Abstract
Development of the eye is closely associated with neural crest cell migration and specification. Eye development is extremely complex, as it requires the working of a combination of local factors, receptors, inductors, and signaling interactions between tissues such as the optic cup and periocular mesenchyme (POM). The POM is comprised of neural crest-derived mesenchymal progenitor cells that give rise to numerous important ocular structures including those tissues that form the optic cup and anterior segment of the eye. A number of genes are involved in the migration and specification of the POM such as PITX2, PITX3, FOXC1, FOXE3, PAX6, LMX1B, GPR48, TFAP2A and TFAP2B. In this review we will discuss the relevance of these genes in the development of the POM and how mutations and defects result in rare ocular diseases.
Keywords: Neural crest, ocular disease, anterior segment dysgenesis, genetics, animal models, development
INTRODUCTION
Embryonic Origins of the Neural Crest
Neural crest cells (NCCs) are multipotent stem cells with migratory ability that arise from the dorsal neural tube during embryonic development (Beebee, 2000; Williams, 2015). When the neuroectoderm invaginates to form the neural tube and detaches from the surface ectoderm, neural crest cells migrate out laterally from the junction between the neural tube and surface ectoderm (Beebee, 2000). Neural crest cells can be divided into the cranial neural crest, the heart neural crest and the trunk neural crest, wherein cranial neural crest cells provide the major contribution to ocular development (Beebee, 2000).
Ocular Structures Derived from the Neural Crest
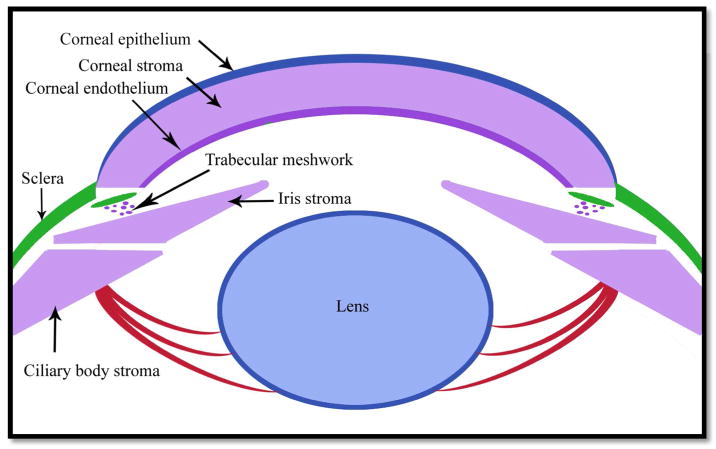
The major cranial neural crest contribution to ocular development includes the periocular mesenchyme (POM), which are migratory mesenchymal cells composed of neural crest cells and paraxial mesoderm cells (Gage, 2005). The POM is derived specifically from the forebrain and undergoes three migratory waves that give rise to various structures in the eye (Williams, 2015). The first wave migrates into the region between the surface ectoderm and the newly invaginated optic vesicle, eventually condensing to form the corneal endothelium (Cvekl, 2004; Williams, 2015). The second wave migrates between the corneal epithelium and corneal endothelium, giving rise to the corneal stroma (Cvekl, 2004; Williams, 2015). Finally, the third wave migrates into the space adjacent to the anterior rim of the developing optic cup, contributing to the stroma of the ciliary body and iris, as well as the trabecular meshwork (Cvekl, 2004; Williams, 2015).
The differences and similarities between zebrafish, avian and murine neural crest cell contributions to ocular structures have been extensively studied. Particularly, fate mapping in wild-type zebrafish has shown that 24 hours post fertilization (hpf), expression of crestin, a neural crest cell marker, was widespread around the dorsal and posterior region of the eye cup, and at 30 hpf, neural crest cells had migrated towards the anterior part of the eye cup into the region between the corneal epithelium and future retina (Langenberg, 2008). Additionally, studies in which quail neural crest cells were transplanted into the chick showed that neural crest cells give rise to the iris stroma, muscles of the iris, and the ciliary muscle (Creuzet, 2005). Similarly, murine neural crest cells give rise to the corneal endothelium and stroma, ciliary body muscle and stroma, iris stroma, as well as cells of the aqueous outflow structures, including the trabecular meshwork (Gage, 2005) (Figure 1).
Figure 1. Neural crest cell specification.
Structures derived from neural crest cells are given in purple, such as the corneal stroma, corneal endothelium, ciliary body stroma and iris stroma, whereas mesoderm-derived structures, like Schlemm’s canal (adjacent to the trabecular meshwork) and sclera, are given in green, and the surface ectoderm-derived lens and corneal epithelium are given in blue.
Neural crest cells also give rise to the connective fascia of extraocular muscles, as observed in fate mapping studies, wherein neural crest cells were found to be present in the future extraocular muscle region, along with mesoderm tissue that eventually give rise to muscle fibres in the mature structure (Gage, 2005). With respect to vasculature within the eye, both neural crest cells and mesoderm give rise to the early hyaloid artery that nourishes the lens and retina during development, but the endothelial cells of the hyaloid artery at later stages receives contributions only from the mesoderm, while the pericytes clinging to the endothelial cells are primarily derived from neural crest cells (Gage, 2005). Similarly, the endothelial cells of the blood vessel network found in the choroid region are derived from the mesoderm, while the pericytes are derived from the neural crest cells (Gage, 2005).
In this review, we will focus on how abnormalities in cranial neural crest cell development result in rare ocular diseases. The effects on other craniofacial tissues, and the genes implicated in development will also be discussed.
RARE HUMAN OCULAR DISORDERS AND THE NEURAL CREST
Anterior Segment Dysgenesis
Anterior segment dysgenesis (ASD) is a group of developmental disorders in which structures found in the anterior segment of the eye, many of which receive neural crest contributions, develop abnormally. These ASDs can be divided into the following subsections (Table I).
Table I.
