronal degeneration that characterize many neuronal degenerative diseases associated with oxidative stress.
3. MATERIALS AND METHODS
3.1 Antibodies
The monoclonal antibodies specific to the β isotypes of tubulin (βI, βII, βIII) were prepared as previously described (Banerjee et al., 1988; Banerjee et al., 1990; Banerjee et al., 1992; Roach et al., 1998). Briefly, hybridoma supernatants containing antibodies to βI (SAP.4G5), βII (JDR.3B8), βIII (SDL.3D10), and βIV (ONS.1A6) were passed through a protein A-agarose column and washed with PBS. Bound antibody was eluted with 0.1 M glycine-HCl (pH 2.3) and fractions were collected and their absorbance at 280 nm determined. Fractions containing the antibodies were pooled and dialyzed overnight at 4°C with PBS containing 0.02% NaN3. The antibodies were stored at −20°C until use. The human GAPDH (glyceraldehyde-3-phosphate dehydrogenase) antibody was purchased from Invitrogen.
3.2 Cell culture
Human SK-N-SH neuroblastoma cells were provided by Dr. Consuelo Walss-Bass. The cells were plated and grown at 37°C in a humidified atmosphere of 5% CO2, 95% O2 in Minimum Essential Medium with Earle’s salts and L-glutamine (MEM, Cellgro) plus 10% fetal calf serum, 1 mM sodium pyruvate, 1% non-essential amino acids, penicillin-streptomycin-fungisone antibiotics and 1.5 g/L sodium bicarbonate. For cell differentiation, cells were plated in normal medium for 24hr before being induced to differentiate by addition of retinoic acid (RA, Sigma) to the culture medium to a final concentration of 30 μM. The medium was changed on alternate days, and cultures were allowed to differentiate for 1-2 weeks. After 14 days with or without retinoic acid treatment, the cells were treated with 500 μM glutamate, 100 μM glycine, and 2 mM CaCl2 at incremental time points. The “control” cells are those that have not been treated with glutamate/glycine. After incubation, the cells were washed with PBS and harvested for Western blot assay and measurement of ROS.
3.3 RNA interference
To knock down the β isotypes specifically, both the βII and βIII siRNAsiGENOMESMARTpool reagents were obtained from Dharmacon Research, Inc. The non-targeting siRNA obtained from Ambion was used in all experiments as a negative control. Cells were transfected with β isotype-specific siRNA at a final concentration of 100nM using lipofectamine 2000 (Invitrogen) following the manufacturer’s instructions (Invitrogen).
3.4 Measurement of Reactive Oxygen Species (ROS)
The generation of ROS was evaluated using 2′, 7′-dichlorodihydrofluorescein-diacetate (DCFH-DA) (Invitrogen Molecular Probes). The method is based on the ability of DCFH-DA to enter cells freely and hydrolyze to non-membrane permeable 7′-dichloradihydrofluorescein, which in the presence of peroxidases reacts with ROS to form the fluorescent derivative dichlorofluorescein (DCF) (McArdle et al., 2005). Cells were harvested and washed three times with PBS. The cells were then resuspended in 1 ml PBS and incubated for 30 min with 10 μM DCFH-DA at 37°C. After incubation, the cells were washed with PBS, then pelleted and lysed in 100 μl of lysis buffer (50 mMTris (pH 8.0), 120 mM NaCl, 0.5% NP-40, 0.2 mM sodium orthovanadate, 100 mM sodium fluoride, 1% 200 mM PMSF, dissolved in dimethylsulfoxide, 1% Protease Inhibitor Cocktail). The lysed cells were centrifuged at 15,000 × g for 30 min at 4°C. Fluorescence intensities of the supernatants were measured at an excitation wavelength of 450 nm and an emission wavelength of 510 nm.
