Abstract
Some animals have basic culture, but to date there is not much evidence that cultural traits evolve as part of a cumulative process as seen in humans. This may be due to limits in animal physical cognition, such as an inability to compare the efficiency of a novel behavioural innovation with an already existing tradition. We investigated this possibility with a study on a natural tool innovation in wild chimpanzees: moss-sponging, which recently emerged in some individuals to extract mineral-rich liquids at a natural clay-pit. The behaviour probably arose as a variant of leaf-sponging, a tool technique seen in all studied chimpanzee communities. We found that moss-sponges not only absorbed more liquid but were manufactured and used more rapidly than leaf-sponges, suggesting a functional improvement. To investigate whether chimpanzees understood the advantage of moss- over leaf-sponges, we experimentally offered small amounts of rainwater in an artificial cavity of a portable log, together with both sponge materials, moss and leaves. We found that established moss-spongers (having used both leaves and moss to make sponges) preferred moss to prepare a sponge to access the rainwater, whereas leaf-spongers (never observed using moss) preferred leaves. Survey data finally demonstrated that moss was common in forest areas near clay-pits but nearly absent in other forest areas, suggesting that natural moss-sponging was at least partly constrained by ecology. Together, these results suggest that chimpanzees perceive functional improvements in tool quality, a crucial prerequisite for cumulative culture.
Keywords: tool use, efficiency, sponges, field experiment, subculture, Pan troglodytes schweinfurthii
1. Introduction
Over recent decades, social network analyses and experiments in the wild and captivity have produced evidence that some animal behaviour can spread socially [1–4] giving ground for the notion of animal cultures [5]. Yet there is still little compelling evidence for the evolution of cultural traits within groups or populations of animals. Consequently, animal cultures remain seen as stagnant, population-level portfolios of behaviour, much in contrast to what is seen in humans [6–8].
While cultural evolution has become a hot topic in science [9], the term is not uniformly defined, especially when applied to animals. According to some definitions, cultural evolution occurs through stochastic, drift-like processes, as seen in changes in the songs of humpback whales and some birds [10,11]. Other definitions require that cultural evolution entails some sort of functional improvement, similar to natural selection, a process termed ‘cumulative cultural evolution’ (CCE). For instance, Schofield et al. ([12], p. 114) define CCE as ‘a modification […] of a cultural trait (i.e. acquired via social learning) that enhances its complexity, efficiency, security, or convenience’, a definition we use in this article. Importantly, this view of CCE does not mandate incremental changes in the complexity of behavioural traditions, as proposed by other authors [6–8], as this effectively limits the notion of cultural evolution to humans, a perspective we and others [13,14] find unhelpful for evolutionary studies. Cultural evolution, in our view, is equivalent to cultural change, which also broadens the range of relevant research to include, for example, experimental studies of zebra finch songs or route learning in pigeons [15,16].
Whatever definition is adopted, the current literature remains weak on examples of cultural change, particularly in wild animals and for tool use, which is astonishing considering the growing interest in animal innovations and traditions [17]. While all current cultural traits must have started off as innovations, most innovations in animals are not copied by others and remain one-off occurrences (e.g. [18]). This is particularly true for chimpanzees (Pan troglodytes), a species well known for its culturally acquired behaviour [19], where only a few of numerous behavioural innovations have spread through communities [20,21].
This has led to the hypothesis that, compared with humans, animals experience fundamental limitations in the types of social learning required for the high-fidelity spread of novel behaviours, which some authors consider a precondition for CCE [6–8]. For example, while there is a consensus that chimpanzees are avid social learners, they may achieve this by stimulus enhancement, local enhancement or emulation [22], but not through imitation or teaching [6,23]. As a result, chimpanzees may not truly understand the behaviours they learn from others but need to re-invent the wheel anew from one generation to the next [6–8]. A similar point has been made for New Caledonian crows (Corvus moneduloides), a species for which there is evidence for local and stimulus enhancement, but not for imitation regarding behaviour transmission between conspecifics and with humans [24,25]. Nevertheless, more work is needed in both species to identify the specific social learning mechanisms that contribute to the transmission of tool designs. In addition, others have argued that imitation and teaching are not necessary for CCE to occur, neither in animals nor in humans [26,27], suggesting that an exclusive focus on social learning mechanisms may prevent a deeper understanding of CCE.
Another hypothesis for low levels of cultural evolution in animals is based on limitations in physical cognition (e.g. [28]). Individuals may be unable to recognize that a novel behaviour is more suited for a given task compared with a pre-existing one, and thus fail to experience a motivation to adopt the new behaviour, even if it is more advantageous. Animals, in other words, may simply lack the cognitive ability to understand the functional consequences of physical actions upon the environment, which consequently prevents them from improving previously acquired cultural behaviours [29,30].
This view is controversial, however, as chimpanzees and other species in the wild have demonstrated some understanding of the physical properties of their tools (e.g. western chimpanzees, P. t. verus [31]; capuchin monkeys, Sapajus libidinosus [32]). For example, most chimpanzees use sticks to fish for termites, but central chimpanzees (P. t. troglodytes) also manufacture more efficient brush-tipped sticks [33], suggesting that the Central African technique emerged from the unmodified technique. Interestingly, migrating female western chimpanzees adopt a less efficient nut-cracking technique to conform to the prevalent behaviour of their new social group at the cost of personal efficiency [34]. Among non-primates, New Caledonian crows manufacture probing tools to capture invertebrates in trees from the long-barbed edges of palm-like Pandanus leaves, but designs differ across groups of animals, suggesting CCE [35]. In addition, hooked stick tools may also have evolved from unmodified stick tools due to CCE [36,37].
Causal understanding of tool properties has also been demonstrated in captivity, notably for all great apes [38,39] and New Caledonian crows [40]. For example, chimpanzees can change from one technique to another if there is a notable improvement in efficiency [41,42]. As always with captive studies, the concern remains that capacities demonstrated by subjects may be a by-product of conditions absent in natural environments. One solution is to carry out controlled experiments with wild-born animals under laboratory conditions [43], as demonstrated for wild-caught New Caledonian crows that discriminate differences in design features of hooked stick tools in captivity [44].
In sum, the current literature is unable to provide a clear picture regarding the question of whether culturally acquired behaviour in animals can change in directed ways. While captive studies have demonstrated the ability of animals to improve both individually and socially learned techniques, these findings may be artefacts of captive conditions and, as such, of limited value to understand the cultural repertoires described in the wild. Similarly, while field studies have documented naturally occurring changes in behavioural traditions, sometimes with differences in complexity, we are not aware of any documented transition in a cultural trait changing from a less to a more efficient variant, which would provide strong evidence for CCE in wild cultures.
