Abstract
Circulating tumor cells (CTCs) are a promising biomarker for several cancers. We streamlined the experimental procedure of CTC immunofluorescent staining. We encountered a 72-year-old woman with metastatic right renal cell carcinoma (RCC) (clinical stage: T4N0M1), whose CTC number rapidly increased after the administration of sunitinib and then gradually decreased. The change in the CTC number appeared to coincide with laboratory data and hypertension, suggesting that a CTC analysis may be useful for promptly monitoring the treatment response. Our data provided the first evidence of an association between the CTC numbers and the treatment response in a metastatic RCC patient.
Keywords: circulating tumor cells, renal cell carcinoma, sunitinib
Introduction
Radiological examinations and serum tumor markers are mainstays for disease monitoring. Therefore, the absence of specific tumor markers for renal cell carcinoma (RCC) together with the limitations of imaging scans make real-time monitoring of RCC progression challenging, giving rise to the need for a new detection method in clinical practice.
Recently, several molecular targeting agents, such as tyrosine kinase inhibitors (TKIs) and mammalian target of rapamycin inhibitors, have become widely available for metastatic RCC. However, the selection of an appropriate drug depends on the clinician's experience rather than biological evidence.
Several reports have shown that circulating tumor cells (CTCs) are a promising biomarker for several cancers (1-4), as CTCs appear to represent cancer characteristics and are associated with the clinical course. In addition, serial CTC monitoring is expected to be a predictive biomarker of treatment response in other cancer types (5-7).
At our institution, we streamlined the experimental procedure of CTC immunofluorescent staining so that it can be completed within a day. In the present study, we evaluated whether or not a CTC analysis is useful as a new clinical biomarker in progressive metastatic RCC.
Case Report
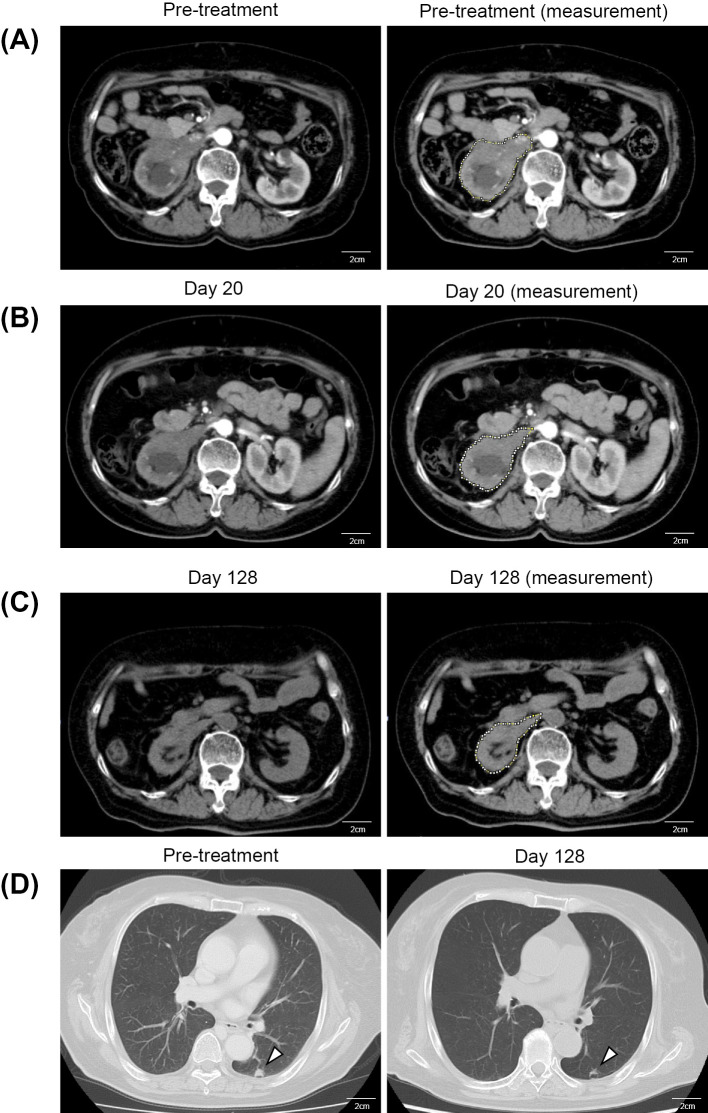
A 72-year-old woman was referred to our hospital because of weight loss and jaundice on 25 April 2016. Because of jaundice, she presented with yellowish discoloration of the bulbar conjunctiva and the skin. Computed tomography (CT) revealed right RCC. The size of the tumor was 30.3 cm2 in area measured, as by the Image-J software program, and directly invaded the inferior vena cava (IVC), the liver, and the right adrenal gland and metastasized to the lung (Fig. 1A and D). The clinical stage was T4N0M1. Notably, a radiological report indicated that the tumor did not show typical characteristics of clear cell RCC, such as early strong enhancement and a washout pattern. Instead, other histological types, particularly papillary type 2 RCC, were more likely because of the robust local invasion.
