Abstract
Temporal processing is fundamental for an accurate synchronization between motor behaviour and sensory processing. Here, we investigate how motor timing during rhythmic tapping influences perception of visual time. Participants listen to a sequence of four auditory tones played at 1 Hz and continue the sequence (without auditory stimulation) by tapping four times with their finger. During finger tapping, they are presented with an empty visual interval and are asked to judge its length compared to a previously internalized interval of 150 ms. The visual temporal estimates show non-monotonic changes locked to the finger tapping: perceived time is maximally expanded at halftime between the two consecutive finger taps, and maximally compressed near tap onsets. Importantly, the temporal dynamics of the perceptual time distortion scales linearly with the timing of the motor tapping, with maximal expansion always being anchored to the centre of the inter-tap interval. These results reveal an intrinsic coupling between distortion of perceptual time and production of self-timed motor rhythms, suggesting the existence of a timing mechanism that keeps perception and action accurately synchronized.
Keywords: timing, time perception, sensorimotor, finger tapping, action–perception coupling
1. Introduction
Humans and many other biological organisms experience the passage of time and make use of this capability to generate highly regulated behaviours. Temporal processing plays a crucial role at multiple levels of action instantiation, from the fine coordination of muscle activations, to the programming of complex action sequences. Ultimately, actions need to be temporally tuned to the relevant sensory events in the external world to achieve an efficient sensorimotor control.
Traditionally, the study of sensory and motor timing has been addressed separately [1,2]. This partly reflects the long-standing debate about whether there exists a unique and dedicated timing network or time is processed locally, within functionally distinct neural systems [1,3,4]. Growing evidence now suggests that the ‘motor brain’ may be part of a core, amodal, network supporting time-keeping functions [5]. Motor structures, at both the cortical and sub-cortical level, seem to be invariably recruited during timing tasks, even when no overt motor response is required; these include in particular the supplementary and pre-supplementary motor areas (SMA and preSMA) [2,6,7] and the basal ganglia [3,8,9].
Evidence in favour of a prominent role of the motor system in timing is not solely based on its consistent recruitment in temporal tasks. Indeed, action shapes multiple aspects of sensory temporal processing. For example, rhythmic motor acts synchronize temporal fluctuations of attention, enhancing perceptual sensitivity to on-beat (target) auditory tones and suppressing the influence of off-beat (distractor) tones [10].
Not only implicit timing (e.g. temporal attention/prediction) but also explicit temporal judgements are strongly modulated by motor performance. Both compression and expansion of perceived time have been documented at different moments around motor execution and for different types of stimuli and movements [5,11–15]. Remarkably, some of these distortions of time have proved to be amodal, i.e. they occur even for sensory modalities that are (anatomically) disconnected from the relevant motor effector, as the case of visual stimuli during arm movements [13,15–17] or of tactile stimuli during eye movements [18].
Although these findings point to the existence of an amodal clocking mechanism, possibly grounded on motor processes and capable of synchronizing perceptual to motor time, compelling evidence in support of this hypothesis is still limited. In fact, in all the above-mentioned studies the temporal demands of the task are confined to the sensory [11–17] or to the motor domain alone [10].
Another set of studies specifically addressed whether perceptual and motor timing rely on distinct or common mechanisms. Nevertheless, they did so by testing perceptual and motor timing in separate tasks, either looking for correlations in task performance [19–22] or for transfer of temporal learning from one domain to the other [23,24]. Perceptual and action systems are, however, inherently coupled in time [25], even for tasks that do not require explicitly their functional coordination [26,27].
In the present study, we investigated the influence of motor timing performance on the perception of visual time. To this aim, we employed a dual task which combines self-timed rhythmic finger tapping with visual interval estimation. Importantly, the two task components (sensory versus motor) are completely unrelated to each other. This allows us to assess automatic, not task-mediated, interactions between perceptual and motor timing.
2. Methods
(a). Subjects
A total of 16 healthy participants took part in the study, eight in the main (five females; 27.7 ± 3.8 years; mean ± s.d.) and eight in the control experiment (see electronic supplementary material, methods). Subjects were all naïve with respect to the aims of the study and were all paid (€10/h) for their participation. All subjects had a normal or corrected-to-normal vision and were right-handed by self-report. Participants provided informed consent in accordance with the Declaration of Helsinki.
(b). Apparatus
Participants sat in a dark room with their right index finger resting on a small circular button (diameter 0.7 cm, thickness 0.3 cm) placed on a rigid support 7 cm beneath a table. The subjects could not see their hand and were instructed to keep fixation on a small, red light-emitting diode (LED; diameter 0.5 cm) that was placed on the upper surface of the table and gave only minimal illumination of the surroundings. Visual stimulations were provided by means of a yellow LED (diameter 1 cm, stimulus duration 5 ms). The yellow LED was placed 3.5 cm below the red fixation LED and 10.5 cm from the lower edge of the table (figure 1a). The auditory tones were presented with two loudspeakers placed on the table approximately 10 cm from each other and approximately 40 cm from the participant.
Figure 1.
Experimental set-up and procedure. (a) Participants sat in front of a table where two LEDs were positioned, one providing the fixation point (FIX. LED, red) and the other delivering the visual stimulus (STIM. LED, yellow). Participants kept their right index finger on a small button laying on a rigid support below the table. The auditory tones were delivered by two loudspeakers placed in front of the participants. (b) Trial started with the FIX. LED being lit. Four auditory tones (800 Hz, 50 ms) were then played at 1 Hz (inter-sound interval: 1 000 ms; marked in blue). Participants were asked to continue the sequence of tones by pressing the button four times with their right index finger at the same rate as the sound presentation. At random times between the 3rd and the 4th button press (marked in orange), two visual flashes (5 ms each) were presented separated by a variable temporal interval centred on 150 ms (probe; marked in yellow). Participants were required to report verbally whether the probe interval was shorter or longer compared with the standard interval (150 ms, presented at the beginning of each block; not shown).
To accurately set the timing of the visual stimulations, the LEDs were operated by means of a National Instrument data acquisition board (NI-USB 6211, sampling rate 1 000 Hz) controlled with custom-made Matlab code. The voltage signals deriving from the loudspeakers, the button, and the LEDs were all acquired with the same data acquisition device (i.e. NI-USB 6211, sampling rate 1 000 Hz) allowing an accurate alignment in time of the most important events in each trial (i.e. auditory tones, finger taps, and visual stimuli).
