Abstract
Periodontal disease is very common during pregnancy. Although it has been linked to adverse pregnancy outcomes, systematic reviews have reached discrepant conclusions on these links. Therefore, we conducted a systematic overview of systematic reviews studying the association between periodontal disease and adverse pregnancy outcomes. We searched 6 online databases up to November 2016 and hand-searched references and citations of eligible papers. Systematic reviews of studies comparing pregnancy outcomes among women with and without periodontal disease were eligible for inclusion. Primary outcomes were maternal mortality, preterm birth, and perinatal mortality. Two reviewers extracted data and assessed risk of bias of individual systematic reviews. Findings are described in tabular and narrative form. Twenty-three systematic reviews (including between 3 and 45 studies) were included. None reported the association between periodontal disease and maternal or perinatal mortality. Systematic reviews with the lowest risk of bias consistently demonstrated positive associations between periodontal disease and preterm birth (relative risk, 1.6; 95% confidence interval, 1.3 to 2.0; 17 studies, 6,741 participants), low birth weight (LBW; relative risk, 1.7; 95% CI, 1.3 to 2.1; 10 studies, 5,693 participants), preeclampsia (odds ratio, 2.2; 95% CI, 1.4 to 3.4; 15 studies, 5,111 participants), and preterm LBW (relative risk 3.4; 95% CI, 1.3 to 8.8; 4 studies, 2,263 participants). Based on these figures, estimated population-attributable fractions for periodontal disease were 5% to 38% for preterm birth, 6% to 41% for LBW, and 10% to 55% for preeclampsia. In terms of limitations, as several primary studies did not adjust for confounding, meta-analyses may have overestimated the strength of the associations under study. Due to substantial overlap in included primary studies, we could not aggregate results across reviews. Consistent evidence from systematic reviews with low risk of bias indicates that pregnant women with periodontal disease are at increased risk of developing preeclampsia and delivering a preterm and/or LBW baby (PROSPERO: CRD42015030132).
Knowledge Transfer Statement: This study highlights that periodontal disease is an important risk factor for several common adverse pregnancy outcomes. Clinicians should be aware of this link to guide risk selection. Research is needed to develop novel preventive and treatment strategies.
Keywords: periodontitis, gingivitis, maternal mortality, preeclampsia, preterm birth, low birth weight
Introduction
Periodontal disease is an inflammatory and/or infectious disease of the tissues surrounding and supporting the teeth (Armitage 2004; Pihlstrom et al. 2005). Periodontal disease can consist of gingivitis (reversible gingival inflammation) and periodontitis (gingivitis with gingival recession accompanied by loss of connective tissue and alveolar bone; Armitage 2004). The prevalence of gingivitis varies between 50% to 90% among all adults worldwide (Pihlstrom et al. 2005). In a recent evaluation of >3,500 U.S. citizens aged >30 years, >45% had periodontitis, the majority being of moderate severity (Eke et al. 2012). Severe periodontitis was estimated to affect >10% of the world’s population in 2010 (Marcenes et al. 2013).
Several studies have investigated the occurrence of periodontal disease during pregnancy, yielding a wide variation in prevalences (11% to 100%; Ifesanya et al. 2010; Piscoya et al. 2012). Pregnant women with periodontal disease have been reported to be at increased risk of adverse pregnancy outcomes (Pihlstrom et al. 2005), including preeclampsia (Kunnen et al. 2007; Siqueira et al. 2008), preterm delivery (Jarjoura et al. 2005; Offenbacher et al. 2006), and low birth weight (LBW; Marin et al. 2005; Martins Moliterno et al. 2005). However, many other studies failed to confirm these associations (Moore et al. 2004; Gomes-Filho et al. 2006; Bassani et al. 2007).
Given the global disease burden of periodontal disease and the range of adverse pregnancy outcomes that have been associated with it, it is important to clarify their relationship (Chang et al. 2013; GBD 2013 Mortality and Causes of Death Collaborators 2015). This in turn will inform prioritization of the development of preventive and therapeutic interventions to reduce the occurrence of adverse pregnancy outcomes among women with periodontal disease, if relevant. Several systematic reviews have therefore been conducted to clarify the association between periodontal disease and adverse pregnancy outcomes. However, in keeping with the apparently contrasting findings of individual studies, these systematic reviews also have important discrepancies in their conclusions. Given the global health relevance of the topic, it is important that the pertaining literature—which is thus currently clouded—is thoroughly assessed to identify possible sources of the apparently heterogeneous findings and to try to reach more consistent conclusions, if possible. We therefore performed a comprehensive synthesis of findings from systematic reviews assessing the link between periodontal disease and a broad range of adverse pregnancy outcomes, focusing on interpreting findings from the highest-quality reviews. Our primary objective was to assess whether differences in adverse pregnancy outcomes—in particular maternal mortality, perinatal mortality, and preterm birth—exist between 1) women diagnosed with periodontal disease within 6 mo prior to or during pregnancy and 2) women without periodontal disease.
Methods
Protocol and Registration
The revised PRISMA-P guidelines (Preferred Reporting Items for Systematic Reviews and Meta-analyses Protocols) were used to guide reporting of this systematic review (Shamseer et al. 2015). The protocol for this study has been peer reviewed and published (Vanterpool et al. 2016) and was registered with the PROSPERO prospective register of systematic reviews (CRD42015030132) prior to undertaking literature searches.
Information Sources, Search Strategy, and Eligibility Criteria
The electronic databases Cochrane Database of Systematic Reviews, MEDLINE (PubMed), EMBASE, World Health Organization Global Health Library (covers African Index Medicus, Latin American and Caribbean Health Science Literature, Index Medicus for the Eastern Mediterranean Region, Index Medicus for South-East Asia Region, Western Pacific Region Index Medicus, and SciELO in addition to MEDLINE), and Google Scholar were searched on August 20, 2015, from their earliest records. The search was updated November 30, 2016. PROSPERO was searched for unpublished, ongoing, and recently completed systematic reviews. The search was complemented by hand-searching reference lists of the included systematic reviews and performing a citation search for any additional eligible systematic reviews. Two reviewers independently performed the searches.
A search strategy was developed for MEDLINE (Appendix Fig.) and amended for the other electronic databases as necessary. No limitations were applied regarding publication date, publication status, or language. Translation was sought for any reviews published in languages other than English.
Study Selection
Systematic reviews were eligible for inclusion 1) if a systematic search strategy, which included a search of several databases with specific search terms, was performed; 2) if data were included from prospective/retrospective cohort studies, cross-sectional studies, and/or case-control or nested case-control studies; and 3) if they included studies that compared pregnancy outcomes between a) preconceptional (i.e., within 6 mo before conception) or pregnant women with periodontal disease (identified before [i.e., <6 mo] or during pregnancy) and b) preconceptional or pregnant women without periodontal disease. Periodontal disease could consist of gingivitis and/or periodontitis (Armitage 2004), which was diagnosed on the basis of commonly used clinical parameters (e.g., clinical attachment loss, clinical attachment level, probing depth, or bleeding on probing; Highfield 2009).
Outcomes of Interest
Adverse pregnancy outcomes were defined as a maternal, fetal, and/or neonatal complication during pregnancy, labor and delivery, or the postpartum period (up to 6 wk after delivery). We selected the following primary and secondary outcomes of interest based on their severity and/or prevalence.
Primary Outcomes
Maternal mortality: death during pregnancy or within 42 d after termination of pregnancy
Preterm delivery: delivery of a live-born baby before 37 completed wk of gestation
Perinatal mortality: stillbirth (intrauterine death after 20 wk of gestation) or neonatal mortality (death within 28 d after live birth)
Secondary Outcomes
Miscarriage (spontaneous abortion): fetal death before 20-wk gestational age
Preterm prelabor rupture of membranes: leakage of amniotic fluid in the absence of uterine activity before 37-wk gestation
Pregnancy-induced hypertension: onset of hypertension (blood pressure ≥140/90 mm Hg) in the second half of pregnancy in the absence of proteinuria or other markers of preeclampsia
Preeclampsia: hypertension and proteinuria
Clinical chorioamnionitis: clinical evidence of intra-amniotic infection with or without laboratory signs of infection
Histologic chorioamnionitis: diagnosed by histologic examination of the placenta by a pathologist
Stillbirth: intrauterine death after 20 wk of gestation
Very preterm delivery: delivery of a live-born baby before 32 completed wk of gestation
LBW: birth weight <2,500 g
Small for gestational age: birth weight <10th percentile for gestational age
Early onset neonatal sepsis: clinical evidence of sepsis with laboratory signs of infection within 72 h after birth
Neonatal death: death of a live-born baby occurring within the first 28 d of life
Additional relevant outcome measures that were investigated in the included systematic reviews in relation to periodontal disease exposure but not prespecified were also taken into account and are marked as post hoc analyses.
