Abstract
Why share when access to benefits is uncertain is crucial to our understanding of the evolution of humans' extensive cooperation. Here, we investigated some of the different human sharing hypotheses and potential neuroendocrine mechanisms, in one of our closest living relatives, chimpanzees. The strongest predictor of sharing across food types was the presence of enduring and mutually preferred grooming partners, more than harassment, direct signalling, or trade. Moreover, urinary oxytocin levels were higher after the sharing of both individually and jointly acquired resources compared with controls. We conclude that the emotional connection inherent in social bonds was a key factor determining sharing patterns, with the oxytocinergic system potentially facilitating long-term cooperative exchanges. Testing for the role of social bonds in increasing predictability of sharing behaviour, a feature frequently overlooked, may help us to identify the evolutionary drivers of resource sharing and mechanisms that sustain delayed reciprocity between non-kin.
Keywords: Pan troglodytes, cooperation, friendship, oxytocin, harassment
1. Background
Resource acquisition and sharing are thought to play a central role in human collaborative foraging, the evolution of human sociality, and our propensity to cooperate with kin and non-kin [1]. Sharing of resources is a universal human trait, unusual among animals in that it extends beyond kin relationships, or social networks of reciprocating partners [2]. While ‘kin selection’ is proposed to explain food exchange between related individuals [2], the evolutionary mechanisms favouring exchanges between non-kin, in which there is no immediate gain to actors, are highly debated [2,3]. The ‘tolerated theft’ [4] or ‘sharing under pressure’ [5,6] hypotheses propose that possessors share food to decrease energy expenditure-related costs created by beggars, and that the costs of defending a resource, or resource holding potential should predict asymmetries in food distribution patterns. The ‘costly signalling’ [7,8] hypothesis posits sharing as an honest signal advertising the donor's phenotypic quality, and is usually applied to male possessors. Here, food donors gain access to benefits later, not as a payback through trade, but as a result of the donor being an attractive mating partner or social ally. Individuals can broadcast, either generally to bystanders, or specifically to attractive partners via directed food transfers (i.e. directed signalling), and can maximize broadcast efficiency by acquiring costly and widely shared resources (e.g. meat). The ‘reciprocal altruism’ [9] hypothesis states that food is exchanged for delayed benefits, either as food or as another ‘currency’, such as social support, grooming, or mating opportunities. Sharing can also serve as trade-based reciprocity for labour as posited in the ‘cooperative acquisition’ hypothesis [3]. Here, sharing is more predominant among individuals that participated in the food acquisition task, and may facilitate future participation in similar tasks.
These hypotheses represent some of the major proposed hypotheses for human sharing, they are not mutually exclusive and address the motives behind sharing in terms of costs and benefits. However, while the ‘sharing under pressure’ hypothesis assumes immediate benefits to food donors, the other hypotheses involve delayed, socially based benefits [2,10,11]. Given the important role of sharing in human social evolution [1], and the rarity of sharing between unrelated adults outside of a sexual context in other species [12], examining this phenomenon in one of our closest living relatives, chimpanzees, may offer further evolutionary perspective and insights into human food sharing mechanisms.
Wild chimpanzees often engage in group hunting and subsequent sharing of meat as well as other valued, monopolizable food items with both related and unrelated conspecifics [5,12–17]. Several food sharing hypotheses have been applied in chimpanzee research to examine variation in and drivers of chimpanzee food sharing behaviour, with variable results across studies and populations. In line with the ‘sharing under pressure’ hypothesis, harassment by beggars decreased possessors' consumption rates and increased sharing probability in Gombe, thereby supporting the idea that possessors shared meat to reduce harassment costs [5]. In Taï, chimpanzee meat sharing depends on participation in the food acquisition task independent of begging pressure, such that hunters gain more access to meat than non-hunters despite similar motivation to access meat [18], supporting the ‘cooperative acquisition’ hypothesis. Other evidence suggests that chimpanzee food sharing is motivated by contingent service exchange over long periods of time [19], whether via coalitionary support [15,16], mating opportunities [20], food or grooming in captive [21–23] and wild [15,24] populations, but in other wild populations the effect of grooming [5] and mating opportunities [5,25] was not found. These studies emphasize the highly variable nature of chimpanzee sharing, depending on ecological, energetic, demographic, and social factors [10,13,15,18,22]. Nonetheless, as these hypotheses are not mutually exclusive, it is crucial to test them in a single analysis in order to evaluate the independent contribution of each hypothesis, which has rarely been done in wild populations, where cooperation has fitness consequences. Studies investigating adult–adult sharing behaviour in wild populations have mainly focused on meat exchange following hunting (although see [17,26] for adult–adult non-meat sharing in wild populations), whereas studies in captivity investigate the sharing of provisioned foods [19,21–23]. Since human sharing networks are suggested to differ according to the quantity, quality, and difficulty in attaining certain food resources, investigating drivers of both individually or jointly acquired resources [27] in a single population is essential for understanding the evolution of non-kin sharing.
It is suggested that cognitive, emotional, and/or socio-psychological strategies, such as punishment or reward, may jointly enable delayed reciprocal exchange in humans [9,28,29]. Due to assumed cognitive constraints, the notion of calculated reciprocal exchange among non-human animals has been questioned [30] (although see [31]). Rather there is the possibility that emotions play a role in maintaining animals' reciprocal relationships in general, and food sharing in particular. Neuroendocrinological mechanisms, such as the oxytocinergic system, likely facilitate service exchange [12,32,33], by linking cooperative acts with the dopaminergic reward system [34,35], reinforcing partner preferences and the likelihood of interacting again [12]. This offers the potential to establish a system of delayed reciprocal exchange with emotional mechanisms, involving oxytocinergic activity, supporting cognitive ones. Studies across mammals repeatedly show that oxytocinergic involvement is central in promoting maternal nurturing behaviour, parturition and lactation (a primary form of food sharing), as well as partner preferences and social bonds in adult relationships [34–37]. However, the oxytocinergic system is multiplex and has been implicated in a range of psychological states, from anxiolytic effects to stress and aggression [37–39], emphasizing that social context and individual factors influence oxytocinergic effects [35–37]. Furthermore, an increasing number of studies show that oxytocin is associated with trust, coordination, tolerance, and cohesion outside of the reproductive context [12,35,36,40–43], important factors for joint resource acquisition and sharing. In non-human animals, oxytocin and its analogues have been positively linked with adult–adult food sharing behaviour in chimpanzees [12], vampire bats, [44] and pinyon jays [45], but negatively with adult–adult sharing in capuchin monkeys [46]. Accordingly, considering oxytocinergic system involvement in maintaining emotional reciprocity during resource acquisition and sharing appears promising for further understanding of the proximate mechanisms facilitating kin and non-kin sharing behaviour.