An interesting consequence of within-group changes in socially acquired behaviour is the establishment of cultural subgroups, defined here as parts of a group engaging in socially acquired behavioural patterns different from the rest of the group [45]. As has been argued for animal culture in general, a key point is that any eventual cultural subgroup is not the result of shared genetics or shared ecology alone [46]. Socially learned subcultures, in other words, are evidence for diversification within cultures and are important to investigate cultural evolution [47]. Over longer time periods, the behavioural variant that defines the subculture may continue its cultural sweep, to the effect that it becomes part of the entire group's culture. Alternatively, it may remain restricted to parts of the group [48].
In this study, we address the question of CCE in animals by capitalizing on recent observations in the Sonso chimpanzee community (P. t. schweinfurthii) of Budongo Forest, Uganda [21]. In 2011, a behavioural innovation, moss-sponging, naturally spread within a subset of the community [21]. Moss-sponging is an alternative to commonly found leaf-sponging, a behaviour present in all wild chimpanzee communities studied so far. While leaf-sponging is often referred to as a ‘cultural universal’ in chimpanzees [19], its widespread presence may also suggest a genetic basis; studies examining the likelihood of its spontaneous emergence are thus needed [49]. Well before the advent of moss-sponging [50], most members of the Sonso community habitually manufactured leaf-sponges to extract various types of liquids from cavities and rivers. Moss-sponging is probably a variant of leaf-sponging as both consist of harvesting a handful of leafy vegetation or clumps of moss, respectively, subsequently shaped in the mouth into a sponge approximately the size of a golf ball. The sponges are then dipped into the liquid and reinserted and squeezed in the mouth. Moss-sponging was first seen at one specific location in the community's home range, a clay-pit, which consisted of two waterholes in clay ground, filled with mineral-rich suspensions [51]. Immediately after its appearance, the new behaviour spread within a week across seven individuals via proximity-based observational learning [21]. In the subsequent 3 years, moss-sponging propagated further throughout the community, albeit now mainly within the matrilines of cohort members that initially learned the technique [52]. These two studies show that, compared with leaf-sponging, social learning must have contributed strongly to the spread of moss-sponging. In the meantime, moss-sponging was also observed in the Waibira community of Budongo Forest, which has an overlapping home range with the Sonso community (C. Hobaiter 2018, personal communication).
The fact that moss-sponging continued to spread through the community, despite the presence of an already existing technique for absorbing liquids (leaf-sponging), led us to hypothesize that the spread may have been caused by a difference in efficiency between the two types of sponge materials. However, one puzzling fact was that, since its emergence, moss-sponging was almost only observed at the site of its original invention, the clay-pit, with only six observations elsewhere in the forest, despite uninterrupted daily focal follows over several years by field assistants and researchers. Leaf-sponging, instead, continued to be observed in a range of contexts and throughout the forest, including at the clay-pit.
A more parsimonious hypothesis may thus have been that moss-sponging was nothing but a context-specific behaviour, triggered by special ecological conditions present at clay-pits, but that chimpanzees did not perceive the more general functional properties of moss as sponge material. In other words, moss-sponging chimpanzees may have simply used moss at the clay-pit in response to ecological (e.g. clay water) or social (e.g. competition) factors encountered at the location, but not because moss-sponging was part of an enriched cultural repertoire.
To distinguish between these two hypotheses, we collected three sets of data. First, we tested whether moss-sponging was indeed more efficient than leaf-sponging, a crucial prerequisite for any argument based on physical cognition. We were interested in two dimensions of efficiency: absorbency (amount of liquid a sponge could contain) and effectiveness (manufacturing and deployment time).
Second, to test whether moss-savvy (but not moss-ignorant) individuals preferred moss-sponging over leaf-sponging, we tested subjects with a standardized field experiment. The experiment consisted of giving subjects a choice between both sponge materials, leaves and moss, presented on a portable log with an artificial cavity filled with natural rainwater [53]. Not all members of the community had been observed using moss-sponges at the time of the experiment, suggesting some were ‘moss-ignorant’. We thus classified subjects as either ‘moss-spongers’ (i.e. individuals who had been observed manufacturing a moss-sponge at the clay-pit but continued to use leaf-sponges in other contexts, including also at the clay-pit) or ‘leaf-spongers’ (individuals who had never been observed manufacturing moss-sponges but had manufactured leaf-sponges). If moss-sponges are more efficient than leaf-sponges and if chimpanzees can compare tools in terms of efficiency, we predicted that the proportion of moss choices would be higher among known moss-spongers than among leaf-spongers.
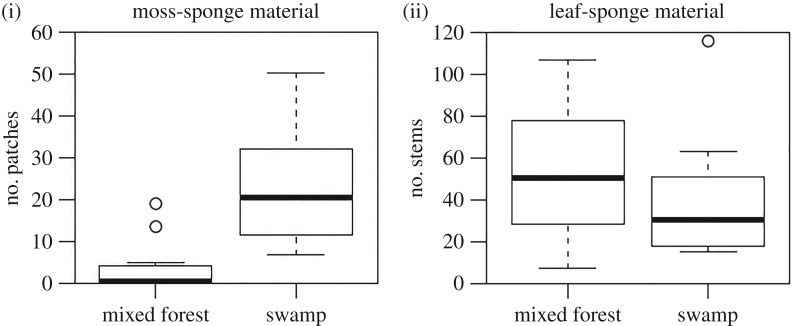
Third, we investigated whether the lack of moss-sponging by moss-savvy individuals throughout most of the forest was a by-product of uneven moss distribution as chimpanzees generally manufacture their tools near the location of use. To evaluate the ecological correlates of moss-sponging, we conducted a survey of leaf and moss distribution at known chimpanzee sponging locations throughout the forest, including areas of mixed forest where rainwater-filled tree holes were located and swamps where clay-pits were located.
2. Material and methods
(a). Study site and subjects
The study was conducted in Budongo Forest Reserve in western Uganda (1°37′–2°00 ′N, 31°22′–31°46′ E) with the Sonso chimpanzee community (P. t. schweinfurthii). The reserve consists mainly of moist semi-deciduous tropical forest, at a mean altitude of 1100 m. The Sonso community's home range is approximately 7 km2 and members have been habituated to human presence since the mid-1990s [54]. At the time of the study, the community consisted of 68 individuals.