3.5 Co-immunoprecipitation
Protein lysates from human SK-N-SH cells were subjected to immunoprecipitation using monoclonal antibodies against βI, βII, and βIII to study the potential association of these β tubulin isotypes with glutathione. Cells were washed in PBS, and then incubated with 50 mM NEM (N-ethylmaleimide) in PBS for 5 min at room temperature. Pelleted cells were washed with PBS and lysed in ice-cold lysis buffer (50 mM Tris (pH = 8.0), 120 mM NaCl, 0.5% NP-40, 0.2 mM sodium orthovanadate, 100 mM sodium fluoride, 1% 200 mM phenylmethylsulfonyl fluoride [PMSF, dissolved in dimethyl sulfoxide (DMSO)], 1% Protease Inhibitor Cocktail] including 10 mM NEM). Protein lysates were incubated at 4°C for 30 min, vortexed every 10 min, and then centrifuged at 12,000 × g for 30 min at 4°C. 300μg of protein extracts were precleared by incubation with 20 μl of 50% slurry of protein G-agarose beads (Amersham Biosciences) for 2hr at 4°C on an orbital shaker. Equal amounts of protein extracts (300 μg per immunoprecipitation) were incubated with equal amount of monoclonal antibodies against βI, βII, and βIII overnight at 4 °C with constant rotation, respectively. Control samples were extracts from cells that had not been incubated with glutamate/glycine. Subsequently, 20μl of a 50% slurry of protein G-agarose beads was added to each sample and incubated for 2hr at 4 °C with mild agitation. Beads were collected by centrifugation. After three washes with cold lysis buffer, pelleted beads were quenched in protein sample buffer and boiled. Samples including protein G-sepharose beads were loaded onto SDS-PAGE gels, and then analyzed on Western blots with different antibodies as needed. Anti-glutathione antibody was purchased from Chemicon (Millipore, MA).
3.6 Statistical analysis
All statistics were performed by one-way ANOVA compared with a control within each group. In all the figures, * indicates ρ <0.05; ** indicates ρ <0.01; and *** indicates ρ <0.001. All data are presented as mean ± S.D.
Acknowledgments
Financial assistance was provided by grants W81XWH-05-1-0238 and W81XWH-10-1-0903 from the Department of Defense Breast Cancer Research Program to R.F. Ludueña and 2 P30 CA054174-17 from the Cancer Therapy and Research Center at the University of Texas Health Science Center at San Antonio through the NCI Cancer Center to R.F. Ludueña. We thank Mrs. Veena Prasad for skilled technical assistance. The research reported here was part of the doctoral dissertation of JG; the suggestions and comments of her committee members, Drs. Fernando Cabral, Lee McAlister-Henn, Consuelo Walss-Bass, Susan Weintraub, and the late Donald McEwen, are gratefully acknowledged.
Footnotes
Conflict of Interest: Dr. Ludueña owns 500,000 shares in the company Oncovista Innovative Therapies, Inc.
References
- Bai RL, Lin CM, Nguyen NY, Liu TY, Hamel E. Identification of the cysteine residue of beta-tubulin alkylated by the antimitotic agent 2,4-dichlorobenzyl thiocyanate, facilitated by separation of the protein subunits of tubulin by hydrophobic column chromatography. Biochemistry. 1989;28:5606–5612. doi: 10.1021/bi00439a040. [DOI] [PubMed] [Google Scholar]
- Banan A, Fields JZ, Decker H, Zhang Y, Keshavarzian A. Nitric oxide and its metabolites mediate ethanol-induced microtubule disruption and intestinal barrier dysfunction. J Pharmacol Exp Ther. 2000;294:997–1008. [PubMed] [Google Scholar]
- Banerjee A, Jordan MA, Luduena RF. Interaction of reduced glutathione with bovine brain tubulin. Biochem Biophys Res Commun. 1985;128:506–512. doi: 10.1016/0006-291x(85)90075-0. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Roach MC, Trcka P, Luduena RF. Increased microtubule assembly in bovine brain tubulin lacking the type III isotype of beta-tubulin. J Biol Chem. 1990;265:1794–1799. [PubMed] [Google Scholar]
