Abstract
It is widely acknowledged that in most species sexual selection continues after mating. Although it is generally accepted that females play an important role in generating paternity biases (i.e. cryptic female choice, CFC), we lack a quantitative understanding of the relative importance of female-controlled processes in influencing variance in male reproductive fitness. Here, we address this question experimentally using the guppy Poecilia reticulata, a polyandrous fish in which pre- and postcopulatory sexual selection jointly determine male reproductive fitness. We used a paired design to quantify patterns of paternity for pairs of rival males across two mating contexts, one in which the female retained full control over double (natural) matings and one where sperm from the same two males were artificially inseminated into the female. We then compared the relative paternity share for a given pair of males across both contexts, enabling us to test the key prediction that patterns of paternity will depend on the extent to which females retain behavioural control over matings. As predicted, we found stronger paternity biases when females retained full control over mating compared with when artificial insemination (AI) was used. Concomitantly, we show that the opportunity for postcopulatory sexual selection (standardized variance in male reproductive success) was greater when females retained control over double matings compared with when AI was used. Finally, we show that the paternity success of individual males exhibited higher repeatability across successive brood cycles when females retained behavioural control of matings compared with when AI was used. Collectively, these findings underscore the critical role that females play in determining the outcome of sexual selection and to our knowledge provide the first experimental evidence that behaviourally moderated components of CFC increase the opportunity for sexual selection.
Keywords: total sexual selection, mate choice, sperm competition, opportunity for selection
1. Introduction
Females commonly mate with two or more males during a single reproductive episode (polyandry [1]), and consequently, sexual selection will often continue after mating in the form of sperm competition and cryptic female choice (CFC) (postcopulatory sexual selection [2]). Sperm competition, for example, occurs when ejaculates from rival males compete to fertilize a female's eggs—a phenomenon first described in insects [3] but since found to be ubiquitous among most sexually reproducing species [4]. CFC, on the other hand, occurs when females moderate the outcome of sperm competition to suit their own reproductive interests [5,6]. Being ‘cryptic’, CFC is notoriously difficult to demonstrate empirically [7], although a growing number of experimental studies have reported evidence that females can differentially manipulate the transfer, storage and/or uptake of sperm depending on the perceived (experimentally manipulated) characteristics of their mates [8–10]. More direct support for the CFC hypothesis comes from studies showing that such female-moderated processes generate biases in fertilization or paternity success [11–15].
In many species, the ability of females to exert CFC depends on their perception of male characteristics (e.g. size, attractiveness, social dominance, relatedness) occurring before, during or after mating. Examples of such behaviourally mediated mechanisms of CFC include differential patterns of sperm ejection in the feral fowl (Gallus gallus domesticus), which depend on the female's perception of male social status [9], and differential sperm storage by female crickets (Teleogryllus oceanicus) based on perceived relatedness [10]. The strong behavioural component of CFC in many species presents the opportunity of experimentally partitioning behavioural elements of CFC from other sources of variance in sperm competition, for example, through the use of artificial fertilization techniques that deny females the opportunity of assessing male attractiveness [7]. In this way, we can compare the relative opportunities for sexual selection (i.e. standardized variances in reproductive success; reviewed in [16]) across matings that include and exclude the possibility of behaviourally mediated CFC. Despite the intuitive appeal of such an approach, we know of no other studies that have evaluated how female control over mating, and thus critical components of CFC that depend on the female's assessment of male quality, increases the opportunity for postcopulatory sexual selection.
The guppy Poecilia reticulata provides a uniquely suitable study system for isolating the influence of behavioural components of CFC on the opportunities for postcopulatory sexual selection, and hence the variation in male reproductive fitness. Guppies are polyandrous livebearing fish that are established models for studying pre- and postcopulatory sexual selection [17–19]. Female choice is well established in this system, with females typically preferring males that are relatively colourful, with high courtship rates, and unfamiliar as mates [18,20]. The development of artificial insemination (AI) in this system allows researchers to experimentally separate precopulatory mating biases from postcopulatory fertilization biases [21]. AI also prevents females from evaluating males prior to mating, thus effectively eliminating mechanisms of cryptic female choice that depend on the female's perception of male quality (e.g. females may exert differential control over sperm transfer through the behavioural manipulation of copulation duration [8,22]). In guppies, the female's perception of male sexual attractiveness is a critical precursor for the differential uptake of sperm from preferred males [8], and therefore the use of AI provides a useful experimental tool for manipulating female control over mating. Importantly, when females are afforded control over successive double matings, the ensuing patterns of paternity have been shown to be strongly bimodal; either the first or second male dominates paternity of the subsequent brood (e.g. [23–25]). By contrast, when AI is used to deliver competing ejaculates (thus undermining female control over mating), the resulting paternity distribution is more uniform (i.e. paternity biases are weaker [21,26]). These striking differences in paternity outcomes between mating contexts have been interpreted as evidence for the importance of behaviourally moderated CFC in this system [17], but this has not been verified empirically within a single study. Indeed, to the best of our knowledge, the relative importance of female behavioural control over matings, in terms of generating variance in male reproductive success, has never been quantified in any species.