(b). Tool features
Tool efficiency was assessed in terms of absorbency, defined as the weight of liquid that a leaf-sponge or a moss-sponge could carry, the assumption being that the more water it could absorb, the more efficient it was. ‘Leaf-sponging’ was defined as using a wad of crumpled or folded leaves to absorb and consume liquid; ‘moss-sponging’ as using a clump of moss or mixture of moss and leaves for the same purpose (figure 1). Sponges manufactured by chimpanzees during daily follows and experiments were collected whenever possible and their absorbency measured. Over 153 days of focal follows and experiments between January 2013 and February 2015, we collected 96 sponges on 48 separate days from 28 identified and three unidentified individuals. We measured the absorbency for n = 62 of them for whom the manufacturer was identified (n = 48 leaf-sponges; n = 14 moss-sponges), collected during natural sponging at the clay-pit, tree holes and rivers (n = 44) and during experiments (n = 18). Absorbency was determined by dipping the sponge in water and then squeezing it, comparing the weight before and after squeezing with a scale (Factory weigh PRO-VA1234, precision: 0.01 g). Each sponge was tested within a few hours after being collected in the forest, ruling out systematic environmental effects (e.g. [55]). Each sponge was then submerged in a container with rainwater, removed, weighed, squeezed until water stopped dripping and weighed again. This procedure was repeated 10 times for each sponge, following Biro and colleagues [56]. To account for possible degradation between repeated squeezes, we included measurement number as a covariate in the statistical analyses. While we measured weight of absorbed liquid, for simplicity we refer to absorbency as volume.
Figure 1.
Two examples of sponge tools manufactured during a log experiment. (a) Leaf-sponge made of Alchornea floribunda. (b) Moss-sponge made of Orthostichella welwitschii. (Online version in colour.)
(c). Availability
We carried out a survey to assess the availability of sponging material (leaves and moss) around locations where chimpanzees had been observed sponging. The prediction was that swamp areas where clay-pits are located contained more moss than mixed forest areas where natural tree holes are located. To this end, in December 2016, we surveyed all locations where chimpanzees had previously been observed sponging from tree holes or water holes (28 locations, n = 8 in swamp areas and n = 20 in mixed forest areas). The survey zone was a 5 m radius around the water source, up to 3 m off the ground. To assess leaf availability, we counted all stems of Acalypha spp. and Lasiodiscus mildraedii, the species most frequently picked by the chimpanzees to manufacture leaf-sponges. We considered a stem as a plant axis that carried at least four leaves. To assess moss availability, we calculated the surface covered by moss in the survey zone. As moss species, we were able to identify Orthostichella welwitschii (mostly hanging from tree branches), Porotrichum elongatum and Plagiochila spp. (a liverwort). We assessed moss coverage by using surfaces of 20 cm × 20 cm, using a cardboard reference unit. If the whole surface was covered by moss, we attributed a value of 1; if half, 0.5; a quarter, 0.25; otherwise 0.
(d). Experiment
To investigate what tool ‘leaf-spongers’ and ‘moss-spongers’ would select if given the choice of the two materials in a controlled context, we manufactured a portable log (length: 33.5 cm; diameter: 14 cm; electronic supplementary material, figure S10) with an artificial cavity drilled in the centre (opening: 8.0 × 8.5 cm; depth: 8.0 cm), filled with 20 ml of rainwater. The apparatus was a modified version of a honey-trap apparatus used in previous experiments [53]. To minimize the risk of disease transmission from humans to chimpanzees, we boiled rainwater collected from tin roofs prior to each experiment. We chose rainwater rather than mineral suspensions to remove any potential inherent advantage that moss might have over leaves in absorbing minerals [21]. We positioned the apparatus in the absence of any individual and supplied tool material at an equal distance from the hole (electronic supplementary material, figure S10) in the form of two clumps of moss (Orthostichella welwitschii) and two leafy branches of Acalypha spp.
We aimed to test subjects in isolation to rule out social influence or competitive pressure. We thus targeted specific individuals by anticipating their travel direction, presenting the apparatus when they were alone (except for mothers with dependent offspring). The choice of subjects was therefore opportunistic and not blind. Since individuals were unconstrained in their daily movement patterns, it was unavoidable that, in some trials (8 of 20), the subject arrived at the apparatus while another individual was already engaged with it. In another case, the subject joined two group members already engaging with the log (electronic supplementary material). If both materials were still available when the subject arrived, we included its choices in the analysis. If an individual participated several times, we only took the first trial into account. Trials had to be repeated occasionally, with at least 24 h intermissions, if the subject interacted with the log but did not manufacture a sponge. All trials were filmed by two experimenters (N.L. and her field assistant) with Panasonic HC-X909 video cameras to get two different angles of the scene. Data included the identity of the subject and eventual bystanders, whether the subject had been seen moss-sponging before and the technique used to retrieve the water from the hole.
There were two experimental periods (January 2014 and January 2015) corresponding to the annual dry season, when chimpanzees are most likely to search for water in tree holes. 20 individuals participated in the experiment, all of which had been observed manufacturing leaf-sponges prior to the experiment: six adult females, five adult males, two subadult females, one subadult male, four juvenile females and two juvenile males. Nine of 20 individuals were classified as ‘moss-spongers’ as they had moss-sponged at least once before the experiment (electronic supplementary material, table S1), while the remaining 11 were classified as ‘leaf-spongers’ by default [52].
The absorbency of the sponges (nine moss-sponges and nine leaf-sponges) manufactured during the experiment was measured as described above. We additionally evaluated efficiency by extracting manufacturing time (latency between first touching the material and removing the fabricated sponge from the mouth) and deployment time (latency between touching the sponge material, fabricating the sponge and transferring the liquid-filled sponge into the mouth) from videos recorded during the experiments for n = 17 leaf-sponges and n = 8 moss-sponges. For both measures, the assumption was that the faster a tool could be manufactured and used, the more efficient it was.
(e). Statistical analyses
To assess differences in sponge absorbency, we fitted a linear mixed model (LMM) with Gaussian error distribution with the lme4 package in R v. 3.4.0 [57,58]. The response variable was the volume of water a given moss absorbed. Type of material (moss/leaf), context (natural observation/experiment) and sponge weight were entered as fixed effects. In addition, we fitted measurement number as a control variable to account for the possibility that absorbency degraded within a sponge over repeated squeezes. Our main interest was the effect of the sponge material. As the degradation effect of repeated squeezes could differ between the two materials or the effects of material differ between the two contexts, we included two 2-way interactions in our model: (i) material and measurement number and (ii) material and context. We fitted sponge ID (due to multiple measurements per sponge) nested in manufacturer ID as a random intercept. Finally, we fitted material and context as uncorrelated random slopes in manufacturer ID. Model fit was assessed visually (distribution and homogeneity of residuals) and numerically (variance inflation factors), and neither check indicated severe violations of assumptions (electronic supplementary material). We also fitted a null model with the material (our factor of primary interest) removed but random effects structure unchanged. The difference between full and null model was assessed using a likelihood ratio test (LRT) [59].
To assess differences in manufacturing and deployment time during the experiment, we fitted two LMMs with material (moss/leaf) as a fixed effect, sponge manufacturer as random intercept, and material as uncorrelated random slope in manufacturer ID. In the first model, we used manufacturing time as the response variable. In the second model, we used deployment time as the response variable. As with the absorbency models, we removed the major factor of interest (material) of these full models to fit corresponding null models, which were also tested with LRTs. We also fitted both models as generalized linear mixed models with Poisson error and log-link function.
