Abstract
Late-delayed radiation-induced brain injury (RIBI) is a major adverse effect of fractionated whole-brain irradiation (fWBI). Characterized by progressive cognitive dysfunction, and associated cerebrovascular and white matter injury, RIBI deleteriously affects quality of life for cancer patients. Despite extensive morphological characterization of the injury, the pathogenesis is unclear, thus limiting the development of effective therapeutics. We previously reported that RIBI is associated with increased gene expression of the extracellular matrix (ECM) protein fibronectin (FN1). We hypothesized that fibronectin contributes to perivascular ECM, which may impair diffusion to the dependent parenchyma, thus contributing to the observed cognitive decline. The goal of this study was to determine the localization of fibronectin in RIBI and further characterize the composition of perivascular ECM, as well as identify the cell of origin for FN1 by in situ hybridization. Briefly, fibronectin localized to the vascular basement membrane of morphologically normal blood vessels from control comparators and animals receiving fWBI, and to the perivascular space of edematous and fibrotic vascular phenotypes of animals receiving fWBI. Additional mild diffuse parenchymal staining in areas of vascular injury suggested bloodbrain-barrier disruption and plasma fibronectin extravasation. Perivascular ECM lacked amyloid and contained lesser amounts of collagens I and IV, which localized to the basement membrane. These changes occurred in the absence of alterations in microvascular area fraction or microvessel density. Fibronectin transcripts were rarely expressed in control comparators, and were most strongly induced within cerebrovascular endothelial and vascular smooth muscle cells after fWBI. Our results demonstrate that fibronectin is produced by cerebrovascular endothelial and smooth muscle cells in late-delayed RIBI and contributes to perivascular ECM, which we postulate may contribute to diffusion barrier formation. We propose that pathways that antagonize fibronectin deposition and matrix assembly or enhance degradation may serve as potential therapeutic targets in RIBI.
INTRODUCTION
Approximately 200,000 patients per year receive fractionated whole-brain irradiation (fWBI) for the treatment of intracranial neoplasia (1). Of patients who survive long enough to develop late-delayed radiation-induced brain injury [RIBI (>six months)], 50–90% will demonstrate higher order cognitive dysfunction, which adversely affects patient day-to-day function and quality of life (2–4). This loss of function is associated with progressive cerebrovascular and white matter injury (5, 6). Cerebrovascular manifestations of injury include perivascular edema suggestive of ongoing injury, as well as perivascular extracellular matrix (ECM) accumulation (fibrosis) indicative of chronicity. Despite numerous studies characterizing the morphology of RIBI (7–12), the pathogenesis and contributing mechanisms remain unclear, thus limiting the development of effective therapies for affected patients.
Studies investigating the pathogenesis of RIBI in humans are confounded by the concurrent presence of neoplasia or other comorbid conditions such as diabetes mellitus and hypertension (13, 14). We and others have shown that rhesus macaques (Macaca mulatta) that receive fWBI, a total dose of 40–80 Gy, 2–5 Gy per fraction, recapitulate the histologic features and progressive cognitive impairment (7, 8, 15–17) observed in humans with RIBI. While rodent models have been used to investigate the pathogenesis and mitigation of RIBI, rodents differ in neuroanatomic (18) and cerebrovascular structure (19) compared to primates.Moreover, rodents do not develop white matter necrosis after fWBI (20, 21), which may limit translatability of findings. Therefore, the NHP model is a justified alternative in which to investigate the pathogenesis of RIBI.
One of the hallmark histopathologic lesions of RIBI is multifocal, spatially stochastic, perivascular ECM accumulation with predilection for white matter (9, 22–24). Similar perivascular fibrosis is associated with impaired blood flow in heart disease (25) and contributes to impaired gas exchange in interstitial lung disease (26–28). Thus, we postulate that perivascular ECM accumulation may be a key event in the pathogenesis of RIBI, resulting in diffusion barrier formation and subsequent ischemic injury to dependent white matter. Necrosis and disruption of white matter neurotransmission negatively affects cognition.
