Abstract
Transforming growth factor‐β1 (TGF‐β1) is widely used in an active recombinant form to stimulate the chondrogenic differentiation of mesenchymal stem cells (MSCs). Recently, it has been shown that the application of multiaxial load, that mimics the loading within diarthrodial joints, to MSCs seeded in to fibrin‐poly(ester‐urethane) scaffolds leads to the endogenous production and secretion of TGF‐β1 by the mechanically stimulated cells, which in turn drives the chondrogenic differentiation of the cells within the scaffold. The work presented in this short communication provides further evidence that the application of joint mimicking multiaxial load induces the secretion of TGF‐β1 by mechanically stimulated MSCs. The results of this work also show that joint‐like multiaxial mechanical load activates latent TGF‐β1 in response to loading in the presence or absence of cells; this activation was not seen in non‐loaded control scaffolds. Despite the application of mechanical load to scaffolds with different distributions/numbers of cells no significant differences were seen in the percentage of active TGF‐β1 quantified in the culture medium of scaffolds from different groups. The similar level of activation in scaffolds containing different numbers of cells, cells at different stages of differentiation or with different distributions of cells suggests that this activation results from the mechanical forces applied to the culture system rather than differences in cellular behaviour. These results are relevant when considering rehabilitation protocols after cell therapy or microfracture, for articular cartilage repair, where increased TGF‐β1 activation in response to joint mobilization may improve the quality of developing cartilaginous repair material. © 2016 The Authors Journal of Tissue Engineering and Regenerative Medicine Published by John Wiley & Sons Ltd
Keywords: cartilage, regeneration, endogenous TGF, growth factor, mechanobiology, paracrine
1. Introduction
Transforming growth factor β (TGF‐β) is considered to be one of the key factors involved in the chondrogenic differentiation of mesenchymal stem cells (MSCs) and is required for the classical chondrogenic differentiation of MSCs in vitro (Johnstone et al., 1998). Transforming growth factor β is secreted by cells in an inactive, latent form in which the active TGF‐β peptide is bound to the latency associated peptide (LAP). For TGF‐β to bind to, and activate, a target receptor the mature TGF‐β peptide must first be released from the LAP (Robertson and Rifkin, 2013). This TGF‐β activation can occur in a variety of ways, including protease degradation (e.g. the serine protease plasmin), mechanical stimulation, deglycosylation or the application of a number of physiochemical stimuli such as heat, extremes of pH and ultraviolet (UV) light (Lyons et al., 1990; Robertson and Rifkin, 2013). Mechanical forces, in a number of different forms, have been shown to activate TGF‐β. Work by Annes et al. (2004) and Wipff et al. (2007) has shown that integrin binding and subsequent cell generated traction forces are involved in the activation of TGF‐β that is bound to extracellular matrix via Latent tgfb binding protein (LTBP), while the mechanical activation of TGF‐β has also been demonstrated in fluid environments in response to the application of fluid shear stress or stirring forces (Annes et al., 2004; Wipff et al., 2007; Ahamed et al., 2008; Albro et al., 2012). Work by this group, using a custom built bioreactor, has shown that the application of a combination of shear and compression load, which mimics the load of a diarthrodial joint, induces the chondrogenesis of human MSCs via the induction of TGF‐β1 secretion in stimulated cells (Li et al., 2010). This work aimed to further investigate the effect of joint like load on TGF‐β1 by quantifying not only the effect of multiaxial load on the overall production of TGF‐β1 but also its activation. Cell seeding distribution was either uniform, as is commonly performed during tissue engineering studies, or scaffolds contained the same number of cells but with a layer seeded directly on top of the scaffold in order to mimic a superficial zone, as seen in cartilage. Cells seeded on top of the scaffolds only was also included as a control.
2. Materials and methods
Human mesenchymal stem cells were isolated, with full ethical approval, from bone marrow from four donors via density centrifugation separation and plastic adhesion. The marrow aspirates used were either collected from vertebral bodies (two females aged 18 years and 49 years, one male aged 22 years) or from the tibial plateau (one male aged 48 years). Following isolation, MSCs were expanded to passage four before being seeded into fibrin–poly(ester‐urethane) scaffolds as described previously (Li et al., 2009). The scaffolds were seeded with cells in three different ways; group 1 scaffolds were evenly seeded with 4 million cells; group 2 scaffolds had 3.6 million cells seeded evenly within the scaffold and 400 000 seeded on the loaded surface of the scaffold; and group 3 scaffolds were seeded with just 400 000 cells on the loaded surface of the scaffold. Different seeding patterns were used to investigate the effect of different distributions and numbers of cells on TGF‐β1 activation. Following seeding, scaffolds were cultured in media consisting of Dulbecco's modified Eagle's medium 4.5 g/l glucose (Gibco, Carlsbad, CA, USA), sodium pyruvate 0.11 g/l (Sigma‐Aldrich, Buchs, SG, Switzerland), l‐ascorbic acid 2‐phosphate sesquimagnesium salt hydrate 50 μg/ml (Sigma‐Aldrich), dexamethasone 1 × 10−7 m (Sigma‐Aldrich), insulin transferrin and selenium 1% (Cyanogen, Guangzhou, China), non‐essential amino acids 1% (Gibco), Primocin 0.1% (Invitrogen, San Diego, CA, USA) and 6‐aminocaproic acid 5 μm (Sigma‐Aldrich).
