Abstract
Mortality is higher in septic patients with a history of alcohol use disorder than in septic patients without a history of chronic alcohol usage. We have previously described a model of chronic alcohol ingestion followed by sepsis from cecal ligation and puncture in which alcohol-fed septic mice have higher mortality than water-fed septic mice, associated with altered gut integrity and increased production of TNF and IFNγ by splenic CD4+ T cells without alterations in CD8+ T cell function. The purpose of this study was to determine whether this represents a common host response to the combination of alcohol and sepsis by creating a new model in which mice with chronic alcohol ingestion were subjected to a different model of sepsis. C57Bl/6 mice were randomized to receive either alcohol or water for 12 weeks and then subjected to Pseudomonas aeruginosa pneumonia. Mice were sacrificed either 24 hours after the onset of sepsis or followed for survival. Alcohol-fed septic mice had significantly higher 7-day mortality than water-fed septic mice (96% vs 58%). This was associated with a 5-fold increase in intestinal apoptosis in alcohol-fed septic animals, accompanied by an increase in the pro-apoptotic protein Bax. Serum IL-6 levels were higher and IL-2 levels were lower in alcohol-fed septic mice. In contrast, CD8+ T cell frequency was lower in alcohol-fed mice than water-fed septic mice, associated with increased production of IFNγ and TNF in stimulated splenocytes. No significant differences were noted in CD4+ T cells, lung injury or bacteremia. Mice with chronic alcohol ingestion thus have increased mortality regardless of their septic insult, associated with changes in both the gut and the immune system.
Keywords: Ethanol, intestine, immune, CD4+ T cell, cytokine, bacteria, mortality
INTRODUCTION
Sepsis is life threatening organ dysfunction secondary to a dysregulated host response to infection (1). Sepsis is the leading cause of death among critically ill patients in the United States with at least 270,000 patients dying annually (2). While mortality from sepsis is high, not all patients have the same chance of developing the disease or dying from it, as patients with chronic co-morbidities are at significantly higher risk.
Alcohol use disorder represents a serious public health challenge. Alcohol use disorder affects nearly 33 million people in the United States, where the lifetime prevalence of alcohol abuse is estimated to be 18% (3;4). Notably, deaths from alcohol recently reached a 35 year high (5).
An estimated 20-40% of patients admitted to the hospital have alcohol use disorder and up to one third of patients admitted to the intensive care unit (ICU) have this disease (6–8). Septic patients with alcohol use disorder have a higher mortality than septic patients without a similar medical history and have increased severity of multiple organ dysfunction (9;10). Notably, a recent study of diagnosis trajectories of 120,000 septic patients demonstrated that alcohol abuse carries a greater than 2-fold increased risk of death compared to patients without co-morbidities (11). Further, alcohol use disorder is associated with an increased risk of developing community acquired pneumonia and increased risk of mortality from pneumonia in trauma patients by complex mechanisms including impaired alveolar surfactant production, barrier integrity and macrophage function (12–15).
In an attempt to develop a pre-clinical model of long-term alcohol use followed by sepsis, we previously described a model of chronic alcohol ingestion followed by cecal ligation and puncture (CLP) (16;17). Mice with 12 weeks of alcohol ingestion (referred to as alcohol-fed hereafter) prior to CLP had a higher mortality than mice who drank water (referred to as water-fed hereafter) prior to CLP. This was associated with worsened gut integrity with increased intestinal epithelial apoptosis, decreased proliferation, and increased permeability. In addition, splenic CD4+ T cells isolated from alcohol-fed septic mice had a marked increase in both TNF and IFNγ production following ex vivo stimulation although no difference was noted in stimulated cytokine production in CD8+ T cells. In addition, serum cytokines were generally similar between alcohol-fed and water-fed septic mice although serum IL-6 was lower in alcohol-fed septic mice. Subsequent studies demonstrated that alcohol-fed septic mice had delayed T cell activation and effector function (18).
Despite a greater pre-clinical and clinical understanding of sepsis, outside of targeted antibiotic therapy, current management of sepsis at the bedside is non-specific, mainly consisting of rapid fluid resuscitation and supportive therapy (19). The etiology of the failure of pre-clinical trials to translate to clinical therapy is assuredly multifactorial. However, one of the hypothesized reasons for this failure is that animal studies are performed in homogenous populations that were healthy prior to the onset of sepsis, whereas septic patients are heterogeneous by nature and typically have one or more co-morbidities prior to the onset of sepsis (20). Further, the type of co-morbidity and type of sepsis each have a profound impact on the dysregulated host response (21;22), and it cannot be assumed that a single model of either sepsis or a chronic co-morbidity is fully representative of the population at large. In light of existing knowledge on the impact of a) chronic alcohol in isolation, b) sepsis in isolation, and c) chronic alcohol followed by CLP, this study aimed to understand the interaction between alcohol and sepsis in the setting of a different model of sepsis than has previously been studied in the context of chronic alcohol ingestion. Notably, the pneumonia model of sepsis is clinically relevant since pneumonia is, the most common cause of sepsis, accounting for nearly half of all cases (23), and alcohol use has been shown to increase the risk of developing pneumonia in the community (24).
METHODS
Animals
Six week old male and female C57BL/6 mice were purchased from a commercial vendor (Jackson Laboratories, Bar Harbor, ME). Animals were allowed to acclimatize for one week prior to being randomized to receive water or alcohol ad libidum (details on alcohol feeding below) for 12 weeks. After 12 weeks, mice were again randomized to receive sham operation or intratracheal injection of P. aeruginosa (details on pneumonia below). Animals were sacrificed at 24 hours or were followed 7 days for survival after induction of pneumonia. Experiments were performed in accordance with the National Institutes of Health Guidelines for the Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at Emory University School of Medicine (Protocol DAR-2002473-090316BN). To minimize animal suffering, all animals were given a single dose of buprenex (0.1mg/kg, McKesson Medical, San Francisco, CA) prior to sham surgery or pneumonia. All animals were evaluated twice a day to determine if they were moribund, and if they were, they were sacrificed immediately.
Chronic alcohol ingestion model
Mice were equally randomized to receive either alcohol or water. Mice in the alcohol group were given alcohol by increasing the concentration from 0% to 20% (volume/volume) over the course of two weeks (5% v/v for 5 days, 10% v/v for 5 days, 15% v/v for 5 days) and then received 20% v/v for an additional ten weeks. This model is intended to mimic moderate chronic alcohol intake based upon alcohol levels, and we have previously shown that at the conclusion of 12 weeks of drinking either alcohol or water, mice had similar liver histology, gut integrity and renal function (16;17). In addition, body weights were similar between mice at the conclusion of 12 weeks of drinking either alcohol or water (Supplementary figure 1A). At the conclusion of 12 weeks of alcohol ingestion, mice (n=16) had a blood alcohol concentration of 28 mg/dl.
P. aeruginosa pneumonia
Animals were approximately 19 weeks old at the time of pneumonia induction or sham surgery (purchased at six weeks, one week acclimatization, 12 weeks alcohol or water feeding). Animals randomized to the pneumonia group received an intratracheal injection of P. aeruginosa, the most common cause of Gram negative nosocomial pneumonia. Under isoflurane anesthesia, a 1 cm. midline neck incision was created. After separation of the strap muscles, the trachea was identified and 40uL of P. aeruginosa (Strain ATCC 27853, Manassas, VA) diluted in PBS (2 × 108 CFU/mL) was injected via a 28-gauge syringe (25). After installation of bacteria, the animals were held vertically for 10 seconds to improve bacterial delivery to the lungs. Animals undergoing sham surgery underwent the identical procedure, except they received sterile saline instead of bacteria. The neck was then closed in layers. All animals received 1mL subcutaneous normal saline for fluid resuscitation post-operatively to account for insensible losses. Following recovery from anesthesia, mice received ad libidum water regardless of whether they had previously been randomized to receive alcohol or water to mimic the clinical scenario in which all patients are given hydration in the ICU, but are not given alcohol, regardless of their prior history of alcohol intake.