ECM is a heterogeneous amalgamation of proteins, proteoglycans, glycoproteins and other molecules. Therefore, our gene expression study included preliminary screening of a several ECM contributors in a non-human primate (NHP) model of RIBI (29) as mechanisms of degradation, accumulation and remodeling vary by target ECM molecule and implicate different signaling pathways for future analysis. Of the ECM components evaluated, which included several laminins, proteoglycans and nidogen, only fibronectin (FN1) was differentially regulated in RIBI (29). Thus, we hypothesized that fibronectin is a key contributor to ECM in RIBI. While the majority of fibronectin in the body is produced in the liver and circulates as a component of blood plasma (30), detection of FN1 mRNAs suggested a component of local production. We further hypothesized that the cell of origin would be the cerebral endothelial cell. In the current study we sought to localize fibronectin and further characterize the composition of perivascular ECM and to identify the cell of origin for FN1 transcripts. Additionally, we examined the gene expression of several factors that contribute to fibronectin matrix deposition and assembly in an effort to identify potential molecular mechanisms of RIBI.
MATERIALS AND METHODS
Tissue and Subjects
Quantitative immunohistochemical and gene expression analyses were performed on the same animals as those used by Andrews et al.(29). Whole-brain irradiated animals (n = 4, aged 5.6–10.7 years, 7.5– 8.7 kg) received 40 Gy fWBI 4–7 months prior to necropsy as part of a previous experiment (7, 15). All four whole-brain irradiated animals were euthanized during the study period (4.3–6.6 months postirradiation, mean: 5.6 months) due to the development of severe neurologic signs consisting of progressive ataxia, visual impairment and lethargy or weakness. Late-delayed RIBI was verified by histopathology (29).
Control comparators (n = 3, aged 5.5–6.6 years, 5.3–8.3 kg) had been part of an unrelated study in which animals received a single, 10 Gy thoracic dose, eight months prior to necropsy and subcutaneous injections of vehicle (1 ml sterile phosphate buffered saline), twice daily for four months, ceasing four months prior to necropsy. Neurologically these animals were normal. Respiratory rate, heart rate, pulse oximetry and ejection fraction were within normal limits until euthanasia.
Animal husbandry and experimental procedures are detailed elsewhere (7). All procedures were approved by the Wake Forest University School of Medicine Institutional Animal Care and Use Committee (IACUC) and were in accordance with the Guide for Care and Use of Laboratory Animals. The Wake Forest University School of Medicine is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) and adheres to all state and federal animal welfare laws.
Irradiation
Irradiation procedures for both groups of animals are described elsewhere (29). Animals receiving fWBI were administered a total of 40 Gy mid-plane (8 fractions × 5 Gy/fraction; 2 fractions/week × 4 weeks; nominal dose rate of 4.9–5.4 Gy/min; biological effective dose for α/β of × Gy, BED3, of 106.7 Gy) using a clinical linear accelerator and opposed lateral fields of 6 MV X rays. These fields were designed to match human whole-brain radiation fields, enclosing the cranial contents (nominal field size of 1137 cm2, field edge tangential to the base of skull) with the central axis of each lateral beam placed at the respective outer canthus. The eyes and olfactory region were shielded from radiation by cylindrical eye blocks. fWBI is originally described in Robbins et al. (15) and further described in Hanbury et al.(7). NHP receiving fWBI were sedated with ketamine HCl (15 mg/kg body weight, intramuscularly) and maintained on isoflurane gas (3% induction, 1.5% maintenance) in 100% oxygen during irradiation. Control comparators received 10 Gy single dose thoracic irradiation delivered to the anterior-posterior thoracic mid-plane as part of a previous study. The maximum dose administered to the brain is estimated to be less than 0.1 Gy (<1%). X rays (6 MV) were delivered with parallel opposed anterior-posterior fields from a clinical linear accelerator at a nominal dose rate of 4 Gy/min. The irradiated volume included the heart, mediastinum and superior, lateral and inferior lung fields extending 4 cm below the xyphoid.
