Abstract
Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) and cryoglobulinemic vasculitis (CV) rarely coexist. An 83-year-old woman was admitted with rapidly progressive renal failure, gastrointestinal hemorrhage and purpura with myeloperoxidase (MPO)-ANCA positivity and cryoglobulinemia. Despite intensive immunosuppressive treatment, she died of aspergillus pneumonia. Autopsy revealed necrotizing crescentic glomerulitis in the majority of the glomeruli, accompanied by partially membranoproliferative-like glomerular changes. Immunofluorescence staining revealed the presence of neutrophil extracellular trap (NET) formation in the glomeruli and cutaneous arteries. These pathological findings suggested that MPO-AAV and/or CV caused NET formation, leading to lethal systemic vasculitis.
Keywords: ANCA, anti-neutrophil cytoplasmic antibody, cryoglobulin, vasculitis, neutrophil extracellular traps, NETs
Introduction
Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a systemic vasculitis that is probably caused by a circulating autoantibody named ANCA, which targets small- to medium-sized blood vessels and which may affect any organ in the body. Cryoglobulinemic vasculitis (CV) is another type of systemic vasculitis caused by cryoglobulin immune-complex deposits that precipitate in vivo at temperatures of less than 37℃ (1), and which also affects small- to medium-sized vessels (2). Both diseases are independent of each other but may-on rare occasions-be complicated. In such cases, it is difficult to distinguish between the diseases based on the clinical or pathological findings.
Neutrophil extracellular traps (NETs) are chromatin filaments that are coated with histones, protease, and granular proteins, including myeloperoxidase (MPO). They are produced and released by neutrophils and contribute to endothelial injury and death (3). NET formation can be visualized by immunofluorescence staining for Cit-H3, co-localization with extracellular DNA, and MPO (4). Several studies have indicated that NETs play an important role in endothelial injury and the necrosis of vessels in various organs of patients with AAV (3, 5). NET formation was recently observed in other autoimmune diseases, including systemic lupus erythematous and rheumatoid arthritis (6); however, the role of NET formation in these diseases has not been fully elucidated. In this report, we describe a case of lethal systemic vasculitis in a patient with high serum MPO-ANCA and cryoglobulin titers. The etiology of the vasculitis is discussed based on the histological findings.
Case Report
An 83-year-old Japanese woman was admitted to our hospital for the evaluation of malaise, gastrointestinal hemorrhage, and weakness of the legs. Her medical history included chronic kidney disease and hypertension, but no family history of note. She reported an episode of melena three months before her admission, which had resolved spontaneously. Thereafter, she developed malaise, loss of appetite, and weakness of her legs, and presented to our hospital.
On admission, a physical examination revealed the following findings: blood pressure, 107/64 mmHg, heart rate, 98 beats per minute; and body temperature, 36.4℃, with mild weakness and distal sensory impairment of the legs. Respiratory and skin examinations revealed no abnormal findings, and her level of consciousness was normal.
Laboratory testing revealed hematuria (>100 red blood cells/high power field), proteinuria (3.53 g/gCr), severe anemia (hemoglobin: 6.2 g/L), and increased serum creatinine (the baseline level of 1.19 mg/dL increased to 7.53 mg/dL). Her serum C-reactive protein (CRP) level was increased to 2.17 mg/dL, her complement levels were decreased: complement 3 (C3), 38 mg/dL; complement 4 (C4), 11 mg/dL; complement H50 (CH50), <10 U/mL, and serum cryoglobulin (type III) was detected. Her MPO-ANCA level (208 U/mL) was elevated (normal range: <3.5 U/mL) and her rheumatoid factor level was increased to 63 mg/dL (normal range: <15 IU/mL). No autoantibodies, including proteinase 3 (PR3)-ANCA, antinuclear antibody, SS-A and SS-B antibody, hepatitis B virus (HBV), hepatitis C virus (HCV), and human immunodeficiency virus (HIV) antibody, were detected, and no monoclonal antibodies or amyloid were detected in her serum. The patient's Birmingham Vasculitis Activity Score was 17 (7). There was no evidence of thrombotic microangiopathy, including an increased serum lactate dehydrogenase level or a decreased platelet count. Computed tomography showed chronic sinusitis without polyps, but no evidence of malignancy. An endoscopic examination of the upper gastrointestinal tract showed numerous bleeding ulcers in the stomach and duodenum.