Data Collection Process
Two reviewers (S.F.V. and K.T.) independently screened titles and abstracts of studies identified by the search strategy. Full-text reports of potential eligible systematic reviews were obtained and evaluated against the eligibility criteria independently by 2 reviewers (L.A.D. and B.V.W.). Any discrepancies were resolved through discussion, with arbitration by a third reviewer (J.V.B.) if consensus could not be reached.
Two reviewers (L.A.D. and B.V.W.) independently performed data extraction using a customized form developed a priori. This form (Appendix Table 1) is based on the Cochrane Effective Practice and Organization of Care (2002) checklist and the PRISMA statement (Shamseer et al. 2015).
Assessment of Risk of Bias
Risk of bias of the included systematic reviews was assessed independently by 2 reviewers (L.A.D. and B.V.W.) using the AMSTAR checklist (Assessment of Multiple Systematic Reviews; Higgins and Green 2009; Pieper et al. 2012). The AMSTAR checklist consists of 11 assessment criteria, each rated by 1 point (Shea et al. 2007; Shea et al. 2009). Disagreement was resolved through discussion between the 2 reviewers and arbitration by a third reviewer (J.V.B.) when consensus was not reached. To evaluate selective reporting within systematic reviews, the reported outcomes in the protocols (if available) were compared with the published reports of the systematic reviews. If a systematic review protocol was not available, the outcomes reported in the Methods and Results sections of the published systematic review were compared.
Risk of bias across systematic reviews was assessed with the use of a citation matrix that cross-links all systematic reviews (columns) with all the primary studies included in the systematic reviews (rows; Pieper et al. 2014). Separate citation matrices were created for each outcome measure. The degree of overlap (i.e., the repeated occurrences of the primary studies) was quantified by calculating the corrected covered area (CCA) in this citation matrix (Pieper et al. 2014). We aimed to also include all relevant data from unpublished systematic reviews.
Synthesis of Results
The characteristics and main findings of the included systematic reviews are presented in tabular form and summarized through a narrative synthesis. We particularly aimed to explore underlying reasons for discrepancies among findings of individual reviews, taking into account potential sources of bias. No meta-analysis was performed, due to overlap of primary studies in the meta-analyses included in our review. A meta-meta-analysis would thus provide a distorted and biased overview of the association between periodontal disease and adverse pregnancy outcomes (Pieper et al. 2014).
Given the effect estimate of the review with the lowest risk of bias (i.e., highest AMSTAR score), we calculated a population-attributable fraction (PAF) to estimate the contribution of periodontal disease to the global burden of each adverse pregnancy outcome, using the following formula:
where Ppop is the proportion of the population (i.e., pregnant women) exposed (i.e., to periodontal disease) and RR is the relative risk of the outcome in the exposed versus the nonexposed. Given the wide range of published estimates of the incidence of periodontal disease during pregnancy (i.e., 11% to 100%), we used both extremes to estimate PAFs. Consequently, the actual PAF is likely to lie within the range between these extremes.
Results
Study Selection
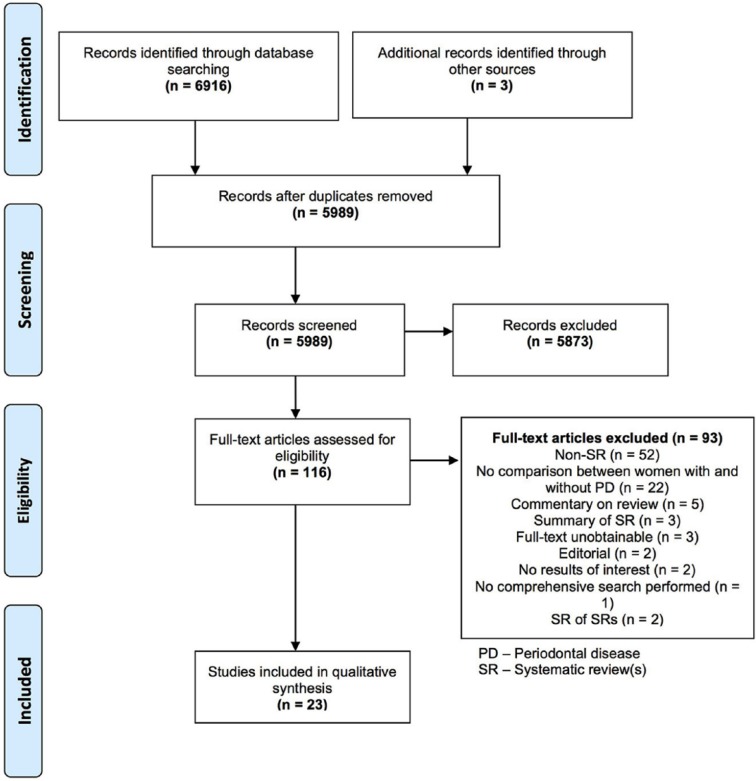
The electronic search yielded 6,916 potentially relevant titles. After removal of duplicates and screening of titles and abstracts, 113 studies were screened to assess eligibility for final inclusion (Fig.). Of these, 14 studies were not published in English language and therefore translated. Appendix Table 2 provides an overview of the excluded studies at this stage.
Figure.
Flow diagram of study selection.
Study Characteristics
Twenty-three systematic reviews were included in this systematic overview (Appendix: Additional Reference List; Table 1). These reported on a total of 120 individual studies; the number of primary studies included varied from 3 to 45 per review (Appendix Table 3). The primary studies were performed in 37 countries, with the majority conducted in Brazil.
Table 1.
Study Characteristics.
