Abstract
A 37-year-old woman with systemic lupus erythematosus presented with gait disturbance and cognitive dysfunction. Brain magnetic resonance imaging (MRI) revealed small, punctate, T2-/fluid-attenuated inversion recovery-hyperintense and T1-hypointense lesions without gadolinium enhancement, which is atypical for progressive multifocal leukoencephalopathy (PML). On a pathological examination of biopsied brain tissues, JC virus-infected cells were hardly detected via immunohistochemistry but were certainly detected via in situ hybridization, conclusively verifying the PML diagnosis. After tapering off the immunosuppressant and mefloquine administration, the MRI findings revealed gradual improvement, and she has been stable for over 18 months. A punctate MRI pattern is not specific to natalizumab-associated PML but may be a ubiquitous early sign useful for the early diagnosis of PML.
Keywords: progressive multifocal leukoencephalopathy (PML); systemic lupus erythematosus (SLE); punctate pattern, pathological examination; early diagnosis; mefloquine
Introduction
Progressive multifocal leukoencephalopathy (PML) is a fatal demyelinating disease caused by the reactivation of latent JC virus (JCV) infection. Although the life cycle and molecular pathogenesis of JCV have not been completely elucidated yet, nonpathogenic “archetype” JCV may occasionally transform into the “neurotropic type (PML-type)”, which is thought to cause PML according to the “archetype hypothesis” (1-3).
This disease was previously known as a complication of AIDS, but at present, PML development due to the use of immunomodulatory drugs, such as natalizumab (NTZ) for multiple sclerosis (MS), is a serious concern (4). PML can be comorbid with autoimmune disorders, such as systemic lupus erythematosus (SLE), when the patient is under immunosuppressant therapy (5). Brain magnetic resonance imaging (MRI) in cases of PML typically shows large subcortical lesions with an increased T2-weighted signal and correlates with rapid progression that usually leads to a poor prognosis (6). Recently, in NTZ-treated MS patients, atypical MRI findings with a punctate pattern have been reported as an early sign of PML (7, 8-10), but whether or not these images are also indicative of early PML lesions in non-MS patients is unclear.
We herein report a case of pathologically-proven PML in a patient with SLE, who showed a punctate MRI pattern and a good clinical course. The pathology suggested a relatively low viral replication in the infected cells that may be indicative of the early demyelinating stage, supporting a better prognosis.
Case Report
A 37-year-old woman had been diagnosed with SLE at 12 years of age. SLE recurred several times despite combination therapy with high-dose intravenous methylprednisolone, oral prednisolone (PSL), azathioprine (AZA), and 6-mercaptopurine. At 16 and 17 years of age, plasmapheresis and high-dose intravenous cyclophosphamide therapy (IVCY) were introduced, respectively, to control the disease. At 17 years of age, she experienced insomnia, auditory hallucinations, and convulsive seizures, indicating neuropsychiatric SLE (NPSLE). Thus, an antiepileptic drug was temporarily administered. At 18 years of age, she had severe lupus nephritis with nephrotic syndrome. As the lupus nephritis was refractory, the treatment was changed from PSL+AZA+IVCY to PSL+cyclosporine+IVCY at 20 years of age. At 23 years of age, she underwent hemodialysis for about 8 months, and LDL apheresis was introduced. As she experienced convulsive seizures again, NPSLE was suspected and an antiepileptic drug was temporarily administered. At 27 years of age, immunoadsorption plasmapheresis was performed. From 28 years of age, her SLE symptoms were suppressed with PSL+cyclosporine. At 32 years of age, cyclosporine was switched to mycophenolate mofetil (MMF) for better control of the disease. Since then, SLE and lupus nephritis had been relatively well controlled with PSL 8-10 mg/day+MMF 1,000-1,250 mg/day despite a long history of refractory SLE.