Summary of phenotypes for rare ocular diseases of the neural crest, and genes suspected to be involved.
| Disease | Phenotype | Genes (Expressed in NC/POM (Y/N) |
|---|---|---|
| Axenfeld-Rieger Syndrome | Defects in the iris (polycoria, corectopia), cornea and iridocorneal angle, and posterior embryotoxon; high intraocular pressure. | Pitx2 (Y) Foxc1 (Y) |
| Peters anomaly | Iridocorneal and corneolenticular adhesions, cataracts and embryotoxon | Pitx3 (N) Foxe3 (N) |
| Aniridia | Absence of iris or iris hypoplasia. | Pax6 (N) |
| Nail Patella Syndrome | Optic nerve damage, increased intraocular pressure sometimes, loss of outer neuroretinal tissue. | Lmx1b (Y) |
| Branchio-Oculo-Facial Syndrome | Craniofacial defects, coloboma, microphthalmia. | Tfap2a (Y) |
| CHARGE Syndrome | Coloboma, heart deficits, choanal atresia, retarded development, genital defects and ear abnormalities. | Chd7 (N) PBAF (N) |
| Waardenburg Syndrome | Iris heterochromia, laterally displaced canthus. | Pax3 (Y) Sox10 (Y) MITF (N) |
| Treacher-Collins Syndrome | Abnormalities in craniofacial bone, problems with the auditory system; eyelid coloboma, palpebral fissures being angled downward and a reduction in eyelashes in medial eyelid region. | Tcof1 (Y) p53 (Y) |
| Char Syndrome | Facial abnormalities like increased width between the eyes; patent ductus arteriosus. | Tfap2b (Y) |
Axenfeld-Rieger Syndrome
Axenfeld-Rieger Syndrome is an ASD that is typified by defects in the iris, cornea and iridocorneal angle tissue (Tümer, 2009; Volkmann, 2011). Problems with the iris include hypoplasia, polycoria, corectopia and iris strands that connect the iridocorneal angle to the trabecular meshwork, in addition to posterior embryotoxon, sclerocornea and corneal opacities (Tümer, 2009). Patients with Axenfeld-Rieger Syndrome also exhibit glaucomatous features such as elevated intraocular pressure, which usually begins in adulthood (Tümer, 2009). Paired Like Homeodomain 2 (Pitx2) transcription factor has been well-established as playing an important role in anterior segment development and mutations in this gene in humans have been reported to result in Axenfeld-Rieger Syndrome (Tümer, 2009). Pitx2 is a transcription factor, which in mice is expressed in the POM by embryonic day (E) 11.5 (Gage, 2005). Mice in which conditional deletion of Pitx2 in the cranial neural crest have been created and exhibit ocular defects that are initiated as early as E12.5, including absence of corneal endothelium and corneal stroma. By E16.5, the mutant eyes were observed as being deeply embedded in the head, with eyes that were directly connected to the hypothalamus (Evans AL, 2005). Further, while control mice displayed a typically developing and lengthening optic stalk, mutant optic stalks remained thick and short (Evans AL, 2005). Even heterozygous null Pitx2 mutants demonstrated clinical features of Axenfeld-Rieger Syndrome. For example, by 3 weeks of age, the mutants had reduced central corneal thickness, iridocorneal adhesions, and about half of the mutants showed iris defects such as hypoplasticity (Chen, 2016). Finally, a large portion of the adult mutants showed high intraocular pressure, while also demonstrating optic nerve cupping and a spectrum of retinal ganglion cell loss when compared to controls (Chen, 2016).
G-protein coupled receptor 48 (GPR48) is widely expressed in surface ectoderm and optic cup derivatives, and is thought to be important for eye development through regulation of PITX2 (Weng, 2008; Van Schoore, 2005). Homozygous mutants for Gpr48 demonstrate anterior segment defects, including but not limited to a smaller eye size, varying degrees of iris hypoplasia accompanied with reduced expression of α-smooth muscle actin in the iris, increased pupil size, and adhesions between the iris and cornea (Weng, 2008). Almost half of the mutants displayed corneal opacities, while the majority had vascularization of the cornea, in addition to corneal keratopathy and thinning of corneal epithelium, the latter of which began as early as postnatal day (P) 10 (Weng, 2008). Electron microscopy further revealed disorganized collagen fibrils in the mutant corneal stroma as compared to controls and a reduction of several extracellular matrix proteins within the cornea (Weng, 2008). Cataracts were also present in some of mutants, along with disorganized fibre cells, and after 6 months of age, less than half of mutants presented with a reduction in retinal ganglion cell layer thickness (Weng, 2008). Interestingly, at P0, Pitx2 expression was greatly reduced in mutant animals, and follow-up experiments using chromatin immunoprecipitation (ChIP) suggest that Gpr48 regulates Pitx2 through interaction with cAMP response element binding protein (CREB) (Weng, 2008).
Individuals with Forkhead Box C1 (Foxc1) mutations also show classic features of Axenfeld-Rieger syndrome, with associated iris hypoplasia and polycoria, similar to Pitx2 mutations, as well as posterior embryotoxon, corneal opacities, aniridia and congenital glaucoma (Tümer, 2009). At early embryonic stages in mice Foxc1 is expressed in the POM, as well as the region surrounding the optic stalk and in some parts of the paraxial mesoderm, whereas Forkhead Box C2 (Foxc2) is expressed only in the neural crest region without any expression found in the mesoderm region (Gage, 2005). Conditional Foxc2 neural crest cell knockouts have been shown to demonstrate microphthalmia, misshapen irises, and vascularization and opacity in the cornea (Seo, 2017). These Foxc2 conditional knockouts also showed thickening of the peripheral cornea and conjunctiva, while the corneal epithelium of these mutants contained mesenchymal cells rather than epithelial cells normally found in this structure (Seo, 2017). Furthermore, at E15.5, double neural crest conditional knockouts for Foxc1 and Foxc2 showed that the corneal epithelium adhered to the surface ectoderm, and by postnatal day (P) 0, these animals had misshapen irises and smaller pupils compared to controls (Seo, 2017). By E14.5, double mutants also had reduced expression of Pitx2 and Activating protein 2 beta (AP-2β), although expression was unchanged in single Foxc1 and Foxc2 knockouts, suggesting that both are required for maintaining Pitx2 expression during development (Seo, 2017).