In this study, we employ a paired experimental design to compare and quantify patterns of paternity for pairs of rival males across two mating contexts, one in which females retain full control over double (natural) matings and one where sperm from the two competing males are artificially inseminated into females. Importantly, our paired experimental design ensures that in each replicate, we compare the relative paternity share for a given pair of males in both contexts. This design enables us to test the key prediction that patterns of paternity will depend on the extent to which females retain behavioural control over matings. Specifically, we expect to see stronger paternity biases (i.e. a bimodal paternity distribution) when females are afforded full control over mating compared with when AI is used. Consequently, we predict that the opportunity for postcopulatory sexual selection (i.e. standardized variance in male reproductive success) will be greater when females are afforded full control over double matings compared with when AI is used. Our support for both predictions in this paper underscores the important role that females play in determining the outcome of sexual selection in this system.
2. Material and methods
(a). Fish maintenance and experimental overview
The guppies used in this experiment were laboratory-reared descendants of wild-caught fish from the Alligator Creek River, Queensland, Australia. Fish were maintained in tanks with approximately equal sex ratios on a 12 L : 12 D cycle at 26 (±1)°C and fed with a mix of Artemia nauplii and commercial dry food. Experimental males were selected haphazardly from a stock population whose age ranged between six and 10 months, while females were aged six months, approximately matched for size (standard length; distance between the snout and the tip of the caudal peduncle; mean ± s.e. = 26.1 ± 0.08 mm) and raised in single sex tanks to ensure virginity (i.e. this ensured that females were both sexually receptive and did not have sperm stored from previous matings). All females were assigned haphazardly to either the natural double-mating treatment (hereafter, ‘NAT’) or the AI treatment. Our paired design ensured that in each replicate the same pair of competing males was used in both treatments (i.e. NAT and AI).
(b). Mating trials (natural double-mating treatment)
To obtain natural double matings, each female was placed in an observation tank (35 × 19 cm, filled to 13 cm) containing gravel and left to acclimatize overnight. In the morning, a male was gently placed into the observation tank and observed until he mated once with the female through consensual mating. After the first mating, the male was removed from the tank and the female was left for 10 min before a second male was added to the tank. If the female refused to mate with the second male within 10 min, the male was replaced and so on until the female mated consensually with a second male. For both first and second matings, all recorded copulations were successful, as confirmed by the ensuing postcopulatory jerks performed by the male, which signal successful sperm transfer [27]. We obtained a total of 25 double-mated females. For each mating, we recorded the latency to mate (the time taken for the female to mate with that particular male) as a proxy for female mating preference, and noted the time between the first and the second matings.
(c). Artificial inseminations treatment
After taking part in the mating trials, each of the focal males within each replicate (i.e. n = 25 pairs) was isolated individually for 7 days before being used in the AI trials. In each AI trial, the ejaculates from the two males (which were arbitrarily labelled as ‘male 1’ and ‘male 2’) were stripped artificially by applying pressure to the abdomen (see [28] for a detailed description of this procedure). The sperm from the two males were mixed in equal proportions (see below) and artificially inseminated into a sedated female (a different, unrelated female from the one used in the mating trial) using a standard protocol (for more details, see [28]). In guppies, sperm are packaged in spermatozeugmata (sperm bundles), each containing approximately 21 000 sperm cells. In each AI trial, a total of 40 sperm bundles (20 from each male) were inseminated into each virgin female. After sperm extraction, we took a tissue sample from each male's caudal fin and stored these in absolute ethanol until required for the paternity analyses (below).