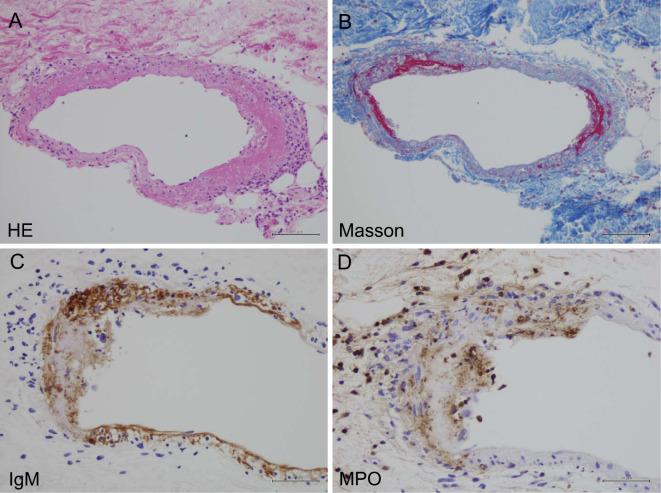
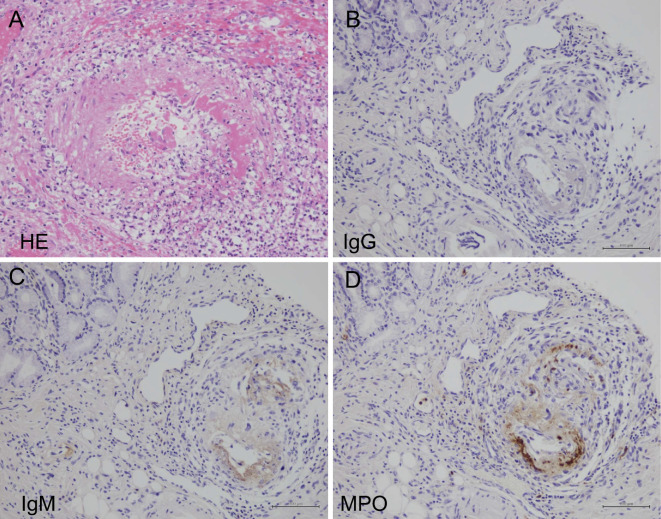
On the fourth day after admission, purpura developed on the patient's legs and hips. Skin biopsy of the purpura revealed necrotizing vasculitis in the dermal small arteries (Fig. 1A and B). Immunohistochemistry revealed IgG and IgM, and the extracellular deposition of MPO on the necrotic vascular wall (Fig. 1C and D).
Figure 1.
Light microscopy of a skin purpura biopsy specimen. Light microscopy of a skin purpura biopsy specimen revealed fibrinoid necrosis with neutrophils infiltrating the cutaneous small arteries. (A) Hematoxylin and Eosin staining (×400). (B) Masson staining (×400). Immunostaining revealed (C) IgM and (D) MPO on the vascular walls.
A clinical diagnosis of systemic vasculitis caused by type III CV or AAV, which was suspected to represent microscopic polyangiitis (MPA), was made according to the European Medicines Agency algorithm (8). She was treated with maintenance hemodialysis, three sessions of double filtration plasmapheresis, and bolus methylprednisolone (500 mg for three days), followed by a maintenance dose of methylprednisolone (40 mg/day). However, she did not recover and finally died of aspergillus pneumonia 31 days after hospital admission.