Peters anomaly
Paired Like Homeodomain 3 (Pitx3) transcription factor is important for lens development and has also been found to be associated with Peters anomaly, a condition involving the presence of iridocorneal and corneolenticular adhesions, as well as cataracts and a displaced Schwalbe’s line (Summers, 2008). Studies using aphakia mice deficient in Pitx3 show that this gene is expressed in wild-type mice at E9.5 in the lens placode, similar to the expression pattern of Foxe3 in the lens, although the two genes do not interact to affect lens development (Medina-Martinez, 2009). Later at E11.5, Pitx3 expression is found in the anterior lens epithelium in wild-type mice, which is missing in homozygous aphakia mice, while Pitx3 is not normally expressed in the fibre cell region of control mice, with expression found in this region in mutant mice (Medina-Martinez, 2009). Homozygous aphakia mice with Pitx3 deficiency also develop small lenses with an elongated shape (Medina-Martinez, 2009).
Previous studies have also shown an association between mutations in the Forkhead Box Protein E3 (Foxe3) gene, and abnormal ocular phenotypes including Peters anomaly, microphthalmia, anophthalmia, and iris and retinal coloboma, in addition to sclerocornea (Garcia-Montalvo, 2014). Foxe3 is expressed in the head ectoderm and lens epithelium of mice and adult mice that are homozygous null for Foxe3 show many features of other aforementioned genetic mutations, including a reduced lens size and a thicker anterior lens epithelium layer compared to controls (Medina-Martinez, 2005; Kerr, 2014). In addition, some mutants displayed corneolenticular adhesions and abnormally shaped nuclei of fibre cells within the lens, as well as retinal folding, missing corneal endothelium, disarrayed corneal stroma and a reduction in thickness of the corneal epithelium (Medina-Martinez, 2005). The invaginated surface ectoderm at E10.5 is smaller in mutants compared to controls, and vacuoles begin to form in the lens by E14.5 (Medina-Martinez, 2005). Moreover, cell proliferation in the anterior lens epithelium is greatly reduced at E14.5 in mutants when compared to controls, and differentiation of anterior lens epithelium occurs much earlier in mutant animals (Medina-Martinez, 2005)
Aniridia
Another form of ASD is aniridia, which involves absence of the iris, and studies in patient populations with aniridia have revealed several mutations within the Paired Box Protein 6 (Pax6) gene that were associated with this phenotype (Chograni, 2014). PAX6 is normally expressed in the surface ectoderm and neuroectoderm-derived tissue, such as the retina. In addition to iris hypoplasia, Pax6 heterozygous null mutant mice showed variable expressivity of other ocular defects, such as corneal opacities, and some animals displayed sclerization of the cornea and vasculature in the periphery of the cornea (Kanakubo, 2006). Although Pax6 is not expressed in the POM, at E11.5, heterozygous Pax6 mutants showed an increase in the number of temporal neural crest cells in the lens, likely stemming from improper migration of neural crest cells, and eyes of these mutants also had an accumulation of neural crest cells in the vitreal region (Kanakubo, 2006). The presence of neural crest cells in anterior chamber was associated with corneal opacity, whereas misplacement of neural crest cells in posterior eye region was associated with coloboma (Kanakubo, 2006). There were also fewer neural crest cells present in the hypoplastic iris and degenerate ciliary in the mutants compared to controls, all of which suggests that ocular abnormalities in the Pax6 heterozygous mutants partially arise from abnormal migration and misplacement of neural crest cells during embryonic development (Kanakubo, 2006). Despite the fact that Pax6 is only expressed in the surface ectoderm-derived and neuroectoderm-derived tissue, this molecule may be involved in signaling with other factors present in the neural crest, thereby resulting in the secondary effects on the neural crest cells where Pax6 is not expressed.
Nail Patella Syndrome
Nail Patella Syndrome (NPS) is another form of ASD in which patients display abnormalities of the eye, such as optic nerve damage, increased intraocular pressure in some cases, loss of outer neuroretinal tissue, as well as deteriorating vision (Romero P, 2011). Further molecular analysis revealed the presence of missense mutations within the LIM Homeobox Transcription Factor 1 Beta (Lmx1b) gene, suggesting that this gene plays a role in human eye development with deficits leading to glaucomatous changes (Romero P, 2011). Lmx1b is a gene thought to be involved in development of neural crest-derived structures, with a role in etiology of NPS (Liu, 2010). In mice, its expression has been detected in the POM by E10.5 and then in the presumptive cornea beyond P21 (Liu, 2010). Conditional knockout mice with Lmx1b deleted in neural crest cells show several anterior segment anomalies, including microphthalmia, iris hypoplasia, and reduced anterior chamber size, as well as corneal vascularization and reduced expression of corneal endothelium marker, NCAM (Liu, 2010). These mutant mice also had a thicker anterior lens epithelium layer, a slightly smaller lens, hypoplasia of corneal endothelium, and reduced expression of myocilin, a protein marker of the trabecular meshwork, which is a porous structure responsible for aqueous humor outflow from the eye (Liu, 2010). When Lmx1b was deleted only in adult mice using a temporal knockout system, these mutants showed corneal opacities, vascularization in the cornea, a thinner corneal epithelium, and a reduction in corneal stroma keratocytes, along with disorganized collagen fibrils in the corneal stroma (Liu, 2010).
Coloboma
Colobomas are holes that can be found in a number of ocular structures that either receive neural crest cell contributions or are derived from tissues like the surface ectoderm and neuroectoderm that participate in signaling interactions with the neural crest. Coloboma can occur in the eyelid, iris, ciliary body, retina, optic nerve and choroid that negatively affect visual acuity, with this condition sometimes being a part of genetic syndromes (Nakamura, 2011). Specific subtypes include coloboma affecting the anterior segment, such as iridial coloboma, as well as coloboma affecting the posterior segment, such as chorioretinal coloboma and optic disc coloboma (Nakamura, 2011). Developmentally, optic fissure closure defects are thought to lead to posterior segment coloboma in particular (Nakamura, 2011).