- Banerjee A, Roach MC, Trcka P, Luduena RF. Preparation of a monoclonal antibody specific for the class IV isotype of beta-tubulin. Purification and assembly of alpha beta II, alpha beta III, and alpha beta IV tubulin dimers from bovine brain. J Biol Chem. 1992;267:5625–5630. [PubMed] [Google Scholar]
- Banerjee A, Roach MC, Wall KA, Lopata MA, Cleveland DW, Luduena RF. A monoclonal antibody against the type II isotype of beta-tubulin. Preparation of isotypically altered tubulin. J Biol Chem. 1988;263:3029–3034. [PubMed] [Google Scholar]
- Beckman JS, Chen J, Ischiropoulos H, Crow JP. Oxidative chemistry of peroxynitrite. Methods Enzymol. 1994;233:229–240. doi: 10.1016/s0076-6879(94)33026-3. [DOI] [PubMed] [Google Scholar]
- Bibert S, Liu CC, Figtree GA, Garcia A, Hamilton EJ, Marassi FM, Sweadner KJ, Cornelius F, Geering K, Rasmussen HH. FXYD proteins reverse inhibition of the Na+-K+ pump mediated by glutathionylation of its beta1 subunit. J Biol Chem. 2011;286:18562–18572. doi: 10.1074/jbc.M110.184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britto PJ, Knipling L, Wolff J. The local electrostatic environment determines cysteine reactivity of tubulin. J Biol Chem. 2002;277:29018–29027. doi: 10.1074/jbc.M204263200. [DOI] [PubMed] [Google Scholar]
- Britto PJ, Knipling L, McPhie P, Wolff J. Thiol-disulphide interchange in tubulin: kinetics and the effect on polymerization. Biochem J. 2005;389:549–558. doi: 10.1042/BJ20042118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carre M, Andre N, Carles G, Borghi H, Brichese L, Briand C, Braguer D. Tubulin is an inherent component of mitochondrial membranes that interacts with the voltage-dependent anion channel. J Biol Chem. 2002;277:33664–33669. doi: 10.1074/jbc.M203834200. [DOI] [PubMed] [Google Scholar]
- Chen YR, Chen CL, Pfeiffer DR, Zweier JL. Mitochondrial complex II in the post-ischemic heart: oxidative injury and the role of protein S-glutathionylation. J Biol Chem. 2007;282:32640–32654. doi: 10.1074/jbc.M702294200. [DOI] [PubMed] [Google Scholar]
- Cotgreave IA. Analytical developments in the assay of intra- and extracellular GSH homeostasis: specific protein S-glutathionylation, cellular GSH and mixed disulphide compartmentalisation and interstitial GSH redox balance. Biofactors. 2003;17:269–277. doi: 10.1002/biof.5520170126. [DOI] [PubMed] [Google Scholar]
- Cumming RC, Andon NL, Haynes PA, Park M, Fischer WH, Schubert D. Protein disulfide bond formation in the cytoplasm during oxidative stress. J Biol Chem. 2004;279:21749–21758. doi: 10.1074/jbc.M312267200. [DOI] [PubMed] [Google Scholar]
- Dalle-Donne I, Giustarini D, Rossi R, Colombo R, Milzani A. Reversible S-glutathionylation of Cys 374 regulates actin filament formation by inducing structural changes in the actin molecule. Free Radic Biol Med. 2003;34:23–32. doi: 10.1016/s0891-5849(02)01182-6. [DOI] [PubMed] [Google Scholar]
- Demasi M, Silva GM, Netto LE. 20 S proteasome from Saccharomyces cerevisiae is responsive to redox modifications and is S-glutathionylated. J Biol Chem. 2003;278:679–685. doi: 10.1074/jbc.M209282200. [DOI] [PubMed] [Google Scholar]
- Derry WB, Wilson L, Khan IA, Luduena RF, Jordan MA. Taxol differentially modulates the dynamics of microtubules assembled from unfractionated and purified beta-tubulin isotypes. Biochemistry. 1997;36:3554–3562. doi: 10.1021/bi962724m. [DOI] [PubMed] [Google Scholar]
- Dong Z, Thoma RS, Crimmins DL, McCourt DW, Tuley EA, Sadler JE. Disulfide bonds required to assemble functional von Willebrand factor multimers. J Biol Chem. 1994;269:6753–6758. [PubMed] [Google Scholar]