We used two tests to assess subjects' choices during the experiment. First, we ran a proportion test to address the hypothesis that, given their presumed differences in knowledge, moss-spongers were more likely to choose moss than leaf-spongers and that leaf-spongers were more likely to choose leaves than moss-spongers. Because this was a directed hypothesis, we opted to provide a one-tailed p-value here. In addition, if effects were significant, but opposite to what we predicted, we would consider the result as non-significant (i.e. the same interpretation as if accepting the null hypothesis [60]).
Second, we addressed the same question but framed the problem as correlational (i.e. how strongly material choice was correlated with presumed knowledge). For this, we investigated the correlation between the likelihood of individuals to use moss in the experiment (yes = 1; no = 0) and their presumed knowledge of the moss-sponging technique (yes = 1; no = 0). This coding allows the calculation of repeatability R (intra-class correlation coefficient) between the choice of material during the experiment and presumed knowledge [61,62]. This metric can be interpreted as the proportion of total variance accounted for by differences between individuals [61]. At its highest (R = 1), there is no within-subject variance, so in our case the matching between choices during the experiment and subjects’ knowledge would be perfect. We computed a null distribution of expected R-values based on 2000 permuted datasets and assessed statistical significance as the proportion of R-values from these permuted datasets that were larger or equal to our observed R-value [62].
Finally, we compared the frequencies of materials to manufacture sponges between different locations/forest types using a Mann–Whitney U-test.
3. Results
(a). Absorbency
The model assessing the absorbency of moss-sponges manufactured by chimpanzees in both natural and experimental contexts differed significantly from the null model (LMM, LRT: 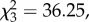 p < 0.0001). We found that sponges made of moss absorbed significantly more liquid than sponges made of leaves, and this difference was more pronounced for the sponges manufactured in the experimental context (LRT,
p < 0.0001). We found that sponges made of moss absorbed significantly more liquid than sponges made of leaves, and this difference was more pronounced for the sponges manufactured in the experimental context (LRT, 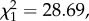 p < 0.0001; table 1, figure 2). In the natural context, moss-sponges absorbed an average of 13.1 ml; leaf-sponges absorbed an average of 8.4 ml of liquid (figure 2). In the experimental context, moss-sponges absorbed an average of 26.3 ml; leaf-sponges absorbed an average of 9.5 ml of liquid (figure 2). Not surprisingly, heavier sponges, independently of the material used to manufacture them, absorbed more liquid than lighter sponges (1 g increase in weight corresponded to 0.85 ml more liquid absorbed; table 1).
p < 0.0001; table 1, figure 2). In the natural context, moss-sponges absorbed an average of 13.1 ml; leaf-sponges absorbed an average of 8.4 ml of liquid (figure 2). In the experimental context, moss-sponges absorbed an average of 26.3 ml; leaf-sponges absorbed an average of 9.5 ml of liquid (figure 2). Not surprisingly, heavier sponges, independently of the material used to manufacture them, absorbed more liquid than lighter sponges (1 g increase in weight corresponded to 0.85 ml more liquid absorbed; table 1).
Table 1.
Results of the LMM testing differences in absorbency. Each sponge was measured 10 times.
| beta | s.e. | t | |
|---|---|---|---|
| intercept | 2.58 | 0.86 | 2.99 |
| material (moss or leaves) | 16.87 | 3.17 | 5.32 |
| context (experimental or natural) | −1.03 | 0.92 | −1.12 |
| measurement number (10 dips) | −0.38 | 0.06 | −5.92 |
| weight (g) | 0.85 | 0.07 | 12.09 |
| material × measurement number | −0.06 | 0.13 | −0.45 |
| material × context | −12.23 | 2.02 | −6.06 |
Figure 2.
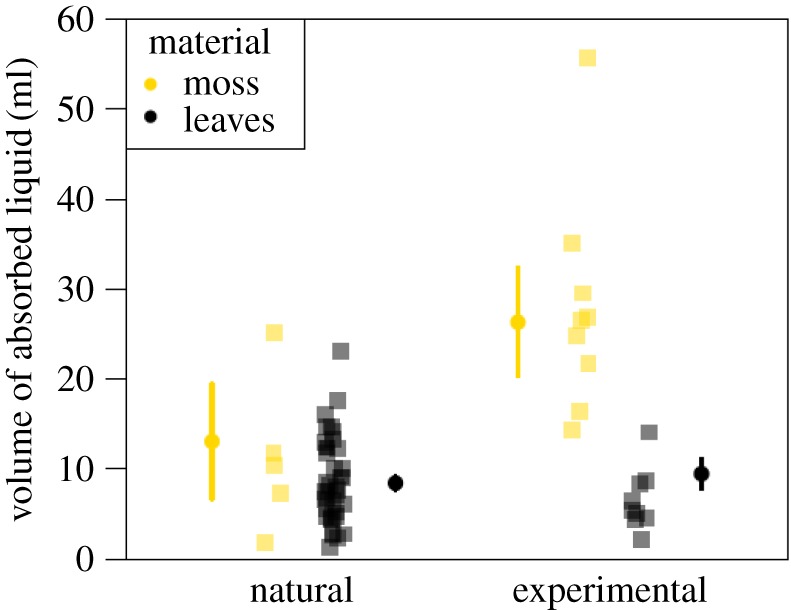
Comparison of absorbency for natural and experimental sponges. Each square represents the mean volume absorbed by one sponge across 10 repeated measurements. Circles represent model predictions. Lines are 95% confidence intervals. (Online version in colour.)
(b). Manufacturing and deployment time
The model comparing manufacturing time only between experimentally manufactured moss-and leaf-sponges was marginally significantly different from the null model (LMM: n = 25 sponges by 15 individuals, LRT: 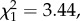 p = 0.0635; table 2 and figure 3). Moss-sponges took on average 7.2 s to manufacture while leaf-sponges took on average 11.2 s.
p = 0.0635; table 2 and figure 3). Moss-sponges took on average 7.2 s to manufacture while leaf-sponges took on average 11.2 s.
Table 2.
Results of the LMMs testing differences in manufacturing and deployment time between moss and leaf-sponges.
| beta | s.e. | t | |
|---|---|---|---|
| manufacturing time | |||
| intercept | 11.18 | 1.56 | 7.16 |
| material (moss versus leaf) | −3.99 | 2.00 | −2.00 |
| deployment time | |||
| intercept | 12.79 | 1.56 | 8.20 |
| material (moss versus leaf) | −3.80 | 1.68 | −2.26 |
Figure 3.