Branchio-Oculo-Facial Syndrome
Branchio-Oculo-Facial Syndrome (BOFS) is characterized by craniofacial defects accompanied by ocular abnormalities, such as coloboma and microphthalmia (Gestri, 2009). The Tfap2a gene has previously been linked to optic fissure closure defects and retinal abnormalities associated with coloboma, along with coloboma in the retina and choroid, as well as microphthalmia, iris coloboma and sclerocornea in humans (Gestri, 2009). Tfap2a is a human homolog of AP-2α, which is part of the activating protein 2 (AP-2) family of transcription factors, consisting of a range of retinoic acid responsive proteins that includes AP-2α, AP-2β, AP-2δ, AP-2γ and AP-2ε (Bassett, 2007, 2010, 2012; Eckert, 2005; Kerr, 2014; West-Mays, 1999). By E8.75, AP-2α is expressed in the POM surrounding the optic vesicle of wild-type mice, although expression ceases in this region after E10.5 (Bassett, 2010). Mice that were homozygous null for AP-2α displayed the inability of the lens to pinch off from the surface ectoderm, and did not form an anterior lens epithelium when compared to wild-type animals, in addition to showing variable expressivity of lens abnormalities (West-Mays, 1999). The persistent attachment of the lens to the cornea is reminiscent of Peters anomaly, discussed earlier. However, specific mutations in AP-2α in humans have yet to be linked with this disorder. The AP-2α null mice also failed to fully develop a retinal pigment epithelium in the dorsal region of the eye, with the structure being replaced by a pseudo-neural retina at this stage, and mutants also showed improper development of ciliary body and iris when compared to wild-type animals (West-Mays, 1999). When experiments were conducted to determine the underlying etiology of the observed defects, it was discovered that the optic vesicle faced the periocular mesenchyme to a greater extent as opposed to facing the surface ectoderm, when compared to controls (Bassett, 2010). The AP-2α null mice also displayed absence of expression of class III β-tubulin in the optic stalk region, suggesting optic nerve defects, while also displaying absence of optic fissure closure at E13.5 when compared to wild-type animals (Bassett, 2010).
CHARGE Syndrome
A human syndrome that includes coloboma as a key feature but also shows non-ocular deficits would be CHARGE syndrome, with the acronym representing the varied defects observed, including coloboma, heart deficits, choanal atresia, retarded development, genital defects and ear abnormalities (Bajpai, 2010). Chromodomain-helicase-DNA-binding protein 7 (CHD7) is a factor expressed in the neural ectoderm and surface ectoderm of the eye and is believed to be responsible in part for the etiology of CHARGE syndrome (Bajpai, 2010). Individuals with mutations in CHD7 consistently showed abnormalities in the temporal bone and hearing problems, while most individuals also showed coloboma, heart defects or developmental issues (Zetner, 2010). With respect to ocular defects, the major deficiency commonly observed was coloboma associated with the retina, choroid, as well as the optic nerve, while coloboma associated with the iris and eyelid were also observed, albeit at a lower frequency among patients with CHD7 mutations (Zetner, 2010). Microphthalmia and strabismus were also a less common occurrence, affecting visual acuity (Zetner, 2010).
Polybromo-Associated BAF (PBAF) is a chromatin remodeling complex that binds to CHD7, suggesting an important role for the interaction of PBAF with CHD7 in affecting ocular phenotypes (Bajpai, 2010). When cells from neuroectoderm-based neuronal spheres were transfected with small hairpin RNA that reduced expression of CHD7, these neuroectoderm neurospheres displayed reduced formation of neural crest cell-like cells. Moreover, the neural crest cell-like cells that did form had a lower ability to migrate and a reduction in expression of TWIST1, which is normally expressed in multipotent and migratory neural crest cell populations (Bajpai, 2010). In particular, disrupting the ATPase domain of CHD7 by introducing a point mutation into Xenopus embryos showed that neural crest cell migration was negatively affected, suggesting this domain plays a key role in neural crest migration patterns (Bajpai, 2010). Further analysis in Xenopus tadpoles of different stages containing the same point mutation revealed the presence of vestibular defects, coloboma in the eye, craniofacial cartilage abnormalities and deficits in heart structures derived from neural crest (Bajpai, 2010). While mouse models of CHARGE syndrome are restricted to defects of neuroectoderm tissues like the retina, the aberrant neural crest cell migration seen in Xenopus CHD7 mutants suggests that CHD7 may interact inductively with neuroectoderm to participate in etiology of CHARGE syndrome in mice and humans (Sperry, 2014; Gage, 2015).
Waardenburg Syndrome
Waardenburg Syndrome is another rare neural crest disease and constitutes 4 different types. Individuals with Waardenburg Syndrome show iris heterochromia, and a laterally displaced canthus, with all patients displaying mutations in the Pax3 gene, encoding a transcription factor expressed during embryonic development in the neural crest (Maczkowiak F., 2010; Wang, 2010). Similarly, another study showed that some patients displaying signs of this disorder also had either heterozygous or homozygous mutations for Sox10, another transcription factor expressed in the neural crest (Bondurand, 2007; Trost, 2013). Preliminary experimental analysis using a human immortalized cell line has revealed that when the Pax3 gene is co-transfected with the promoter for Microphthalmia-associated Transcription Factor (MITF) with the luciferase reporter gene downstream of this promoter, there is heavy luciferase activity, suggestive of the interaction between Pax3 and MITF, which is expressed in the developing RPE (Bondurand, 2000; Heavner, 2012). In addition, co-transfection of both Pax3 and Sox10 led to a 1500 fold increase in expression of luciferase, implicating the synergistic effects of Pax3 and Sox10 on increasing MITF expression, possibly leading to ocular deficits in Waardenburg syndrome (Bondurand, 2000).