Figure 1.
Computed tomography (CT) images during the clinical course. (A) Contrast-enhanced abdominal CT image before the treatment. The area of the tumor was 30.3 cm2, measured at the level of the branching of the right renal artery. The analysis was done using the Image-J software program. This image shows that tumor directly invaded the right renal vein and the inferior vena cava (IVC). Scale bars, 2 cm. (B) Contrast-enhanced abdominal CT image on Day 20 after the treatment. The area of the tumor was 26.1 cm2, measured at the level of the branching of the right renal artery. Scale bars, 2 cm. (C) Plain abdominal CT image on Day 128 after treatment. The area of the tumor was 17.6 cm2, measured at the level of the branching of the right renal artery. The analysis was done using the Image-J software program. This image shows a 42% reduction in tumor size compared with pretreatment. Scale bars, 2 cm. (D) Chest CT image of lung metastasis. The arrowheads indicate the lung metastasis. The left image shows pre-treatment left lung metastasis (9.8×8.8 mm). The right image shows the same metastasis on Day 128 after the treatment with sunitinib. Scale bars, 2 cm.
At the initial visit, the serum total bilirubin (T-Bil: 4.29 mg/dL) and serum lactate dehydrogenase (LDH: 161 U/L) exceeded the upper normal limits. Among the adverse prognostic factors of the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) model, this case presented only “less than one year lapsed from the diagnosis to treatment (one month)”. Thus, the disease status was classified as intermediate risk (8). Given the findings suggestive of non-clear RCC, a systemic treatment for metastatic RCC, sunitinib (37.5 mg/day for 2 weeks followed by 1 week off schedule to reduce side effects) (9), was administered for the first time on 27 April 2016. Sunitinib is a multi-receptor-targeted TKI that is recommended for non-clear RCC therapy in ESMO Guideline 2016 (10).
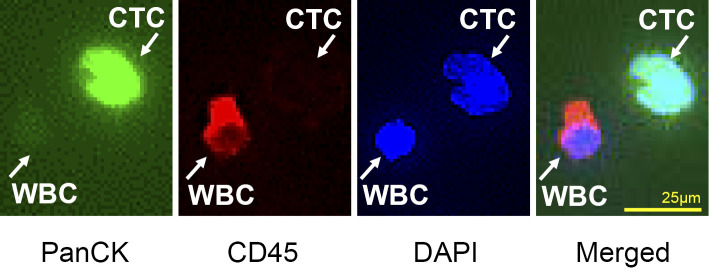
To assess the treatment efficacy, we performed a CTC analysis by immunofluorescent staining. Research involving human samples was approved by the institutional review board. The patient provided her written informed consent. The detection of CTC was done using a CytoQuest CR (Abnova, Taipei, Taiwan) (Fig. 2) in accordance with the manufacturer's protocol. The Cytoquest CR positively selects CTCs using anti-epithelial-cell-adhesion molecule (EpCAM)-capturing antibody. First, 4.0 mL of blood was drawn into an EDTA-2Na collection tube. Next, buffy coat containing CTCs was separated by gradient cell separation. After washing with RPMI medium, the prepared buffy coat was injected into an EpCAM-coated microfluidic slide. The captured cells were immunostained with the antibody cocktail containing anti-pan-cytokeratin (PanCK) antibody (FITC), anti-CD45 antibody (PE), and DAPI. Stained cells were observed with a fluorescence microscope (Keyence, Osaka, Japan). The CTCs were defined as PanCK (+), CD45 (-), and DAPI (+) cells, whereas white blood cells (WBCs) were defined as PanCK (-), CD45 (+), and DAPI (+) cells (Fig. 3). The CTC count was done manually before sunitinib administration and on Days 2, 4, 10, and 20 during treatment to monitor the change in CTC number.
