Abstract
Corticosterone plays a central role in maintaining homeostasis, promoting energy acquisition, and regulating the stress response in birds. Exposure to elevated levels of corticosterone during development can profoundly alter offspring behaviour and physiology, but the effects of elevated maternal corticosterone on offspring development remain poorly understood.
We tested two competing hypotheses concerning the effect of maternally derived corticosterone on growth and development of free-living house wrens: (i) elevated maternal corticosterone causes damaging effects on nestling phenotype and fitness (collateral damage hypothesis) and (ii) increased maternal corticosterone enhances offspring fitness by preparing nestlings for the environment experienced by their mother (environmental/maternal-matching hypothesis).
We used a non-invasive means to increase maternal corticosterone by providing females with corticosterone-injected mealworms prior to and during egg production in the absence of any overt pre-natal maternal stress. To disentangle pre- and post-natal effects of this elevation in maternal corticosterone, we cross-fostered young in two experiments: (i) nestlings of control and experimental females were reared by unmanipulated, natural females in a uniform maternal environment; (ii) a split-brood design that enabled us to assess the interaction between the mother’s corticosterone treatment and that of the nestlings.
There were significant pre-natal effects of increased maternal corticosterone on nestling growth and survival. Offspring of females experiencing experimentally increased corticosterone were heavier and larger than offspring of control females. There also was a significant interaction between maternal corticosterone treatment and the corticosterone treatment to which young were exposed within the egg in their effect on nestling survival while in the nest; experimental young exhibited greater survival than control young, but only when reared by control mothers. There was also a significant effect of maternal corticosterone treatment on nestling stress reactivity and, in both experiments, on the eventual recruitment of offspring as breeding adults in the local population.
These patterns are broadly consistent with the environmental/maternal-matching hypothesis, and highlight the importance of disentangling pre- and post-natal effects of manipulations of maternal hormone levels on offspring phenotype.
Keywords: birds, corticosterone, cross-fostering, development, HPA axis, glucocorticoid, maternal effects, stress reactivity
INTRODUCTION
Glucocorticoids play an important role in mediating the physiological and behavioural responses of organisms to changes in their environment (Scheuerlein, Van’t Hof & Gwinner, 2001; Schoech, Rensel, Bridge, Boughton, & Wilcoxen, 2009; Bartoš, Schams, Bubenik, Kotrba, & Tomanek, 2010; Boonstra, 2013; Paitz et al., 2014; Bebus, Small, Jones, Elderbrock, & Schoech, 2016). Corticosterone, a glucocorticoid and the primary metabolic steroid in birds (Hau, Casagrande, Ouyang, & Baugh, 2016), is secreted by the adrenal gland, and elevated in response to the metabolic demands arising during energetically demanding life-history events such as migration and breeding, but also in response to unpredictable changes in the environment, including decreased food availability and increased predation risk (Wingfield, Suydam, & Hunt, 1994; Scheuerlein, Van’t Hof, & Gwinner, 2001; Pereyra & Wingfield, 2003; Jenni-Eiermann, Glaus, Gruebler, Schwabl, & Jenni, 2008; Schoech, Rensel, & Heiss, 2011; Krause et al., 2016). Regarded as the avian ‘stress hormone’, corticosterone is often used by investigators as a proxy to assess stress levels, condition, health, and fitness of individuals and populations (Bonier, Martin, Moore, & Wingfield, 2009a; Bonier, Moore, Martin, & Robertson, 2009b; Lothery, Thompson, Lawler, & Sakaluk, 2014; Crino, Van Oorschot, Johnson, Malisch, & Breuner, 2011).
In birds, females exposed to stressful situations during egg formation elevate their plasma corticosterone levels, leading to increased corticosterone levels in the yolk and albumen of their eggs (Hayward & Wingfield, 2004; Navara & Pinson, 2010; Almasi et al., 2012; Bowers, Bowden, Sakaluk, & Thompson, 2015a). Development of avian embryos and neonates in an environment with increased maternal corticosterone can often have profound, long-lasting effects on their adult phenotype (e.g. Bonier et al., 2009a; Schoech et al., 2011; Bowers, Bowden, Thompson, & Sakaluk, 2016a). Compared with the well-documented effects of corticosterone on adults (e.g. Astheimer, Buttemer, & Wingfield 1992; Love, Chin, Wynne-Edwards, & Williams, 2005; Lõhmus, Sundström, & Moore, 2006; Adkins-Regan, Banerjee, Correa, & Schweitzer, 2013; Jimeno, Briga, Hau, & Verhulst, 2018) and nestlings and fledglings (e.g. Kitaysky, Wingfield, & Piatt, 2001; Pereya & Wingfield, 2002; Wada & Breuner, 2008; Butler, Leppert, & Dufty Jr., 2009; Crino et al., 2011; Patterson, Winkler, & Breuner 2011; Strange, Bowden, Thompson, & Sakaluk, 2016), considerably less is known about effects of maternally derived corticosterone in the egg on the development of avian embryos (but see reviews in Henriksen, Rettenbacher, & Groothuis, 2011; Williams & Groothuis, 2015). This paucity of information persists despite the potential for all aspects of pre-natal (embryonic) and even post-natal (nestling, fledgling) development to be affected by the presence of maternal corticosterone in the egg. Recognition of the range of possible effects on nestlings, adults, and, presumably by extension, embryos, has led to the development of two competing hypotheses, one focused on the potential harm inflicted on young via increased maternal corticosterone, and the other on the possibility that an increase in maternally derived corticosterone may actually benefit offspring.
The conventional view has focused on the short-term effects of elevated maternal corticosterone on offspring phenotype, which often results in lower mass, delayed growth, greater stress reactivity, and reduced immunocompetence of nestlings (Love et al., 2005; Saino, Romano, Ferrari, Martinelli, & Møller, 2005; Hayward, Richardson, Grogan, & Wingfield, 2006). These effects are generally regarded as a harmful and an unavoidable consequence of pre-natal maternal stress and the consequent exposure of the embryo to maternally derived corticosterone, which we designate as the collateral damage hypothesis. Negative correlations between corticosterone levels and body condition or habitat quality are consistent with this hypothesis (Müller et al., 2007; Jenni-Eiermann, Glaus, Gruebler, Schwabl, & Jenni, 2008), as are reports of negative effects of maternally derived corticosterone on nestling growth and development (Henriksen, Rettenbacher, & Groothuis, 2011; Schoech et al., 2011). In contrast to this traditional view, an emerging body of empirical research suggests that maternal effects can be a powerful tool by which mothers shape the phenotype of their offspring so that they are optimally suited to the environment in which they develop, the environmental/maternal-matching hypothesis (Henriksen et al., 2011; Sheriff & Love, 2013; Merrill & Grindstaff, 2015; Crino & Breuner, 2015; Grindstaff, 2016; Sheriff et al., 2017). This hypothesis posits a positive relationship between an increase in maternally derived corticosterone and offspring fitness, particularly if the corticosterone that females transfer to their offspring via the egg prepares offspring for success under the stressful conditions that their mother experienced (Groothuis, Müller, von Engelhardt, Carere, & Eising, 2005; Chin et al., 2009).
One of the difficulties in testing these hypotheses, particularly in an altricial bird species, is that any experimental manipulation of maternal, and, therefore, egg corticosterone levels could directly affect the developing embryo and resulting nestling, as well as maternal incubation behaviour (pre-natal effects), or indirectly affect them through an alteration of nestling brooding and provisioning by mothers (post-natal effects). In this study, we experimentally manipulated maternal corticosterone levels by providing females with corticosterone-injected mealworms (as in Bowers et al., 2016a) to test the collateral damage and environmental/maternal-matching hypotheses, employing cross-fostering of young to disentangle pre- and post-natal effects. Here, cross-fostering served to disassociate the corticosterone state of the mother laying the eggs from the corticosterone state of the mother providing post-natal care (see also Bowers et al., 2015a). We conducted two experiments: (i) one in which clutches of eggs were transferred among nests such that unmanipulated, ‘natural’ females reared offspring from eggs that had been produced either by experimental or control females (testing for a pre-natal maternal effect via the egg), whereas experimental and control females reared offspring from eggs that had been produced by natural females (testing for a post-natal maternal effect via maternal behaviour); and (ii) an experiment involving reciprocal cross-fostering of nestlings that resulted in control and experimental females rearing split broods comprising nestlings hatching from eggs produced by both experimental and control females. The first experiment focuses on the pre- and post-natal maternal effects when nestlings of control and experimental females are reared in a uniform maternal environment, whereas the second enables us to assess the interaction of the mother’s corticosterone state and that of the nestlings in assessing effects of experimentally elevated maternal corticosterone.
MATERIALS AND METHODS
Study species, general methods, and site
House wrens are small (10–12 g), secondary cavity-nesting, insectivorous passerines that breed in north-central Illinois, USA, from late April to early August (see Figure 3 in Johnson 2014). House wrens in the migratory study population readily accept nestboxes in lieu of natural cavities as breeding sites, and are double-brooded, with early-season broods initiated in late April/early May and late-season broods in late June/early July (Drilling & Thompson, 1991; Bowers, Sakaluk, & Thompson, 2012a; Bowers et al., 2012b; Bowers et al., 2016b). Females typically lay 6–8 eggs (modal clutch size = 7 eggs) during the early season and 4–7 eggs (modal clutch size = 6 eggs) during the late season. The incubation period lasts about 12 d after the last egg in the clutch has been laid, with the day the first egg of the clutch hatches being termed brood-day 0. Incubation of eggs and brooding of nestlings is performed exclusively by the female, but both parents provision nestlings with food; males rarely, if ever, feed females during incubation (Johnson, 2014). During the incubation and nestling stages, house wrens are tolerant of the disturbances associated with daily nest visits without any obvious fitness-related consequences (Styrsky, Dobbs, & Thompson, 2000). Fledging occurs on brood-day 15–17. See Johnson (2014) for additional information on house wren biology.