Tissue Collection and Histopathology
Tissue collection, processing and archival procedures were the same for all subjects. At the time of necropsy, the animals were humanely euthanized in accordance with the American Veterinary Medical Association’s Guidelines on Euthanasia (31) by deep anesthesia with pentobarbital followed by exsanguination and perfusion of the vascular system with 2 l of cold normal saline. The brain was removed intact, and sectioned coronally in 4-mm intervals using a stainless steel brain matrix with cutting guides. Once removed from the matrix, all slices were photographed. Alternating sections were either immediately frozen on dry ice or immersed in 4% cold paraformaldehyde for 24 h.
Fixed tissues were embedded in paraffin and sectioned coronally at 4 μm, then stained with hematoxylin and eosin (H&E). All animals were assessed by a board certified veterinary pathologist (JMC) who was blinded to the irradiation status of all animals. Each animal received a full histopathologic assessment in accordance with the Society for Toxicologic Pathology’s Recommended Practices for Sampling and Processing the Nervous System (32) with the addition of a section of prefrontal cortex containing Brodmann area 46. A minimum of eight tissue sections were reviewed, and structures included but were not limited to: cerebral cortex, subcortical and deep white matter structures, striatum, hippocampus, thalamus, midbrain, cerebellum and brainstem. The presence of necrosis, inflammatory changes and vascular lesions was tabulated and scored. Histopathologic scoring is detailed elsewhere (29). In summary, white matter lesions were scored from 0–4 as follows: absent (0), minimal (1 = inflammatory or vascular changes without disruption of the neuropil or clear neuronal loss); mild (2 = focal vascular injury and inflammation with loss of neuropil or neurons and microglial activation); moderate (3¼extensive or multifocal vascular injury, hemorrhage, disruption of the neuropil, neuronal loss and microglial activation); or severe (4 = extensive or multifocal vascular injury, hemorrhage, disruption of the neuropil, neuronal loss and microglial activation, with additionally extensive zones of necrosis within neural tissue). Vascular lesions within regions were tabulated as follows: a: endothelial hypertrophy; b: perivascular extracellular matrix deposition, c: perivascular edema, d: haphazard angiogenesis.
Special Stains, Immunohistochemistry and RNAScopet®
Formalin-fixed, paraffin-embedded brain tissues containing deep white matter (centrum semiovale) were sectioned coronally at 4 μm. Immunohistochemistry (IHC) and RNAScope in situ hybridization assays were performed on a Leica Bond RX (Leica Biosystems, Buffalo Grove, IL) automated stainer. Sections from animals receiving fWBI and control comparators were stained concurrently.
The composition of perivascular ECM was evaluated by IHC for fibronectin (rabbit polyclonal; 1:100), collagens I (rabbit monoclonal; 1:1,000) and IV (rabbit polyclonal; 1:300), all acquired from Abcamt, (Cambridge, MA). Slides were air-dried overnight and loaded onto the Leica Bond RX. All sections were dewaxed, rinsed and underwent heat-induced epitope retrieval for 20 min at 99°C in the following solutions: fibronectin, citrate buffer (pH 6.0); collagen-I and IV, EDTA buffer (pH 8.0). Endogenous peroxidase activity was blocked by 10-min incubation with IHC/ISH Super Blocking Novocastra solution (Leica Biosystems). All slides were incubated with the primary antibody for 15 min and then rinsed. Secondary antibody and chromogen application were as according to manufacturer’s specifications (Bond Polymer Refine Red Detection Kit; Leica Biosystems). Slides were then removed from the instrument, dehydrated, cleared with xylene and coverslipped.
