Abstract
There is a critical need to identify patients with radiation-resistant tumors early after treatment commencement. In this study, we use diffuse reflectance spectroscopy (DRS) to investigate changes in vascular oxygenation and total hemoglobin concentration in A549 radiation-sensitive and resistant tumors treated with a clinically relevant dose fraction of 2 Gy. DRS spectra were acquired before, immediately after, 24, and 48 hours after radiation. Our data reveals a significantly higher reoxygenation (sO2) in the radiation-resistant tumors 24 and 48h after treatment, and provides promising evidence that DRS can discern between the reoxygenation trends of radiation-sensitive and resistant tumors.
1. Introduction
Radiation therapy is typically delivered in several fractions to treat cancers because dose fractionation is believed to reoxygenate and hence radiosensitize the tumor. The central hypothesis of this theory is that the death of oxygenated cells in response to a radiation dose fraction improves the oxygenation of previously hypoxic cells, thus radiosensitizing these cells to subsequent radiation fractions [1]. The oxygen-sensing pO2 microelectrode has established a wealth of knowledge related to tumor oxygenation before and during radiation therapy, and its association with treatment outcome [2–7]. Gatenby et al. assessed pretreatment oxygen tension in advanced head and neck tumors in the clinical setting and observed that complete responders were characterized by a significantly higher oxygen content compared to those in the non-responder group. Their findings strongly suggested that hypoxia within metastatic lesions was linked to a poor prognosis after radiotherapy [5]. Similar findings were later reported by Brizel et al. [2], who showed that hypoxia adversely affected the short term clinical response to radiation, disease-free survival and overall survival in patients with head and neck squamous cell carcinoma (HNSCC). Ressel et al. monitored changes in pO2 in HNSCC patients, and found that tumors with the best long-term outcome displayed a significant increase in the median pO2 during therapy [8]. In paclitaxel-treated MCA-4 murine mammary carcinoma tumors, Milas et al. observed that reoxygenation was the dominant mechanism by which paclitaxel greatly enhanced tumor radioresponse [9]. Although these studies and others [10,11] have offered compelling evidence that tumor reoxygenation before and between radiation fractions is critically associated with treatment response, others have shown that reoxygenation after radiation is also associated with tumor recurrence [12,13]. In a clinical study, Dietz et al. measured pO2 in cervical lymph node metastases of 37 patients with advanced HNSCC before and one week after chemoradiation. Their findings revealed that a higher degree of reoxygenation was correlated with a poor treatment outcome, and also suggested that pretreatment hypoxia held a poor prognostic significance for advanced HNSCC [12].
Given the invasive nature of the oxygen-sensing electrode and its inability to perform frequent longitudinal measurements of the same tissue, we sought to determine if a non-invasive approach involving diffuse reflectance spectroscopy (DRS) was sensitive to radiation-induced changes in tumor oxygenation, and whether such changes were different in radiation-sensitive and resistant tumor xenografts. DRS is an optical fiber-based technique that uses visible or near-infrared light to interrogate tissue; the sampling depth within tissue is dependent on the separation of source and detector optical fibers, and the reflected light is used to quantify the underlying scatterers and absorbers, a combination of which is used for recognition of tissue pathology. The vascular oxygenation (sO2) in tumors can be quantified by determining the individual contributions of major absorbers, such as oxygenated (HbO2) and deoxygenated (dHb) hemoglobin within the blood vessels [14–17]. We recently demonstrated that vascular oxygenation was inversely correlated with immunohistochemical quantification of tumor hypoxic fraction [18]. In addition, such measurements have been shown to be concordant with microelectrode-based determinations of tumor pO2 [19]. DRS has previously been shown to be sensitive to radiation dose-dependent changes in oxygenation during therapy [13]. Furthermore, such optical measurements of oxygenation were shown to be predictive of local control and treatment failure after radiation in a human head and neck tumor xenograft model [13,20]. However, studies examining the sensitivity of optical spectroscopy to changes in oxygenation after clinically relevant radiation dose fractions have been lacking. The goal of this study was to determine the sensitivity of DRS to changes in tumor oxygenation in response to dose fractions that were closer to the clinical paradigm. To perform these investigations, we used a matched model of radiation resistance to generate an isogenic radiation-resistant cell line from human A549 lung cancer cells. Whereas most investigations studying the physiologic and molecular changes associated with radiotherapy establish responder and non-responder groups following a high radiation dose, this study was conducted with tumor xenografts generated from a pair of isogenic radiation-resistant and sensitive cancer cell lines, where the radioresistance had already been established. The tumor xenografts developed from these cell lines in athymic mice were radiated with 4 doses of 2 Gy X-ray radiation therapy. DRS spectra were acquired every day during the course of treatment, allowing the evaluation of short-term changes in tumor oxygenation, and the cumulative effects of radiation following each dose. Our results point to radiation-induced changes in oxygenation in both sensitive and resistant tumors.