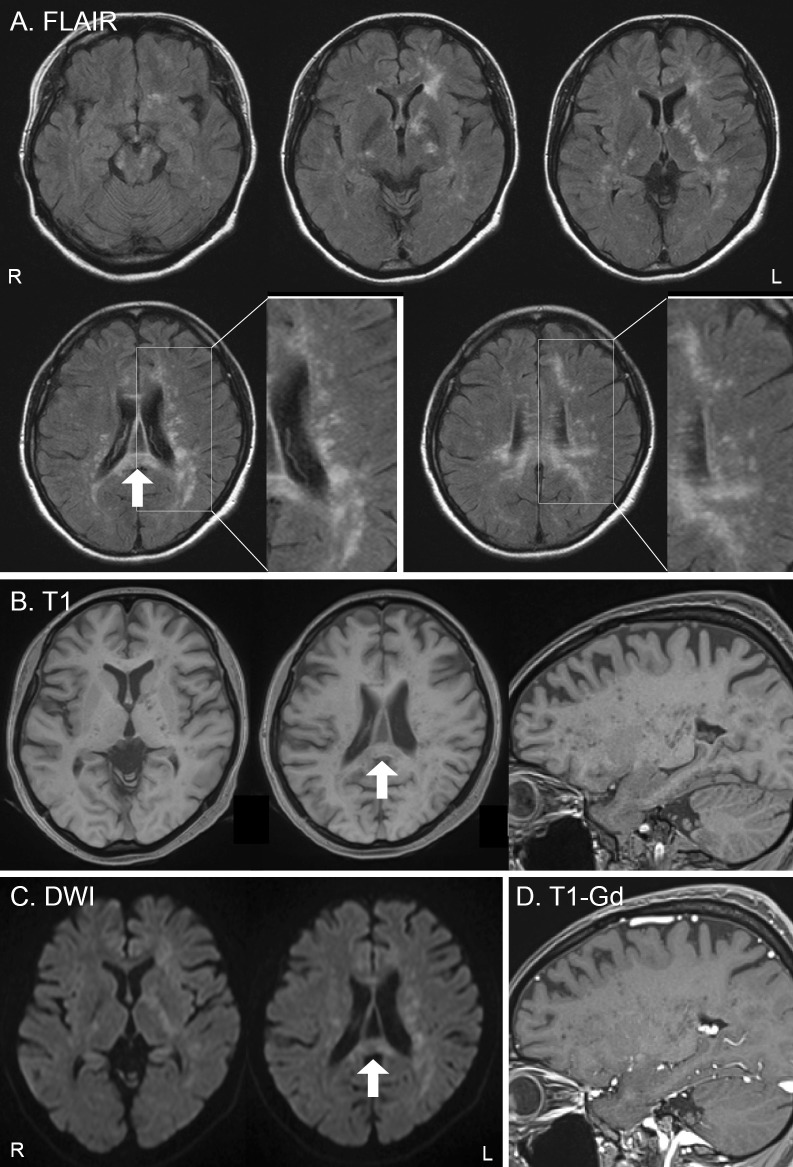
Approximately five months before the initial visit to our department, she began experiencing gait disturbance and cognitive dysfunction, and these symptoms slowly became exacerbated. At the initial visit, brain MRI revealed small, punctate, T2-/fluid-attenuated inversion recovery (FLAIR)-hyperintense and T1-hypointense lesions without gadolinium (Gd) enhancement in the bilateral cerebral peduncles, internal capsule, corpus callosum, and deep white matter of the left frontal lobe and bilateral periventricular area (Fig. 1). SLE-related vasculitis was suspected, as she had a long history of refractory SLE and lupus nephritis with NPSLE, and the size and distribution of MRI lesions resembled those reported in patients with NPSLE (11, 12). However, blood tests showed no exacerbation of SLE, and Gd enhancement was not apparent on brain MRI. We also considered PML as a differential diagnosis; however, PML was thought to be unlikely due to the lack of U-fiber involvement and the atypical MR images.
Figure 1.