Amyloid was detected with Putchler’s modification of Bennhold’s Congo Red stain. Paraffin-embedded, formalin-fixed brain tissues were sectioned coronally at 10 μm. Positive identification of amyloid was defined as green birefringence under polarized light.
Fibronectin transcripts were visualized by in situ hybridization RNAScope 2.5 Assay, with concurrent visualization of the cerebral microvasculature by anti-GLUT1 IHC. In situ hybridization was performed according to manufacturer’s specifications, using a RNAScope 2.5 LS Reagent Kit - Brown [Advanced Cell Diagnostics (ACD™), Newark, CA) and standard Leica reagents as follows. Slides were loaded onto the Leica Bond RX, then dewaxed, followed by heat-induced epitope retrieval in EDTA buffer pH 8.0 at 958C for 15 min. Enzyme-induced epitope retrieval was completed in ACD protease III for 15 min at 40°C, followed by quenching of endogenous peroxidase by 10-min incubation in hydrogen peroxide at room temperature. A custom-made probe optimized for the rhesus macaque FN1 sequence (1289–2251 of XM_015110901.1) was applied for 2 h at 428C followed by an amplification series at 42°C and subsequent probe labeling at room temperature. Slides were stained with Bond Polymer Refine Detection (ACD) for 20 min then rinsed. Slides were counterstained with hematoxylin for 5 min and rinsed, followed by ACD Bluing for 2 min.
Antibodies were stabilized in preparation for IHC by 10-min incubation with IHC/ISH Super Blocking (Leica Biosystems). Slides were then incubated for 15 min with a rabbit polyclonal anti-glucose transporter GLUT1 antibody (rabbit polyclonal, 1:1,000; Abcam) and rinsed. The secondary antibody and chromogen were visualized with the Bond Polymer Refine Red Detection Kit or when run without concurrent in situ hybridization, Bond Polymer Refine Detection and applied according to manufacturer specifications. Slides were then removed from the instrument, dehydrated, cleared with xylene and coverslipped.
Histomorphometry
Digital images were captured using an Olympus BX61VS virtual slide microscope with VS120 software at 20× magnification. Microvessel density, cross-sectional microvascular area fraction, and in situ hybridization probe density and probe area were quantified on the same tissue section. All parameters were quantified from a single tissue section within deep white matter (centrum ovale) within a rectangular region of interest (ROI) no less than 15 mm2 (equivalent to a minimum of sixty 40× high-power fields). Microvessel density and cross-sectional microvascular area fraction were computed in VisioPharm version 6.5.0.2303 (Broomfield, CO) using the following modifications to APP no. 10022 (‘CD31, Tumor Neovascularization’) and an unbiased counting frame. The microvasculature was identified using an AEC_DAB-DAB-input channel refined to exclude brown objects and the recognition algorithm was thresholded to exclude all background objects. Microvessel density was defined as the number of microvascular profiles per mm2. Cross-sectional microvascular area fraction was defined as the total area occupied by blood vessels including lumina divided by the total area (area within blood vessels/total area of the ROI) and expressed as percentage area (microvascular area fraction * 100). The crosssectional area for each microvessel profile was also recorded, and analyses are detailed in the statistical methods.
Microvascular profiles generated by the previous analysis were converted to a single ROI (vascular) with the remaining surrounding area defined as parenchyma. Fibronectin in situ hybridization probe foci were identified using a Fast Red_DAB-DAB-input channel and the recognition algorithm was thresholded to exclude all nonbrown objects. Probe density was calculated as the number of probe foci per mm2. Cumulative probe area was defined as (cumulative probe area/ anatomic subcompartment ROI area * 100) μm2. The cross-sectional area for each probe aggregate was also recorded. The assessor was blinded to the irradiation status of the animals.