Western blots
Segments of jejunum were snap-frozen in liquid nitrogen at time of sacrifice and stored at −80°C. These segments were subsequently weighed and added to 5× volume-to-weight ice-cold lysis buffer (50 mM Tris HCl; 10 mM EDTA; 100 mM NaCl; 0.5% Triton X-100, 10% SDS) that also contained a protease inhibitor cocktail (Complete Mini, EDTA-free, Roche, Indianapolis, IN) for tissue homogenization and protein extraction. After homogenization and 30 minutes incubating on ice, homogenates were centrifuged at 10,600 × g for 10 minutes at 4°C. Sample supernatant total protein concentration was determined using the Pierce 660 nm protein assay (Thermo Scientific, Rockford, IL). Comparative protein analysis was performed using 40 μg of protein which was added to an equal volume of Laemmli buffer and then boiled for 5 minutes. Protein was separated by SDS-PAGE on a 4-15% gradient stain-free gel (BioRad, Hercules, CA) at 120V for 75 minutes. Stain free gel technology adds an ultraviolet (UV) tag to all the tryptophan residues in the protein and allows for visualization of the protein on the gel after UV activation without requiring staining of the gel itself (26). The stain-free gel was activated for 5 minutes and protein was transferred to PVDF membranes using a semi-wet method via a Transblot Turbo (BioRad) at 25V for 10 minutes. After transfer, membranes were blocked in 5% non-fat milk in TBS with 0.1% Tween-20 for 1 hour at room temperature. Membranes were then incubated overnight at 4°C with rabbit anti-caspase 3 (1:1000), rabbit anti-Bax (1: 1000), rabbit anti-Bcl-2 (1:1000), rabbit anti-Bid (1:1000), rabbit anti-PUMA (1:1000, all from Cell Signaling Technologies, Danvers, MA), rabbit anti-TNFR-1 (1:200) or rabbit anti-Fas-L (1:200, Santa Cruz Technologies, Santa Cruz, CA). In the morning after washing in TBS 0.1% Tween-20, membranes were incubated for 1 hour at room temperature in anti-rabbit antibody linked to horseradish peroxidase (1: 1000, Cell Signaling). Proteins were detected with a chemiluminescent system (GE Healthcare, Buckinghamshire, UK) and visualized with a charged coupled device (ChemiDoc Touch, BioRad). Resulting bands were analyzed using intensity quantification software (ImageLab 5.2, BioRad). Linear dynamic detection range with stain-free technology was used for lane protein normalization and comparisons (27). Data are presented as relative protein expression compared to the water-fed sham group which was given a value of 1.
Crypt proliferation
Mice received an intraperitoneal injection of 5-bromo-2′deoxyuridine (BrdU, 5 mg/mL diluted in normal saline; Sigma-Aldrich, St. Louis, MO) 90 minutes prior to sacrifice to label cells in S-phase. Whole intestinal tissue was harvested at sacrifice and paraffin-embedded. Sections were serially deparaffinized and rehydrated, incubated in hydrogen peroxide, exposed to Antigen Decloaker and boiled. Sections were then blocked for 30 minutes with Protein Block (Dako, Carpinteria, CA) and incubated in a humidified container with rat monoclonal anti-BrdU (1:500; Accurate Chemical & Scientific, Westbury, NY) overnight at 4° C. Slides were then incubated at room temperature with goat anti-rat secondary antibody (1:500; Accurate Chemical & Scientific) for 30 minutes and then incubated with streptavidin-horseradish peroxidase (1:500; Dako) for 30 minutes. Samples were developed with diaminobenzidine, followed by hematoxylin counterstaining. S-phase cells were quantified in jejunal crypts of 100 contiguous well-oriented crypt-villus units.
Intestinal permeability
Five hours prior to sacrifice, animals received an oral gavage of 0.5mL of 22 mg/mL fluorescein isothiocyanate conjugated dextran (FD-4, molecular mass 4400D, Sigma-Aldrich). Whole blood collected at sacrifice was centrifuged at 10,600 × g at 4°C for 10 minutes. An aliquot of plasma was diluted equally with PBS and the concentration of FD-4 was determined using fluorospectrometry (Synergy HT, BioTek, Winooski, VT) at an excitation wavelength of 485nm and emission wavelength of 528 nm. All samples were run in duplicate.
Lung assays
Myeloperoxidase (MPO) activity was measured on snap frozen lung sections. Each sample was weighed and mechanically homogenized. Working substrate buffer containing o-dianisidine (0.167 mg/mL, Sigma) and 3% H2O2 was added to each sample. After addition of buffer, absorbance at 460 nm was measured every 30 seconds for each sample over 6 minutes (Synergy HT, Bio-Tek). MPO activity was calculated as ΔOD/minute (U) per mg of lung tissue.
A separate cohort of animals had lung tissue sectioned and stained for H&E. Qualitative histopathologic assessment of lung injury was performed by an examiner blinded to sample identity.
BAL and blood cultures
Bronchoalveolar lavage (BAL) fluid was obtained by cannulating the trachea with a 22-gauge catheter, and lavaging the lungs with 1 ml of PBS. Whole blood was collected via cardiac puncture into EDTA-lined tubes at the time of sacrifice. Samples were plated and serially diluted on sheep’s blood agar plates and incubated at 37°C in 5% CO2. After 24 hours, colony counts were measured.
Liver injury
Aspartate aminotransferase (AST) and Alanine aminotransferase (ALT) were measured on a Beckman AU480 chemistry auto-analyzer (Beckman Diagnostics, LaBrea, CA) according to manufacturer instructions.
Serum cytokines
Whole blood was centrifuged at 10,600 × g for 10 minutes. Plasma cytokine concentrations were determined using a multi-plex magnetic bead cytokine assay kit (Bio-Rad) according to manufacturer protocol.
Phenotypic flow cytometric analysis
Spleens were harvested and single-cell suspensions were prepared from each animal. Samples were stained with anti-CD3 (17A2, Biolegend, San Diego, CA), anti-CD4 (RM4.5, BD Bioscience), anti-CD8 (MCD0830, Invitrogen, Waltham, MA), anti-Gr-1 (RB6.8C5, BD Pharmingen, San Jose, CA), anti-B220 (RM2630, Invitrogen), anti-CD19 (CD5, Biolegend), anti-CD11b (M1-79, Biolegend), anti-CD11c-PE (HL3, eBioscience, Waltham, MA), anti-MHCII (M5/114.15.2, eBiosciences), anti-F4/80 (BM8, Biolegend), anti-CD25 (PC61, Biolegend), anti-FoxP3 (FJK-16S, eBioscience), anti-CD44 (IM7, Biolegend), and anti-CD62L (MEL-14, eBioscience). Similar techniques were used for flow cytometry on lung tissue. Samples were run on an LSR II flow cytometer (BD Biosciences) and analyzed using FlowJo software version10.0.7 (TreeStar, Ashland, OR).
Intracellular cytokine staining
Single cell splenocyte suspensions were incubated in RPMI 1640 culture medium and stimulated ex vivo for four hours with phorbol 12-myristate 13-acetate (PMA, 30ng/mL) and ionomycin (400ng/mL) with Brefeldin-A (10ug/mL) at 37°C. After stimulation, cells were stained for the follow extracellular and intracellular markers: anti-CD4 (RM4.5, BD Biosciences), anti-CD8 (MCD0830, Invitrogen), anti-TNF (MP6-XT22, BioLegend), anti-IFNγ (XMG1.2, BD Pharmingen), and anti-IL-2 (5336636, BD Pharmingen). Data were analyzed with FlowJo software.
Immune cell apoptosis
Single cell splenic suspensions were processed according to the manufacturer’s instructions (BioLegend) for staining of Annexin V (B206041) and 7-AAD (B204597). Cells were also stained for all the phenotypic markers described above.
Cytokine blockade
A separate group of septic animals received anti-mouse IFNγ antibody (100uL of 1mg/mL solution, clone XMG1.2, BioXcell, West Lebanon, NH), anti-mouse TNF antibody (250uL of 1mg/mL solution, clone XT3.11, BioXcell) or isotype control. Anti-IFNγ and isotype control were given at the time of pneumonia induction whereas anti-TNF was given at 12 hours after pneumonia induction based upon published literature on kinetics of production of each cytokine (28;29). Animals were then followed 7 days for survival.