| Systematic Review | Search Strategy | Definitions | Included Studies | Meta-Analysis Performed |
|---|---|---|---|---|
| Chambrone (2011) | Databases: CENTRAL, EMBASE, MEDLINE, OpenSIGLE Other approaches: Reference lists Search period: FER/2010-10-10 Limitations: No limitations mentioned |
Periodontal disease: Slight/mild periodontitis (1-2mm
CAL)Moderate periodontitis (3-4mm CAL)Severe periodontitis
(≥5mm CAL) Outcomes: - LBW: <2500g - PTB: <37wk GA - PTB/LBW: <37wk GA/<2500g |
11 studies (11 prospective cohort): 12,173
women Countries: Fiji; Madagascar; Malaysia; Spain; Sri Lanka; UK; USA (5) |
Y |
| Conde-Agudelo (2008) | Databases: CINAHL, EMBASE, LILACS, MEDLINE,
POPLINE Other approaches: Abstracts international meetings, Contact investigators, Reference lists, Textbooks Search period: FER/2007-06-30 Limitations:No language restrictions |
Periodontal disease: Not reported Outcomes: - PE: Hypertension and proteinuria after 20 weeks GA |
9 studies (2 cohort; 7 CC): 3,912 women Countries: Argentina; Brazil; Colombia; Israel (2); Jordan; Netherlands; Turkey; USA |
Y |
| Corbella (2012a) | Databases: CENTRAL, EMBASE, MEDLINE Other approaches: None Search period:1970/2009-12 Limitations: Language restriction: English; French; German |
Periodontal disease:Not reported Outcomes: - LBW: <2500g - PTB: <37wk GA - Spontaneous abortion/Stillbirth: no definition reported |
26 studies (16 cohort; 10 CC): 23,069
women Countries: Austria; Brazil; Canada; Chile; Fiji; Finland; Hungary (2); Iceland; Jordan; Pakistan; Spain; Sri Lanka; Turkey; UK (2); USA (9); Venezuela |
N |
| Corbella (2012b) | Databases: MEDLINE Other approaches: Hand search, Reference lists Search period: 1965/2011-01 Limitations: Search limited to human studies Language restriction: English; French; German |
Periodontal disease:Not reported Outcomes: - LBW: <2500g - PLBW: no definition reported - PTB: <37wk GA |
17 studies (5 cohort; 11 CC; 1 CS): 10,148
women Countries: Brazil (5); Canada; Chile; France; Hungary (2); South-Korea; Madagascar; Spain; Tanzania; USA (3) |
Y |
| Corbella (2016) | Databases: Cochrane, EMBASE, MEDLINE, SCOPUS Other approaches: Hand search, Reference lists Search period: 1965/2015-01 Limitations: Search limited to human studies No language restrictions |
Periodontal disease:Not reported Outcomes: - LBW: no definition reported - PLBW: no definition reported - PTB: no definition reported |
22 studies (8 cohort; 12 CC; 2 CS): 17,053
women Countries: Brazil (7); Canada; Chile; France; Hungary (2); Italy; South-Korea; Madagascar; Malaysia; Spain (2); Tanzania; USA (3) |
Y |
| Huang (2014) | Databases: EMBASE, PUBMED Other approaches: Reference lists Search period: FER/2013-03 Limitations: Language restriction: English; Chinese |
Periodontal disease: Either the combination of a specific PD
or the presence of CAL or the occurrence of
BOP Outcomes: PE: Blood pressure ≥140/90 mmHg on two separate occasions after 20 weeks GA and ≥1+ proteinuria |
11 studies (3 cohort; 8 CC): 3,916 women Countries: Brazil (3); Canada; Colombia; India (2); South-Korea; Thailand; Turkey; USA |
Y |
| Ide (2013) | Databases: CENTRAL, EMBASE, MEDLINE, WEB OF
SCIENCE Other approaches: Hand search, Reference lists Search period: FER/2012-05 Limitations: No language restrictions |
Periodontal disease: Ranging from gingivitis through
aggressive periodontitisOutcomes: - LBW: <2500g - Miscarriage: no definition reported - PE: maternal hypertension and proteinuria >20wk GA - PTB: <37wk GA - SGA: restricted growth at any given time during gestation - Stillbirth: no definition mentioned - VLBW: <1500g - VPTB: <32wk GA |
38 studies (14 cohort; 22 CC; 2 CS): 25,816
women Countries: Brazil (13); Canada (2); Colombia; Croatia; Finland; France; Hungary; Italy; Japan; Jordan (2); Madagascar; Malaysia; Spain; Thailand; Turkey (3); UK (3); USA (4) |
Y |
| Khader (2005) | Databases: MEDLINE Other approaches: Reference lists Search period: 1966/2002-08 Limitations: Language restriction: English |
Periodontal disease:Not reported Outcomes: - PLBW: <2500g and ≥1 of: GA <37wk, preterm labor or PROM - PTB: <37wk GA |
5 studies (2 cohort; 3 CC): 2,369 women Countries: Chile; USA (4) |
Y |
| Konopka (2012) | Databases: MEDLINE, PUBMED, Polish Medical
Bibliography Other approaches: None Search period: 1966/2010 Limitations: Language restriction: English; German; Polish |
Periodontal disease:Not reported Outcomes: - LBW: <2500g - PLBW: no definition reported - PTB: <37wk GA |
22 studies (6 cohort; 15 CC; 1 CS): 12,047
women Countries: Austria; Brazil (5); Chile; Colombia; Croatia; Germany; Hungary; Jordan; Poland (2); Spain; Sri Lanka; Turkey; UK; USA (4) |
Y |
| Kunnen (2010) | Databases: CINAHL, EMBASE, MEDLINE Other approaches: Reference lists Search period: CINAHL: 2003/2010-08-15 EMBASE: 1974/2010-08-15 MEDLINE:2003/2010-08-15 Limitations: Language restriction: Dutch; English; French; German; Spanish |
Periodontal disease: Not reported Outcomes: - PE: no definition reported |
12 studies (2 cohort; 9 CC; 1 CS): 6,244
women Countries: Argentina; Brazil; Colombia; France; India; Jordan; Netherlands; Thailand; Turkey (2); USA (2) |
N |
| Madianos (2002) | Databases: EMBASE, MEDLINE Other approaches: Hand search, Reference lists Search period: FER/2001-10 Limitations: Search limited to human studies Language restriction: English |
Periodontal disease:Not reported Outcomes: - LBW: <2500g - PLBW: no definition reported - PTB: <37wk GA |
5 studies (1 cohort; 4 CC): 1,588 women Countries: USA (5) |
N |
| Oliveira (2009) | Databases: LILACS, PUBMED, ScIELO Other approaches: None Search period: 1988-10/2007-07) Limitations: Language restriction: English |
Periodontal disease:Not reported Outcomes: - LBW: no definition reported - PTB: no definition reported |
23 Studies (8 cohort; 15 CC): 8,177 women Countries: Brazil (4); Chile; Croatia; Germany; Hungary (2); Saudi Arabia; Spain; Thailand; UK (3); USA (8) |
N |
| Rustveld (2008) | Databases: Cochrane, EMBASE, MEDLINE Other approaches: Reference lists Search period: 1964/2009-08 Limitations: Language restriction: English, German, Spanish |
Periodontal disease:Not reported Outcomes: - PE: no definition reported |
3 studies (1 cohort; 2 CC): 1,305 women Countries: Colombia; Turkey; USA |
N |
| Sanchez (2004) | Databases: MEDLINE, PUBMED Other approaches: Reference lists Search period:1960/2003-05 Limitations: Language restriction: English |
Periodontal disease: Not reported Outcomes: - PLBW: Birth weight less <2500g and GA <37 weeks |
9 studies (3 cohort; 6 CC): 4, 086 women Countries: Chile (2); UK; USA (6) |
N |
| Scannapieco (2003) | Databases: CENTRAL, MEDLINE, MEDLINE Daily
Update Other approaches:Reference lists Search period: FER/2002-09 Limitations: Search limited to human studies |
Periodontal disease:Not reported Outcomes: - LBW: no definition reported - PLBW: no definition reported - PTB: no definition reported |
9 studies (6 CC; 3 CS): 4,383 women Countries: UK; USA (9); Venezuela |
N |
| Sgolastra (2013) | Databases: CDSR, CENTRAL, CINAHL, DARE, ISI WoK, MEDLINE,
Science Direct, SCOPUS Other approaches: Manual search, reference lists Search period: FER/2013-03-24 Limitations: No limitations applied with regard to publication year and language |
Periodontal disease: Not reported Outcomes: - PE: no definition reported |
15 studies (3 cohort; 12 CC): 5,111 women Countries: Brazil (3); Canada; Chile; Japan; South-Korea; Netherlands; India (2); Thailand; Turkey (2); USA (2) |
Y |
| Teshome (2016) | Databases: AMED, CINAHL, Cochrane, EMBASE, Google Scholar,
MEDLINE Other approaches: Reference lists Search period: 2000/2015-09 Limitations: Language restriction: English |
Periodontal disease: Not
reported Outcomes: - LBW: <2500g - PTB: <37wk GA |
10 studies (10 CC): 2,423 women Countries: Argentina; Brazil (2); India (3); Iran; Jordan; Senegal; Tanzania |
N |
| Vergnes (2007) | Databases: BIOSIS, EMBASE, LILACS, MEDLINE.
