Abstract
BACKGROUND
Cerebral glutathione (GSH), a marker of oxidative stress, has been quantified in neurodegenerative diseases and psychiatric disorders using proton magnetic resonance spectroscopy (MRS). Using a reproducible MRS technique is important as it minimizes the impact of measurement technique variability on the study results and ensures that other studies can replicate the results.
HYPOTHESIS
We hypothesized that very short echo time (TE) acquisitions would have comparable reproducibility to a long TE MEGA-PRESS acquisition, and that the short TE PRESS acquisition would have the poorest reproducibility.
STUDY TYPE
Prospective
SUBJECTS/PHANTOMs
Ten healthy adults were scanned during two visits, and six metabolite phantoms containing varying concentrations of GSH and metabolites with resonances that overlap with GSH were scanned once.
FIELD STRENGTH/SEQUENCE
At 3T, we acquired MRS data using four different sequences: PRESS, SPECIAL, PR-STEAM, and MEGA-PRESS
ASSESSMENT
Reproducibility of each MRS sequence across two visits was assessed.
STATISTICAL TESTS
Mean coefficients of variation (CV) and mean absolute difference (AD) were used to assess reproducibility. Linear regressions were performed on data collected from phantoms to examine the agreement between known and quantified levels of GSH.
RESULTS
Of the four techniques, PR-STEAM had the lowest mean CV and AD (5.4% and 7.5% respectively), implying excellent reproducibility, followed closely by PRESS (5.8% and 8.2%) and SPECIAL (8.0 and 10.1%), and finally by MEGA-PRESS (13.5% and 17.1%). Phantom data revealed excellent fits (R2 ≥ 0.98 or higher) using all methods.
DATA CONCLUSION
Our data suggest that GSH can be quantified reproducibly without the use of spectral editing.
Keywords: glutathione, magnetic resonance spectroscopy, reproducibility, phase rotation STEAM, SPECIAL, MEGA-PRESS
INTRODUCTION
Using proton magnetic resonance spectroscopy at 3 Tesla (T), cerebral glutathione (GSH), a marker of oxidative stress, has been quantified in a multitude of neurodegenerative diseases and psychiatric disorders such as schizophrenia (1), bipolar disorder (2–4), depression (5), obsessive compulsive disorder (6), Alzheimer’s disease (7), and amyotrophic lateral sclerosis (8). Historically, GSH has been difficult to quantify due to the overlapping resonances of other metabolites within the human brain such as myo-Inositol, creatine, glutamate, glutamine, glucose, and γ-aminobutyric acid (9). However, with the advent of improved hardware, higher field strengths, and improved localization techniques, quantification of cerebral GSH has been possible. Currently, the most commonly utilized MRS localization techniques to quantify GSH are: PRESS (2,10,11), PR-STEAM (12–15), SPECIAL (3,5,16), and MEGA-PRESS (17–19).
Here, we quantified GSH using all four techniques in ten healthy volunteers twice in order to assess and compare reproducibility of the aforementioned techniques. We also quantified GSH using all four techniques in phantoms that contained varying concentrations of GSH as well as several other overlapping metabolites. Previous comparison studies focused on GSH often compared localization techniques or echo times (TE). One study showed that GSH quantification was a comparable between 68ms TE MEGA-PRESS and a very short 5 ms TE STEAM sequence in humans at 4T (20), while another showed that GSH measured with PRESS (TE = 35 ms) were inconsistent with actual GSH levels in phantoms, especially at low GSH levels, and inferior to MEGA-PRESS at TE = 130 ms (21). Two other studies showed that GSH spectral editing using longer TEs (120–130 ms) was superior to shorter TEs (68–70 ms) in phantoms and humans (22,23). Therefore, we hypothesized that the very short TE acquisitions (i.e., PR-STEAM and SPECIAL) would have comparable reproducibility to a 120ms TE MEGA-PRESS acquisition, and that the short 30 ms TE PRESS acquisition would have the poorest reproducibility.