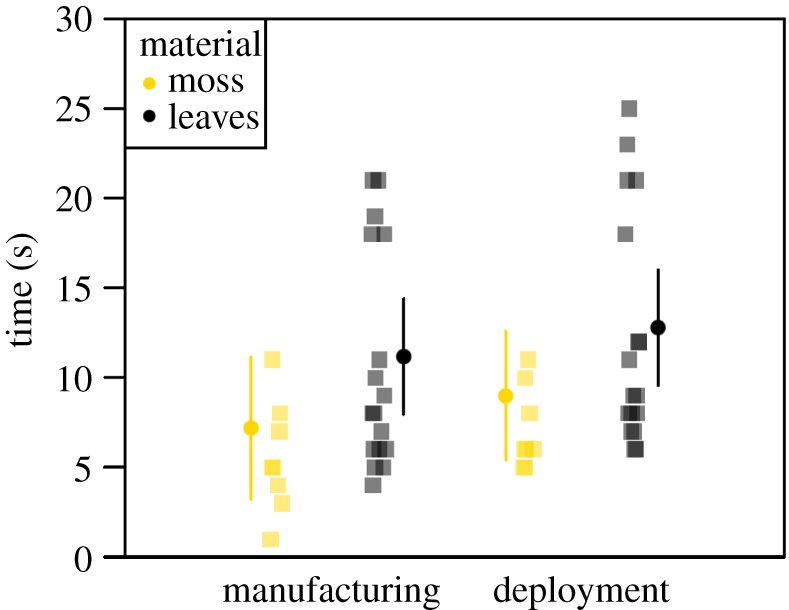
Comparison of moss- and leaf-sponges manufacturing (i) and deployment (ii) time. Raw data are shown as squares and model estimates as circles with 95% confidence intervals. (Online version in colour.)
The model comparing deployment time (manufacturing plus first use) between experimentally manufactured moss-and leaf-sponges differed significantly from the null model (LRT: 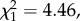 p = 0.0347; table 2, figure 3). Here, the combined time was on average 9.0 s for moss-sponges and on average 12.8 s for leaf-sponges.
p = 0.0347; table 2, figure 3). Here, the combined time was on average 9.0 s for moss-sponges and on average 12.8 s for leaf-sponges.
In both cases, GLMMs with Poisson error structure revealed very similar results (see electronic supplementary material).
(c). Experiment
We tested 20 individuals. In line with our predictions, the proportion of individuals that used moss for sponge production was higher among known moss- than leaf-spongers (proportion test: 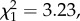 one-tailed p = 0.0361, moss-spongers: 7/9, leaf-spongers: 3/11; electronic supplementary material, table S1).
one-tailed p = 0.0361, moss-spongers: 7/9, leaf-spongers: 3/11; electronic supplementary material, table S1).
To assess the correlation between presumed knowledge and choice during the experiment, we calculated the repeatability of the material chosen. The repeatability estimate was R = 0.52 (p = 0.009, range of permuted R: 0.00–0.81; see electronic supplementary material, figure S8). These results indicate that individuals were more likely to choose the material in the experiment that corresponded to their presumed knowledge.
(c). Availability
We found that both Acalypha spp. and Lasiodiscus mildraedii were more readily available around tree-hole sponging locations in mixed forest areas (n = 20 locations) than in swamp areas (n = 8 locations) although this difference was not statistically significant (Mann–Whitney test, W = 105.5, p = 0.2034; figure 4). However, there was significantly less moss material available at known sponging locations in the mixed forest than in the swamp areas, where the clay-pits were located (Mann–Whitney test, W = 5, p < 0.001; figure 4).
Figure 4.
Availability of sponge material across forest types.
4. Discussion
We tested experimentally whether the spread of moss-sponging, first observed in the Sonso chimpanzees of Budongo Forest in 2011, could be connected to differences in efficiency between this behavioural innovation and the ancestral leaf-sponging variant, and whether this led to the establishment of a new subculture in the community. We report three sets of findings that are directly relevant to this question and to the topic of cultural evolution more generally. In the first set, we found that moss-sponges represented a functional improvement compared to ancestral leaf-sponges. Moss-sponges were both more effective in absorbing rainwater and fabricated and used more quickly than leaf-sponges. Our results are thus in line with an ongoing discussion on tool efficiency as an indicator of cumulative culture, exemplified by data on New Caledonian crows whose hooked tools are more efficient than non-hooked tools [36,37] and central African chimpanzees whose brush-tipped termite fishing tools are more efficient than non-brushed tools [33].
Our second finding was to show experimentally that chimpanzees who already had experience with moss-sponges preferred moss over leaves as material to fabricate sponges when presented with a novel problem unrelated to the original socio-ecological context of moss-sponging (i.e. independent of location, liquid type and social competition). By contrast, individuals that had never been observed moss-sponging mainly chose leaves, suggesting they did not perceive moss as a suitable sponge material in this novel situation. These results demonstrate that moss-sponging is not tied to a particular ecological condition but generally available to individuals who have learned the novel technique beforehand. Our experimental results are also supported by the natural observations of Sonso individuals using moss-sponges outside the context of the clay-pit, which suggest that moss-sponging is in the process of being applied more widely.
In a third set of findings, we reported that the most likely reason natural moss-sponging was not seen outside its original clay-pit context was the uneven availability of moss throughout the forest. Survey data showed that the two most common plant species to manufacture leaf-sponges were abundant throughout the forest and present at the 28 locations where chimpanzees had been observed leaf-sponging. By contrast, moss was rare in the forest, except in swamp areas where clay-pits are located, which effectively prevented moss-spongers from executing their behaviour because of a lack of opportunities [63,64]. Nevertheless, chimpanzees do not transport moss-sponges from moss-rich areas to moss-depleted ones, suggesting that the functional improvements may not be enough to modify chimpanzees' preference entirely.
The core evidence for cultural evolution was the result of our field experiment, which essentially suggested the presence of a cultural subgroup in tool use within the Sonso community. Our experiment did not specifically address the role of social learning in sponge manufacturing, as this was done in previous studies [21,52]. More importantly, the current study suggests that most leaf-spongers did not perceive moss as a potential sponge material [29], suggesting a lack of underlying cultural knowledge.
Nevertheless, 3 of 11 classified leaf-spongers (electronic supplementary material, table S1) chose moss to manufacture a sponge during the experiment, which requires some explanation. For one individual, KH, we cannot exclude that she was socially influenced by observing an individual before her using moss. However, this argument does not apply for other trials, such as when ST, roughly the same age, chose moss, even after having observed an individual before her using a leaf-sponge (electronic supplementary material). It is also possible that the three new moss-spongers (i) were simply oblivious to the choices offered, (ii) recognized the advantages of moss as sponge material in situ or (iii) were curious to try out its properties in the absence of any prior social learning. Generally, however, we find explanations based on ad hoc trial and error experimenting less plausible because multiple studies with this community have already shown a remarkable resistance to using novel tools in experimental situations [63,65]. The most likely explanation, in our view, is that these three individuals had acquired the moss-sponging behaviour prior to the experiment, but never showed it during observer presence. It would be important to monitor these previously unidentified moss-spongers to check whether moss-sponging remains present. We have observations for one individual, KH, who was subsequently observed moss-sponging at the clay-pit.