Statistics
Survival studies were analyzed using the log-rank test. Two-way comparisons were tested for normality using the D’Agostino-Pearson omnibus normality test. Data with a normal distribution were compared using the Students t-test, and data that did not have a normal distribution were compared using the Mann Whitney test. Multi-group comparisons were analyzed with one-way ANOVA, followed by Tukey post-test. All data were analyzed using Prism 6.0 (GraphPad San Diego, CA) and are presented as mean ± SEM. A p value of <0.05 was considered to be statistically significant throughout.
RESULTS
Mice were first randomized to receive either 12 weeks of ad libitum chronic alcohol ingestion or water ingestion. Mice were then randomized again to receive either sepsis via P. aeruginosa or sham surgery. This resulted in four groups: a) water-fed sham, b) alcohol-fed sham, c) water-fed septic, and d) alcohol-fed septic. Animals were then followed for survival or were sacrificed at a single timepoint (24 hours) after either sham surgery or pneumonia.
Effect of chronic alcohol and sepsis on mortality
Mice that were alcohol-fed prior to the onset of sepsis had a significantly higher mortality following sepsis than mice that were water-fed prior to the onset of sepsis (96% vs 58%, Fig. 1).
FIG. 1. Effect of alcohol and sepsis on survival.
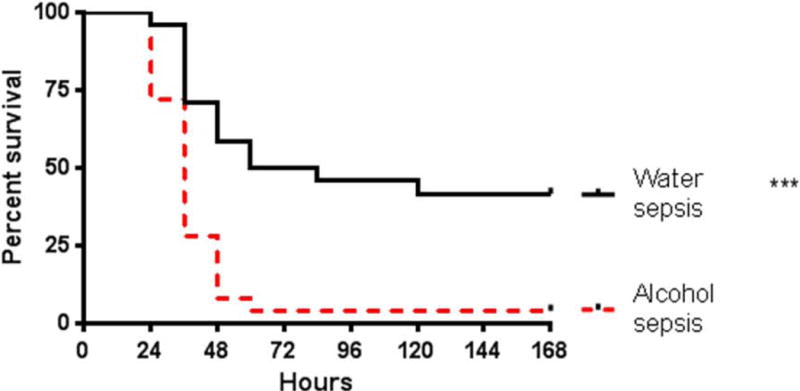
Alcohol-fed septic mice had significantly higher 7-day mortality than water-fed septic mice (p<0.0001, n=24-25/group).
Effect of chronic alcohol and sepsis on intestinal integrity
Jejunal levels of the apoptotic executioner active caspase 3 were similar in water-fed sham, alcohol-fed sham mice and water-fed septic mice (Fig. 2A). In contrast, active caspase 3 levels were 5.6-fold higher in alcohol-fed septic mice than water-fed septic mice (Fig 2A). To delineate the specific pathways contributing to this increase in intestinal apoptosis, multiple mitochondrial and receptor-mediated apoptosis pathway proteins were investigated. Within the mitochondrial pathway, levels of the pro-apoptotic protein Bax were 6.3-fold higher in alcohol-fed septic mice compared to the water-fed septic mice (Fig. 2B). In contrast, mitochondrial pathway proteins Bcl-2, Bid, and PUMA were not statistically significant different between mice, regardless of whether they received alcohol or were septic (Fig 2C–E). Similarly no differences were detected in the receptor mediated pathway proteins Fas-L and TNFR-1 (Fig 2F, G).
FIG. 2. Effect of alcohol and sepsis on intestinal apoptosis.
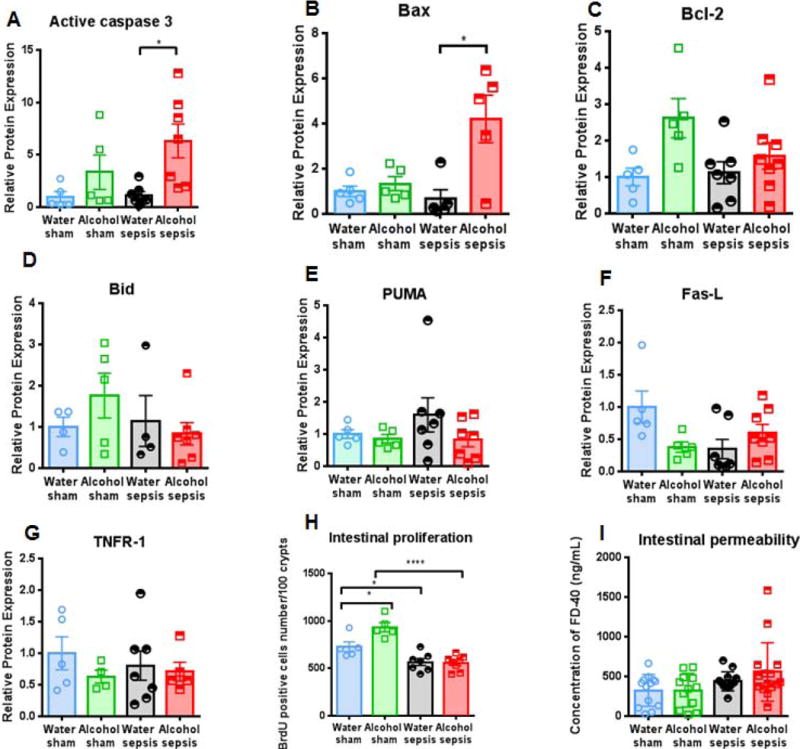
While active caspase 3 levels were similar in water-fed sham mice, alcohol-fed sham mice and water-fed septic mice, active caspase 3 levels were significantly higher in alcohol-fed septic mice than water-fed septic mice (A, p=0.039, n=5-7/groups). Levels of the pro-apoptotic protein Bax were also higher in alcohol-fed septic mice than water-fed septic mice (B, p=0.038, n=5/group). In contrast, there were no differences in protein levels of Bcl-2, Bid, PUMA, Fas-L, or TNFR-1 (C-G, p=0.71, p>0.99, p=0.91, p=0.82, p>0.99 respectively, n=5-8/group). Alcohol, in isolation, increased intestinal proliferation (H, p=0.02) while sepsis, in isolation, decreased intestinal proliferation (H, p=0.048). However, there was no synergistic effect as alcohol-fed septic mice had similar intestinal proliferation as water-fed septic mice (H, p=0.99, n=5-8/group). Permeability was not different between alcohol-fed septic mice and water-fed septic mice (I, p=0.61, n=12-15/group).
Alcohol in isolation increased crypt proliferation as the number of BrdU positive cells was higher in alcohol-fed sham mice than water-fed sham mice (Fig. 2H). In contrast, sepsis in isolation decreased proliferation as the number of BrdU positive cells was lower in water-fed septic mice than water-fed sham mice and was also lower in alcohol-fed septic mice than alcohol-fed sham mice. Despite alcohol-fed sham mice having higher proliferation than water-fed sham mice, there was no difference in the number of BrdU positive cells between water-fed septic mice and alcohol-fed septic mice. (Fig. 2H). Neither alcohol nor sepsis (or the combination) altered intestinal permeability, although there was a trend toward increased permeability in alcohol-fed septic mice (Fig. 2I). Additionally, there was no statistically significant difference in markers of liver injury (AST and ALT) between water-fed septic mice and alcohol-fed septic mice (Supplemental figure 1B, C).