MATERIALS AND METHODS
Acquisition
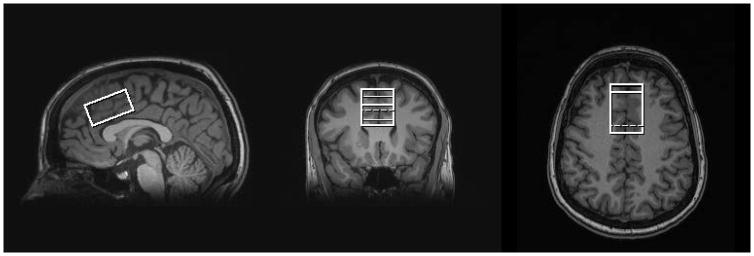
All studies were conducted on a 3T Siemens TIM Trio MR system with a 32-channel head coil. The study protocol was approved by the local Institutional Review Board, and all participants provided written informed consent. MP-RAGE anatomical images were acquired and utilized for prescription of a 24-cm3 spectroscopic voxel in the medial frontal lobe, which included the anterior cingulate (Figure 1). Ten participants (3 males, 7 females) with a mean age of 26 (3.3) years completed the study. Each participant completed two MRS sessions with a short break (approximately 15 minutes in length on average) between sessions. During the break, each participant was removed from the scanner. Automatic shimming was performed using the Siemens “Advanced” shimming option, followed-up by manual adjustments if needed. Each MRS session included the following acquisitions: PRESS (TE = 30ms, NEX = 256, 16-step phase cycle), MEGA-PRESS (TE = 120ms, 128 ‘ON’ and 128 ‘OFF’ scans, editing pulse bandwidth = 62.7Hz, ‘ON’ editing pulse frequency = 4.56ppm, refocusing pulse bandwidth = 1kHz, 16-step phase cycle (same as PRESS)), PR-STEAM (TM/TE = 10/6.5ms, NEX = 256, RF phases: φ1 = 135°, φ2 = 22.5°, φ13 = 112.5°, φADC = 0°), and SPECIAL (TE = 8ms, NEX = 256, 8-step phase cycle) applied in a randomized order. A TE of 120 ms for the MEGA-PRESS GSH acquisition was chosen based on a recent study comparing GSH quantification by MEGA-PRESS at different TEs in humans (23). The following parameters were identical for all four sequences: TR = 2000 ms, spectral width = 2.5kHz, and 2048 complex points. To minimize chemical shift dispersion, the offset frequency was set to −2.7ppm for the non-editing sequences (PRESS, SPECIAL, and PR-STEAM) and −1.7ppm for MEGA-PRESS. To ensure consistent spectral quality throughout the scan, the shim values were checked between each sequence and adjusted manually when necessary. A water reference (NEX = 16) was acquired with each of the 4 sequences for phase and eddy current correction as well as quantification.
Figure 1.
Three orthogonal T1-weighted images in a human showing voxel placement within the medial frontal lobe including bilateral anterior cingulate.
Analysis
The Siemens TWIX data were exported for each of the four sequences. PRESS and SPECIAL data were frequency and phase corrected prior to quantification using FID-A (24), while PR-STEAM data were frequency and phase corrected using in-house Matlab code. Basis sets for PRESS, SPECIAL, and PR-STEAM were simulated using ideal RF pulses and included the following metabolites: alanine (Ala), aspartate (Asp), creatine (Cr), γ-aminobuytric acid (GABA), glucose (Glc), glutamate (Glu), glutamine (Gln), GSH, glycine (Gly), glycerophosphocholine (GPC), lactate (Lac), myo-Inositol (mI), N-acetylaspartate (NAA), N-acetylaspartylglutamate (NAAG), phosphocholine (PCh), phosphocreatine (PCr), phosphoroylethanolamine (PE), scyllo-Inositol (sI), and taurine (Tau). These basis sets were imported into LCModel (version 6.3-0I) and used for quantification (25). Macromolecule signals in the short TE sequences were handled using LCModel, which automatically includes a basis set of macromolecules and accounts for their presence within the spectrum. In LCModel, wconc was set to 55556 for all sequences so that the results were referenced to tissue water. Also in LCModel, atth2o was set to 0.92 for PR-STEAM and SPECIAL and 0.687 for PRESS, respectively. The phantom data were also analyzed using vitro=T per the LCModel manual. After fitting in LCModel, only GSH levels with Cramér Rao Lower Bounds (CRLB) ≤ 20% were included in statistical analyses. For MEGA-PRESS, data were analyzed using Gannet 3.0 (26), which applied frequency and phase correction to the data prior to fitting. Only GSH/Water ratios with fit errors below 15%, similar to GABA (27), were used for further analyses. GSH levels from all four sequences were corrected for the proportion of gray matter, white matter, and CSF within the spectroscopic voxel using Matlab code based directly on the work of Gasparovic et al. (28). All GSH levels are reported in institutional units.
To test the accuracy of GSH quantification of each of the four sequences, six phantoms were built with varying concentrations of GSH: 0 mM, 1 mM, 1.75 mM, 2.5 mM, 5 mM, and 10 mM. Due to their overlapping resonances with GSH, the following metabolites were also included at neurobiological concentrations as suggested by Govindaraju et al. (9): Asp, choline (Cho), Cr, GABA, Glc, Gln, Gln, mI, and NAA. Each of the six phantoms was scanned with the same localization sequences: PRESS, STEAM, SPECIAL, and MEGA-PRESS in a randomized order. The following parameters were identical for all 4 sequences: TR = 10000 ms, VOI = 24 cm3, NEX = 64, spectral width = 2.5 kHz, and 2048 complex points. All other scan parameters were identical to those used in the human experiments listed previously including a water reference acquisition to be used for phase and eddy current correction as well as quantification. All phantom data were apodized by a 2 Hz exponential function prior to processing in LCModel (PRESS, STEAM, and SPECIAL) or Gannet (MEGA-PRESS). Linear regressions assumed to pass through the origin were computed for each sequence to determine how well the known concentrations of GSH matched with the quantified GSH concentrations from either LCModel or Gannet.
Statistical Analysis
To show that spectral quality was maintained across sequences within and between sessions, a coefficient of variation (CV in %) of shim full-width half maximum (FWHM) values was computed for each spectrum and then, a Wilcoxon test on the paired CVs for session 1 and session 2 was performed to test for differences in spectral quality between sessions with significance set to p<0.05. Differences in GSH levels were assessed via one-way ANOVA with main effect of sequence (significance set at p<0.05). Reproducibility between sessions was assessed via mean CV (in %) and mean absolute difference (AD in %).