To investigate mechanisms behind individually and jointly acquired resource sharing among adult chimpanzees, we tested some of the aforementioned hypotheses, in two chimpanzee groups of the Taï National Park, Côte d'Ivoire. We considered social and non-social attributes of possessors and beggars to formulate predictions and apply them to the proposed hypotheses (table 1). Specifically, we predicted that if food sharing is driven by ‘sharing under pressure’, the number of beggars, begging duration, and occurrence of harassment should positively predict sharing. Furthermore, resource holding potential (as measured by coercion ability through dominance status and history of aggressive interactions), a method used in human [3], vampire bat [47], and chimpanzee [19] studies, should determine asymmetries in resource distribution [3].
Table 1.
Predictions for the different food sharing hypotheses.
| Hypotheses and their predictions (expected effect on sharing in parentheses) |
|---|
| Sharing under pressure – Begging pressure and resource holding potential predict food distribution |
| 1. Longer begging duration and greater number of beggars (positive) |
| 2. Harassment occurrence (positive) |
| 3. Large rank difference in favour of food possessors (negative) or beggars (positive) |
| 4. Aggression potential from beggars towards possessors (positive) |
| 5. Previous grooming provided by partner (no effect) |
| Reciprocal altruism – Sharing of resource reflects long-term reciprocation |
| 1. Rank (no effect) |
| 2. Aggression potential towards the possessor (no effect) |
| 3. Longer begging duration (negative) |
| Trade dependent: |
| 4. Former grooming provided by partner (positive) |
| Bonding dependent: |
| 5. Mutual and stable partner preference in grooming (positive) |
| Costly signalling (direct) – Sharing of resource reflects attractiveness of partner |
| 1. Rank of partner within and between the sexes (positive) |
| 2. For male possessors, fully tumescent female partners (positive) |
| 3. Aggression potential towards the possessor (no effect) |
| 4. Grooming provided by partner or relationship quality (no effect) |
The ‘costly signalling’ hypothesis is quality dependent, such that individuals can maximize broadcast efficiency by both acquiring and sharing valuable resources, but also by specifically signalling to attractive partners via directed food transfers. In accordance with the direct signalling hypothesis, sharing behaviour should be partner dependent and vary between the sex combinations, independently from past grooming or agonistic experience. It provides clear predictions for male possessors who should direct sharing behaviour towards cycling females (potential mate partners) or high-ranking males (potential social allies), but is more ambiguous in explaining female-led sharing. Nonetheless, we expect that female chimpanzees likewise benefit from enhanced social status or potential access to future resources, thus may use food sharing as an honest signalling tool. If so, females should target higher-ranking individuals, whether males or females.
Partner choice in biological markets is an important mechanism of reciprocity [48], and mutual and stable partner choice over long time periods may result in an emotional connection between partners (social bond) [48]. If food sharing behaviour is driven by long-term social factors via ‘reciprocal altruism’ we expect exchange of affiliative behaviour to positively influence sharing likelihood. The exchange may be a result of pure economic trade, in which we expect high rates of grooming received to increase the likelihood of food sharing [23], or as a result of social bonds, by which a stable, mutual preference in grooming, will determine sharing behaviour. Furthermore, if past positive exchange promotes sharing among partners, we should expect sharing to occur after short begging durations compared to duration of begging which does not lead to sharing.
One of the objectives of this study was to examine motivations for the sharing of jointly acquired as well as individually acquired foods. Thus, as the cooperative acquisition hypothesis only accounts for the sharing of jointly acquired resources (i.e. meat) but not for the sharing of individually acquired foods, we do not directly test this hypothesis here (but see [18]).
Furthermore, we examine the oxytocinergic system as a potential underlying mechanism involved in facilitating cooperation during chimpanzee sharing and hunting behaviour. If food acquisition and sharing in chimpanzees are prosocial acts (i.e. voluntary actions that may benefit others) that extend beyond economic trade, and are not driven by harassment, we would expect a positive link between all sharing acts and oxytocinergic system activity. Conversely, if physical effort or stressful situations are the main causes of oxytocinergic system activation [38,49], we would expect urinary oxytocin levels to be high after hunting and the sharing of meat, activities that are associated with exercise and cortisol secretion [50], but not after the sharing of non-meat items. Furthermore, if oxytocinergic activation is dependent on the identity of the interaction partner, rather than influenced by prosocial or stressful acts, we would expect higher urinary oxytocin levels only after sharing behaviour with bond partners, whether this involves meat or non-meat items.
2. Methods
(a). Data collection
We conducted fieldwork at the Taï National Park (5°45′ N, 7°07′ W), Côte d'Ivoire, between October 2013–May 2014 and September 2014–May 2015, observing two well-habituated neighbouring chimpanzee (Pan troglodytes verus) groups, South and East.
Behavioural data and urine samples for oxytocin measurements were collected using all day focal animal sampling [51], following 20 individuals including all adult (more than 12 years) males and most frequently observed females (five males and five parous females in each group). We continuously documented all changes in sub-group composition and size, activity, and social interactions involving the focal individual using the CyberTracker software (v. 3.389). Moreover, we collected ad libitum recordings of hunting and sharing behaviour of 40 adult individuals (10 males and 30 females) using a HD Panasonic camcorder. Overall, we recorded total of 2 278 observation hours in the East group and 2 271 in the South group during 557 focal days, with a total of 78 instances of successful hunts (at a rate of 1 every 7 days for both groups together).
(b). Dominance ranks and social relationships
We used focal-follow data collected by researchers (2013–2015) as well as trained local observers (South: 1999–2016; East: 2007–2016) to determine changes in social and dominance relationships over time.
We applied a likelihood-based adaptation of the Elo rating approach [52,53], using uni-directional submissive pant grunt vocalizations to estimate the dominance hierarchy within each of the sexes separately (as dominance rank and sex are confounded variables in chimpanzee societies, with females subordinate to adult males), standardized to a range from 0 to 1.
We used a full 4-year period (2012–2015) of focal-follow data collection to evaluate two separate dyadic scores of grooming and aggression and their changes over time, while accounting for directionality. We implemented a method similar to the Elo rating, the Dynamic Dyadic Sociality Index [53,54], in which each grooming positively and each aggression negatively affected the respective dyadic score. This provided two separated continuous directed daily measures for grooming and aggression, assessed by the accumulative history of interactions such that each interaction led to an update of the respective dyadic score, and accounts for stability in partner choice over time (see electronic supplementary material for details).
(c). Social bonds
The directed grooming scores were used to assess relationship quality, evaluating for each individual the top (i.e. preferred) male and female grooming partner until the day preceding the sharing bout. A dyad scored 1 only when both individuals were each other's top grooming partners, and scored 0 otherwise. Therefore, a score of 1 reflected dyads with long-lasting stable and mutual grooming relationships, hereafter referred to as social bond partners.
