Abstract
The clinical efficacy and outcomes of pazopanib treatment for metastatic extraosseous Ewing sarcoma remain unclear. We herein report a case of heavily pre-treated metastatic extraosseous Ewing sarcoma in which pazopanib treatment achieved a significant improvement. A 17-year-old girl was referred to our hospital due to metastatic extraosseous Ewing sarcoma. The initial cytotoxic chemotherapy was temporarily effective, however, her disease eventually progressed, and she was subsequently treated with pazopanib. The recurrent tumor showed a marked response to pazopanib therapy; the therapeutic effect has lasted for more than 26 months. The present case suggests that pazopanib may be a therapeutic option for extraosseous Ewing sarcoma.
Keywords: extraosseous Ewing sarcoma, pazopanib
Introduction
Ewing sarcoma commonly occurs in the long bones and deep soft tissues of the extremities in children or adolescents. Multidisciplinary therapy, such as surgery, radiation therapy, and chemotherapy, generally improves the symptoms and outcomes of Ewing sarcoma; however, metastatic Ewing sarcoma is usually difficult to control. Pazopanib is a novel multi-target tyrosine kinase inhibitor that primarily inhibits vascular endothelial growth factor receptor (VEFGR) -1, -2, and -3, platelet-derived growth factor receptor (PDGFR) -α, and -β, and c-kit (1). Pazopanib is approved for the treatment of soft tissue sarcoma after standard chemotherapy in patients with refractory disease (2). Little is known about the clinical activity of pazopanib for Ewing sarcoma, and there have been no reports on the long-term efficacy of pazopanib in its treatment. We herein report a case in which metastatic extraosseous Ewing sarcoma was successfully treated with pazopanib.
Case Report
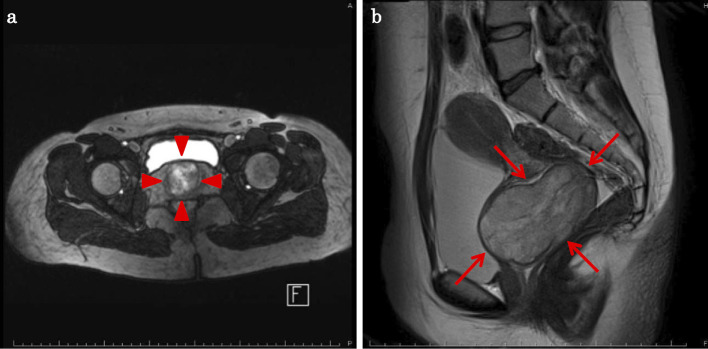
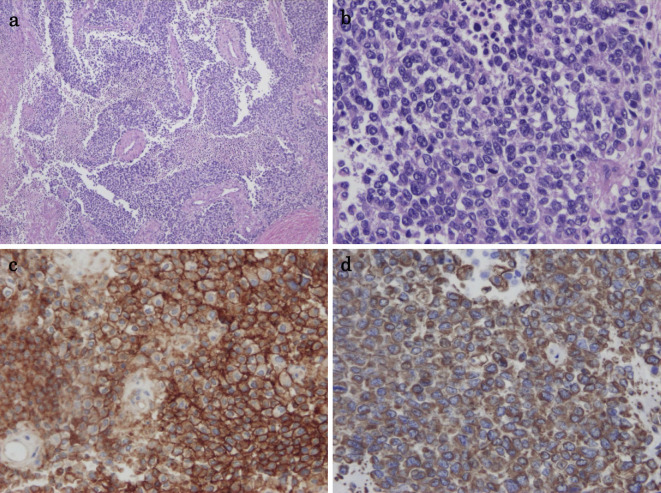
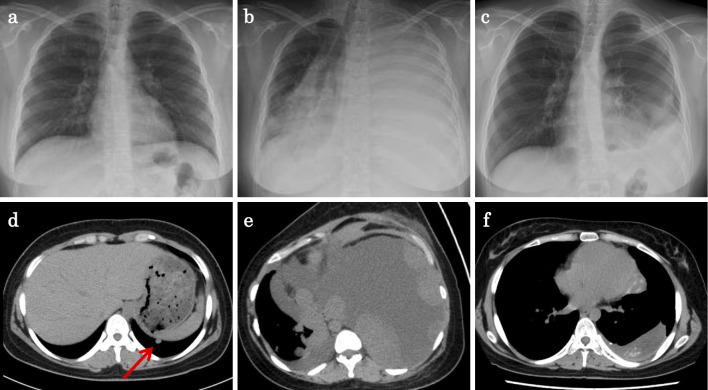
A 17-year-old girl with no relevant medical history was incidentally diagnosed with a vaginal tumor during a routine obstetric examination at 20 weeks of gestation. Magnetic resonance imaging (MRI) demonstrated a solid mass of 40 mm in size in the vaginal wall (Fig. 1a). At 37 weeks of gestation, she presented with genital bleeding and underwent an incident-free emergency cesarean section resulting in the delivery of a healthy male infant. At three months post-delivery, MRI showed that the vaginal wall mass had increased in size to 70 mm (Fig. 1b). Tumor resection was strongly recommended; however, the patient refused this treatment. She was subsequently lost to follow-up. At four months post-delivery, she presented with a loss of consciousness due to anemia and bleeding from a vaginal tumor. Emergency tumor resection was performed. The post-operative pathological examination revealed a monotonous solid and sheet-like proliferation of small round cells (Fig. 2a and b). Immunohistochemical