This study was conducted on the 130-ha Mackinaw Study Area (40.665°N, 88.89°W) in McLean County, Illinois, during the 2016 breeding season. The study site’s 700 identical nestboxes are uniformly spaced (5.4 boxes/ha) throughout an isolated tract of secondary oak-hickory-maple bottomland and upland forest surrounded by intensively cultivated land (DeMory, Thompson, & Sakaluk, 2010). Nestboxes are mounted on 1.5-m sections of electrical-line conduit, with a 48.3-cm diameter aluminium predator baffle mounted under the nestbox to discourage nest predators. Details on construction materials and nestbox dimensions can be found in Appendix 1 of Lambrechts et al. (2010).
In both experiments, we ringed adults and nestlings with uniquely numbered leg bands for individual identification. We also used a portable digital scale (Acculab Pocket Pro PP-401, Sartorius Group, Bohemia, NY, USA) to weigh (± 0.1 g) adults upon capture and nestlings upon hatching and during post-natal development. We obtained wing (straightened and flattened) and tail measurements using a stopped rule (± 0.1 mm) and tarsus length using dial callipers (± 0.1 mm). Nestlings were ringed, weighed, and had their tarsus length measured on brood-day 11, at which point body mass has reached an asymptote and positively predicts recruitment and future reproductive success in the study population (Bowers et al., 2014; Bowers, Thompson, & Sakaluk 2015b).
In both experiment 1 (conducted on first broods early in the breeding season) and experiment 2 (conducted later in the breeding season on a different set of nests comprising a mixture of second broods, renests after nest failure, and females breeding for the first time that season), nestboxes were visited at least twice weekly to determine when females began nest-building. Males initiate nest construction by bringing small sticks to the nestbox and building a crude, concave platform to which females add softer plant materials to form the nest-cup (Johnson 2014; further details in Bowers et al., 2016a). Treatments were assigned prior to clutch initiation by attaching a small plastic cup to the predator baffle to contain mealworms (Tenebrio molitor; see Figure 1 in Bowers et al., 2016a). We injected mealworms with peanut oil using a 19-gauge needle, discarding any that leaked upon injection. We injected control mealworms with 20 μL of peanut oil and experimental mealworms with 20 μL of peanut oil containing 0.5 mg/ml corticosterone (product number 27840-500MG, Sigma-Aldrich Corp. St. Louis, MO, USA), corresponding to the lower of two concentrations used to manipulate maternal corticosterone in an earlier study (Bowers et al., 2016a). As in that study, we provided five freshly injected mealworms each morning beginning a few days before clutch initiation, whether or not the mealworms from the previous day were consumed (dead mealworms left over from the previous day were discarded). In both experiments, we continued providing mealworms each morning until clutch completion.
An earlier study on our house wren population that used this non-invasive technique found that most females ate at least some, if not all, of the proffered corticosterone- and vehicle-injected mealworms (Bowers et al., 2016a). Because corticosteroids are lipophilic (Moore & Johnston, 2008), the yolks of eggs produced by females given corticosterone-injected mealworms resulted in a yolk concentration of about 10 ng/g compared with 4 ng/g in control eggs (Bowers et al., 2016a). These experimentally increased levels are similar to those reported in other studies (e.g. Love et al., 2005; Haussmann, Longenecker, Marchetto, Juliano, & Bowden, 2012). Because capturing and sampling blood from females during or shortly after egg laying dramatically increases nest-abandonment rates (Lothery et al., 2014), it was not feasible to document the circulating corticosterone levels in the plasma of females. However, studies on other species have shown that injected mealworms with concentrations in this range produce a transient elevation of circulating corticosterone levels in adults within a physiologically relevant range, similar to levels that occur in response to a capture-restraint protocol, which is the level of effect we wanted to achieve (Breuner, Greenberg, & Wingfield, 1998; Merrill, Angelier, O’Loghlen, Rothstein, & Wingfield, 2012; Henderson, Evans, Heidinger, Adams, & Arnold, 2014).
Experiment 1: Cross-fostering in a uniform maternal environment
When the first nest with evidence of nest building by females was found, a treatment was randomly assigned (experimental or control, based on a coin toss) beginning 3.9 ± 0.3 days (mean ± SE) prior to clutch initiation. Thereafter, experimental and control treatments were alternated with natural, unmanipulated nests for cross-fostering such that, whenever a treatment or control was assigned, it was then paired with a natural nest as closely matched as possible in clutch-initiation date and clutch size. There was no difference between control (n = 22) and experimental nests (n = 19) in the number of days elapsed between the beginning of the treatment and clutch initiation (F1, 35 = 0.37, p = 0.547). In a few cases, nests at which we assigned a treatment never had a clutch of eggs, so sample sizes are not perfectly balanced.
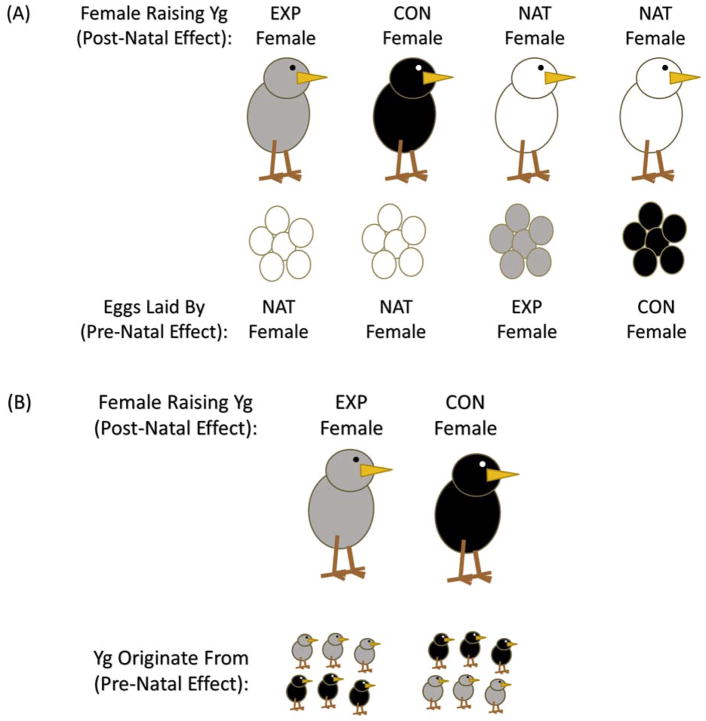
To assess pre- and post-natal effects of maternal corticosterone treatment, we matched experimental and control clutches with unmanipulated, natural clutches produced at the same time for cross-fostering, such that natural females reared the biological offspring of control and experimental females (assessing pre-natal maternal effects via the egg) and control and experimental females each reared offspring of natural females (assessing post-natal maternal effects via maternal care) (see Figure 1a). Clutches were swapped on the fourth day of full incubation. Nests within each dyad were matched as closely as possible according to clutch initiation (mean difference from natural nests ± SE: control = 0.28 ± 0.35 days, experimental = 0.50 ± 0.35 days) and clutch size (mean difference from natural nests ± SE: control = −0.18 ± 0.17 eggs, experimental = −0.11 ± 0.17 eggs). Thus, there was no statistical difference in either timing or clutch size between control and experimental nests and the natural nests with which they were matched (clutch-initiation date: F1,34 = 0.20, P = 0.654; clutch size: F1, 33 = 0.08, P = 0.785).
Figure 1.
Schematic representation of treatment groups in (A) Experiment 1 and (B) Experiment 2. Pre-natal effects would be manifested through effects within nests. Post-natal effects would be manifested through effects between nests.
On brood-day 11, in addition to ringing and measuring nestlings, we also administered a capture-and-restraint protocol to assess hypothalamic-pituitary-adrenal (HPA) axis reactivity following Strange et al. (2016), using three randomly selected nestlings pulled blind from each brood. From one of these three nestlings, we drew a baseline blood sample (mean ± SE time from capture to collection of a complete sample = 2.15 ± 0.10 min, n = 49 samples within 3 min plus one sample that required 4.5 min to obtain). There was no relationship between the time to obtain a blood sample and our measure of baseline corticosterone (r48 = 0.140, p = 0.334). We obtained samples from the second nestling after 15 min of restraint (n = 51) and from the third after 30 min (n = 50). We collected blood using brachial venepuncture with heparinised capillary tubes (product number 22-362566, Fisher Scientific, Chicago, IL, USA). After returning these nestlings to their nest, we subsequently made daily visits to check for fledging. We stored nestling blood samples on ice in the field until we returned to the laboratory, where they were immediately placed in a refrigerator prior to centrifuging them in a HemataStat II haematocrit centrifuge (Separation Technology Inc., Sanford, FL, USA) at 1,930 × g for 60 sec to separate plasma from red blood cells; the majority of samples were processed within 5 hours of collection and 2 hours of refrigeration. We measured haematocrit (a commonly used measure of health state and oxygen-carrying capacity; Bowers et al., 2014) as the mean of three measures of the packed red blood cells in the capillary tube, and then extracted the plasma from the tube using a 100-μL Hamilton syringe. Plasma was stored in a microcentrifuge tube at −20 °C until further analyses.
We quantified nestling plasma corticosterone concentrations using a commercially produced enzyme-linked immunoassay kit (Cayman Chemical 501320; Ann Arbor, MI) that we validated for use in our study species (see Appendix S1 for further details). Plasma was thawed at 4 °C, diluted 20× (baseline samples) or 100× (15 or 30 min samples) with ELISA buffer, and assayed in duplicate, following the manufacturer’s instructions. Absorbance was measured at 405 nm using a MRX Revelation plate reader (Thermo LabSystems; Philadelphia, PA) and Revelation software (Version 4.22; Dynex Technologies; Chantilly, VA). As needed, samples were rerun at different dilutions (20× – 320×) to obtain values within the range of 37–80% binding (i.e. the range yielding ≥90% corticosterone recovery, as determined during assay validation, see Figure S2). Samples were rerun when the coefficient of variation (CV) between replicates was ≥20%. Average intra-assay and inter-assay CVs were 2.8 ± 2.8% and 15.1 ± 7.8%, respectively. Corticosterone concentrations were measured blind to treatment.