PASCAL Other approaches: Reference lists Search period: BIOSIS, LILACS, PASCAL:1990/2005-09 EMBASE:1980/2005-09 MEDLINE:1966/2005-09 Limitations: No limitations applied with regard to publication and language; Search limited to human studies |
Periodontal disease: Not reported Outcomes: - LBW: <2500g - PLBW: <37wk GA and/or <2500g - PTB: <37wk GA |
17 studies (4 cohort; 11 CC; 2 CS): 7,151
women Countries: Austria; Brazil (5); Germany; Hungary; Peru; Poland; Saudi Arabia; Sri Lanka; UK; USA (4) |
Y |
| Vettore (2006) | Databases: CAPES thesis, LILACS, PUBMED, ScIELO Other approaches:Reference lists Search period: FER/2005-12-21 Limitations: Search limited to human studies Language restriction: English; Portuguese |
Periodontal disease: Not reported Outcomes: - LBW: no definition reported - PTB: no definition reported |
33 studies (6 cohort; 27 CC): 12,191 women Countries: Austria; Brazil (6); Chile; France; Germany; Hungary; Iceland; Italy; Japan; Poland; Saudi Arabia; Spain; Sri Lanka; Turkey; UK (3); USA (10) |
N |
| Wei (2013) | Databases: EMBASE, PUBMED Other approaches: None Search period: FER/2013-02-10 Limitations: No limitations mentioned |
Periodontal disease: Not reported Outcomes: - PE: no definition reported |
15 studies (2 cohort; 13 CC): 4,711 women Countries: Argentina; Brazil (4); Canada; India; Iran; South-Korea; Netherlands; Thailand; Turkey (2); USA (2) |
Y |
| Wimmer (2008) | Databases: CINAHL, EMBASE, MEDLINE, PASCAL Other approaches: Reference lists Search period: FER/2008-02-01 Limitations: No limitations mentioned |
Periodontal disease:Not reported Outcomes: - BW: no definition reported - GA: no definition reported - IUGR: no definition reported - LBW: no definition reported - PLBW: no definition reported - PTB: no definition reported |
45 studies (23 cohort; 22 CC): 23,780
women Countries: Austria; Brazil (9); Canada; Chile; Croatia; Denmark; Finland (2); Germany; Hungary (2); Iceland; Poland; Saudi Arabia; Spain (2); Sri Lanka; Thailand; Turkey (2); UK (5); USA (11); Venezuela |
N |
| Xiong (2006) | Databases: CINAHL, Current Contents, EMBASE,
MEDLINE Other approaches: Reference lists Search period: 1966-01/2005-03 Limitations: No limitations mentioned |
Periodontal disease:Not reported Outcomes: - BW: no definition reported - GA: no definition reported - LBW: no definition reported - Miscarriage: no definition reported - PE: no definition reported - PTB: no definition reported - SGA: no definition reported |
22 studies (9 cohort; 13 CC): 10,234 women Countries: Austria; Brazil; Canada; Chile; Hungary; Iceland; Saudi Arabia; Senegal; Sri Lanka; Thailand; Turkey (2); UK (3); USA (6); Venezuela |
N |
| Xiong (2007) | Databases: CINAHL, Current Contents, EMBASE,
MEDLINE Other approaches: Reference lists Search period:1966-01/2006-12 Limitations: No limitations mentioned |
Periodontal disease:Not reported Outcomes: - BW: no definition reported - GA: no definition reported - Gestational Diabetes: no definition reported - LBW: no definition reported - Miscarriage: no definition reported - PE: no definition reported - PTB: no definition reported - SGA: no definition reported |
39 studies (13 cohort; 26 CC): amount of participants not
reported Countries: Argentina; Austria; Brazil (4); Canada (2); Chile; Colombia; Croatia; Denmark; Germany; Hungary (2); Iceland; Israel; Saudi Arabia; Senegal; Spain; Sri Lanka; Thailand; Turkey (2); UK (4); USA (10); Venezuela |
N |
AMED, Allied and Complementary Medicine; BOP, bleeding on probing; BW, birth weight; CAL, clinical attachment loss; CC, case control; CDSR, Cochrane Database of Systematic Reviews; CENTRAL, Cochrane Central Register of Controlled Trials; CS, cross sectional; DARE, Database of Abstracts of Reviews of Effects; FER, from earliest records; GA, gestational age; ISI WoK, ISI Web of Knowledge; IUGR, intrauterine growth restriction; LBW, low birth weight; N, no; OpenSIGLE, Open system for Information on Grey Literature in Europe; PD, probing depth; PE, pre-eclampsia; PLBW, preterm low birth weight; PROM, preterm rupture of membranes; PTB, preterm birth; PTB/LBW, combination of preterm birth and/or low birth weight; SGA, small for gestational age; UK, United Kingdom; USA, United States of America; VLBW, very low birth weight; VPTB, very preterm birth; Y, yes.
Risk of Bias within Reviews
None of the included systematic reviews reached the maximum score of 11 points on the AMSTAR checklist (Appendix Table 4). Scores varied between 1 and 9 points. None of the included systematic reviews provided information about a previously published protocol for the review. Additionally, none of the reviews provided information about conflicts of interest relevant to the primary included studies. Systematic reviews that included meta-analyses generally had a higher AMSTAR score than those that did not.
Synthesis of Results
None of the included systematic reviews reported information about the association between periodontal disease and maternal or perinatal mortality. Seventeen systematic reviews reported on the association between periodontal disease and preterm birth (Table 2), of which 7 performed a meta-analysis. Reviews that included meta-analyses consistently had higher AMSTAR scores. Six meta-analyses showed a statistically significant positive association between periodontal disease and preterm birth: odds ratios (ORs) and/or relative risks (RRs) ranging from 1.6 to 3.9. The seventh showed borderline significance (RR, 1.7; 95% confidence interval [95% CI], 1.0 to 2.8) but also provided evidence for a dose-response association with more severe periodontal disease being associated with the strongest risk of preterm birth (RR, 2.0; 95% CI, 1.3 to 2.9). The systematic review by Corbella et al. 2016 (hereinafter, all italicized citations refer to the Additional Reference List) had the highest score on the AMSTAR checklist and reported an RR of 1.6 (95% CI, 1.3 to 2.0; based on 17 studies with 6,741 participants). The association between periodontal disease and preterm birth was consistent in sensitivity meta-analyses restricted to primary studies with the lowest risk of bias, which were performed in 3 reviews.
Table 2.
Evidence of the Association between Periodontal Disease and Primary Outcome Preterm Birth.
| Systematic Review (Year); No. of Studies (Participants) | Main Findings1. Results of Meta-Analysis: or/RR (95%-Ci)2. Results of Subgroup Analysis: or/RR (95%-Ci) | Risk of Bias Assessment | Summary of Findings |
|---|---|---|---|
| Chambrone (2011) 8 (10,804) |
All studies RR 1.7 (1.0-2.8) Studies of high methodological quality RR 1.8 (1.0-3.1) All studies PD defined by PPD and CAL: RR 1.4 (1.1-1.8) PD defined by CAL alone: RR 3.1 (0.2-45.1) PD defined by other methods: RR 1.3 (0.5-3.0) Studies of high methodological quality PD defined by PPD and CAL: RR 1.4 (1.1-1.8) PD defined by CAL alone: RR 3.1 (0.2-45.1) Mild PD defined by PPD and CAL: RR 1.3 (1.0-1.7) Moderate-severe PD defined by PPD and CAL: RR 2.0 (1.3-2.9) Jeffcoat (2001) was not included in the meta-analysis. This cohort study found a positive association between PD and PTB, with the following ORs: 4.5 (2.2-9.2) (<37 weeks of GA), 5.3 (2.1-13.6) (<35 weeks of GA) and 7.1 (1.7-27.4) (<32 weeks of GA). |
Individual studies: NOS-scale (max. 14.0) Mean: 11.9 Range: 9.0-13.0 Systematic review: AMSTAR = 7 |
Overall analysis did not show a significant association between PD and PTB. PD was associated with PTB in studies where PD was defined by PPD and CAL. There was evidence of a dose-response association between severity of PD and risk of PTB. |
| Chambrone (2012b) 14 (8,588) |
OR 1.8 (1.6-2.0) No subgroup analyses performed |
Individual studies: Not reported Systematic review: AMSTAR = 6 |
PD showed a significant association with PTB. |
| Corbella (2016) 17 (6,741) |
RR 1.6 (1.3-2.0) Low risk of bias studies RR 1.7 (1.3-2.2)Moderate risk of bias studies: RR 1.5 (1.1-2.1) |
Individual studies: Cochrane Bias Methods Group (max. 5.0) Mean: 4.5 Range: 4.0-5.0 Systematic review: AMSTAR = 8 |
PD showed a significant association with PTB, which was consistent in studies with low and moderate risk of bias. |
| Ide(2013)24 (18,626) | CC studies reporting PD as a categorical variable: OR 2.5