staining was positive for tumor markers, CD99 (Fig. 2c) and vimentin (Fig. 2d). Fluorescence in situ hybridization (FISH) revealed a EWS (Ewing sarcoma) gene split in the tumor cells, indicating EWS gene rearrangement (Fig. 3). MRI also revealed a solid tumor of 16 mm in size in the right sacral ala. A chest X-ray at the initial presentation showed no obvious abnormalities (Fig. 4a); however, computed tomography (CT) revealed a micronodular lesion in the left lower lung field (Fig. 4d). She was finally diagnosed with primary vaginal Ewing sarcoma with metastasis to the bone and lung, and was referred to our hospital for post-operative treatment. She received 6 cycles of chemotherapy with vincristine (2 mg/m2), doxorubicin (75 mg/m2) [or actinomycin D (1.25 mg/m2)] and cyclophosphamide (1,200 mg/m2) [VDC(A)] on day 1 alternating with 5 cycles of chemotherapy with ifosfamide (1,800 mg/m2) and etoposide (100 mg/m2) (IE) on days 1-5 every 3 weeks. Actinomycin D (1.25 mg/m2) was substituted for doxorubicin after the cumulative dose of doxorubicin reached 375 mg/m2. She declined surgical resection due to loss of pregnancy and comorbidities. She subsequently underwent radiotherapy (50 Gy in 25 fractions) for the residual pelvic tumors. Repeat CT and MRI demonstrated partial disease regression; however, she refused further chemotherapy because of intractable nausea and fatigue. At six months after the discontinuation of the initial treatments, metastases developed in the left chest (Fig. 4b and e). She underwent chemotherapy consisting of one cycle of gemcitabine (675 mg/m2 on days 1 and 8) and docetaxel (75 mg/m2 on day 8) (GEM/DTX) and one cycle of carboplatin (300 mg/m2 on days 1-2) and etoposide (100 mg/m2 on days 1-5) (CBDCA/ETP); however, the recurrent tumors showed no response. After 2 months of treatment, her dyspnea was rapidly exacerbated, and she subsequently consented to receiving pazopanib therapy (800 mg/day). The tumors showed remarkable regression (Fig. 4c and f) and her dyspnea improved. After 9 months of pazopanib treatment, Positron emission tomography (PET) revealed the complete regression of tumor. At the time of writing, she has been treated with pazopanib for 26 months. In this time there has been no tumor recurrence, and the only adverse events have been mild hand-foot syndrome and fatigue (grade 2).
Figure 1.
(a) Magnetic resonance imaging (MRI) demonstrated a solid mass of 40 mm in size located at the vaginal wall at 20 weeks of gestation (arrowheads). (b) At three months post-delivery, MRI showed that the vaginal wall mass had increased in size to 70 mm (arrows).
Figure 2.
Low- (a) and high-power (b) views of a specimen of the vaginal tumor (Hematoxylin and Eosin staining) showing a monotonous solid and sheet-like proliferation of small round cells. Immunohistochemical staining was positive for CD99 (c) and vimentin (d).
Figure 3.