Effect of chronic alcohol and sepsis on lung injury and bacteremia
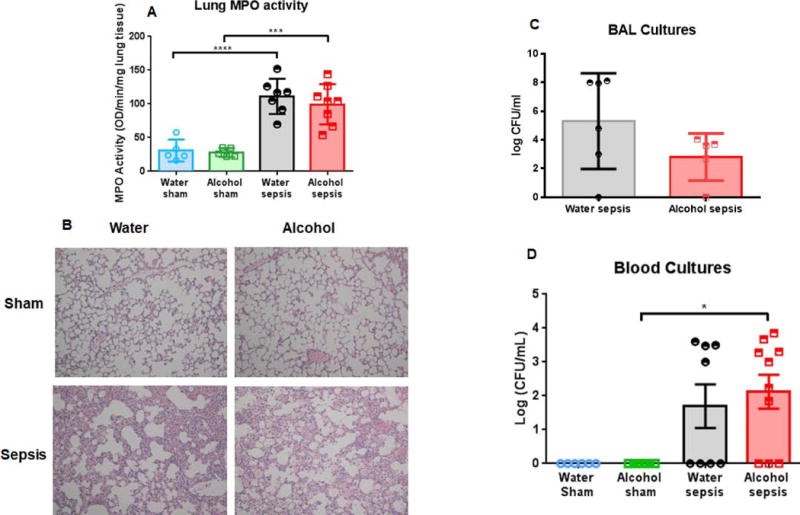
Alcohol, in isolation had no impact on lung neutrophil activation as measured by lung MPO (Fig. 3A). In contrast, sepsis from a pulmonary source led to a marked increase in lung neutrophil activation as MPO levels were significantly higher in water-fed septic mice than water-fed sham mice. There was not a synergistic effect of alcohol to lung injury as lung MPO levels were similar between water-fed septic mice and alcohol-fed septic mice. Histologic images of lung sections were consistent with these results, with greater inflammation and septal wall thickening in septic mice, independent of chronic alcohol ingestion (Fig 3B). Similar levels of bacteria were detectable in BAL fluid in water-fed septic mice and alcohol-fed septic mice (Fig. 3C). Flow cytometry of lung showed similar numbers of CD4+ T cells, CD8+ T cells, neutrophils, macrophages and dendritic cells in water-fed septic mice and alcohol-fed septic mice (Supplementary figure 2).
FIG. 3. Effect of alcohol and sepsis on lung injury and bacteremia.
Water-fed septic mice had significantly higher lung MPO activity than water-fed sham mice (A, p<0.0001), and alcohol-fed septic mice had significantly higher lung MPO activity than alcohol-fed sham mice (A, p=0.0002). However, there was no difference in lung MPO activity between alcohol-fed septic mice and water-fed septic mice (A, p=0.77, n=5-8/group). Representative histomicrographs are shown, magnification 100× (B). BAL cultures had similar amounts of bacterial detectable between water-fed septic mice and alcohol-fed septic mice (C, p=0.19, n=5-6/group). Blood cultures were sterile in sham animals, regardless of whether they drank alcohol. Systemic bacterial burden was similar between alcohol-fed septic mice and water-fed septic mice (D, p>0.99, n=6-10/group).
Blood cultures were sterile in sham mice, regardless of whether they drank water or alcohol (Fig. 3D). Pneumonia induced bacteremia 24 hours after the onset of sepsis, but this was independent of chronic alcohol ingestion as bacterial load was similar between water-fed septic mice and alcohol-fed septic mice.
Effect of chronic alcohol and sepsis on serum cytokines
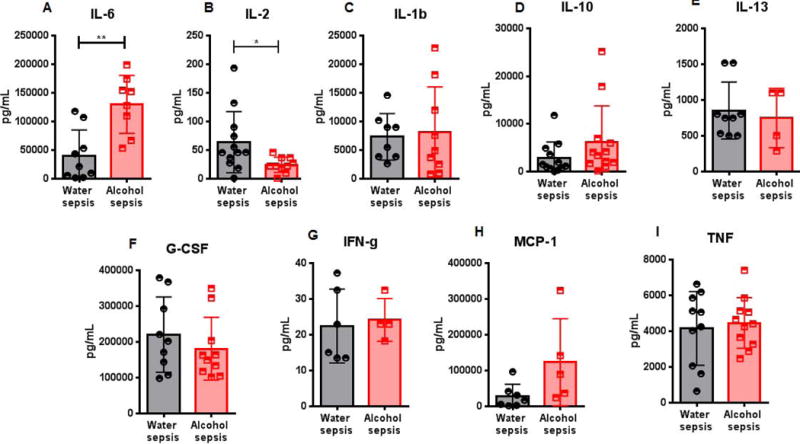
IL-6 levels were higher in alcohol-fed septic mice compared to water-fed septic mice (Fig. 4A). In contrast, IL-2 levels were lower in alcohol-fed septic mice compared to water-fed septic mice (Fig. 4B). No differences were noted in the serum concentration of IL-1β, IL-10, IL-13, G-CSF, IFNγ, MCP-1 or TNF between the groups (Fig 4C–I).
FIG. 4. Effect of alcohol and sepsis on systemic cytokines.
IL-6 levels were higher in alcohol-fed septic mice than water-fed septic mice (A, p=0.0015). IL-2 levels were lower in alcohol-fed septic mice than water-fed septic mice (B, p=0.019). No differences were note in IL-1β, IL-10, IL-13, G-CSF, IFNγ, MCP-1 and TNF between alcohol-fed septic mice and water-fed septic mice (C-I, p=0.65, p=0.11, p=0.69, p=0.39, p=0.64, p=0.07, p=0.69, n=8-12 all groups except n=5-7 for MCP-1 and n=4 for alcohol-septic mice for IL-13).
Effect of chronic alcohol and sepsis on CD4+ T cells
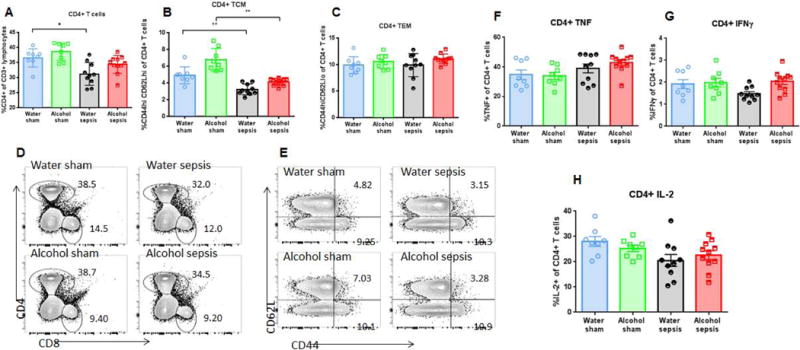
There was no difference in splenic weights between water-fed septic mice and alcohol-fed septic mice (Supplementary figure 1D). Splenic CD4+ T cell frequency was similar in water-fed and alcohol-fed sham animals. Sepsis induced a decrease in CD4+ T cell frequency as percentage of CD4+ T cells was lower in water-fed septic animals than water-fed sham animals. However, this was independent of alcohol as there was no difference between in the frequency of CD4+ T cells between water-fed septic mice and alcohol-fed septic mice (Figure 5A, D).
FIG. 5. Effect of alcohol and sepsis on CD4+ T cells.
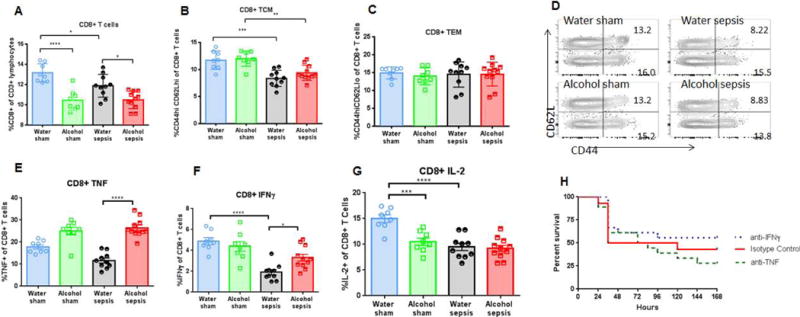
While sepsis in isolation decreased frequency of CD4+ T cells (p=0.03), there was no difference in CD4+ T cell frequency between alcohol-fed septic mice and water-fed septic mice (A, p=0.89). Representative flow cytometry plot for CD4+ T cells is shown (D). Sepsis, in isolation also decreased frequency of central memory CD4+ T cells in both water-fed (p=0.008) and alcohol-fed mice (p=0.005), but there was no difference in central memory CD4+ T cell frequency between alcohol-fed septic mice and water-fed septic mice (B, p=0.39). Effector memory CD4+ T cell frequency was similar in all groups (C, p=0.33 comparing alcohol-septic to water-septic). Representative flow cytometry plot for both central memory and effector memory CD4+ T cells is shown (E). Stimulated cytokine production from CD4+ T cells was similar between alcohol-fed septic mice and water-fed septic mice for TNF (F, p>0.99), IFNγ (G, p=0.052), and IL-2 (H, p=0.84), n=8-11/group for all panels.
