Abstract
Body fat distribution contributes to obesity-related metabolic and cardiovascular disorders. Visceral fat is more detrimental than subcutaneous fat. However, the mechanisms underlying visceral fat-mediated cardiometabolic dysregulation are not completely understood. Localized increases in expression of the renin angiotensin system (RAS) in adipose tissue (AT) may be implicated. We therefore investigated mRNA and protein expression of RAS components in visceral versus subcutaneous AT using paired samples from individuals undergoing surgery (N = 20, body mass index: 45.6 ± 6.2 kg/m2, and age: 44.6 ± 9.1 years). We also examined RAS-related proteins in AT obtained from individuals on renin angiotensin aldosterone system (RAAS) targeted drugs (N = 10, body mass index: 47.2 ± 9.3 kg/m2, and age: 53.3 ± 10.1 years). Comparison of protein expression between subcutaneous and visceral AT samples showed an increase in renin (p = 0.004) and no change in angiotensinogen (p = 0.987) expression in visceral AT. Among proteins involved in angiotensin peptide generation, angiotensin converting enzyme (p = 0.02) was increased in subcutaneous AT while chymase (p = 0.001) and angiotensin converting enzyme-2 (p = 0.001) were elevated in visceral fat. Furthermore, visceral fat expression of angiotensin II type-2 receptor (p = 0.007) and angiotensin II type-1 receptor (p = 0.031) was higher, and MAS receptor (p < 0.001) was lower. Phosphorylated-p53 (p = 0.147), AT fibrosis (p = 0.138) and average adipocyte size (p = 0.846) were similar in the two depots. Nonetheless, visceral AT showed increased mRNA expression of inflammatory (TNFα, p < 0.001; IL-6, p = 0.001) and oxidative stress markers (NOX2, p = 0.038; NOX4, p < 0.001). Of note, mRNA and protein expression of RAS components did not differ between subjects taking or not taking RAAS related drugs. In summary, several RAS related proteins are differentially expressed in subcutaneous versus visceral AT. This differential expression may not alter AngII but likely increases Ang1-7 generation in visceral fat. These potential differences in active angiotensin peptides and receptor expression in the two depots suggest that localized RAS may not be involved in differences in visceral vs subcutaneous AT function in obese individuals. Our findings do not support a role for localized RAS differences in visceral fat-mediated development of cardiovascular and metabolic pathology.
Keywords: obesity, fat distribution, visceral fat, renin-angiotensin system, angiotensin, chymase, Mas receptor
Introduction
Obesity results in heightened vulnerability to cardiovascular and metabolic disorders (Saydah et al., 2014). Among the obesity-related factors which contribute to adverse cardiometabolic effects, accumulation of visceral fat is critical. The role of visceral fat in cardiovascular and metabolic disease is suggested by cross-sectional studies and supported by experimental studies of weight gain (Fox et al., 2007; Orr et al., 2008; Mahabadi et al., 2009; Parikh et al., 2009; Liu et al., 2010; Romero-Corral et al., 2010; Chandra et al., 2014; Covassin et al., 2018). However, the molecular mechanisms mediating the pathological effects of visceral fat are not completely understood (Tchernof and Despres, 2013).
The renin angiotensin system (RAS) is a systemic hormone system which regulates blood pressure and plays a role in energy homeostasis (Marcus et al., 2013; Littlejohn and Grobe, 2015; Chappell, 2016). RAS constitutes a complex cascade of pathways through which AGT is converted to form bioactive peptides, AngII and angiotensin 1–7 (Ang1-7), via multistep-enzymatic processes including renin, ACE, chymase, and ACE homolog-2 (ACE2) (Chappell, 2016). These peptides mediate their cellular effects through AngII type-1 receptor (AT1R), AngII type-2 receptor (AT2R), and MASR. Of note, the AngII and Ang1-7 peptides have effects which are antagonistic to each other. AngII is proinflammatory, profibrotic and has vasoconstrictive effects, while Ang1-7 is anti-inflammatory, anti-fibrotic and has vasodilatory effects (Simoes e Silva et al., 2013). Similar contrasting effects of AngII peptide are also evident in cells based on activation of AT1R versus AT2R (Akazawa et al., 2013). Among the cellular signaling pathways, p53 plays a prominent role in RAS. Activation of p53 increases AGT and AT1R expression (Leri et al., 1998, 2000; Fiordaliso et al., 2001). Conversely, AngII activates p53 pathway to mediate its downstream cellular effects (Grishko et al., 2003; Liu et al., 2009; Guan et al., 2013). Furthermore, p53 has been shown to play an important role in oxidative stress, insulin signaling, angiogenesis, apoptosis, inflammation, and fibrosis in different cells including AT (Minamino et al., 2009; Shimizu et al., 2012; Ghosh et al., 2013; Gogiraju et al., 2015).
Obesity is associated with increased systemic RAS activation (Sarzani et al., 2008). Several animal studies demonstrate increases in RAS with high fat feeding and development of metabolic disorders (Favre et al., 2015; Littlejohn and Grobe, 2015). Conversely, blocking RAS via targeting AT1R, ACE1 activity, and/or (pro) renin receptor induces weight loss along with decreases in visceral obesity (Lee et al., 2008; Mathai et al., 2008; de Kloet et al., 2009; Favre et al., 2015; Littlejohn and Grobe, 2015; Tan et al., 2016; Azushima et al., 2017). Indeed, weight loss in humans is also associated with reduced RAS activity (Engeli et al., 2005; Wang et al., 2012). However, use of RAS targeted drugs has been largely shown to have no effect on body weight in clinical trials (Nedogoda et al., 2013; Littlejohn and Grobe, 2015). Of note, the role of AT1R in obesity-related pathology is evident in studies showing that RAS inhibitors improve glucose metabolism and reduce diabetes incidence in patients with metabolic syndrome (Investigators et al., 2006; Group et al., 2010). Among the factors contributing to increased RAS in obesity, the paracrine contribution of AT to systemic RAS has been suggested (Sarzani et al., 2008; Marcus et al., 2013). Several RAS components are present in AT where they locally regulate the micro-environment and thereby alter AT function (Sarzani et al., 2008; Marcus et al., 2013). AngII activation of the AT1R causes increases in adipocyte size, AT inflammation, oxidative stress, fibrosis, and decreases insulin sensitivity (Furuhashi et al., 2004; Azushima et al., 2017) while AngII activation of the AT2R can cause browning of the white AT, and decreases inflammation (Than et al., 2017). Similarly, increasing circulating Ang1-7 has been also shown to improve glucose and lipid metabolism, attenuate AT inflammation induced by a high fat diet, and also decreases abdominal fat mass (Santos et al., 2010, 2012; Liu et al., 2012; Schuchard et al., 2015).