RESULTS
Representative spectra acquired from each of the four sequences are shown in Figure 2. Data from all the sequences were of excellent quality. The mean shim FWHM (± standard deviation) for visit 1 and visit 2 were 16.1 ± 0.9 Hz and 16.4 ± 1.1 Hz, respectively, and the CV of the shim FWHM across sequences was 0.60% (range: 0–1.2%) for session 1 and 0.73% (range: 0.33–1.68%) for session 2. The CVs between sessions were not significantly different (p = 0.441); thus, spectral quality was consistent across sessions. Table 1 summarizes mean GSH levels and reproducibility metrics for the four sequences. MEGA-PRESS GSH levels were comparable to PRESS GSH levels (p=0.096), and PR-STEAM and SPECIAL GSH levels were comparable (p=0.90). MEGA-PRESS and PRESS GSH levels were significantly lower (p<0.05) than PR-STEAM and SPECIAL GSH levels. Mean GSH CRLBs (± standard deviations) reported by LCModel were 5.4 ± 0.7% and 5.3 ± 0.4% for PRESS during sessions 1 and 2, 5.2 ± 0.6% and 5.3 ± 0.8% for SPECIAL during sessions 1 and 2, and 4.9 ± 0.3% for PR-STEAM during both sessions. Fit errors reported by Gannet for GSH were 5.6 ± 2.3% for session 1 and 5.6 ± 1.8% for session 2. No GSH data from these three sequences were excluded due to CRLB, and all MEGA-PRESS GSH data were included as well.
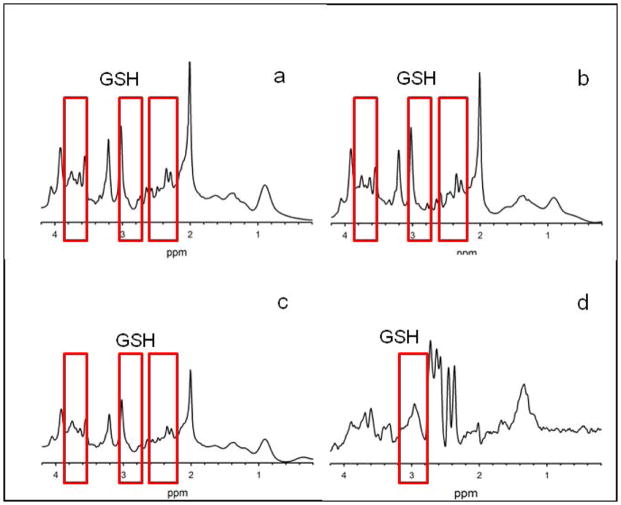
Figure 2.
Representative spectra from one human subject acquired in one session using SPECIAL (a), PRESS (b), PR-STEAM (c), and MEGA-PRESS (d). SPECIAL, PRESS, and PR-STEAM are all scaled identically. Since MEGA-PRESS’s signal is much smaller than the other sequences, the spectrum is scaled in order to maximize the visibility of the peaks within the spectrum (zoomed in x10,000). Data acquired using PRESS, SPECIAL, and PR-STEAM were analyzed using LCModel, and the MEGA-PRESS data was analyzed using Gannet 3.0. A single GSH resonance is observable at 2.95ppm in MEGA-PRESS spectrum (d, highlighted with a red box); whereas, the four GSH resonances at 2.15, 2.53, 2.95, and 3.77 ppm are present in the other three spectra (a–c). These resonances are highlighted with red boxes surrounding the locations for GSH at 3.77pm, 2.95ppm, and a combined box for peaks at 2.15 and 2.53 ppm.
Table 1.
Reproducibility Assessments of GSH for the 4 sequences
| Mean GSH ± SD | Mean CV (%) | Mean AD (%) | ||
|---|---|---|---|---|
| PR-STEAM | 1 | 2.32 ± 0.14 | 5.4 | 7.5 |
| 2 | 2.26 ± 0.18 | |||
| SPECIAL | 1 | 2.37 ± 0.13 | 8.0 | 10.1 |
| 2 | 2.23 ± 0.41 | |||
| PRESS | 1 | 1.69 ± 0.18 | 5.8 | 8.2 |
| 2 | 1.70 ± 0.09 | |||
| MEGA-PRESS | 1 | 1.99 ± 0.32 | 13.5 | 17.1 |
| 2 | 1.76 ± 0.41 |
AD – absolute difference, CV – coefficient of variation, SD – standard deviation
In terms of mean CV, PR-STEAM had the lowest CV of 5.4% followed by PRESS of 5.8%, SPECIAL of 8%, and finally MEGA-PRESS with a CV of 13.5%. For mean AD, PR-STEAM had the lowest AD of 7.5% followed by PRESS (8.2%) and SPECIAL (10.1%). MEGA-PRESS had the highest mean AD of 17.1%.