CD4+ T cell subsets were next evaluated. The frequency of CD44+ CD62L+ central memory cells was similar between water-fed sham mice and alcohol-fed sham mice. Sepsis decreased CD4+ T central memory cells as frequencies were lower in water-fed septic mice than water-fed sham mice as well as alcohol-fed septic mice compared to alcohol-fed sham mice. However, this was independent of alcohol as no differences were detected between the two septic groups (Fig 5B, E). Frequencies of CD4+ CD44+ CD62L− T effector memory cells were similar in all four groups of mice (Fig 5C, E). In addition, no differences were noted in TNF, IFNγ or IL-2 production when splenic CD4+ lymphocytes were stimulated ex vivo for cytokine production (Fig 5 F–H).
Effect of chronic alcohol and sepsis on CD8+ T cells
Alcohol in isolation decreased splenic CD8+ T cell frequency, which was lower in alcohol-fed sham mice than water-fed sham mice. Sepsis alone had a similar effect as CD8+ T cell frequency was lower in water-fed septic mice than water-fed sham mice. In addition, alcohol-fed septic mice had lower CD8+ T cell frequency than water-fed septic mice (Fig 6A, Fig. 5D).
FIG. 6. Effect of alcohol and sepsis on CD8+ T cells.
Both alcohol in isolation (p<0.0001) and sepsis in isolation decreased frequency of CD8+ T cells (p=0.048). Further, CD8+ T cell frequency was lower in alcohol-fed septic mice than water-fed septic mice (A, p=0.012). Representative flow cytometry plot for CD8+ T cells is shown in figure 5D. Sepsis in isolation also decreased frequency of central memory CD8+ T cells in both water-fed (p=0.002) and alcohol-fed mice (p=0.004), but there was no difference in central memory CD8+ T cell frequency between alcohol-fed septic mice and water-fed septic mice (B, p=0.41). Effector memory CD8+ T cell frequency was similar in all groups (C, p=0.99 comparing alcohol-septic to water-septic). Representative flow cytometry plot for both central memory and effector memory CD8+ T cells is shown (D). Stimulated cytokine production from CD8+ T cells showed increased TNF (E, p<0.0001) and IFNγ (F, p=0.02) in alcohol-septic mice compared to water-septic mice without a change in IL-2 (G, p=0.98), n=8-11/group for all panels. Neither anti-TNF nor anti-IFNγ altered mortality following alcohol ingestion and sepsis compared to isotype control (H, p=0.49 and p=0.66 respectively). n=8-11/group for all panels except survival where n=14-18/group.
CD8+ T cell subsets were next evaluated. The frequency of CD44+ CD62L+ central memory cells was similar between water-fed sham mice and alcohol-fed sham mice. Sepsis decreased CD8+ T central memory cells as frequencies were lower in water-fed septic mice than water-fed sham mice as well as alcohol-fed septic mice compared to alcohol-fed sham mice. However, this was independent of alcohol as no differences were detected between the two septic groups (Fig 6B, D). Frequencies of CD8+ CD44+ CD62L− T effector memory cells were similar in all four groups of mice (Fig 6C, D). Stimulated CD8+ T cells demonstrated an increase in TNF production in alcohol-fed septic mice compared to water-fed septic controls (Fig 6E). IFNγ production was decreased in water-fed septic animals compared to water-fed sham injected animals, and IFNγ production was increased in alcohol-fed septic mice compared to the water-fed septic mice (Fig 6F). Both alcohol in isolation and sepsis in isolation decreased IL-2 production from stimulated CD8+ T cells as levels in both alcohol-fed sham mice and water-fed septic mice were lower than water-fed sham mice. However, the combination of alcohol and sepsis was not synergistic as alcohol-fed septic mice were not different from either alcohol-fed sham mice or water-fed septic mice (Fig 6G). Since TNF and IFNγ production were both higher in stimulated CD8+ T cells of septic alcohol-fed animals, each was individually blocked using systemic monoclonal antibodies directed against each. Survival was similar in alcohol-fed septic mice regardless of whether they received anti-TNF, anti-IFNγ or vehicle (Fig. 6H).
Effect of chronic alcohol and sepsis on frequency of other immune cells
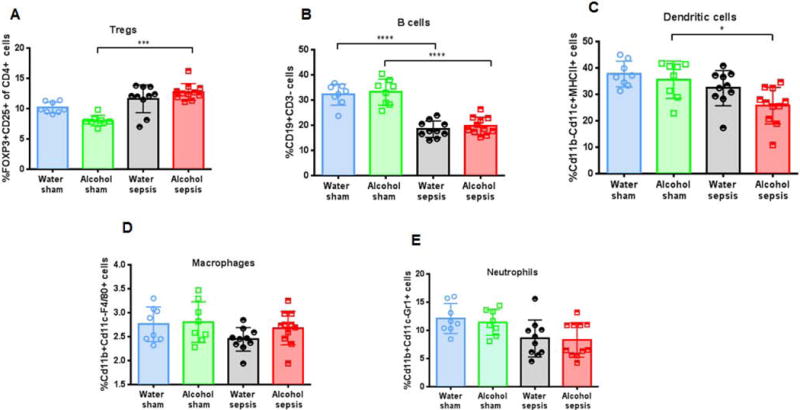
Neither alcohol alone nor sepsis alone altered frequency of CD4+ Foxp3+ regulatory T cells (Treg) as levels were similar between water-fed sham mice, alcohol-fed sham mice and water-fed septic mice. Treg frequency was higher in alcohol-fed septic mice than alcohol-fed sham mice but was not different than water-fed septic mice (Fig 7A). Sepsis decreased the frequency of splenic B cells in both water-fed and alcohol-fed mice, but this was independent of alcohol as B cell frequency was similar between water-fed and alcohol-fed sham mice and water-fed and alcohol-fed septic mice (Fig 7B). No statistically significant differences were detected between water-fed septic mice and alcohol-fed septic mice in dendritic cells, macrophages or neutrophils (Fig. 7 C–E).
FIG. 7. Effect of alcohol and sepsis on other immune cells.
Treg frequency was similar between alcohol-fed and water-fed septic mice (A, p>0.99). Sepsis decreased B cell frequency in both water-fed (p<0.0001) and alcohol-fed mice (p<0.0001), but there was no difference in B cell frequency between alcohol-fed septic mice and water-fed septic mice (B, p=0.87). Frequencies of dendritic cells (C, p=0.11), macrophages (D, p=0.42) and neutrophils (E, p= 0.99) were similar between alcohol-fed septic mice and water-fed septic mice. n=8-11/group for all panels.
Effect of chronic alcohol and sepsis on immune cell apoptosis
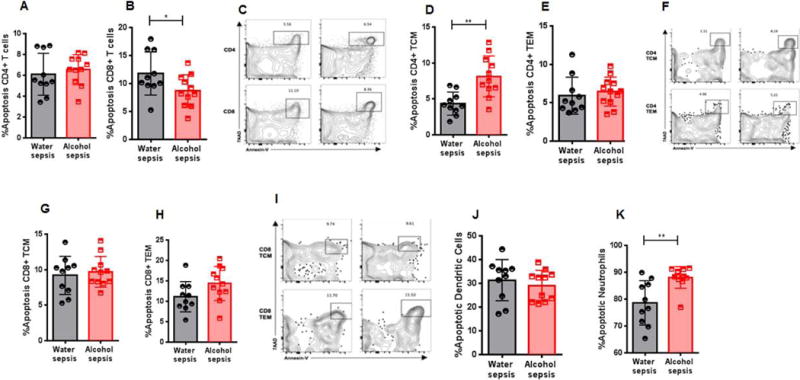
Apoptosis was similar in CD4+ T cells between water-fed and alcohol-fed septic mice (Fig 8A, C). Apoptosis was higher in central memory CD4+ T cells but was unchanged in effector memory CD4+ T cells (Fig. 8D–F). In contrast, apoptosis was slightly lower in CD8+ T cells from alcohol-fed septic mice compared to water-fed septic mice (Fig. 8B, C) but no differences were noted in either central memory or effector memory CD8+ T cells (Fig. 8G–I). Dendritic cell apoptosis was similar between water-fed septic mice and alcohol-fed septic mice (Fig. 8J), and there was increased neutrophil apoptosis in alcohol-fed septic mice (Fig. 8K).