2. Methods
2.1 Cell culture and development of tumor xenografts
A549 lung cancer cells were obtained from American Type Culture Collection (ATCC; CCL185). A matched radiation-resistant clone (rA549) was developed from the A549 cells by repeated exposure to radiation (25 fractions of 2 Gy each every 3 days) as described previously [21]. Swiss athymic (nu/nu) mice that were 8-10 weeks old and weighing between 20 and 25 g were injected on the left or right flank with a subcutaneous bolus (10 million cells suspended in serum- and media-free saline) of A549 or rA549 cells to grow tumor xenografts. Once tumor volumes reached 200 mm3, mice were assigned to 1 of 4 groups depending on whether they received radiation therapy (A549-XT and rA549XT) or sham radiation (A549-NT and rA549-NT). A total of 30 mice and 32 tumors were used across all four groups. The number of mice allocated to each group can be seen in Table 1. Tumor volumes were monitored using Vernier calipers calculated according to the equation V = (π/6) x (length) x (width) x (height).
Table 1. Tumor distribution among control and experimental groups.
| Group | Cell Line | No. of mice | No. of tumors |
|---|---|---|---|
| Radiation-sensitive tumors (NT) | A549 | 8 | 9 |
| Radiation-sensitive tumors (XT) | A549 | 7 | 7 |
| Radiation-resistant tumors (NT) | rA549 | 7 | 8 |
| Radiation-resistant tumors (XT) | rA549 | 8 | 8 |
|
| |||
| Total No. of Tumors | 30 | 32 | |
2.2 Fractionated radiation therapy of tumor xenografts
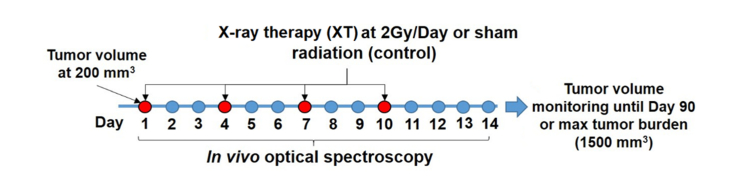
Mice in the XT groups were administered radiation therapy at a dose of 2 Gy twice a week for two weeks [Fig. 1], for a total dose of 8 Gy as described in previous studies [22]. Radiotherapy was performed in the X-RAD 320 animal cabinet (Precision X-Ray, North Branford, CT) by placing the animals on a platform 50 mm away from the X-ray source. A collimated X-ray beam with a dose rate of 0.88 Gy/min delivered a 2 Gy dose to the tumors. Mice were anesthetized using a mixture of isofluorane and room air (1.5% v/v) introduced into the radiator through an access port. All parts of the animal except the tumor were shielded using lead blocks. Mice were monitored daily and tumors were excised either at Day 90 post-radiation or when tumor volume reached 1500 mm3.
Fig. 1.
Timeline for study design. Red time points indicate days of radiation administration at a dose of 2 Gy. DRS spectra were collected before and after each radiation dose. The blue time points indicate collection of DRS spectra during the course of radiation therapy, and after radiation therapy on days 11-14.
2.3 Diffuse reflectance spectroscopy
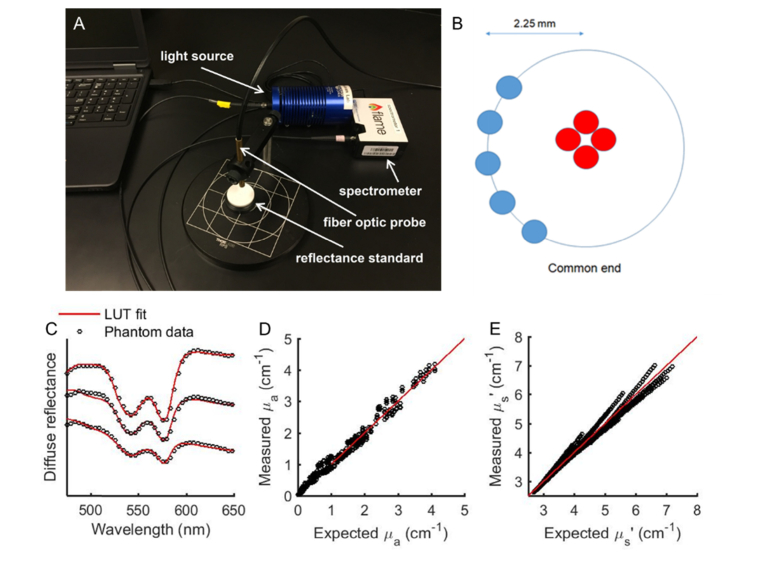
The DRS setup consisted of a tungsten halogen lamp (HL-2000, Ocean Optics; Dunedin, Florida), a fiber optic spectrometer (Flame, Ocean Optics; Dunedin, Florida), and a fiber optic probe (dia. = 200 μm, NA = 0.22; FiberTechOptica, Ontario, Canada) with source-detector separation of 2.25 mm [Fig. 2(a)]. The probe consists of four central fibers for illumination and five peripheral fibers for collecting the diffusely reflected light from tissue [Fig. 2(b)]. An 80% reflectance standard was used to correct the wavelength-dependent daily changes in lamp throughout and calculate diffuse reflectance. DRS spectra were acquired in the wavelength range of 480 to 600 nm. Pre-radiation DRS measurements were performed at 50, 100, 150, and 200 mm3. DRS spectra were acquired prior to and immediately after radiation, on days 1, 4, 7, and 10, as indicated by the red time points shown in Fig. 1. DRS spectra were also acquired on intervening days (blue time points in Fig. 1) when radiation therapy was not performed to obtain data from 24 and 48 hours post-radiation, and also following the end of radiation therapy, on days 11 through 14.