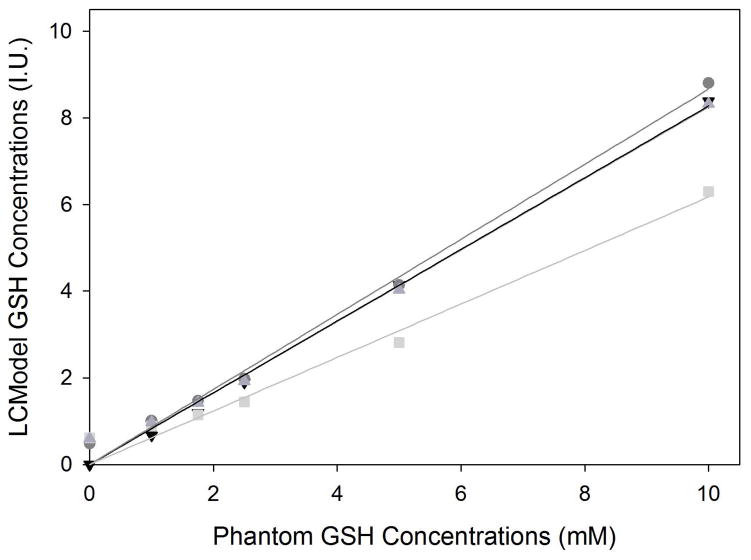
For the phantom experiments, the mean shim FWHM for the six phantoms was 3.5 ± 0.1 Hz. Actual GSH concentrations versus quantified GSH concentrations and resultant linear regressions are shown for SPECIAL, PRESS, and PR-STEAM, and MEGA-PRESS in Figure 3. Plots of the phantom data acquired using the four different sequences are shown in Figure 4. As expected, CRLBs decreased as the concentration of GSH increased. PRESS had the largest deviations between actual and quantified GSH levels while PR-STEAM, SPECIAL, and MEGA-PRESS were comparable in terms of the actual versus quantified GSH levels with one exception at 0 mM. At this concentration, PRESS, PR-STEAM, and SPECIAL all detected trace amounts of GSH whereas MEGA-PRESS reported no GSH concentration. Overall, linear fits between actual GSH concentration and quantified GSH concentration were excellent (see Table 2). MEGA-PRESS linear regression fits were the best out of the four sequences with R2=0.998 followed by SPECIAL, PRESS, and PR-STEAM R2 values of 0.996, 0.987, and 0.995, respectively.
Figure 3.
Plots of actual versus quantified GSH levels from phantoms with varying concentrations of GSH are shown for each of the 4 sequences: SPECIAL (
 ), PR-STEAM (
), PR-STEAM (
 ), PRESS (
), PRESS (
 ), and MEGA-PRESS (▼). At higher GSH levels, the sequences were similar in their ability to detect GSH. From the 0mM GSH phantom, MEGA-PRESS was the only sequence to report 0mM while the other non-edited sequences reported trace levels of GSH. The linear regressions for SPECIAL, MEGA-PRESS and PR-STEAM were similar such that MEGA-PRESS and PR-STEAM linear regression lines overlapped. Overall, linear fits to the data were excellent with R2 values of 0.996, 0.995, 0.987, and 0.998, respectively.
), and MEGA-PRESS (▼). At higher GSH levels, the sequences were similar in their ability to detect GSH. From the 0mM GSH phantom, MEGA-PRESS was the only sequence to report 0mM while the other non-edited sequences reported trace levels of GSH. The linear regressions for SPECIAL, MEGA-PRESS and PR-STEAM were similar such that MEGA-PRESS and PR-STEAM linear regression lines overlapped. Overall, linear fits to the data were excellent with R2 values of 0.996, 0.995, 0.987, and 0.998, respectively.
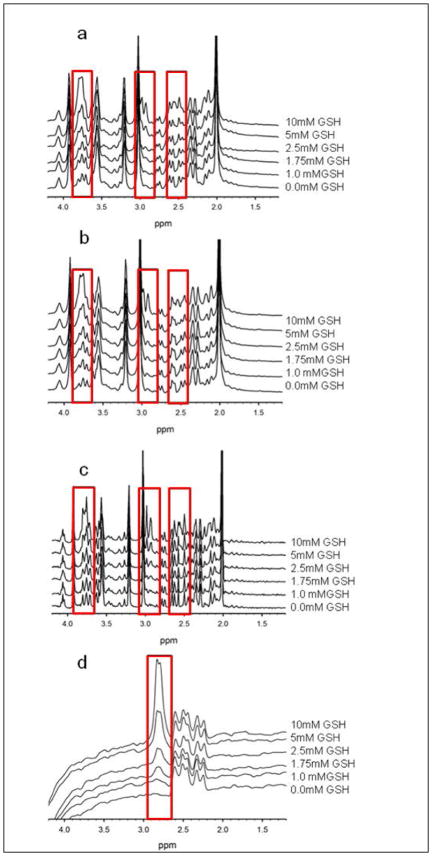
Figure 4.
Spectra from each phantom with GSH levels of 0.0mM, 1mM, 1.75mM, 2.5mM, 5mM, and 10mM acquired using the following sequences: SPECIAL (a), PRESS (b), PR-STEAM (c), and MEGA-PRESS (d). The red boxes indicate the location of the multiple GSH resonances detectable in SPECIAL, PR-STEAM, and PRESS and the single GSH resonance observed in MEGA-PRESS. These boxes further highlight the visible increase in GSH levels with increasing GSH concentration with the phantoms.
Table 2.