Experiment 2: Split-brood cross-fostering
In this experiment, the first nest with evidence of nest building by females was randomly assigned either to a control or to an experimental treatment. Thereafter, assignment of treatment alternated as new nests were initiated, and we provided mealworms each morning beginning 2.2 ± 0.3 days (mean ± SE) prior to clutch initiation. With the exception of the number of nests (experimental = 23 nests, control = 23 nests), we used the same protocol and timing used in experiment 1.
We then identified dyads of experimental and control nests at the same stage of development (based on when eggs hatched) and used a split-brood protocol to cross-foster nestlings between them on brood-day 2. During the swap, nestlings were marked with a unique pattern of toenail clippings that identified their nest of origin as well as their individual identity. After the swap, each nest had a modal brood of six nestlings (three each from control and experimental females). Nestlings were then reared by the control or the experimental female that initiated the nest, with the result that each female reared three of her own offspring and three offspring of another female assigned to the opposite treatment (Figure 1b). Thus, any differences in size, condition, or health state between control and experimental nestlings within the nest would be caused by pre-natal effects manifested through the egg. Differences between nests of experimental and control females, however, would be attributable to differences in post-natal care. Following the application of treatments, we recorded hatching success and nestling survival to brood-days 4, 11 and fledging; nestling mass was recorded on brood-day 0 (or brood-day 1 if a nestling hatched after the daily nest check occurred) and also on brood-days 2, 4 and 11. Tarsus and wing length were measured on brood-day 11.
Statistical analysis
We used SAS (version 9.4) for all analyses and all tests were two-tailed (α = 0.05). We used Satterthwaite’s degrees-of-freedom approximation, which can result in non-integer denominator degrees of freedom. We first analysed clutch sizes in relation to treatment for control and experimental females pooled across both experiments using a linear mixed model (PROC MIXED), with treatment as a main effect and clutch-initiation date (reflecting time of season) as a covariate. In this pooled sample, two females produced a brood in each of the two experiments, but including maternal identity as a random effect to account for the non-independence of these nests resulted in an overly conservative reduction in denominator degrees of freedom. Thus, we omitted the second nest produced by each of these two females to conduct an analysis in which each nest was produced by a different female; results obtained using this approach are qualitatively similar to those obtained with the full dataset and random effect of maternal ID (data not shown).
In experiment 1, we analysed nestling asymptotic body mass (307 nestlings from 53 broods) in a linear mixed model including treatment as a fixed effect, brood-day 0 and tarsus length as covariates, and nest as a random effect. Body mass adjusted for tarsus length (a measure of skeletal size) is generally reflective of body condition (e.g. García-Berthou, 2001; but see Barnett, Suzuki, Sakaluk, & Thompson, 2015). We used a similar model to analyse nestling haematocrit (149 nestlings from 51 broods) and HPA reactivity (151 nestlings from 51 broods). For our analysis of haematocrit, we included body mass at this age and brood-day 0 as covariates, and, for our analysis of HPA reactivity, we included blood-sampling time (baseline, 15, and 30 min) and the interaction between treatment and time as fixed effects, in addition to brood size. There were no post-natal effects of maternal corticosterone on either nestling body condition, haematocrit, or survival after hatching in experiment 1 (see Results); thus, we included the natural, unmanipulated nestlings in our analysis of pre-natal effects to assess any potential effect of mealworm supplementation on these traits. We also analysed the recruitment of offspring as breeding adults in the subsequent year in relation to pre- and post-natal maternal effects using a generalized linear mixed model in PROC GENMOD with a binary response (1 = recruited, 0 = did not recruit), including nestlings as repeated observations within nests to account for the statistical non-independence of nestlings in the same nest.
In experiment 2, we used a split-plot ANCOVA in PROC MIXED with complete randomisation of whole plots to examine the effects of corticosterone treatment on nestling growth. Pre- and post-natal maternal effects, as described above, were included as fixed effects, with the former applied at the level of the individual nestling (split plot unit) and the latter applied to the whole brood (whole plot unit). Nest was included as a random effect to account for the statistical non-independence of nestlings within a brood, and maternal identity was included as a random effect (nested within nestbox) to account for the statistical non-independence of siblings within split plots. Brood-day 0 was also included as a covariate to control for seasonal effects, as offspring condition is contingent on time of year. We used a repeated measures ANCOVA to analyse the compiled mass measurements from brood-days 2, 4, and 11. To assess the effects of corticosterone treatment on nestling survival following cross-fostering (i.e. the number of initial nestlings in split broods that fledged), we used a generalized linear mixed model (PROC GLIMMIX) with a binary response (fledged or did not), with pre- and post-natal treatments as crossed main effects and nest as a random effect. We also analysed the recruitment of offspring as breeding adults in the subsequent year in relation to pre- and post-natal maternal effects, including their interaction, as described for experiment 1 above.
RESULTS
Effects on clutch size
As females in both experiments were treated the same way through egg laying, we were able compare clutch sizes of control and experimental females pooled across both experiments. When controlling for clutch-initiation date, the consumption of corticosterone-injected mealworms significantly increased clutch size by about 0.5 eggs (estimate ± SE = 0.445 ± 0.207, F1, 80 = 4.60, p = 0.035; Figure 2). Clutch size declined over the course of the breeding season (estimate ± SE = −0.023 ± 0.003, F1, 80 = 23.98, p < 0.001), but there was no interaction between treatment and clutch-initiation date in their effect on clutch size (F1, 21.8 = 0.03, p = 0.865; removed from model). Thus, increased maternal corticosterone was associated with a slight increase in clutch size regardless of breeding date.
Figure 2.
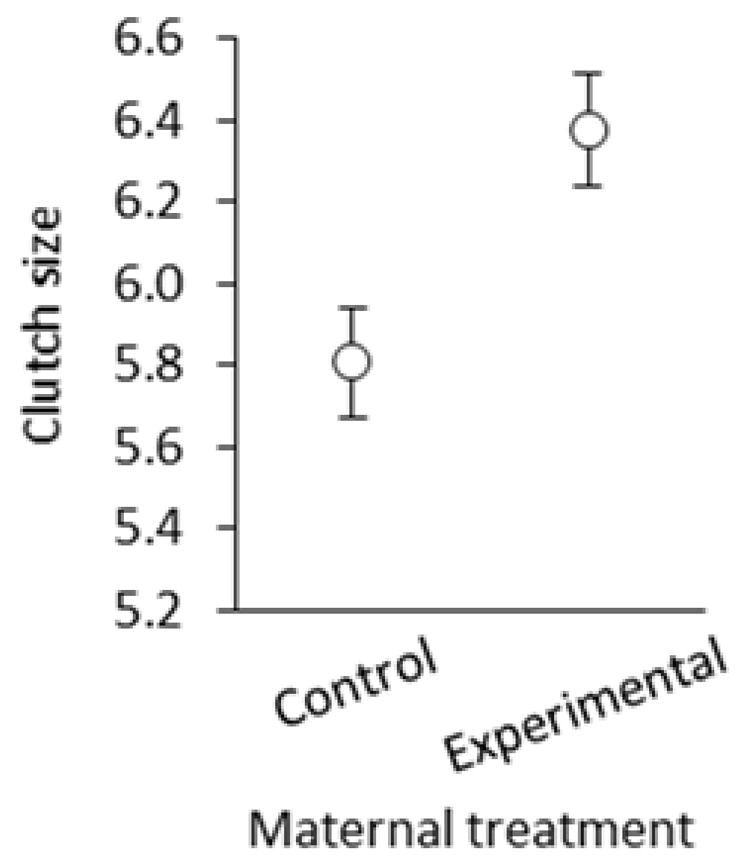
Clutch size in relation to maternal corticosterone treatment (least-squares mean ± SE).
Experiment 1: Cross-fostering in a uniform maternal environment
There were no pre-natal or post-natal effects of corticosterone treatment on nestling survival (pre-natal: F2, 47 = 1.35, p = 0.269; post-natal: F2, 47 = 1.75, p = 0.185) or haematocrit (Table 1). However, maternal access to corticosterone-injected mealworms affected nestling development via the egg, as evidenced by a pre-natal effect of maternal corticosterone on nestling size-adjusted body mass (Table 1; Figure 3). There was, however, no effect of maternal corticosterone treatment on size-adjusted mass of natural nestlings reared by control and experimental females (Table 1), nor was there any significant pre- or post-natal effect on nestling tarsus length (pre-natal: F2, 46.6 = 1.78, p = 0.179; post-natal: F2, 45.3 = 2.92, p = 0.064).
Table 1.