Considering the varied and opposing roles of RAS in AT, differential expression of RAS components in regional fat depots may underlie the pathological effects of visceral obesity (Marcus et al., 2013; Littlejohn and Grobe, 2015). Hence we first examined the expression of RAS components in paired abdominal subcutaneous versus visceral AT. We hypothesized that compared to subcutaneous AT, visceral fat will have increased expression of RAS proteins involved in the pro-fibrotic AngII/AT1R pathway but attenuated expression of proteins mediating the anti-fibrotic Ang1-7/MASR pathway. We also determined the activation of the p53 signaling pathway, fibrosis, inflammation, oxidative stress, and adipocyte size in the subcutaneous versus visceral fat depots to evaluate potential detrimental downstream effects of RAS activation. Since RAAS targeted drugs are therapeutically used for hypertension, we next evaluated the effects of RAAS-targeted drugs on AT expression of RAS-related proteins in subcutaneous and visceral fat. We hypothesized that use of RAAS-targeted drugs will attenuate the expression of proteins associated with detrimental effects of RAS in AT.
Materials and Methods
Human Subjects
Adipose tissue samples obtained from 20 subjects (19 females) undergoing bariatric surgery were used to test our hypothesis. Pre-surgery clinical records were reviewed to obtain demographics and clinical information. Presence of diabetes, hypertension, and dyslipidemia was identified by clinical notes or medications. Sleep apnea was identified by overnight oximetry, polysomnography or clinical notes related to sleep apnea treatment. Additional AT samples from 10 subjects (6 females) undergoing bariatric surgery and prescribed RAAS targeted drugs were examined for depot specific alterations in AT associated with RAAS targeted therapy. Of these 10 subjects, 4 subjects were prescribed an ACE inhibitor (lisinopril), 4 subjects were taking an angiotensin receptor blocker (losartan), and 2 subjects were prescribed an aldosterone receptor antagonist (spironolactone). Demographics of the study population are detailed in Table 1. The study was approved by the Institutional Review Board and written informed consent was obtained from all subjects.
Table 1.
Characteristics of the study subjects.
| No prescription of RAAS related drugs (N = 20) | Prescribed RAAS targeted drugs (N = 10) | |
|---|---|---|
| Age [years] | 44.6 ± 9.1 | 53.3 ± 10.1∗ |
| Weight [kg] | 126.5 ± 22.8 | 132.8 ± 37.8 |
| Body Mass Index [kg/m2] | 45.6 ± 6.2 | 47.2 ± 9.3 |
| Diabetes, n [%] | 7 [35%] | 6 [60%] |
| Hypertension, n [%] | 10 [50%] | 10 [100%]# |
| Dyslipidemia, n [%] | 12 [60%] | 5 [50%] |
| Sleep Apnea, n [%] | 14 [70%] | 9 [90%] |
For continuous variables, data presented as mean ± SD. ∗P < 0.05 as determined by Wilcoxon Rank Sums Test. #P < 0.05 as determined by 2-tailed Fisher’s Exact Test.
Visceral (omental) and subcutaneous fat samples were endoscopically obtained during surgery. Biopsy samples were immediately brought to the lab, aliquoted for mRNA analysis, Western blot analysis and paraffin sectioning, and stored at -80°C for batched analysis.
mRNA Analysis
Transcription of RAS components, inflammation and oxidative stress markers in the two fat depots was determined by standard reverse-transcription PCR using commercially available TaqMan probes (Applied Biosystems, Foster City, CA, United States). Briefly, AT was homogenized in Trizol reagent (Life Technologies, Carlsbad, CA, United States) and centrifuged to remove lipid layer. RNA containing aqueous phase was collected after treatment with chloroform. Total RNA from the aqueous phase was precipitated using ethanol and purified using PureLink RNA isolation kit (Life Technologies, Carlsbad, CA, United States). cDNA library was created using high-capacity cDNA reverse transcription kit (Applied Biosystems) for semi-quantitative determination of specific mRNA using the following commercial TaqMan probes per manufacturer instructions: AGT (Hs01586213_m1), renin (Hs00982555_m1), ACE (Hs00174179_m1), chymase (Hs01095979_g1), ACE2 (Hs01085333_m1), AT1R (Hs00258938_m1), AT2R (Hs02621316_s1), MASR (Hs00267157_s1), pro-renin receptor (Hs00997145_m1), p53 (Hs01034249_m1), IL-6 (Hs00174131_m1), TNFα (Hs00174131_m1), NOX2 (Hs00166163_m1), NOX4 (Hs01379108_m1), and GAPDH (Hs02786624_g1). Data were analyzed by comparative CT method using GAPDH as endogenous control (Schmittgen and Livak, 2008).
Western Blot Analysis
The expression of RAS related proteins in the two depots was determined by standard Western blot analysis. Briefly, AT was pulverized in liquid nitrogen, suspended in RIPA buffer (Thermo Fisher Scientific Inc., Waltham, MA, United States) containing protease inhibitor cocktail (Millipore Sigma, Burlington, MA, United States) followed by centrifugation to remove lipid layer and insoluble pellet. Equal amount of protein from each sample was separated by PAGE and transferred onto PVDF membrane. The membrane were blocked with 5% non-fat milk and incubated overnight at 4°C with specific primary antibody for chymase (sc-59589, Santa Cruz Biotechnology, Dallas, TX, United States), AGT (origene-CF804670, OriGene Technologies Inc., Rockville, MD, United States), renin (sc-22752), ACE (ab28311, Abcam, Cambridge, MA, United States), ACE2 (MABN59, Millipore Sigma), AT2R (sc-9040), AT1R (ab124734), MASR (sc-54848, phospho-p53 (ab1431), p53 (9282s, Cell Signaling Technology, Danvers, MA, United States), and GAPDH (endogenous control, ab8245). After incubation with specific primary antibody, the membrane was washed and incubated with appropriate secondary antibodies, washed and developed using Luminata Forte Western HRP substrate (WBLUF0100, Millipore Sigma). Images were acquired with Odyssey Fc Image System (LI-COR Corporate, Lincoln, NE, United States) and analyzed using LI-COR Image studio (version 4.0). Protein expression was normalized to loading control (GAPDH). Abdominal subcutaneous and visceral fat samples from each subject were run on the same gel to minimize variations.
Measurement of Fibrosis and Adipocyte Size
Adipose tissue samples were paraffin embedded, sectioned and stained for fibrosis using Masson’s trichrome staining at the Mayo Clinic core facility (Arizona) following standard protocols. Quantitation of fibrosis was done using Image J software using standard instructions1. For each tissue sample, percent fibrosis was determined in 5 random images and average value was used for further analysis. Furthermore, adipocyte size was determined using Aperio Image Scope software (Leica Biosystems, Buffalo grove, IL, United States) in 8 random areas per sample.