Also relevant is that two of nine known moss-spongers opted for the traditional leaf-sponging technique in the experiment. This might have been the result of individual differences in conservatism, manufacturing skills or taste. For example, some individuals may prefer the technique they are more used to, even if they understand differences in efficiency [53]. Leaves are the more habitual material to manufacture a sponge, which may have hindered some individuals from seeing the more efficient moss solution [29]. Social conformity may also cause some chimpanzees to opt for a less efficient technique [34]. In sum, while we showed consistency between attributed prior knowledge and choice in the experiment, our results suggest that context and individual differences interact with each other and determine an individual's choice of tool material, even in controlled situations [65].
Overall, these results provide, to our knowledge, the first evidence that wild chimpanzees can switch from an older, less efficient variant towards a newer, more efficient, socially learned technique. Whether or not moss-spongers preferentially chose moss because they understood and compared the physical properties of the two materials seems very plausible but can ultimately not be decided by our data. While it is possible that experience with moss led to an understanding that moss is more efficient than leaves, moss-spongers may have simply become more familiar with moss compared with other chimpanzees, such that differences in habits were ultimately responsible for our findings. We do not find this a very strong argument because all individuals, including the moss-spongers, continued to use leaf-sponges regularly outside the context of the clay-pit over the years following the appearance of moss-sponging, predicting that all subjects should have chosen leaves in the experiment.
In sum, our findings are consistent with the interpretation that the innovation and social spread of moss-sponging effectively led to the formation of a tool-related cultural subgroup in the Sonso community. This outcome may be based on a cognitive ability to perceive and compare the functional properties and efficiency of tools. Cognitively ‘less demanding’ explanations, for example, that chimpanzees simply chose the more locally abundant material, were ruled out by our experiment, which controlled for the availability of tool materials. Our data further highlight a potential role of efficiency as a driver of cultural evolution, insofar as more efficient traits are favoured and eventually come to dominate, while less efficient traits are neglected and eventually abandoned. In our case, one reason why moss-sponging did not spread as much as its efficiency suggested might be the mere lack of available resources. There is no doubt ecological factors generally have a strong influence on the emergence and maintenance of cultural behaviour [63]. The Sonso chimpanzees had been observed for over 20 years before moss-sponging appeared, with dozens of chimpanzees visiting the swamp forest but no one innovating the behaviour before 2011. One explanation for this is that other nutrient resources, such as Raphia pith [66], became less abundant due to human activities, forcing chimpanzees to look for alternative sources, such as mineral-rich water found in clay-pits [51]. Moss-sponges then spread socially in a subgroup of the current generation of chimpanzees, who adopted the more efficient form compared to the ancestral trait. It will be interesting to see how new generations of Sonso chimpanzees, regularly exposed to moss-sponging demonstrators, magistrate between the old tradition, leaf-sponging, and the more recent tradition, moss-sponging, in tool-assisted drinking contexts.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Samuel Adue for his invaluable help in the field, Dr Michelle Price for moss identification and Dr Caroline Asiimwe for her advice regarding experimental design. We thank UNCST and UWA for permission to conduct our research in Budongo Forest Reserve and the Royal Zoological Society of Scotland for supporting BCFS. We thank Andy Whiten for comments on an earlier draft. We thank Alex Thornton, Josh Firth and one anonymous reviewer for their useful comments on an earlier version of the article.
Ethics
Field protocols were reviewed and permission to conduct this research was given by the Uganda Wildlife Authority (UWA), the Ugandan National Council for Science and Technology (UNCST), the National Forestry Authority (NFA) and the resident veterinary section at Budongo Conservation Field Station (BCFS).
Data accessibility
All data and code used in this study are available in the electronic supplementary material.
Authors' contributions
Data collection: N.L. and J.G.; statistical analysis: C.N. and N.L.; experimental design: N.L., T.G. and K.Z.; manuscript writing: N.L., T.G., C.N. and K.Z.; funding: K.Z.
Competing interests
We declare we have no competing interests.
Funding
This work was funded by the European Research Council (FP7/2007-2013/ERC n°283871) and the Swiss National Science Foundation (grants 310030_143359 to K.Z.; CR13I1_162720 and P300PA_164678 to T.G.).
References
- 1.Aplin LM, Farine DR, Morand-Ferron J, Cockburn A, Thornton A, Sheldon BC. 2015. Experimentally induced innovations lead to persistent culture via conformity in wild birds. Nature 518, 538–541. ( 10.1038/nature13998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boogert NJ, Giraldeau L-A, Lefebvre L. 2008. Song complexity correlates with learning ability in zebra finch males. Anim. Behav. 76, 1735–1741. ( 10.1016/j.anbehav.2008.08.009) [DOI] [Google Scholar]
- 3.Alem S, Perry CJ, Zhu X, Loukola OJ, Ingraham T, Søvik E, Chittka L. 2016. Associative mechanisms allow for social learning and cultural transmission of string pulling in an insect. PLoS Biol. 14, e1002564 ( 10.1371/journal.pbio.1002564) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ladds Z, Hoppitt W, Boogert NJ. 2017. Social learning in otters. R. Soc. open sci. 4, 170489 ( 10.1098/rsos.170489) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whiten A, Goodall J, McGrew WC, Nishida T, Reynolds V, Sugiyama Y, Tutin C.EG, Wrangham RW, Boesch C. 1999. Cultures in chimpanzees. Nature 399, 682–685. ( 10.1038/21415) [DOI] [PubMed] [Google Scholar]