Brain MRI findings at the initial visit (A: FLAIR images, B: T1-weighted images, C: diffusion-weighted images, D: T1-weighted images with gadolinium enhancement). FLAIR images (A) show high-intensity areas in the bilateral cerebral peduncles, internal capsule, corpus callosum, and deep white matter of the left frontal lobe and bilateral periventricular area. These lesions show a small punctate pattern without U-fiber involvement (A: enlarged view). These punctate lesions are scattered in the vicinity of the larger merged lesion in the left frontal lobe. Small punctate lesions are clearly visualized as low-intensity signals on T1-weighted images (B: axial and sagittal) in the deep white matter and internal capsule. The lesions appear as slightly high-intensity signals on diffusion-weighted images (C) and do not show any enhancement after gadolinium administration on T1-weighted images (D). The arrows in the FLAIR image, T1-weighted image, and diffusion-weighted images show the biopsied area (A, B, and C). FLAIR: fluid-attenuated inversion recovery, DWI: diffusion-weighted images, Gd: gadolinium
Polymerase chain reaction (PCR) of the cerebrospinal fluid (CSF) revealed the presence of JCV DNA (at most 500 copies/mL), but repeated trials were sometimes JCV-negative. The serum was positive for anti-JCV antibody (Index 2.24), which is associated with an increased risk of PML in patients with a history of NTZ treatment (4, 13), although this patient had not been treated with NTZ. The CD4+ T cell count was 148 cells/μL. T-CD4+ lymphopenia is reported to be associated with a risk of PML in patients with SLE (14). We reduced the dose of PSL from 8 to 7 mg and carefully observed the clinical symptoms, MRI findings, and JCV DNA in the CSF. Brain MRI may have shown a slight improvement, and gait disturbance and cognitive disorder were slightly exacerbated over the next four months. Although we detected JCV DNA in the CSF, it did not increase for more than four months.
The inconsistent detection of JCV DNA in the CSF, atypical brain MRI findings, and the clinical course prevented us from confirming the diagnosis in the present patient. As the treatment for PML is quite different from that for SLE-related vasculitis, it is important to verify the diagnosis. Because SLE-related vasculitis could not be ruled out, a brain biopsy of the corpus callosum (Fig. 1, arrow) was performed. We also examined the nucleotide sequences of the JCV genome in the CSF and brain tissues to clarify whether or not the JCV was PML-type.
Pathological detection of JCV-infected glial cells
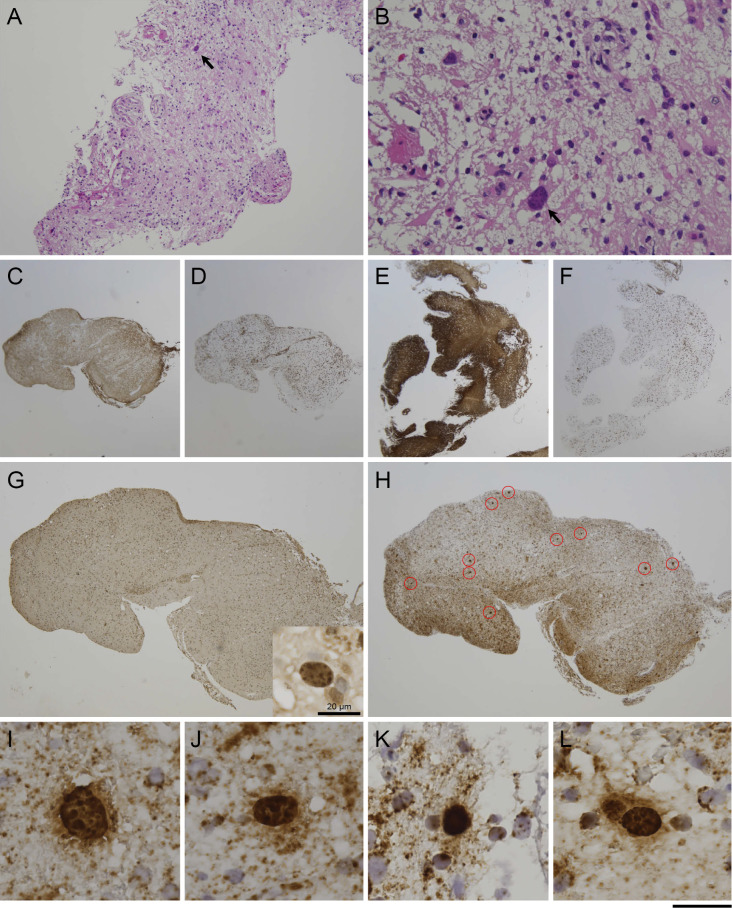