(2.2-2.8) CC studies reporting PD as a continuous variable (PD): WMD 0.04 (0.01-0.06) CC studies reporting PD as a continuous variable (CAL): WMD -0.04 (-0.07-0.02) CC studies reporting PD as a continuous variable (BOP): WMD 4.7 (2.8-6.7) Prospective cohort studies reporting PD as a categorical variable: RR 1.2 (0.9-1.5) Prospective cohort studies reporting PD as a continuous variable (PD): WMD 0.01 (-0.00-0.02) Prospective cohort studies reporting PD as a continuous variable (CAL): WMD -0.02 (-0.03, -0.01) Prospective cohort studies reporting PD as a continuous variable (POB): WMD -0.00 (-0.6-0.6) |
Individual studies: NOS (max 8.0): Mean: 5.5 Range: 4-7 Systematic review: AMSTAR = 6 |
PD showed a positive association with preterm birth. |
| Khader (2005) 4 (2,156) |
OR 3.9 (2.1-7.0) PTB regardless of BW: OR 4.3 (2.6-7.0) PTB regardless of BW excluding the study with the lowest quality score: OR 4.3 (2.5-7.4) |
Individual studies: Margetts et al.a (max 100%) Mean: 60 Range: 35-71 Systematic review: AMSTAR = 7 |
PD showed a positive association with PTB. |
| Konopka (2012) 7 (3,253) |
OR 2.7 (2.1-3.6) No subgroup analyses performed |
Individual studies: Margetts et al.a (max 100%) Mean: 46 Range: 31-68 Systematic review: AMSTAR = 6 |
PD showed a positive association with PTB. |
| Vergnes (2007) Not reported |
OR 2.3 (1.1-4.9) No subgroup analyses performed |
Individual studies: Margetts et al.a (max. 100%) Mean: 54.9 Range: 30.0-82.0 Systematic review: AMSTAR = 7 |
PD showed a positive association with PTB. |
| Corbella (2012a) 25 (19,493) |
Fifteen studies found a significant positive association between PD and PTB (OR/RR 1.8-20), of which seven did not report an OR/RR, or reported an OR/RR without 95%-CI (OR/RR 1.1-1.9). Two studies found a significant association between PD and moderate-severe PTB only. Eight studies found no significant association (OR/RR 0.7-1.9). | Individual studies: Not reported Systematic review: AMSTAR = 2 |
The vast majority of included studies identified a positive association between PD and PTB, albeit with highly variable OR/RRs. |
| Madianos (2002) 1 (1,313) |
One cohort study included which found a positive association between PD and PTB: OR 4.5 (2.2-9.2) (<37 weeks of GA), 5.3 (2.1-13.6) (<35 weeks of GA) and 7.1 (1.7-27.4) (<32 weeks of GA). | Individual studies: Not reported Systematic review: AMSTAR = 4 |
One cohort study included which showed a positive association between PD and PTB. |
| Oliveira (2009) 11 (4,982) |
8/11 studies reported a positive association between PD and PTB (OR/RR 2.0-8.1), no 95%-CI were reported. Three studies showed no association. | Individual studies: Not reported Systematic review: AMSTAR = 2 |
The vast majority of included studies identified a positive association between PD and PTB. |
| Sanchez (2004)1 (1,313) | One cohort study included which reported a positive association between PD and PTB, with the following ORs: 4.5 (2.2-9.2) (<37 weeks of GA), 5.3 (2.1-13.6) (<35 weeks of GA) and 7.1 (1.7-27.4) (<32 weeks of GA). | Individual studies: Not reported Systematic review: AMSTAR = 1 |
One study included which showed a positive association between PD and PTB. |
| Scannapieco (2003)1 (1,313) | One cohort study included which reported a positive association between PD and PTB, with the following ORs: 4.5 (2.2-9.2) (<37 weeks of GA), 5.3 (2.1-13.6) (<35 weeks of GA) and 7.1 (1.7-27.4) (<32 weeks of GA). | Individual studies: Not reported Systematic review: AMSTAR = 3 |
One study included which showed a positive association between PD and PTB. |
| Teshome (2016) 4 (809) |
Three studies reported a significant positive association between PD and PTB (4.2-137.5). One study found no association. | Individual studies: NIH checklist Mean: 10.3 Range: 9-12 Systematic review: AMSTAR = 5 |
The vast majority of included studies identified a positive association between PD and PTB. |
| Vettore (2006) 12 (7,370) |
Four studies reported a significant positive association between PD and PTB (OR/RR 2.2-7.1). Another two studies reported a positive association between PD and PTB but OR/RR was not provided. Six studies showed no association. | Individual studies: Not reported Systematic review: AMSTAR = 4 |
Half of the included studies identified a positive association between PD and PTB |
| Wimmer (2008) 28 (15,822) |
16/28 studies reported a positive association between PD and PTB (OR/RR 1.1-20). 12 studies showed no association. | Individual studies: Not reported Systematic review: AMSTAR = 2 |
The majority of included studies identified a positive association between PD and PTB. |
| Xiong (2006) 11 (7,629) |
Seven studies reported a significant positive association between PD and PTB (OR/RR 2.1 to 20). One study reported only a significant association between moderate/severe PD and PTB (2.1 [1.3-3.4]) and not between mild PD and PTB (1.2 [0.9-1.7]). Four studies showed no association. | Individual studies: Not reported Systematic review: AMSTAR = 3 |
The vast majority of included studies identified a positive association between PD and PTB. |
| Xiong (2007) 20 (13,246) |
10/20 studies reported a positive association between PD and PTB (OR/RR 2.1 to 20.0), whereas 10 found no association. | Individual studies: Not reported Systematic review: AMSTAR = 3 |
Half of the included studies identified a positive association between PD and PTB |
BOP, bleeding on probing; BW, birth weight; CAL, clinical attachment loss; CC, case-control; CI, confidence interval; GA, gestational age; NIH, National Institutes of Health; NOS, Newcastle-Ottawa scale; OR, odds ratio; PD, periodontal disease; PPD, probing pocket depth; PTB, preterm birth; RR, relative risk; WMD, weighed mean difference.
Reviews in which meta-analyses were performed are listed first, followed by reviews in which no meta-analysis was performed.
Margetts BM, Thompson RL, Key T, et al. Development of a scoring system to judge the scientific quality of information from case-control and cohort studies of nutrition and disease. Nutr Cancer 1995;24:231-239.
Nine systematic reviews reported on the association between periodontal disease and preeclampsia (Appendix Table 5). Of 5 reviews in which a meta-analysis was performed, 4 found a significant association between periodontal disease and preeclampsia, with ORs/RRs ranging from 2.2 to 2.8. The meta-analysis of Sgolastra et al. 2013 had the highest AMSTAR score and reported a significant association between periodontal disease and preeclampsia based on 15 studies with 5,111 participants (OR, 2.2; 95% CI, 1.4 to 3.4). This finding was however not consistent in subgroup and sensitivity analyses, possibly due to lack of power. There was some evidence from a review by Huang et al. 2014 that the positive association between periodontal disease and preeclampsia was consistent when meta-analyses were restricted to studies with lower risk of bias and that the association was stronger in studies that adjusted for confounding. Interestingly, this review also indicated that the risk of preeclampsia was larger when periodontal disease was diagnosed earlier in pregnancy. Kunnen et al. 2010 did not perform a meta-analysis but had a high AMSTAR score and reported a positive association between periodontal disease and preeclampsia in the majority of the included studies.
Sixteen systematic reviews reported on the association between periodontal disease and LBW (Appendix Table 6). The 6 reviews that performed a meta-analysis consistently had higher AMSTAR scores than those that did not. Each of the former found a significant positive association between periodontal disease and LBW, with ORs/RRs ranging from 1.3 to 4.0. The review by Corbella et al. 2016 had the highest AMSTAR score and found a significant positive association based on data from 10 studies including 5,693 participants (RR, 1.7; 95% CI, 1.3 to 2.1). In sensitivity analyses within this review, the association was strongest for studies with the lowest risk of bias.
Only 1 systematic review, which had a low AMSTAR score (2 points), investigated the association between periodontal disease and small for gestational age (Appendix Table 7). Two primary studies were identified in this review: whereas both studies reported a point estimate indicative of a positive association, this was statistically significant in only 1 study (RR, 2.3; 95% CI, 1.1 to 4.7). A meta-analysis was not performed.
No systematic reviews reporting on any of the other predefined secondary outcomes were identified.