- 6.Tennie C, Call J, Tomasello M. 2009. Ratcheting up the ratchet: on the evolution of cumulative culture. Proc. R. Soc. B 364, 2045–2415. ( 10.1098/rstb.2009.0052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyd R, Richerson PJ. 1996. Why culture is common but cultural evolution is rare. Proc. Br. Acad. 88, 77–93. [Google Scholar]
- 8.Dean LG, Vale GL, Laland KN, Flynn E, Kendal RL. 2014. Human cumulative culture: a comparative perspective. Biol. Rev. Camb. Philos. Soc. 89, 284–301. ( 10.1111/brv.12053) [DOI] [PubMed] [Google Scholar]
- 9.Brewer J, Gelfand M, Jackson JC, MacDonald IF, Peregrine PN, Richerson PJ, Turchin P, Whitehouse H, Wilson DS. 2017. Grand challenges for the study of cultural evolution. Nat. Ecol. Evol. 1, 70 ( 10.1038/s41559-017-0070). [DOI] [PubMed] [Google Scholar]
- 10.Lynch A, Baker AJ. 1993. A population memetics approach to cultural-evolution in chaffinch song: meme diversity within populations. Am. Nat. 141, 597–620. ( 10.1086/285493) [DOI] [PubMed] [Google Scholar]
- 11.Filatova OA, Burdin AM, Hoyt E. 2013. Is killer whale dialect evolution random? Behav. Processes 99, 34–41. ( 10.1016/j.beproc.2013.06.008) [DOI] [PubMed] [Google Scholar]
- 12.Schofield DP, McGrew WC, Takahashi A, Hirata S. 2018. Cumulative culture in nonhumans: overlooked findings from Japanese monkeys? Primates 59, 113–122. ( 10.1007/s10329-017-0642-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whiten A. In press. A second inheritance system: the extension of biology through culture. Royal Society Interface Focus7, 20160142. [DOI] [PMC free article] [PubMed]
- 14.McGrew WC. 2017. Ourselves explained. Human Ethol. Bull. 3, 141–144. (doi:10.22330/heb/323/141-144) [Google Scholar]
- 15.Fehér O, Wang H, Saar S, Mitra PP, Tchernichovski O. 2009. De novo establishment of wild-type song culture in the zebra finch. Nature 459, 564–568. ( 10.1038/nature07994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sasaki T, Biro D. 2017. Cumulative culture can emerge from collective intelligence in animal groups. Nat. Commun. 8, 15049 ( 10.1038/ncomms15049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reader SM, Morand-Ferron J, Flynn E. 2016. Animal and human innovation: novel problems and novel solutions. Proc. R. Soc. B 371, 20150182 ( 10.1098/rstb.2015.0182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamamoto S, Yamakoshi G, Humle T, Matsuzawa T. 2008. Invention and modification of a new tool use behavior: ant-fishing in trees by a wild chimpanzee (Pan troglodytes verus) at Bossou, Guinea. Am. J. Primatol. 70, 699–702. ( 10.1002/ajp.20544) [DOI] [PubMed] [Google Scholar]
- 19.Whiten A, Goodall J, McGrew WC, Nishida T, Reynolds V, Sugiyama Y, Tutin C.EG, Wrangham RW, Boesch C. 2001. Charting cultural variation in chimpanzees. Behaviour 138, 1481–1516. ( 10.1163/156853901317367717) [DOI] [Google Scholar]
- 20.Nishida T, Matsusaka T, McGrew WC. 2009. Emergence, propagation or disappearance of novel behavioral patterns in the habituated chimpanzees of Mahale: a review. Primates 50, 23–36. ( 10.1007/s10329-008-0109-y) [DOI] [PubMed] [Google Scholar]
- 21.Hobaiter C, Poisot T, Zuberbühler K, Hoppitt W, Gruber T. 2014. Social network analysis shows direct evidence for social transmission of tool use in wild chimpanzees. PLoS Biol. 12, e1001960 ( 10.1371/journal.pbio.1001960) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagell K, Olguin RS, Tomasello M. 1993. Processes of social-learning in the tool use of chimpanzees (Pan troglodytes) and human children (Homo sapiens). J. Comp. Psychol. 107, 174–186. ( 10.1037/0735-7036.107.2.174) [DOI] [PubMed] [Google Scholar]
- 23.Whiten A, McGuigan N, Marshall-Pescini S, Hopper LM. 2009. Emulation, imitation, over-imitation and the scope of culture for child and chimpanzee. Proc. R. Soc. B 364, 2417–2428. ( 10.1098/rstb.2009.0069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Logan CJ, Breen AJ, Taylor AH, Gray RD, Hoppitt WJ.E. 2016. How New Caledonian crows solve novel foraging problems and what it means for cumulative culture. Learn. Behav. 44, 18–28. ( 10.3758/s13420-015-0194-x) [DOI] [PubMed] [Google Scholar]
- 25.Kenward B, Rutz C, Weir A.AS, Kacelnik A. 2006. Development of tool use in New Caledonian crows: inherited action patterns and social influences. Anim. Behav. 72, 1329–1343. ( 10.1016/j.anbehav.2006.04.007) [DOI] [Google Scholar]
- 26.Caldwell CA, Millen AE. 2009. Social learning mechanisms and cumulative cultural evolution: is imitation necessary? Psychol. Sci. 20, 1478–1483. ( 10.1111/j.1467-9280.2009.02469.x) [DOI] [PubMed] [Google Scholar]
- 27.Zwirner E, Thornton A. 2015. Cognitive requirements of cumulative culture: teaching is useful but not essential. Sci. Rep. 5, 16781 ( 10.1038/srep16781) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Povinelli DJ. 2000. Folk physics for apes: the chimpanzee's theory of how the world works. Oxford, UK: Oxford University Press. [Google Scholar]
- 29.Gruber T, Zuberbühler K, Clément F, van Schaik CP. 2015. Apes have culture but may not know that they do. Front. Psychol. 6, 91 ( 10.3389/fpsyg.2015.00091). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Csibra G, Gergely G. 2011. Natural pedagogy as evolutionary adaptation. Proc. R. Soc. B 366, 1149–1157. ( 10.1098/rstb.2010.0319) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakura O, Matsuzawa T. 1991. Flexibility of wild chimpanzee nut-cracking behavior using stone hammers and anvils: an experimental analysis. Ethology 87, 237–248. ( 10.1111/j.1439-0310.1991.tb00249.x) [DOI] [Google Scholar]
- 32.Visalberghi E, Adessi E, Truppa V, Spagnoletti N, Ottoni E, Izar P, Fragaszy D. 2009. Selection of effective stone tools by wild bearded capuchin monkeys. Curr. Biol. 19, 213–217. ( 10.1016/j.cub.2008.11.064) [DOI] [PubMed] [Google Scholar]
- 33.Sanz CM, Call J, Morgan D. 2009. Design complexity in termite-fishing tools of chimpanzees (Pan troglodytes). Biol. Lett. 5, 293–296. ( 10.1098/rsbl.2008.0786) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luncz LV, Sirianni G, Mundry R, Boesch C. 2018. Costly culture: differences in nut-cracking efficiency between wild chimpanzee groups. Anim. Behav. 137, 63–73. ( 10.1016/j.anbehav.2017.12.017) [DOI] [Google Scholar]
- 35.Hunt GR, Gray RD. 2003. Diversification and cumulative evolution in New Caledonian crow tool manufacture. Proc. R. Soc. Lond. B 270, 867–874. ( 10.1098/rspb.2002.2302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sugasawa S, Klump BC, St Clair JJH, Rutz C. 2017. Causes and consequences of tool shape variation in New Caledonian crows. Curr. Biol. 27, 3885–3890. ( 10.1016/j.cub.2017.11.028) [DOI] [PubMed] [Google Scholar]