(d). Food sharing
We defined sharing whenever individual B received access to food in possession of A (electronic supplementary material, videos S1–S3) [5,12]. Food transfers usually followed begging behaviour (see electronic supplementary material) and involved both passive sharing, without any facilitation of the transfer by the possessor and active sharing, whenever the possessor facilitated the transfer by cutting or tearing a piece and laying it in front of the beggar or by releasing or handing over a piece into the beggar's hand or mouth. A food sharing bout was defined from the first sharing instance of a certain food resource until the end of feeding time. Within each bout we defined partner-specific food sharing events, in which we considered all food exchanges within each dyad as a single data point, accounting for directionality. Sharing included one jointly acquired resource (i.e. meat), and six different individually acquired resource types (i.e. fruit, seeds, nuts, insects, honey, and tools). We used the complete subset of bouts with comprehensive video recordings of all begging interactions, whether leading to sharing or not. We then tested the effects of begging duration and occurrence of harassment on sharing during dyadic begging events (see electronic supplementary material). We defined harassment as any begging interaction that reduced possessors' feeding efficiency or increased their energy expenditure [5].
(e). Urine sample collection and analysis
We collected all possible urine samples from focal subjects during daily follows. We measured samples collected 15–60 min after the target behaviours of group hunting, meat and food sharing, according to estimates of the oxytocin clearance rate [33,55]. As a control, we used a conservative time period of 90 min of feeding in proximity to others without positive social interactions, begging or sharing behaviour, to ensure that control sample values would not be affected by social interactions. Controls occurred either on the same or different days as hunting and sharing behaviour, thus, we accounted for potential variation in the social environment in the statistical analysis. Sample collection, extraction, and analysis followed the protocol used by Samuni et al. [41] (see electronic supplementary material). Urinary oxytocin levels were measured using a commercially available enzyme immunoassay kit (Assay Designs, catalogue no. 901-153A-0001). We measured creatinine levels in all urine samples and expressed urinary oxytocin values as pg/mg creatinine, to control for variation in urine volume and concentration [56]. Overall, 246 urine samples (mean ± s.d.: 12.3 ± 6.2 samples/individual; 116 control samples and 130 hunting and sharing samples) were included in the statistical analysis.
(f). Statistical analysis
We conducted two Generalized Linear Mixed Models (GLMM [57]) with binomial error structure and logit link function, to investigate variables affecting sharing likelihood among adults.
First, we examined the sharing under pressure hypothesis [5] by testing the effect of three measures of begging pressure, that is the number of beggars, begging duration, and occurrence of harassment, on sharing likelihood (begging model; see electronic supplementary material). Our dataset for the begging model included 255 begging events (138 resulted in sharing) of 30 beggars and 16 possessors, involving 93 dyads from two groups and 38 bouts.
Second, we used a decision-making approach to examine what influences the likelihood of a possessor to share with one individual over the other (sharing model). This was done by determining for each food sharing bout all pairs involving the possessor with all other adult individuals present in the sub-group and whether they shared or not (0/1). We only included sharing bouts with complete information of all sharing events and with at least two partners present (resulting in 245 sharing events). This approach models equal opportunities to share with each partner present as the null hypothesis. Our test predictors for this model were the directed grooming and aggression scores, social bonds (as described above), and a three-way interaction between the dominance rank of the possessor and partner with the sex combination as described from the perspective of the potential sharing partner (i.e. F-F, M-M, M-F, F-M). Including an interaction between the dominance ranks considers the rank difference between the possessor and partners but still retains information on the respective position of the two within the dominance hierarchy. We estimated maternal kinship by means of genetic and pedigree data (see electronic supplementary material), to control for relatedness. We also controlled for group membership (East and South), food type (i.e. meat or non-meat), and for male possessors whether potential female partners were fully tumescent. We included the log of the inverted number of adult individuals present in the sub-group (not including the food possessor) as an offset term to account for differences in sharing likelihood due to sub-group size. The identity of the bout, possessor, partner, and dyad were included as random effects with random slopes to account for specific identities driving sharing likelihood (see electronic supplementary material). Our dataset for the sharing model included 718 data points of 40 beggars and 23 possessors, of 186 dyads (among them 89 dyads which shared food and 11 dyads with a social bond) from two groups and 120 bouts.
To investigate the involvement of the oxytocinergic system in chimpanzee sharing behaviour, we fitted a Linear Mixed Model (LMM; oxytocin model) [57] with Gaussian error structure and identity link function, and log-transformed the response variable, urinary oxytocin levels (pg/mg creatinine). Our test predictor for the oxytocin model was the type of event: (i) participation in group hunting of monkeys [13], either unsuccessfully or without meat sharing behaviour (five males and four females, 23 samples of 17 events), (ii) sharing of meat (10 males and seven females, 58 samples of 34 events), (iii) sharing of non-meat food resource (six males and seven females, 49 samples of 32 events), and control of (iv) feeding in proximity to others without begging behaviour or sharing, and without positive social interactions, except for vocalizations (10 males and 10 females, 116 samples of 103 events). Here, we separated samples collected after the sharing of meat from sharing of non-meat since only meat exchange follows hunting behaviour, a coordinated act known to influence oxytocinergic system activity [18,41], and cortisol secretion [50]. We also controlled for variables that may affect oxytocin secretion, and included random effects with random slopes (see electronic supplementary material). Our dataset for the oxytocin model included 246 samples from 20 individuals from 186 events. In an additional analysis with a subset of the samples included in the oxytocin model, we fitted a LMM to investigate whether urinary oxytocin levels after sharing differ between food donors and possessors, and whether levels are higher after sharing with a social bond partner in comparison to a non-bond partner (see electronic supplementary material).
We fitted the models in R (v. 3.3.0 [58]) using the R package lme4 [59] and compared the fit of both full models with those of a respective null model lacking the test predictors, but identical to the respective full model in all other terms [60], using a likelihood ratio test. Prior to fitting the models, we assessed model stability, collinearity, and deviation from model assumptions (see electronic supplementary material).