Treacher-Collins syndrome
Treacher-Collins syndrome is another rare ocular disease of the neural crest stemming from mutations within the Tcof1 gene that codes for a specific phosphoprotein found within the nucleus called Treacle, with wide phenotypic variability, wherein some patients hardly display any defects (Trainor, 2009). Characteristics of this condition in humans include developmental abnormalities in craniofacial bone, problems with the auditory system, in addition to ocular defects that include eyelid coloboma, palpebral fissures that are angled downward and a reduction in eyelashes in medial regions of the eyelid (Trainor, 2009). De novo Tcof1 mutations are also quite common in patients presenting with this syndrome, in addition to the variability in genotype and phenotype (Trainor, 2009). In normal mice, TCOF1 is expressed in the neuroectoderm that gives rise to the neural crest, as well as migrating neural crest cells (Trainor, 2009). Studies using a Treacher-Collins syndrome mouse model having Tcof1 mutations show that at embryonic stages, neural crest cell numbers are reduced by a quarter the amount found in controls, with increased apoptosis found in the neuroectoderm, along with a reduction in cell proliferation of the migrating neural crest cells (Trainor, 2009). This suggests that Tcof1 mutations result in an increase in apoptosis of neuroectoderm cells and a decrease in neural crest cell proliferation, possibly resulting in the Treacher-Collins phenotype (Trainor, 2009). Intriguing new findings also indicate that either genetic or pharmacological reduction of p53, a signaling molecule whose upregulation increases the severity of Treacher-Collins syndrome, leads to a reduction in apoptosis within neuroectoderm cells that give rise to neural crest cells, ameliorating the abnormal craniofacial phenotype at later stages in embryos (Jones, 2008).
Non-Syndromic Ocular Defects
Previous work has pinpointed many genes implicated in ocular defects not characterized by a syndrome. One example of such a gene encodes retinaldehyde dehydrogenase (RALDH) enzyme that synthesizes retinoic acid (RA), which is a derivative of retinol and is involved in neural crest signaling important for eye development. Specifically, 2 isoforms exist for RALDH, which are RALDH1 and RALDH3, and both are both present in the retina and corneal epithelium (Matt, 2005). At E12.5, single mutants for RALDH3 deletion had a thicker POM between the retina and surface ectoderm compared to wild-type animals, while also showing absence of TUNEL expression in the ventral presumptive retina, whereas wild-type animals showed TUNEL expression in both the dorsal and ventral retina (Matt, 2005). Mice with mutations in both forms of the enzyme had severe ocular defects in the eyelid, anterior segment, as well as in the lens, primary vitreous and retina (Matt, 2005). At E11.5 in the double mutants that had both RALDH1 and RALDH3 deletions, the POM was thicker than in controls, with this tissue type replacing the eyelid and cornea (Matt, 2005). Ventral retinal defects observed in double mutants were much more severe than in the single RALDH3 mutants (Matt, 2005). Not only did double mutant animals show posterior segment defects, but at E18.5, they also showed anterior segment defects, specifically absence of the corneal and iris stroma, as well as absence of anterior chamber formation (Matt, 2005). The same defects were observed in mutants with retinoic acid receptor β (RARβ) and retinoic acid receptor γ (RARγ) deleted specifically from the neural crest, providing support for the hypothesis that retinoic acid in the developing neural retina interacts with RAR found in the POM, influencing eye development by affecting the latter target tissue (Matt, 2005).
Char Syndrome
Char syndrome is a neural crest disease where patients present with facial abnormalities, including increased width between the eyes and a heart condition called patent ductus arteriosus (Zhao, 2001). This condition is associated with mutations in the Tfap2b gene, a human analog of AP-2β in the mouse, although ocular defects have yet to be associated with this disorder in humans (Zhao, 2001). In the mouse, AP-2β is expressed in the lens placode, as well as the neural crest during development (Barzago, 2017; Bassett, 2007; West-Mays, 1999). Previous work using conditional deletion of AP-2β in cranial neural crest cells using the Wnt1Cre-LoxP system resulted in anterior segment defects (Martino, 2016). In 2–3 month-old mutant mice, defects of neural crest-derived structures were evident, including absence of a corneal endothelium, corneal vascularization and iridocorneal adhesions. Secondary defects were also observed, including corneolenticular adhesions, with the ciliary body containing fewer folds than control animals (Martino, 2016). Follow-up studies of intraocular pressure measurement demonstrated a 3-fold increase in adult mutant animals compared to controls, possibly stemming from the aqueous blockade resulting from iridocorneal adhesions. This spike in intraocular pressure corresponded with retinal ganglion cell loss and a reduction in retinal ganglion axon myelination (Martino, 2016). Further work on this mouse model has shown that AP-2β is important for normal development and morphology of the ciliary body and trabecular meshwork. For example, by P7, healthy animals displayed properly folded ciliary processes, whereas the knockout demonstrated a reduction in the amount of ciliary process folding, in addition to abnormal placement of neural crest cells that normally give rise to aqueous outflow structures like the trabecular meshwork (Akula, 2017). Furthermore, control eye sections of adult mice were found to have normal expression of myocilin, a protein marker of the trabecular meshwork, whereas knockout eye sections displayed a reduction in expression (Akula, 2017), all of which suggests that differentiation in these knockout mice is affected in the ciliary body and trabecular meshwork, both of which receive neural crest cell contributions.
Oligogenic Effects
A number of the ocular syndromes discussed above exhibit variability in penetrance and expressivity. For example individuals with TFAP2A mutations or deletions show extensive variability in phenotype including microphthalmia or anophthalmia, cataract, coloboma, strabismus and ptosis. The differences in phenotype may be attributed to the type of mutation with which they are associated. For example, BOFS patients who have a deletion of one copy of the TFAP2A gene typically have a milder phenotype than those with missense mutations in one allele (Milunsky, 2008). However, growing evidence in animal models has suggested that oligogenic effects may contribute to the variability in phenotype in many of these ocular diseases. For example, studies in zebrafish have shown that partial deletion of Tfap2a can affect the expressivity of ocular phenotypes in knockdown models of bone morphogenic protein 4 (Bmp4) and transcription factor 7-like1a (Tcf711a). Knockdown of either of these genes alone did not result in any ocular phenotype, but when combined with a partial knockdown of Tfap2a multiple ocular anomalies were observed, including coloboma, anophthalmia and microphthalmia (Gestri, 2009). These findings illustrate a genetic interaction between Tfap2a and these two genes. In mice, a genetic interaction between Tfap2a and Pax6 in eye development has also been demonstrated, with double heterozygote mice exhibiting much more severe ocular defects that single heterozygotes (Makhani, 2007). Thus, the variation in phenotype and expressivity observed in some rare human ocular diseases is likely the result of genetic backgrounds consisting of unidentified modifier genes that are also important in regulating ocular development. Indeed, patients with multiple gene mutations are at a higher risk of having offspring with severe ocular disorders. Thus, future studies investigating the genetic interaction between the cranial neural crest cell genes outlined in this review will help to further understand these complex diseases.