- 37.St Clair JJH, Klump BC, Sugasawa S, Higgott CG, Colegrave N, Rutz C. 2018. Hook innovation boosts foraging efficiency in tool-using crows. Nat. Ecol. Evol. 2, 441–444. ( 10.1038/s41559-017-0429-7) [DOI] [PubMed] [Google Scholar]
- 38.Manrique HM, Gross AN-M, Call J. 2010. Great apes select tools on the basis of their rigidity. J. Exp. Psychol. Anim. Behav. Process 36, 409–422. ( 10.1037/a0019296) [DOI] [PubMed] [Google Scholar]
- 39.Lehner SR, Burkart JM, van Schaik CP. 2011. Can captive orangutans (Pongo pygmaeus abelii) be coaxed into cumulative build-up of techniques? J. Comp. Psychol. 125, 446–455. ( 10.1037/a0024413) [DOI] [PubMed] [Google Scholar]
- 40.Chappell J, Kacelnik A. 2002. Tool selectivity in a non-primate, the New Caledonian crow (Corvus moneduloides). Anim. Cogn. 5, 71–78. ( 10.1007/s10071-002-0130-2) [DOI] [PubMed] [Google Scholar]
- 41.Yamamoto S, Humle T, Tanaka M. 2013. Basis for cumulative cultural evolution in chimpanzees: social learning of a more efficient tool-use technique. PLoS ONE 8, e55768 ( 10.1371/journal.pone.0055768) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davis SG, Vale GL, Schapiro SJ, Lambeth SP, Whiten A. 2016. Foundations of cumulative culture in apes: improved foraging efficiency through relinquishing and combining witnessed behaviors in chimpanzees (Pan troglodytes). Sci. Rep. 6, 35953 ( 10.1038/srep35953) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gruber T, Singleton I, van Schaik CP. 2012. Sumatran orangutans differ in their cultural knowledge but not in their cognitive abilities. Curr. Biol. 22, 2231–2235. ( 10.1016/j.cub.2012.09.041) [DOI] [PubMed] [Google Scholar]
- 44.St Clair JJH, Rutz C. 2013. New Caledonian crows attend to multiple functional properties of complex tools. Proc. R. Soc. B 368, 20120415 ( 10.1098/rstb.2012.0415) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mann J, Stanton MA, Patterson EM, Bienenstock EJ, Singh LO. 2012. Social networks reveal cultural behaviour in tool-using dolphins. Nat. Commun. 3, 980 ( 10.1038/ncomms1983) [DOI] [PubMed] [Google Scholar]
- 46.Laland KN, Janik VM. 2006. The animal cultures debate. Trends Ecol. Evol. 21, 542–547. ( 10.1016/j.tree.2006.06.005) [DOI] [PubMed] [Google Scholar]
- 47.Newson L, Richerson PJ, Boyd R. 2007. Cultural evolution and the shaping of cultural diversity. In Handbook of cultural psychology (eds Kitayama S, Cohen D), pp. 454–476. New York, NY: The Guilford Press. [Google Scholar]
- 48.Matsuzawa T. 2003. Koshima monkeys and Bossou chimpanzees: Long-term research on culture in nonhuman primates. In Animal social complexity (eds de Waal FBM, Tyack P), pp. 374–387. Cambridge, MA: Harvard University Press. [Google Scholar]
- 49.Bandini E, Tennie C. 2017. Spontaneous reoccurrence of ‘scooping’, a wild tool-use behaviour, in naïve chimpanzees. PeerJ 5, e3814 ( 10.7717/peerj.3814) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gruber T, Muller MN, Strimling P, Wrangham RW, Zuberbühler K. 2009. Wild chimpanzees rely on cultural knowledge to solve an experimental honey acquisition task. Curr. Biol. 19, 1806–1810. ( 10.1016/j.cub.2009.08.060) [DOI] [PubMed] [Google Scholar]
- 51.Reynolds V, et al. 2015. Mineral acquisition from clay by Budongo Forest chimpanzees. PLoS ONE 10, e0134075 ( 10.1371/journal.pone.0134075) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lamon N, Neumann C, Gruber T, Zuberbühler K. 2017. Kin-based cultural transmission of tool use in wild chimpanzees. Sci. Adv. 3, e1602750 ( 10.1126/sciadv.1602750) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gruber T, Muller MN, Reynolds V, Wrangham RW, Zuberbühler K. 2011. Community-specific evaluation of tool affordances in wild chimpanzees. Sci. Rep. 1, 128 ( 10.1038/srep00128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reynolds V. 2005. The chimpanzees of the Budongo forest: ecology, behaviour, and conservation. Oxford, UK: Oxford University Press. [Google Scholar]
- 55.Songok J, Salminen P, Toivakka M. 2014. Temperature effects on dynamic water absorption into paper. J. Colloid Interface Sci. 418, 373–377. ( 10.1016/j.jcis.2013.12.017) [DOI] [PubMed] [Google Scholar]
- 56.Biro D, Sousa C, Matsuzawa T. 2007. Ontogeny and cultural propagation of tool use by wild chimpanzees at Bossou, Guinea: case studies in nut cracking and leaf folding. In Cognitive development in chimpanzees (eds Matsuzwa T, Tomonaga M, Tanaka M), pp. 476–508. Berlin, Germany: Springer. [Google Scholar]
- 57.R Development Core Team. 2015. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://wwwr-projectorg [Google Scholar]
- 58.Bates D, Maechler M, Bolker B, Walker S. 2014. lme4: Linear mixed-effects models using Eigen and S4. R package version 1.1–7.
- 59.Dobson AJ. 2002. An introduction to generalized linear models. Boca Raton, FL: Chapman and Hall. [Google Scholar]
- 60.Ruxton GD, Neuhäuser M. 2010. When should we use one-tailed hypothesis testing? Methods Ecol. Evol. 1, 114–117. ( 10.1111/j.2041-210X.2010.00014.x) [DOI] [Google Scholar]
- 61.Nakagawa S, Schielzeth H. 2010. Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol. Rev. Camb. Philos. Soc. 85, 935–956. [DOI] [PubMed] [Google Scholar]
- 62.Stoffel MA, Nakagawa S, Schielzeth H. 2017. rptR: repeatability estimation and variance decomposition by generalized linear mixed-effects models. Methods Ecol. Evol. 8, 1639–1644. ( 10.1111/2041-210X.12797) [DOI] [Google Scholar]
- 63.Gruber T, Zuberbühler K, Neumann C. 2016. Travel fosters tool use in wild chimpanzees. eLife 5, e16371 ( 10.7554/eLife.16371) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sanz CM, Morgan DB. 2013. Ecological and social correlates of chimpanzee tool use. Proc. R. Soc. B 368, 20120416 ( 10.1098/rstb.2012.0416) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gruber T. 2016. Great apes do not learn novel tool use easily: conservatism, functional fixedness, or cultural influence? Int. J. Primatol. 37, 296–316. ( 10.1007/s10764-016-9902-4) [DOI] [Google Scholar]
- 66.Reynolds V, Lloyd AW, Babweteera F, English CJ. 2009. Decaying Raphia farinifera palm trees provide a source of sodium for wild chimpanzees in the Budongo Forest, Uganda. PLoS ONE 4, e6194 ( 10.1371/journal.pone.0006194) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and code used in this study are available in the electronic supplementary material.