Figure 2.

The semi-automatic CytoQuest CR to enrich CTC. The CytoQuest CR captures CTC from buffy coat in EpCAM-coated slide. CTC: circulating tumor cell, EpCAM: epithelial-cell-adhesion molecule
Figure 3.
Immunofluorescent staining of CTCs and WBCs. Immunofluorescent images of CTCs and WBCs collected by CytoQuest CR are shown. The CTCs are captured on an EpCAM-coated slide. The CTCs stain positive for PanCK, an epithelial maker, and negative for CD45, a hematopoietic marker. The WBCs stain negative for PanCK and positive for CD45. CTCs: circulating tumor cells, EpCAM: epithelial-cell-adhesion molecule, PanCK: pan-cytokeratin, WBC: white blood cells
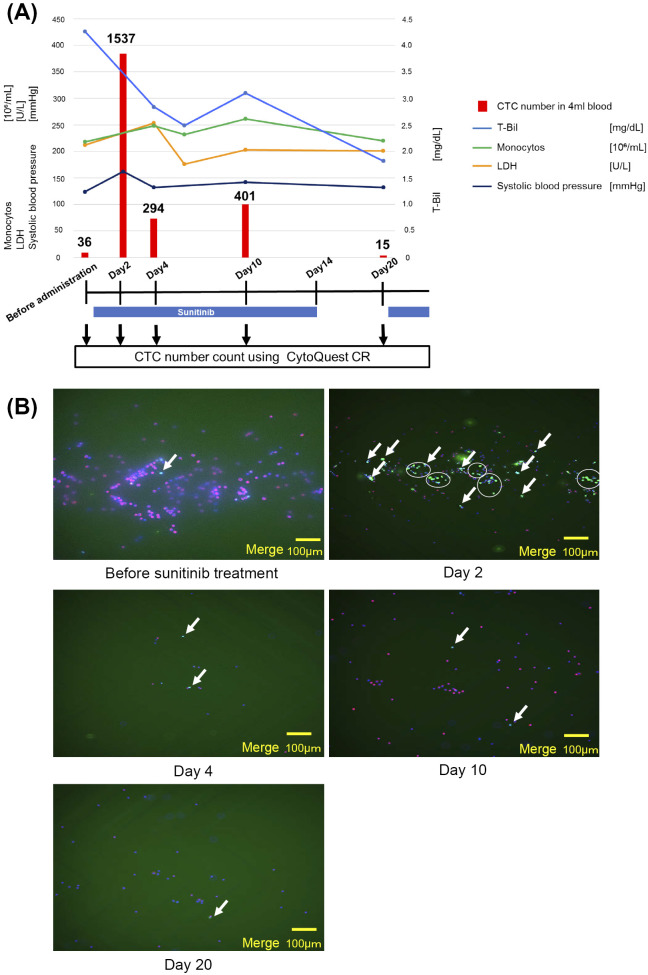
We detected CTCs before sunitinib treatment and on Days 2, 4, 10, and 20 during the treatment. Details on the CTC number, blood pressure, and laboratory data such as LDH, monocyte and T-Bil are shown in (Fig. 4). Following the administration of sunitinib, the CTC number increased rapidly on Day 2, presumably due to an oncolytic event, and then decreased gradually from Day 4 until Day 20, followed by a slight and transient increase on Day 10. Regarding the laboratory data, immediately after the administration of sunitinib, there were transient increases in the LDH and monocyte values. T-Bil decreased soon after the treatment but increased transiently on Day 10, similar to the CTC number and other laboratory data. On Day 20, the CTC number and LDH dropped below the pre-treatment levels, and T-Bil declined to 2.5 mg/dL These improvements in data allegedly occurred as a result of the relief of tumor occlusion in the IVC.
Figure 4.
