Abstract
We herein report a patient with Miller Fisher syndrome mimicking Tolosa-Hunt syndrome. A 47-year-old man presented with right orbital pain and diplopia. On a neurological examination, he had right oculomotor nerve palsy and diminished deep tendon reflexes. Brain magnetic resonance imaging failed to show any parenchymal lesions; however, the bilateral oculomotor nerves were gadolinium-enhanced. The presence of a triad of orbital pain, ipsilateral oculomotor nerve palsy, and a rapid response to steroid therapy met the diagnostic criteria for Tolosa-Hunt syndrome. After discharge, antibodies against GQ1b and GT1a were reported to be positive only with phosphatidic acid. The present case was ultimately diagnosed as an incomplete phenotype of Miller Fisher syndrome.
Keywords: Miller Fisher syndrome, Tolosa-Hunt syndrome, antiganglioside antibodies, phosphatidic acid
Introduction
Miller Fisher syndrome (MFS) is characterized by a triad of ataxia, ophthalmoparesis, and areflexia and recognized as a variant of Guillain-Barré syndrome (GBS) (1), with antiganglioside antibodies playing a major role in its pathogenesis (2). Tolosa-Hunt syndrome (THS) is characterized by orbital pain accompanied by diplopia, with a granulomatous inflammatory process pathophysiologically involved (3,4).
We herein report a case of MFS presenting with unilateral ophthalmoparesis and orbital pain resembling the clinical features of THS. An assay of antiganglioside antibodies with a mixture of phosphatidic acid was useful for the detection of antiganglioside antibodies, resulting in the ultimate diagnosis of MFS.
Case Report
A 47-year-old man was admitted to our hospital because of diplopia and pulsating headache. The patient had been well until two weeks before the admission, when he had a fever for several days. Ten days prior to the admission, he became aware of a tingling sensation in his right first and second fingers. He had been bitten on the left leg by an unidentified insect at the same time. He was evaluated at another clinic, where carpal tunnel syndrome was suspected. Three days prior to the admission, he developed diplopia and right orbital pain accompanied by bilateral throbbing headache.
On an examination, his body temperature was 36.5℃; his other vital signs were normal. Erythema was noted on the left thigh. On a neurological examination, ptosis and restriction of the ocular movement only in the right eye were noted. The ocular movement was restricted in abduction, elevation, and depression; the abduction was mostly restricted (Fig. 1). No anisocoria was noted, and the light reflex was prompt in both eyes. Other cranial nerves were intact. Weakness of the extremities was not noted, although the deep tendon reflexes were diminished in the upper extremities and absent in the lower extremities. The other findings of the neurological examination were normal.
Figure 1.
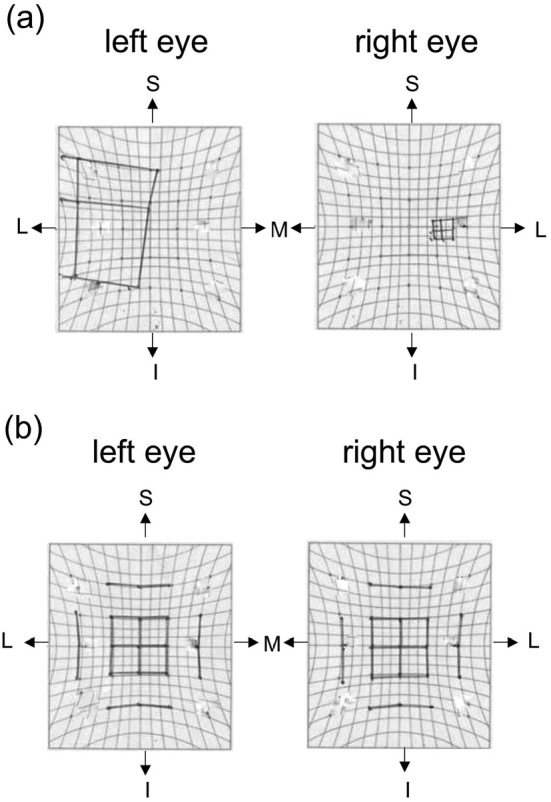
Hess charts plotted on admission (a) and two months later (b). The Hess chart on admission showed underactivity of the right medial rectus, superior rectus, and inferior rectus muscles, compatible with the right oculomotor nerve palsy (a). Two months later, the right oculomotor nerve palsy was improved (b). S: superior, L: lateral, M: medial, I: inferior, R: right
The findings of a complete blood count and metabolic panel were normal. Tests for acetylcholine receptor antibody, HIV antibody, myeloperoxidase anti-neutrophil cytoplasmic antibody, and antinuclear antibody were all negative. The serum levels of vitamin B1 and B12, hemoglobin A1c, and angiotensin-converting enzyme were normal. The thyroid function was normal. A lumbar puncture was performed. There was no pleocytosis; however, the protein content and IgG index were increased to 67 mg/dL and 0.67, respectively. Nerve conduction studies as well as the F-wave response were all normal. Brain magnetic resonance imaging (MRI) showed normal findings except for the enhancement of both oculomotor nerves by gadolinium-enhanced T1-weighted image (Fig. 2). The enhancement was more apparent in the right oculomotor nerve than in the left one (Fig. 2). Enhanced lumbar MRI showed no enhancement of the cauda equina. A biopsy of the erythema on the left leg was performed under suspicion of Lyme disease; the findings were negative.