Statistical Analysis
Data distribution was examined. Normally distributed data are reported as mean ± SD. Non-normally distributed data are reported as median along with interquartile range. The differences between the abdominal subcutaneous and visceral AT, and drug usage were assessed using repeated ANOVA. Prior to the analysis, skewed variables were log transformed to approximate normal distribution. All analyses were performed using JMP Pro 13.0.0 and P < 0.05 was considered statistically significant.
Results
Angiotensin Peptide Generation Pathways
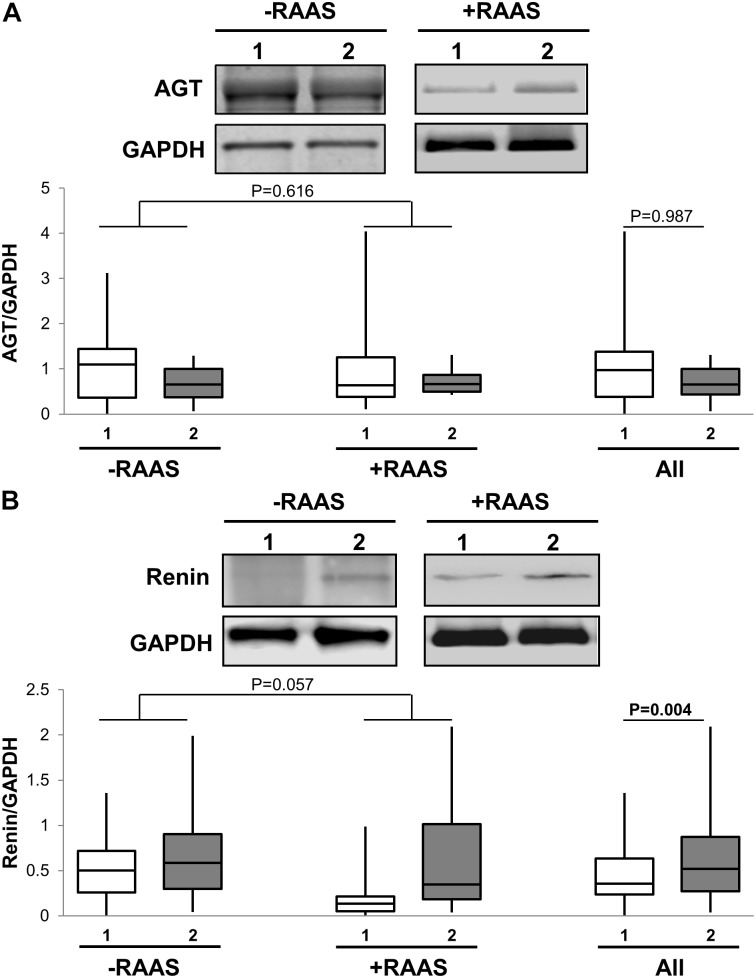
The first step in generation of bioactive angiotensin peptides involves conversion of inactive AGT to AngI by enzymatic renin activity. Therefore, we examined the mRNA and protein expression of both AGT and renin in subcutaneous versus visceral fat depots. Increased transcription of AGT in visceral fat was evident (Table 2). However, mRNA for renin was not detected in most study samples. At the protein level, we showed that AGT expression was not different between the two depots while renin expression was increased in visceral fat (Figure 1). Additionally, the mRNA and protein expression of AGT and renin did not vary with use of RAAS targeted drug and there were no interactions between drug use and depots (protein expression: AGT p = 0.959; renin p = 0.162).
Table 2.
Transcription of RAS components in AT.
| mRNA ratio | -RAAS (n = 20) |
+RAAS (n = 10) |
P-Value |
||||
|---|---|---|---|---|---|---|---|
| Subcutaneous | Visceral | Subcutaneous | Visceral | Depot | Drug | Depot∗Drug | |
| AGT/GAPDH | 0.0059 (0.0122-0.0025) | 0.0286 (0.0391-0.0162) | 0.0046 (0.0107-0.0032) | 0.0226 (0.0354-0.0134) | <0.001 | 0.55 | 0.7817 |
| RENIN/GAPDH | ND | ND | ND | ND | - | - | - |
| ACE/GAPDH | 0.0056 (0.0129-0.0045) | 0.0287 (0.0452-0.0168) | 0.0062 (0.0223-0.0029) | 0.024 (0.0415-0.0116) | <0.001 | 0.887 | 0.661 |
| Chymase/GAPDH | 0.0051 (0.0076-0.0015) | 0.0106 (0.0191-0.0052) | 0.0036 (0.0148-0.0004) | 0.0076 (0.0135-0.0057) | 0.001 | 0.522 | 0.614 |
| ACE2/GAPDH | 0.0002 (0.0005-0.0002) | 0.0016 (0.0034-0.0011) | 0.0002 (0.0013-0.0001) | 0.0028 (0.004-0.0015) | <0.001 | 0.242 | 0.93 |
| AT1R/GAPDH | 0.0011 (0.0017-0.0004) | 0.0017 (0.0032-0.001) | 0.0015 (0.0017-0.0012) | 0.002 (0.0025-0.0011) | 0.03 | 0.595 | 0.269 |
| AT2R/GAPDH | 0.0003 (0.0008-0.0002) | 0.001 (0.0013-0.0007) | 0.0002 (0.0008-0.0002) | 0.0012 (0.0025-0.0005) | <0.001 | 0.956 | 0.265 |
| MASR/GAPDH | 0.0005 (0.0012-0.0002) | 0.0009 (0.0014-0.0003) | 0.0004 (0.0011-0.0001) | 0.0013 (0.0067-0.0004) | 0.011 | 0.786 | 0.058 |
| P53/GAPDH | 0.0036 (0.0454-0.0015) | 0.0456 (0.0673-0.034) | 0.0039 (0.0785-0.0015) | 0.0607 (0.0786-0.0318) | <0.001 | 0.642 | 0.6613 |
| Pro-renin receptor/GAPDH | 0.1402 (0.1838-0.0339) | 0.0874 (0.1518-0.0533) | 0.1283 (0.1926-0.061) | 0.0589 (0.1311-0.0401) | 0.498 | 0.343 | 0.735 |
Data presented as median and interquartile range. –RAAS refers to measures obtained from AT of subjects not taking any RAAS related drugs. +RAAS refers to measures obtained from AT of subjects prescribed RAAS targeted drugs. P-values were determined using repeated ANOVA. ND: not detected. Significant P-values are bolded.
FIGURE 1.
Angiotensin I Generation Pathway. Representative Western blots and graphs showing expression of AGT, (A) and renin (B) in abdominal subcutaneous (1, white bars) and visceral (2, gray bars) fat depots. Data are presented as median and interquartile range. Fiskars depict minimum and maximum values. –RAAS: data from AT of subjects not taking any RAAS related drugs; +RAAS: data from AT of individuals taking RAAS targeted drugs. P-values were determined using repeated ANOVA.