Pre- and post-natal effects on nestling body condition, haematocrit, and HPA reactivity in experiment 1.
| Pre-natal effects | Post-natal effects | |||||||
|---|---|---|---|---|---|---|---|---|
| Estimate ± SE | F | df | P | Estimate ± SE | F | df | P | |
|
|
|
|||||||
| Body condition (size-adjusted body mass) | ||||||||
| Treatment | 4.88 | 2, 43.5 | 0.012 | 2.36 | 2, 44.9 | 0.106 | ||
| Experimentala | 0.297 ± 0.096 | −0.172 ± 0.100 | ||||||
| Controla | 0.056 ± 0.100 | −0.195 ± 0.108 | ||||||
| Brood-day 0 | −0.015 ± 0.010 | 2.07 | 1, 43.8 | 0.157 | −0.011 ± 0.011 | 1.03 | 1, 45.4 | 0.316 |
| Tarsus length | 0.374 ± 0.045 | 68.08 | 1, 280.0 | < 0.001 | 0.361 ± 0.046 | 60.9 | 1, 292.0 | < 0.001 |
| Intercept | 5.528 ± 1.892 | 5.327 ± 1.977 | ||||||
| Haematocrit | ||||||||
| Treatment | 0.35 | 2, 47.3 | 0.707 | 1.95 | 2, 47.0 | 0.153 | ||
| Experimentala | 0.823 ± 0.263 | 0.706 ± 1.918 | ||||||
| Controla | 0.056 ± 0.100 | −3.556 ± 2.073 | ||||||
| Brood-day 0 | 0.139 ± 0.098 | 0.31 | 1, 47.4 | 0.578 | 0.138 ± 0.209 | 0.43 | 1, 47.4 | 0.513 |
| Body mass | 2.039 ± 0.967 | 4.45 | 1, 140.0 | 0.037 | 2.078 ± 0.959 | 4.70 | 1, 141.0 | 0.032 |
| Intercept | 1.408 ± 36.40 | −0.159 ± 34.85 | ||||||
| HPA reactivity (plasma corticosterone concentration) | ||||||||
| Treatment | 0.52 | 1, 22.9 | 0.477 | 1.78 | 1, 19.8 | 0.198 | ||
| Controlb | 4.913 ± 3.042 | 8.817 ± 3.037 | ||||||
| Time | 34.98 | 2, 47.4 | < 0.001 | 59.13 | 2, 41.6 | < 0.001 | ||
| 0–3 minc | −12.914 ± 2.645 | −15.642 ± 2.492 | ||||||
| 15 minc | −1.254 ± 2.645 | −2.227 ± 2.437 | ||||||
| Treatment × Time | −5.305 ± 3.845 | 1.19 | 2, 47.4 | 0.314 | −9.127 ± 3.727 | 3.86 | 2, 41.6 | 0.029 |
| Brood size | −1.568 ± 1.065 | 2.17 | 1, 26.1 | 0.153 | −2.327 ± 1.088 | 4.58 | 1, 20.1 | 0.045 |
| Intercept | 25.913 ± 6.735 | 32.156 ± 6.761 | ||||||
relative to natural, unmanipulated offspring;
relative to experimental offspring;
relative to corticosterone concentrations after 30 min of restraint
Figure 3.
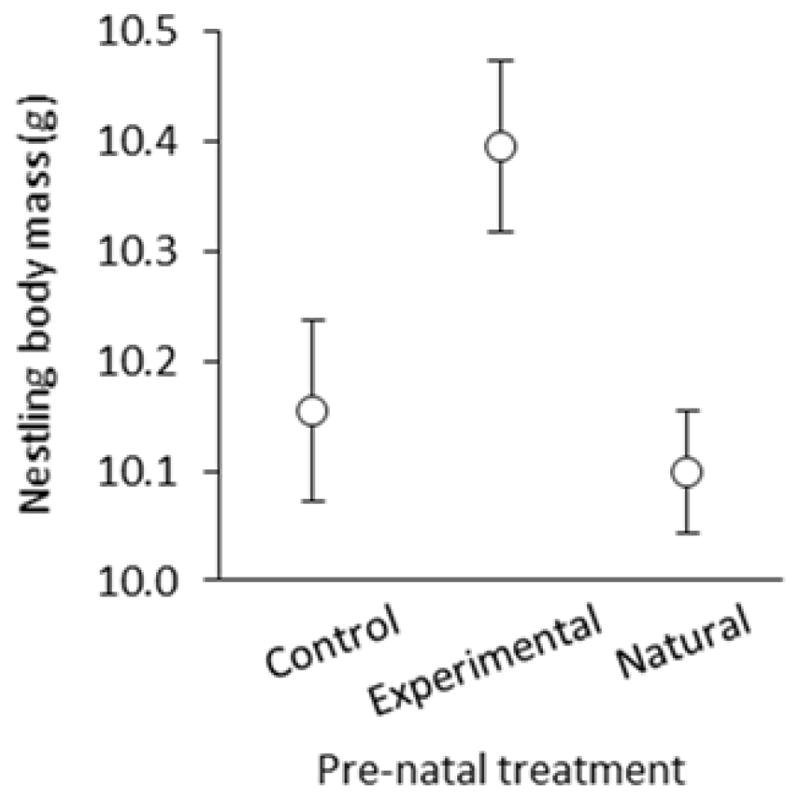
Pre-natal effects on nestling body mass in relation to maternal corticosterone treatment (least-squares mean ± SE). Nestlings in control and experimental treatments were reared by unmanipulated, natural mothers, whereas nestlings in the natural treatment were reared by control or experimental mothers.
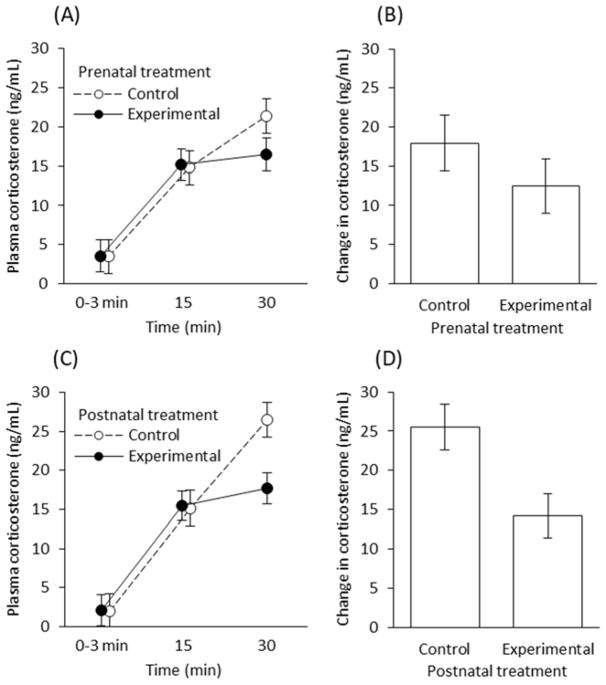
There were no differences in baseline corticosterone concentrations among any pre-natal or post-natal treatment group (pre-natal: F1, 64.3 = 0.00, p = 0.982, post-natal: F1, 56.2 = 0.00, p = 0.971; Figure 4). Among pre-natal treatments, there was no difference in the timing (i.e. no interaction between treatment and time; Table 1) or magnitude (F1, 22 = 1.22, p = 0.281; Figure 4B) of corticosterone increase following restraint (Table 1, FFigure 4A,B); however, there was a non-significant tendency for experimental nestlings to have reduced corticosterone concentrations at 30 min (1, 64.8 = 2.61, p = 0.111; Fig 4A). Brood size did not affect the magnitude or timing of the corticosterone response among pre-natal treatment groups (estimate SE = −0.991 ± 3.029, F1, 22 = 0.11, p = 0.747). Maternal corticosterone treatment did, however, induce a post-natal effect on nestling HPA reactivity, as indicated by an interaction between the post-natal treatment and the duration of restraint in their effect on nestling corticosterone (Table 1; Figure 4C). Among post-natal treatments, natural nestlings raised by experimental females had lower corticosterone concentrations after 30 min of restraint compared with those raised by control females (Table 1; Figure 4C,D), and a diminished overall stress response (F1, 20 = 7.60, p = 0.012). Additionally, nestlings in larger broods had reduced overall corticosterone responses relative to broods with fewer young (estimate SE = −5.899 ± 2.213, F1, 20 = 7.11, p = 0.015).
Figure 4.
Nestling corticosterone in relation to pre- and post-natal treatments. (A) and (B) depict nestlings hatched from eggs produced by control and experimental females but reared by unmanipulated females (pre-natal maternal effects via the egg); (C) and (D) depict nestlings hatched from eggs produced by natural, unmanipulated females but reared either by control or experimental females (post-natal maternal effects via differences in parental care after hatching). Time in (A) and (C) reflect minutes since a nestling was removed from the nest and held under restraint. (B) and (D) reflect the difference in corticosterone between baseline (time = 0–3 min) and stress-induced individuals (after 30 min of restraint) within broods. Plotted are least-squares means ± SE.
Experiment 2: Split-brood cross-fostering
There was a significant pre-natal effect of maternal corticosterone treatment on nestling tarsus length, as nestlings hatching from eggs produced by experimental females had significantly longer tarsi than those hatching from eggs produced by control females (F1, 31.5 = 4.19, p = 0.0491; Figure 5), regardless of the treatment of the mother that raised them. Nestling tarsus length also increased significantly with time-of-season (estimate ± SE = 0.023 ± 0.010, F1, 49.3 = 5.16, p = 0.0276). There was, however, no significant post-natal effect on tarsus length of the nestlings (F1, 31.2 = 0.12, P = 0.7289). There were no significant effects of maternal corticosterone treatment on nestling wing length (pre-natal: F1, 27.9 = 0.34, p = 0.5641; post-natal: F1, 37.9 = 0.05, p = 0.8254), size-adjusted body mass (pre-natal: F1, 28.2 = 1.58, p = 0.2190; post-natal: F1, 24 = 0.95, p = 0.3407), or nestling growth (pre-natal: F1, 32.9 = 0.00, p = 0.9787; post-natal: F1, 36.2 = 0.29, p = 0.5933).
Figure 5.