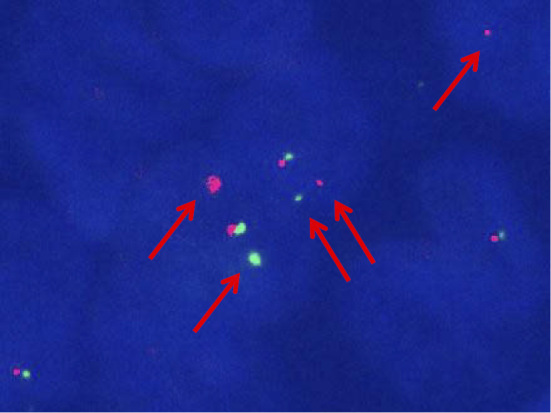
A fluorescence in situ hybridization (FISH) analysis of the Ewing sarcoma (EWS) gene showed the splitting of the green and red signals, indicating EWS gene rearrangement (arrows).
Figure 4.
The imaging findings over the course of treatment. Chest X-ray revealed no obvious abnormalities at the initial presentation (a), a homogenous opacity in the left hemithorax before the initiation of pazopanib, (b) and the decreased tumor size at 11 months after the initiation of pazopanib treatment (c). Computed tomography (CT) showed a micronodular lesion in the left lower lung field on presentation (arrow) (d), massive pleural effusion and multiple tumors in the left hemithorax before the initiation of pazopanib (e), and the decreased tumor size at eleven months after the initiation of pazopanib treatment (f).
Discussion
Ewing sarcoma commonly occurs in the long bones and deep soft tissues of the extremities of children or adolescents. Multidisciplinary therapy consisting of combined chemotherapy and local therapy (surgery and/or radiotherapy) improves the prognosis of Ewing sarcoma; however, metastatic Ewing sarcoma remains difficult to control. The standard chemotherapy for Ewing sarcoma is VDC/IE [vincristine, doxorubicin, cyclophosphamide (VDC), and dactinomycin alternating with IE)]; however, this treatment is often associated with intolerable adverse events (3).
The chimeric fusion oncogene EWS-FLI1 is considered a therapeutic target for Ewing sarcoma based on the causal association with the translocation of the EWS gene. Many novel agents that inhibit the EWS-FLI1 fusion protein have been tested to investigate possible therapeutic applications, with largely unsatisfactory results. New targets for Ewing sarcoma include the angiogenic pathway, the mammalian target of rapamycin (mTOR) and insulin-like growth factor-1 (IGF-1) pathways, the bone microenvironment, the poly ADP-ribose polymerase 1 (PARP1) pathway, and the GD2 ganglioside pathways (4, 5). Previous preclinical studies indicated that vascular endothelial growth factor A (VEGF-A) and platelet-derived growth factor (PDGF) were promising therapeutic targets for Ewing sarcoma (6-8), which suggests that the angiogenic pathway is a potential target.
Pazopanib is not currently a standard chemotherapy for Ewing sarcoma. There are only three reports of successful treatment in the literature, and there are no reports of Ewing sarcoma patients attaining long-term remission with pazopanib treatment. Table summarizes the case reports (including the present case) on the treatment of metastatic Ewing sarcoma with pazopanib. Yamamoto et al. (9) reported a case of recurrent extraosseous Ewing sarcoma of the retroperitoneum that responded well to pazopanib for 3 months. Alcindor (10) reported a case of recurrent metastatic Ewing sarcoma that was refractory to several lines of cytotoxic chemotherapy, and which responded to pazopanib for 12 weeks. Finally, Attia et al. (11) reported a case of metastatic extraosseous Ewing sarcoma that responded to pazopanib for several weeks. In our case, the symptoms of Ewing sarcoma showed a dramatic improvement after the initiation of pazopanib treatment, with the complete regression of the tumor observed on a PET scan at for more than 26 months after the start of treatment, with minimum side effects in a patient who was heavily pretreated by chemotherapy and radiotherapy.
Table.