FIG. 8. Effect of alcohol and sepsis on immune cell apoptosis.
No difference was noted in apoptosis in of CD4+ T cells between alcohol-fed septic mice and water-fed septic mice (A, p=0.56). In contrast, apoptosis was lower in CD8+ T cells of alcohol-fed septic mice (B, p=0.049). Representative flow cytometry plot is shown for CD4+ T cells and CD8+ T cells (C). Apoptosis was higher in central memory CD4+ T cells (D, p=0.001) in alcohol-fed septic mice but was not different in effector memory CD4+ T cells (E, p=0.37). Representative flow cytometry plot is shown (F). Apoptosis was not different in either central memory CD8+ T cells (G, p=0.63) or effector memory CD8+ T cells (H, p=0.08) in alcohol-fed septic mice and water-fed septic mice. Representative flow cytometry plot for is shown (I). Apoptosis was similar in dendritic cells in alcohol-fed septic mice (J, p=0.51) but was increased in neutrophils (K, p=0.002), n=10-11 mice/group for all panels.
DISCUSSION
Chronic alcohol ingestion followed by pneumonia-induced sepsis led to higher mortality in mice than drinking water prior to the onset of pneumonia-induced sepsis. While both alcohol alone and sepsis alone impacted numerous organs, the combination of alcohol and sepsis led to synergistic abnormalities in a subset of cells that were greater than could be predicted by either variable is isolation. Parameters that were disproportionately altered by alcohol/sepsis may represent potential mechanisms explaining why mortality is higher in alcohol/sepsis than water/sepsis. In this context, increased mortality in alcohol-fed septic mice was associated with increased gut apoptosis (accompanied by elevated Bax levels), elevated IL-6 levels, decreased IL-2 levels, and lower CD8+ T cell frequency with decreased apoptosis and increased TNF and IFNγ production in stimulated CD8+ T cells. In contrast, intestinal proliferation, intestinal permeability, lung MPO levels, systemic bacterial burden, CD4+ T cells (frequency including subsets and cytokine production), and frequency of other immune cells (Treg, B cells, dendritic cells, macrophages or neutrophils) were not different between alcohol-fed septic mice and water-fed septic mice.
These results should be interpreted in the context of previous studies of alcohol and sepsis. We have previously published on a model of alcohol followed by sepsis using the identical chronic ingestion protocol used herein followed by CLP (16–18). Those studies showed a similar increase in mortality to that demonstrated by alcohol followed by pneumonia. In addition, alcohol-fed mice subjected to CLP had worsened gut integrity (increased intestinal apoptosis, increased permeability, decreased proliferation) and changes in splenic CD4+ T cells with increased TNF and IFNγ production following ex vivo stimulation. In contrast, no difference was noted in stimulated cytokine production in CD8+ T cells, and serum cytokines were generally similar between alcohol-fed septic and water-fed septic mice (although serum IL-6 was lower in the former) as was liver histology. An additional study of alcohol and sepsis by Barros et al. examined male Wistar rats who received four weeks of alcohol (5% v/v for the first week, 10% v/v for the other three weeks) followed by intraperitoneal injection of feces (30). This study demonstrated a similar marked increase in mortality in alcohol-fed septic rats, associated with decreased IL-6 and TNF, decreased glucose and increased creatinine (gut and immune endpoints were not examined) between rats that drank alcohol alone compared to rats that drank alcohol and then were made septic (sepsis alone vs. alcohol plus sepsis was not described). In contrast, no differences were detected in IL-4 or IL-13 levels. The same group also examined female Wistar rats using a similar model (31) and found similar mortality differences. This was associated with increased TNF, decreased IL-6 and MIF, increased AST between alcohol-fed septic rats and water-fed septic rats. In contrast, there was no difference in serum levels of IL-10, TGF-β or IL-13.
It is difficult to find a common thread that ties the current results to all prior studies of alcohol followed by sepsis due to differences in alcohol model, sepsis model, gender and endpoints examined. However, there are some inferences that can be drawn. Our prior work – which approximates the current study most closely– demonstrated that the gut and the immune system are both altered by the combination of alcohol and sepsis, moreso than would be predicted by examining either variable in isolation. Similarly, cytokine alterations have been found in all studies with the combination of alcohol and sepsis. Notably, some of these findings are similar in alcohol/CLP and alcohol/pneumonia such as increased gut epithelial apoptosis. However, the specifics appear to be dependent, at least in part, on the specifics of the sepsis model used (and either the alcohol protocol used or species used). For instance, alcohol/CLP predominantly affects CD4+ T cells whereas alcohol/pneumonia predominantly affects CD8+ T cells. Finally, the exact opposite effect is seen with serum IL-6 which is decreased in alcohol/CLP and alcohol/feces injection but increased in alcohol/pneumonia. This suggests that the host response to alcohol and sepsis has some common elements and some model-specific elements. This is important since the concept of a common host response in sepsis is controversial (32;33). Further, it is important to notice that septic hosts respond differently in the setting of different co-morbidities, such that mice with chronic alcohol ingestion prior to sepsis respond differently than aged ones or those with cancer (21;22;34).
Gut epithelial apoptosis is increased following both alcohol in isolation and sepsis in isolation, but the combination leads to a synergistic increase in intestinal cell death regardless of the model of sepsis. This suggests that gut epithelial apoptosis is potentially important in the pathophysiology of mortality from the combination of alcohol and sepsis, which is supported by the observation that administration of systemic epidermal growth factor (which improves gut integrity including decreasing apoptosis) improves survival in alcohol-fed mice subjected to CLP (17). This is also consistent with the observation that the combination of alcohol and hypoxia/reoxygenation induces apoptosis in gut epithelial cells in vitro (35). The mechanisms through which alcohol and sepsis induce apoptosis appear distinct, at least in part, from sepsis in isolation. P. aeruginosa pneumonia-induced sepsis is associated with increased Bcl-2 in the mitochondrial pathway without changes in Bax, Bid, or Bcl-xL. Similarly, the same model is associated with increased TNF-R1 and decreased Fas in the receptor-mediated pathway without changes in Fas-L FADD, pFADD or TRADD expression (36). While alcohol increases gut apoptosis in vitro, the mechanisms behind this have yet to be defined (37). In contrast, the combination of alcohol and sepsis induces an upregulation of Bax in the mitochondrial pathway without changes in Bcl-2, Bid, or PUMA and without changes in Fas-L or TNFR-1 in the receptor-mediated pathway. Of note, the link between Bax and alcohol is limited, although Bax is associated with neuronal apoptosis following acute alcohol ingestion in infant mice, and alcohol increases Bax expression in Jurkat cells, associated with increased apoptosis (38;39).
The majority of the immune alterations in alcohol-fed mice followed by pneumonia-induced sepsis occurred in the CD8+ T cell compartment. CD8+ T cell numbers are decreased in both alcohol alone and sepsis alone (40;41). Notably, the combination of alcohol and pneumonia led to lower CD8+ T cell frequency with decreased apoptosis and increased TNF and IFNγ production in stimulated CD8+ T cells. In theory, decreased apoptosis might be expected to lead to an increase in CD8+ T cell frequency, although sepsis-induced changes in CD4+ T cell apoptosis (independent of alcohol) could change CD8+ T cell frequency by a relative change in absolute numbers. The findings that the combination of alcohol and sepsis led to increased stimulated TNF and IFNγ production in CD8+ T cells was surprising, since cytokine production is generally felt to be beneficial in survival from sepsis. However, the importance of these findings is unclear given our finding that survival was similar in alcohol-fed septic mice regardless of whether they received anti-TNF, anti-IFNγ or vehicle.