Clinical course, changes in the laboratory data and CTC number. The CTC analysis and routine blood tests were conducted before the treatment and on Days 2, 4, 10, and 20 during the treatment. Down-pointing arrows indicate CTC analysis points. Sunitinib administration is indicated with blue bars (37.5 mg/day for 2 weeks followed by 1 week off). The vertical axis on the left shows the values of monocytes, lactate dehydrogenase (LDH), and systolic blood pressure. The vertical axis on the right shows the values of total bilirubin (T-Bil). The CTC numbers are indicated with red bars with the actual numbers on the top. (B) Low-magnitude images of CTCs before sunitinib administration and at Days 2, 4, 10, and 20 after treatment. The white arrows indicate CTCs, and aggregated CTCs are circled. Only merged images are shown in this figure. CTCs: circulating tumor cells, LDH: lactate dehydrogenase
In terms of adverse events, grade 3 asymptomatic hypertension according to the Criteria for Adverse Events (NCI CTCAE, version 4.0) occurred on Days 2 and 10, and grade 2 hypothyroidism occurred on Day 87 (TSH 35.9 μIU/mL, FT3 2.5 pg/mL, FT4 1.0 ng/dL). An antihypertensive drug (amlodin 5 mg) was prescribed on Day 2, and the blood pressure returned to normal after Day 10. Thus, sunitinib was continued without dose reduction.
A CT examination on Day 128 showed a 14% reduction in the size of the primary lesion with shrinkage of the lung metastasis (Fig. 1D). In addition, the primary lesion further shrank by 28% (Fig. 1A-C). The area of the tumor was measured at the level of branching of the right renal artery using the Image-J software program.
Discussion
In the present patient, we performed a CTC analysis along with a routine blood examination and CT for real-time monitoring of the disease status. In order to capture rare CTCs in the peripheral blood, we employed a CTC detection device, the CytoQuest CR, which enabled the detection of CTCs within a day after blood collection. The CTC number rapidly increased after sunitinib administration and gradually decreased afterwards. Given the concurrent adverse events and concordant fluctuations in some laboratory data, the changes in the CTC number may reflect the treatment efficacy.
T-Bil served as a biomarker of the treatment response in the present patient, as the direct tumor invasion to the liver resulted in a deteriorated liver function. The concordant fluctuation of the CTC number with the T-Bil value indicated that the monitoring of the CTC number is a promising biomarker for predicting the treatment response.
Regarding adverse events, a rise in blood pressure (Day 2) appeared to coincide with an increase in the CTC number, in addition to hypothyroidism that occurred in the late course of treatment. Since the occurrence of adverse events is reported to be associated with an improved clinical outcome in patients receiving sunitinib (11), we surmise that sunitinib exerted anti-cancer effects on the tumor, including the one projecting into the IVC, leading to the influx of dissociated cancer cells into the circulation. Together, the adverse events and increase in CTC number may be indicative of a favorable outcome. In line with our hypothesis, the tumor size decreased in a time-dependent manner (Fig. 1B and C). Consistent with our findings, several earlier studies reported that CTCs more promptly reflect the treatment response than current imaging techniques (12).
However, CTC detection is generally difficult in RCC patients due to the low expression of EpCAM, especially in clear cell RCC, which accounts for the majority of RCC cases (13). In our study, despite the fact that EpCAM was targeted to capture RCC-derived CTCs, we were still able to capture a number of CTCs, possibly due to the pathological features of this case of RCC, as our patient did not present with the typical characteristics of clear cell RCC on CT. Indeed, an earlier study reported that chromophobe and papillary type RCC express more EpCAM than clear cell type (13).
Regarding the utility of CTC as a novel biomarker, Blumke et al. reported that the detection of cytokeratin-positive CTC in metastatic RCC patients was a poor independent prognostic factor (14). Furthermore, in other types of cancers, such as prostate cancer and colorectal cancer, intensive studies have shown that CTC enumeration using the CellSearch system is reflective of the treatment response and prognosis (15, 16). However, the real-time monitoring of the CTC number following treatment was not performed in earlier studies. Although we agree that the presence of CTC per se reflects disease aggressiveness, our results suggest that an increase in the CTC number as a result of an oncolytic event may be a favorable biomarker. In addition, single cell pick-up from the slide followed by whole-genome amplification allows for the detection of loss-of-function mutations in key genes, such as CDKN2A and fumarate hydratase, in our assay (17).
Several limitations associated with this study must be considered. First, there may be some observer bias when counting the CTC number. To eliminate this bias, observers were not notified of the treatment course. Second, the positive selection of CTCs requires EpCAM expression on the surface of CTCs, which, in turn, eliminates EpCAM-negative CTCs that underwent epithelial-mesenchymal transition. Furthermore, due to the lack of a consensus on RCC markers, a molecular analysis of CTC could not be performed in this study. Finally, a pathological diagnosis was not made in this case, since surgical resection was not performed due to an inoperable tumor burden.