(d). Gestation length and number of broods produced
After each female was mated (through natural matings or AI), she was isolated in a 2 l plastic tank containing gravel and plastic plants until she gave birth to a brood (after approx. one month, see §3). The day of parturition was noted and used to calculate the time (in days) taken to produce offspring (hereafter ‘gestation length’). Offspring within each brood were counted to estimate brood size and then preserved in absolute ethanol until required for the paternity analyses. After producing a brood, females were left in their respective containers to produce subsequent broods. In guppies, females can store sperm for several months and will continue to produce successive broods [18]. All subsequent broods were similarly preserved for paternity analyses (see below). Once a female stopped producing offspring (more than 50 days without producing offspring or showing signs of pregnancy), she was sedated in order to collect a tissue sample from her caudal fin, which was preserved for the paternity analyses.
(e). Paternity analyses
DNA was extracted using a tissue kit (EDNA HISPEX, Fisher Biotec) and five microsatellites (TTA, AGAT11, Kond15, Kond21, Pret46; GenBank accession nos. AF164205, BV097141, AF368429, AF368430, AF127242) were amplified using standard PCR protocols (for details, see [28]). Paternity was then assigned using CERVUS (v. 3.0.7, available at http://www.fieldgenetics.com) with 95% strict confidence.
(f). Data analysis
All analyses were performed using R v. 3.3.2 [29]. Means are reported with their respective standard errors (s.e.). We initially compared the opportunities for sexual selection (standardized variances in paternity success, calculated by dividing the variances by their squared means) between the NAT and AI treatments. To test this, we used a randomization approach, as implemented by Devigili et al. [30], to determine whether the difference in the variance in paternity share between the two treatments was larger than expected by chance in the first brood. This approach was necessary because differences in paternity success may arise owing to the binomial error associated with small brood sizes. To this end, a Monte Carlo simulation was run in Windows Excel using PopTools (v. 3.2) in which we simulated (10 000 times) expected paternity scores given the observed brood sizes. We then derived a p-value by calculating the proportion of times that the simulated statistic was larger than the observed one. To further evaluate differences in the opportunities for sexual selection in each treatment, we ran a linear mixed-effects model using the observed standardized variances as our dependent variable, treatment (NAT or AI) as a fixed factor and pair ID as a random factor (to account for the non-independence of data owing to the paired nature of our experiment). The significance of fixed factors was calculated from the F-statistic with the lmerTest package using Satterthwaite's approximation for the denominator degrees of freedom.
We also expected females to favour the preferred male when given the possibility of exerting CFC through behavioural processes (for example, by increasing the duration of copulation; [22]). To test this prediction, we determined whether relative latency to mate predicted paternity success in the NAT group. We used a generalized linear mixed-effect model (‘glmer’ function with a binomial distribution in the lme4 package) in which the number of offspring in each brood was included as a weighting factor, and the relative differences in latency to mate between the two competing males (male 2 − male 1) was fitted as a predictor variable. Some females in the NAT treatment rejected some males between the first and the second male they mated with, and therefore the time between the two successful matings differed among females. Only 2 out of the 25 females mated on different (but consecutive) days, while in the 23 remaining cases, the average time from one copulation to the second was less than 1 h (mean 49.7 ± 6.4 min, see electronic supplementary material, figure S1). Including this variable (time between successive matings) into the model did not change the results, so it was not included in the final model.
Next, we tested whether the number of broods and the number of offspring produced by each female differed between treatments. To address these questions, we used a generalized linear mixed-effects model in which we specified a Poisson distribution. In the model analysing the number of broods, treatment was included as the fixed factor and pair ID was fitted as a random effect. To analyse the number of offspring, we included treatment, brood number and their interaction as fixed factors and female ID (to account for multiple broods from the same female) and pair ID as random effects. Female standard length did not differ significantly between the two groups (p = 0.546) and including this term in the models did not change the results, so it was excluded from our final models. The significance of fixed factors was assessed using the ‘Anova’ function of the package car. Log-transformed gestation length was analysed using a linear mixed-effects model (‘lmer’ function), with treatment, brood number and their interaction included as fixed factors, and both female ID and pair ID as random factors.