Figure 2.
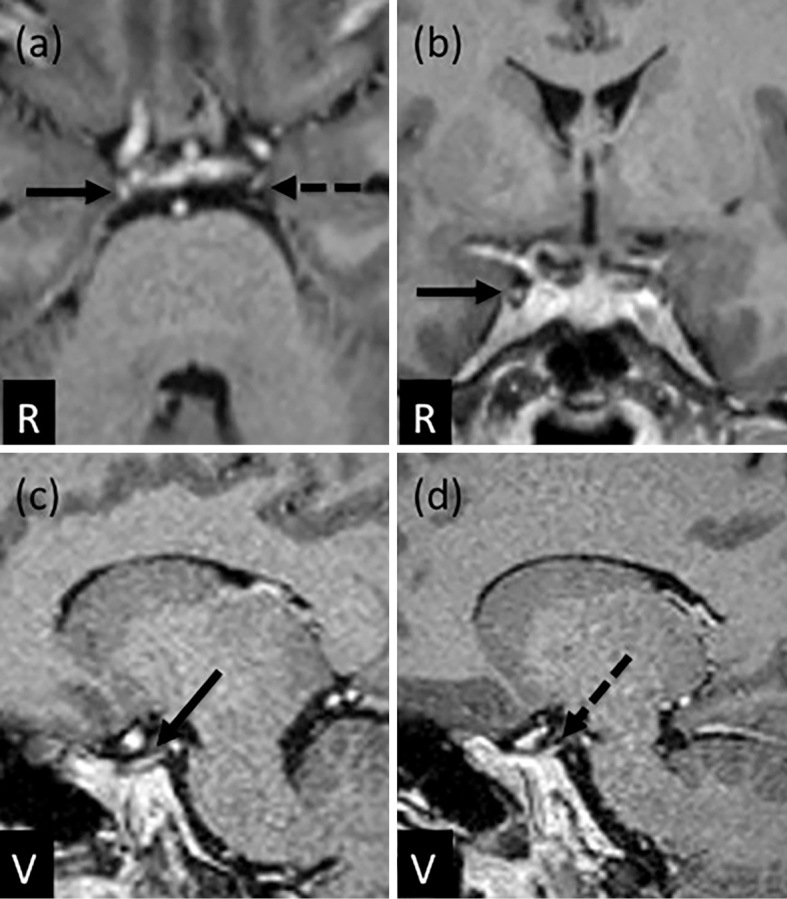
Gadolinium-enhanced T1-weighted magnetic resonance imaging: axial (a), coronal (b), and sagittal (c, d) views. The right oculomotor nerve was enhanced clearly in axial (a: arrow), coronal (b: arrow), and sagittal (c: arrow) views. The enhancement of the left oculomotor nerve was noted in axial (a: dotted arrow) and sagittal (d: dotted arrow) views. R: right, V: ventral
A combination of unilateral oculomotor nerve palsy and ipsilateral orbital pain made THS the most likely diagnosis. While the decreased tendon reflex and disturbed extraocular movement suggested MFS, the laterality of oculomotor nerve involvement and accompanying throbbing headache were not consistent with the typical clinical features of MFS. A tentative diagnosis of THS was established, and prednisolone (PSL) at 40 mg/day was started. The orbital pain and ptosis were markedly improved within 48 hours after the administration of PSL. This prompt response to corticosteroid as well as the orbital pain and deficit in the third nerve met the international headache society criteria for THS. He was discharged with a daily intake of 30 mg of PSL, which was tapered over 2 months. Over this two-month period, no change in the deep tendon reflexes was noted, with reflexes still diminished in the upper extremities and absent in the lower extremities. After his discharge, the results of antiganglioside antibodies were obtained: antibodies against GQ1b and GT1a were positive only with phosphatidic acid (PA). His ophthalmoplegia fully recovered by two months later (Fig. 1). The patient was ultimately diagnosed with MFS.