Our data provided the first evidence of an association between the number of CTCs and the treatment response in a metastatic RCC patient. A large-scale study is warranted for the validation of these findings.
Author's disclosure of potential Conflicts of Interest (COI).
Shigeo Horie: Research funding, Astellas and Sanofi Aventis.
Acknowledgement
We thank the nurses at the Department of Urology, Juntendo University Hospital for collecting the blood samples and the staff at Kyodo-ken for their technical assistance.
References
- 1. Bidard FC, Peeters DJ, Fehm T, et al. . Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol 15: 406-414, 2014. [DOI] [PubMed] [Google Scholar]
- 2. Krebs MG, Sloane R, Priest L, et al. . Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J Clin Oncol 29: 1556-1563, 2011. [DOI] [PubMed] [Google Scholar]
- 3. Cohen SJ, Punt CJ, Iannotti N, et al. . Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol 26: 3213-3221, 2008. [DOI] [PubMed] [Google Scholar]
- 4. Antonarakis ES, Lu C, Luber B, et al. . Androgen receptor splice variant 7 and efficacy of taxane chemotherapy in patients with metastatic castration-resistant prostate cancer. JAMA Oncol 1: 582-591, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maheswaran S, Sequist LV, Nagrath S, et al. . Detection of mutations in EGFR in circulating lung-cancer cells. New Engl J Med 359: 366-377, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bardia A, Haber DA. Solidifying liquid biopsies: can circulating tumor cell monitoring guide treatment selection in breast cancer? J Clin Oncol 32: 3470-3471, 2014. [DOI] [PubMed] [Google Scholar]
- 7. Smerage JB, Barlow WE, Hortobagyi GN, et al. . Circulating tumor cells and response to chemotherapy in metastatic breast cancer: SWOG S0500. J Clin Oncol 32: 3483-3489, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ko JJ, Xie W, Kroeger N, et al. . The International Metastatic Renal Cell Carcinoma Database Consortium model as a prognostic tool in patients with metastatic renal cell carcinoma previously treated with first-line targeted therapy: a population-based study. Lancet Oncol 16: 293-300, 2015. [DOI] [PubMed] [Google Scholar]
- 9. Lee JL, Kim MK, Park I, et al. . RandomizEd phase II trial of Sunitinib four weeks on and two weeks off versus Two weeks on and One week off in metastatic clear-cell type REnal cell carcinoma: RESTORE trial. Ann Oncol 26: 2300-2305, 2015. [DOI] [PubMed] [Google Scholar]
- 10. Escudier B, Porta C, Schmidinger M, et al. . Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 27: v58-v68, 2016. [DOI] [PubMed] [Google Scholar]
- 11. Rini BI, Cohen DP, Lu DR, et al. . Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst 103: 763-773, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Budd GT, Cristofanilli M, Ellis MJ, et al. . Circulating tumor cells versus imaging--predicting overall survival in metastatic breast cancer. Clin Cancer Res 12: 6403-6409, 2006. [DOI] [PubMed] [Google Scholar]
- 13. Patriarca C, Macchi RM, Marschner AK, Mellstedt H. Epithelial cell adhesion molecule expression (CD326) in cancer: a short review. Cancer Treat Rev 38: 68-75, 2012. [DOI] [PubMed] [Google Scholar]
- 14. Bluemke K, Bilkenroth U, Meye A, et al. . Detection of circulating tumor cells in peripheral blood of patients with renal cell carcinoma correlates with prognosis. Cancer Epidemiol Biomarkers Prev 18: 2190-2194, 2009. [DOI] [PubMed] [Google Scholar]
- 15. Matsusaka S, Suenaga M, Mishima Y, et al. . Circulating tumor cells as a surrogate marker for determining response to chemotherapy in Japanese patients with metastatic colorectal cancer. Cancer Sci 102: 1188-1192, 2011. [DOI] [PubMed] [Google Scholar]
- 16. Okegawa T, Nutahara K, Higashihara E. Prognostic significance of circulating tumor cells in patients with hormone refractory prostate cancer. J Urol 181: 1091-1097, 2009. [DOI] [PubMed] [Google Scholar]
- 17. Linehan WM, Spellman PT, Ricketts CJ, et al. ; The Cancer Genome Atlas Research Network. . Comprehensive molecular characterization of papillary renal-cell carcinoma. N Engl J Med 374: 135-145, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]