Linear Regression Coefficients from GSH Phantom Data
| βGSH ± SE | t | p | R2 | |
|---|---|---|---|---|
| PR-STEAM | 0.825 ± 0.025 | 33.294 | <0.001 | 0.995 |
| SPECIAL | 0.866 ± 0.023 | 38.212 | <0.001 | 0.996 |
| PRESS | 0.617 ± 0.029 | 21.621 | <0.001 | 0.987 |
| MEGA-PRESS | 0.827 ± 0.014 | 59.566 | <0.001 | 0.998 |
SE – standard error
DISCUSSION
In this study, we compared the reproducibility of four commonly used techniques to quantify cerebral GSH in vivo. Of the four techniques, PR-STEAM had the lowest mean CV and AD followed by PRESS, SPECIAL, MEGA-PRESS. Short TE sequences had better reproducibility in humans compared to spectral editing since MEGA-PRESS had the highest mean CV and AD. Phantom data showed excellent linear regression fits for all four sequences with MEGA-PRESS having the best overall fit and PRESS having the lowest fit. Overall, these data suggest that GSH can be accurately and reproducibly quantified using any of the methods tested, either with or without the use of spectral editing.
The choice to use spectral editing for GSH detection is generally based on the notion that spectral editing will improve reproducibility over conventional methods – such as PRESS – wherein the GSH peaks are not visible to the naked eye. The findings of this study suggest the opposite: that unedited short-TE MRS approaches provide more reproducible GSH quantification compared to spectral editing. The improved reproducibility is likely attributable to two factors. Firstly, the use of short TEs simultaneously reduces J-coupling dependent phase dispersion and T2 relaxation, which improves SNR. Second, while spectral editing optimizes the detection of a single GSH resonance at 2.95 ppm, conventional MRS allows efficient detection of four distinct GSH resonances (at 2.15, 2.53, 2.95 and 3.77 ppm). This effectively improves SNR further, while also improving the linear independence of the GSH signal from the multiple overlapping resonances.
Similar to a previous study (21), we also found notable differences in GSH measurements from the metabolite phantoms using four different localization sequences. In the MEGA-PRESS phantom data, no observable GSH was quantified in the phantom containing 0mM of GSH. The MEGA-PRESS sequence is optimized to detect only the 2.95ppm resonance of GSH and to remove all overlapping resonances or macromolecules; thus, when GSH is absent, there are no signals at 2.95 ppm, and the fitting software correctly reports no concentration. In contrast, the three non-spectral editing techniques detected GSH of ~0.5mM in the phantom containing 0mM of GSH, suggest systematic measurement errors when the actual GSH concentrations are very low. The analysis of these three short-TE sequences involves fitting the entire spectrum which contains resonances from GSH at 2.15, 2.53, 2.95, and 3.77 ppm, as well as resonances from many other metabolites (in phantoms and humans) and macromolecules (in humans only) that overlap with GSH. Fitting multiple peaks theoretically improves the detection and fitting accuracy as evidenced by low CRLB. In this study, we opted to keep GSH in the LCModel basis set when analyzing phantom data even though GSH was not present in the 0mM phantom for consistency. However, LCModel will attempt to fit all metabolites in the provided basis set and adjust the fits of other metabolites to do so. This potentially led to the overestimation of GSH in the 0mM GSH phantom and highlights a potential measurement bias at lower levels of GSH. This may also be partly due to the fact that it is not possible for LCModel to return negative concentration values; so, whereas LCModel can either over- or under-estimate the concentration in the non-zero GSH phantoms, in the somewhat unrealistic scenario of a zero GSH concentration, LCModel can, in the presence of errors, only overestimate GSH levels. This fact is supported by the observation that, at all other GSH concentrations, GSH measurements were comparable between MEGA-PRESS, SPECIAL, and PR-STEAM with PRESS being the poorest. The potential implication of these results is that when using a MRS technique to study to a specific illness or disease, one may consider the dynamic range of how GSH levels are expected to change. Based on the phantom work, potential changes that may result in substantially depleted GSH or an absolute measurement of GSH is required may be best detected using MEGA-PRESS even with the slightly worse reproducibility. Despite the systematic errors inherent to the non-editing techniques at very low GSH concentrations, all four sequences particularly very short TE sequences may be considered for other conditions where GSH is expected to increase or decrease.
While all four sequences had excellent linear fits of R2 ≥ 0.98 for the phantom data, there were some differences between them in terms of slope. As noted previously, at lower concentrations of GSH, MEGA-PRESS was able to correctly identify 0mM of GSH within a phantom, but at higher GSH concentrations, all four sequences were comparable. When the linear regression model was restricted to pass through the origin such that the actual 0mM GSH concentration resulted in no quantifiable GSH, the slopes or βGSH for MEGA-PRESS, SPECIAL, and PR-STEAM were very similar (0.827, 0.866, and 0.825). PRESS, on the other hand, had a slope or βGSH of 0.617, suggesting that it may be less sensitive to detecting changes in GSH. If we did not restrict the y-intercept and examine the three sequences that overestimated 0mM GSH concentration, the y-intercepts were all not significant (p>0.26); thus, the initial starting value had little influence on the slope or potentially the sensitivity of the measurements. Overall, while the three non-edited sequences overestimate 0mM GSH, the bias in the GSH measurements appears to only be at non-physiological concentrations of GSH. At more physiologically relevant concentrations of GSH, MEGA-PRESS, SPECIAL, and PR-STEAM appear to be more sensitive than PRESS in phantoms.