Scaffolds were kept in free‐swelling culture or were exposed to 20 1‐h cycles of 10% compression superimposed on a 10% pre‐strain and shear loading (± 25°) at 1 Hz for 1 h a day five times a week for 4 weeks. The culture media was collected three times a week and pooled by week before being stored at −20°C for analysis.
To establish the direct effect of the mechanical environment, cell‐free scaffolds were loaded with the same loading protocol for 6 h in culture medium containing 20 ng/ml of latent TGF‐β (R&D Systems, Minneapolis, MN, USA).
The Human TGF‐β 1 DuoSet ELISA (enzyme‐linked immunosorbent assay) kit (R&D Systems) was used according to the manufacturer's instructions to quantify the amount of TGF‐β1 in the samples before and after activation in order to determine the percentage of active TGF‐β1 in the samples.
Statistical analyses were performed using GraphPad Prism 6 software (GraphPad Software Inc., La Jolla, CA, USA). Cell free activation was assessed using the Student's t‐test. The Shapiro–Wilk test was used to determine normality and the Kruskal–Wallis and Dunn's multiple comparison tests used to determine significance, which was determined as p ≤ 0.05.
3. Results and discussion
The quantification results for TGF‐β1 show that mechanical load leads to a significant increase in the total amount of TGF‐β1 in culture media compared with control scaffolds in group 1 between weeks 2 and 4 of culture. Increased levels of total media TGF‐β1 are also seen in response to load group 2 loaded scaffolds compared with controls; however, this was not significant because of raised levels of total media TGF‐β1 in group 2 control scaffolds (Figure 1a–d). No change in media TGF‐β1 content was seen in group 3 in response to load over the 4‐week culture period.
Figure 1.

Enzyme‐linked immunosorbent assay (ELISA) data of endogenous transforming growth factor (TGF)‐β1 generated in serum‐free culture medium under static or multiaxial loading conditions. (a–d) Total endogenous TGF‐β1 secreted into condition culture media from week 1 to week 4. (e–h) Activated TGF‐β1 as a percentage of total TGF‐β1 from week 1 to week 4. Statistical significance was defined as p ≤ 0.05 and determined using the Kruskal–Wallis and Dunn's multiple comparison tests. *represents P ≤ 0.05, **represents P ≤ 0.01, ***represents P ≤ 0.001 and ****represents P ≤ 0.0001 (n = 4 donors)
Quantification of the active TGF‐β1 in each sample show that in groups 1, 2 and 3 the percentage of active TGF‐β1 in each loaded group was significantly increased above the levels in all three control groups between weeks 2 and 4 of culture (Figure 1e–h). This clearly shows that the application of joint mimicking load in this system not only leads to an increase in the total production of TGF‐β1 but also induces the activation of the latent TGF‐β released by the MSCs.
Over the course of the 4 weeks in culture no significant differences were observed in the percentage of active TGF‐β1 between the three loaded groups, despite there being different distributions of cells within the scaffolds of different groups, the scaffolds of the three groups containing different numbers of cells and the cells in the scaffolds of different groups being at different stages of chondrogenic differentiation. This consistency in the degree of TGF‐β1 activation, regardless of the variation in the cell populations within the scaffolds suggests that the increase in the amount of active TGF‐β1 in response to load results from a physical activation similar to that seen by others in response to mechanical load, particularly shear, rather than a result of enzymatic activation owing to activity of the cells within the scaffold (Ahamed et al., 2008, Albro et al., 2012). The inclusion in the media of the protease inhibitor 6‐aminocaproic acid in order to prevent fibrin degradation during culture also suggests that this activation is unlikely to be caused by the activity of plasmin as it is one of the enzymes inhibited by 6‐aminocaproic acid (Kupcsik et al., 2009). To investigate this further, latent TGF‐β was added into a cell‐free system and the same loading protocol applied for 6 h. No active TGF‐β was measured in the unloaded control (Figure 2). However, application of multiaxial load did lead to activation of TGF‐β, with the percentage of activated TGF‐β relative to total TGF‐β detected in the medium being comparable to that seen in the cell‐containing system (Figure 2b).
Figure 2.