(c). Procedure
Before starting the experiment, participants familiarized themselves with the visual interval estimation task (see electronic supplementary material, methods). The main experiment consisted of separate blocks of 45 trials each. At the beginning of each block, participants were presented with 10 repetitions of the standard interval which was delivered by the yellow LED while they maintained fixation on the red LED. The standard interval was marked by two brief visual flashes of 5 ms each and had a fixed length of 150 ms. Each repetition of the standard interval was separated by a random pause drawn from a uniform distribution ranging from 1.1 to 2.7 s. Once the sequence of standard intervals was completed, the block of trials was run. Each trial was structured as follows. The fixation LED was turned on and four auditory tones (800 Hz; 50 ms) were played at a temporal rate of 1 Hz. Participants were asked to continue the sequence of tones by pressing the button four times with their right index finger at the same temporal rate as the sound presentation (figure 1b). During the finger tapping, the auditory stimulation was extinguished, and participants were required to keep their finger in contact with the button (i.e. to not lift their finger) to minimize hand movements. At random times between the 3rd and the 4th button press (i.e. during the last inter-tap interval) the probe interval was presented by the yellow LED and participants were asked to verbally report at the end of the trial (i.e. when the fixation LED was turned off) whether it was shorter or longer than the standard interval. The probe interval varied randomly on a trial-by-trial basis from approximately 70 to 300 ms in steps of approximately 10 ms. To optimize data sampling, the exact range of stimulus variation and its grain was adjusted by the experimenter according to the subjects' performance (for details, see the electronic supplementary material, methods).
Stimulus presentation times were randomly extracted from a uniform distribution with 1 s width and mean centred on the estimated half of the 4th inter-tap interval. To maximize stimulus sampling within the 4th inter-tap interval, the mean of the distribution was adjusted online by an automatic algorithm according to individual tapping performance (see the electronic supplementary material).
Participants completed on average 26.75 ± 0.9 blocks of trials (mean ± s.e.), yielding a total of 1 076 ± 48 trials per subject (mean ± s.e.).
(d). Data analysis
Stimulus presentation times were expressed with respect to the central point of the probe interval, calculated as the difference between the onset of the second and first flash. Stimulus latencies were then computed as the difference between the stimulus presentation time and the onset of the 4th finger tap.
Individual data were binned (bin size, 150 ms) according to stimulus latency. For each bin, data were fitted with cumulative Gaussian functions, estimated by means of the Maximum Likelihood method. Both the Point of Subjective Equality (PSE) and the Just Noticeable Difference threshold (corresponding to the standard deviation (SD) of the fitted cumulative Gaussian function) were derived from the psychometric function parameters. The standard errors (SEs) of the PSEs and SDs were estimated by bootstrap (100 iterations). For each latency bin, psychometric functions were never fitted to less than 30 data points. For illustration purposes, the individual PSEs were ‘baseline-corrected’ before averaging them across subjects and expressed as deviations from the (individual) mean PSE across the entire time window. To evaluate statistically the influence of stimulus latency on visual temporal judgments, both the PSEs and SDs were analysed with a repeated-measures ANOVA, with latency (−950, −800, −650, −500, −350, −200, −50 ms) as a within-subject factor.
We further characterized the effects that were previously established at the group level by pooling the single-trial data across all subjects, generating aggregate (super-subject) data. Prior to data aggregation, we de-biased the participant-specific data by subtracting the individual PSE computed over all trials from the probe values (i.e. the visual intervals presented). This procedure has the only effect of removing the participant-specific bias in perceived duration, preserving instead any modulation in perceived duration as a function of stimulus latency.
The aggregate data were then binned (bin size, 50 ms) according to stimulus latency and fitted with cumulative Gaussian functions to derive temporally resolved estimates of the PSE, as previously described for the single-subject data. For this analysis, psychometric functions were only fitted to datasets including at least 160 trials. To overcome the temporal discretization imposed by the binning procedure and derive a continuous estimate of the latency of maximal time dilation (corresponding to negative values of the PSE), we fitted a Gaussian function to the time course of the PSE aligned to the 4th tap (from −850 to −150 ms). The choice of using a Gaussian model was prompted by the bell-shaped profile of the data and further legitimated by its high goodness of fit (adjusted-R2 = 0.71). The mean of the best-fitting Gaussian function was used as an estimate of the latency at which the visual interval was perceived as maximally dilated.
The aggregate data were also analysed depending on the motor timing performance. Trials were split into three categories (each containing an almost equal number of trials) according to the length of the last inter-tap interval (i.e. between the onset of the 4th and 3rd tap): (1) short, corresponding to the 0.25 quantile of the pooled distribution of inter-tap intervals (2 156 trials), (2) accurate, inter-taps between the 0.35 and the 0.65 quantile (2 528 trials), and (3) long, inter-taps equal or larger than the 0.75 quantile (2 147 trials). Data belonging to each category were then binned (bin size, 100 ms) and fitted with psychometric functions (when including at least 60 trials), yielding three separate time courses of the perceived visual duration aligned to the 4th tap onset. We evaluated the difference in the latency of maximal time dilation between the short and the long inter-tap category by using a non-parametric statistical approach based on permutations ([28]; see the electronic supplementary material). Finally, we estimated the 90% confidence intervals of the latencies of maximal time dilation for the three inter-tap categories by means of bootstrap (1 000 iterations of bootstrap with replacement). Two-sided confidence intervals were calculated based on the quantiles (0.05 and 0.95) of the bootstrap distribution.