Discussion
MFS is a syndrome classically characterized by a triad of ophthalmoplegia, areflexia, and ataxia (1). Diverse clinical features are known to occur in patients with MFS (2,5,6); however, the clinical features of unilateral ophthalmoplegia and its rapid response to corticosteroid are not typical of classical MFS. In the present case, rapid improvements in the right orbital pain as well as ptosis of the right side were obtained by corticosteroid administration.
The pathophysiological mechanisms underlying MFS have yet to be precisely elucidated; however, complement activation is considered to play a major role in developing anti-ganglioside antibody-meditated neuropathy (7). Willison et al. advocated the complement-mediated distal motor nerve and motor nerve terminal injury in association with anti-GQ1b, anti-DG1a, and anti-GM1 ganglioside antibodies (7). This model of anti-GQ1b and pore-forming membrane attack complex-mediated injury depends on an exogenous source of complements (7). Steroid reduces the production of and deactivates complements (8). As the turnover duration of compliments is as short as half a day (8), the rapid improvement of oculomotor nerve injury noted in this case can be attributed to the effects of corticosteroid. To our knowledge, there have been four reported cases of MFS in which steroid was effective (9-11). All four cases were reported to be recurrent MFS. Steroid treatment was performed in their second attack in all four cases, and a rapid response to steroids was observed within a few days (9-11). This suggests that there may be a small subset of patients in whom steroids are effective.
In the present case, antibody against GQ1b was detected only with PA. Kusunoki et al. reported that the binding activity of anti-GM1 immunoglobulin G antibody was increased when the wells of a plate were coated with ganglioside and PA by enzyme-linked immunosorbent assay (12). This greater binding activity was considered to be derived from a possible conformational epitope formed by ganglioside and PA and/or a more effective way of GM1 presentation with PA (12).
The neuroradiological findings in patients with THS include mass lesions in or dilatation of the cavernous sinus (13). In the present case, MRI showed the enhancement of both oculomotor nerves, although only the right oculomotor nerve was clinically affected. The enhancement was observed proximal as well as distal to the cavernous sinus and was more apparent in the right oculomotor nerve than in the left one. Although the number of reported cases is small, the abnormal gadolinium enhancement of cranial nerves on MRI has been reported in patients with MFS (14-18). Most of the reported enhanced cranial nerves were symptomatic; however, one study reported the enhancement of clinically silent cranial nerves in patients with MFS. Recently, Malhotra et al. reviewed 17 pediatric patients with MFS and reported that the gadolinium enhancement of clinically silent optic nerves was noted in 3 patients (18). This suggests that the MRI findings do not always correlate with the clinical manifestations of cranial nerve involvement in MFS cases (18). Alternatively, steroid treatment may have prevented the left oculomotor nerve from becoming clinically affected.
The present case developed retro-orbital pain and throbbing headache in association with right oculomotor nerve palsy. Headache is not a commonly reported symptom in patients with MFS. However, two of three patients in Fisher's original report were reported to have headache (1). The pathogenesis of headache in patients with MFS has yet to be elucidated; however, possible explanations include the effects of increased cerebrospinal fluid protein and the activation of the trigeminovascular pain pathway elicited by the demyelination of cranial nerves. Friedman et al. reported a 35-year-old man with MFS, who had headache at the onset (19). They suggested that the headache was attributable in part to antibodies against either GD3 or GQ1b.
In conclusion, we herein report a case of MFS mimicking THS. For patients with ipsilateral oculomotor nerve with orbital pain in whom THS is suspected, an assay of antiganglioside antibodies should be considered.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We thank Dr. Kusunoki (Kindai University) for measuring the antiganglioside antibodies.