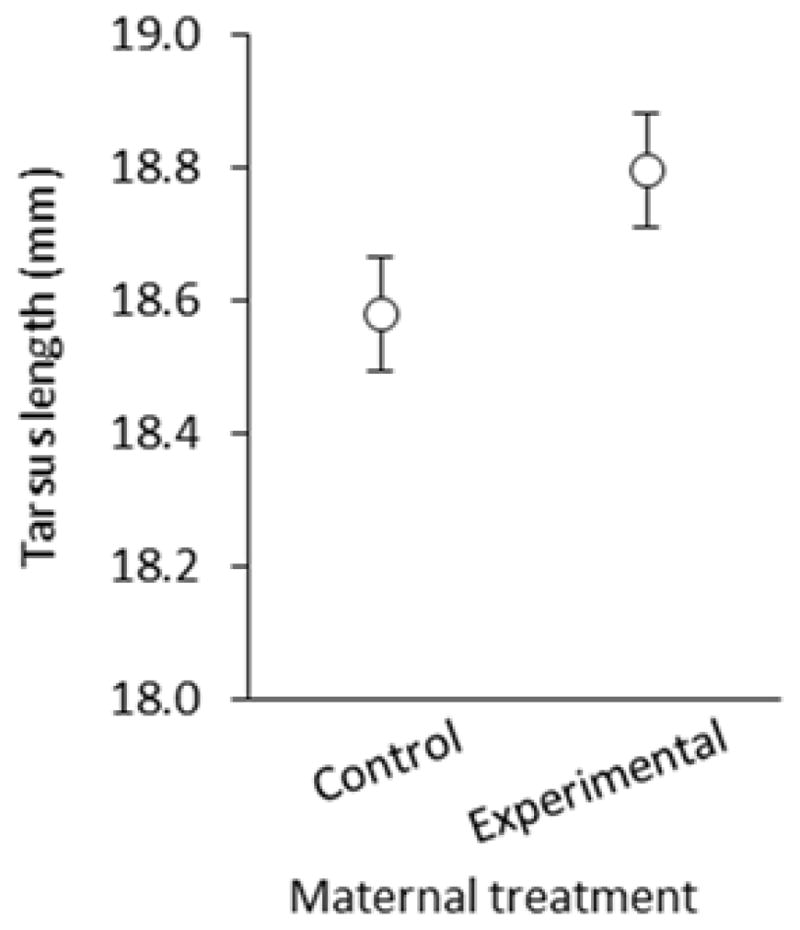
Pre-natal effects on nestling tarsus length in relation to maternal corticosterone treatment (least-squares means ± SE).
There was a significant interaction between the corticosterone treatment to which the young had been exposed in the egg via their mother (pre-natal effect) and the corticosterone treatment of the female rearing the young (post-natal effect) in their effect on nestling fledging success (F1, 261 = 6.12, p = 0.014; Figure 6). Follow-up tests revealed no difference in the survival of control and experimental young when reared by experimental mothers (F1, 261 = 2.15, p = 0.14), but when reared by control mothers, experimental young exhibited significantly higher survival than control young (F1, 261 = 4.15, p = 0.0428; Figure 6).
Figure 6.
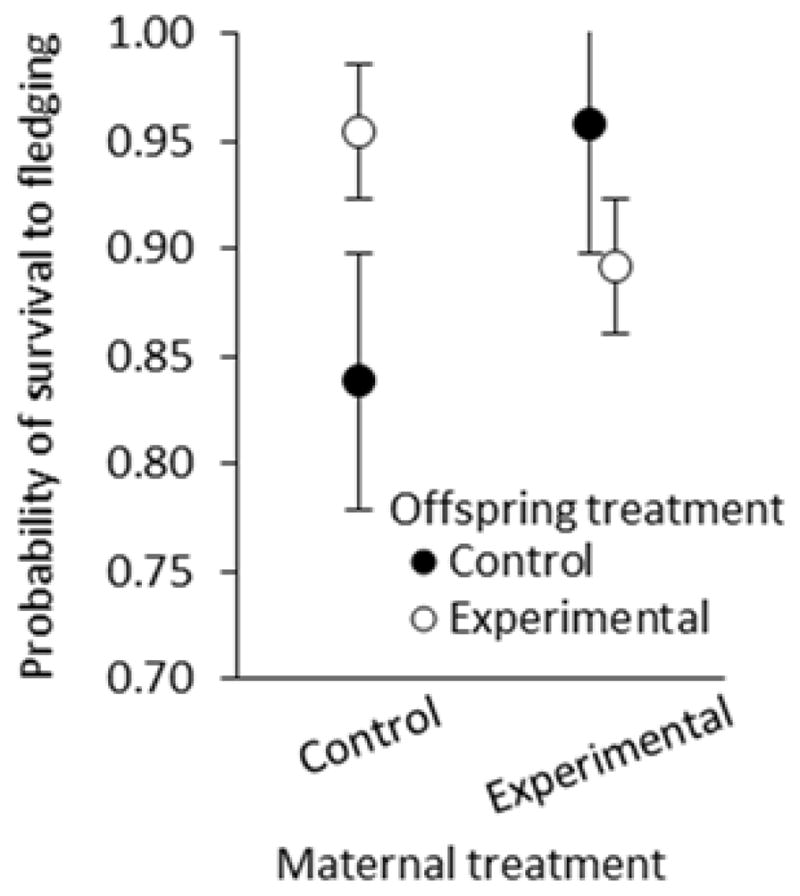
Survival of nestlings in split broods (number that fledged following cross-fostering) reared by control mothers and experimental mothers.
Long-term effects on offspring recruitment
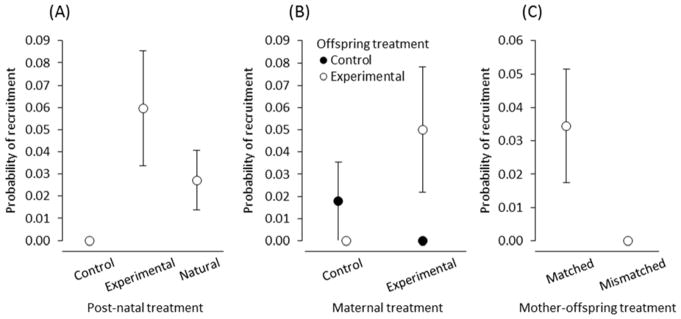
In experiment 1, there was no pre-natal effect of maternal corticosterone within the egg on recruitment ( , p = 0.5598), but there was a post-natal effect ( , p = 0.0131; Figure 7A), as natural offspring reared by experimental females had increased recruitment relative to natural offspring reared by control females (Figure 7A). In experiment 2, there was also a significant effect on recruitment, specifically an interaction between pre- and post-natal effects ( , p = 0.0430; Figure 7B). Follow-up tests to parse this interaction revealed a tendency for experimental offspring reared by experimental females to have increased recruitment compared with (i) experimental offspring reared by control mothers (z1 = 3.16, p = 0.0756) and (ii) control offspring reared by experimental mothers (z1 = 3.16, p = 0.0756); neither of these mismatched treatment groupings resulted in any recruited offspring. Indeed, whenever offspring and their mothers had the same treatment (either both control or both experimental), these matched groupings resulted in increased recruitment compared with nestlings that had a treatment different from the female rearing them ( , p = 0.0436).
Figure 7.
Effects of maternal corticosterone on the eventual recruitment of offspring as breeding adults. (A) Post-natal effect of maternal corticosterone treatment during egg production on the recruitment of unrelated foster offspring (experiment 1). (B) Interaction between pre- and post-natal effects of maternal corticosterone on offspring recruitment (experiment 2). (C) Recruitment of offspring in relation to the match (both mother and offspring are either control or experimental) or mismatch (mother and offspring have different treatments) of pre- and post-natal effects (experiment 2).
DISCUSSION
If adverse conditions lead to an increase in maternal corticosterone during egg formation, and this increased corticosterone is transferred, at least to an extent, to the egg, the collateral damage hypothesis proposes that there will be long-term, negative effects on offspring. In contrast, the environmental/maternal-matching hypothesis posits that transfer of maternal corticosterone to the egg prepares offspring for the stressful environment that they are about to encounter, and is generally beneficial to offspring provided the maternal environment matches the offspring environment (Bonier et al., 2009b; Sheriff et al., 2017). Our results appear to be broadly consistent with the environmental/maternal-matching hypothesis. There were positive, pre-natal effects of increased maternal plasma corticosterone (i.e. via the egg or, less likely, female incubation behaviour) on size-adjusted body mass and structural body size (i.e. tarsus length) of offspring. There also was a significant interaction between the corticosterone treatment of the female rearing young and the corticosterone treatment to which young had been pre-natally exposed via the egg in their effect on nestling fledging success. A parallel, albeit weaker, interaction on offspring recruitment revealed that whenever offspring and their mothers had the same treatment (either both control or both experimental), these matched groupings resulted in higher recruitment compared with nestlings that had a treatment different from the female rearing them; this too is consistent with the environmental/maternal-matching hypothesis. There was also a significant post-natal effect of maternal corticosterone treatment (i.e. via its effect on maternal behaviour) on nestling stress reactivity. Collectively, these results parallel earlier, generally positive effects of pre-natal corticosterone exposure in house wren nestlings in our study system (Strange et al., 2016, Bowers et al., 2016a).
Experimental manipulation of maternal corticosterone had a significant effect on size-adjusted mass and structural body size of nestlings, but solely through pre-natal influences. When nestlings were reared in a uniform maternal environment (experiment 1), increased maternal corticosterone led to an increase in their body condition, a trait positively correlated with their recruitment and subsequent longevity in the study population (Bowers et al., 2014). In the split-brood cross-fostering experiment, nestlings originating from experimental females had longer tarsi, regardless of the treatment of the mother that raised them. Thus, this pre-natal effect on structural size of nestlings parallels the pre-natal effect on body condition in experiment 1. A likely explanation for the increased size of experimental nestlings is that an increase in the corticosterone concentration of yolks of eggs laid by experimental females results in a higher begging rate of nestlings that hatch from these eggs, and this in turn leads to increased provisioning of these nestlings relative to control nestlings (but see Barnett, Clairardin, Thompson, & Sakaluk, 2011). In a previous study, the concentration of corticosterone in egg yolks increased in females fed corticosterone-injected mealworms, and offspring hatching from these eggs begged at a higher rate than control offspring and eventually attained greater size-adjusted mass (Bowers et al., 2016a). However, unlike the current study, this study was unable to disentangle pre- and post-natal contributions of elevated maternal corticosterone to offspring phenotype.