- Finkel T. Oxidant signals and oxidative stress. Curr Opin Cell Biol. 2003;15:247–254. doi: 10.1016/s0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- Gallogly MM, Mieyal JJ. Mechanisms of reversible protein glutathionylation in redox signaling and oxidative stress. Curr Opin Pharmacol. 2007;7:381–391. doi: 10.1016/j.coph.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Gan PP, Pasquier E, Kavallaris M. Class III beta-tubulin mediates sensitivity to chemotherapeutic drugs in non small cell lung cancer. Cancer Res. 2007;67:9356–9363. doi: 10.1158/0008-5472.CAN-07-0509. [DOI] [PubMed] [Google Scholar]
- Ghezzi P, Romines B, Fratelli M, Eberini I, Gianazza E, Casagrande S, Laragione T, Mengozzi M, Herzenberg LA, Herzenberg LA. Protein glutathionylation: coupling and uncoupling of glutathione to protein thiol groups in lymphocytes under oxidative stress and HIV infection. Mol Immunol. 2002;38:773–780. doi: 10.1016/s0161-5890(01)00114-6. [DOI] [PubMed] [Google Scholar]
- Guo J, Walss-Bass C, Luduena RF. The beta isotypes of tubulin in neuronal differentiation. Cytoskeleton (Hoboken) 2010;67:431–441. doi: 10.1002/cm.20455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen JM, Go YM, Jones DP. Nuclear and mitochondrial compartmentation of oxidative stress and redox signaling. Annu Rev Pharmacol Toxicol. 2006;46:215–234. doi: 10.1146/annurev.pharmtox.46.120604.141122. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin and glutaredoxin systems. J Biol Chem. 1989;264:13963–13966. [PubMed] [Google Scholar]
- Huber K, Patel P, Zhang L, Evans H, Westwell AD, Fischer PM, Chan S, Martin S. 2-[(1-methylpropyl)dithio]-1H-imidazole inhibits tubulin polymerization through cysteine oxidation. Mol Cancer Ther. 2008;7:143–151. doi: 10.1158/1535-7163.MCT-07-0486. [DOI] [PubMed] [Google Scholar]
- Kemmerling U, Munoz P, Muller M, Sanchez G, Aylwin ML, Klann E, Carrasco MA, Hidalgo C. Calcium release by ryanodine receptors mediates hydrogen peroxide-induced activation of ERK and CREB phosphorylation in N2a cells and hippocampal neurons. Cell Calcium. 2007;41:491–502. doi: 10.1016/j.ceca.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Khan IA, Luduena RF. Possible regulation of the in vitro assembly of bovine brain tubulin by the bovine thioredoxin system. Biochim Biophys Acta. 1991;1076:289–297. doi: 10.1016/0167-4838(91)90280-d. [DOI] [PubMed] [Google Scholar]
- Khan IA, Luduena RF. Different effects of vinblastine on the polymerization of isotypically purified tubulins from bovine brain. Invest New Drugs. 2003;21:3–13. doi: 10.1023/a:1022946305242. [DOI] [PubMed] [Google Scholar]
- Landino LM, Moynihan KL, Todd JV, Kennett KL. Modulation of the redox state of tubulin by the glutathione/glutaredoxin reductase system. Biochem Biophys Res Commun. 2004;314:555–560. doi: 10.1016/j.bbrc.2003.12.126. [DOI] [PubMed] [Google Scholar]
- Landino LM, Hasan R, McGaw A, Cooley S, Smith AW, Masselam K, Kim G. Peroxynitrite oxidation of tubulin sulfhydryls inhibits microtubule polymerization. Arch Biochem Biophys. 2002;398:213–220. doi: 10.1006/abbi.2001.2729. [DOI] [PubMed] [Google Scholar]
- Landino LM, Brown CM, Edson CA, Gilbert LJ, Grega-Larson N, Wirth AJ, Lane KC. Fluorescein-labeled glutathione to study protein S-glutathionylation. Anal Biochem. 2010;402:102–104. doi: 10.1016/j.ab.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little M, Luduena RF. Structural differences between brain beta 1- and beta 2-tubulins: implications for microtubule assembly and colchicine binding. Embo J. 1985;4:51–56. doi: 10.1002/j.1460-2075.1985.tb02316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H, Berk A, Kaiser CA, Krieger M, Scott MP, Bretscher A, Ploegh H, Matsudaira P. Molecular cell biology. New York: Sara Tenney; 2008. p. 11. [Google Scholar]
- Luduena RF. Multiple forms of tubulin: different gene products and covalent modifications. Int Rev Cytol. 1998;178:207–275. doi: 10.1016/s0074-7696(08)62138-5. [DOI] [PubMed] [Google Scholar]