Six pieces of fragmented brain tissues, up to approximately 1×5 mm in size, were obtained. In hematoxylin/eosin staining, numerous macrophages were apparent (Fig. 2A), compatible with a demyelinating lesion. Although astroglia with large bizarre nuclei were present (Fig. 2B), oligodendroglia-like cells with typical JCV inclusions were hardly detected. Immunohistochemical labeling of two pieces of brain tissues showed decreased GFAP signals with an elevated number of CD68-positive cells (Fig. 2C and D) in contrast to four other pieces with normal GFAP signals and relatively few CD68-positive cells (Fig. 2E and F). The former two pieces were more severely damaged than the latter four, and atypical astrocytes with bizarre nuclei were present.
Figure 2.
Pathology of early PML. A, B: Biopsied brain tissues show atypical astroglia with large irregular nuclei (arrow), but oligodendroglia-like cells with typical JCV inclusions were hardly detectable with Hematoxylin and Eosin staining. C, D: One piece of brain tissue showed decreased GFAP signals with an elevated CD68-positive cell density, suggesting advanced tissue degradation. C: GFAP, D: CD68. E, F: Another piece was rather mildly affected with relatively normal GFAP signals and a lower CD68-positive cell density. E: GFAP, F: CD68. G: Immunohistochemistry (IHC) for JCV capsid proteins. JCV-positive cells were hardly detected with immunohistochemistry, except for one potentially positive cell (inset). H: In situ hybridization (ISH) for JCV DNA. ISH detected more than 20 JCV-positive cells (circled in red), with the largest number of positive cells observed in tissues with higher CD68-positive cell density. I-L: JCV-positive cells with ISH. All cells contained punctate signals in the enlarged nuclei, compatible with clustered JCV virions, represented as dot-shaped inclusions. Scale bars: (A) 200 µm; (B) 50 µm; (C-F) 1 mm; (G, H) 500 µm; (I-L) 25 µm. PML: progressive multifocal leukoencephalopathy, JCV: JC virus
Immunohistochemistry with anti-JCV antibodies (VP1, VP2/VP3C) was performed, and only one potentially JCV-positive cell was detected with the anti-JCV VP1 antibody (Fig. 2G, inset); infected cells were not clearly positive with the anti-JCV VP2/VP3C antibody (data not shown). However, a more sensitive in situ hybridization (ISH) method targeting JCV DNA revealed more than 20 JCV-positive oligodendroglia-like cells. Most JCV-positive cells were present in the two pieces of brain tissue with the higher CD68-positive cell density (Fig. 2H). All JCV-positive cells showed intranuclear punctate signals indicative of clustered JCV progenies at promyelocytic leukemia nuclear bodies (Fig. 2I-L). The host inflammatory response was minimal, and only a few CD3-positive T cells were observed. Nearly equal numbers of CD4- and CD8-positive cells were present. Inflammatory cells of the B-cell lineage were also examined, but immunoreactivity for CD20, CD79a, and CD138 was not detectable (data not shown). These findings argued against SLE-related vasculitis.