Finally, we tested whether the paternity success of individual focal males (those arbitrarily labelled as ‘male 1’ in each pair) was significantly repeatable across successive brood cycles. To test this, we used the ‘rptProportion’ function within the rptR package [31]. Confidence intervals for repeatability estimates were calculated by parametric bootstrapping (1000 iterations) and the statistical significance of the estimates was estimated using likelihood ratio tests. Below we report repeatability values for paternity success between the first and second brood cycles but found that results remained qualitatively similar when we included broods 1, 2 and 3 in the analysis. However, as we derive lower statistical power from the latter tests (because fewer females gave birth to offspring in the third broods), we confine our repeatability analysis to the first two broods.
3. Results
The two treatments generated remarkably different paternity distributions (figure 1). As predicted, the distribution in the naturally mated (NAT) treatment was distinctly bimodal (figure 1a), while in the AI treatment paternity was more evenly distributed between the two competing males (figure 1b). Overall, across all brood cycles, we found that the standardized variances in male reproductive success were highly significantly different between treatments (F1,17.201 = 29.706, p < 0.001), indicating a greater opportunity for sexual selection in the NAT treatment than in the AI treatment. We observed qualitatively similar differences in paternity distributions and standardized variances in paternity success within each successive brood cycle (see electronic supplementary material for broods 2 and 3 and electronic supplementary material, figure S2). Overall, the observed standardized variance in the AI treatment was 0.520 and 1.744 in the NAT treatment (difference NAT − AI = 1.224). The observed difference was significantly larger than expected by chance (mean simulated difference = 0.158, CI: −0.148 to 0.468, comparison of simulated expected paternity scores with observed values: p < 0.001, figure 2).
Figure 1.
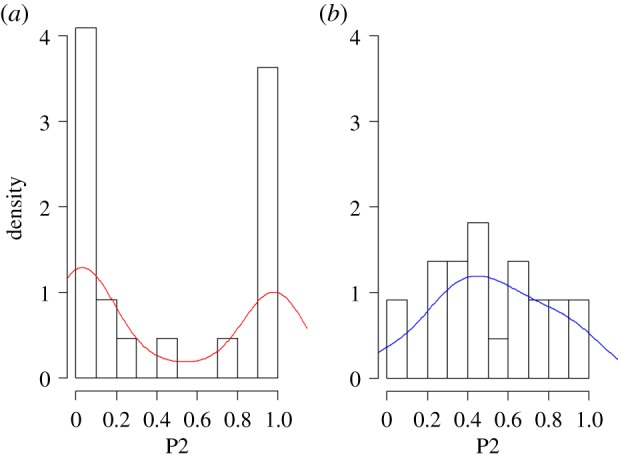
Distributions of paternity (P2) in the two treatments (natural matings (a) and artificial inseminations (b)) in the first brood. Patterns of paternity in successive broods exhibited similar distributions and are reported in electronic supplementary material. (Online version in colour.)
Figure 2.
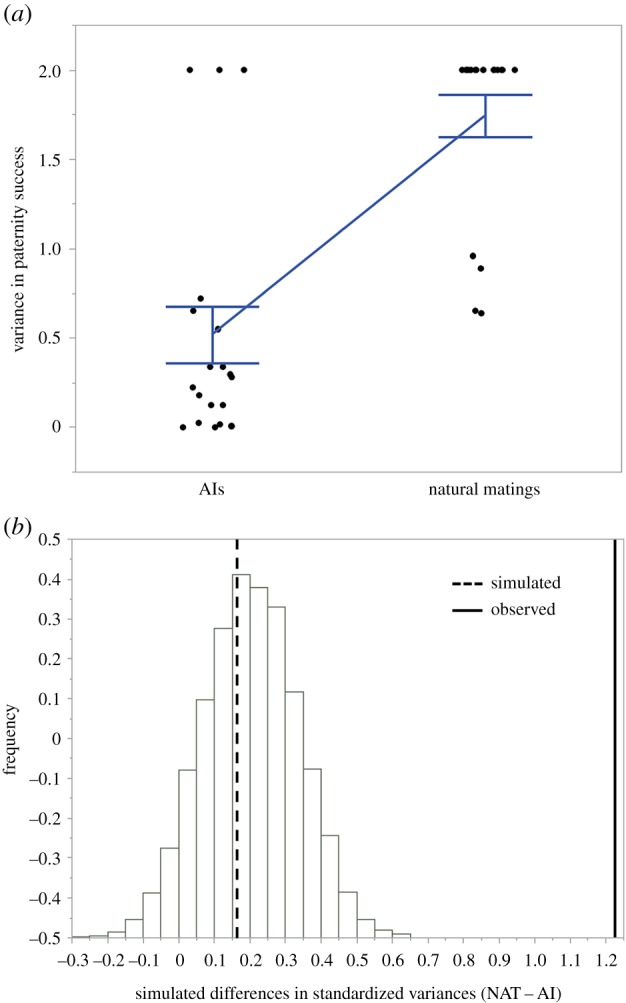
(a) Observed standardized variance between AI and NAT treatments. (b) Simulated versus observed difference in standardized variance between AI and NAT. Vertical lines represent means (dotted line, simulated difference; solid line, observed difference). Positive values indicate that opportunities for postcopulatory sexual selection were greater in the NAT than in the AI treatment. (Online version in colour.)