There are several factors that may contribute to the significantly higher PR-STEAM and SPECIAL GSH levels compared to PRESS. First, the very short TEs of PR-STEAM and SPECIAL leads to less signal loss due to J-coupling and T2 relaxation compared to PRESS, which may lead to the higher GSH levels. Second, as evidenced with the phantom data, PRESS was less sensitive to detecting GSH than PR-STEAM and SPECIAL, which could lead to the lower GSH levels observed with PRESS. Even though PRESS had very good reproducibility which was comparable to PR-STEAM and SPECIAL in humans, it appears as though these measurements may consistently underestimate GSH levels.
While the mean GSH levels reported in this study varied according to the localization sequence used, each sequence has GSH levels similar to previously published studies. When compared to previous studies using PR-STEAM in healthy controls, the GSH levels or GSH/tCr levels are within the 95% confidence intervals of the levels reported (13,14). Even though the voxel size and partial volume correction technique varied, the PRESS GSH levels reported here are similar to those in healthy volunteers in another study with the voxel placed in the ventral anterior cingulate (2), and are slightly lower than the mean GSH levels from the dorsomedial prefrontal cortex in healthy controls (29) or from the left anterior cingulate in healthy controls (21). When calculated relative to total creatine, GSH/tCr levels in this study were similar to or slightly higher than healthy control GSH/tCr levels measured using SPECIAL from the medial prefrontal cortex (3). In terms of MEGA-PRESS GSH values, Chan et al. reported GSH integrals, which were integrals normalized to the summation of the integrals per subject and averaged across all subjects (23) while Sanaei Nezhad et al. reported GSH levels relative to water (21). In contrast, Terpstra et al. reported GSH levels normalized to an assumed NAA concentration of 10 mM (20,30) and An et al. reported GSH levels normalized to 8 mM of creatine (22). Due to differences in concentration referencing (relative to water versus relative to a specific metabolite), voxel placement, basis set modeling, and quantification, direct comparisons between our GSH levels and previously published levels are difficult; however, our GSH levels using MEGA-PRESS are slightly lower than Sanaei Nezhad et al., but still within the nominal range. Nevertheless, the GSH levels reported here are within similar ranges reported by Govindaraju et al. and Cooper et al. (9,31), suggesting that these are valid metrics to quantify GSH.
To our knowledge, there are very few reproducibility studies that reported GSH levels at 3T in the frontal lobe. Three previous studies that assessed reproducibility in the anterior cingulate cortex or frontal lobe using PRESS at 3T did not report GSH levels or reproducibility metrics (32–34). In terms of the PR-STEAM sequence, the mean CV and mean AD for GSH quantified in this study from the medial frontal lobe were very similar to a previous study in the anterior cingulate (14). While not in the anterior cingulate, two other studies using short TE semi-LASER in the hippocampus and posterior cingulate of healthy adults scanned twice either one month or one week apart showed that GSH was reproducibly measured with CVs of approximately 18% and 10%, respectively (35,36). Thus, the mean CVs reported in this study for PRESS, SPECIAL, and PR-STEAM are comparable or slightly better than previously published reports from different brain regions.
Our finding of improved reproducibility of PRESS over MEGA-PRESS in humans contrasts to a previous study (21) that suggested using spectral editing over PRESS to measure GSH at 3T especially for GSH levels below 4mM. There are several major differences between this previous study and our study. First, GSH coupling constants differed such that the previous study used GSH-glycine couplings from Kaiser et al. (37), which suggest simulating this resonance as a doublet. In contrast, this study used the GSH-glycine couplings from Govindaraju et al. (9), which suggests simulating this resonance as a singlet. We quantified the data with a simulated basis set with coupling constants from Kaiser et al. and observed comparable CVs and R2 to those from data fit using a basis set with Govindaraju et al. couplings presented here. Second, two different programs were used to simulate the basis sets used for quantification (NMRSCOPE versus VESPA) and the composition of both basis sets differed. Third, the RF pulse timings, editing pulse bandwidths, and TEs differed as well. Here, we used the Siemens MEGA-PRESS work-in-progress (WIP) sequence whereas the other study used a sequence for a Philips scanner. Fourth, the software used to quantify GSH from MEGA-PRESS differed as well (AMARES versus Gannet). All of these differences could explain why our PRESS phantom fits differed greatly from the other study’s PRESS phantom fits. Despite these differences in findings, this work suggests that short TEs sequences are more reproducible than MEGA-PRESS.
The TE chosen for the MEGA-PRESS acquisition in this study was based on the comparison work of An et al. in phantoms (22) and Chan et al. in both phantoms and humans (23). In phantoms, An et al. showed that the maximal GSH peak amplitude occurred at 131 ms TE and not at 70 ms TE using J-PRESS (22) while Chan et al. showed that the SNR and quantification of GSH was better with a TE of 120 ms, in contrast to the 68 ms TE suggested by Terpstra et al. (20). There is a possibility that the reproducibility of MEGA-PRESS with a TE of 68 ms may be better than a TE of 120ms. However, since we did not acquire MEGA-PRESS with a TE of 68ms, we cannot comment on the reproducibility.