In general, results from previous studies in a variety of avian, reptilian, and mammalian taxa show that exposure pre-natally to corticosterone and cortisol has a negative effect on survival, body condition, and other taxon-specific traits (e.g. Vercken, de Fraipont, Dufty, & Clobert, 2007; Sanderson et al., 2014). However, a number of studies show no significant effects on body size or behaviour, suggesting that pre-natal exposure to increased corticosterone is not always detrimental (e.g. Love & Williams, 2008; Chin et al., 2009; Carter, Paitz, McGhee, & Bowden, 2016). For example, in Japanese quail (Coturnix japonica), offspring of corticosterone-manipulated females grew slower in their first week post-hatching, but were not significantly different in size from control nestlings by adulthood (Hayward & Wingfield, 2004). Similarly, nestlings hatching from corticosterone-injected eggs in our study population were lighter than control nestlings at hatching, but, through compensatory growth, were heavier near the time of nest-leaving (Strange et al., 2016), a likely positive effect; however, there are often long-term costs to compensatory growth (Metcalfe & Monaghan, 2001; Monaghan, 2008; Grace, Froud, Meillère, & Angelier, 2017,). There is evidence of positive effects of pre-natal corticosterone or cortisol exposure in a variety of taxa (Meylan & Clobert, 2005; Capelle, Semeniuk, Sopinka, Heath, & Love, 2016). The immediate cause for these pre-natal effects is most likely the maternal corticosterone and other possible compounds associated with elevated maternal corticosterone that were transferred to the eggs. It is, of course, possible that female incubation behaviour was altered, but we think it unlikely that this caused the pre-natal effects we document here, as previous work on the study population showed no effect of the changes in incubation behaviour (e.g. nest attentiveness, incubation constancy) induced through experimental food supplementation on hatching success or other variables related to breeding productivity (Lothery et al., 2014).
In experiment 1, there was a significant effect of maternal corticosterone on offspring stress reactivity (Figure 4C,D), but, surprisingly, this was manifested as a post-natal effect in unmanipulated, natural nestlings that could only be attributable to an effect of corticosterone on maternal behaviour that persisted to the rearing of nestlings. Asymptotic levels of corticosterone are generally thought to be reached after 30 min in passerines (Wingfield, Suydam, & Hunt, 1994; Rensel, Boughton, & Schoech, 2010), although recent work has often extended measurement time to 40 min or longer (Wada, Hahn, & Breuner, 2007; Haussmann et al., 2012; Baugh, Davidson, Hau, & van Oers, 2017; Wingfield et al., 2018). The difference in stress reactivity of unmanipulated nestlings reared by control and experimental females can only be explained by a change in the behaviour of experimental females during rearing that resulted from corticosterone provided during egg laying. Any such change is undoubtedly linked to the form or magnitude of parental care afforded to the young by the female during the nestling stage, which could involve changes in brooding effort, provisioning, and parental attentiveness, among other components of parental investment. One obvious candidate would be maternal provisioning behaviour, which increases with experimental activation of the female immune system during egg laying and the associated increase in maternal corticosterone (Bowers et al., 2015a). This increased maternal provisioning results in nestlings achieving significantly higher size-adjusted body mass (Bowers et al., 2015a), which could, in theory, influence their stress reactivity. Experimentally increased post-natal corticosterone in nestling Savannah sparrows (Passerculus sandwichensis) led to lower stress reactivity than control nestlings (Pakkala, Norris, Sedinger, & Newman, 2016), so it seems possible that if maternal parental behaviour affects HPA axis function, females could, post-natally, influence stress reactivity of their young. Indeed, in mammals, a number of studies have shown that maternal behaviour can, to some extent, mitigate the effects of any down-regulation of the HPA axis in offspring that arises from adversity experienced early in life (Fish et al., 2004; Weaver et al., 2004).
Whatever the proximate cause of the lower stress reactivity of unmanipulated nestlings reared by experimental females, it would be helpful to know whether this result reflects an effect on female behaviour brought about by increased corticosterone or is, in fact, favoured by selection on the mother, as suggested by the positive effects of maternal corticosterone treatment on the recruitment of offspring as adults. If the latter is true, why might it be beneficial to glucocorticoid-exposed females to produce young with reduced stress reactivity? A general pattern that seems to be emerging in comparative studies of adult birds is that stress reactivity is muted during reproduction, whereas in stressful times outside the breeding period, HPA axis activity is upregulated; likewise, stress reactivity is lower during periods of food abundance, but higher when there may be insufficient food to support reproduction (Krause et al., 2016; Wingfield et al., 2018). These patterns suggest that stress reactivity is linked adaptively to the trade-off between reproduction and self-maintenance, with HPA axis activity down-regulated when resources are differentially allocated to reproduction. If this is true, it might provide a clue as to why females stressed during breeding might benefit by producing young with lower overall stress reactivity: if these young are likely to breed in the same stressful environment as their mother, it might be more advantageous to forgo the survival-enhancing benefits of stress reactivity in favour of current reproduction. Assuming that nestling stress reactivity patterns persist into adulthood, this could represent an indirect form of adaptive programming, mediated by post-natal maternal behaviour, and constituting a form of maternally imposed, transgenerational terminal investment; a recent review suggests that this kind of cryptic terminal investment may be more common than is generally appreciated (Duffield, Bowers, Sakaluk, & Sadd, 2017).
Perhaps the most striking pattern to emerge from the split-brood cross-fostering experiment was the interaction between pre- and post-natal maternal effects on nestling survival through to fledging: experimental nestlings had higher survival than control young when reared by control mothers, but there was no difference in survival when nestlings were reared by experimental mothers. It is easy to understand why survival of nestlings hatching from eggs laid by corticosterone-manipulated mothers might be higher: this study and our earlier work has revealed that an increase in maternally derived corticosterone leads to increased body condition, body size, and enhanced immune responsiveness of nestlings (Bowers et al., 2015a; Bowers et al., 2016a; Strange et al., 2016), traits predictive of offspring recruitment and longevity (Bowers et al., 2014). But why should the effect of maternal corticosterone treatment on nestling survival disappear when nestlings are reared by experimental mothers? We hypothesize that the increased provisioning effort of females that ensues upon experimental elevation of maternal corticosterone (Bowers et al., 2015a) likely mitigates any advantage that might otherwise accrue to the increase in maternally derived corticosterone of experimental nestlings. This interaction between the corticosterone treatment of the female rearing the young and the corticosterone treatment to which the young had been pre-natally exposed might also explain why pre-natal effects of maternal corticosterone on mass and growth of offspring were largely muted, aside from a marginally significant increase in the tarsus length of experimental nestlings. If control young reared by control females are less likely to survive, then those that survive might grow larger because there are fewer competing siblings within the brood, thereby masking any effect of the treatment on offspring size.
In conclusion, our results are broadly consistent with the environmental/maternal-matching hypothesis. Our non-invasive approach for elevating maternal corticosterone resulted in both pre- and post-natal effects on offspring, suggesting that maternally derived corticosterone and, potentially, other compounds transferred to the egg, prepare offspring for the environment their mother encountered, increasing their probability of survival in that environment. Our results further demonstrate the importance of distinguishing between pre- and post-natal effects of maternal hormonal manipulation, as this affects not only the contents of eggs, but also subsequent parental behaviour. If both are not taken into account, then the actual cause of any effects of maternal hormones on offspring could be incorrectly interpreted as exclusively direct effects on the developing embryo (Crino & Breuner, 2015). Cross-fostering of young remains a powerful tool for parsing the effects of maternally derived hormones in the egg and hormonal effects on maternal behaviour that influence offspring phenotype and fitness.
Supplementary Material
Acknowledgments
We thank the 2016 and 2017 Wren Crews for contributing to data collection and the ParkLands Foundation (Merwin Preserve), the Illinois Great Rivers Conference of the United Methodist Church, and the Sears and Butler families for the use of their properties. Financial support was provided by NIH grant R15HD076308; the School of Biological Sciences, Illinois State University; the Beta Lambda Chapter of the Phi Sigma Biological Sciences Honor Society; and the Department of Biological Sciences, University of Memphis. All activities complied with current laws of the United States and with the Illinois State University Institutional Animal Care and Use Committee Protocol 865938, U.S. Geological Survey banding permit 09211, and U.S. Fish and Wildlife Service collecting permit MB692148-0.
Footnotes
AUTHORS’ CONTRIBUTIONS
B.M.W., E.K.B., S.K.S., and C.F.T. designed the study, applied experimental treatments, made all field measurements, and collected blood samples; K.A.T. and J.F.F. conducted plasma corticosterone assays; B.M.W., S.K.S., and E.K.B. conducted statistical analyses; B.M.W. wrote the initial draft of the manuscript, and all authors contributed to subsequent revisions and gave final approval of the manuscript.
DATA ACCESSIBILITY
Data deposited in the Dryad Digital Repository: http://doi:10.5061/dryad.16049f4, (Weber et al., 2018)
Additional supporting information may be found in the online version of this article.