CONCLUSION
During embryonic and postnatal development, cranial neural crest cells derived from the dorsal neural tube contribute to many ocular structures, including the ciliary body, iris, cornea, and trabecular meshwork. As we have outlined in this review, multiple transcription factors play key roles in development of the cranial neural crest and formation of these tissues. Aberrant mutations and gene signaling during development not only lead to primary ocular defects of neural crest-derived structures, but also secondary ocular defects of structures not derived from the neural crest, including the retina, as evidenced by knockout mouse models for these genes. Moreover, such aberrant development is associated with a range of rare ocular diseases in humans, including diseases affecting the anterior eye segment, such as Axenfeld-Rieger Syndrome, Peters anomaly, Aniridia and Nail Patella Syndrome, as well as defects affecting the posterior segment, such as BOFS and CHARGE syndrome, in addition to other rare neural crest diseases like Waardenburg syndrome, Treacher-Collins syndrome and Char syndrome (Table I).
Current studies of the neural crest have thus far focused on characterizing genes that participate in the coordination and development of cranial neural crest derivatives in several model organisms. However, fewer studies have investigated the epigenetic mechanisms, post-transcriptional and post-translational modifications regulating cranial neural crest development and migration at embryonic and postnatal stages (Simoes-Costa, 2013). Novel tracing techniques, such as fate mapping using mice expressing β-galactosidase or an RFP variant in the presence of Cre recombinase, can be used for tracing neural crest cell migration, which will allow researchers to elucidate early embryonic developmental changes that lead to the ocular defects in adult animals (Gage, 2005; Madisen, 2010). In addition to cellular techniques, molecular techniques such as chromatin profiling, ChIP-sequencing and bioinformatics techniques are also useful in probing the epistatic, post-transcriptional and post-translational mechanisms of neural crest cell migration and regulation (Simoes-Costa, 2013). These latter techniques will also be useful in teasing out the complex genetic interaction involved in crest-cell associated diseases such as those that cause rare ocular syndromes.
SIGNIFICANCE STATEMENT.
Rare ocular diseases can develop from abnormalities in a specific tissue type during prenatal human development known as the neural crest, with often debilitating effects. This review summarizes previous work on these rare diseases arising from defects in neural crest development. This work is important in order to achieve a holistic understanding of the various genes and developmental pathways involved in these rare ocular diseases, and this overview can be used as a starting point for background knowledge to investigate these pathways further, and to consider novel and innovative treatment options.
Acknowledgments
This work was supported by the National Eye Institute/National Institutes of Health (EY025789 to J. WM)
This work was completed through helpful advice from Aftab Taiyab, McMaster University.
Footnotes
Conflict of Interest Statement
The authors declare no conflict of interest.
References
- Akula M, Martino VB, Williams T, West-Mays J. Abnormal development and differentiation of the periocular mesenchyme in AP-2β neural crest cell knockout mice. Paper presented at the Association for Research in Vision and Ophthalmology; Baltimore, USA. 2017. May 8, [Google Scholar]
- Bajpai R, Chen DA, Rada-Iglesias A, Zhang J, Xiong Y, Helms J, Chang C, Zhao Y, Swigut T, Wysocka J. CHD7 cooperates with PBAF to control multipotent neural crest formation. Nature. 2010;463:958–962. doi: 10.1038/nature08733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzago MM, Kurosaki M, Fraelli M, Bolis M, Giudice C, Nordio L, Domenici L, Terao M, Garrattini E. Generation of a new mouse model of glaucoma characterized by reduced expression of the AP-2β and AP-2δ proteins. Nature. 2017;7(1) doi: 10.1038/s41598-017-11752-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett EA, Korol A, Deschamps PA, Buettner R, Wallace VA, Williams T, West-Mays JA. Overlapping Expression Patterns and Redundant Roles for AP-2 Transcription Factors in the Developing Mammalian Retina. Developmental Dynamics. 2012;241(4):814–829. doi: 10.1002/dvdy.23762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett EA, Pontoriero GF, Feng W, Marquardt T, Fini ME, Williams T, West-Mays J. Conditional deletion of activating protein-2α (AP-2α) in the developing retina demonstrates non-cell-autonomous roles for AP-2α in optic cup development. Molecular and Cellular Biology. 2007;27(21):7497–7510. doi: 10.1128/MCB.00687-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett EA, Williams T, Zacharias AL, Gage PJ, Fuhrmann S, West-Mays JA. AP-2α knockout mice exhibit optic cup patterning defects and failure of optic stalk morphogenesis. 2010;19(9):1791–1804. doi: 10.1093/hmg/ddq060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebee DC, Coats JM. The lens organizes the anterior segment: specification of neural crest cell differentiation in the avian eye. Dev Bio. 2000;220(2):424–431. doi: 10.1006/dbio.2000.9638. [DOI] [PubMed] [Google Scholar]