3. Results
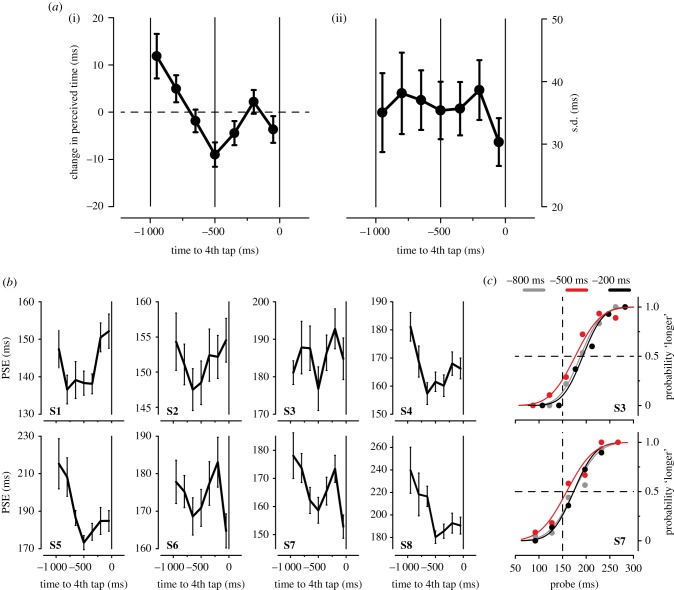
Figure 2a(i) shows the change in visual perceived duration, averaged across subjects, as a function of stimulus presentation time relative to the onset of the 4th finger tap (time zero). Perceived visual time undergoes a complex set of modulations during the rhythmic finger tapping with three major changes: (i) time compression approximately 800 ms before the 4th finger tap (positive values; corresponding to approximately 200 ms after the 3rd finger tap), (ii) time dilation about halfway between the two consecutive finger taps (negative values around approx. −500 ms), and (iii) time compression approximately 200 ms before the 4th finger tap.
Figure 2.
(a) (i) Average perceived duration (expressed as deviation from the mean PSE; see Methods) as a function of stimulus latency (time zero: 4th finger tap onset). Positive values indicate (relative) compression of time, negative values indicate (relative) expansion of time. The dashed horizontal line indicates no (relative) change in perceived duration. Solid vertical lines at −1 000, −500, and 0 ms: beginning, centre, and end of the instructed inter-tap interval (1 000 ms), respectively. Error bars represent s.e.m. (ii) Average (inverse) of the precision of the temporal judgements (s.d.) plotted as a function of stimulus latency (relative to 4th tap onset). Error bars represent s.e.m. (b) Individual PSEs as a function of stimulus latency. (c) Psychometric functions showing the proportion of trials where the probe interval was perceived as longer than the standard interval (150 ms; dashed vertical line). Results for two representative subjects (S3 and S7) are shown separately for probe intervals presented at −800 ms (light grey), −500 ms (red), and −200 ms (black) relative to 4th tap onset. Dots represent binned data points.
This non-monotonic pattern of variation of visual time locked to the finger tapping is highly consistent across subjects, as shown by the single-subject data (figure 2b). The psychometric functions of two representative subjects calculated for the three latencies where visual temporal estimates are maximally distorted are reported in figure 2c (latencies: −800, −500, and −200 ms). Like the majority of the participants (figure 2b), both subjects have a bias, underestimating perceived time (PSE > 150 ms). Nevertheless, apparent time is consistently dilated at the centre of the inter-tap interval (−500 ms) compared to that estimated at −800 as well as at−200 ms (indexed by the relative leftward shift of the red curves). The modulation of visual time perception that unfolds during the rhythmic finger tapping is statistically significant, as indicated by the main effect of stimulus latency on the PSE (F6,42 = 4.514, p = 0.042, Greenhouse–Geisser corrected; one-way ANOVA for repeated-measures).
While visual apparent time shows systematic distortions locked to the finger taps, the precision of temporal judgements (inverse of SD) is not affected by the concurrent motor behaviour (F6,42 = 1.160, p = 0.346, the main effect of stimulus latency; one-way ANOVA for repeated-measures; figure 2a(ii)).
Interestingly, the distortions of perceived time are smaller when the data are aligned to tap offset (maximal difference in perceived time: approx. 10 ms) compared to tap onset (approx. 20 ms), suggesting that they might be linked to the control of tap initiation rather than its release (see electronic supplementary material, figure S4 results).
We exclude that the observed distortions of visual time are due to the embedding of the stimulus in a rhythmic sequence of events, irrespective of their motor origin. In fact, temporal judgements are not significantly modulated when the visual probe is presented during a 1 Hz sequence of four auditory tones (control experiment) as opposed to during finger tapping (see electronic supplementary material, figure S5 results).
To characterize better the fine-grained dynamical modulations of visual time during the rhythmic finger tapping, we pooled single trials across all subjects, generating aggregate data. This procedure allows reducing the bin size given that it increases the number of trials available to fit reliable psychometric functions at each latency. Data aggregation was justified by the small across-subject variability in the pattern of perceptual modulations (figure 2b) as well as in tapping performance (see electronic supplementary material, figure S3 results). The time course of perceived visual duration calculated on the aggregate data follows a smooth, non-monotonic pattern, with (relative) time expansion around the centre of the inter-tap interval and two peaks of (relative) time compression at equidistant latencies (i.e. −800 and −200 ms; figure 3). The (reversed) bell-shaped profile that characterizes the time course of visual perception can be well fitted by a Gaussian function, whose mean provides an estimate of the latency of maximal time expansion. As shown in figure 3, the Gaussian function (fitted to the data from −850 to −150 ms, black symbols) represents a good model of the temporal dynamics of perceived duration (adjusted-R2 = 0.71) and its mean (−495 ms) almost coincides with half of the inter-tap interval (inter-tap = 1 014 ± 73 ms; mean ± s.d.).
Figure 3.
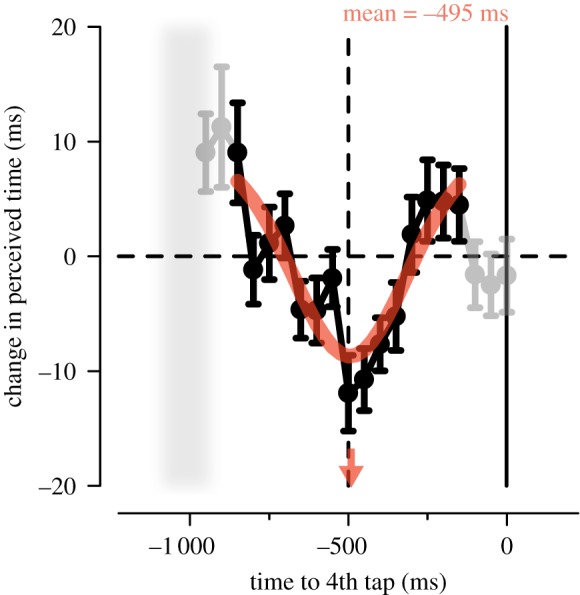
Change in the perceived duration (relative to the PSE estimated on the entire set of trials) aligned to the 4th tap and calculated on the data pooled across subjects. Error bars represent standard errors (s.e.) estimated by bootstrap. The Gaussian function that best fits the data in the range from −850 to −150 ms (black symbols) is plotted in red. Red arrow: mean of the best-fitting Gaussian function (−495 ms, indexing the latency of maximal time expansion). Grey shaded area: onset of the 3rd finger tap (mean ± 1 s.d.).