As expected, when females mated naturally, we found that latency to mate (female willingness to mate) was a significant predictor of paternity success (χ2 = 4.755, p = 0.029). Specifically, we found that the difference in mating latency between the first and second males to mate with the female predicted the relative paternity share of the ensuing brood; the more willing the female was to mate with the second male, the higher was his paternity success.
On average, females produced 2.7 ± 0.2 broods (range: 1–6). We detected no significant effect of treatment on the number of broods produced over time (χ2 = 0.305, p = 0.581; NAT: 2.8 ± 0.3, AI: 2.5 ± 0.26). The number of offspring did not differ between treatments (χ2 = 0.052, p = 0.819) but was affected by brood number (χ2 = 72.401, p < 0.001) and the interaction brood number and treatment (χ2 = 24.944, p < 0.001). The number of offspring produced declined over time, and this decline was sharper in the AI treatment than in the NAT treatment. However, this result needs to be interpreted cautiously as fewer than ten females produced a fourth brood (figure 3). Females assigned to the AI treatment exhibited slightly longer gestation times (34.6 ± 0.88 days) than those in the NAT treatment (32.5 ± 0.75 days; F = 4.1451, p = 0.049), and longer gestation in the first brood compared with subsequent ones (F = 5.0614, p < 0.001). However, no significant brood-by-treatment interaction for gestation length was detected.
Figure 3.
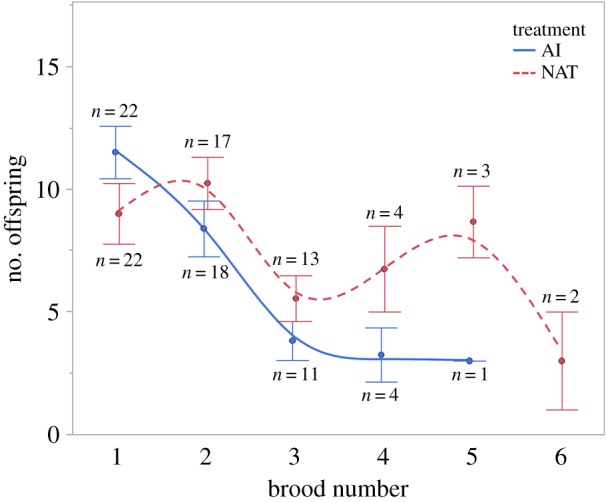
Number of offspring produced (mean ± s.e.) by the two treatments (natural matings and artificially inseminated) in the successive broods. Numbers in the graphs indicate the number of females producing offspring at each given brood. (Online version in colour.)
Finally, our repeatability analyses confirmed that the paternity success of individual focal males (within the same female) was significantly repeatable in both groups, but the estimate was substantially higher in the NAT group (repeatability estimate R = 0.89 [CI = 0.681–0.987], p < 0.001) than in the AI group (R = 0.127 [CI = 0–0.261], p = 0.045).
4. Discussion
We found striking differences in paternity distributions, and hence the opportunities for sexual selection, between mating treatments. When females mated naturally with two successive males, the ensuing paternity distribution was highly skewed towards one of the males. By contrast, when AI was used, paternity was more equally distributed between the two males. These findings thereby underscore the critical role that behavioural components of CFC [8,22,25] have on the opportunity for (postcopulatory) sexual selection. Our findings from the natural mating treatment support this conclusion by showing that the female's preferred male at the precopulatory stage (as indicated by latency to mate) was also the one that fertilized most of the eggs. However, when we experimentally precluded female control over mating through AI, the strong paternity bias disappeared and the opportunity for sexual selection was reduced.