This study has a number of limitations. All animals not followed for survival were sacrificed at a single timepoint (24 hours). As such, we cannot conclude that variables that were not different between alcohol-fed septic mice and water-fed septic mice were unimportant in mediating mortality in alcohol/sepsis without a more comprehensive timecourse. Since sepsis is a dynamic disease, we emphasize that measuring multiple parameters at only a single timepoint following pneumonia limits conclusions that can be drawn from our study. Further, while numerous parameters were higher or lower in alcohol-fed septic mice than water-fed septic mice, association is not the same as causation, and we cannot conclude that any parameters that were different are mechanistically responsible for difference in mortality. Although we do not know the cause of the increased morality in alcohol/sepsis, we speculate that it may, in fact, relate to the differences identified between water/septic mice and alcohol/septic mice. Specifically, increased gut epithelial apoptosis has been shown to be mechanistically associated with mortality in mouse models of both CLP and pneumonia, since preventing sepsis-induced gut apoptosis via overexpression of the anti-apoptotic protein Bcl-2 improves survival in both models of sepsis. The mechanism through which increased gut apoptosis changes survival is not entirely understood but is associated with increased permeability through the unrestricted pathway (as opposed to the leak pathway which is measured by FD4) as well as changing the host inflammatory response and the host microbiome. It is also possible that a more altered inflammatory response as evidenced by elevated serum IL-6 levels is responsible for the elevated mortality in alcohol/sepsis mice. It is also certainly possible that the altered immune response seen with decreased CD8+ T cell frequency and increased production of IFNγ and TNF in stimulated splenocytes played a significant role although the absence of changes in bacterial burden and the IFNγ and TNF blocking experiments make this less likely. Another limitation is that a few of the multiple parameters examined gave different results for either alcohol in isolation or sepsis in isolation than published literature (for instance, gut apoptosis was not elevated following pneumonia in isolation) (42) which could make interpreting the combination of alcohol and sepsis more difficult. Finally, some of the endpoints shown to be different in a rat model of shorter term alcohol followed by a different model of sepsis (30;31) were not examined in this study, so we are unable to draw any conclusions as to their importance.
Despite these limitations, this study demonstrates that chronic alcohol ingestion followed by sepsis increases mortality. Similar to a model of chronic alcohol ingestion followed by CLP, the gut and immune system appear to be disproportionately affected. The relative contributions of alcohol, sepsis and model of sepsis appear to be nuanced, as there appears to be a partially generic host response that is catalyzed by the combination of alcohol and sepsis and a more individualized host response that is differentially impacted by different models of sepsis. Future studies are required to determine the mechanisms responsible for increased mortality in alcohol/sepsis and how generalizable these may be.
Supplementary Material
SUPPLEMENTAL FIG. 1 No difference was detected in body weights between mice following 12 weeks of alcohol ingestion or water ingestion (A, p=0.43). AST (B, p=0.052) and ALT (C, p=0.18) levels were not statistically significantly different between water-fed septic mice and alcohol-fed septic mice. Splenic weights were also similar between water-fed septic mice and alcohol-fed septic mice (D, p=0.97), n=5-6 for all panels.
SUPPLEMENTAL FIG. 2 Flow cytometry on lung tissue showed similar numbers of CD4+ T cells (A, p=0.33), CD8+ T cells (B, p=0.25), neutrophils (C, p=0.12), macrophages (D, p=0.25) and dendritic cells (E, p=0.18) between water-fed mice and alcohol-fed mice, n=5-6/group for all.
Acknowledgments
Source of Funding: This work was supported by funding from the National Institutes of Health (GM072808, GM095442, GM104323, GM109779, GM113228, GM117895).
Footnotes
Conflicts of Interest: None of the authors has a conflict of interest to disclose.
References
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rhee C, Dantes R, Epstein L, Murphy DJ, Seymour CW, Iwashyna TJ, Kadri SS, Angus DC, Danner RL, Fiore AE, Jernigan JA, Martin GS, Septimus E, Warren DK, Karcz A, Chan C, Menchaca JT, Wang R, Gruber S, Klompas M. Incidence and Trends of Sepsis in US Hospitals Using Clinical vs Claims Data, 2009-2014. JAMA. 2017;318:1241–1249. doi: 10.1001/jama.2017.13836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B, Hasin DS. Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757–66. doi: 10.1001/jamapsychiatry.2015.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatr. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- 5.Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in Drug and Opioid Overdose Deaths–United States, 2000-2014. MMWR Morb Mortal Wkly Rep. 2016;64:1378–1382. doi: 10.15585/mmwr.mm6450a3. [DOI] [PubMed] [Google Scholar]
- 6.de WM, Jones DG, Sessler CN, Zilberberg MD, Weaver MF. Alcohol-use disorders in the critically ill patient. Chest. 2010;138:994–1003. doi: 10.1378/chest.09-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de WM, Wan SY, Gill S, Jenvey WI, Best AM, Tomlinson J, Weaver MF. Prevalence and impact of alcohol and other drug use disorders on sedation and mechanical ventilation: a retrospective study. BMC Anesthesiol. 2007;7:3. doi: 10.1186/1471-2253-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smothers BA, Yahr HT, Ruhl CE. Detection of alcohol use disorders in general hospital admissions in the United States. Arch Intern Med. 2004;164:749–756. doi: 10.1001/archinte.164.7.749. [DOI] [PubMed] [Google Scholar]
- 9.O’Brien JM, Jr, Lu B, Ali NA, Martin GS, Aberegg SK, Marsh CB, Lemeshow S, Douglas IS. Alcohol dependence is independently associated with sepsis, septic shock, and hospital mortality among adult intensive care unit patients. Crit Care Med. 35:345–350, 35. doi: 10.1097/01.CCM.0000254340.91644.B2. [DOI] [PubMed] [Google Scholar]
- 10.Moss M, Parsons PE, Steinberg KP, Hudson LD, Guidot DM, Burnham EL, Eaton S, Cotsonis GA. Chronic alcohol abuse is associated with an increased incidence of acute respiratory distress syndrome and severity of multiple organ dysfunction in patients with septic shock. Crit Care Med. 2003;31:869–877. doi: 10.1097/01.CCM.0000055389.64497.11. [DOI] [PubMed] [Google Scholar]
- 11.Beck MK, Jensen AB, Nielsen AB, Perner A, Moseley PL, Brunak S. Diagnosis trajectories of prior multi-morbidity predict sepsis mortality. Sci Rep. 2016;6:36624. doi: 10.1038/srep36624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de RA, Cavalcanti M, Marcos MA, Garcia E, Ewig S, Mensa J, Torres A. Impact of alcohol abuse in the etiology and severity of community-acquired pneumonia. Chest. 2006;129:1219–1225. doi: 10.1378/chest.129.5.1219. [DOI] [PubMed] [Google Scholar]
- 13.Bochicchio GV, Joshi M, Bochicchio K, Tracy K, Scalea TM. A time-dependent analysis of intensive care unit pneumonia in trauma patients. J Trauma. 2004;56:296–301. doi: 10.1097/01.TA.0000109857.22312.DF. [DOI] [PubMed] [Google Scholar]
- 14.Mehta AJ. Alcoholism and critical illness: A review. World J Crit Care Med. 2016;5:27–35. doi: 10.5492/wjccm.v5.i1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joshi PC, Guidot DM. The alcoholic lung: epidemiology, pathophysiology, and potential therapies. Am J Physiol Lung Cell Mol Physiol. 2007;292:L813–L823. doi: 10.1152/ajplung.00348.2006. [DOI] [PubMed] [Google Scholar]