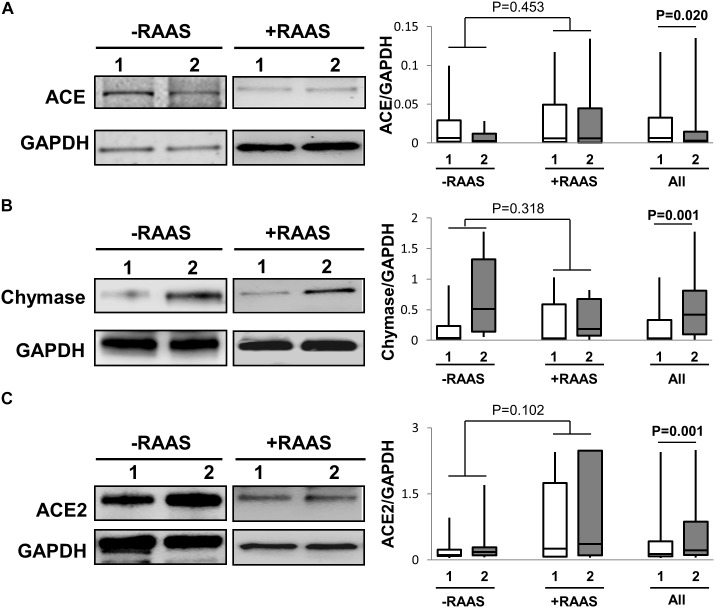
Further, the predominant classical RAS pathway involves hydrolysis of AngI to AngII by the metallopeptidase ACE. However, the conversion AngI to AngII is also enabled by the enzyme chymase. Hence, we determined the expression of both ACE and chymase. While ACE protein expression was lower, chymase protein expression was higher in visceral fat (Figure 2). Unlike protein expression, increased mRNA for both ACE and chymase were seen in visceral AT (Table 2). The alternate counter-regulatory RAS axis (Ang1-7/MASR) requires ACE2 activity to generate Ang1-7. The expression of ACE2 protein (Figure 2) and mRNA (Table 2) was elevated in visceral fat. The mRNA and protein expression of ACE, chymase, and ACE2 did not vary with use of RAAS targeted drugs and no interaction between depots and drug usage was apparent (protein expression: ACE p = 0.500; chymase p = 0.204; ACE2 p = 0.867).
FIGURE 2.
Pathways to AngII and Ang1-7 Peptide Generation. Representative Western blots and graphs showing expression of ACE, (A), chymase (B) and ACE2, (C) in abdominal subcutaneous (1, white bars) and visceral (2, gray bars) fat depots. Data are presented as median and interquartile range. Fiskars depict minimum and maximum values. –RAAS: data from AT of subjects not taking any RAAS related drugs; +RAAS: data from AT of individuals taking RAAS targeted drugs. P-values were determined using repeated ANOVA.
Cellular RAS Receptors
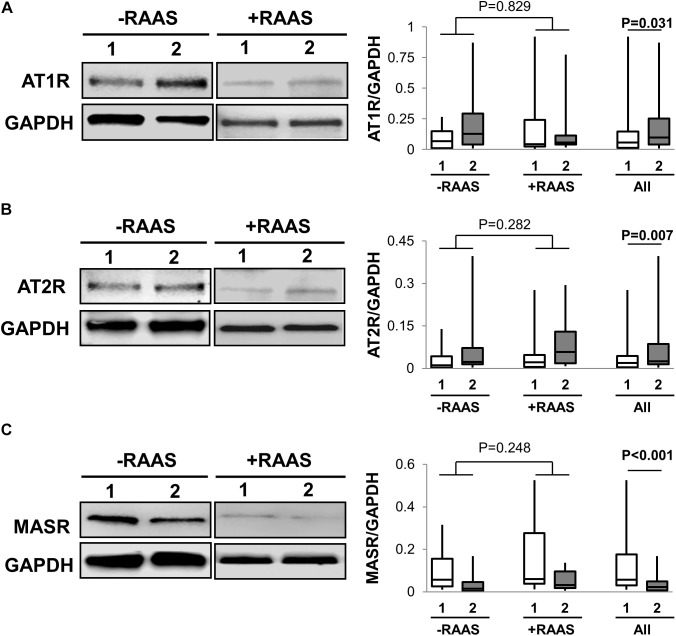
Classical RAS mediates its contrasting cellular action through mainly AT1R and AT2R while the alternate RAS axis requires MASR for its downstream cellular effects. The mRNA and protein expression of AT1R and AT2R was increased in visceral fat (Table 2 and Figure 3). However, MASR mRNA expression was higher while MASR protein expression was lower in visceral AT. We also showed that the transcription of pro-renin receptor was not different between the two depots (Table 2). Similar to proteins involved in angiotensin peptide generation, the mRNA and protein expression of cellular RAS receptors did not show any alterations related to RAAS targeted drug use or interactions of drug use with depots (protein expression: AT1R p = 0.187; AT2R p = 0.57; MASR p = 0.198).
FIGURE 3.
Cellular Receptors for Angiotensin Peptides. Representative Western blots and graphs showing expression of AT1R, (A), AT2R, (B) and MASR in (C) abdominal subcutaneous (1, white bars) and visceral (2, gray bars) fat depots. Data are presented as median and interquartile range. Fiskars depict minimum and maximum values. –RAAS: data from AT of subjects not taking any RAAS related drugs; +RAAS: data from AT of individuals taking RAAS targeted drugs. P-values were determined using repeated ANOVA.