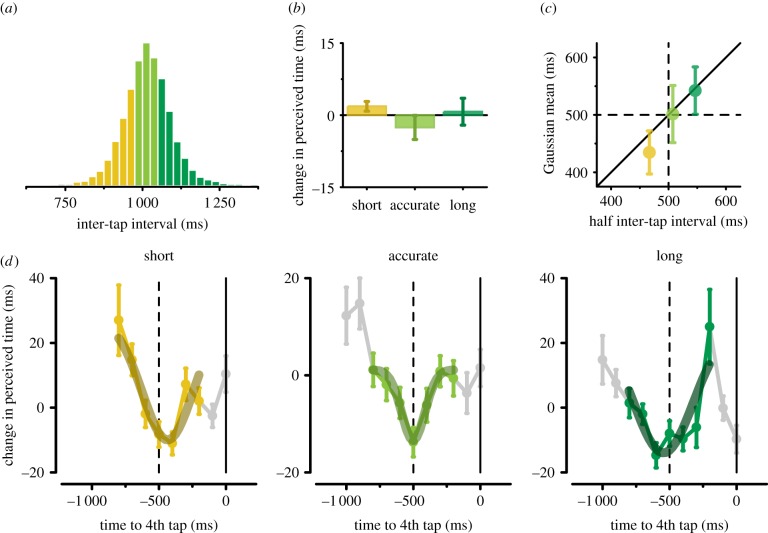
The peculiar modulation of visual time perception locked to the rhythmic motor behaviour suggests an intriguing hypothesis: maximal perceived time expansion might indeed be anchored to the centre of the inter-tap interval, independently of the finger tapping rate. Alternatively, expansion might occur at a fixed latency before (or after) the onset of the finger movement (i.e. approx. 500 ms). To distinguish between these two options, we exploited the natural trial-by-trial variability in motor timing performance. We therefore defined three trial categories on the basis of the (aggregate) distribution of inter-tap intervals (figure 4a; see also Methods): (i) short inter-taps, i.e. trials in which participants tapped at a faster rate (yellow), (ii) accurate inter-taps, i.e. trials in which participants tapped at the instructed rate (light green), and (iii) long inter-taps, i.e. trials in which participants tapped at a slower rate (dark green). Although the modulations in perceived duration show a very similar bell-shaped profile for all three sets of trials (confirming the robustness of the observed distortions), they do not perfectly overlap in time (figure 4d). Strikingly, the latency bin corresponding to maximal time expansion varies across the three inter-tap categories in an orderly fashion (−400, −500, and −600 ms for short, accurate, and long, respectively). This systematic change in latency bin is further confirmed by the best-fitting Gaussian functions, whose means closely match half the length of the inter-tap intervals in the three trial categories (half inter-tap interval: 467, 507, and 546 ms, MEDIAN; Gaussian mean: −435, −501, and −543 ms, for short, accurate, and long, respectively; figure 4c,d).
Figure 4.
(a) Distribution of the (last) inter-tap interval (data pooled across subjects). Trials are split into three categories: (i) short inter-taps (0.25 quantile of the distribution, yellow), (ii) accurate inter-taps (between the 0.35 and the 0.65 quantile, light green), and (iii) long inter-taps (≥0.75 quantile, dark green). (b) Average perceived duration (expressed as deviation from the mean PSE) for the short, accurate, and long trial categories (data collapsed across stimulus latencies). Error bars represent s.e.m. (c) The mean of the best-fitting Gaussian function is plotted against half of the inter-tap interval (median values) for the short (yellow), accurate (light green), and long (dark green) categories of trials. Error bars represent 90% confidence intervals calculated by bootstrap. The diagonal indicates that maximal perceived time expansion occurs halfway between the two consecutive finger taps. (d) Perceived visual duration aligned with the 4th tap and best-fitting Gaussian functions for the short, accurate, and long trials. Coloured symbols indicate the data used to fit the Gaussian functions (i.e. from −800 to −200 ms); data marked in grey were not used for the Gaussian modelling. Error bars represent s.e. estimated by bootstrap.
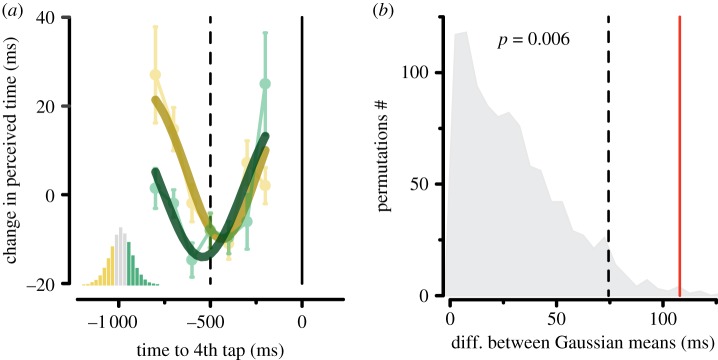
The consistent shift in the time courses of visual perception according to the motor timing performance is evident when superimposing the results for the short and the long inter-taps (figure 5a): the difference between the latencies of maximal time expansion (estimated as the mean of the best-fitting Gaussian functions) for these two sets of trials is 108 ms. This value is well above the 0.95 quantile of the permutation-based distribution (74 ms), yielding a p-value of 0.006 (figure 5b). On the contrary, mean perceived time does not co-vary with the length of the inter-tap interval, as shown by the non-significant difference of the PSE across the three trial categories (F2,14 = 0.692, p = 0.455, Greenhouse–Geisser corrected; figure 4b), suggesting the lack of a direct (monotonic) relationship between perceived and reproduced time.
Figure 5.
(a) Best-fitting Gaussian functions for the short (yellow) and long (dark green) inter-tap categories superimposed to the time courses in perceived duration aligned to the 4th tap (same data shown in figure 4d). (b) Distribution of the difference between the means of the best-fitting Gaussian functions (indexing the latencies of maximal time expansion) obtained by randomly permuting (1 000 iterations) the trials belonging to the short and to the long categories. Dashed line: 0.95 quantile of the distribution. Red line: difference between the means of the Gaussian functions fitted to the short and long inter-tap trials.