References
- Adkins-Regan E, Banerjee SB, Correa SM, Schweitzer C. Maternal effects in quail and zebra finches: behavior and hormones. General and Comparative Endocrinology. 2013;190:34–41. doi: 10.1016/j.ygcen.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Almasi B, Rettenbacher S, Müller C, Brill S, Wagner H, Jenni L. Maternal corticosterone is transferred into the egg yolk. General and Comparative Endocrinology. 2012;178:139–144. doi: 10.1016/j.ygcen.2012.04.032. [DOI] [PubMed] [Google Scholar]
- Astheimer LB, Buttemer WA, Wingfield JC. Interactions of corticosterone with feeding, activity and metabolism in passerine birds. Ornis Scandinavica. 1992;23:355–365. [Google Scholar]
- Barnett CA, Clairardin SG, Thompson CF, Sakaluk SK. Turning a deaf ear: a test of the manipulating androgens hypothesis in house wrens. Animal Behaviour. 2011;81:113–120. [Google Scholar]
- Barnett CA, Suzuki TN, Sakaluk SK, Thompson CF. Mass-based condition measures and their relationship with fitness: in what condition is condition? Journal of Zoology. 2015;296:1–5. doi: 10.1111/jzo.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoš L, Schams D, Bubenik G, Kotrba R, Tománek M. Relationship between rank and plasma testosterone and cortisol in red deer males (Cervus elaphus) Physiology & Behavior. 2010;101:628–634. doi: 10.1016/j.physbeh.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Baugh AT, Davidson SC, Hau M, van Oers K. Temporal dynamics of the HPA axis linked to exploratory behavior in a wild European songbird (Parus major) General and Comparative Endocrinology. 2017;250:104–112. doi: 10.1016/j.ygcen.2017.06.011. [DOI] [PubMed] [Google Scholar]
- Bebus SE, Small TW, Jones BC, Elderbrock EK, Schoech SJ. Associative learning is inversely related to reversal learning and varies with nestling corticosterone exposure. Animal Behaviour. 2016;111:251–260. [Google Scholar]
- Bonier F, Martin PR, Moore IT, Wingfield JC. Do baseline glucocorticoids predict fitness? Trends in Ecology & Evolution. 2009a;24:634–642. doi: 10.1016/j.tree.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Bonier F, Moore IT, Martin PR, Robertson RJ. The relationship between fitness and baseline glucocorticoids in a passerine bird. General and Comparative Endocrinology. 2009b;163:208–213. doi: 10.1016/j.ygcen.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Boonstra R. Reality as the leading cause of stress: rethinking the impact of chronic stress in nature. Functional Ecology. 2013;27:11–23. [Google Scholar]
- Bowers EK, Sakaluk SK, Thompson CF. Experimentally increased egg production constrains future reproduction of female house wrens. Animal Behaviour. 2012a;83:495–500. [Google Scholar]
- Bowers EK, Smith RA, Hodges CJ, Zimmerman LM, Thompson CF, Sakaluk SK. Sex-biased terminal investment in offspring induced by maternal immune challenge in the house wren (Troglodytes aedon) Proceedings of the Royal Society B. 2012b;279:2891–2898. doi: 10.1098/rspb.2012.0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers EK, Hodges CJ, Forsman AM, Vogel LA, Masters BS, Johnson BGP, … Sakaluk SK. Neonatal body condition, immune responsiveness, and hematocrit predict longevity in a wild bird population. Ecology. 2014;95:3027–3034. doi: 10.1890/14-0418.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers EK, Bowden RM, Sakaluk SK, Thompson CF. Immune activation generates corticosterone-mediated terminal reproductive investment in a wild bird. American Naturalist. 2015a;185:769–783. doi: 10.1086/681017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers EK, Thompson CF, Sakaluk SK. Persistent sex-by-environment effects on offspring fitness and sex-ratio adjustment in a wild bird population. Journal of Animal Ecology. 2015b;84:473–486. doi: 10.1111/1365-2656.12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers EK, Bowden RM, Thompson CF, Sakaluk SK. Elevated corticosterone during egg production elicits increased maternal investment and promotes nestling growth in a wild song bird. Hormones and Behavior. 2016a;83:6–13. doi: 10.1016/j.yhbeh.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers EK, Grindstaff JL, Soukup SS, Drilling NE, Eckerle KP, Sakaluk SK, Thompson CF. Spring temperatures influence selection on breeding date and the potential for phenological mismatch in a migratory bird. Ecology. 2016b;97:2880–2891. doi: 10.1002/ecy.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuner CW, Greenberg AL, Wingfield JC. Noninvasive corticosterone treatment rapidly increases activity in Gambel’s white-crowned sparrows (Zonotrichia leucophrys gambelii) General Comparative Endocrinology. 1998;111:386–394. doi: 10.1006/gcen.1998.7128. [DOI] [PubMed] [Google Scholar]
- Butler MW, Leppert LL, Dufty AM., Jr Effects of small increases in corticosterone levels on morphology, immune function, and feather development. Physiological and Biochemical Zoology. 2009;83:78–86. doi: 10.1086/648483. [DOI] [PubMed] [Google Scholar]
- Capelle PM, Semeniuk CAD, Sopinka NM, Heath JW, Love OP. Prenatal stress exposure generates higher early survival and smaller size without impacting developmental rate in a Pacific salmon. Journal of Experimental Zoology. 2016;325A:641–650. doi: 10.1002/jez.2058. [DOI] [PubMed] [Google Scholar]
- Carter AW, Paitz RT, McGhee KE, Bowden RM. Turtle hatchlings show behavioral types that are robust to developmental manipulations. Physiology and Behavior. 2016;155:46–55. doi: 10.1016/j.physbeh.2015.11.034. [DOI] [PubMed] [Google Scholar]
- Chin EH, Love OP, Verspoor JJ, Williams TD, Rowley K, Burness G. Juveniles exposed to embryonic corticosterone have enhanced flight performance. Proceedings of the Royal Society B. 2009;276:499–505. doi: 10.1098/rspb.2008.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crino OL, Van Oorschot BK, Johnson EE, Malisch JL, Breuner CW. Proximity to a high traffic road: glucocorticoid and life history consequences for nestling white-crowned sparrows. General and Comparative Endocrinology. 2011;173:323–332. doi: 10.1016/j.ygcen.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Crino OL, Breuner CW. Developmental stress: evidence for positive phenotypic and fitness effects in birds. Journal of Ornithology. 2015;156:S389–S398. [Google Scholar]
- DeMory ML, Thompson CF, Sakaluk SK. Male quality influences male provisioning in house wrens independent of attractiveness. Behavioral Ecology. 2010;21:1156–1164. [Google Scholar]
- Drilling NE, Thompson CF. Mate switching in multibrooded house wrens. Auk. 1991;108:60–70. [Google Scholar]
- Duffield KR, Bowers EK, Sakaluk SK, Sadd BM. A dynamic threshold model for terminal investment. Behavioral Ecology and Sociobiology. 2017;71:185. doi: 10.1007/s00265-017-2416-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish EW, Shahrokh D, Bagot R, Caldji C, Bredy T, Szyf M, Meaney MJ. Epigenetic programming of stress responses through variations in maternal care. Annals of the New York Academy of Sciences. 2004;1036:167–180. doi: 10.1196/annals.1330.011. [DOI] [PubMed] [Google Scholar]
- García-Berthou E. On the misuse of residuals in ecology: testing regression residuals vs. the analysis of covariance. Journal of Animal Ecology. 2001;70:708–711. [Google Scholar]
- Grace JK, Froud L, Meillère A, Angelier F. House sparrows mitigate growth effects of post-natal glucocorticoid exposure at the expense of longevity. General and Comparative Endocrinology. 2017;253:1–12. doi: 10.1016/j.ygcen.2017.08.011. [DOI] [PubMed] [Google Scholar]
- Grindstaff JL. Developmental immune activation programs adult behavior: insight from research on birds. Current Opinion in Behavioral Sciences. 2016;7:21–27. doi: 10.1016/j.cobeha.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groothuis TGG, Müller W, von Engelhardt N, Carere C, Eising C. Maternal hormones as a tool to adjust offspring phenotype in avian species. Neuroscience & Biobehavioral Reviews. 2005;29:329–352. doi: 10.1016/j.neubiorev.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Hau M, Casagrande S, Ouyang JQ, Baugh AT. Glucocorticoid-mediated phenotypes in vertebrates: multilevel variation and evolution. Advances in the Study of Behavior. 2016;48:41–115. [Google Scholar]
- Haussmann MF, Longenecker AS, Marchetto NM, Juliano SA, Bowden RM. Embryonic exposure to corticosterone modifies the juvenile stress response, oxidative stress and telomere length. Proceedings of the Royal Society B. 2012;279:1447–1456. doi: 10.1098/rspb.2011.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward LS, Wingfield JC. Maternal corticosterone is transferred to avian yolk and may alter offspring growth and adult phenotype. General and Comparative Endocrinology. 2004;135:365–371. doi: 10.1016/j.ygcen.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Hayward LS, Richardson JB, Grogan MN, Wingfield JC. Sex differences in the organizational effects of corticosterone in the egg yolk of quail. General and Comparative Endocrinology. 2006;146:144–148. doi: 10.1016/j.ygcen.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Henderson LJ, Evans NP, Heidinger BJ, Adams A, Arnold KE. Maternal condition but not corticosterone is linked to offspring sex ratio in a passerine bird. PLoS ONE. 2014;9:e110858. doi: 10.1371/journal.pone.0110858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen R, Rettenbacher S, Groothuis TGG. Prenatal stress in birds: pathways, effects, function and perspectives. Neuroscience and Biobehavioral Reviews. 2011;35:1484–1501. doi: 10.1016/j.neubiorev.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Jenni-Eiermann S, Glaus E, Grüebler M, Schwabl H, Jenni L. Glucocorticoid response to food availability in breeding barn swallows (Hirundo rustica) General and Comparative Endocrinology. 2008;155:558–565. doi: 10.1016/j.ygcen.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Jimeno B, Briga M, Hau M, Verhulst S. Male but not female zebra finches with high plasma corticosterone have lower survival. Functional Ecology. 2018;32:713–721. [Google Scholar]
- Johnson LS. House wren (Troglodytes aedon) In: Rodewald PG, editor. The Birds of North America Online. Ithaca: Cornell Lab of Ornithology; 2014. Retrieved from the Birds of North America: https://birdsna.org/Species-Account/bna/species/houwre. [DOI] [Google Scholar]