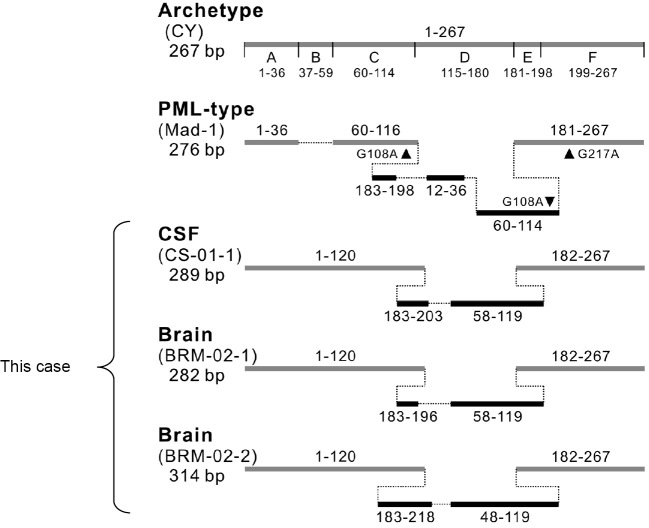
The JCV genome in the CSF and brain tissues was cloned, and the nucleotide sequences were examined. Apparent mutations (deletion and insertions) characteristic of the PML-type virus were found in the non-coding control region (NCCR) (Fig. 3). Based on these pathological findings and the nucleotide sequence analysis, the diagnosis of PML was confirmed.
Figure 3.
A comparison of the JCV non-coding control region (NCCR) sequence pattern. The NCCR sequence patterns in the CSF and brain tissues from this patient were compared with the archetype (CY) and PML-type NCCRs (Mad-1). The horizontal gray lines indicate the DNA fragments identical to the archetype NCCR (5' and 3' nucleotide positions 1-267 within the JCV genotype). The black lines indicate the duplicated sequences inserted into the deleted region. The nucleotide numbers corresponding to the archetype NCCR are shown above or below the solid lines. CSF: cerebrospinal fluid, PML: progressive multifocal leukoencephalopathy
Clinical course after the brain biopsy
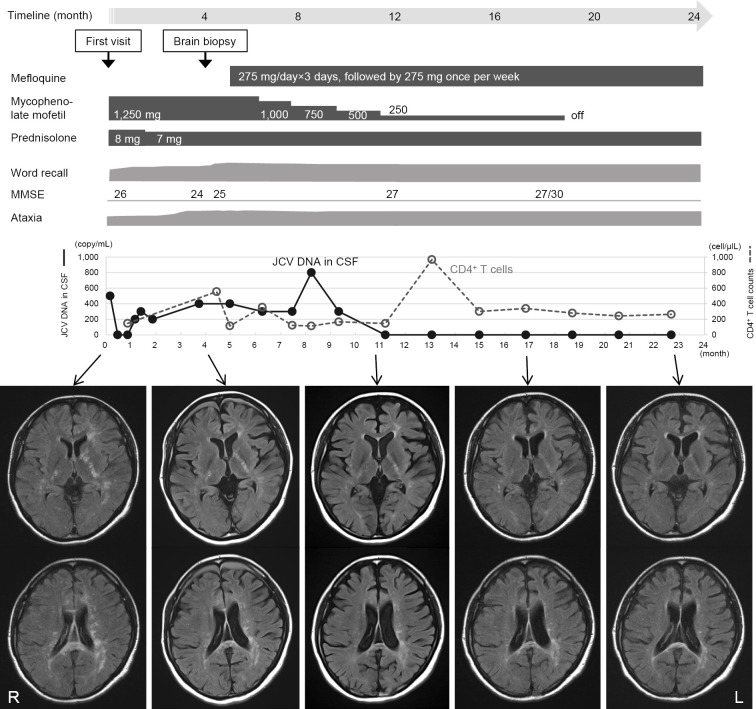
Once the diagnosis was confirmed, MMF was gradually tapered off, and mefloquine was administered (loading dose of 275 mg for 3 days, followed by 275 mg once per week). Brain MRI revealed a gradual improvement in the lesions, and no new lesions developed after these therapeutic interventions. The PCR analysis of the CSF was negative for JCV DNA, 7 months after the brain biopsy, and it has been consistently negative for the past 12 months. The number of CD4+ T cells changed slightly, but no definite trend has been observed so far. Cognitive dysfunction improved slightly without ataxic deterioration (Fig. 4). The patient has been stable for over 18 months.