Transmission Electron Microscopy (TEM)
The cerebral microvasculature was visualized in archived, formalinfixed, 4-mm coronal sections of brain tissue by transillumination on a dissecting microscope. The surrounding 3 mm3 were sub-dissected then post-fixed in 2% osmium tetroxide in phosphate buffer for 1 h, followed by dehydration in EtOH. Samples were prepared for resin infiltration by incubation in propylene oxide then gradually infiltrated with 1:1, 1:2 and pure solutions of Spurr’s resin, and cured in a 70°C oven overnight. Sections (90 nm) were obtained using a Reichert-Jung Ultracut E ultramicrotome (Reichert-Jung; Vienna, Austria), stained with lead citrate and uranyl acetate and viewed with an FEI Tecnaie Spirit TEM (Hillsboro, OR) operating at 80 kV. Images were acquired with an AMT 2Vu CCD camera (Advanced Microscopy Techniques; Woburn, MA).
Gene Expression Analyses
Relative gene expression was evaluated by RT-qPCR within three brain regions [dorsolateral prefrontal cortex (Brodmann area 46), hippocampus, and deep temporal white matter (centrum semiovale)] from all animals and conducted on the same samples as in Andrews et al. (29). Additional factors were selected for their involvement in ECM deposition and remodeling, and RAAS or Rho/ROCK signaling (Table 1). Gene names are reported in accordance with the recommendations of the HUGO Gene Nomenclature Committee (HGNC).
TABLE 1.
Classification of Markers in RT-qPCR Profiler Gene Expression Array
| Renin-angiotensin system | Rho/ROCK | Extracellular matrix deposition and remodeling | |
|---|---|---|---|
| HGNC | ACE | ROCK1 | MMP9 |
| Symbol | ACE2 | ROCK2 | CTGF |
| AGT | RHOA | ITGAV | |
| AGTR1 | CDC42 | ITGB1 | |
| MME | RAC1 | ACTA2 | |
| MAS1 | COL1A1 |
RNA was extracted using a modified QIAGEN® RNeasy Mini kit, purification, quality control and RT-qPCR procedures are detailed in Andrews et al. (29). RT-qPCR was performed on an ABI 7500 Fast system (Applied Biosystemst, Carlsbad, CA) using a QIAGEN Custom RT2 Profiler Array utilizing Rhesus macaque specific primers. Cycle thresholds (Ct) were acquired for all target and housekeeping genes per sample; the limit of detection was set to 35 Ct. Relative gene expression (ΔCt) for each target gene was determined by normalization of the target gene Ct to the geometric mean of two housekeeping genes (β-actin and hypoxanthine-guanine phosphoribosyltransferase). Relative expression was calculated as 2–(Target gene – geometric mean of housekeeping genes). Calculation of fold change (2–ΔΔCt) was as described by Livak (33).
Statistical Analyses
Statistical analyses were performed under the guidance of a statistician and using GraphPad version 7.03 (StataCorp LP, College Station, TX) unless otherwise noted. Normality and equality of variance were assessed by Shapiro-Wilk and Levene’s tests, respectively. The effect of fWBI on cumulative microvascular area fraction and microvessel density was compared using Student’s t test.
Two separate, 2 × 2 repeated-measures analysis of variance (ANOVA) were conducted to compare the main effects of irradiation status and anatomic location (vascular and parenchymal) and the interaction effect between irradiation status and anatomic location on in situ hybridization probe focus density and cumulative probe focus area, respectively. Samples were matched by subject with respect to anatomic location (e.g., vascular and parenchymal compartments were measured within the same animal). Post hoc comparisons were performed to assess the effect of radiation within regions using Bonferroni’s procedure. Multiplicity adjusted P values are reported and P < 0.05 was considered significant.
As values for individual subjects were similarly distributed within irradiation groups, individual probe focus areas and individual microvascular profile areas were pooled within groups with respect to irradiation status. Differences in distribution between groups were confirmed by Kolmogorov-Smirnov test. The effect of radiation was compared between groups by Mann-Whitney U due to right-skew. Individual probe focus area data were stratified by region. P values < 0.05 were considered significant.