The relative importance of CFC in sexual selection has long been a source of debate, and only in recent years, with the development of new techniques and powerful experimental approaches, are we becoming more aware of its evolutionary significance [7]. Despite this progress, however, we generally lack a clear understanding of the mechanisms underlying female-moderated biases in paternity. Where data do exist, the results from several species indicate that females may exert control over the number of sperm that compete for fertilization, for example, by manipulating the number of sperm transferred at copulation, ejected after insemination or differentially retained in storage (e.g. [6,8,13,32]). The results from our experiment, coupled with previous research on guppies, similarly invoke female-moderated changes in sperm numbers as the proximate mechanism underlying paternity biases in this system (see also [26]). In guppies, females can manipulate the number of sperm received from the male during mating by adjusting the duration of copulations [22]. This behavioural regulation of sperm transfer likely accounts for the previous finding that when the female's perception of male attractiveness is experimentally manipulated, females will accept more sperm from males they perceive to be relatively attractive [8]. Given the importance of relative sperm number in predicting fertilization success in guppies [26], we can therefore attribute the increased skew in paternity distribution in the NAT group to female control mechanisms that bias the number of sperm received in favour of relatively attractive males.
As we report above, we found that the female's preferred male (i.e. those with the shortest mating latencies) sired most of the ensuing brood. This evidence further supports our conclusion that paternity biases are attributable, at least in part, to females manipulating sperm retention to favour attractive males. Interestingly, in the present experiment, we show that the paternity patterns in both treatment groups (natural matings and AI) were highly consistent across successive broods produced by the same female. In the NAT group, paternity distributions were consistently bimodal across successive brood cycles, while those for the AI group exhibited consistently uniform distributions across brood cycles (see electronic supplementary material, figure S2). Moreover, we found that the level of repeatability in individual paternity success differed between treatments; in the NAT group, the paternity success of individual focal males was highly repeatable across successive brood cycles, while the success of those same males was far less repeatable in the AI treatment. This latter finding suggests that behaviourally moderated processes that influence sperm uptake/retention can be predictive of longer-term patterns of sperm storage that confer an advantage towards preferred males also in subsequent broods. In short, by manipulating copulations to favour preferred males in the short term, females are able to influence patterns of sperm storage and competitive fertilization success well into the future. As far as we are aware, this is the first evidence revealing a causal link between behaviourally moderated mechanisms of CFC and paternity outcomes following periods of prolonged sperm storage.
Overall, our findings corroborate the role that CFC plays in biasing postcopulatory success among competing males. We know from prior work on guppies and other species that sperm competition, attributable to male-driven processes that determine the success of competing ejaculates, is a potent form of sexual selection on male traits (e.g. see review by Simmons & Fitzpatrick [33]). However, the importance of female roles in postcopulatory selection is less clear, and to our knowledge, this has never been quantified formally within an experimental setting. Our findings for guppies address this question by revealing the critical role that females play in determining the total opportunity for sexual selection, which is often manifested by the complete domination of paternity by a single male. Clearly, other aspects of the mating system, such as the operational sex ratio [34], population structure [35] and a range of physiological process [36] will further influence the total opportunity for sexual selection (reviewed in [16]). We advocate for further experimental work designed to understand how these factors interact with behaviourally modulated processes of CFC to alter the dynamics of sexual selection in this and other systems.
Supplementary Material
Acknowledgement
We thank Ryan Dosselli for his invaluable help with microsatellite analyses and Cameron Duggin for his support with fish husbandry. We also thank two anonymous reviewers for their constructive feedback on the earlier version of the manuscript.
Ethics
This project was conducted under the approval of the University of Western Australia's Animal Ethics Committee (approval no. RA/3/100/1376).
Data accessibility
The data for this study are available on Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.57d120b [37].
Authors' contributions
C.G. and J.P.E. conceived and designed the experiment; C.G. carried out all practical components; both authors contributed towards the statistical analysis and co-drafted and approved the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This research was funded by an ARC DECRA to C.G.