Reported Cases of Metastatic Ewing Sarcoma Treated with Pazopanib.
| Case | Age/Sex | Site | Pretreatment | Dose of pazopanib | Outcome | Reference |
|---|---|---|---|---|---|---|
| 1 | 62/M | retroperitoneum | None | 800 mg daily | Died 8 months after diagnosis | (9) |
| 2 | 24/F | paravertebral | VDC(A)/IE, RT, CPM/TPT, CPT-11/TMZ, IFM/ETP, temsirolimus | 800 mg daily | Recurrence 12 weeks after treatment | (10) |
| 3 | 69/M | S2 nerve root | Surgery, VDC/IE, RT, CPM/TPT, CPT-11/TMZ | 800 mg daily | Died several weeks after treatment | (11) |
| 4 | 17/F | vagina | VDC(A)/IE, RT, GEM/DTX, CBDCA/ETP | 800mg daily | Alive 26 months after treatment | Present case |
M: male, F: female, VDC(A): vincristine, doxorubicin, cyclophosphamide, (actinomycin D), IE: ifosfamide, etoposide, RT: radiotherapy, CPM: cyclophosphamide, TPT: topotecan, CPT-11: irinotecan, TMZ: temozolomide, IFM: ifosfamide, ETP: etoposide, GEM: gemcitabine, DTX: docetaxel, CBDCA: carboplatin
The present case is also rare in that the tumor was located in the female genital tract; Ewing sarcomas are usually located in the long bones of the extremities and the pelvic bones, while extraosseous Ewing sarcoma is commonly located in the deep soft tissues of the extremities, retroperitoneum, and thorax. Although there have been some case reports of Ewing sarcoma in the ovary or uterus, primary vaginal Ewing sarcoma is extremely rare. Of note, primary vaginal Ewing sarcoma also seems to have a more favorable prognosis in comparison to other sites, possibly because of the initial diagnosis is typically made at an earlier stage and as a result of easier anatomic resection (12).
In summary, we reported a case of metastatic extraosseous Ewing sarcoma that was successfully treated with pazopanib and strongly suggest that further investigations should be undertaken to determine the mechanism underlying the efficacy of pazopanib in Ewing sarcoma. In particular, biomarkers predicting the therapeutic efficacy of pazopanib are needed.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Schutz FA, Choueiri TK, Sternberg CN. Pazopanib: Clinical development of a potent anti-angiogenic drug. Crit Rev Oncol Hematol 77: 163-171, 2011. [DOI] [PubMed] [Google Scholar]
- 2. van der Graaf WT, Blay JY, Chawla SP, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 379: 1879-1886, 2012. [DOI] [PubMed] [Google Scholar]
- 3. Grier HE, Krailo MD, Tarbell NJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med 348: 694-701, 2003. [DOI] [PubMed] [Google Scholar]
- 4. Gaspar N, Hawkins DS, Dirksen U, et al. Ewing sarcoma: current management and future approaches through collaboration. J Clin Oncol 33: 3036-3046, 2015. [DOI] [PubMed] [Google Scholar]
- 5. Biswas B, Bakhshi S. Management of Ewing sarcoma family of tumors: current scenario and unmet need. World J Orthop 7: 527-538, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ikeda AK, Judelson DR, Federman N, et al. ABT-869 inhibits the proliferation of Ewing Sarcoma cells and suppresses platelet-derived growth factor receptor beta and c-KIT signaling pathways. Mol Cancer Ther 9: 653-660, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ackermann M, Morse BA, Delventhal V, Carvajal IM, Konerding MA. Anti-VEGFR2 and anti-IGF-1R-Adnectins inhibit Ewing's sarcoma A673-xenograft growth and normalize tumor vascular architecture. Angiogenesis 15: 685-695, 2012. [DOI] [PubMed] [Google Scholar]
- 8. Hamdan R, Zhou Z, Kleinerman ES. Blocking SDF-1alpha/CXCR4 downregulates PDGF-B and inhibits bone marrow-derived pericyte differentiation and tumor vascular expansion in Ewing tumors. Mol Cancer Ther 13: 483-491, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yamamoto Y, Nozawa M, Shimizu N, Minami T, Yoshimura K, Uemura H. Pazopanib for recurrent extraosseous Ewing's sarcoma of the retroperitoneum. Int J Urol 21: 1183-1184, 2014. [DOI] [PubMed] [Google Scholar]
- 10. Alcindor T. Response of refractory Ewing sarcoma to pazopanib. Acta Oncol 54: 1063-1064, 2015. [DOI] [PubMed] [Google Scholar]
- 11. Attia S, Okuno SH, Robinson SI, et al. Clinical activity of pazopanib in metastatic extraosseous ewing sarcoma. Rare Tumors 7: 5992, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bancalari E, de Alava E, Tardio JC. Primary vaginal Ewing sarcoma: case report and review of the literature. Int J Surg Pathol 20: 305-310, 2012. [DOI] [PubMed] [Google Scholar]