Individual probe focus areas and individual microvascular profiles are reported as median with 95% confidence intervals (CI). Microvascular area fraction, microvessel density and cumulative probe focus area are reported as mean with 95% CI. Probe focus density data were square-root-transformed to achieve normality, then back-transformed. Geometric mean and 95% CI are reported.
Gene expression analyses with respect to irradiation status were stratified by region and were evaluated as follows: non-parametric data were compared by Mann Whitney U. Normally distributed data were compared by Student’s t and Welch’s tests for data with equal and unequal variances, respectively. Significance was determined at P < 0.01.
RESULTS
Histopathology
All animals receiving fWBI developed multifocal cerebrovascular and white matter injury consistent with latedelayed RIBI. Notable lesions included perivascular edema with serum protein extravasation, perivascular ECM aggregation and white matter necrosis. The cerebrovascular lesions were multifocal, spatially-stochastic and occurred primarily within white matter. Multifocal accumulation of perivascular ECM was observed in all animals receiving fWBI. These lesions were noted in the white matter (4/4 animals), cortical gray matter (1/4 animals), hippocampus (1/4 animals), thalamus (2/3 animals), midbrain (2/4 animals), brainstem (2/3 animals) and spinal cord (1/3 animals); a denominator less than 4 indicates the tissue section was not available for analysis. Full histopathologic assessment is detailed elsewhere (29). No significant lesions were present in control comparators.
Characterization of Perivascular ECM
Fibronectin preferentially localized to the pia mater, arachnoid mater and basement membrane zone of morphologically normal blood vessels in both groups (Figs. 1A–B and 2D). Additional irregular, regionally-extensive parenchymal fibronectin immunoreactivity was observed in areas of vascular injury in animals receiving fWBI (Fig. 1B), and interpreted as vascular leakage. Fibronectin was a major contributor to the perivascular material in edematous (Fig. 2E) and fibrotic vascular morphologies (Fig. 2F). In edematous vascular morphologies, the material within the perivascular space was also immunoreactive for collagen-I (Fig. 2H) and collagen-IV (Fig. 2K).
FIG. 1.
Distribution of perivascular ECM contributors in radiation-induced brain injury (RIBI; low magnification). Panel A: Fibronectin IHC; control. Frontal cortex and subtending white matter. Fibronectin (red) localized to the vascular basement membrane zone, pia and arachnoid. Panel B: Fibronectin IHC; fWBI. Frontal cortex and subtending white matter. The distribution of fibronectin (red) was similar to that in the control animals (panel A), with additional regionally extensive parenchymal staining adjacent to foci of necrosis and associated abnormal vasculature (black arrows). Panel C: Collagen-I IHC; control. Subcortical white matter, temporal cortex. The microvasculature lacked collagen-I (red). Panel D: Collagen-I IHC; fWBI. Subcortical white matter, temporal cortex. Collagen-I (red) localized to the basement membrane and wall of occasional small caliber arterioles and capillaries (black arrows), and was absent in others (white arrows). Panels E and F: Collagen-IV IHC; control and fWBI, respectively. Subcortical white matter, temporal cortex. Collagen-IV (red) localized to the basement membrane zone of the cerebral microvasculature, and the distribution was comparable between control and fWBI groups. All slides were counterstained with hematoxylin.
FIG. 2.