References
- 1.Parker GA, Birkhead TR. 2013. Polyandry: the history of a revolution. Proc. R. Soc. B 368, 20120335 ( 10.1098/rstb.2012.0335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birkhead TR, Pizzari T. 2002. Postcopulatory sexual selection. Nat. Rev. Gen. 3, 262–273. ( 10.1038/nrg774) [DOI] [PubMed] [Google Scholar]
- 3.Parker GA. 1970. Sperm competition and its evolutionary consequences in the insects. Biol. Rev. 45, 525–567. ( 10.1111/j.1469-185X.1970.tb01176.x) [DOI] [Google Scholar]
- 4.Birkhead TR, Møller AP. 1998. Sperm competition and sexual selection. San Diego, CA: Academic Press. [Google Scholar]
- 5.Eberhard WG. 1996. Female control: sexual selection by cryptic female choice. Princeton, NJ: Princeton University Press. [Google Scholar]
- 6.Thornhill R. 1983. Cryptic female choice and its implications in the scorpionfly Harpobittacus nigricepts. Am. Nat. 122, 765–788. ( 10.1086/284170) [DOI] [Google Scholar]
- 7.Firman RC, Gasparini C, Manier MK, Pizzari T. 2017. Postmating female control: 20 years of cryptic female choice. Trends Ecol. Evol. 32, 368–382. ( 10.1016/j.tree.2017.02.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pilastro A, Simonato M, Bisazza A, Evans JP. 2004. Cryptic female preferences for colorful males in guppies. Evolution 58, 665–669. ( 10.1111/j.0014-3820.2004.tb01690.x) [DOI] [PubMed] [Google Scholar]
- 9.Pizzari T, Birkhead TR. 2000. Female feral fowl eject sperm of subdominant males. Nature 405, 787–789. ( 10.1038/35015558) [DOI] [PubMed] [Google Scholar]
- 10.Tuni C, Beveridge M, Simmons LW. 2013. Female crickets assess relatedness during mate guarding and bias storage of sperm towards unrelated males. J. Evol. Biol. 26, 1261–1268. ( 10.1111/jeb.12118) [DOI] [PubMed] [Google Scholar]
- 11.Alonzo SH, Stiver KA, Marsh-Rollo SE. 2016. Ovarian fluid allows directional cryptic female choice despite external fertilization. Nat. Commun. 7, 12452 ( 10.1038/ncomms12452) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gasparini C, Pilastro A. 2011. Cryptic female preference for genetically unrelated males is mediated by ovarian fluid in the guppy. Proc. R. Soc. B 278, 2495–2501. ( 10.1098/rspb.2010.2369) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edvardsson M, Arnqvist G. 2000. Copulatory courtship and cryptic female choice in red flour beetles Tribolium castaneum. Proc. R. Soc. Lond. B 267, 559–563. ( 10.1098/rspb.2000.1037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lymbery RA, Kennington WJ, Evans JP. 2017. Egg chemoattractants moderate intraspecific sperm competition. Evol. Lett. 1, 317–327. ( 10.1002/evl3.34) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lüpold S, Pitnick S, Berben KS, Blengini CS, Belote JM, Manier MK. 2013. Female mediation of competitive fertilization success in Drosophila melanogaster. Proc. Natl Acad. Sci. USA 110, 10 693–10 698. ( 10.1073/pnas.1300954110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans JP, Garcia-Gonzalez F. 2016. The total opportunity for sexual selection and the integration of pre- and post-mating episodes of sexual selection in a complex world. J. Evol. Biol. 29, 2338–2361. ( 10.1111/jeb.12960) [DOI] [PubMed] [Google Scholar]
- 17.Evans JP, Pilastro A. 2011. Postcopulatory sexual selection. In The ecology and evolution of poeciliid fishes (eds Evans JP, Pilastro A, Schlupp I), pp. 197–208. Chicago, IL: Chicago University Press. [Google Scholar]
- 18.Houde AE. 1997. Sex, color, and mate choice in guppies. Princeton, NJ: Princeton University Press. [Google Scholar]
- 19.Rios-Cardenas O., Morris M.. 2011. Precopulatory sexual selection. In Ecology and evolution of poeciliid fishes (eds Evans JP, Pilastro A, Schlupp I), pp. 187–196. Chicago, IL: Chicago University Press. [Google Scholar]
- 20.Magurran AE. 2005. Evolutionary ecology: the Trinidadian guppy. Oxford, UK: Oxford University Press. [Google Scholar]