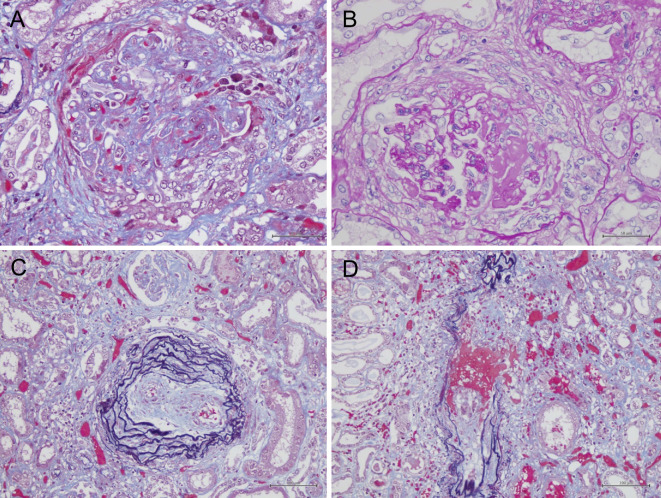
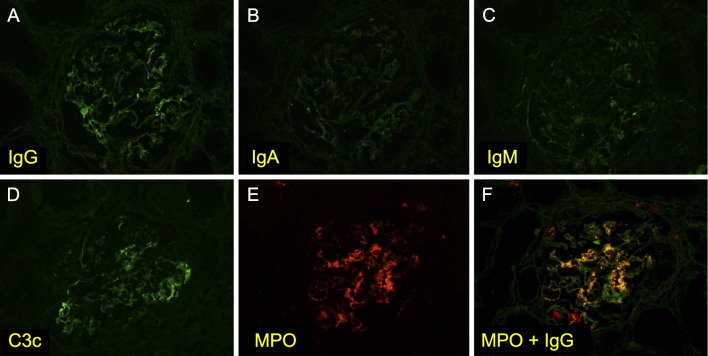
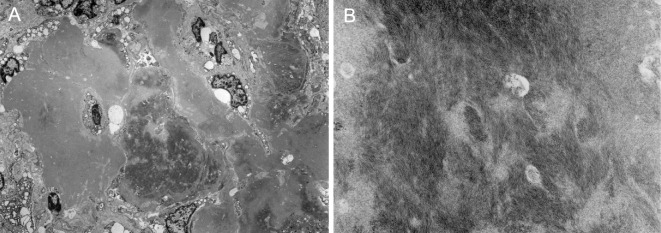
An autopsy was performed. The kidneys were mildly atrophic and showed global glomerular sclerosis in 50% of the total glomeruli, and cellular or fibrocellular crescentic glomerulitis in more than 50% of the remaining glomeruli (Fig. 2A). Some glomeruli had proliferative changes that were compatible with a membranoproliferative glomerulonephritis (MPGN)-like lesion (Fig. 2B). Interlobular arteries showed unusual intimal fibrosis (Fig. 2C), and the arterioles were affected by fibrinoid necrosis with neutrophilic infiltration (Fig. 2D). The interstitial fibrosis, tubular atrophy, and disruption of the elastic lamina were graded as severe. Immunofluorescence of the glomeruli showed partial and slight staining of IgG, C3, and MPO (Fig. 3). Electron microscopy demonstrated fibrillary deposits in the glomerular sclerotic lesion, indicating the possible presence of cryoglobulin (Fig. 4).
Figure 2.
Light microscopy of a kidney specimen. At autopsy, a pathological examination of the kidney revealed global sclerosis in 50% of the total glomeruli with (A) cellular or fibrocellular crescents and (B) MPGN-like lesions in some parts of the glomeruli. (C, D) The interlobular arteries and arterioles were affected by fibrinoid necrosis with neutrophilic infiltration.
Figure 3.
Immunofluorescent staining of the kidney. Immunofluorescent staining of the glomeruli revealed (A) IgG, (D) C3, and (E) MPO positivity. (F) MPO and IgG were co-stained in the glomerular capillary walls.
Figure 4.