Preterm LBW is a combination of preterm birth (delivery of a live-born baby before 37 wk of gestation) and/or LBW (birth weight <2,500 g). This outcome measure was not prespecified in our review protocol, but since we identified a large number of systematic reviews that evaluated this outcome, we decided to add a post hoc interpretation of the systematic reviews that reported on this outcome. Seventeen reviews investigated the association between periodontal disease and preterm LBW (Appendix Table 8), of which 7 performed a meta-analysis. These 7 reviews consistently had higher AMSTAR scores than those that did not perform meta-analysis. All reviews that performed a meta-analysis reported a significant positive association between periodontal disease and preterm LBW, with ORs/RRs varying between 2.1 and 5.3. The meta-analysis of Corbella et al. 2016 had the highest AMSTAR score and found a significant positive association between periodontal disease and preterm LBW in the overall analysis based on data from 4 studies including 2,263 participants (RR, 3.4; 95% CI, 1.3 to 8.8). Whereas the point estimate was highest among studies with low risk of bias within this review, this association was not statistically significant. Two systematic reviews (Vergnes et al. 2007 and Konopka et al. 2012) included data from considerably more primary studies but had slightly lower AMSTAR scores than the meta-analysis of Corbella et al. 2016. The pooled ORs of both meta-analyses were quite similar to that reported in the review by Corbella et al. 2016: 2.3 (95% CI, 1.9 to 2.9; 22 studies, 12,047 participants) and 2.8 (95% CI, 2.0 to 4.1; 17 studies, 7,151 participants), respectively.
Additional Analyses
Overall interpretation of the findings across systematic reviews is likely biased by the degree of overlap in the included primary studies. To assess this, we prepared citation matrices that cross-linked all systematic reviews (columns) with all primary included studies (rows). The degree of overlap was quantified by calculating the CCA (Pieper et al. 2014). Across the 23 systematic reviews included in this overview, 120 primary studies were included (Appendix Table 3). The degree of overlap was high, CCA = 11.3%. As the degree of overlap may be underestimated when combining reviews that explored different outcomes measures, we constructed individual citation matrices for each outcome measure with the exception of small for gestational age, since this was examined in only 1 systematic review. Seventeen systematic reviews on periodontal disease and preterm birth included 70 primary studies with high overlap: CCA = 11.6% (Appendix Table 9). Respective figures for the other outcomes all indicate moderate to high degrees of overlap: preeclampsia, 9 reviews with 23 studies, CCA = 30.4% (Appendix Table 10); LBW, 16 reviews with 48 studies, CCA = 20.8% (Appendix Table 11); and preterm LBW, 17 reviews, including 51 studies, CCA = 10% (Appendix Table 12).
Estimates of the incidence of periodontal disease during pregnancy vary widely, from 11 (95% CI, 9.1 to 13.4) to 100% (Ifesanya et al. 2010; Piscoya et al. 2012). Thus, for each outcome, we calculated 2 PAFs, 1 for each extreme (i.e., 9.1% and 100%). Using RRs of the systematic reviews with the highest AMSTAR scores for each adverse pregnancy outcome, we calculated the following PAFs (the true PAF is likely to lie within the range of these 2 extremes): preterm birth, 5.2% (95% CI, 3.7% to 6.7%) and 37.5% (95% CI, 32.8% to 42.2%); preeclampsia, 9.8% (95% CI, 7.8% to 11.8%) and 54.5% (95% CI, 49.7% to 59.3%); LBW, 6.0% (95% CI, 4.4% to 7.6%) and 41.2% (95% CI, 36.4% to 46.0%); preterm LBW, 17.9% (95% CI, 15.3% to 20.5%) and 70.6% (95% CI, 69.4% to 71.8%).
Discussion
Summary of Evidence
This comprehensive overview of 23 systematic reviews found strong evidence for an association between periodontal disease and various adverse pregnancy outcomes. Systematic reviews that performed a meta-analysis generally had the lowest risk of bias. Given aggregate estimates from the highest-quality reviews, we estimated PAFs. Although these should be interpreted with caution in terms of causality, they appear to indicate that periodontal disease during pregnancy contributes importantly to the overall risks of preterm births, LBW, and preeclampsia. This highlights the pivotal global health relevance of periodontal disease as well as the urgent need to identify the underlying mechanisms and accordingly develop preventive strategies aimed at reducing its considerable disease burden.
This highly comprehensive review is the first to systematically synthesize the available evidence from published systematic reviews on the association between periodontal disease and various adverse pregnancy outcomes. Following a detailed prespecified and peer-reviewed protocol (Vanterpool et al. 2016), we used a highly comprehensive search strategy, which included probing a large number of electronic literature databases, screening references and citations, as well as identifying ongoing systematic reviews. High-quality systematic reviews and meta-analyses were available to clarify the association between periodontal disease and preterm birth, LBW, preeclampsia, and preterm LBW. Findings clearly support strong positive associations between periodontal disease and these outcomes and were highly consistent across systematic reviews with low risk of bias. For a number of outcomes, there was additional evidence from within reviews that higher-quality primary studies supported this link. We provide evidence that inconsistency in the systematic review literature on periodontal disease and adverse pregnancy outcomes primarily stems from existing reviews with moderate to high risk of bias.
Strength and Limitations
Our systematic overview also has limitations. Despite extensive efforts, including consulting interlibrary loan systems, approaching libraries internationally, and emailing authors and coauthors, we were unable to retrieve full texts for a small number of records that were potentially eligible for inclusion based on title and abstract screening. Although this may have introduced a degree of bias, we do not believe that it substantially influenced our findings. Several included systematic reviews reported that primary included studies did not adjust for confounders in a consistent manner. Several factors, such as smoking, socioeconomic status, maternal age, and ethnicity, potentially confound the association between periodontal disease and adverse pregnancy outcomes. Lack of adjustment for important confounders may have resulted in an overestimation of the association measures between periodontal disease and adverse pregnancy outcomes. There was variation in the definition of periodontal disease used in the primary studies included in the systematic reviews that we identified, and only 3 systematic reviews actually reported a definition of periodontal disease. Furthermore, definitions of outcome measures preterm birth, LBW, and preterm LBW were sometimes used interchangeably among systematic reviews leading to inconsistency in the primary studies included in systematic reviews reporting on preterm birth, LBW, and preterm LBW. Given the close biological relationship between gestational age and birth weight, the association between periodontal disease and LBW may in part result from the impact of periodontal disease on length of gestation. Several systematic reviews therefore grouped both outcomes as preterm LBW. As the associations between periodontal disease and preterm birth, LBW, and preterm LBW were all positive, periodontal disease is likely to affect birth weight and length of gestation. Both associations may in part be due to the link between periodontal disease and preeclampsia, as preeclampsia is a known risk factor for (indicated) preterm delivery as well as for intrauterine growth retardation, resulting in LBW.
Comparison with Existing Literature
We are aware of 1 previous overview of systematic reviews in this field, which was published in 2011 (Ramchandani et al. 2011). Drawing evidence from 6 published systematic reviews on periodontal disease and preterm LBW only, Ramchandani et al. concluded that “there is an association between maternal periodontal disease and adverse pregnancy outcomes” although they considered their conclusion “tentative” and highlighted the need for further studies in this field. Using a considerably more exhaustive search strategy, we were able to draw on evidence from 17 systematic reviews on periodontal disease and preterm LBW. Our current overview furthermore adds significantly to the evidence base in this field by evaluating a much broader range of clinically relevant pregnancy outcomes in relation to periodontal disease.