- Kitaysky AS, Wingfield JC, Piatt JF. Corticosterone facilitates begging and affects resource allocation in the black-legged kittiwake. Behavioral Ecology. 2001;12:619–625. [Google Scholar]
- Krause JS, Pérez JH, Chmura HE, Meddle SL, Hunt KE, Gough L, … Wingfield JC. The stress response is attenuated during inclement weather in parental, but not in pre-parental, Lapland longspurs (Calcarius lapponicus) breeding in the Low Arctic. Hormones and Behavior. 2016;83:68–74. doi: 10.1016/j.yhbeh.2016.05.018. [DOI] [PubMed] [Google Scholar]
- Lambrechts MM, Adriaensen F, Ardia DR, Artemyev AV, Atiénzar F, Bańbura J, … Ziane N. The design of artificial nestboxes for the study of secondary hole-nesting birds: a review of methodological inconsistencies and potential biases. Acta Ornithologica. 2010;45:1–26. [Google Scholar]
- Lõhmus M, Sundström LF, Moore FR. Non-invasive corticosterone treatment changes foraging intensity in red-eyed vireos Vireo olivaceus. Journal of Avian Biology. 2006;37:523–526. [Google Scholar]
- Lothery CJ, Thompson CF, Lawler ML, Sakaluk SK. Food supplementation fails to reveal a trade-off between incubation and self-maintenance in female house wrens. PLoS ONE. 2014;9:e106260. doi: 10.1371/journal.pone.0106260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love OP, Chin EH, Wynne-Edwards KE, Williams TD. Stress hormones: a link between maternal condition and sex-biased reproductive investment. American Naturalist. 2005;166:751–766. doi: 10.1086/497440. [DOI] [PubMed] [Google Scholar]
- Love OP, Wynne-Edwards KE, Bond L, Williams TD. Determinants of within- and among-clutch variation in yolk corticosterone in the European starling. Hormones and Behavior. 2008;53:104–111. doi: 10.1016/j.yhbeh.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Love OP, Williams TD. The adaptive value of stress-induced phenotypes: effects of maternally derived corticosterone on sex-biased investment, cost of reproduction, and maternal fitness. American Naturalist. 2008;172:E135–E149. doi: 10.1086/590959. [DOI] [PubMed] [Google Scholar]
- Madliger CL, Semeniuk CAD, Harris CM, Love OP. Assessing baseline stress physiology as an integrator of environmental quality in a wild avian population: implications for use as a conservation biomarker. Biological Conservation. 2015;192:409–417. [Google Scholar]
- Metcalfe NB, Monaghan P. Compensation for a bad start: grow now, pay later? Trends in Ecology & Evolution. 2001;16:254–260. doi: 10.1016/s0169-5347(01)02124-3. [DOI] [PubMed] [Google Scholar]
- Monaghan P. Early growth conditions, phenotypic development and environmental change. Philosophical Transactions of the Royal Society B. 2008;363:1635–1645. doi: 10.1098/rstb.2007.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meylan S, Clobert J. Is corticosterone-mediated phenotype development adaptive? Maternal corticosterone treatment enhances survival in male lizards. Hormones and Behavior. 2005;48:44–52. doi: 10.1016/j.yhbeh.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Merrill L, Angelier F, O’Loghlen AL, Rothstein SI, Wingfield JC. Sex-specific variation in brown-headed cowbird immunity following acute stress: a mechanistic approach. Oecologia. 2012;170:25–38. doi: 10.1007/s00442-012-2281-4. [DOI] [PubMed] [Google Scholar]
- Merrill L, Grindstaff JL. Pre and post-natal antigen exposure can program the stress axis of adult zebra finches: evidence for environment matching. Brain, Behavior, and Immunity. 2015;45:71–79. doi: 10.1016/j.bbi.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan P, Spencer KA. Stress and life history. Current Biology. 2014;24:R408–R412. doi: 10.1016/j.cub.2014.04.017. [DOI] [PubMed] [Google Scholar]
- Moore MC, Johnston GIH. Toward a dynamic model of deposition and utilization of yolk steroids. Integrative and Comparative Biology. 2008;48:411–418. doi: 10.1093/icb/icn079. [DOI] [PubMed] [Google Scholar]
- Müller C, Jenni-Eiermann S, Blondel J, Perret P, Caro SP, Lambrechts MM, Jenni L. Circulating corticosterone levels in breeding blue tits Parus caeruleus differ between island and mainland populations and between habitats. General and Comparative Endocrinology. 2007;154:128–136. doi: 10.1016/j.ygcen.2007.05.031. [DOI] [PubMed] [Google Scholar]
- Navara KJ, Pinson SE. Yolk and albumen corticosterone concentrations in eggs laid by white versus brown caged laying hens. Poultry Science. 2010;89:1509–1513. doi: 10.3382/ps.2009-00416. [DOI] [PubMed] [Google Scholar]
- Paitz RT, Clairardin SG, Gould AC, Hicke JW, Zimmerman LM, Bowden RM. Corticosterone levels during the nesting process in red-eared sliders (Trachemys scripta) Journal of Herpetology. 2014;48:567–570. [Google Scholar]
- Pakkala JJ, Norris DR, Sedinger JS, Newman AEM. Experimental effects of early-life corticosterone on the hypothalamic-pituitary-adrendal axis and pre-migratory behaviour in a wild songbird. Functional Ecology. 2016;30:1149–1160. [Google Scholar]
- Patterson SH, Winkler DW, Breuner CW. Glucocorticoids, individual quality and reproductive investment in a passerine bird. Animal Behaviour. 2011;81:1239–1247. [Google Scholar]
- Pereyra ME, Wingfield JC. Changes in plasma corticosterone and adrenocortical response to stress during the breeding cycle in high altitude flycatchers. General and Comparative Endocrinology. 2003;130:222–231. doi: 10.1016/s0016-6480(02)00568-3. [DOI] [PubMed] [Google Scholar]
- Rensel MA, Boughton RK, Schoech SJ. Development of the adrenal stress response in the Florida scrub-jay (Aphelocoma coerulescens) General and Comparative Endocrinology. 2010;165:255–261. doi: 10.1016/j.ygcen.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Saino N, Romano M, Ferrari RP, Martinelli R, Møller AP. Stressed mothers lay eggs with high corticosterone levels which produce low-quality offspring. Journal of Experimental Zoology. 2005;303A:998–1006. doi: 10.1002/jez.a.224. [DOI] [PubMed] [Google Scholar]
- Sanderson JL, Young AJ, Hodge SJ, Kyabulima S, Walker SL, Cant MA. Hormonal mediation of a carry-over effect in a wild cooperative mammal. Functional Ecology. 2014;28:1377–1386. [Google Scholar]
- Scheuerlein A, Van’t Hof TJ, Gwinner E. Predators as stressors? Physiological and reproductive consequences of predation risk in tropical stonechats (Saxicola torquata axillaris) Proceedings of the Royal Society of London B. 2001;268:1575–1582. doi: 10.1098/rspb.2001.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoech SJ, Rensel MA, Bridge ES, Boughton RK, Wilcoxen TE. Environment, glucocorticoids, and the timing of reproduction. General and Comparative Endocrinology. 2009;163:201–207. doi: 10.1016/j.ygcen.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Schoech SJ, Rensel MA, Heiss RS. Short- and long-term effects of developmental corticosterone exposure on avian physiology, behavioral phenotype, cognition, and fitness: a review. Current Zoology. 2011;57:514–530. [Google Scholar]
- Sheriff MJ, Love OP. Determining the adaptive potential of maternal stress. Ecology Letters. 2013;16:271–280. doi: 10.1111/ele.12042. [DOI] [PubMed] [Google Scholar]
- Sheriff MJ, Bell A, Boonstra R, Dantzer B, Lavergne SG, McGhee KE, … Love OP. Integrating ecological and evolutionary context in the study of maternal stress. Integrative and Comparative Biology. 2017;57:437–449. doi: 10.1093/icb/icx105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange MS, Bowden RM, Thompson CF, Sakaluk SK. Pre- and post-natal effects of corticosterone on fitness-related traits and the timing of endogenous corticosterone production in a songbird. Journal of Experimental Zoology. 2016;325A:347–359. doi: 10.1002/jez.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styrsky JD, Dobbs RC, Thompson CF. Food-supplementation does not override the effect of egg mass on fitness-related traits of nestling house wrens. Journal of Animal Ecology. 2000;69:690–702. [Google Scholar]
- Vercken E, de Fraipont M, Dufty AM, Jr, Clobert J. Mother’s timing and duration of corticosterone exposure modulate offspring size and natal dispersal in the common lizard (Lacerta vivipara) Hormones and Behavior. 2007;51:379–386. doi: 10.1016/j.yhbeh.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Wada H, Hahn TP, Breuner CW. Development of stress reactivity in white-crowned sparrow nestlings: total corticosterone response increases with age, while free corticosterone response remains low. General and Comparative Endocrinology. 2007;150:405–413. doi: 10.1016/j.ygcen.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Wada H, Breuner CW. Transient elevation of corticosterone alters begging behavior and growth of white-crowned sparrow nestlings. Journal of Experimental Biology. 2008;211:1696–1703. doi: 10.1242/jeb.009191. [DOI] [PubMed] [Google Scholar]
- Weaver ICG, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behaviour. Nature Neuroscience. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Weber BM, Bowers EK, Terrell KA, Falcone JF, Thompson CF, Sakaluk SK. Data from: Pre- and post-natal effects of experimentally manipulated maternal corticosterone on growth, stress reactivity, and survival of nestling house wrens. Dryad Digital Repository. 2018 doi: 10.1111/1365-2435.13126. http://doi:10.5061/dryad.16049f4. [DOI] [PMC free article] [PubMed]
- Williams TD, Groothuis TGG. Egg quality, embryonic development, and post-hatching phenotype: an integrated perspective. In: Deeming DC, Reynolds SJ, editors. Nest, eggs, & incubation. Oxford, UK: Oxford University Press; 2015. pp. 113–126. [Google Scholar]
- Wingfield JC, Suydam R, Hunt K. The adrenocortical responses to stress in snow buntings (Plectrophenax nivalis) and Lapland longspurs (Calcarius lapponicus) at Barrow, Alaska. Comparative Biochemistry and Physiology Part C: Pharmacology, Toxicology and Endocrinology. 1994;108:299–306. [Google Scholar]
- Wingfield JC, Hau M, Boersma PD, Romero LM, Hillgarth N, Ramenofsky M, … Wikelski M. Effects of El Niño and La Niña Southern Oscillation events on the adrenocortical responses to stress in birds of the Galapagos Islands. General and Comparative Endocrinology. 2018;259:20–33. doi: 10.1016/j.ygcen.2017.10.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.