Electron microscopy of the kidney. Electron microscopy revealed fibrillary deposits in a lesion of glomerular sclerosis (A; ×1,500, B; ×15,000).
The gastric and duodenal ulcers were accompanied by fibrinoid necrotic vasculitis in the submucosal small arteries, with the infiltration of neutrophils and positive staining of IgM and MPO on the vascular walls (Fig. 5).
Figure 5.
Light microscopy of the gastric mucosa. Light microscopy of gastric and duodenal ulcers revealed fibrinoid necrosis, with neutrophils infiltrating the submucosal small arteries in (A) Hematoxylin and Eosin staining (×400), with positive staining of (C) IgM and (D) MPO on the vascular walls.
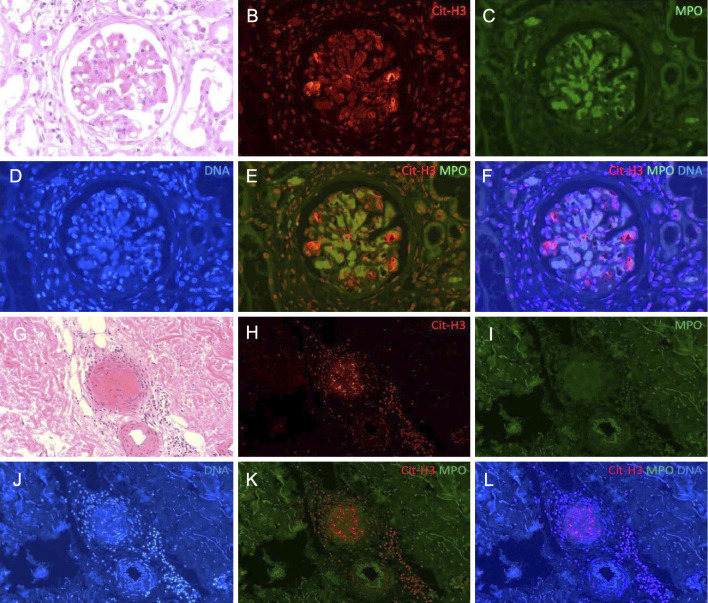
We used immunofluorescence to detect citrullinated histone 3 (Cit-H3) using anti-Cit-H3 (Abcam, ab5103) and goat anti-rabbit IgG H&L (Abcam, ab150080), DNA stained with 4'6-diamidino-2-phenylindole (DAPI), and MPO stained with anti-MPO (Gene Tex, GTX11729) to visualize NET formation in the skin biopsy and autopsy specimens (4). Partial staining of Cit-H3 was observed with extracellular DNA in the glomerulus (Fig. 6A-F) and cutaneous arteries (Fig. 6G-L). Although co-localization was seen with MPO staining in a few parts of the glomeruli (<10%) (Fig. 6F), it was not seen in the cutaneous arteries (Fig. 6L).
Figure 6.
The immunofluorescence findings of NETs in the kidney and skin purpura. The other lesions of glomerulitis (A-F) and cutaneous small arteries of the skin purpura (G-L). (A, G) Hematoxylin and Eosin staining; (B, H) Cit-H3 staining with anti-Cit-H3 and Alexa Fluor 594-conjugated goat anti-rabbit IgG H&L (red); (C, I) MPO staining with FITC-conjugated anti-MPO (green); (D, J) DNA staining with DAPI (blue). Cit-H3 and extracellular DNA were co-localized with MPO in the glomeruli (F), but not in the cutaneous arteries (L). The small arteries of the gastric and duodenal ulcers were negative for Cit-H3.
These findings suggest that the systemic vasculitis in this case involved NET formation caused by MPO-ANCA and/or cryoglobulin.
Discussion
We investigated a case of systemic vasculitis affecting the kidneys, skin, and gastrointestinal tract with serological MPO-ANCA and cryoglobulin. It was difficult to distinguish AAV from CV based on the clinical findings of this case because the clinical signs and symptoms were common to both diseases (9). It was also challenging to determine the etiology of this case using pathological approaches.