Adipose Tissue p53, Fibrosis, Adipocyte Size, Oxidative Stress, and Inflammation
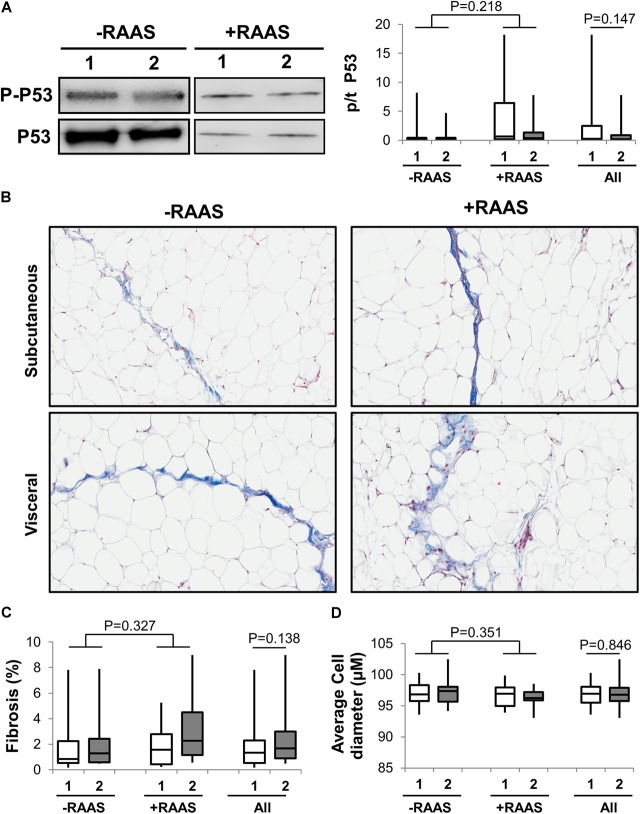
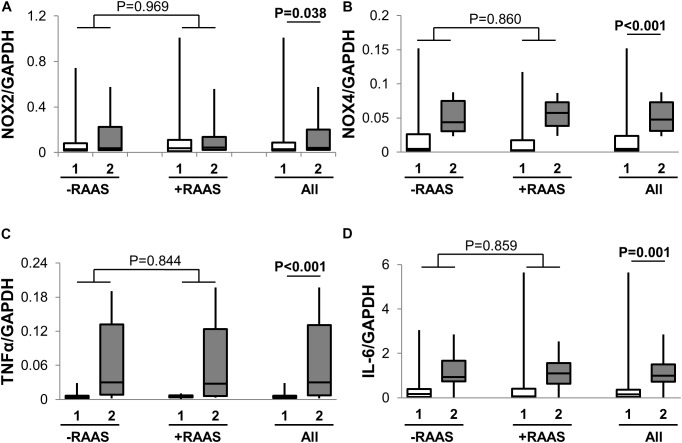
Considering that several of the detrimental cellular effects of AngII are mediated via activation of the p53 pathway, we examined the expression of phosphorylated and total p53 in visceral and subcutaneous fat samples. Phosphorylated p53 and total p53 did not differ between the two fat depots (Figure 4). However, increased transcription of p53 mRNA was observed in visceral fat (Table 2). Next, we quantified fibrosis in visceral and subcutaneous AT as it is considered a surrogate marker for AT dysfunction and a downstream detrimental effect of RAS (Sun et al., 2013). Tissue fibrosis was similar in the two fat depots in our study subjects irrespective of RAAS drug usage and no interactions between depots and drug use was seen (p = 0.606, Figure 4). To gain further insights, we also examined average adipocyte size along with mRNA expression of inflammatory (IL-6 and TNFα) and oxidative stress markers (NOX2 and NOX4). Adipocyte size was not different in the two fat depots (Figure 4) and was not altered by RAAS drug usage. However, increased transcription of IL-6 and TNFα was observed in visceral fat along with increases in NOX2 and NOX4 mRNA (Figure 5). Also, no interactions with use of RAAS targeted drug were seen (IL-6 p = 0.783; TNFα p = 0.491; NOX4 p = 0.594; NOX2 p = 0.570)
FIGURE 4.
Adipose Tissue p53 Activation, Fibrosis and Cell Size. Representative Western blots and graph showing expression of phosphorylated p-53 (A) in abdominal subcutaneous (1, white bars) and visceral (2, gray bars) fat depots. Representative trichrome stained images (B) and graphs quantifying fibrosis (C) and average adipocyte size (D) from subcutaneous and visceral fat depots. Data are presented as median and interquartile range. Fiskars depict minimum and maximum values. –RAAS: data from AT of subjects not taking any RAAS related drugs. +RAAS:data from AT of individuals taking RAAS targeted drugs. P-values were determined using repeated ANOVA.
FIGURE 5.
Adipose Tissue Oxidative Stress and Inflammation. Graph showing transcription of NOX2 (A), NOX4 (B), TNFα (C), and IL-6 (D) in abdominal subcutaneous (1, white bars) and visceral (2, gray bars) fat depots. Data are presented as median and interquartile range. Fiskars depict minimum and maximum values. –RAAS: data from AT of subjects not taking any RAAS related drugs. +RAAS: data from AT of individuals taking RAAS targeted drugs. P-values were determined using repeated ANOVA.
Discussion
The main finding of our study is that even though several components of the renin-angiotensin pathway are differentially expressed in the subcutaneous and visceral fat depots, the overall effects of these alterations do not appear likely to contribute to increased RAS activity in visceral fat. This is contradictory to our hypothesis that visceral fat would have increased expression of AngII/AT1R but decreased expression of Ang1-7/MASR and AT2R. This is the first study, in humans, to examine the expression of RAS related proteins and mRNA including both the traditional as well as the alternate RAS axis in paired visceral and subcutaneous AT. Previous studies only examined the transcription of the proteins comprising the traditional RAS axis (AGT/AngII/AT1R) (Dusserre et al., 2000; Giacchetti et al., 2000, 2002).
Renin angiotensin system is a complex system with multiple functional arms which may counterbalance each other. AngI generation is the first step toward formation of bioactive angiotensin peptides. On the one hand, we observed an increased transcription of AGT and renin in visceral AT. On the other hand we show that AGT protein expression was similar in the two fat depots but renin protein expression was increased in visceral fat. Nonetheless, the increases in renin protein expression in visceral fat may suggest a differential increase in AngI peptide in visceral fat depots. However, conclusive evidence can be only drawn from measuring renin activity which was beyond the scope of this manuscript. Our mRNA findings are consistent with the previous studies showing increased AGT mRNA expression in visceral fat (Dusserre et al., 2000; Giacchetti et al., 2000, 2002). The difference in AGT mRNA and protein expression may result from secretion of AGT peptide thereby resulting in no change in intracellular AGT protein levels. The discrepancies in mRNA and protein data related to AGT expression could also suggest post-transcription/translational mechanisms which may reduce stability of AGT protein expression and requires further investigation. Similar inconsistencies between mRNA and protein data were also observed for ACE, MASR and p53.
Among enzymes involved in AngII generation, we show that while ACE protein expression is higher in subcutaneous AT, chymase protein expression is higher in visceral fat. Hence even though differences in ACE and chymase protein expression exist, these differences may be redundant and lead to similar levels of AngII in the two fat depots. Nevertheless, our data suggest that since AngII generation in visceral fat may be more dependent on chymase activity, the beneficial therapeutic effects of ACE inhibitors in reducing AngII levels in visceral fat may be less effective. Of note, overall expression of RAS related proteins in AT of individuals taking and not taking RAAS directed therapeutics was similar, and no interaction between depot differences and drug use was observed. Several beneficial effects of RAS are mediated through the alternate axis comprising Ang1-7/MASR. Ang1-7 generation is dependent on the enzymatic actions of ACE2. Importantly, ACE2 enzymatic activity serves a dual purpose. First, it reduces the availability of AngII, and second, it forms Ang1-7. We demonstrate that ACE2 expression is higher in visceral AT, which suggests that Ang1-7 generation may also be higher and this could also likely lower AngII in visceral fat. These findings highlight the need for future studies examining angiotensin peptides in regional fat depots.