Figure 4.
A summary of the clinical course of the diagnostic and therapeutic procedures with corresponding MR images. The timeline shown above represents the clinical course (24 months) after the first visit. Of note, punctate lesions in the deep white matter and internal capsule on FLAIR images gradually disappeared, and no new lesions have developed since the tapering-off of mycophenolate mofetil and the administration of mefloquine. The CSF was negative for JCV DNA at 11 months and has remained negative for the past 12 months. The ataxia has not worsened, and cognitive dysfunction has improved slightly. The patient has been stable for more than 18 months since the brain biopsy. JCV: JC virus, CSF: cerebrospinal fluid, MMSE: Mini Mental State Examination, FLAIR: fluid- attenuated inversion recovery
Discussion
Recently, PML development resulting from the use of immunomodulatory drugs has become a serious concern, and JCV in particular is known to reactivate with disease-modifying MS therapies, such as NTZ. The early diagnosis of PML is crucial, and rare MR images of a punctate pattern in the deep white matter have been described as a promising early sign for the diagnosis of NTZ-associated PML (7-10). Contrast enhancement with the punctate pattern may suggest PML-immune reconstitution inflammatory syndrome or productive JCV infection (8, 15). However, whether or not this unique MR pattern is also indicative of PML in non-MS patients has been unclear, and the relevance of this pattern to the underlying histopathology also remains to be elucidated. This is the first case in which a punctate pattern was observed on MRI in a patient with SLE, and an early stage of PML was pathologically proven.
MRI in the present case revealed small, punctate, T2-/FLAIR-hyperintense and T1-hypointense lesions in the bilateral cerebral peduncles, internal capsule, corpus callosum, and deep white matter of the left frontal lobe and bilateral periventricular area. Because these images were completely different from those observed in previously reported PML cases with SLE (5, 16, 17), it was difficult to diagnose this case with PML based on the MRI findings alone, and it was possible to suspect SLE-related vasculitis (11, 12). Although PCR showed that the CSF was positive for JCV DNA (500 copies/mL) at the initial visit, the brain MRI findings and the clinical course were atypical for PML. The commonly used PCR method for detecting JCV DNA targets the large T antigen of JCV and cannot distinguish PML-type JCV from archetype JCV. The CSF of our patient may have contained archetype JCV. Thus, the detection of JCV DNA in the CSF by PCR was not sufficient to establish a definite diagnosis in an atypical case such as this.
In our patient, punctate MRI lesions were scattered in the vicinity of the larger merged lesion in the left frontal lobe, resembling the “milky way appearance” reportedly observed at the early stages in patients with NTZ-associated PML (8, 18). We therefore considered that this MRI pattern might similarly indicate an early stage of PML in our patient with SLE. However, the atypical clinical course and MRI findings alone were insufficient to confirm the diagnosis. We therefore performed a brain biopsy, resulting in a definite diagnosis of PML. Of note, this unique punctate MRI pattern was observed in a case of non-NTZ-associated PML. Some PML cases in patients with psoriasis were reported to display punctate lesions on MRI, although the MRI findings were not well-described (19, 20). The punctate pattern may not be specific for NTZ-associated PML but instead ubiquitous for PML.