- 21.Evans JP, Zane L, Francescato S, Pilastro A. 2003. Directional postcopulatory sexual selection revealed by artificial insemination. Nature 421, 360–363. ( 10.1038/nature01367) [DOI] [PubMed] [Google Scholar]
- 22.Pilastro A, Mandelli M, Gasparini C, Dadda M, Bisazza A. 2007. Copulation duration, insemination efficiency and male attractiveness in guppies. Anim. Behav. 74, 321–328. ( 10.1016/j.anbehav.2006.09.016) [DOI] [Google Scholar]
- 23.Evans JP, Magurran AE. 2001. Patterns of sperm precedence and predictors of paternity in the Trinidadian guppy. Proc. R. Soc. Lond. B 268, 719–724. ( 10.1098/rspb.2000.1577) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gasparini C, Pilastro A, Evans JP. 2011. Male genital morphology and its influence on female mating preferences and paternity success in guppies. PLoS ONE 6, e22329 ( 10.1371/journal.pone.0022329) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pitcher TE, Neff BD, Rodd FH, Rowe L. 2003. Multiple mating and sequential mate choice in guppies: females trade up. Proc. R. Soc. Lond. B 270, 1623–1629. ( 10.1098/rspb.2002.2280) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boschetto C, Gasparini C, Pilastro A. 2011. Sperm number and velocity affect sperm competition success in the guppy (Poecilia reticulata). Behav. Ecol. Sociobiol. 65, 813–821. ( 10.1007/s00265-010-1085-y) [DOI] [Google Scholar]
- 27.Liley NR. 1966. Ethological isolating mechanisms in four sympatric species of poeciliid fishes. Behaviour 13(Suppl.), 1–197. [Google Scholar]
- 28.Gasparini C, Dosselli R, Evans JP. 2017. Sperm storage by males causes changes in sperm phenotype and influences the reproductive fitness of males and their sons. Evol. Lett. 1, 16–25. ( 10.1002/evl3.2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.R. Core Team. 2016. A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.R-project.org/. [Google Scholar]
- 30.Devigili A, Di Nisio A, Grapputo A, Pilastro A. 2016. Directional postcopulatory sexual selection is associated with female sperm storage in Trinidadian guppies. Evolution 70, 1829–1843. ( 10.1111/evo.12989) [DOI] [PubMed] [Google Scholar]
- 31.Stoffel MA, Nakagawa S, Schielzeth H. 2017. rptR: repeatability estimation and variance decomposition by generalized linear mixed-effects models. Meth. Ecol. Evol. 8, 1639–1644. ( 10.1111/2041-210x.12797) [DOI] [Google Scholar]
- 32.Bloch MC, Herbeck JT, Lewis SM. 1996. Mechanisms of sperm transfer and storage in the red flour beetle (Coleoptera, Tenebrionidae). Ann. Ent. Soc. Am. 89, 892–897. ( 10.1093/aesa/89.6.892) [DOI] [Google Scholar]
- 33.Simmons LW, Fitzpatrick JL. 2012. Sperm wars and the evolution of male fertility. Reproduction 144, 519–534. ( 10.1530/rep-12-0285) [DOI] [PubMed] [Google Scholar]
- 34.Janicke T, Morrow EH. 2018. Operational sex ratio predicts the opportunity and direction of sexual selection across animals. Ecol. Lett. 21, 384–391. ( 10.1111/ele.12907) [DOI] [PubMed] [Google Scholar]
- 35.McDonald GC, Pizzari T. 2018. Structure of sexual networks determines the operation of sexual selection. Proc. Natl Acad. Sci. USA 115, E53–E61. ( 10.1073/pnas.1710450115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kekäläinen J, Evans JP. 2018. Gamete-mediated mate choice: towards a more inclusive view of sexual selection. Proc. R. Soc. B 285, 20180836 ( 10.1098/rspb.2018.0836) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gasparini C, Evans J. 2018. Data from: Female control over multiple matings increases the opportunity for postcopulatory sexual selection Dryad Digital Repository. ( 10.5061/dryad.57d120b) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Gasparini C, Evans J. 2018. Data from: Female control over multiple matings increases the opportunity for postcopulatory sexual selection Dryad Digital Repository. ( 10.5061/dryad.57d120b) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The data for this study are available on Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.57d120b [37].