Furthermore, we show that AT1R and AT2R have higher expression in visceral fat, and MASR has higher expression in subcutaneous fat. An increase in AT1R mRNA in visceral fat has been previously reported; (Giacchetti et al., 2002), however, expression of AT2R and MASR has not been examined before. The potential benefits of increases in Ang 1-7 generation (suggested by increased ACE2 expression) in visceral fat would likely be counteracted by decreases in MASR expression in visceral fat. It is also likely that the lower expression of MASR in visceral fat may be a reflection of increased Ang1-7 generation. Indeed, Ang1-7 is shown to cause internalization of MASR which may potentiate endosomal degradation (Gironacci et al., 2011).
The overall absence of RAS related differences in proteins involved in AngII generation along with decreases in MASR (possibly in conditions of increased Ang1-7) in visceral fat tissue is supported by similar levels of cellular p53 pathway activation, fibrosis, and adipocyte size. However, increased mRNA of proteins related to inflammation and oxidative stress in visceral fat was observed. Our findings are concordant with previous studies showing similar fibrosis between regional fat depots (Divoux et al., 2010; Muir et al., 2016). However, this is in contrast to a previous study which showed increased collagen deposition in visceral fat (Michaud et al., 2016). The lack of difference in AT fibrosis in the regional fat depots in our study may be related to our study population, which only includes obese individuals, and the method used to measure fibrosis. Importantly, previous studies, (Divoux et al., 2010; Michaud et al., 2016) reported a positive correlation with the amounts of fibrosis in omental and subcutaneous fat, suggesting that similar central mechanisms may be contributing to concomitant deposition of extracellular matrix in the two fat depots. This suggests that while increased RAS in obesity may still contribute to AT fibrosis, this may be related to central/systemic effects of RAS rather than localized regional differences in AT.
The strength of our study is in the comprehensive measurement of RAS including the classic as well as the alternate beneficial arm, at the mRNA and protein level. Our study also includes assessment of paired subcutaneous AT from individuals taking RAAS targeted drugs which provides insights into differential effects of these drugs on proteins of the RAS pathway in visceral versus subcutaneous tissue. Limitations include our study population which comprises mostly morbidly obese individuals with several comorbidities, and chronic usage of medications. Also, the lack of comparison with AT samples from normal-weight subjects limits our ability to comment on the impact of obesity on AT RAS. On the other hand, it is the morbidly obese population which is at greatest risk for visceral fat mediated cardiovascular and metabolic disease, and is the population of greatest interest regarding mechanisms through which visceral fat may elicit adverse consequences. Importantly, our population includes a large proportion of females which prevents generalization to males. Our study is also limited by the lack of availability of specific AT2R antibody (Hafko et al., 2013). To overcome this limitation, we have also provided mRNA data which, consistent with protein quantification, also show preferential increases in visceral AT.
In summary, even though there is differential expression of RAS proteins in subcutaneous versus visceral AT, these differences appear unlikely to contribute to changes in AT function and to cardiometabolic pathophysiology associated with visceral adiposity.
Author Contributions
PS, YZ, KS, CB, AA, and TK conceptualized and designed the study. YZ, KS, CB, KP, and MP collected and analyzed the tissue samples. YZ and PS performed the data analysis. YZ, KS, CB, KP, MP, AA, TK, NC, and PS interpreted the data, drafted the manuscript, and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
- ACE
angiotensin converting enzyme
- AGT
angiotensinogen
- AngII
angiotensin II
- Ang1-7
angiotensin 1-7
- AT
adipose tissue
- AT1R
angiotensin II type-1 receptor
- AT2R
angiotensin II type-2 receptor
- BMI
body mass index
- MASR
Mas receptor
- NOX
NADPH oxidase
- RAS
renin-angiotensin system
- RAAS
renin-angiotensin aldosterone system
Funding. This work was supported by American Physiological Society Stride Summer Undergraduate Research Fellowships [NHLBI 1 R25 HL115473-01 to KS and MP]; the American Heart Association [16POST30260005, KP; 16POST27210011, CB; 16SDG27250156, NC; and17GRNT33660138, PS] and NIDDK grant DK115594 to AA.
References
- Akazawa H., Yano M., Yabumoto C., Kudo-Sakamoto Y., Komuro I. (2013). Angiotensin II type 1 and type 2 receptor-induced cell signaling. Curr. Pharm. Des. 1917 2988–2995. 10.2174/1381612811319170003 [DOI] [PubMed] [Google Scholar]
- Azushima K., Ohki K., Wakui H., Uneda K., Haku S., Kobayashi R., et al. (2017). Adipocyte-specific enhancement of angiotensin ii type 1 receptor-associated protein ameliorates diet-induced visceral obesity and insulin resistance. J. Am. Heart Assoc. 6:e004488. 10.1161/JAHA.116.004488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra A., Neeland I. J., Berry J. D., Ayers C. R., Rohatgi A., Das S. R., et al. (2014). The relationship of body mass and fat distribution with incident hypertension: observations from the Dallas Heart Study. J. Am. Coll. Cardiol. 6410 997–1002. 10.1016/j.jacc.2014.05.057 [DOI] [PubMed] [Google Scholar]
- Chappell M. C. (2016). Biochemical evaluation of the renin-angiotensin system: the good, bad, and absolute? Am. J. Physiol. Heart Circ. Physiol. 3102 H137–H152. 10.1152/ajpheart.00618.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covassin N., Sert-Kuniyoshi F. H., Singh P., Romero-Corral A., Davison D. E., Lopez-Jimenez F., et al. (2018). Experimental weight gain increases ambulatory blood pressure in healthy subjects: implications of visceral fat accumulation. Mayo Clin. Proc. 935 618–626. 10.1016/j.mayocp.2017.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet A. D., Krause E. G., Kim D. H., Sakai R. R., Seeley R. J., Woods S. C. (2009). The effect of angiotensin-converting enzyme inhibition using captopril on energy balance and glucose homeostasis. Endocrinology 1509 4114–4123. 10.1210/en.2009-0065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divoux A., Tordjman J., Lacasa D., Veyrie N., Hugol D., Aissat A., et al. (2010). Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diab. Metab. Res. Rev. 5911 2817–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusserre E., Moulin P., Vidal H. (2000). Differences in mRNA expression of the proteins secreted by the adipocytes in human subcutaneous and visceral adipose tissues. Biochim. Biophys. Acta 15001 88–96. 10.1016/S0925-4439(99)00091-5 [DOI] [PubMed] [Google Scholar]