3. Results
Overall, we observed 312 adult–adult sharing events with 40 partners, which occurred 0.56 times per observation day (0.07 per observation hour). Approximately 65% of all sharing bouts and 55% of all sharing events in our study involved non-meat items, with males as the possessors in 69% of cases (79% for the possession of meat). On average, male and female possessors shared with 38.8% and 35.2% of all adult individuals present during the sharing bout, respectively (48% for male and female possessors during meat exchange). In 39% of sharing bouts, a social bond partner of food possessors was present, and possessors shared with 35% of their non-bond partners and 62% of their bond partners. The average begging duration per event (seconds) was 104.50 ± 139.44 between non-bond partners and 41.32 ± 27.32 between bond partners. Active begging behaviour (i.e. reach hand, hold, hand to mouth) preceded 87% of sharing acts, and 13% of food transfer occurred when beggars were in proximity but without any gestures directed towards the possessor. Harassment occurred at 12% of begging events, resulted in sharing 50% of the time, and was especially rare among social bond partners, occurring at a rate of 0.03%. Retaliation (any type of aggressive interaction) by possessors was uncommon and occurred at 1.5% of begging events, all consisting of arm wave gesture. Active sharing occurred in 10% of all sharing events, and in 21% when only considering large and highly divisible food items (i.e. meat and Treculia africana), with an occurrence of 17% and 42% of active sharing among non-bond and bond partners, respectively.
Contradictory to the predictions of the sharing under pressure hypothesis, we found a significant negative effect of begging pressure on sharing likelihood (full-null model comparison likelihood ratio test: χ2 = 19.725, d.f. = 3, p < 0.001; electronic supplementary material, table S2). Both the number of beggars (estimate ± s.e.: −0.749 ± 0.214; p = 0.002) and begging duration (−0.855 ± 0.287; p = 0.002; electronic supplementary material, figure S1) negatively affected sharing likelihood, while harassment did not affect sharing (0.506 ± 0.497; p = 0.373). We also found higher sharing probability in individuals of East compared to South (p = 0.009). There was no effect of food type or sex combination on sharing probability. Furthermore, sharing probability dropped from a maximum of 0.67 in the first begging event to 0 by the fourth begging event, and no sharing occurred beyond the third begging event (electronic supplementary material, figure S2).
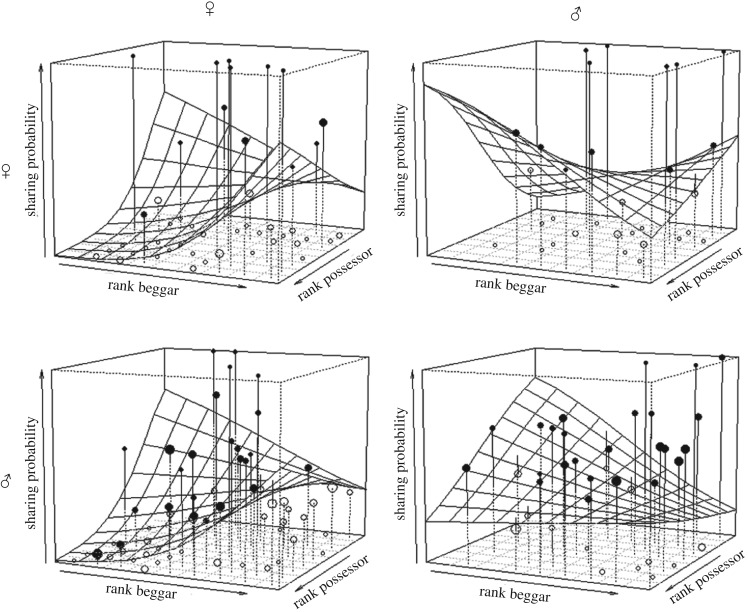
We analysed how the likelihood to share with some adult individuals but not with others varied across food possessors and all potential adult partners present. Therefore, we only included cases in which we had full information of whom the possessor shared with (see methods). Overall, the full-null model comparison was significant (likelihood ratio test: χ2 = 43.623, d.f. = 18, p < 0.001; electronic supplementary material, table S3). Specifically, we found a significant effect of relationship quality, such that mutually preferred grooming partners were more likely to share (1.076 ± 0.473; p = 0.030). Both received grooming (trade; 0.288 ± 0.178; p = 0.106), and aggressions (−0.013 ± 0.233; p = 0.896) had no significant effect on the likelihood to share. The three-way interaction between dominance ranks and sex combinations significantly affected sharing likelihood (p = 0.027; figure 1). Specifically, low-ranking males more frequently shared with low-ranking partners of both sexes, while high-ranking males shared more frequently with high-ranking partners of both sexes, although the effect of male–male high rank sharing was less pronounced. Female–female sharing as well occurred more frequently between females of similar rank, while low-ranking females shared more frequently with high-ranking males and high-ranking females shared more with low-ranking males. Moreover, the likelihood to share meat was significantly higher than the likelihood to share non-meat (p < 0.001). These effects were not driven by group membership, kinship, or by dyads involving males and fully tumescent females. We also found a non-significant interaction between relationship quality and food type (p = 0.500, see electronic supplementary material), highlighting that for both types of food sources individuals shared more frequently with mutually preferred partners.
Figure 1.
Effect of the interaction between the rank of the possessor, rank of beggar and the sex combination from the perspective of the food possessor on the likelihood to share. Sex symbols on the top and left side represent beggars and possessors, respectively. Shown are the observed probabilities to share food (larger point volumes denote a larger number of observations), as well as the model results (surface).
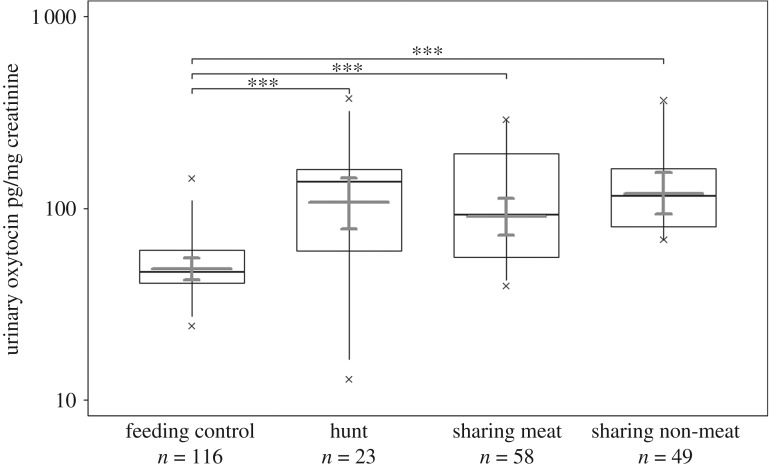
We then investigated individual variation in oxytocin reactivity during chimpanzee food acquisition and sharing behaviour compared with control contexts using event-related sampling [33]. Overall, we found an effect of the behavioural events sampled on variation in urinary oxytocin levels (full-null model comparison - likelihood ratio test: χ2 = 42.033, d.f. = 3, p < 0.001; figure 2, table 2). Specifically, individuals had higher urinary oxytocin levels after hunting, sharing meat, and sharing non-meat than after the control context (p < 0.001), but did not significantly differ among hunting and the two types of sharing (electronic supplementary material, table S1). We found females had higher (p = 0.005) urinary oxytocin levels than males, but found no effects of group membership, sub-group size, or dominance rank on urinary oxytocin levels. An additional analysis with a subset of the oxytocin sharing data (full-null model comparison: χ2 = 0.440, d.f. = 2, p = 0.802; table S4; see electronic supplementary material) revealed no significant differences in urinary oxytocin levels among food donors and recipients (p = 0.526), and no significant effect of sharing with a bond partner in comparison to a non-bond partner (p = 0.859).