4. Discussion
The present study shows that perceived visual time is modulated during rhythmic finger tapping. This perceptual modulation is time locked to the onset of the finger tap and evolves with a non-monotonic profile during the approximately 1 s interval between the two consecutive taps. Remarkably, the temporal dynamics of this perceptual modulation scales linearly with the motor timing performance, so that maximal time expansion is always experienced at the centre of the produced inter-tap interval. Overall, this study provides evidence for an intrinsic link between perceptual and motor timing, showing that perceptual time can be rigidly anchored to motor time.
Previous studies examined the influence of action on perceptual time. However, perceptual timing has not been directly related to motor timing performance, as in all these studies, movement execution was not subjected to any explicit temporal demand [11–17]. A more recent electrophysiological study in monkeys combined sensory and motor timing into a single task to investigate their possible interrelation [29]. Monkeys were presented with a time interval demarcated by two flashes and were trained to reproduce that interval with a saccadic eye movement. The neural representations of sensory and motor time were indeed found to be linked. Neural responses in the lateral intraparietal cortex during the presentation of visual stimuli (sensory time) predicted, on a trial-by-trial basis, the responses observed in the same neurons during motor reproduction (motor time), as well as accuracy in the behavioural performance (reproduced time). However, because movement timing directly depended on sensory timing, the association between the neural responses in the two task epochs could arise from the task design itself. Indeed, to fulfill the task, sensory time needed to be translated into motor time, i.e. into a temporally structured motor plan. Differently, in our study, we asked participants to perform two concurrent, yet independent, sensory and motor timing tasks. Such experimental design allowed us to reveal the inherent (not task-mediated) coupling between perceptual and motor time.
Participants performed self-timed rhythmic finger tapping—a task that has been largely used both in humans and non-human primates to investigate motor timing at the behavioural and neurophysiological level [30]. The auditory stimulation was extinguished during the finger tapping, making our task equivalent to the continuation phase of the classical synchronization-continuation paradigm described by Wing & Kristofferson [31]. Motor timing performance in this task entails a memory component as it depends exclusively on an internally generated time signal whose errors cannot be corrected online by (sensory-)motor synchronization to an external metronome. This kind of task engages an explicit timing mechanism. Indeed, both humans [32] and monkeys [33] seem to control the temporal interval between two consecutive movements rather than the kinematics of the finger movement (e.g. velocity). Interestingly, visual distortions of time were more strongly locked to tap onsets rather than offsets, suggesting that they might indeed be linked to movement initiation—whose timing is likely under explicit temporal control—as opposed to movement release—whose timing reflects most probably motor implementation processes. The implicit timing component embedded in movement execution was further minimized in our experiment, as participants performed isometric-like button presses, which did not require lifting the finger. Therefore, both the perceptual and the motor task used in the present study relied on explicit rather than implicit timing functions, which may be realized by distinct mechanisms [34,35]. Moreover, both tasks relied to a large degree on memory, i.e. on an internalized time interval, which in the motor task provided the primary time signal, while in the perceptual task served as a reference signal (the standard) against which evaluating the sensory-encoded signal (the probe).
The perceptual versus motor distinction has often been adopted in the timing literature [1,2], but its anatomical and functional validity is still highly controversial. For example, the time-dependent component of performance variability is comparable in perceptual and motor tasks, pointing to a shared sensory-motor clock [21]. Similarly, individual performance correlates across interval estimation and finger tapping tasks [20] and temporal learning generalizes from the sensory to the motor domain [23,24]. Conversely, psychophysical research has revealed that time estimates (and their precision) vary across sensory modalities [36–38], they are vulnerable to changes in many low-level features of the stimuli [39–41], and they undergo spatially selective adaptation [42,43], indicating that timing functions might be embedded within local, sensory-specific, processing. Neurophysiological investigations also provide mixed results, showing evidence of both distinct as well as shared neural timers for perception and action [2,44]. Yet, movement can bias perceived time [11–17], counteract sensory-induced temporal illusions [45], and improve sensory event timing [46]. Thus, if time is intricately linked with early sensory functions, the motor system should at least modulate the sensory activity that carries the relevant information for perceptual timing.
Increasing evidence suggests that the motor system may play a crucial role in instantiating temporal predictions, by dynamically tuning the ongoing sensory activity to the incoming input with resulting behavioural benefits [47,48]. In fact, when effective sensory-motor synchronization is enabled by rhythmic stimulation, overt movements have been shown to improve perceptual sensitivity [10,48]. Indeed, rhythmic contexts strongly engage the motor system, inducing periodicity in motor preparedness (as indexed by response speed) [49] and also when rhythm-based predictions are actually detrimental for task performance [50]. In our study, the visual stimuli were randomly presented with respect to the rhythmic finger tapping, impeding de facto their temporal prediction. Nevertheless, despite visual information itself lacking a regular structure, the rhythmic issue of motor commands could have endogenously driven the ongoing visual activity, with functional consequences for visual processing, and possibly for visual temporal estimates. This appears plausible in light also of recent evidence for the existence of an early and automatic mechanism of visuomotor coupling based on oscillatory synchronization [26,27,51]. Slow brain oscillations (approx. 4 Hz) not only predict visual performance on a trial-by-trial basis, but also synchronize with the hand movement [27], generating, at the behavioural level, movement-locked rhythmicity in visual contrast sensitivity [26,27,52] and, possibly, in audio-visual simultaneity judgements [53]. A motor account for the current results is further corroborated by the fact that rhythmic auditory stimulation (the sensory counter-part of finger tapping) was ineffective in driving similar modulations of perceptual time, ruling out that the latter was merely caused by the rhythmic context in which the visual stimulus was embedded.
The present findings demonstrate that visual and motor timing performance are coupled, but they do not allow us to pinpoint the neural mechanisms and substrates which are responsible for this coupling. Two options appear possible: a common timer for perception and action or two distinct timing mechanisms, whose functioning is nevertheless coupled. The fact that there was no actual link between the perceptual and motor timing tasks in the current study, except their temporal contingency, suggests that even if separate, the sensory and motor clocks must be in most circumstances finely synchronized. One feature of the current pattern of results sets an important constraint for the possible underlying timing mechanism. We did not find any direct relationship between perceived and reproduced (motor) time such that, for example, perceived dilation of the visual stimulus was associated with shorter inter-tap intervals (and vice versa), as it is expected based on a single accumulator of time for perception and action. Rather, visual time changed dynamically, and the dynamics of these changes was rigidly coupled to motor time; this points to a more complex mechanism, possibly based on a time-varying (non-monotonic) process which is capable of modulating the timing of both perceptual and motor events.