- Engeli S., Bohnke J., Gorzelniak K., Janke J., Schling P., Bader M., et al. (2005). Weight loss and the renin-angiotensin-aldosterone system. Hypertension 453 356–362. 10.1161/01.HYP.0000154361.47683.d3 [DOI] [PubMed] [Google Scholar]
- Favre G. A., Esnault V. L., Van Obberghen E. (2015). Modulation of glucose metabolism by the renin-angiotensin-aldosterone system. Am. J. Physiol. Endocrinol. Metab. 3086 E435–E449. 10.1152/ajpendo.00391.2014 [DOI] [PubMed] [Google Scholar]
- Fiordaliso F., Leri A., Cesselli D., Limana F., Safai B., Nadal-Ginard B., et al. (2001). Hyperglycemia activates p53 and p53-regulated genes leading to myocyte cell death. Diabetes Metab. Res. Rev. 5010 2363–2375. [DOI] [PubMed] [Google Scholar]
- Fox C. S., Massaro J. M., Hoffmann U., Pou K. M., Maurovich-Horvat P., Liu C. Y., et al. (2007). Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 1161 39–48. 10.1161/CIRCULATIONAHA.106.675355 [DOI] [PubMed] [Google Scholar]
- Furuhashi M., Ura N., Takizawa H., Yoshida D., Moniwa N., Murakami H., et al. (2004). Blockade of the renin-angiotensin system decreases adipocyte size with improvement in insulin sensitivity. J. Hypertens. 2210 1977–1982. 10.1097/00004872-200410000-00021 [DOI] [PubMed] [Google Scholar]
- Ghosh A. K., Quaggin S. E., Vaughan D. E. (2013). Molecular basis of organ fibrosis: potential therapeutic approaches. Exp. Biol. Med. (Maywood) 2385 461–481. 10.1177/1535370213489441 [DOI] [PubMed] [Google Scholar]
- Giacchetti G., Faloia E., Mariniello B., Sardu C., Gatti C., Camilloni M. A., et al. (2002). Overexpression of the renin-angiotensin system in human visceral adipose tissue in normal and overweight subjects. Am. J. Hypertens. 155 381–388. 10.1016/S0895-7061(02)02257-4 [DOI] [PubMed] [Google Scholar]
- Giacchetti G., Faloia E., Sardu C., Camilloni M. A., Mariniello B., Gatti C., et al. (2000). Gene expression of angiotensinogen in adipose tissue of obese patients. Int. J. Obes. Relat. Metab. Disord. 24(Suppl. 2), S142–S143. 10.1038/sj.ijo.0801305 [DOI] [PubMed] [Google Scholar]
- Gironacci M. M., Adamo H. P., Corradi G., Santos R. A., Ortiz P., Carretero O. A. (2011). Angiotensin (1-7) induces MAS receptor internalization. Hypertension 582 176–181. 10.1161/HYPERTENSIONAHA.111.173344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogiraju R., Xu X., Bochenek M. L., Steinbrecher J. H., Lehnart S. E., Wenzel P., et al. (2015). Endothelial p53 deletion improves angiogenesis and prevents cardiac fibrosis and heart failure induced by pressure overload in mice. J. Am. Heart Assoc. 42:e001770. 10.1161/JAHA.115.001770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grishko V., Pastukh V., Solodushko V., Gillespie M., Azuma J., Schaffer S. (2003). Apoptotic cascade initiated by angiotensin II in neonatal cardiomyocytes: role of DNA damage. Am. J. Physiol. Heart Circ. Physiol. 2856 H2364–H2372. 10.1152/ajpheart.00408.2003 [DOI] [PubMed] [Google Scholar]
- Group N. S., McMurray J. J., Holman R. R., Haffner S. M., Bethel M. A., Holzhauer B., et al. (2010). Effect of valsartan on the incidence of diabetes and cardiovascular events. N. Engl. J. Med. 36216 1477–1490. [DOI] [PubMed] [Google Scholar]
- Guan A., Gong H., Ye Y., Jia J., Zhang G., Li B., et al. (2013). Regulation of p53 by jagged1 contributes to angiotensin II-induced impairment of myocardial angiogenesis. PLoS One 810:e76529. 10.1371/journal.pone.0076529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafko R., Villapol S., Nostramo R., Symes A., Sabban E. L., Inagami T., et al. (2013). Commercially available angiotensin II At(2) receptor antibodies are nonspecific. PLoS One 87:e69234. 10.1371/journal.pone.0069234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Investigators D. T., Bosch J., Yusuf S., Gerstein H. C., Pogue J., Sheridan P., et al. (2006). Effect of ramipril on the incidence of diabetes. N. Engl. J. Med. 35515 1551–1562. [DOI] [PubMed] [Google Scholar]
- Lee M. H., Song H. K., Ko G. J., Kang Y. S., Han S. Y., Han K. H., et al. (2008). Angiotensin receptor blockers improve insulin resistance in type 2 diabetic rats by modulating adipose tissue. Kid. Int. 747 890–900. 10.1038/ki.2008.313 [DOI] [PubMed] [Google Scholar]
- Leri A., Claudio P. P., Li Q., Wang X., Reiss K., Wang S., et al. (1998). Stretch-mediated release of angiotensin II induces myocyte apoptosis by activating p53 that enhances the local renin-angiotensin system and decreases the Bcl-2-to-Bax protein ratio in the cell. J. Clin. Invest. 1017 1326–1342. 10.1172/JCI316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leri A., Fiordaliso F., Setoguchi M., Limana F., Bishopric N. H., Kajstura J., et al. (2000). Inhibition of p53 function prevents renin-angiotensin system activation and stretch-mediated myocyte apoptosis. Am. J. Pathol. 1573 843–857. 10.1016/S0002-9440(10)64598-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlejohn N. K., Grobe J. L. (2015). Opposing tissue-specific roles of angiotensin in the pathogenesis of obesity, and implications for obesity-related hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 30912 R1463–R1473. 10.1152/ajpregu.00224.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Lv X. H., Li H. X., Cao X., Zhang F., Wang L., et al. (2012). Angiotensin-(1-7) suppresses oxidative stress and improves glucose uptake via Mas receptor in adipocytes. Acta Diabetol. 494 291–299. 10.1007/s00592-011-0348-z [DOI] [PubMed] [Google Scholar]
- Liu J., Fox C. S., Hickson D. A., May W. D., Hairston K. G., Carr J. J., et al. (2010). Impact of abdominal visceral and subcutaneous adipose tissue on cardiometabolic risk factors: the Jackson Heart Study. J. Clin. Endocrinol. Metab. 9512 5419–5426. 10.1210/jc.2010-1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Wang G., Zhou G., Tan Y., Wang X., Wei W., et al. (2009). Angiotensin II-induced p53-dependent cardiac apoptotic cell death: its prevention by metallothionein. Toxicol. Lett. 191 314–320. 10.1016/j.toxlet.2009.09.015 [DOI] [PubMed] [Google Scholar]