There are several limitations to this study. The macromolecule background is observable in the very short and short TE sequences used here. Individual metabolite-nulled spectra were not acquired for each sequence in each person in the interest of time available to complete the study and to maintain subject comfort throughout the study. However, macromolecule signals are taken into account since LCModel incorporates a macromolecule basis set when fitting. Further, studies by Schaller et al and Cudalbu et al concluded that using a simulated basis set is adequate for quantification (38,39). Future studies are warranted to study the impact of individualized macromolecule spectra on fitting. Another limitation is that reproducibility was only assessed in one brain region and may not translate to other regions of the brain. A third limitation is that different software packages were used to quantify GSH. Gannet was used for the MEGA-PRESS data, and LCModel was used for all other data since these two packages are frequently used in the field of MRS for edited and non-edited data, respectively. By using two different quantification packages, it is unclear from our data whether the accurate detection of 0 mM GSH in a phantom by MEGA-PRESS and over-estimation of GSH by the three other sequences is due to the localization technique or the quantification software. More studies are needed to compare fitting algorithms. Reassuringly, the overall linear fits for actual versus quantified GSH concentrations across the four sequences are very similar, especially at neurobiologically relevant concentrations of GSH.
In conclusion, reproducible quantification of GSH can be achieved without the use of spectral editing techniques, and in fact, the data presented here suggests that non-editing techniques are comparable or better than a spectral editing technique.
Acknowledgments
Grant Support: This study was supported by the National Institute Health: R01MH094520
References
- 1.Wijtenburg SA, Wright SN, Korenic SA, et al. Altered glutamate and regional cerebral blood flow levels in schizophrenia: A 1H-MRS and pCASL study. Neuropsychopharmacology. 2017;42:562–571. doi: 10.1038/npp.2016.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lagopoulos J, Hermens DF, Tobias-Webb J, et al. In vivo glutathione levels in young persons with bipolar disorder: A magnetic resonance spectroscopy study. J Psychiatr Res. 2013;47:412–417. doi: 10.1016/j.jpsychires.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Godlewska BR, Yip SW, Near J, Goodwin GM, Cowen PJ. Cortical glutathione levels in young people with bipolar disorder: a pilot study using magnetic resonance spectroscopy. Psychopharmacology. 2014;231:327–332. doi: 10.1007/s00213-013-3244-0. [DOI] [PubMed] [Google Scholar]
- 4.Soeiro-de-Souza MG, Pasterello BF, da Leite CC, Henning A, Moreno RA, Garcia Otaduy MC. Dorsal anterior cingulate lactate and glutathione levels in euthymic bipolar I disorder: 1H-MRS Study. Int J Neuropsychopharmacol. 2016;19:1–8. doi: 10.1093/ijnp/pyw032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Godlewska BR, Near J, Cowen PJ. Neurochemistry of major depression: a study using magnetic resonance spectroscopy. Psychopharmacology. 2015;232:501–507. doi: 10.1007/s00213-014-3687-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brennan BP, Jensen JE, Perriello C, et al. Lower posterior cingulate cortex glutathione levels in obsessive-compulsive disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1:116–124. doi: 10.1016/j.bpsc.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mandal PK, Saharan S, Tripathi M, Murari G. Brain glutathione levels--a novel biomarker for mild cognitive impairment and Alzheimer’s disease. Biol Psychiatry. 2015;78:702–710. [Google Scholar]
- 8.Weiduschat N, Mao X, Hupf J, et al. Motor cortex glutathione deficit in ALS measured in vivo with the J-editing technique. Neurosci Lett. 2014;570:102–107. doi: 10.1016/j.neulet.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 9.Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13:129–153. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 10.Bottomley PA. Spatial localization in NMR spectroscopy in vivo. Ann N Y Acad Sci. 1987;508:333–348. doi: 10.1111/j.1749-6632.1987.tb32915.x. [DOI] [PubMed] [Google Scholar]
- 11.Duffy SL, Lagopoulos J, Cockayne N, Hermens DF, Hickie IB, Naismith SL. Oxidative stress and depressive symptoms in older adults: A magnetic resonance spectroscopy study. J Affect Disord. 2015;180:29–35. doi: 10.1016/j.jad.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Hennig J. The application of phase rotation for localized in vivo proton spectroscopy with short echo times. J Magn Reson. 1992;96:40–49. [Google Scholar]
- 13.Wijtenburg SA, Knight-Scott J. Very short echo time improves the precision of glutamate detection at 3T in 1H magnetic resonance spectroscopy. J Magn Reson Imaging. 2011;34:645–652. doi: 10.1002/jmri.22638. [DOI] [PubMed] [Google Scholar]
- 14.Wijtenburg SA, Gaston FE, Spieker EA, et al. Reproducibility of phase rotation STEAM at 3T: Focus on glutathione. Magn Reson Med. 2014;72:603–609. doi: 10.1002/mrm.24959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knight-Scott J, Shanbhag DD, Dunham SA. A phase rotation scheme for achieving very short echo times with localized stimulated echo spectroscopy. Magn Reson Imaging. 2005;23:871–876. doi: 10.1016/j.mri.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Mekle R, Mlynarik V, Gambarota G, Hergt M, Krueger G, Gruetter R. MR spectroscopy of the human brain with enhanced signal intensity at ultrashort echo times on a clinical platform at 3T and 7T. Magn Reson Med. 2009;61:1279–1285. doi: 10.1002/mrm.21961. [DOI] [PubMed] [Google Scholar]