- 16.Yoseph BP, Breed E, Overgaard CE, Ward CJ, Liang Z, Wagener ME, Lexcen DR, Lusczek ER, Beilman GJ, Burd EM, Farris AB, Guidot DM, Koval M, Ford ML, Coopersmith CM. Chronic alcohol ingestion increases mortality and organ injury in a murine model of septic peritonitis. PLoS ONE. 2013;8:e62792. doi: 10.1371/journal.pone.0062792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klingensmith NJ, Yoseph BP, Liang Z, Lyons JD, Burd EM, Margoles LM, Koval M, Ford ML, Coopersmith CM. Epidermal Growth Factor Improves Intestinal Integrity and Survival in Murine Sepsis Following Chronic Alcohol Ingestion. Shock. 2017;47:184–192. doi: 10.1097/SHK.0000000000000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Margoles LM, Mittal R, Klingensmith NJ, Lyons JD, Liang Z, Serbanescu MA, Wagener ME, Coopersmith CM, Ford ML. Chronic Alcohol Ingestion Delays T Cell Activation and Effector Function in Sepsis. PLoS ONE. 2016;11:e0165886. doi: 10.1371/journal.pone.0165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit Care Med. 2017;45:486–552. doi: 10.1097/CCM.0000000000002255. [DOI] [PubMed] [Google Scholar]
- 20.Stortz JA, Raymond SL, Mira JC, Moldawer LL, Mohr AM, Efron PA. Murine Models of Sepsis and Trauma: Can We Bridge the Gap? ILAR. 2017;21:1–16. doi: 10.1093/ilar/ilx007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fox AC, Robertson CM, Belt B, Clark AT, Chang KC, Leathersich AM, Dominguez JA, Perrone EE, Dunne WM, Hotchkiss RS, Buchman TG, Linehan DC, Coopersmith CM. Cancer causes increased mortality and is associated with altered apoptosis in murine sepsis. Crit Care Med. 2010;38:886–893. doi: 10.1097/CCM.0b013e3181c8fdb1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lyons JD, Mittal R, Fay KT, Chen CW, Liang Z, Margoles LM, Burd EM, Farris AB, Ford ML, Coopersmith CM. Murine Lung Cancer Increases CD4+ T Cell Apoptosis and Decreases Gut Proliferative Capacity in Sepsis. PLoS ONE. 2016;11:e0149069. doi: 10.1371/journal.pone.0149069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Marik PE. The clinical features of severe community-acquired pneumonia presenting as septic shock. Norasept II Study Investigators. J Crit Care. 2000;15:85–90. doi: 10.1053/jcrc.2000.16460. [DOI] [PubMed] [Google Scholar]
- 25.Yoseph BP, Klingensmith NJ, Liang Z, Breed ER, Burd EM, Mittal R, Dominguez JA, Petrie B, Ford ML, Coopersmith CM. Mechanisms of Intestinal Barrier Dysfunction in Sepsis. Shock. 2016;46:52–59. doi: 10.1097/SHK.0000000000000565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilda JE, Gomes AV. Western blotting using in-gel protein labeling as a normalization control: stain-free technology. Methods Mol Biol. 2015;1295:381–391. doi: 10.1007/978-1-4939-2550-6_27. [DOI] [PubMed] [Google Scholar]
- 27.Klingensmith NJ, Yoseph BP, Liang Z, Lyons JD, Burd EM, Margoles LM, Koval M, Ford ML, Coopersmith CM. Epidermal Growth Factor Improves Intestinal Integrity and Survival in Murine Sepsis Following Chronic Alcohol Ingestion. Shock. 2017;47:184–192. doi: 10.1097/SHK.0000000000000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romero CR, Herzig DS, Etogo A, Nunez J, Mahmoudizad R, Fang G, Murphey ED, Toliver-Kinsky T, Sherwood ER. The role of interferon-gamma in the pathogenesis of acute intra-abdominal sepsis. J Leukoc Biol. 2010;88:725–735. doi: 10.1189/jlb.0509307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eskandari MK, Bolgos G, Miller C, Nguyen DT, DeForge LE, Remick DG. Anti-tumor necrosis factor antibody therapy fails to prevent lethality after cecal ligation and puncture or endotoxemia. J Immunol. 1992;148:2724–2730. [PubMed] [Google Scholar]
- 30.Barros FR, Castro-Faria-Neto HC, Castro CL, Aguiar Nemer AS, Rocha EM, Silva Fonseca VA. Effects of chronic ethanol consumption in experimental sepsis. Alcohol Alcohol. 2012;47:677–682. doi: 10.1093/alcalc/ags081. [DOI] [PubMed] [Google Scholar]
- 31.Castro CL, Aguiar-Nemer AS, Castro-Faria-Neto HC, Barros FR, Rocha EM, Silva-Fonseca VA. Effect of chronic ethanol consumption in female rats subjected to experimental sepsis. Braz J Med Biol Res. 2013;46:1033–1039. doi: 10.1590/1414-431X20133189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fry DE. The generic septic response. Crit Care Med. 2008;36:1369–70. doi: 10.1097/CCM.0b013e31816a11e9. [DOI] [PubMed] [Google Scholar]
- 33.McConnell KW, McDunn JE, Clark AT, Dunne WM, Dixon DJ, Turnbull IR, Dipasco PJ, Osberghaus WF, Sherman B, Martin JR, Walter MJ, Cobb JP, Buchman TG, Hotchkiss RS, Coopersmith CM. Streptococcus pneumoniae and Pseudomonas aeruginosa pneumonia induce distinct host responses. Crit Care Med. 2010;38:223–241. doi: 10.1097/CCM.0b013e3181b4a76b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inoue S, Sato T, Suzuki-Utsunomiya K, Komori Y, Hozumi K, Chiba T, Yahata T, Nakai K, Inokuchi S. Sepsis-induced hypercytokinemia and lymphocyte apoptosis in aging-accelerated Klotho knockout mice. Shock. 2013;39:311–316. doi: 10.1097/SHK.0b013e3182845445. [DOI] [PubMed] [Google Scholar]
- 35.Amin PB, Diebel LN, Liberati DM. The synergistic effect of ethanol and shock insults on Caco-2 cytokine production and apoptosis. Shock. 2008;29:631–635. doi: 10.1097/SHK.0b013e318157ec2e. [DOI] [PubMed] [Google Scholar]
- 36.Perrone EE, Jung E, Breed E, Dominguez JA, Liang Z, Clark AT, Dunne WM, Burd EM, Coopersmith CM. Mechanisms of methicillin-resistant Staphylococcus aureus pneumonia-induced intestinal epithelial apoptosis. Shock. 2012;38:68–75. doi: 10.1097/SHK.0b013e318259abdb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Asai K, Buurman WA, Reutelingsperger CP, Schutte B, Kaminishi M. Low concentrations of ethanol induce apoptosis in human intestinal cells. Scand J Gastroenterol. 2003;38:1154–1161. doi: 10.1080/00365520310006252. [DOI] [PubMed] [Google Scholar]
- 38.Young C, Klocke BJ, Tenkova T, Choi J, Labruyere J, Qin YQ, Holtzman DM, Roth KA, Olney JW. Ethanol-induced neuronal apoptosis in vivo requires BAX in the developing mouse brain. Cell Death Differ. 2003;10:1148–1155. doi: 10.1038/sj.cdd.4401277. [DOI] [PubMed] [Google Scholar]
- 39.Kapasi AA, Patel G, Goenka A, Nahar N, Modi N, Bhaskaran M, Reddy K, Franki N, Patel J, Singhal PC. Ethanol promotes T cell apoptosis through the mitochondrial pathway. Immunology. 2003;1008:313–320. doi: 10.1046/j.1365-2567.2003.01584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saad AJ, Jerrells TR. Flow cytometric and immunohistochemical evaluation of ethanol-induced changes in splenic and thymic lymphoid cell populations. Alcohol Clin Exp Res. 1991;15:796–803. doi: 10.1111/j.1530-0277.1991.tb00603.x. [DOI] [PubMed] [Google Scholar]
- 41.Danahy DB, Anthony SM, Jensen IJ, Hartwig SM, Shan Q, Xue HH, Harty JT, Griffith TS, Badovinac VP. Polymicrobial sepsis impairs bystander recruitment of effector cells to infected skin despite optimal sensing and alarming function of skin resident memory CD8 T cells. PLoS Pathog. 2017;13:e1006569. doi: 10.1371/journal.ppat.1006569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coopersmith CM, Stromberg PE, Dunne WM, Davis CG, Amiot DM, Buchman TG, Karl IE, Hotchkiss RS. Inhibition of intestinal epithelial apoptosis and survival in a murine model of pneumonia-induced sepsis. JAMA. 2002;287:1716–1721. doi: 10.1001/jama.287.13.1716. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTAL FIG. 1 No difference was detected in body weights between mice following 12 weeks of alcohol ingestion or water ingestion (A, p=0.43). AST (B, p=0.052) and ALT (C, p=0.18) levels were not statistically significantly different between water-fed septic mice and alcohol-fed septic mice. Splenic weights were also similar between water-fed septic mice and alcohol-fed septic mice (D, p=0.97), n=5-6 for all panels.
SUPPLEMENTAL FIG. 2 Flow cytometry on lung tissue showed similar numbers of CD4+ T cells (A, p=0.33), CD8+ T cells (B, p=0.25), neutrophils (C, p=0.12), macrophages (D, p=0.25) and dendritic cells (E, p=0.18) between water-fed mice and alcohol-fed mice, n=5-6/group for all.