We first analyzed the pathology of early-stage PML showing a punctate MRI pattern. Despite the presence of relatively large T2-/FLAIR-hyperintense lesions, JCV-infected oligodendroglia-like cells were hardly detected with histological staining and immunolabeling; however, more than 20 JCV-positive cells were detected with sensitive ISH. These data indicate that regardless of the number of JCV-infected cells, the viral replication rate in each cell was relatively low, resulting in only mild tissue degeneration. JCV DNA in the CSF was not always detected in repeated PCR trials, which may indicate low viral replication, consistent with the pathological findings. Biopsied brain tissue specimens are usually tiny, and the JCV-infected oligodendroglia-like cells were scattered in the samples we obtained. Our samples may therefore not have contained any affected lesions simply due to technical limitations. In our patient, one of the biopsied brain tissues fortunately contained a sufficient number of JCV-positive cells to confirm the diagnosis. We cannot deny the possibility that biopsied samples from other areas with strongly hypointense signals on T1-weighted images might have revealed more severe pathological changes with abundant populations of JCV-positive cells. Although early cytopathological changes, including the dot-shaped inclusions in JCV-infected cells, have been studied (3, 21), most of our knowledge about PML pathology is derived from autopsied cases typically associated with HIV infection (22). Thus, the present case is important for its novelty. Furthering our understanding of early PML pathology will aid in the diagnosis of biopsied brain tissues.
When the immunosuppressant was tapered off and mefloquine was administered, the T2-/FLAIR-hyperintense lesions gradually diminished in size, and the patient has remained stable over the long term. A punctate MRI pattern is not specific to NTZ-associated PML, and an awareness of this unique MRI pattern will aid in the early diagnosis of PML, leading to the early treatment and a better prognosis.
The treatment with mefloquine was approved by our hospital Ethics Committee. We obtained written informed consent from the patient.
Financial Support
This work was supported in part by JSPS KAKENHI (Grand Number 17K09768 and 15K06759) and by a Grant-in-Aid for the Research Committee of Prion Disease and Slow Virus Infection, Research on Policy Planning and Evaluation for Rare and Intractable Diseases from the Ministry of Health, Labor and Welfare of Japan (Grant Number H29-Nanchitou (Nan)-Ippan-036).
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We thank Takayuki Funatsu for performing the brain biopsy, Souichi Nukuzuma and Masayuki Saijo for the analysis of JCV in the CSF, and Kenta Takahashi for the analysis of JCV in the biopsied brain tissues.
References
- 1. Tan CS, Koralnik IJ. Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: clinical features and pathogenesis. Lancet Neurol 9: 425-437, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. White MK, Khalili K. Pathogenesis of progressive multifocal leukoencephalopathy--revisited. J Infect Dis 203: 578-586, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shishido-Hara Y. Progressive multifocal leukoencephalopathy and promyelocytic leukemia nuclear bodies: a review of clinical, neuropathological, and virological aspects of JC virus-induced demyelinating disease. Acta Neuropathol 120: 403-417, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schwab N, Schneider-Hohendorf T, Melzer N, Cutter G, Wiendl H. Natalizumab-associated PML: challenges with incidence, resulting risk, and risk stratification. Neurology 88: 1197-1205, 2017. [DOI] [PubMed] [Google Scholar]
- 5. Henegar CE, Eudy AM, Kharat V, Hill DD, Bennett D, Haight B. Progressive multifocal leukoencephalopathy in patients with systemic lupus erythematosus: a systematic literature review. Lupus 25: 617-626, 2016. [DOI] [PubMed] [Google Scholar]