- Bondurand N, Dastot-Le Moal F, Stanchina L, Collot N, Baral V, Marlin S, Attie-Bitach T, Giurgea I, Skopinski L, Reardon W, Toutain A, Sarda P, Echaieb A, Lackmy-Port-Lis M, Touraine R, Amiel J, Goossens M, Pingault V. Deletions at the SOX10 gene locus cause Waardenburg syndrome types 2 and 4. Am J Hum Genet. 2007;81:1169–1185. doi: 10.1086/522090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondurand N, Pingault V, Goerich DE, Lemort N, Sock E, Le Caignec C, Wegner M, Goossens M. Interaction among SOX10, PAX3 and MITF, three genes altered in Waardenburg syndrome. Human Molecular Genetics. 2000;9(13):1907–1917. doi: 10.1093/hmg/9.13.1907. [DOI] [PubMed] [Google Scholar]
- Chen L, Gage PJ. Heterozygous Pitx2 null mice accurately recapitulate the ocular features of Axenfeld-Rieger Syndrome and Congenital Glaucoma. Investigative Ophthalmology and Visual Science. 2016;57(11):5023–5030. doi: 10.1167/iovs.16-19700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chograni M, Derouiche K, Chaabouni M, Lariani I, Bouhamed HC. Molecular analysis of the PAX6 gene for aniridia and congenital cataracts in Tunisian families. Human Genome Var. 2014:1. doi: 10.1038/hgv.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creuzet S, Vincent C, Couly G. Neural crest derivatives in ocular and periocular structures. Int J Dev Biol. 2005;49:161–165. doi: 10.1387/ijdb.041937sc. [DOI] [PubMed] [Google Scholar]
- Cvekl A, Tamm ER. Anterior eye development and ocular mesenchyme: New insights from mouse models and human diseases. BioEssays9: News and Reviews in Molecular, Cellular and Developmental Biology. 2004;26(4):374–386. doi: 10.1002/bies.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert D, Buhl S, Weber S, Jäger R, Schorle H. The AP-2 family of transcription factors. Genome Biology. 2005;6(13):246. doi: 10.1186/gb-2005-6-13-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans ALGP. Expression of the homeobox gene Pitx2 in neural crest is required for optic stalk and ocular anterior segment development. Hum Mol Genet. 2005;14:3347–3359. doi: 10.1093/hmg/ddi365. [DOI] [PubMed] [Google Scholar]
- Gage PJ, Hurd EA, Martin DM. Mouse Models for the Dissection of CHD7 Functions in Eye Development and the Molecular Basis for Ocular Defects in CHARGE Syndrome. IOVS. 2015;56(13):7923–7930. doi: 10.1167/iovs.15-18069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage PJ, Rhoades W, Prucka SK, Hjalt T. Fate maps of neural crest and mesoderm in the mammalian eye. IOVS. 2005;46:4200–4208. doi: 10.1167/iovs.05-0691. [DOI] [PubMed] [Google Scholar]
- Garcia-Montalvo IA, Pelcastre-Luna E, Nelson-Mora J, Buentello-Volante B, Miranda-Duarte A, Zenteno JC. Mutational Screening of FOXE3, GDF3, ATOH7, and ALDH1A3 in Congenital Ocular Malformations. Possible Contribution of the FOXE3 p.VAL201MET Variant to the Risk of Severe Eye Malformations. Ophthalmic Genetics. 2014;35(3):190–192. doi: 10.3109/13816810.2014.903983. [DOI] [PubMed] [Google Scholar]
- Gestri G, Osborne RJ, Wyatt AW, Gerrelli D, Gribble S, Stewart H, Fryer A, Bunyan DJ, Prescott K, Collin JR, Fitzgerald T, Robinson D, Carter NP, Wilson SW, Ragge NK. Reduced TFAP2A function causes variable optic fissure closure and retinal defects and sensitizes eye development to mutations in other morphogenetic regulators. Hum Genet. 2009;126(6):791–803. doi: 10.1007/s00439-009-0730-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gestri G, Osborne RJ, Wyatt AW, Gerrelli D, Gribble S, Stewart H, Fryer A, Bunyan DJ, Prescott K, Collin JR, Fitzgerald T, Robinson D, Carter NP, Wilson SW, Ragge NK. Reduced TFAP2A function causes variable optic fissure closure and retinal defects and sensitizes eye development to mutations in other morphogenetic regulators. Hum Genet. 2009;126(6):791–803. doi: 10.1007/s00439-009-0730-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heavner W, Pevny L. Eye development and retinogenesis. Cold Spring Harb Perspect Biol. 2012;4(12):1–17. doi: 10.1101/cshperspect.a008391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones NC, Lynn ML, Gaudenz K, Sakai D, Aoto K, Rey JP, Glynn EF, Ellington L, Du C, Dixon J, Dixon MJ, Trainor PA. Prevention of the neurocristopathy Treacher Collins syndrome through inhibition of p53 function. Nat Med. 2008;14:125–133. doi: 10.1038/nm1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanakubo S, Nomura T, Yamamura K, Miyazaki J, Tamai M, Osumi N. Abnormal migration and distribution of neural crest cells in Pax6 heterozygous mutant eye, a model for human eye diseases. Genes Cells. 2006;11(8):919–933. doi: 10.1111/j.1365-2443.2006.00992.x. [DOI] [PubMed] [Google Scholar]
- Kerr CL, Zaveri MA, Robinson ML, Williams T, West-Mays JA. AP-2α is required after lens vesicle formation to maintain lens integrity. Dev Dyn. 2014;243:1298–1309. doi: 10.1002/dvdy.24141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenberg T, Kahana A, Wszalek JA, Halloran MC. The eye organizes neural crest cell migration. Dev Dyn. 2008;237(6):1645–1652. doi: 10.1002/dvdy.21577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Johnson R. Lmx1b Is Required for Murine Trabecular Meshwork Formation and for Maintenance of Corneal Transparency. Developmental Dynamics. 2010;239:2161–2171. doi: 10.1002/dvdy.22347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maczkowiak FMS, Wang E, Roche D, Harland R, Monsoro-Burq AH. The Pax3 and Pax7 paralogs cooperate in neural and neural crest patterning using distinct molecular mechanisms, in Xenopus laevis embryos. Dev Biol. 2010;340:381–396. doi: 10.1016/j.ydbio.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makhani LF, Williams T, West-Mays JA. Genetic analysis indicates that transcription factors AP-2alpha and Pax6 cooperate in the normal patterning and morphogenesis of the lens. Mol Vis. 2007;13:1215–1225. [PubMed] [Google Scholar]