Fig. 2.
(a) Experimental DRS setup shows fiber optic probe, spectrometer, light source, and reflectance standard (b) Optical probe design with source-detector separation of 2.25mm. (c) DRS spectra and respective LUT-fits from 3 representative tissue-simulating phantoms with varying levels of scattering and absorption. (d) and (e) Scatter plots of known versus measured values of µa and µs’, where solid line indicates perfect agreement.
2.4 Quantification of tissue optical properties
An empirical lookup table (LUT)-based inverse model was used to quantify vascular oxygenation (sO2), total hemoglobin concentration (THb), and tissue scattering from DRS spectra [Figs. 1(c)-1(e)] [14,15]. Validation studies in tissue-simulating phantoms of known optical properties found that the LUT model had an error of less than 10% for absorption and less than 2% for scattering for the source-detector separation used in this study 2.25 mm [Figs. 2(c)-2(e)]. The tissue phantoms were created with scattering (µ’s(λ) = 1.93-10.92 cm−1) and absorption parameters (µa(λ) = 0-2.35 cm−1) in a physiologically relevant range. Additionally, based on measurements in optical phantoms, the sensing depth was determined to be in the range of 1.5 to 1.8 mm [15].
2.5 Statistical analysis
All statistical analysis was performed using JMP (SAS, Cary, NC). Nested, two-way analysis of variance (ANOVA) was used to determine statistical significant differences in sO2, and THb between the different groups. The tumor group and radiation dose were considered fixed effects while the mice nested within each group were considered random effects. Interactions between all effects were also considered. Post-hoc Tukey HSD tests were used to evaluate statistical significance between specific groups.
3. Results
3.1 Representative DRS spectra and extracted absorption before and after radiation
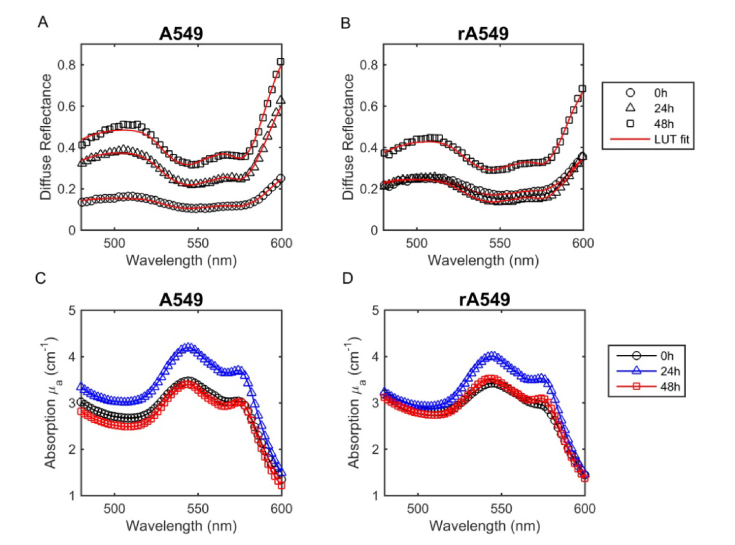
Figures 3(a) and 3(b) present DRS spectra from representative radiation-sensitive (A549) and radiation-resistant (rA549) tumors. These spectra were collected at three different time points: before radiation (0h), 24 hours post-radiation (24h), and 48 hours post-radiation (48h). The symbols indicate the in vivo measurements while the solid lines indicate the LUT model fit. Based on the LUT model fits to the DRS spectra, Figs. 3(c) and 3(d) present the corresponding wavelength-dependent absorption coefficients. In both radiation-sensitive (A549) and resistant (rA549) tumors, the flatter HbO2 absorption bands before radiation (0h) indicate a lower oxygenation when compared to the 24h and 48h time points. Post-radiation, the absorption peaks of oxygenated hemoglobin appear more prominent, indicating an increase in sO2 in both tumors. We also observed a higher magnitude in the absorption coefficient 24h after radiation in the radiation-sensitive tumors, when compared to the pre-radiation and 48h time points. Based on these apparent differences, we proceeded to quantify vascular oxygenation and THb in both the radiation-sensitive and resistant groups.
Fig. 3.
Representative in vivo DRS spectra and absorption spectra prior to radiation (0h), 24 hours post-radiation, and 48 hours post-radiation. Spectra collected from A549 parental tumors (a,c) and rA549 radiation-resistant tumors (b,d). The measured diffuse reflectance and absorption are presented as symbols and the solid lines indicate the LUT model fit.