The fundamental dichotomy between centralized and distributed timing has been partly overcome in recent years by the idea of a core amodal network for timing which interacts with local processes in a context-dependent fashion [54]. The exact nature of this interaction and its operating principles are still unclear. Time-related neuronal activity in two of these core structures—the medial premotor cortex and the putamen—have been extensively investigated in monkeys during rhythmic finger tapping [55–58], and their critical involvement in this task is supported also by neuroimaging evidence in humans [59]. Responses of cells in these areas are locked to the movements and encode the passage of time with respect either to the preceding or to the upcoming finger tap in the sequence [55]. Importantly, neuronal responses have amodal properties, showing similar interval-tuning irrespective of the modality of the stimuli that drive the motor timing [56]. Interestingly, the medial premotor cortex both encodes the passage of time as well as the categorical boundaries used for temporal judgement (short versus long), and is primarily engaged during the continuation phase of finger tapping [59,60]; this suggests its possible role in maintaining an internal representation of time and in evaluating this representation against a subjective criterium. Besides these context-independent and amodal timing structures, another set of evidence suggests that many timing functions are mediated by multiple clocks, operating in parallel within and across modalities [61]. These have been demonstrated when single tasks are performed (e.g. [42,62]). However, when the brain has to deal with multiple tasks, like in our case, that necessarily requires keeping track of different times, a motor clock may absolve the higher-level function of synchronizing the multitude of local clocks. This master ‘clock’ may thus keep different ongoing brain processes time-consistent and synchronized.
We believe that the future combination of neurophysiological measurements with behavioural tasks, whose different components (e.g. sensory versus motor) are simultaneously engaged but functionally de-coupled, could be a powerful approach to clarify the relative contribution of a central driving clock as compared to local, but mutually interacting, clocks.
5. Conclusion
Perception of visual time is distorted during rhythmic motor behaviour, but not during rhythmic sensory stimulation: the modulation of perceptual timing is locked to the motor acts and follows a temporal dynamic which scales linearly with motor timing.
These findings reveal a link between visual and motor timing despite the absence of external temporal cues that could drive visuomotor temporal synchronization. This link is therefore an intrinsic property of brain functioning which could possibly participate in the construction of a unified sense of time, rooted in early sensorimotor functions.
Supplementary Material
Supplementary Material
Ethics
The study and experimental procedures were approved by the local ethics committee (ASL 3 Genova and Comitato Etico della Provincia di Ferrara for the main and control experiment, respectively). Participants provided written, informed consent after explanation of the task and experimental procedures, in accordance with the Declaration of Helsinki and the local ethics committee.
Data accessibility
Data are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.h9v504t [63].
Authors' contributions
A.T., T.V., and M.C.M. conceived of the study and designed the experiments; A.T., T.V., and F.T. collected data; A.T. carried out data analysis and drafted the manuscript; all authors revised the manuscript and gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the European Research Council (ECSPLAIN-FPT7 Grant no. 338866 to M.C.M.).
References
- 1.Mauk MD, Buonomano DV. 2004. The neural basis of temporal processing. Annu. Rev. Neurosci. 27, 307–340. ( 10.1146/annurev.neuro.27.070203.144247) [DOI] [PubMed] [Google Scholar]
- 2.Wiener M, Turkeltaub P, Coslett HB. 2010. The image of time: a voxel-wise meta-analysis. Neuroimage 49, 1728–1740. ( 10.1016/j.neuroimage.2009.09.064) [DOI] [PubMed] [Google Scholar]
- 3.Buhusi CV, Meck WH. 2005. What makes us tick? Functional, neural mechanisms of interval timing. Nat. Rev. Neurosci. 6, 755–765. ( 10.1038/nrn1764) [DOI] [PubMed] [Google Scholar]
- 4.Ivry RB, Spencer RM. 2004. The neural representation of time. Curr. Opin Neurobiol. 14, 225–232. ( 10.1016/j.conb.2004.03.013) [DOI] [PubMed] [Google Scholar]
- 5.Merchant H, Yarrow K. 2016. How the motor system both encodes and influences our sense of time. Curr. Opin. Behav. Sci. 8, 22–27. ( 10.1016/j.cobeha.2016.01.006) [DOI] [Google Scholar]
- 6.Macar F, Coull J, Vidal F. 2006. The supplementary motor area in motor and perceptual time processing: fMRI studies. Cogn. Process. 7, 89–94. ( 10.1007/s10339-005-0025-7) [DOI] [PubMed] [Google Scholar]
- 7.Schubotz RI, Friederici AD, von Cramon DY. 2000. Time perception and motor timing: a common cortical and subcortical basis revealed by fMRI. Neuroimage 11, 1–12. ( 10.1006/nimg.1999.0514) [DOI] [PubMed] [Google Scholar]
- 8.Harrington DL, Zimbelman JL, Hinton SC, Rao SM. 2010. Neural modulation of temporal encoding, maintenance, and decision processes. Cereb. Cortex. 20, 1274–1285. ( 10.1093/cercor/bhp194) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coull JT, Cheng RK, Meck WH. 2011. Neuroanatomical and neurochemical substrates of timing. Neuropsychopharmacology 36, 3–25. ( 10.1038/npp.2010.113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morillon B, Schroeder CE, Wyart V. 2014. Motor contributions to the temporal precision of auditory attention. Nat. Commun. 5, 5255 ( 10.1038/ncomms6255) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomassini A, Gori M, Baud-Bovy G, Sandini G, Morrone MC. 2014. Motor commands induce time compression for tactile stimuli. J. Neurosci. 34, 9164–9172. ( 10.1523/JNEUROSCI.2782-13.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morrone MC, Ross J, Burr D. 2005. Saccadic eye movements cause compression of time as well as space. Nat. Neurosci. 8, 950–954. ( 10.1038/nn1488) [DOI] [PubMed] [Google Scholar]