References
- 1. Fisher M. An unusual variant of acute idiopathic polyneuritis (syndrome of ophthalmoplegia, ataxia and areflexia). N Engl J Med 255: 57-65, 1956. [DOI] [PubMed] [Google Scholar]
- 2. Teener JW. Miller Fisher's syndrome. Semin Neurol 32: 512-516, 2012. [DOI] [PubMed] [Google Scholar]
- 3. Tolosa E. Periarteritic lesions of the carotid siphon with the clinical features of a carotid infraclinoidal aneurysm. J Neurol Neurosurg Psychiatry 17: 300-302, 1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hunt WE, Meagher JN, Lefever HE, Zeman W. Painful opthalmoplegia. Its relation to indolent inflammation of the carvernous sinus. Neurology 11: 56-62, 1961. [DOI] [PubMed] [Google Scholar]
- 5. Mori M, Kuwabara S, Fukutake T, Yuki N, Hattori T. Clinical features and prognosis of Miller Fisher syndrome. Neurology 56: 1104-1106, 2001. [DOI] [PubMed] [Google Scholar]
- 6. Tatsumoto M, Odaka M, Hirata K, Yuki N. Isolated abducens nerve palsy as a regional variant of Guillain-Barré syndrome. J Neurol Sci 243: 35-38, 2006. [DOI] [PubMed] [Google Scholar]
- 7. Willison HJ, Halstead SK, Beveridge E, et al. . The role of complement and complement regulators in mediating motor nerve terminal injury in murine models of Guillain-Barré syndrome. J Neuroimmunol 201-202: 172-182, 2008. [DOI] [PubMed] [Google Scholar]
- 8. Kondo M. [Complementology for clinicians]. Nihon Naika Gakkai Zassi (J Jpn Soc Intern Med) 89(Suppl): 67-69, 2000(in Japanese). [Google Scholar]
- 9. Madhavan S, Geetha , Bhargavan PV. Recurrent Miller Fisher syndrome. J Assoc Physicians India 52: 582-584, 2004. [PubMed] [Google Scholar]
- 10. Toru S, Ohara M, Hane Y, Ishiguro T, Kobayashi T. Successful steroid treatment for recurrent Miller Fisher syndrome. Muscle Nerve 45: 763-764, 2012. [DOI] [PubMed] [Google Scholar]
- 11. Grosso S, Verrotti A, Tei M, Cornacchione S, Giannini F, Balestri P. Recurrent Miller Fisher syndrome in children. Pediatr Neurol 50: 269-271, 2014. [DOI] [PubMed] [Google Scholar]
- 12. Kusunoki S, Morita D, Ohminami S, Hitoshi S, Kanazawa I. Binding of immunoglobulin G antibodies in Guillain-Barré syndrome sera to a mixture of GM1 and a phospholipid: possible clinical implications. Muscle Nerve 27: 302-306, 2003. [DOI] [PubMed] [Google Scholar]
- 13. de Arcaya AA, Cerezal L, Canga A, Polo JM, Berciano J, Pascual J. Neuroimaging diagnosis of Tolosa-Hunt syndrome: MRI contribution. Headache 39: 321-325, 1999. [DOI] [PubMed] [Google Scholar]
- 14. Nagaoka U, Kato T, Kurita K, et al. . Cranial nerve enhancement on three-dimensional MRI in Miller Fisher syndrome. Neurology 47: 1601-1602, 1996. [DOI] [PubMed] [Google Scholar]
- 15. Pedotti R, Carpo M, Lucchi S, Righini A, Scarlato G, Nobile-Orazio E. Lumbosacral root and facial nerve enhancement in Miller Fisher syndrome. J Neurol 245: 753-754, 1998. [DOI] [PubMed] [Google Scholar]
- 16. Hattori M, Takada K, Yamada K, Kamimoto K, Mitake S. A case of Miller Fisher syndrome with gadolinium-enhancing lesions in the cranial nerves and the cauda equina on magnetic resonance imaging. Rinsho Shinkeigaku (Clin Neurol) 39: 1054-1058, 1999(in Japanese). [PubMed] [Google Scholar]
- 17. Garcia-Rivera CA, Rozen TD, Zhou D, et al. . Miller Fisher syndrome: MRI findings. Neurology 57: 1755, 2001. [DOI] [PubMed] [Google Scholar]
- 18. Malhotra A, Zhang M, Wu X, Jindal S, Durand D, Makhani N. MRI findings of optic pathway involvement in Miller Fisher syndrome in 3 pediatric patients and a review of the literature. J Clin Neurosci 39: 63-67, 2017. [DOI] [PubMed] [Google Scholar]
- 19. Friedman DI, Potts E. Headache associated with Miller Fisher syndrome. Headache 47: 1347-1348, 2007. [DOI] [PubMed] [Google Scholar]