3.2 Radiation-induced changes in vascular oxygenation across all radiation doses
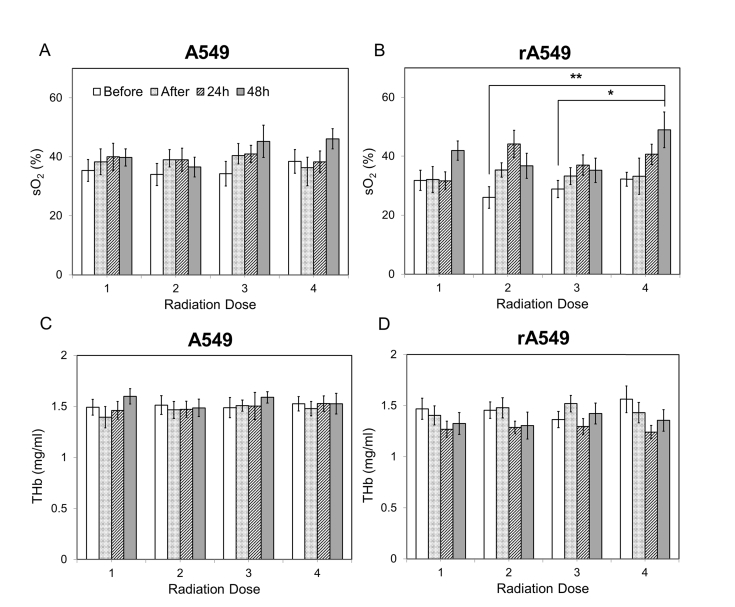
Figure 4 illustrates mean vascular oxygenation and THb values across all four doses for all four groups. For each dose, data for the following time points is illustrated: before radiation (0h), 1 hour after, 24 hours after, and 48 hours after radiation. Statistical analysis of the sO2 data revealed a significant main effect of time for the radiation-sensitive tumors (p = 0.04). For the radiation-resistant tumors, we observed a more pronounced and highly significant increase in sO2 over time (P = 0.0006). More specifically, for the radiation-resistant group, we observed that vascular oxygenation 48h after the 4th dose was significantly greater than sO2 at the pre-radiation time point for both 2nd (p = 0.008) and 3rd (p = 0.04) radiation doses [Fig. 4(b)]. The data also indicated that sO2 immediately before each radiation dose always returned to the pre-treatment sO2 levels of the previous dose. No statistically significant main effects or interactions were found in THb for both tumor groups. However, a trend towards decreased THb after each dose was observed in the radiation-resistant tumors. To further investigate the time-dependent changes post-radiation, we grouped all four doses into four different time points to observe the short-term changes in sO2 and THb.
Fig. 4.
Changes in vascular oxygenation and THb across all four doses of radiation for both radiation-sensitive (a,c) and resistant tumors (b,d). Each radiation dose presents the mean sO2 and THb values for the following time points: 0h, immediately after, 24h, and 48h post radiation. Data shown as mean ± SEM. * indicates p<0.05 and ** p<0.01.
3.3 Short term changes in vascular oxygenation
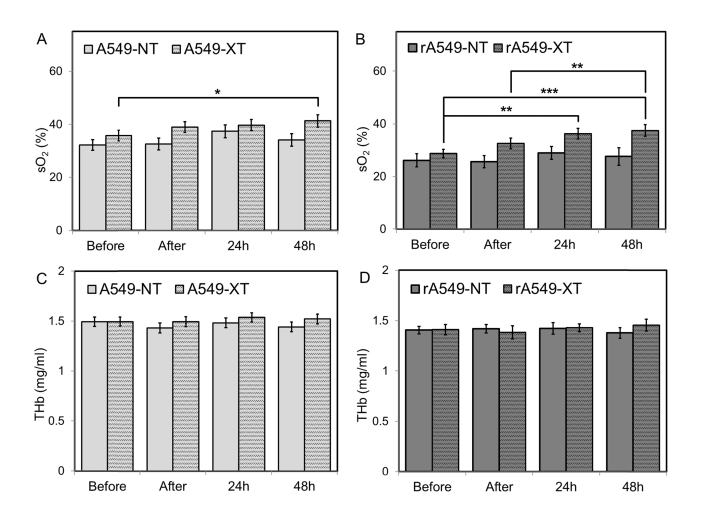
Figure 5 illustrates sO2 and THb values between treated and non-treated groups for both radiation-sensitive and resistant tumors. Each bar illustrates the mean value computed across all four radiation doses for each respective time point (before, 1h after, 24h, or 48h). Oxygenation was found to be significantly different between treated and non-treated groups for radiation-resistant tumors and as a function of time. Post-hoc analysis revealed that sO2 48h post-radiation was significantly greater, when compared to before radiation (p = 0.02) in the A549 tumors [Fig. 5(a)]. For the rA549-XT group, there was a statistically significant increase in sO2 24h (p = 0.003) and 48h (p = 0.0001) post-radiation when compared with pre-radiation measurements [Fig. 5(b)]. In addition, sO2 was also higher 48h after radiation when compared with the 1h post-radiation measurements (p = 0.002). However, no significant changes were found in THb across time or between untreated and treated groups.
Fig. 5.