- Mahabadi A. A., Massaro J. M., Rosito G. A., Levy D., Murabito J. M., Wolf P. A., et al. (2009). Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. Eur. Heart J. 307 850–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus Y., Shefer G., Stern N. (2013). Adipose tissue renin-angiotensin-aldosterone system (RAAS) and progression of insulin resistance. Mol. Cell. Endocrinol. 378 1–14. 10.1016/j.mce.2012.06.021 [DOI] [PubMed] [Google Scholar]
- Mathai M. L., Naik S., Sinclair A. J., Weisinger H. S., Weisinger R. S. (2008). Selective reduction in body fat mass and plasma leptin induced by angiotensin-converting enzyme inhibition in rats. Int. J. Obes. (Lond.) 3210 1576–1584. 10.1038/ijo.2008.126 [DOI] [PubMed] [Google Scholar]
- Michaud A., Tordjman J., Pelletier M., Liu Y., Laforest S., Noel S., et al. (2016). Relevance of omental pericellular adipose tissue collagen in the pathophysiology of human abdominal obesity and related cardiometabolic risk. Int. J. Obes. (Lond.) 4012 1823–1831. 10.1038/ijo.2016.173 [DOI] [PubMed] [Google Scholar]
- Minamino T., Orimo M., Shimizu I., Kunieda T., Yokoyama M., Ito T., et al. (2009). A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat. Med. 159 1082–1087. 10.1038/nm.2014 [DOI] [PubMed] [Google Scholar]
- Muir L. A., Neeley C. K., Meyer K. A., Baker N. A., Brosius A. M., Washabaugh A. R., et al. (2016). Adipose tissue fibrosis, hypertrophy, and hyperplasia: correlations with diabetes in human obesity. Obesity (Silver Spring) 243 597–605. 10.1002/oby.21377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedogoda S. V., Ledyaeva A. A., Chumachok E. V., Tsoma V. V., Mazina G., Salasyuk A. S., et al. (2013). Randomized trial of perindopril, enalapril, losartan and telmisartan in overweight or obese patients with hypertension. Clin. Drug Investig. 338 553–561. 10.1007/s40261-013-0094-9 [DOI] [PubMed] [Google Scholar]
- Orr J. S., Gentile C. L., Davy B. M., Davy K. P. (2008). Large artery stiffening with weight gain in humans: role of visceral fat accumulation. Hypertension 516 1519–1524. 10.1161/HYPERTENSIONAHA.108.112946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh N. I., Keyes M. J., Larson M. G., Pou K. M., Hamburg N. M., Vita J. A., et al. (2009). Visceral and subcutaneous adiposity and brachial artery vasodilator function. Obesity (Silver Spring) 1711 2054–2059. 10.1038/oby.2009.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Corral A., Sert-Kuniyoshi F. H., Sierra-Johnson J., Orban M., Gami A., Davison D., et al. (2010). Modest visceral fat gain causes endothelial dysfunction in healthy humans. J. Am. Coll. Cardiol. 568 662–666. 10.1016/j.jacc.2010.03.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos S. H., Braga J. F., Mario E. G., Porto L. C., Rodrigues-Machado Mda G., Murari A., et al. (2010). Improved lipid and glucose metabolism in transgenic rats with increased circulating angiotensin-(1-7). Arterioscler. Thromb. Vasc. Biol. 305 953–961. 10.1161/ATVBAHA.109.200493 [DOI] [PubMed] [Google Scholar]
- Santos S. H., Fernandes L. R., Pereira C. S., Guimaraes A. L., de Paula A. M., Campagnole-Santos M. J., et al. (2012). Increased circulating angiotensin-(1-7) protects white adipose tissue against development of a proinflammatory state stimulated by a high-fat diet. Regul. Pept. 178 64–70. 10.1016/j.regpep.2012.06.009 [DOI] [PubMed] [Google Scholar]
- Sarzani R., Salvi F., Dessi-Fulgheri P., Rappelli A. (2008). Renin-angiotensin system, natriuretic peptides, obesity, metabolic syndrome, and hypertension: an integrated view in humans. J. Hypertens. 265 831–843. 10.1097/HJH.0b013e3282f624a0 [DOI] [PubMed] [Google Scholar]
- Saydah S., Bullard K. M., Cheng Y., Ali M. K., Gregg E. W., Geiss L., et al. (2014). Trends in cardiovascular disease risk factors by obesity level in adults in the United States, NHANES 1999-2010. Obesity (Silver Spring) 228 1888–1895. 10.1002/oby.20761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen T. D., Livak K. J. (2008). Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 36 1101–1108. 10.1038/nprot.2008.73 [DOI] [PubMed] [Google Scholar]
- Schuchard J., Winkler M., Stolting I., Schuster F., Vogt F. M., Barkhausen J., et al. (2015). Lack of weight gain after angiotensin AT1 receptor blockade in diet-induced obesity is partly mediated by an angiotensin-(1-7)/Mas-dependent pathway. Br. J. Pharmacol. 17215 3764–3778. 10.1111/bph.13172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu I., Yoshida Y., Katsuno T., Tateno K., Okada S., Moriya J., et al. (2012). p53-induced adipose tissue inflammation is critically involved in the development of insulin resistance in heart failure. Cell Metab. 151 51–64. 10.1016/j.cmet.2011.12.006 [DOI] [PubMed] [Google Scholar]
- Simoes e Silva A. C., Silveira K. D., Ferreira A. J., Teixeira M. M. (2013). ACE2, angiotensin-(1-7) and Mas receptor axis in inflammation and fibrosis. Br. J. Pharmacol. 1693 477–492. 10.1111/bph.12159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K., Tordjman J., Clement K., Scherer P. E. (2013). Fibrosis and adipose tissue dysfunction. Cell Metab. 184 470–477. 10.1016/j.cmet.2013.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan P., Blais C., Nguyen T. M., Schiller P. W., Gutkowska J., Lavoie J. L. (2016). Prorenin/renin receptor blockade promotes a healthy fat distribution in obese mice. Obesity (Silver Spring) 249 1946–1954. 10.1002/oby.21592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchernof A., Despres J. P. (2013). Pathophysiology of human visceral obesity: an update. Physiol. Rev. 931 359–404. 10.1152/physrev.00033.2011 [DOI] [PubMed] [Google Scholar]
- Than A., Xu S., Li R., Leow M. S., Sun L., Chen P. (2017). Angiotensin type 2 receptor activation promotes browning of white adipose tissue and brown adipogenesis. Sig. Transduct. Target Ther. 2:17022. 10.1038/sigtrans.2017.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Holst C., Wodzig W. K., Andersen M. R., Astrup A., van Baak M. A., et al. (2012). Circulating ACE is a predictor of weight loss maintenance not only in overweight and obese women, but also in men. Int. J. Obes. (Lond.) 3612 1545–1551. 10.1038/ijo.2011.278 [DOI] [PubMed] [Google Scholar]