Figure 2.
Effects of hunting and sharing on urinary oxytocin levels in wild chimpanzees in the East and South groups (n = 246 samples; 20 subjects; 186 events). Shown are medians (thin horizontal lines), quartiles (boxes), percentiles (2.5 and 97.5%; vertical lines), minimum and maximum (laying crosses) as well as the fitted model and its 95% confidence intervals (thick grey lines and error bars). ***p < 0.001.
Table 2.
Effect of hunting, and the sharing of meat and non-meat on urinary oxytocin levels (log transformed).
| terma | coded level | estimate | s.e. | CIlower | CIupper | χ2 | d.f. | p |
|---|---|---|---|---|---|---|---|---|
| intercept | 3.271 | 0.126 | 3.018 | 3.516 | — | — | ||
| test predictor levels | ||||||||
| hunt | 0.791 | 0.181 | 0.459 | 1.182 | <0.001 | |||
| event (control) | sharing meat | 0.636 | 0.136 | 0.358 | 0.889 | 42.034 | 3 | <0.001 |
| sharing non-meat | 0.904 | 0.141 | 0.648 | 1.172 | <0.001 | |||
| control predictors | ||||||||
| group (East) | South | −0.084 | 0.106 | −0.309 | 0.116 | 0.515 | 1 | 0.473 |
| sex (female) | male | −0.287 | 0.100 | −0.495 | −0.095 | 7.720 | 1 | 0.005 |
| dominance rankb | 0.008 | 0.050 | −0.092 | 0.106 | 0.013 | 0.911 | ||
| sub-group sizec | 0.017 | 0.050 | −0.090 | 0.108 | 0.115 | 0.734 | ||
| data collection period (first) | second | 1.277 | 0.103 | 1.065 | 1.494 | 108.352 | 1 | <0.001 |
aReference categories of factors are indicated in parenthesis.
bz-transformed, mean ± s.d. of the original variables: 0.69 ± 0.25 (range 0-1 with 1 being the highest social rank in each sex category).
cz-transformed, mean ± s.d. of the original variables: 11.77 ± 5.87.
4. Discussion
Our results emphasize the complex and variable nature of chimpanzee food sharing behaviour, and particularly provide strong evidence for the involvement of long-term social factors. Across both jointly and individually acquired food types, possessors directed transfers towards partners with whom grooming was more mutual and enduring, regardless of relatedness, and despite the rarity of harassment. In this respect, our results are similar to the relationship quality effect found for sharing in some captive chimpanzees [19], and the effect of long-term mutual exchange of grooming and food found in vampire bats, interpreted as an effect of social bonds on sharing [47]. We did not find an effect of grooming received on sharing likelihood, suggesting that food was not traded for grooming and emphasizing the importance of long-term and mutual grooming relationships.
In our study we considered two different approaches to investigate the sharing under pressure hypothesis. The first, a direct measure of begging pressure to evaluate restrictions on the possessor's movements by partners or feeding rate reduction, and the second, an assessment of coercion ability to investigate resource holding potential [3,19,47]. Incorporating both approaches, we found no support for sharing under pressure as the main driver for sharing in Taï chimpanzees. On the contrary, the majority of sharing occurred during the first begging bout, and sharing was more likely when begging duration was short and the number of beggars low. Furthermore, directed aggression scores from partners towards food possessors, reflecting coercion ability, did not affect the likelihood to share. The fact that social bonds were a strong predictor in chimpanzee sharing behaviour, and that harassment had no significant effect on sharing, points to selectivity in sharing, suggesting that chimpanzees can control food distribution to gain additional benefits [11]. If so, possessors may use targeted sharing behaviour to form new relationships or to maintain social bonds [12], the latter being associated with fitness benefits [61].
In addition, the effect of rank difference as a measure of both harassment potential and attractiveness of partners indicated little support for sharing under pressure or the signalling-led food sharing hypotheses, as food transfers from relatively lower-ranking possessors to higher-ranking partners were uncommon despite ample opportunity. Conversely, possessors of both sexes shared more frequently with individuals of similar rank. One exception was low-ranking females sharing more with high-ranking male partners, a result that more strongly supports the predictions of direct signalling, as harassment has no significant effect on sharing in Taï as opposed to other chimpanzee populations [5]. Increased sharing predictability between closely ranked individuals emphasizes that factors other than resource holding potential, or directed signalling played a role in sharing. Possible explanations for sharing with closely ranked partners not tested here include other forms of reciprocity, such as exchange of food for coalitionary support [15,16] or food itself [15,24], broadcasting honest information to the wider audience [7], or the existence of additional bonded dyads not identified by our conservative social bond measure.
When investigating the effect of attractive partners in the context of mating, fully tumescent females did not elicit higher rates of male sharing towards these females, supporting findings from other chimpanzee populations [5,25], although we did not consider long-term reproductive opportunities previously shown to have an effect on sharing [20]. When considering all adult bystanders as potential sharing partners, thus not accounting for the possibility that certain bystanders may already possess food, we found that sharing likelihood was greater for meat compared to non-meat (e.g. nuts, seeds, fruits, tools). However, when restricting the analysis to large, divisible, and sparsely distributed nutrient packages, such as meat (in 84% of cases chimpanzees hunted a single monkey) and Treculia africana, and when considering only beggars rather than all bystanders, this result disappeared, emphasizing that the breadth of sharing networks is based on supply and demand. This result is consistent with findings in humans showing that nonsynchronous acquisitions of large packages, as in the case of meat, predict wider sharing networks than for synchronous acquisition, such as the majority of plant food [3].
Collectively, our findings highlight that several factors drive food exchanges, and most strongly support the bonding-based reciprocal altruism hypothesis. We observed five cases in which the alpha male or a group of males travelled large distances (200–800 m) after a successful hunt, while holding a monkey carcass without feeding until fusing with a different, larger sub-group and sharing the meat (L Samuni 2015–2018, personal observation). This is especially puzzling when considering the sharing under pressure hypothesis, given that such fusions likely led to lower per capita meat intake for the meat possessor and hunters. However, such observations are concordant with our findings that social components facilitate food sharing in Taï. Chimpanzee sharing behaviour appears to vary across populations and subspecies, providing need for comparative studies to corroborate or contradict population differences, and for investigations to determine potential selective pressures that lead to the observed differences, where they exist.