Short term changes in vascular oxygenation (a, b) and THb (c,d) before, immediately after, 24 hours, and 48h post radiation for all four groups. Data shown as mean ± SEM. * indicates p < 0.05, ** p< 0.01, *** p< 0.001.
3.4 Short term changes in oxygenated and deoxygenated hemoglobin
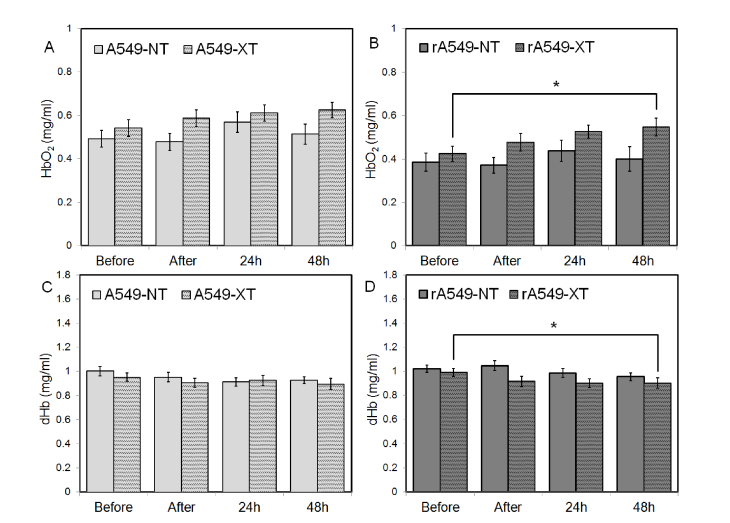
Given the absence of any significant short-term changes in THb, we investigated the changes in oxygenated (HbO2) and deoxygenated hemoglobin (dHb) at the same time points (before, 1h after, 24h, and 48h) to identify the source responsible for driving the changes in sO2. Figure 6 presents mean values of HbO2 and dHb between the treated and untreated groups for both radiation-sensitive and resistant tumors. We found that radiation caused a significant change in in HbO2 and dHb in the radiation-resistant tumors. Specifically we noted that HbO2 was significantly greater 48h post-radiation compared with the pre-radiation measurement (p = 0.04) [Fig. 6(b)]. We also observed that dHb was significantly lower 48h post-radiation when compared with the pre-radiation measurement. (p = 0.047) [Fig. 6(d)]. No significant changes in either HbO2 or dHb were observed in the untreated and treated radiation-sensitive tumors [Figs. 6(a) and 6(c)].
Fig. 6.
Short term changes in oxygenated (HbO2) (a,b) and deoxygenated (dHb) (c,d) hemoglobin before, immediately after, 24 hours, and 48h post radiation for all four groups. Data shown as mean ± SEM. * indicates p < 0.05.
4. Discussion
Although previous studies have investigated tumor reoxygenation following radiation in pre-clinical animal models, the data available covers tumor oxygenation changes following either significantly high radiation doses on consecutive days, or a single high dose [13,20]. Our findings demonstrate that diffuse optical spectroscopy is sensitive to radiation-induced changes in vascular oxygenation when therapeutically relevant doses are administered. Previous studies have shown that reoxygenation depends on both tumor model and radiation dose [23,24]. For the first time, we report changes in reoxygenation kinetics measured in tumors established from a matched model of radiation resistance following a 2Gy radiation dose. By generating a radiation-resistant cancer cell line through the repeated exposure of a parental line to radiation, we avoid the underlying genetic differences present between two different tumor models, and further reduce the contributions that intrinsic cellular properties of different cell lines could be making to the changes we observed.
The mechanism of reoxygenation due to fractionated radiotherapy has been attributed to various processes over the years. Kallman et al. in 1972 proposed four mechanisms by which reoxygenation occurs: reduced O2 metabolism, improved circulation, shrinkage, and migration of surviving cells [25]. Subsequent studies have further investigated the factors responsible for reoxygenation and found that a decrease in oxygen consumption by the tumor cells, and/or an increase in oxygen delivery play a role in reoxygenation [26]. We did not observe significant difference in tumor growth between the radiation-sensitive and radiation-resistant tumors (data not shown). This was most likely due to the lower radiation dose used and the frequency of administration (every 72 hours). However, even when significant changes in tumor size were not present, we were still able to detect functional changes within the tumor with DRS, following such a low radiation dose.
We observed significant differences in oxygenation for the radiation-resistant tumors at time points that we did not observe in the radiation-sensitive tumors. Specifically, we noted a significant increase in oxygenation in the resistant tumors 24 hours after radiation (Fig. 5). An increase in reoxygenation was also observed in the radiation-sensitive tumors; however, this increase was not significant. These findings are in agreement with the studies showing that reoxygenation after radiation can also be a characteristic of radiation-resistant tumors [12,13,20]. In a clinical study with advanced HNSCC patients, Dietz et al. [12] investigated the link between reoxygenation during therapy and the clinical outcome, and observed that the lower the degree of reoxygenation, the better the initial response and clinical outcome; i.e. a higher degree of reoxygenation was more likely to lead to a poor outcome. This is similar to the reoxygenation trend we observed, where the radiation-resistant tumors would be analogous to the poor clinical outcome observed in their study. Similarities in reoxygenation trends between the radiation-resistant and sensitive tumors were also observed. Forty-eight hours after radiation, both radiation-sensitive and resistant tumors display a significant increase in reoxygenation, although the radiation-sensitive tumors present a rather marginal increase in reoxygenation at this time point compared to the resistant tumors [Fig. 5].