- 6. Sahraian MA, Radue EW, Eshaghi A, Besliu S, Minagar A. Progressive multifocal leukoencephalopathy: a review of the neuroimaging features and differential diagnosis. Eur J Neurol 19: 1060-1069, 2012. [DOI] [PubMed] [Google Scholar]
- 7. Phan-Ba R, Lommers E, Tshibanda L, et al. . MRI preclinical detection and asymptomatic course of a progressive multifocal leucoencephalopathy (PML) under natalizumab therapy. J Neurol Neurosurg Psychiatry 83: 224-226, 2012. [DOI] [PubMed] [Google Scholar]
- 8. Hodel J, Darchis C, Outteryck O, et al. . Punctate pattern: a promising imaging marker for the diagnosis of natalizumab-associated PML. Neurology 86: 1516-1523, 2016. [DOI] [PubMed] [Google Scholar]
- 9. Wijburg MT, Witte BI, Vennegoor A, et al. . MRI criteria differentiating asymptomatic PML from new MS lesions during natalizumab pharmacovigilance. J Neurol Neurosurg Psychiatry 87: 1138-1145, 2016. [DOI] [PubMed] [Google Scholar]
- 10. Hodel J, Outteryck O, Dubron C, et al. . Asymptomatic progressive multifocal leukoencephalopathy associated with natalizumab: diagnostic precision with MR imaging. Radiology 278: 863-872, 2016. [DOI] [PubMed] [Google Scholar]
- 11. Bertsias GK, Boumpas DT. Pathogenesis, diagnosis and management of neuropsychiatric SLE manifestations. Nat Rev Rheumatol 6: 358-367, 2010. [DOI] [PubMed] [Google Scholar]
- 12. Jennings JE, Sundgren PC, Attwood J, McCune J, Maly P. Value of MRI of the brain in patients with systemic lupus erythematosus and neurologic disturbance. Neuroradiology 46: 15-21, 2004. [DOI] [PubMed] [Google Scholar]
- 13. Plavina T, Subramanyam M, Bloomgren G, et al. . Anti-JC virus antibody levels in serum or plasma further define risk of natalizumab-associated progressive multifocal leukoencephalopathy. Ann Neurol 76: 802-812, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brandao M, Damasio J, Marinho A, et al. . Systemic lupus erythematosus, progressive multifocal leukoencephalopathy, and T-CD4+ lymphopenia. Clin Rev Allergy Immunol 43: 302-307, 2012. [DOI] [PubMed] [Google Scholar]
- 15. Wattjes MP, Verhoeff L, Zentjens W, et al. . Punctate lesion pattern suggestive of perivascular inflammation in acute natalizumab-associated progressive multifocal leukoencephalopathy: productive JC virus infection or preclinical PML-IRIS manifestation? J Neurol Neurosurg Psychiatry 84: 1176-1177, 2013. [DOI] [PubMed] [Google Scholar]
- 16. Fredericks CA, Kvam KA, Bear J, Crabtree GS, Josephson SA. A case of progressive multifocal leukoencephalopathy in a lupus patient treated with belimumab. Lupus 23: 711-713, 2014. [DOI] [PubMed] [Google Scholar]
- 17. Beppu M, Kawamoto M, Nukuzuma S, Kohara N. Mefloquine improved progressive multifocal leukoencephalopathy in a patient with systemic lupus erythematosus. Intern Med 51: 1245-1247, 2012. [DOI] [PubMed] [Google Scholar]
- 18. Sinnecker T, Othman J, Kuhl M, et al. . 7T MRI in natalizumab-associated PML and ongoing MS disease activity: a case study. Neurol Neuroimmunol Neuroinflamm 2: e171, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gieselbach RJ, Muller-Hansma AH, Wijburg MT, et al. . Progressive multifocal leukoencephalopathy in patients treated with fumaric acid esters: a review of 19 cases. J Neurol 264: 1155-1164, 2017. [DOI] [PubMed] [Google Scholar]
- 20. Bartsch T, Rempe T, Wrede A, et al. . Progressive neurologic dysfunction in a psoriasis patient treated with dimethyl fumarate. Ann Neurol 78: 501-514, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shishido-Hara Y, Yazawa T, Nagane M, et al. . JC virus inclusions in progressive multifocal leukoencephalopathy: scaffolding promyelocytic leukemia nuclear bodies grow with cell cycle transition through an S-to-G2-like state in enlarging oligodendrocyte nuclei. J Neuropathol Exp Neurol 73: 442-453, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Berger JR, Aksamit AJ, Clifford DB, et al. . PML diagnostic criteria: consensus statement from the AAN Neuroinfectious Disease Section. Neurology 80: 1430-1438, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]