From an evolutionary perspective, resource competition is an important driver of natural selection processes [62]. Thus, the idea of resource sharing between non-kin outside of a reproductive context and with no immediate benefit is puzzling. Here, understanding neurophysiological mechanisms may inform evolutionary cooperation theory. Overall, oxytocinergic system activation was positively associated with chimpanzee meat and non-meat sharing and hunting behaviour in comparison to a social feeding control. Furthermore, as urinary oxytocin levels did not differ between hunting, and the sharing of meat or non-meat, oxytocin secretion is unlikely a mere artefact of stress [38,49], but is rather associated with the cooperative act of sharing. Moreover, urinary oxytocin levels were similar in both food donors and recipients independent of the social relationship between the sharing partners, emphasizing that it is the prosocial act rather than partner identity that is associated with oxytocin increase. An earlier, less comprehensive study in a different chimpanzee subspecies also found urinary oxytocin activity in both donors and recipients during food sharing events, irrespective of bonding status [12], indicating that these results are robust and replicable. Note, we found that sharing behaviour in Taï is selective and is driven by social factors. Thus, potential bias in sample collection towards sharing cases between partners with pre-established strong relationships, may have contributed to the observed results. The involvement of the oxytocinergic system in food sharing and hunting suggests a powerful positive feedback mechanism is at play: the release of oxytocin in association with prosocial acts [34,35] may increase the likelihood to positively interact again with partners [33]. Oxytocinergic system activity during resource acquisition and sharing in chimpanzees provides further support that emotional reciprocity [32] is an important mechanism facilitating long-term cooperative exchange of both jointly and individually acquired resources. The oxytocin results fit with those of the behavioural models in which we found that social factors, more than harassment, increased sharing.
We found a strong contingency between the act of sharing and balanced and enduring grooming, key aspects of strong social bonds in some primate species [33,61,63]. This emphasizes that sharing is a cooperative act in chimpanzees, given that in the long term it provides benefits to both actor and recipient [9]. We cannot exclude that the observed contingency is an artefact of bystanders being more likely to approach food possessors that are their closer social partners, potentially increasing the predictability of sharing. Nonetheless, such repeated interactions, reinforced by neuroendocrinological processes, may lead to beneficial contingent exchanges and the formation of social bonds.
Given that established partner preferences, an indication of social bonds, increase the likelihood to share, sharing between preferred partners is more predictable than between dyads which do not share a bond. In humans, it has been shown that sharing acts are concentrated and stable over time within small clusters of households [64]. Such preferred sharing clusters are thought to include a variety of important cooperative relationships (i.e. kin, mate partners, and non-kin) needed to buffer the risks of the unpredictable human foraging niche [64], although whether these relationships constitute social bonds is rarely discussed. We propose that one function of maintaining preferred sharing relationships—or social bonds in chimpanzees—is to facilitate predictability of delayed cooperative exchanges, especially when occurring between non-kin outside of a reproductive context, in which no inclusive fitness benefits are gained. Furthermore, the intrinsic emotional connection, sustained by neurobiological mechanisms such as the oxytocinergic system, and inherent in a social bond, is likely key for supporting delayed reciprocity. We suggest accounting for strong and enduring social connections in future studies will be key to understanding the evolution of cooperation, in both humans and in non-human animals.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank the Centre Suisse de Recherches Scientifiques and staff members of the Taï Chimpanzee Project for support. We thank Roger Mundry for statistical support and Anne Pisor, Adrian Jaeggi, Claudio Tennie, Jorg Massen, and two anonymous reviewers for helpful comments.
Ethics
All methods used in this study were non-invasive and were approved by the Ministries of Research and Environment of Côte d'Ivoire, and Office Ivoirien des Parcs et Réserves. All aspects of the study comply with the ethics policy of both the Max Planck Society and the Department of Primatology of the Max Planck Institute for Evolutionary Anthropology, Germany, and the American Society of Primatologists Principles for the Ethical Treatment of Non-Human Primates.
Data accessibility
Data used in the models are available as electronic supplementary material, dataset S1.
Authors' contributions
L.S., A.P., and A.M. carried out the field data collection, L.S. and A.P. conducted endocrine laboratory work, L.S. conducted behavioural analyses, and L.S., T.D., C.C., and R.M.W. designed the study. All authors contributed to drafts of the manuscript and gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
This study was funded by the Max Planck Society, the Minerva Foundation, Leakey Foundation, the Wenner Gren Foundation (grant no. 9095), and the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (Grant Agreement no 679787).
References
- 1.Kaplan HS, Hooper PL, Gurven M. 2009. The evolutionary and ecological roots of human social organization. Phil. Trans. R. Soc. B 364, 3289–3299. ( 10.1098/rstb.2009.0115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaplan HS, Gurven M. 2005. The natural history of human food sharing and cooperation A review and a new multi-individual approach to the negotiation of norms. In Moral sentiments and material interests the foundations of cooperation in economic life (eds Gintis H, Bowles S, Boyd R, Fehr E), pp. 75–113. Cambridge, MA: MIT Press. [Google Scholar]