- 13.Hagura N, Kanai R, Orgs G, Haggard P. 2012. Ready steady slow: action preparation slows the subjective passage of time. Proc. R. Soc. B 279, 4399–4406. ( 10.1098/rspb.2012.1339) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yarrow K, Haggard P, Heal R, Brown P, Rothwell JC. 2001. Illusory perceptions of space and time preserve cross-saccadic perceptual continuity. Nature 414, 302–305. ( 10.1038/35104551) [DOI] [PubMed] [Google Scholar]
- 15.Iwasaki M, Tomita K, Noguchi Y. 2017. Non-uniform transformation of subjective time during action preparation. Cognition 160, 51–61. ( 10.1016/j.cognition.2016.12.011) [DOI] [PubMed] [Google Scholar]
- 16.Tomassini A, Morrone MC. 2016. Perceived visual time depends on motor preparation and direction of hand movements. Sci. Rep. 6, 27947 ( 10.1038/srep27947) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yokosaka T, Kuroki S, Nishida S, Watanabe J, Cropper SJ. 2015. Apparent time interval of visual stimuli is compressed during fast hand movement. PLoS ONE 10, e0124901 ( 10.1371/journal.pone.0124901) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitazawa S, et al. 2008. Reversal of subjective temporal order due to sensory and motor integrations. In Sensorimotor foundations of higher cognition attention and performance (eds Haggard P, Rossetti Y, Kawato M), pp. 73–97. Oxford, UK: Oxford University Press. [Google Scholar]
- 19.Merchant H, Zarco W, Prado L. 2008. Do we have a common mechanism for measuring time in the hundreds of millisecond range? Evidence from multiple-interval timing tasks. J. Neurophysiol. 99, 939–949. ( 10.1152/jn.01225.2007) [DOI] [PubMed] [Google Scholar]
- 20.Keele SW, Pokorny RA, Corcos DM, Ivry R. 1985. Do perception and motor production share common timing mechanisms: a correctional analysis. Acta Psychol. 60, 173–191. ( 10.1016/0001-6918(85)90054-X) [DOI] [PubMed] [Google Scholar]
- 21.Ivry RB, Hazeltine RE. 1995. Perception and production of temporal intervals across a range of durations: evidence for a common timing mechanism. J. Exp. Psychol. Hum. Percept. Perform. 21, 3–18. ( 10.1037/0096-1523.21.1.3) [DOI] [PubMed] [Google Scholar]
- 22.O'Regan L, Spape MM, Serrien DJ. 2017. Motor timing and covariation with time perception: investigating the role of handedness. Front. Behav. Neurosci. 11, 147 ( 10.3389/fnbeh.2017.00147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Planetta PJ, Servos P. 2008. Somatosensory temporal discrimination learning generalizes to motor interval production. Brain Res. 1233, 51–57. ( 10.1016/j.brainres.2008.07.081) [DOI] [PubMed] [Google Scholar]
- 24.Meegan DV, Aslin RN, Jacobs RA. 2000. Motor timing learned without motor training. Nat. Neurosci. 3, 860–862. ( 10.1038/78757) [DOI] [PubMed] [Google Scholar]
- 25.Schroeder CE, Wilson DA, Radman T, Scharfman H, Lakatos P. 2010. Dynamics of active sensing and perceptual selection. Curr. Opin Neurobiol. 20, 172–176. ( 10.1016/j.conb.2010.02.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomassini A, Spinelli D, Jacono M, Sandini G, Morrone MC. 2015. Rhythmic oscillations of visual contrast sensitivity synchronized with action. J. Neurosci. 35, 7019–7029. ( 10.1523/JNEUROSCI.4568-14.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tomassini A, Ambrogioni L, Medendorp WP, Maris E. 2017. Theta oscillations locked to intended actions rhythmically modulate perception. Elife 6, 225 ( 10.7554/eLife.25618) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maris E, Oostenveld R. 2007. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods 164, 177–190. ( 10.1016/j.jneumeth.2007.03.024) [DOI] [PubMed] [Google Scholar]
- 29.Jazayeri M, Shadlen MN. 2015. A neural mechanism for sensing and reproducing a time interval. Curr. Biol. 25, 2599–2609. ( 10.1016/j.cub.2015.08.038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Repp BH, Su YH. 2013. Sensorimotor synchronization: a review of recent research (2006–2012). Psychon. Bull. Rev. 20, 403–452. ( 10.3758/s13423-012-0371-2) [DOI] [PubMed] [Google Scholar]
- 31.Wing AM, Kristofferson AB. 1973. The timing of interresponse intervals. Percept. Psychophys. 13, 455–460. ( 10.3758/BF03205802) [DOI] [Google Scholar]
- 32.Doumas M, Wing AM. 2007. Timing and trajectory in rhythm production. J. Exp. Psychol. Hum. Percept. Perform. 33, 442–455. ( 10.1037/0096-1523.33.2.442) [DOI] [PubMed] [Google Scholar]
- 33.Donnet S, Bartolo R, Fernandes JM, Cunha JPS, Prado L, Merchant H. 2014. Monkeys time their pauses of movement and not their movement-kinematics during a synchronization-continuation rhythmic task. J. Neurophysiol. 111, 2138–2149. ( 10.1152/jn.00802.2013) [DOI] [PubMed] [Google Scholar]
- 34.Zelaznik HN, Spencer RM, Ivry RB. 2002. Dissociation of explicit and implicit timing in repetitive tapping and drawing movements. J. Exp. Psychol. Hum. Percept. Perform. 28, 575–588. ( 10.1037/0096-1523.28.3.575) [DOI] [PubMed] [Google Scholar]
- 35.Lewis PA, Miall RC. 2003. Distinct systems for automatic and cognitively controlled time measurement: evidence from neuroimaging. Curr. Opin Neurobiol. 13, 250–255. ( 10.1016/S0959-4388(03)00036-9) [DOI] [PubMed] [Google Scholar]
- 36.Harrington DL, Castillo GN, Fong CH, Reed JD. 2011. Neural underpinnings of distortions in the experience of time across senses. Front. Integr. Neurosci. 5, 32 ( 10.3389/fnint.2011.00032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wearden JH, et al. 1998. Why ‘sounds are judged longer than lights': application of a model of the internal clock in humans. Q. J. Exp. Psychol. B 51, 97–120. [DOI] [PubMed] [Google Scholar]