Although findings from this review clearly indicate that periodontal disease is associated with various adverse pregnancy outcomes, this may not necessarily confer causality. However, data from human and animal experimental studies indicate that at least part of this link is indeed likely to be causal (Cetin et al. 2012). It was recently established that the placental microbiome exhibits close resemblance to the oral microbiome (Aagaard et al. 2014). Despite the microbial diversity of the oral microbiome, however (Chen and Jiang 2014; Costalonga and Herzberg 2014), intrauterine infection is mainly associated with periodontopathogenic bacteria from the red and orange complexes, such as Fusobacterium nucleatum and Porphyromonas gingivalis (Offenbacher et al. 1998; Dörtbudak et al. 2005). Detection of F. nucleatum in the placenta (Blanc et al. 2015), amniotic cavity (Ercan et al. 2013), chorioamnion (Doyle et al. 2014), cord blood (Wang et al. 2013), and neonatal gastric aspirates (Gonzales-Marin et al. 2011) is associated with preterm delivery, LBW pregnancies, and early-onset neonatal sepsis. Intravenous delivery of F. nucleatum in rodents induces fetal death and premature birth through activation of toll-like receptor–mediated proinflammatory pathways (Liu et al. 2007; Stockham et al. 2015). Dependent on the specific bacterial strain, P. gingivalis dental infection induces preterm delivery and fetal growth restriction with abnormal placental vascularization in mice (Lin et al. 2003; Ao et al. 2015). P. gingivalis DNA in human chorionic villous tissue (Ibrahim et al. 2015) or amniotic fluid (Ercan et al. 2013) has been linked to recurrent early miscarriage or premature labor with preterm LBW, respectively. Presence of P. gingivalis in the placenta or umbilical cord has been associated with preterm birth and preeclampsia (Chaparro et al. 2013; Chen and Jiang 2014).
Published guidelines on oral health care during pregnancy note that it is an underdeveloped area and that the relevance of oral hygiene during pregnancy is insufficiently recognized by dental and obstetric health professionals. The traditional segregation between dental and general medical education and services may in part be responsible for this. Guidelines also differ in their conclusions about the relationship between periodontal disease and adverse pregnancy outcomes (Council on Clinical Affairs 2011; American College of Obstetricians and Gynecologists 2013; Michigan Department of Health and Human Services 2016), while discussion of this aspect of oral health during pregnancy is even absent in some (Northwest Center to Reduce Oral Health Disparities 2009; Oral Health Care During Pregnancy Expert Workgroup 2012). Our overview demonstrates strong evidence for a link between periodontal disease and adverse pregnancy outcomes, with substantial proportions of preeclampsia, preterm birth, and LBW cases being estimated to be attributable to periodontal disease. Given the high global prevalence of periodontal disease and the adverse pregnancy outcomes associated with it, it is pivotal that preventive and therapeutic strategies be developed to prevent adverse pregnancy outcomes via improving oral health. Although randomized trials and systematic reviews of antibiotics and dental procedures during pregnancy generally demonstrate a positive impact on periodontal disease, the evidence for improvement of pregnancy outcomes is conflicting (Offenbacher et al. 2009). There is thus a need to evaluate novel strategies of preventive and therapeutic approaches at earlier stages of pregnancy, perhaps even preconceptionally.
Conclusion
Given the evidence presented in this comprehensive overview of systematic reviews, we propose that the association between periodontal disease and various common and severe adverse pregnancy outcomes is now sufficiently established for the field to start moving beyond conducting additional primary epidemiologic studies and systematic reviews in this area. There is now a need to focus on elucidating the mechanisms underlying the link between periodontal disease and adverse pregnancy outcomes to inform the development of targeted therapies and preventive strategies.
Author Contributions
L.A. Daalderop, B.V. Wieland, contributed to data analysis, drafted the manuscript; K. Tomsin, J.V. Been, contributed to conception, design, and data analysis, critically revised the manuscript; L. Reyes, B.W. Kramer, S.F. Vanterpool, contributed to conception and design, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplementary Material
Acknowledgments
We thank Timor Faber for his assistance during the analyses.
Footnotes
A supplemental appendix to this article is available online.
The authors received no financial support and declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. 2014. The placenta harbors a unique microbiome. Sci Transl Med. 6(237):237ra65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists Women’s Health Care Physicians, Committee on Health Care for Underserved Women. 2013. Committee opinion No. 569: oral health care during pregnancy and through the lifespan. Obstet Gynecol. 122(2 Pt 1):417–22. [DOI] [PubMed] [Google Scholar]
- Ao M, Miyauchi M, Furusho H, Inubushi T, Kitagawa M, Nagasaki A, Sakamoto S, Kozai K, Takata T. 2015. Dental infection of Porphyromonas gingivalis induces preterm birth in mice. PLoS One. 10(8):e0137249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage GC. 2004. Periodontal diagnoses and classification of periodontal diseases. Periodontol 2000. 34:9–21. [DOI] [PubMed] [Google Scholar]
- Bassani D, Olinto M, Kreiger N. 2007. Periodontal disease and perinatal outcomes: a case-control study. J Clin Periodontol. 34(1):31–39. [DOI] [PubMed] [Google Scholar]
- Blanc V, O’Valle F, Pozo E, Puertas A, Leon R, Mesa F. 2015. Oral bacteria in placental tissues: increased molecular detection in pregnant periodontitis patients. Oral Dis. 21(7):905–912. [DOI] [PubMed] [Google Scholar]
- Cetin I, Pileri P, Villa A, Calabrese S, Ottolenghi L, Abati S. 2012. Pathogenic mechanisms linking periodontal diseases with adverse pregnancy outcomes. Reprod Sci. 19(6):633–641. [DOI] [PubMed] [Google Scholar]
- Chang HH, Larson J, Blencowe H, Spong CY, Howson CP, Cairns-Smith S, Lackritz EM, Lee SK, Mason E, Serazin AC, et al. 2013. Preventing preterm births: analysis of trends and potential reductions with interventions in 39 countries with very high human development index. Lancet. 381(9862):223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaparro A, Blanlot C, Ramirez V, Sanz A, Quintero A, Inostroza C, Bittner M, Navarro M, Illanes SE. 2013. Porphyromonas gingivalis, Treponema denticola and toll-like receptor 2 are associated with hypertensive disorders in placental tissue: a case-control study. J Periodontal Res. 48(6):802–809. [DOI] [PubMed] [Google Scholar]
- Chen H, Jiang W. 2014. Application of high-throughput sequencing in understanding human oral microbiome related with health and disease. Front Microbiol. 5:508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane Effective Practice and Organisation of Care. 2002. Data collection checklist. Ottawa: Institute of Population Health, University of Ottawa; [accessed 2016 Oct 9]. http://methods.cochrane.org/bias/sites/methods.cochrane.org.bias/files/uploads/EPOCDataCollectionChecklist.pdf. [Google Scholar]
- Council on Clinical Affairs. Guideline on perinatal oral health care. Chicago (IL): American Academy of Pediatric Dentistry. [Google Scholar]
- Costalonga M, Herzberg MC. 2014. The oral microbiome and the immunobiology of periodontal disease and caries. Immunol Lett. 162(2 Pt A):22–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörtbudak O, Eberhardt R, Ulm M, Persson GR. 2005. Periodontitis, a marker of risk in pregnancy for preterm birth. J Clin Periodontol. 32(1):45–52. [DOI] [PubMed] [Google Scholar]
- Doyle RM, Alber DG, Jones HE, Harris K, Fitzgerald F, Peebles D, Klein N. 2014. Term and preterm labour are associated with distinct microbial community structures in placental membranes which are independent of mode of delivery. Placenta. 35(12):1099–101. [DOI] [PubMed] [Google Scholar]
- Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ; CDC Periodontal Disease Surveillance Workgroup. 2012. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 91(10):914–920. [DOI] [PubMed] [Google Scholar]