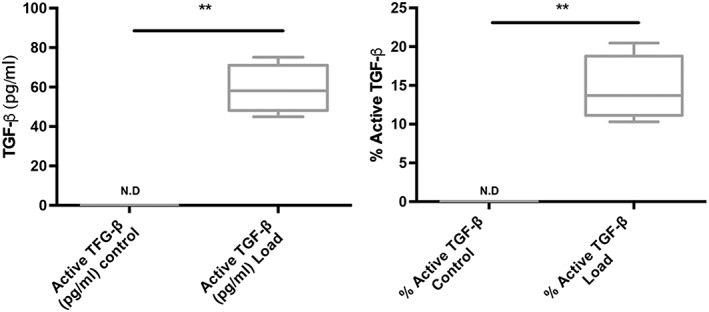
Latent transforming growth factor (TGF)‐β was added to cell‐free fibrin polyurethane scaffolds and multiaxial load was applied for 6 h. (a) Activated TGF‐β was only detected in the loaded samples. (b) The active TGF‐β as a percentage of total TGF‐β detected in the medium reached similar levels to that found in cell containing scaffolds. **p ≤ 0.001 N.D, not detected (n = 4 samples per group)
4. Conclusions
The present study shows that the application of joint mimicking load to cartilage tissue engineering constructs leads not only to the induction of latent TGF‐β1 release into the culture media, but also the activation of the secreted latent TGF‐β1. This activation appears to result from the physical forces applied to the system rather than enzymatic activation, as shown by activation in a cell‐free system. These data are particularly relevant when considering rehabilitation protocols after cell therapy, or microfracture, for articular cartilage repair as this work suggests that early mobilization of the joint may increase TGF‐β1 production and activation and therefore promote chondrogenesis within repair tissue.
Conflict of interest
The authors have declared that there is no conflict of interest.
Acknowledgements
This work was supported by the Acute Cartilage Injury consortium of the AO Foundation and the Swiss National Science Foundation (Grant no: 31003a_146375/1). The authors thank Dr D. Eglin and M. Glarner (Musculoskeletal Regeneration Programme, AO Research Institute Davos, Switzerland) for producing the polyurethane scaffolds and Baxter Biosurgery, Vienna, Austria, for providing the fibrin components.
Gardner, O. F. W. , Fahy, N. , Alini, M. , and Stoddart, M. J. (2017) Joint mimicking mechanical load activates TGFβ1 in fibrin‐poly(ester‐urethane) scaffolds seeded with mesenchymal stem cells. J Tissue Eng Regen Med, 11: 2663–2666. doi: 10.1002/term.2210.
The copyright line for this article was changed on 10 October 2018 after original online publication
References
- Ahamed J, Burg N, Yoshinaga K, et al 2008; In vitro and in vivo evidence for shear‐induced activation of latent transforming growth factor‐beta1. Blood 112: 3650–3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albro MB, Cigan AD, Nims RJ, et al 2012; Shearing of synovial fluid activates latent TGF‐beta. Osteoarthr Cartil 20: 1374–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annes JP, Chen Y, Munger JS, et al 2004; Integrin alphaVbeta6‐mediated activation of latent TGF‐beta requires the latent TGF‐beta binding protein‐1. J Cell Biol 165: 723–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. 1998; In vitro chondrogenesis of bone marrow‐derived mesenchymal progenitor cells. Exp Cell Res 238: 265–272. [DOI] [PubMed] [Google Scholar]
- Kupcsik L, Alini M, Stoddart MJ. 2009; Epsilon‐aminocaproic acid is a useful fibrin degradation inhibitor for cartilage tissue engineering. Tissue Eng Part A 15: 2309–2313. [DOI] [PubMed] [Google Scholar]
- Li Z, Kupcsik L, Yao SJ, et al 2009; Chondrogenesis of human bone marrow mesenchymal stem cells in fibrin‐polyurethane composites. Tissue Eng Part A 15: 1729–1737. [DOI] [PubMed] [Google Scholar]
- Li Z, Kupcsik L, Yao SJ, et al 2010; Mechanical load modulates chondrogenesis of human mesenchymal stem cells through the TGF‐beta pathway. J Cell Mol Med 14: 1338–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons RM, Gentry LE, Purchio AF, et al 1990; Mechanism of activation of latent recombinant transforming growth factor beta 1 by plasmin. J Cell Biol 110: 1361–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson IB, Rifkin DB. 2013; Unchaining the beast; insights from structural and evolutionary studies on TGFbeta secretion, sequestration, and activation. Cytokine Growth Factor Rev 24: 355–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wipff PJ, Rifkin DB, Meister JJ, et al 2007; Myofibroblast contraction activates latent TGF‐beta1 from the extracellular matrix. J Cell Biol 179: 1311–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]