Although we see changes in oxygenation, most pointedly in the radiation-resistant tumors, we do not see changes in THb for either radiation-resistant or sensitive tumors. This is particularly noteworthy because THb has been used as a marker for perfusion [27], and changes in perfusion following radiation therapy were observed by several investigators [26,28]. Previous studies have investigated changes in tumor perfusion and oxygenation following radiation therapy and found that an increase in tumor perfusion takes place 24 and 48h after irradiation [28–30]. Crokart et al. characterized changes in the microenvironment of FSaII tumors after a 2Gy radiation dose and observed an increase in tumor perfusion as early as 4h following irradiation [26]. This is very different from what we observed in our study, given that we failed to see any changes in perfusion (THb). Goda et al. investigated changes in oxygen tension and blood perfusion in MTG-B and RIF-1 tumors before and after a single 20Gy radiation dose, and observed a significant decrease in both oxygen tension and tumor blood perfusion 24h after radiation. This observation led them to conclude that changes in perfusion after radiation are one of the main causes of reoxygenation within the tumor [28]. Considering we administered a 2Gy dose, this difference in dosage could explain why we do not observe the same changes in tumor perfusion that they did 24h post-radiation.
Given that our data did not reveal any changes in THb, we considered possible changes in oxygen consumption. From one of our previous studies investigating the early metabolic changes in the A549 and rA549 cell lines in culture, we observed that the radiation-resistant cells displayed decreased levels of oxygen consumption compared to the radiation-sensitive cells both at baseline and 24h after radiation (2Gy) [21]. Following a 2Gy dose, the radiation-resistant cells appear to decrease their oxygen consumption even more and resort to increased glycolysis to counter-act the ROS-induced toxicity [31]. Furthermore, we found that hypoxia-inducible factor (HIF-1α) protein content was significantly greater in the radiation-resistant cells after radiation compared to the radiation-sensitive cells. Coupling this data from the cells with our in vivo studies, we believe that the reoxygenation observed in the radiation-resistant tumors could be mediated by a decrease in oxygen consumption, which leads to the higher oxygen saturation we were able to detect with DRS. Hu et al. observed very similar results in the inconsistent relationship between THb and changes in sO2. They suggested that the improvement in oxygenation they observed after radiation might be due to the decreased oxygen consumption in the tumor due to tumor cell death. They observed these effects before they noted any anatomical changes in the tumor burden. Their results, just as ours, suggest that changes in perfusion are not directly responsible for changes observed in reoxygenation [13]. The significant changes in HbO2 and dHb in the radiation-resistant tumors [Fig. 6] lead us to believe that the primary drivers of changes in sO2 are local changes in cellular oxygen consumption due to either cell death or as a method to avoid ROS-induced toxicity. Immunohistochemical (IHC) assessments of perfusion, cell death, and DNA damage are necessary to further tease apart these two phenomena, which are part of our ongoing work.
In conclusion, we report the short term reoxygenation trends in a matched model of radiation resistance immediately after, 24h, and 48h after radiation. We demonstrate that DRS was sensitive to changes in oxygenation following a clinically relevant dose of radiation. Although we see a clear distinction in reoxygenation at the 48h post radiation mark between the radiation-resistant and radiation-sensitive tumors, both tumors display a reoxygenation trend following radiation. This suggests that although DRS is sensitive to radiation-induced changes in oxygenation between resistant and sensitive tumors, monitoring of reoxygenation alone may not provide a holistic understanding of the underlying functional changes taking place during radiation therapy to predict therapy response. Other molecular and metabolic markers could shed more light on the pathways involved in the development and maintenance of radiation resistance.
Funding
Arkansas Biosciences Institute (NR); Center for Microbial Pathogenesis and Host Inflammatory Responses (RPMD: NIGMS COBRE P20GM103625).
Disclosures
The authors declare that there are no conflicts of interest related to this article.