- Ercan E, Eratalay K, Deren O, Gur D, Ozyuncu O, Altun B, Kanli C, Ozdemir P, Akincibay H. 2013. Evaluation of periodontal pathogens in amniotic fluid and the role of periodontal disease in pre-term birth and low birth weight. Acta Ondontol Scand. 71(3–41):553–559. [DOI] [PubMed] [Google Scholar]
- GBD 2013 Mortality and Causes of Death Collaborators. 2015. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 385(9963):117–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes-Filho IS, da Cruz SS, Rezende EJ, da Silveira BB, Trindade SC, Passos JS, de Freitas CO, Cerqueira EM, de Souza Teles Santos CA. 2006. Periodontal status as predictor of prematurity and low birth weight. J Public Health Dent. 66(4):295–298. [DOI] [PubMed] [Google Scholar]
- Gonzales-Marin C, Spratt D, Millar M, Simmonds M, Kempley S, Allaker R. 2011. Levels of periodontal pathogens in neonatal gastric aspirates and possible maternal sites of origin. Mol Oral Microbiol. 26(5):277–290. [DOI] [PubMed] [Google Scholar]
- Higgins J, Green S. 2009. Cochrane handbook for systematic reviews of interventions. Version 5.0.2. Chichester (UK): John Wiley & Sons Ltd; [updated 2009. September; accessed 2017 May 15]. http://handbook.cochrane.org/v5.0.2/. [Google Scholar]
- Highfield J. 2009. Diagnosis and classification of periodontal disease. Aust Dent J. 54 Suppl 1:S11–S26. [DOI] [PubMed] [Google Scholar]
- Ibrahim MI, Abdelhafeez MA, Ellaithy MI, Salama AH, Amin AS, Eldakrory H, Elhadad NI. 2015. Can Porphyromonas gingivalis be a novel aetiology for recurrent miscarriage? Eur J Contracept Reprod Health Care. 20(2):119–127. [DOI] [PubMed] [Google Scholar]
- Ifesanya JU, Ifesanya AO, Asuzu MC, Oke GA. 2010. Determinants of good oral hygiene among pregnant women in Ibadan, south-western Nigeria. Ann Ib Postgrad Med. 8(2):95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarjoura K, Devine PC, Perez-Delboy A, Herrera-Abreu M, D’Alton M, Papapanou PN. 2005. Markers of periodontal infection and preterm birth. Am J Obstet Gynecol. 192(2):513–519. [DOI] [PubMed] [Google Scholar]
- Kunnen A, Blaauw J, van Doormaal JJ, van Pampus MG, van der Schans CP, Aarnoudse JG, van Winkelhoff AJ, Abbas F. 2007. Women with a recent history of early-onset pre-eclampsia have a worse periodontal condition. J Clin Periodontol. 34(3):202–207. [DOI] [PubMed] [Google Scholar]
- Lin D, Smith MA, Elter J, Champagne C, Downey CL, Beck J, Offenbacher S. 2003. Porphyromonas gingivalis infection in pregnant mice is associated with placental dissemination, an increase in the placental Th1/Th2 cytokine ratio, and fetal growth restriction. Infect Immun. 71(9):5163–5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Redline RW, Han YW. 2007. Fusobacterium nucleatum induces fetal death in mice via stimulation of TLR4-mediated placental inflammatory response. J Immunol. 179(4):2501–2508. [DOI] [PubMed] [Google Scholar]
- Marcenes W, Kassebaum NJ, Bernabé E, Flaxman A, Naghavi M, Lopez A, Murray CJ. 2013. Global burden of oral conditions in 1990–2010: a systematic analysis. J Dent Res. 92(7):592–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin C, Segura-Egea JJ, Martínez-Sahuquillo Á, Bullón P. 2005. Correlation between infant birth weight and mother’s periodontal status. J Clin Periodontol. 32(3):299–304. [DOI] [PubMed] [Google Scholar]
- Martins Moliterno LF, Monteiro B, Da Silva Figueredo CM, Fischer RG. 2005. Association between periodontitis and low birth weight: a case–control study. J Clin Periodontol. 32(8):886–890. [DOI] [PubMed] [Google Scholar]
- Michigan Department of Health and Human Services. 2016. During pregnancy, the mouth matters: a guide to Michigan perinatal oral health. Ypsilanti (MI): Michigan Department of Health and Human Services; [accessed 2017 Aug 22]. http://www.michigan.gov/documents/mdhhs/Oral_Health_Guidelines_2015_508090_7.pdf [Google Scholar]
- Moore S, Ide M, Coward P, Randhawa M, Borkowska E, Baylis R, Wilson RF. 2004. A prospective study to investigate the relationship between periodontal disease and adverse pregnancy outcome. Br Dent J. 197(5):251–258. [DOI] [PubMed] [Google Scholar]
- Northwest Center to Reduce Oral Health Disparities. 2009. Guidelines for oral health care in pregnancy. Seattle (WA): School of Dentistry, University of Washington. [Google Scholar]
- Offenbacher S, Beck JD, Jared HL, Mauriello SM, Mendoza LC, Couper DJ, Stewart DD, Murtha AP, Cochran DL, Dudley DJ, et al. ; Maternal Oral Therapy to Reduce Obstetric Risk (MOTOR) Investigators. 2009. Effects of periodontal therapy on rate of preterm delivery: a randomized controlled trial. Obstet Gynecol. 114(3):551–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offenbacher S, Boggess KA, Murtha AP, Jared HL, Lieff S, McKaig RG, Mauriello SM, Moss KL, Beck JD. 2006. Progressive periodontal disease and risk of very preterm delivery. Obstet Gynecol. 107(1):29–36. [DOI] [PubMed] [Google Scholar]
- Offenbacher S, Jared H, O’Reilly P, Wells SR, Salvi GE, Lawrence HP, Socransky SS, Beck JD. 1998. Potential pathogenic mechanisms of periodontitis-associated pregnancy complications. Ann Periodontol. 3(1):233–250. [DOI] [PubMed] [Google Scholar]
- Oral Health Care During Pregnancy Expert Workgroup. 2012. Oral health care during pregnancy: a national consensus statement. Washington (DC): National Maternal and Child Oral Health Resource Center; [accessed 2017 Aug 22]. https://www.mchoralhealth.org/PDFs/OralHealthPregnancyConsensus.pdf [Google Scholar]
- Pieper D, Antoine S-L, Mathes T, Neugebauer EA, Eikermann M. 2014. Systematic review finds overlapping reviews were not mentioned in every other overview. J Clin Epidemiol. 67(4):368–375. [DOI] [PubMed] [Google Scholar]
- Pieper D, Buechter R, Jerinic P, Eikermann M. 2012. Overviews of reviews often have limited rigor: a systematic review. J Clin Epidemiol. 65(12):1267–1273. [DOI] [PubMed] [Google Scholar]
- Pihlstrom BL, Michalowicz BS, Johnson NW. 2005. Periodontal diseases. Lancet. 366(9499):1809–1820. [DOI] [PubMed] [Google Scholar]
- Piscoya MD, Ximenes RA, Silva GM, Jamelli SR, Coutinho SB. 2012. Periodontitis-associated risk factors in pregnant women. Clinics. 67(1):27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramchandani M, Siddiqui M, Kanwar R, Lakha M, Phi L, Giacomelli L, Chiapelli F. 2011. Proteomic signature of periodontal disease in pregnancy: predictive validity for adverse outcomes. Bioinformation. 5(7):300–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA; PRISMA-P Group. 2015. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 349:g7647. [DOI] [PubMed] [Google Scholar]
- Shea BJ, Bouter LM, Peterson J, Boers M, Andersson N, Ortiz Z, Ramsay T, Bai A, Shukla VK, Grimshaw JM. 2007. External validation of a measurement tool to assess systematic reviews (AMSTAR). PLoS one. 2(12):e1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea BJ, Hamel C, Wells GA, Bouter LM, Kristjansson A, Grimshaw J, Grimshaw J, Henry DA, Boers M. 2009. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol. 62(10):1013–1020. [DOI] [PubMed] [Google Scholar]
- Siqueira FM, Cota LO, Costa JE, Haddad JPA, Lana AMQ, Costa FO. 2008. Maternal periodontitis as a potential risk variable for preeclampsia: a case-control study. J Periodontol. 79(2):207–215. [DOI] [PubMed] [Google Scholar]
- Stockham S, Stamford JE, Roberts CT, Fitzsimmons TR, Marchant C, Bartold PM, Zilm PS. 2015. Abnormal pregnancy outcomes in mice using an induced periodontitis model and the haematogenous migration of Fusobacterium nucleatum sub-species to the murine placenta. PLoS One. 10(3):e0120050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanterpool SF, Tomsin K, Reyes L, Zimmermann LJ, Kramer BW, Been JV. 2016. Risk of adverse pregnancy outcomes in women with periodontal disease and the effectiveness of interventions in decreasing this risk: protocol for systematic overview of systematic reviews. Syst Rev. 5:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Buhimschi CS, Temoin S, Bhandari V, Han YW, Buhimschi IA. 2013. Comparative microbial analysis of paired amniotic fluid and cord blood from pregnancies complicated by preterm birth and early-onset neonatal sepsis. PLoS One. 8(2):e56131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.