- Luduena RF, Shooter EM, Wilson L. Structure of the tubulin dimer. J Biol Chem. 1977;252:7006–7014. [PubMed] [Google Scholar]
- Luduena RF, A B. The post-translational modifications of tubulin, in cancer drug discovery and development. In: F T, editor. The role of microtubules in cell biology, neurobiology, and oncology. Totowa, NJ: Humana press; 2008. pp. 105–121. [Google Scholar]
- Malorni W, Iosi F, Mirabelli F, Bellomo G. Cytoskeleton as a target in menadione-induced oxidative stress in cultured mammalian cells: alterations underlying surface bleb formation. Chem Biol Interact. 1991;80:217–236. doi: 10.1016/0009-2797(91)90026-4. [DOI] [PubMed] [Google Scholar]
- Mayadas TN, Wagner DD. Vicinal cysteines in the prosequence play a role in von Willebrand factor multimer assembly. Proc Natl Acad Sci U S A. 1992;89:3531–3535. doi: 10.1073/pnas.89.8.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle F, Pattwell DM, Vasilaki A, McArdle A, Jackson MJ. Intracellular generation of reactive oxygen species by contracting skeletal muscle cells. Free Radic Biol Med. 2005;39:651–657. doi: 10.1016/j.freeradbiomed.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Mellon MG, Rebhun LI. Sulfhydryls and the in vitro polymerization of tubulin. J Cell Biol. 1976;70:226–238. doi: 10.1083/jcb.70.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minotti G, Cairo G, Monti E. Role of iron in anthracycline cardiotoxicity: new tunes for an old song? Faseb J. 1999;13:199–212. [PubMed] [Google Scholar]
- Pan S, Berk BC. Glutathiolation regulates tumor necrosis factor-alpha-induced caspase-3 cleavage and apoptosis: key role for glutaredoxin in the death pathway. Circ Res. 2007;100:213–219. doi: 10.1161/01.RES.0000256089.30318.20. [DOI] [PubMed] [Google Scholar]
- Pizzi M, Boroni F, Bianchetti A, Moraitis C, Sarnico I, Benarese M, Goffi F, Valerio A, Spano P. Expression of functional NR1/NR2B-type NMDA receptors in neuronally differentiated SK-N-SH human cell line. Eur J Neurosci. 2002;16:2342–2350. doi: 10.1046/j.1460-9568.2002.02403.x. [DOI] [PubMed] [Google Scholar]
- Porstmann T, Wietschke R, Cobet G, Lorenz K, Grunow R, Jahn S, Bollmann R, Stamminger G, von Baehr R. Immunochemical quantification of Cu/Zn superoxide dismutase in prenatal diagnosis of Down’s syndrome. Hum Genet. 1990;85:362–366. doi: 10.1007/BF00206762. [DOI] [PubMed] [Google Scholar]
- Reynolds IJ, Hastings TG. Glutamate induces the production of reactive oxygen species in cultured forebrain neurons following NMDA receptor activation. J Neurosci. 1995;15:3318–3327. doi: 10.1523/JNEUROSCI.15-05-03318.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinna A, Torres M, Forman HJ. Stimulation of the alveolar macrophage respiratory burst by ADP causes selective glutathionylation of protein tyrosine phosphatase 1B. Free Radic Biol Med. 2006;41:86–91. doi: 10.1016/j.freeradbiomed.2006.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach MC, Boucher VL, Walss C, Ravdin PM, Luduena RF. Preparation of a monoclonal antibody specific for the class I isotype of beta-tubulin: the beta isotypes of tubulin differ in their cellular distributions within human tissues. Cell Motil Cytoskeleton. 1998;39:273–285. doi: 10.1002/(SICI)1097-0169(1998)39:4<273::AID-CM3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Roediger B, Armati PJ. Oxidative stress induces axonal beading in cultured human brain tissue. Neurobiol Dis. 2003;13:222–229. doi: 10.1016/s0969-9961(03)00038-x. [DOI] [PubMed] [Google Scholar]
- Schwarz PM, Liggins JR, Luduena RF. Beta-tubulin isotypes purified from bovine brain have different relative stabilities. Biochemistry. 1998;37:4687–4692. doi: 10.1021/bi972763d. [DOI] [PubMed] [Google Scholar]