- 3.Kaplan HS, Hill K, Cadeliña RV, Hayden B, Hyndman DC, Preston RJ, Smith EA, Stuart DE, Yesner DR. 1985. Food sharing among ache foragers: tests of explanatory hypotheses. Curr. Anthropol. 26, 223–246. ( 10.1086/203251) [DOI] [Google Scholar]
- 4.Blurton JNG. 1984. A selfish origin for human food sharing: tolerated theft. Ethol. Sociobiol. 5, 1–3. ( 10.1016/0162-3095(84)90030-X) [DOI] [Google Scholar]
- 5.Gilby IC. 2006. Meat sharing among the Gombe chimpanzees: harassment and reciprocal exchange. Anim. Behav. 71, 953–963. ( 10.1016/j.anbehav.2005.09.009) [DOI] [Google Scholar]
- 6.Wrangham RW. 1975. Behavioural ecology of chimpanzees in Gombe National Park, Tanzania. Thesis, University of Cambridge; (doi:10.17863/CAM.16415) [PubMed] [Google Scholar]
- 7.Smith EA, Bird RLB. 2000. Turtle hunting and tombstone opening: public generosity as costly signaling. Evol. Hum. Behav. 21, 245–261. ( 10.1016/S1090-5138(00)00031-3) [DOI] [PubMed] [Google Scholar]
- 8.Barclay P. 2013. Strategies for cooperation in biological markets, especially for humans. Evol. Hum. Behav. 34, 164–175. ( 10.1016/j.evolhumbehav.2013.02.002) [DOI] [Google Scholar]
- 9.Trivers RL. 1971. The evolution of reciprocal altruism. Q. Rev. Biol. 46, 35–57. ( 10.2307/2822435) [DOI] [Google Scholar]
- 10.Jaeggi AV, Schaik CPV. 2011. The evolution of food sharing in primates. Behav. Ecol. Sociobiol. 65, 2125–2140. ( 10.1007/s00265-011-1221-3) [DOI] [Google Scholar]
- 11.Jaeggi AV, Gurven M. 2013. Natural cooperators: food sharing in humans and other primates. Evol. Anthropol. 22, 186–195. ( 10.1002/evan.21364) [DOI] [PubMed] [Google Scholar]
- 12.Wittig RM, Crockford C, Deschner T, Langergraber KE, Ziegler TE, Zuberbühler K. 2014. Food sharing is linked to urinary oxytocin levels and bonding in related and unrelated wild chimpanzees. Proc. R. Soc. B 281, 20133096 ( 10.1098/rspb.2013.3096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boesch C. 1994. Cooperative hunting in wild chimpanzees. Anim. Behav. 48, 653–667. ( 10.1006/anbe.1994.1285) [DOI] [Google Scholar]
- 14.Boesch C, Boesch-Achermann H. 2000. The chimpanzees of the Taï Forest: behavioural ecology and evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 15.Mitani JC, Watts DP. 2001. Why do chimpanzees hunt and share meat? Anim. Behav. 61, 915–924. ( 10.1006/anbe.2000.1681) [DOI] [Google Scholar]
- 16.Nishida T, Hasegawa T, Hayaki H, Takahata Y, Uehara S. 1992. Meat-sharing as a coalition strategy by an alpha male chimpanzee. Top. Primatol. 1, 159–174. [Google Scholar]
- 17.Pruetz JD, Lindshield S. 2012. Plant-food and tool transfer among savanna chimpanzees at Fongoli, Senegal. Primates 53, 133–145. ( 10.1007/s10329-011-0287-x) [DOI] [PubMed] [Google Scholar]
- 18.Samuni L, Preis A, Deschner T, Crockford C, Wittig RM. 2018. Reward of labor coordination and hunting success in wild chimpanzees. Commun. Biol. 1, 138 ( 10.1038/s42003-018-0142-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaeggi AV, De Groot E, Stevens JMG, Van Schaik CP. 2013. Mechanisms of reciprocity in primates: testing for short-term contingency of grooming and food sharing in bonobos and chimpanzees. Evol. Hum. Behav. 34, 69–77. ( 10.1016/j.evolhumbehav.2012.09.005) [DOI] [Google Scholar]
- 20.Gomes CM, Boesch C. 2009. Wild chimpanzees exchange meat for sex on a long-term basis. PLoS ONE 4, e5116 ( 10.1371/journal.pone.0005116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaeggi AV, Stevens JMG, Schaik CPV. 2010. Tolerant food sharing and reciprocity is precluded by despotism among bonobos but not chimpanzees. Am. J. Phys. Anthropol. 143, 41–51. ( 10.1002/ajpa.21288) [DOI] [PubMed] [Google Scholar]
- 22.Silk JB, Brosnan SF, Henrich J, Lambeth SP, Shapiro S. 2013. Chimpanzees share food for many reasons: the role of kinship, reciprocity, social bonds and harassment on food transfers. Anim. Behav. 85, 941–947. ( 10.1016/j.anbehav.2013.02.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Waal FBM. 1989. Food sharing and reciprocal obligations among chimpanzees. J. Hum. Evol. 18, 433–459. ( 10.1016/0047-2484(89)90074-2) [DOI] [Google Scholar]
- 24.Mitani JC. 2006. Reciprocal exchange in chimpanzees and other primates. In Cooperation in primates and humans (eds Kappeler PM, van Schaik CP), pp. 107–119. Berlin, Germany: Springer. [Google Scholar]
- 25.Gilby IC, Emery Thompson M, Ruane JD, Wrangham R. 2010. No evidence of short-term exchange of meat for sex among chimpanzees. J. Hum. Evol. 59, 44–53. ( 10.1016/j.jhevol.2010.02.006) [DOI] [PubMed] [Google Scholar]
- 26.Hockings KJ, Humle T, Anderson JR, Biro D, Sousa C, Ohashi G, Matsuzawa T. 2007. Chimpanzees share forbidden fruit. PLoS ONE 2, e886 ( 10.1371/journal.pone.0000886) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaeggi AV, Hooper PL, Beheim BA, Kaplan H, Gurven M. 2016. Reciprocal exchange patterned by market forces helps explain cooperation in a small-scale society. Curr. Biol. 26, 2180–2187. ( 10.1016/j.cub.2016.06.019) [DOI] [PubMed] [Google Scholar]
- 28.Melis AP, Semmann D. 2010. How is human cooperation different? Phil. Trans. R. Soc. B 365, 2663–2674. ( 10.1098/rstb.2010.0157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fehr E, Gächter S. 2002. Altruistic punishment in humans. Nature 415, 137–140. ( 10.1038/415137a) [DOI] [PubMed] [Google Scholar]
- 30.Schino G, Aureli F. 2017. Reciprocity in group-living animals: partner control versus partner choice. Biol. Rev. 92, 665–672. ( 10.1111/brv.12248) [DOI] [PubMed] [Google Scholar]
- 31.Dufour V, Pelé M, Neumann M, Thierry B, Call J. 2009. Calculated reciprocity after all: computation behind token transfers in orang-utans. Biol. Lett. 5, 172–175. ( 10.1098/rsbl.2008.0644) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schino G, Aureli F. 2009. Reciprocal altruism in primates: partner choice, cognition, and emotions. Adv. Stud. Behav. 39, 45–69. ( 10.1016/S0065-3454(09)39002-6) [DOI] [Google Scholar]
- 33.Crockford C, Wittig RM, Langergraber K, Ziegler TE, Zuberbühler K, Deschner T. 2013. Urinary oxytocin and social bonding in related and unrelated wild chimpanzees. Proc. R. Soc. B 280, 20122765 ( 10.1098/rspb.2012.2765) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donaldson ZR, Young LJ. 2008. Oxytocin, vasopressin, and the neurogenetics of sociality. Science 322, 900–904. ( 10.1126/science.1158668) [DOI] [PubMed] [Google Scholar]