- 38.Burr D, Silva O, Cicchini GM, Banks MS, Morrone MC. 2009. Temporal mechanisms of multimodal binding. Proc. R. Soc. B 276, 1761–1769. ( 10.1098/rspb.2008.1899) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanai R, et al. 2006. Time dilation in dynamic visual display. J. Vis. 6, 1421–1430. [DOI] [PubMed] [Google Scholar]
- 40.Terao M, Watanabe J, Yagi A, Nishida S. 2008. Reduction of stimulus visibility compresses apparent time intervals. Nat. Neurosci. 11, 541–542. ( 10.1038/nn.2111) [DOI] [PubMed] [Google Scholar]
- 41.Tomassini A, Gori M, Burr D, Sandini G, Morrone MC. 2011. Perceived duration of visual and tactile stimuli depends on perceived speed. Front. Integr. Neurosci. 5, 51 ( 10.3389/fnint.2011.00051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnston A, Arnold DH, Nishida S. 2006. Spatially localized distortions of event time. Curr. Biol. 16, 472–479. ( 10.1016/j.cub.2006.01.032) [DOI] [PubMed] [Google Scholar]
- 43.Burr D, Tozzi A, Morrone MC. 2007. Neural mechanisms for timing visual events are spatially selective in real-world coordinates. Nat. Neurosci. 10, 423–425. ( 10.1038/nn1874) [DOI] [PubMed] [Google Scholar]
- 44.Bueti D, Walsh V, Frith C, Rees G. 2008. Different brain circuits underlie motor and perceptual representations of temporal intervals. J. Cogn. Neurosci. 20, 204–214. ( 10.1162/jocn.2008.20017) [DOI] [PubMed] [Google Scholar]
- 45.Tomassini A, Gori M, Burr D, Sandini G, Morrone MC. 2012. Active movement restores veridical event-timing after tactile adaptation. J. Neurophysiol. 108, 2092–2100. ( 10.1152/jn.00238.2012) [DOI] [PubMed] [Google Scholar]
- 46.Carlini A, French R. 2014. Visual tracking combined with hand-tracking improves time perception of moving stimuli. Sci. Rep. 4, 5363 ( 10.1038/srep05363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schubotz RI. 2007. Prediction of external events with our motor system: towards a new framework. Trends Cogn. Sci. 11, 211–218. ( 10.1016/j.tics.2007.02.006) [DOI] [PubMed] [Google Scholar]
- 48.Morillon B, Baillet S. 2017. Motor origin of temporal predictions in auditory attention. Proc. Natl Acad. Sci. USA 114, E8913–E8921. ( 10.1073/pnas.1705373114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morillon B, Schroeder CE, Wyart V, Arnal LH. 2016. Temporal prediction in lieu of periodic stimulation. J. Neurosci. 36, 2342–2347. ( 10.1523/JNEUROSCI.0836-15.2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Breska A, Deouell LY. 2014. Automatic bias of temporal expectations following temporally regular input independently of high-level temporal expectation. J. Cogn. Neurosci. 26, 1555–1571. ( 10.1162/jocn_a_00564) [DOI] [PubMed] [Google Scholar]
- 51.Tomassini A, D'Ausilio A. 2018. Passive sensorimotor stimulation triggers long lasting alpha-band fluctuations in visual perception. J. Neurophysiol. 119, 380–388. ( 10.1152/jn.00496.2017) [DOI] [PubMed] [Google Scholar]
- 52.Benedetto A, Spinelli D, Morrone MC. 2016. Rhythmic modulation of visual contrast discrimination triggered by action. Proc. R. Soc. B 283, 20160692 ( 10.1098/rspb.2016.0692) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Benedetto A, Burr DC, Morrone MC. 2018. Perceptual oscillation of audiovisual time simultaneity. eNeuro 5, e0047–18 ( 10.1523/ENEURO.0047-18.2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Merchant H, Harrington DL, Meck WH. 2013. Neural basis of the perception and estimation of time. Annu. Rev. Neurosci. 36, 313–336. ( 10.1146/annurev-neuro-062012-170349) [DOI] [PubMed] [Google Scholar]
- 55.Merchant H, Zarco W, Perez O, Prado L, Bartolo R. 2011. Measuring time with different neural chronometers during a synchronization-continuation task. Proc. Natl Acad. Sci. USA 108, 19 784–19 789. ( 10.1073/pnas.1112933108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Merchant H, Perez O, Zarco W, Gamez J. 2013. Interval tuning in the primate medial premotor cortex as a general timing mechanism. J. Neurosci. 33, 9082–9096. ( 10.1523/JNEUROSCI.5513-12.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bartolo R, Prado L, Merchant H. 2014. Information processing in the primate basal ganglia during sensory-guided and internally driven rhythmic tapping. J. Neurosci. 34, 3910–3923. ( 10.1523/JNEUROSCI.2679-13.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crowe DA, Zarco W, Bartolo R, Merchant H. 2014. Dynamic representation of the temporal and sequential structure of rhythmic movements in the primate medial premotor cortex. J. Neurosci. 34, 11 972–11 983. ( 10.1523/JNEUROSCI.2177-14.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rao SM, Harrington DL, Haaland KY, Bobholz JA, Cox RW, Binder JR. 1997. Distributed neural systems underlying the timing of movements. J. Neurosci. 17, 5528–5535. ( 10.1523/JNEUROSCI.17-14-05528.1997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mendoza G, Méndez JC, Pérez O, Prado L, Merchant H. 2018. Neural basis for categorical boundaries in the primate pre-SMA during relative categorization of time intervals. Nat. Commun. 9, 1098 ( 10.1038/s41467-018-03482-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bruno A, Cicchini GM. 2016. Multiple channels of visual time perception. Curr. Opin. Behav. Sci. 8, 131–139. ( 10.1016/j.cobeha.2016.02.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heron J, Aaen-Stockdale C, Hotchkiss J, Roach NW, McGraw PV, Whitaker D. 2012. Duration channels mediate human time perception. Proc. R. Soc. B 279, 690–698. ( 10.1098/rspb.2011.1131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tomassini A, Vercillo T, Torricelli F, Morrone MC. 2018. Data from: Rhythmic motor behaviour influences perception of visual time Dryad Digital Repository. ( 10.5061/dryad.h9v504t) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Tomassini A, Vercillo T, Torricelli F, Morrone MC. 2018. Data from: Rhythmic motor behaviour influences perception of visual time Dryad Digital Repository. ( 10.5061/dryad.h9v504t) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.h9v504t [63].