AAV is a systemic vasculitis that is probably caused by a circulating autoantibody named ANCA, which targets small- to medium-sized vessels and which may affect any organ in the body. The typical pathological findings in the kidney include pauci-immune necrotizing crescentic glomerulonephritis and fibrinoid necrosis; however, regions of glomerulitis may also be positive for immunoglobulin (10). In observational studies, authors have suggested that NET formation is a key process in the development of organ injury in AAV patients (3, 5).
CV, another type of vasculitis, is caused by cryoglobulins that precipitate at temperatures of <37℃ and which re-dissolve on warming. Cryoglobulinemia induces organ injury through the precipitation of immunoglobulins or immune-complexes consisting of monoclonal or polyclonal IgM and IgG. These cause vascular inflammation through endothelial activation, and vascular occlusion in the microcirculation (11). The pathological findings of CV include inflammatory infiltration in small-to-medium vessels, as well as type I MPGN and hyaline intraluminal thrombi containing IgM, IgG, and C3; however, crescentic glomerulitis or fibrinoid necrosis can also occur (12). The pathological findings in this case were either partially or completely consistent with AAV and/or CV.
Myeloperoxidase is a neutrophil peroxidase enzyme and a major autoantigen that causes tissue injury in patients with MPO-AAV. Several observational studies have reported extracellular MPO staining in patients with MPO-AAV (3, 13, 14). Although extracellular MPO alone is not specific, and irrespective of the cause, neutrophil stimulation may lead to the release of MPO via NET formation or degranulation, and the detection of a large amount of MPO in extracellular lesions is strongly suggestive of MPO-AAV (15).
NET formation is considered to be an important process in the development of tissue injury in AAV patients. Several studies have reported the formation of NETs in the kidney biopsy specimens of these patients (3, 15), as well as patients with lupus nephritis and rheumatoid arthritis. Behnen et al. reported that immobilized immune-complex stimulates the release of NETs in human neutrophils in vitro (16). This indicates the possibility that NET formation may develop in patients with CV; however, the relationship between CV and NETs has not been reported. We believe that the NET formation and strong MPO staining in the present case suggest that the systemic vasculitis was caused by MPO-AAV with the possible involvement of CV.
The relationship between AAV and CV is unknown. The coexistence of ANCA and cryoglobulin is not usually serologically detected; however, there are a few reported cases. Lamprecht et al. reported two cases of CV with increased serum PR3-ANCA (17). The pathological examination of kidney specimens from these cases revealed MPGN and crescentic glomerulitis respectively, and both cases were positive for IgM and C3 deposition. The authors concluded that the pathogenesis of these cases was CV, and that the production of ANCA was secondary. Asai et al. reported a case of systemic vasculitis with an increase in both serum MPO-ANCA and cryoglobulin (18). The examination of a kidney biopsy specimen revealed crescentic glomerulitis with IgG, IgM, and C3 staining and they finally made a diagnosis of AAV. Contrary to our cases, these cases were reported to show a good response to treatment.
In conclusion, our patient developed systemic vasculitis with serological MPO-ANCA and cryoglobulin. The pathological findings included MPO positivity and NET formation in the patient's kidneys and skin. The findings indicated that MPO-AAV and/or CV caused NET formation, leading to systemic vasculitis.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
This case was presented at the 3rd International Renal Pathology Conference 2017 in India. We would like to express our gratitude to the staff of Department of Nephrology, Tokyo Metropolitan Health and Medical Treatment Corporation Okubo Hospital (Tokyo, Japan) for their valuable comments and clinical supports.