- 35.Rilling JK, Young LJ. 2014. The biology of mammalian parenting and its effect on offspring social development. Science 345, 771–776. ( 10.1126/science.1252723) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang Y, Platt ML. 2018. Oxytocin and vasopressin flatten dominance hierarchy and enhance behavioral synchrony in part via anterior cingulate cortex. Sci. Rep. 8, 8201 ( 10.1038/s41598-018-25607-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piva M, Chang SWC. 2018. An integrated framework for the role of oxytocin in multistage social decision-making. Am. J. Primatol. ( 10.1002/ajp.22735) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neumann ID, Slattery DA. 2016. Oxytocin in general anxiety and social fear: a translational approach. Biol. Psychiatry 79, 213–221. ( 10.1016/j.biopsych.2015.06.004) [DOI] [PubMed] [Google Scholar]
- 39.de Jong TR, Neumann ID. 2017. Oxytocin and aggression. In Behavioral pharmacology of neuropeptides: oxytocin (eds Hurlemann R, Grinevich V), pp. 175–192. Vol. 35. Cham, Switzerland: Springer. [Google Scholar]
- 40.Insel TR. 2010. The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron 65, 768–779. ( 10.1016/j.neuron.2010.03.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samuni L, Preis A, Mundry R, Deschner T, Crockford C, Wittig RM. 2017. Oxytocin reactivity during intergroup conflict in wild chimpanzees. Proc. Natl Acad. Sci. USA 114, 268–273. ( 10.1073/pnas.1616812114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang SWC, Brent LJN, Adams GK, Klein JT, Pearson JM, Watson KK, Platt ML. 2013. Neuroethology of primate social behavior. Proc. Natl Acad. Sci. USA 110, 10 387–10 394. ( 10.1073/pnas.1301213110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen X, Gautam P, Haroon E, Rilling JK. 2017. Within vs. between-subject effects of intranasal oxytocin on the neural response to cooperative and non-cooperative social interactions. Psychoneuroendocrinology 78, 22–30. ( 10.1016/j.psyneuen.2017.01.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carter GG, Wilkinson GS. 2015. Intranasal oxytocin increases social grooming and food sharing in the common vampire bat Desmodus rotundus. Horm. Behav. 75, 150–153. ( 10.1016/j.yhbeh.2015.10.006) [DOI] [PubMed] [Google Scholar]
- 45.Duque JF, Leichner W, Ahmann H, Stevens JR. 2018. Mesotocin influences pinyon jay prosociality. Biol. Lett. 14, 20180105 ( 10.1098/rsbl.2018.0105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brosnan SF, Talbot CF, Essler JL, Leverett K, Flemming T, Dougall P, Heyler C, Zak PJ. 2015. Oxytocin reduces food sharing in capuchin monkeys by modulating social distance. Behaviour 152, 941–961. ( 10.1163/1568539X-00003268) [DOI] [Google Scholar]
- 47.Carter GG, Wilkinson GS. 2013. Food sharing in vampire bats: reciprocal help predicts donations more than relatedness or harassment. Proc. R. Soc. B 280, 20122573 ( 10.1098/rspb.2012.2573) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hammerstein P, Noë R. 2016. Biological trade and markets. Phil. Trans. R. Soc. B 371, 20150101 ( 10.1098/rstb.2015.0101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jong TR, et al. 2015. Salivary oxytocin concentrations in response to running, sexual self-stimulation, breastfeeding and the TSST: The Regensburg Oxytocin Challenge (ROC) study. Psychoneuroendocrinology 62, 381–388. ( 10.1016/j.psyneuen.2015.08.027) [DOI] [PubMed] [Google Scholar]
- 50.Sobolewski ME. 2012. The hormonal correlates of male chimpanzee social behavior. PhD thesis, University of Michigan. [Google Scholar]
- 51.Altmann J. 1974. Observational study of behavior: sampling methods. Behaviour 49, 227–266. ( 10.1163/156853974X00534) [DOI] [PubMed] [Google Scholar]
- 52.Neumann C, Duboscq J, Dubuc C, Ginting A, Irwan AM, Agil M, Widdig A, Engelhardt A. 2011. Assessing dominance hierarchies: validation and advantages of progressive evaluation with Elo-rating. Anim. Behav. 82, 911–921. ( 10.1016/j.anbehav.2011.07.016) [DOI] [Google Scholar]
- 53.Mielke A, Samuni L, Preis A, Gogarten JF, Crockford C, Wittig RM. 2017. Bystanders intervene to impede grooming in Western chimpanzees and sooty mangabeys. R. Soc. open sci. 4, 171296 ( 10.1098/rsos.171296) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kulik L. 2015. Development and consequences of social behavior in rhesus macaques (Macaca mulatta). PhD thesis, University of Leipzig. [Google Scholar]
- 55.Seltzer LJ, Ziegler TE. 2007. Non-invasive measurement of small peptides in the common marmoset (Callithrix jacchus): a radiolabeled clearance study and endogenous excretion under varying social conditions. Horm. Behav. 51, 436–442. ( 10.1016/j.yhbeh.2006.12.012) [DOI] [PubMed] [Google Scholar]
- 56.Bahr NI, Palme R, Möhle U, Hodges JK, Heistermann M. 2000. Comparative aspects of the metabolism and excretion of cortisol in three individual nonhuman primates. Gen. Comp. Endocrinol. 117, 427–438. ( 10.1006/gcen.1999.7431) [DOI] [PubMed] [Google Scholar]
- 57.Baayen RH. 2008. Analyzing linguistic data: a practical introduction to statistics using R, 1st edn Cambridge, UK: Cambridge University Press. [Google Scholar]
- 58.R Core Team. 2016. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 59.Bates D, Maechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. (doi:10.18637/jss.v067.i01) [Google Scholar]
- 60.Forstmeier W, Schielzeth H. 2010. Cryptic multiple hypotheses testing in linear models: overestimated effect sizes and the winner's curse. Behav. Ecol. Sociobiol. 65, 47–55. ( 10.1007/s00265-010-1038-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Silk JB. 2007. Social components of fitness in primate groups. Science 317, 1347–1351. ( 10.1126/science.1140734) [DOI] [PubMed] [Google Scholar]
- 62.Trivers RL. 1972. Parental investment and sexual selection. In Sexual selection and the descent of man (ed. Campbell B.), pp. 136–179. Chicago, IL: Aldine. [Google Scholar]
- 63.Mitani JC. 2009. Male chimpanzees form enduring and equitable social bonds. Anim. Behav. 77, 633–640. ( 10.1016/j.anbehav.2008.11.021) [DOI] [Google Scholar]
- 64.Dyble M, Thompson J, Smith D, Salali GD, Chaudhary N, Page AE, Vinicuis L, Mace R, Migliano AB. 2016. Networks of food sharing reveal the functional significance of multilevel sociality in two hunter-gatherer groups. Curr. Biol. 26, 2017–2021. ( 10.1016/j.cub.2016.05.064) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used in the models are available as electronic supplementary material, dataset S1.