- Martino VB, Sabljic T, Deschamps P, Green RM, Akula M, Peacock E, Ball AK, Williams T, West-Mays JA. Conditional deletion of AP-2β in the cranial neural crest results in anterior segment dysgenesis and early-onset glaucoma. Disease Models and Mechanisms. 2016;9:849–861. doi: 10.1242/dmm.025262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matt N, Dupé V, Garnier J, Dennefeld C, Chambon P, Mark M, Ghyselinck NB. Retinoic acid-dependent eye morphogenesis is orchestrated by neural crest cells. Development. 2005;132(21):4789–4800. doi: 10.1242/dev.02031. [DOI] [PubMed] [Google Scholar]
- Medina-Martinez O, Brownell I, Amaya-Manzanares F, Hu Q, Behringer RR, Jamrich M. Severe Defects in Proliferation and Differentiation of Lens Cells in Foxe3 Null Mice. Mol Cell. 2005;25(20):8854–8863. doi: 10.1128/MCB.25.20.8854-8863.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Martinez O, Shah R, Jamrich M. Pitx3 controls multiple aspects of lens development. Dev Dyn. 2009;238(9):2193–2201. doi: 10.1002/dvdy.21924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milunsky JM, Maher TA, Zhao G, Roberts AE, Stalker HJ, Zori RT, Burch MN, Clemens M, Mulliken JB, Smith R, Lin AE. TFAP2A mutations result in branchio-oculo-facial syndrome. Am J Hum Genet. 2008;82(5):1171–1177. doi: 10.1016/j.ajhg.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura KM, Diehl NN, Mohney BG. Incidence, ocular findings, and systemic associations of ocular coloboma: a population-based study. Arch Ophthalmol. 2011;129(1):69–74. doi: 10.1001/archophthalmol.2010.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero PSF, Lopez P, Reyes L, Herrera L. c.194 A>C (Q65P) mutation in the LMX1B gene in patients with nail-patella syndrome associated with glaucoma. Mol Vis. 2011;17:1929–1939. [PMC free article] [PubMed] [Google Scholar]
- Seo S, Chen L, Liu W, Zhao D, Schultz KM, Sasman A, Liu T, Zhang HF, Gage P, Kum T. Foxc1 and Foxc2 in the Neural Crest Are Required for Ocular Anterior Segment Development. IOVS. 2017;58(3):1368–1377. doi: 10.1167/iovs.16-21217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoes-Costa MB, ME Insights into neural crest development and evolution from genomic analysis. Genome Res. 2013;23:1069–1080. doi: 10.1101/gr.157586.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry ED, Hurd EA, Durham MA, Reamer EN, Stein AB, Martin DM. The chromatin remodeling protein CHD7, mutated in CHARGE syndrome, is necessary for proper craniofacial and tracheal development. Dev Dyn. 2014;243(9):1055–1066. doi: 10.1002/dvdy.24156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers KM, Withers SJ, Gole GA, Piras S, Taylor PJ. Anterior segment mesenchymal dysgenesis in a large Australian family is associated with the recurrent 17 bp duplication in PITX3. Molecular Vision. 2008;14:2010–2015. [PMC free article] [PubMed] [Google Scholar]
- Trainor PA, Dixon J, Dixon MJ. Treacher Collins syndrome: etiology, pathogenesis and prevention. Eur J Hum Genet. 2009;17:275–283. doi: 10.1038/ejhg.2008.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trost A, Schroedl F, Lange S, Rivera FJ, Tempfer H, Korntner S, Stolt CC, Wegner M, Bogner B, Kaser-Eichberger A, Krefft K, Runge C, Aigner L, Reitsamer HA. Neural crest origin of retinal and choroidal pericytes. Invest Ophthalmol Vis Sci. 2013;54(13):7910–7921. doi: 10.1167/iovs.13-12946. [DOI] [PubMed] [Google Scholar]
- Tümer Z, Bach-Holm D. Axenfeld–Rieger syndrome and spectrum of PITX2 and FOXC1 mutations. European Journal of Human Genetics. 2009;17(12):1527–1539. doi: 10.1038/ejhg.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schoore G, Mendive F, Pochet R, Vassart G. Expression pattern of the orphan receptor LGR4/GPR48 gene in the mouse. Histochem Cell Biol. 2005;125(1):35–50. doi: 10.1007/s00418-005-0002-3. [DOI] [PubMed] [Google Scholar]
- Volkmann B, Zinkevich NS, Mustonen A, Schilter KF, Bosenko DV, Reis LM, Broeckel U, Link BA, Semina EV. Potential Novel Mechanism for Axenfeld-Rieger Syndrome: Deletion of a Distant Region Containing Regulatory Elements of PITX2. IOVS. 2011;52(3):1450–1459. doi: 10.1167/iovs.10-6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Li S, Xiao X, Wang P, Guo X, Zhang Q. PAX3 mutations and clinical characteristics in Chinese patients with Waardenburg syndrome type 1. Mol Vis. 2010;16:1146–1153. [PMC free article] [PubMed] [Google Scholar]
- Weng J, Luo J, Cheng X, Jin C, Zhou X, Qu J, Tu L, Ai D, Li D, Wang J, Martin JF, Amendt BF, Liu M. Deletion of G protein-coupled receptor 48 leads to ocular anterior segment dysgenesis (ASD) through down-regulation of Pitx2. Proc Natl Acad Sci U S A. 2008;105(16):6081–6086. doi: 10.1073/pnas.0708257105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West-Mays JA, Zhang J, Nottoli T, Hagopian-Donaldson S, Libby D, Strissel KJ, Williams T. AP-2alpha transcription factor is required for early morphogenesis of the lens vesicle. Dev Biol. 1999;206(1):46–62. doi: 10.1006/dbio.1998.9132. [DOI] [PubMed] [Google Scholar]
- Williams A, Bohnsack B. Neural crest derivatives in ocular development: Discerning the eye of the storm. Birth Defects Research Part C: Embryo Today: Reviews. 2015;105(2):87–95. doi: 10.1002/bdrc.21095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetner GE, Layman WS, Martin DM, Scacheri PC. Molecular and phenotypic aspects of CHD7 mutation in CHARGE syndrome. Am J Med Genet A. 2010;152A:674–686. doi: 10.1002/ajmg.a.33323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Satoda M, Licht JD, Hayashizaki Y, Gelb BD. Cloning and characterization of a novel mouse AP-2 transcription factor, AP-2delta, with unique DNA binding and transactivation properties. J Biol Chem. 2001;276:40755–40760. doi: 10.1074/jbc.M106284200. [DOI] [PubMed] [Google Scholar]