References and links
- 1.Withers H. R., “The four r’s of radiotherapy,” Adv. Radiat. Biol. 5, 241–271 (1975). 10.1016/B978-0-12-035405-4.50012-8 [DOI] [Google Scholar]
- 2.Brizel D. M., Sibley G. S., Prosnitz L. R., Scher R. L., Dewhirst M. W., “Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck,” Int. J. Radiat. Oncol. Biol. Phys. 38(2), 285–289 (1997). 10.1016/S0360-3016(97)00101-6 [DOI] [PubMed] [Google Scholar]
- 3.Nordsmark M., Bentzen S. M., Rudat V., Brizel D., Lartigau E., Stadler P., Becker A., Adam M., Molls M., Dunst J., Terris D. J., Overgaard J., “Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study,” Radiother. Oncol. 77(1), 18–24 (2005). 10.1016/j.radonc.2005.06.038 [DOI] [PubMed] [Google Scholar]
- 4.Rudat V., Stadler P., Becker A., Vanselow B., Dietz A., Wannenmacher M., Molls M., Dunst J., Feldmann H. J., “Predictive value of the tumor oxygenation by means of pO2 histography in patients with advanced head and neck cancer,” Strahlenther. Onkol. 177(9), 462–468 (2001). 10.1007/PL00002427 [DOI] [PubMed] [Google Scholar]
- 5.Gatenby R. A., Kessler H. B., Rosenblum J. S., Coia L. R., Moldofsky P. J., Hartz W. H., Broder G. J., “Oxygen distribution in squamous cell carcinoma metastases and its relationship to outcome of radiation therapy,” Int. J. Radiat. Oncol. Biol. Phys. 14(5), 831–838 (1988). 10.1016/0360-3016(88)90002-8 [DOI] [PubMed] [Google Scholar]
- 6.Lartigau E., Lusinchi A., Weeger P., Wibault P., Luboinski B., Eschwege F., Guichard M., “Variations in tumour oxygen tension (pO2) during accelerated radiotherapy of head and neck carcinoma,” Eur. J. Cancer 34(6), 856–861 (1998). 10.1016/S0959-8049(97)10172-1 [DOI] [PubMed] [Google Scholar]
- 7.Hockel M., Schlenger K., Aral B., Mitze M., Schaffer U., Vaupel P., “Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix,” Cancer Res. 56(19), 4509–4515 (1996). [PubMed] [Google Scholar]
- 8.Ressel A., Weiss C., Feyerabend T., “Tumor oxygenation after radiotherapy, chemotherapy, and/or hyperthermia predicts tumor free survival,” Int. J. Radiat. Oncol. Biol. Phys. 49(4), 1119–1125 (2001). 10.1016/S0360-3016(00)01523-6 [DOI] [PubMed] [Google Scholar]
- 9.Milas L., Hunter N. R., Mason K. A., Milross C. G., Saito Y., Peters L. J., “Role of reoxygenation in induction of enhancement of tumor radioresponse by paclitaxel,” Cancer Res. 55(16), 3564–3568 (1995). [PubMed] [Google Scholar]
- 10.Grau C., Overgaard J., “The influence of radiation dose on the magnitude and kinetics of reoxygenation in a C3H mammary carcinoma,” Radiat. Res. 122(3), 309–315 (1990). 10.2307/3577761 [DOI] [PubMed] [Google Scholar]
- 11.Olive P. L., “Radiation-induced reoxygenation in the SCCVII murine tumour: evidence for a decrease in oxygen consumption and an increase in tumour perfusion,” Radiother. Oncol. 32(1), 37–46 (1994). 10.1016/0167-8140(94)90447-2 [DOI] [PubMed] [Google Scholar]
- 12.Dietz A., Vanselow B., Rudat V., Conradt C., Weidauer H., Kallinowski F., Dollner R., “Prognostic impact of reoxygenation in advanced cancer of the head and neck during the initial course of chemoradiation or radiotherapy alone,” Head Neck 25(1), 50–58 (2003). 10.1002/hed.10177 [DOI] [PubMed] [Google Scholar]
- 13.Hu F., Vishwanath K., Salama J. K., Erkanli A., Peterson B., Oleson J. R., Lee W. T., Brizel D. M., Ramanujam N., Dewhirst M. W., “Oxygen and perfusion kinetics in response to fractionated radiation therapy in FaDu head and neck cancer xenografts are related to treatment outcome,” Int. J. Radiat. Oncol. Biol. Phys. 96(2), 462–469 (2016). 10.1016/j.ijrobp.2016.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajaram N., Nguyen T. H., Tunnell J. W., “Lookup table-based inverse model for determining optical properties of turbid media,” J. Biomed. Opt. 13(5), 050501 (2008). 10.1117/1.2981797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nichols B. S., Rajaram N., Tunnell J. W., “Performance of a lookup table-based approach for measuring tissue optical properties with diffuse optical spectroscopy,” J. Biomed. Opt. 17(5), 057001 (2012). 10.1117/1.JBO.17.5.057001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palmer G. M., Ramanujam N., “Monte Carlo-based inverse model for calculating tissue optical properties. Part I: Theory and validation on synthetic phantoms,” Appl. Opt. 45(5), 1062–1071 (2006). 10.1364/AO.45.001062 [DOI] [PubMed] [Google Scholar]