- 17.Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998;11:266–272. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 18.Matsuzawa D, Obata T, Shirayama Y, et al. Negative correlation between brain glutathione level and negative symptoms in schizophrenia: a 3T 1H-MRS study. PLoS One. 2008;3:e1944. doi: 10.1371/journal.pone.0001944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raschke F, Noeske R, Dineen RA, Auer DP. Measuring cerebral and cerebellar glutathione in children using (1)H MEGA-PRESS MRS. Am J Neuroradiol. 2018;39:375–379. doi: 10.3174/ajnr.A5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terpstra M, Vaughan TJ, Ugurbil K, Lim KO, Schulz SC, Gruetter R. Validation of glutathione quantitation from STEAM spectra against edited 1H NMR spectroscopy at 4T: application to schizophrenia. Magma. 2005;18:276–282. doi: 10.1007/s10334-005-0012-0. [DOI] [PubMed] [Google Scholar]
- 21.Sanaei Nezhad F, Anton A, Parkes LM, Deakin B, Williams SR. Quantification of glutathione in the human brain by MR spectroscopy at 3 Tesla: Comparison of PRESS and MEGA-PRESS. Magn Reson Med. 2017;78:1257–1266. doi: 10.1002/mrm.26532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.An L, Zhang Y, Thomasson DM, et al. Measurement of glutathione in normal volunteers and stroke patients at 3T using J-difference spectroscopy with minimized subtraction errors. J Magn Reson Imaging. 2009;30:263–270. doi: 10.1002/jmri.21832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan KL, Puts NA, Snoussi K, Harris AD, Barker PB, Edden RA. Echo time optimization for J-difference editing of glutathione at 3T. Magn Reson Med. 2017;77:498–504. doi: 10.1002/mrm.26122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simpson R, Devenyi GA, Jezzard P, Hennessy TJ, Near J. Advanced processing and simulation of MRS data using the FID appliance (FID-A)-An open source, MATLAB-based toolkit. Magn Reson Med. 2017;77:23–33. doi: 10.1002/mrm.26091. [DOI] [PubMed] [Google Scholar]
- 25.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 26.Edden RA, Puts NA, Harris AD, Barker PB, Evans CJ. Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. J Magn Reson Imaging. 2014;40:1445–1452. doi: 10.1002/jmri.24478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rowland LM, Krause BW, Wijtenburg SA, et al. Medial frontal GABA is lower in older schizophrenia: a MEGA-PRESS with macromolecule suppression study. Mol Psychiatry. 2016;21:198–204. doi: 10.1038/mp.2015.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gasparovic C, Song T, Devier D, et al. Use of tissue water as a concentration reference for proton spectroscopic imaging. Magn Reson Med. 2006;55:1219–1226. doi: 10.1002/mrm.20901. [DOI] [PubMed] [Google Scholar]
- 29.Durieux AM, Horder J, Mendez MA, et al. Cortical and subcortical glutathione levels in adults with autism spectrum disorder. Autism Res. 2016;9:429–435. doi: 10.1002/aur.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Terpstra M, Henry PG, Gruetter R. Measurement of reduced glutathione (GSH) in human brain using LCModel analysis of difference-edited spectra. Magn Reson Med. 2003;50:19–23. doi: 10.1002/mrm.10499. [DOI] [PubMed] [Google Scholar]
- 31.Cooper AJ, Kristal BS. Multiple roles of glutathione in the central nervous system. Biol Chem. 1997;378:793–802. [PubMed] [Google Scholar]
- 32.Mullins PG, Chen H, Xu J, Caprihan A, Gasparovic C. Comparative reliability of proton spectroscopy techniques designed to improve detection of J-coupled metabolites. Magn Reson Med. 2008;60:964–969. doi: 10.1002/mrm.21696. [DOI] [PubMed] [Google Scholar]
- 33.Wellard RM, Briellmann RS, Jennings C, Jackson GD. Physiologic variability of single-voxel proton MR spectroscopic measurements at 3T. Am J Neuroradiol. 2005;26:585–590. [PMC free article] [PubMed] [Google Scholar]
- 34.de Matos NM, Meier L, Wyss M, et al. Reproducibility of neurochemical profile quantification in pregenual cingulate, anterior midcingulate, and bilateral posterior insular subdivisions measured at 3 Tesla. Front Hum Neurosci. 2016;10:300. doi: 10.3389/fnhum.2016.00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bednarik P, Moheet A, Deelchand DK, et al. Feasibility and reproducibility of neurochemical profile quantification in the human hippocampus at 3 T. NMR Biomed. 2015;28:685–693. doi: 10.1002/nbm.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Terpstra M, Cheong I, Lyu T, et al. Test-retest reproducibility of neurochemical profiles with short-echo, single-voxel MR spectroscopy at 3T and 7T. Magn Reson Med. 2016;76:1083–1091. doi: 10.1002/mrm.26022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaiser LG, Marjanska M, Matson GB, et al. (1)H MRS detection of glycine residue of reduced glutathione in vivo. J Magn Reson. 2010;202:259–266. doi: 10.1016/j.jmr.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schaller B, Xin L, Cudalbu C, Gruetter R. Quantification of the neurochemical profile using simulated macromolecule resonances at 3 T. NMR Biomed. 2013;26:593–599. doi: 10.1002/nbm.2896. [DOI] [PubMed] [Google Scholar]
- 39.Cudalbu C, Mlynarik V, Xin L, Gruetter R. Quantification of in vivo short echo-time proton magnetic reosnance spectra at 14. 1 T using two different approaches of modeling the macromolecule spectrum. Meas Sci Technol. 2009;20:104034. [Google Scholar]