References
- 1. Brouet JC, Clauvel JP, Danon F, Klein M, Seligmann M. Biologic and clinical significance of cryoglobulins: a report of 86 cases. Am J Med 57: 775-788, 1974. [DOI] [PubMed] [Google Scholar]
- 2. Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised international chapel hill consensus conference nomenclature of vasculitides. Arthritis Rheum 65: 1-11, 2013. [DOI] [PubMed] [Google Scholar]
- 3. Kessenbrock K, Krumbholz M, Schönermarck U, et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med 15: 623-625, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li P, Li M, Lindberg MR, Kennett MJ, Xiong N, Wang Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med 207: 1853-1862, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Söderberg D, Kurz T, Motamedi A, Hellmark T, Eriksson P, Segelmark M. Increased levels of neutrophil extracellular trap remnants in the circulation of patients with small vessel vasculitis, but an inverse correlation to anti-neutrophil cytoplasmic antibodies during remission. Rheumatology (Oxford) 54: 2085-2094, 2015. [DOI] [PubMed] [Google Scholar]
- 6. Gupta S, Kaplan MJ. The role of neutrophils and NETosis in autoimmune and renal diseases. Nat Rev Neph 12: 402-413, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Suppiah R, Mukhtyar C, Flossmann O, et al. A cross-sectional study of the Birmingham Vasculitis Activity Score version 3 in systemic vasculitis. Rheumatology (Oxford) 50: 899-905, 2011. [DOI] [PubMed] [Google Scholar]
- 8. Watts R, Lane S, Hanslik T, et al. Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann Rheum Dis 66: 222-227, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jennette JC, Ronald JF. Small-vessel vasculitis. N Eng J Med 337: 1512, 1997. [DOI] [PubMed] [Google Scholar]
- 10. Haas M, Eustace JA. Immune complex deposits in ANCA-associated crescentic glomerulonephritis: a study of 126 cases. Kidney Int 65: 2145-2152, 2004. [DOI] [PubMed] [Google Scholar]
- 11. Sansonno D, Tucci FA, Ghebrehiwet B, et al. Role of the receptor for the globular domain of C1q protein in the pathogenesis of hepatitis C virus-related cryoglobulin vascular damage. J Immunol 183: 6013-6020, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Beddhu S, Bastacky S, Johnson JP. The clinical and morphologic spectrum of renal cryoglobulinemia. Medicine (Baltimore) 81: 398-409, 2002. [DOI] [PubMed] [Google Scholar]
- 13. Brouwer E, Huitema MG, Mulder AH, et al. Neutrophil activation in vitro and in vivo in Wegener's granulomatosis. Kidney Int 45: 1120-1131, 1994. [DOI] [PubMed] [Google Scholar]
- 14. Saeki T, Kuroda T, Morita T, Suzuki K, Arakawa M, Kawasaki K. Significance of myeloperoxidase in rapidly progressive glomerulonephritis. Am J Kidney Dis 26: 13-21, 1995. [DOI] [PubMed] [Google Scholar]
- 15. O'Sullivan KM, Lo CY, Summers SA, et al. Renal participation of myeloperoxidase in antineutrophil cytoplasmic antibody (ANCA)-associated glomerulonephritis. Kidney Int 88: 1030-1046, 2015. [DOI] [PubMed] [Google Scholar]
- 16. Behnen M, Leschczyk C, Möller S, et al. Immobilized immune complexes induce neutrophil extracellular trap release by human neutrophil granulocytes via FcγRIIIB and Mac-1. J Immunol 193: 1954-1965, 2014. [DOI] [PubMed] [Google Scholar]
- 17. Lamprecht P, Schmitt WH, Gross WL. Mixed cryoglobulinaemia, glomerulonephritis, and ANCA: essential cryoglobulinaemic vasculitis or ANCA -associated vasculitis? Nephrol Dial Transplant 13: 213-221, 1998. [DOI] [PubMed] [Google Scholar]
- 18. Asai O, Nakatani K, Yoshimoto S, et al. A case of MPO-ANCA-related microscopic polyangiitis with mixed cryogulobulinemia. Nihon Jinzo Gakkai Shi 48: 377-384, 2006(in Japanese, Abstract in English). [PubMed] [Google Scholar]