- 17.Brown J. Q., Wilke L. G., Geradts J., Kennedy S. A., Palmer G. M., Ramanujam N., “Quantitative optical spectroscopy: a robust tool for direct measurement of breast cancer vascular oxygenation and total hemoglobin content in vivo,” Cancer Res. 69(7), 2919–2926 (2009). 10.1158/0008-5472.CAN-08-3370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dadgar S., Troncoso J. R., Rajaram N., “Optical spectroscopic sensing of tumor hypoxia,” J. Biomed. Opt. 23(6), 1–6 (2018). 10.1117/1.JBO.23.6.067001 [DOI] [PubMed] [Google Scholar]
- 19.Palmer G. M., Viola R. J., Schroeder T., Yarmolenko P. S., Dewhirst M. W., Ramanujam N., “Quantitative diffuse reflectance and fluorescence spectroscopy: tool to monitor tumor physiology in vivo,” J. Biomed. Opt. 14(2), 024010 (2009). 10.1117/1.3103586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vishwanath K., Klein D., Chang K., Schroeder T., Dewhirst M. W., Ramanujam N., “Quantitative optical spectroscopy can identify long-term local tumor control in irradiated murine head and neck xenografts,” J. Biomed. Opt. 14(5), 054051 (2009). 10.1117/1.3251013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alhallak K., Jenkins S. V., Lee D. E., Greene N. P., Quinn K. P., Griffin R. J., Dings R. P. M., Rajaram N., “Optical imaging of radiation-induced metabolic changes in radiation-sensitive and resistant cancer cells,” J. Biomed. Opt. 22(6), 060502 (2017). 10.1117/1.JBO.22.6.060502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stein A. P., Swick A. D., Smith M. A., Blitzer G. C., Yang R. Z., Saha S., Harari P. M., Lambert P. F., Liu C. Z., Kimple R. J., “Xenograft assessment of predictive biomarkers for standard head and neck cancer therapies,” Cancer Med. 4(5), 699–712 (2015). 10.1002/cam4.387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koutcher J. A., Alfieri A. A., Devitt M. L., Rhee J. G., Kornblith A. B., Mahmood U., Merchant T. E., Cowburn D., “Quantitative changes in tumor metabolism, partial pressure of oxygen, and radiobiological oxygenation status postradiation,” Cancer Res. 52(17), 4620–4627 (1992). [PubMed] [Google Scholar]
- 24.Rofstad E. K., DeMuth P., Fenton B. M., Sutherland R. M., “31P nuclear magnetic resonance spectroscopy studies of tumor energy metabolism and its relationship to intracapillary oxyhemoglobin saturation status and tumor hypoxia,” Cancer Res. 48(19), 5440–5446 (1988). [PubMed] [Google Scholar]
- 25.Kallman R. F., “The phenomenon of reoxygenation and its implications for fractionated radiotherapy,” Radiology 105(1), 135–142 (1972). 10.1148/105.1.135 [DOI] [PubMed] [Google Scholar]
- 26.Crokart N., Jordan B. F., Baudelet C., Ansiaux R., Sonveaux P., Grégoire V., Beghein N., DeWever J., Bouzin C., Feron O., Gallez B., “Early reoxygenation in tumors after irradiation: determining factors and consequences for radiotherapy regimens using daily multiple fractions,” Int. J. Radiat. Oncol. Biol. Phys. 63(3), 901–910 (2005). 10.1016/j.ijrobp.2005.02.038 [DOI] [PubMed] [Google Scholar]
- 27.Sunar U., Quon H., Durduran T., Zhang J., Du J., Zhou C., Yu G., Choe R., Kilger A., Lustig R., Loevner L., Nioka S., Chance B., Yodh A. G., “Noninvasive diffuse optical measurement of blood flow and blood oxygenation for monitoring radiation therapy in patients with head and neck tumors: a pilot study,” J. Biomed. Opt. 11(6), 064021 (2006). 10.1117/1.2397548 [DOI] [PubMed] [Google Scholar]
- 28.Goda F., Bacic G., O’Hara J. A., Gallez B., Swartz H. M., Dunn J. F., “The relationship between partial pressure of oxygen and perfusion in two murine tumors after X-ray irradiation: a combined gadopentetate dimeglumine dynamic magnetic resonance imaging and in vivo electron paramagnetic resonance oximetry study,” Cancer Res. 56(14), 3344–3349 (1996). [PubMed] [Google Scholar]
- 29.Sonveaux P., Dessy C., Brouet A., Jordan B. F., Grégoire V., Gallez B., Balligand J. L., Feron O., “Modulation of the tumor vasculature functionality by ionizing radiation accounts for tumor radiosensitization and promotes gene delivery,” FASEB J. 16(14), 1979–1981 (2002). 10.1096/fj.02-0487fje [DOI] [PubMed] [Google Scholar]
- 30.Dewhirst M. W., Oliver R., Tso C. Y., Gustafson C., Secomb T., Gross J. F., “Heterogeneity in tumor microvascular response to radiation,” Int. J. Radiat. Oncol. Biol. Phys. 18(3), 559–568 (1990). 10.1016/0360-3016(90)90061-N [DOI] [PubMed] [Google Scholar]
- 31.Lee D. E., Alhallak K., Jenkins S. V., Vargas I., Greene N. P., Quinn K. P., Griffin R. J., Dings R. P. M., Rajaram N., “A radiosensitizing inhibitor of HIF-1 alters the optical redox state of human lung cancer cells in vitro,” Sci. Rep. 8(1), 8815 (2018). 10.1038/s41598-018-27262-y [DOI] [PMC free article] [PubMed] [Google Scholar]