Distribution of perivascular ECM contributors in vascular pathomorphologies in radiation-induced brain injury (high magnification). Left-side column shows morphologically normal microvessels (control). Center column shows edematous vascular pathomorphology, fWBI. Material within the perivascular space was immunoreactive for fibronectin (panel E), collagen-I (panel H) and collagen-IV (panel K). Right-side column shows perivascular fibrosis, fWBI. Panels A–C: Morphology of vascular injury in RIBI (H&E stained). Panel A: Normal microvessel, control. Minimal expansion of the perivascular space (black arrows, glia limitans). Panel B: Edematous vascular morphology, fWBI. Prominent expansion of the perivascular space (black arrows, glia limitans) by eosinophilic material interpreted as plasma extravasation. The adjacent neuropil is edematous and contains several reactive (gemistocytic) astrocytes (asterisk). Panel C: Fibrotic vascular morphology, fWBI. Prominent expansion of the perivascular space (black arrows, glia limitans) by loose, fibrillar extracellular matrix. Panels D–F: Fibronectin immunohistochemistry. Fibronectin (red) localized to the basement membrane zone in morphologically normal microvessels (panel D) and was present throughout the perivascular space in edematous (panel E) and fibrotic vascular phenotypes (panel F) in animals receiving fWBI. Panels G–I: Collagen-I immunohistochemistry. Collagen-I (red) localized adjacent to the basement membrane; it was scant to absent in control comparators (panel G) and diffuse in animals receiving fWBI (panel I). Panels J–L: Collagen-IV immunohistochemistry. Collagen-IV localized to the vascular wall and basement membrane in all vascular phenotypes (panels J–L); distribution was comparable between fWBI animals and control comparators
Collagen-I localized adjacent to the basement membrane zone and was scant to absent in the subcortical white matter of control animals (Figs. 1C and 2G). Diffuse, basement membrane zone staining for collagen-I was present in scattered, otherwise normal-appearing small caliber arterioles and capillaries (Fig. 1D) and fibrotic vascular phenotypes (Fig. 2I) of animals receiving fWBI.
Collagen-IV localized to the microvascular wall and basement membrane zone of the cerebral vasculature in both groups (Figs. 1E, F and 2J, L). The distribution was comparable between both groups. TEM of affected vessels demonstrated medium electron-dense, fibrillar material without regular periodicity within Virchow-Robbin’s space (Fig. 3). This material did not stain for amyloid.
FIG. 3.
Expansion of Virchow-Robin space by perivascular ECM. Panel A: Transmission electron micrograph (TEM) indicating abundant fibrillar extracellular material surrounding a blood vessel (*). This fibrillar material lacks the regular periodicity of type I collagen. In addition, Virchow-Robbin’s space also contains a few lymphocytes and macrophages containing phagocytosed debris consistent with hemosiderin (M). Panel B: H&E stain, corresponding histopathology indicating aggregated perivascular ECM, as well as perivascular inflammatory cells, as in panel A. Black arrows indicate the glia limitans.
Localization of Fibronectin mRNAs by In Situ Hybridization
fWBI resulted in a prominent induction of FN1 expression in the vascular compartment (Fig. 4B, endothelial cells Fig. 4C, and vascular smooth muscle cells Fig 4D) with a 1,259-fold increase in anatomic subcompartment fractional area (P < 0.006), a 206-fold increase in mean probe focus density (P < 0.005) and a twofold individual probe aggregate area (P < 0.002; Fig. 4E). FN1 expression was also increased within in the parenchyma (glia) after fWBI, albeit to a lesser extent, with increases in mean probe focus density (18-fold increase, P < 0.03; Fig. 4E) and median probe aggregate area (1.3-fold increase, P < 0.0001; Fig. 4E), but no difference in cumulative anatomic subcompartment fractional area. There was a significant interaction effect between irradiation status and anatomic subregion for both probe focus number (P < 0.03) and cumulative percent area (P < 0.04).
FIG. 4.
fWBI was associated with prominent induction of FN1 expression with predilection for the vascular compartment. Panel A: Control comparator; normal microvessels, fibronectin transcripts (brown) are rare. Panel B: fWBI-receiving animal; fibronectin transcripts primarily localized to the vascular compartment. Panel C: fWBI-receiving animal; fibronectin transcripts localized to cerebrovascular endothelial cells (black arrows; red: GLUT-1 IHC), and to (panel D) vascular smooth muscle cells (white arrows; red: α-smooth muscle actin). Panel E: Within the vascular compartment, fWBI was associated with a 1,259-fold increase in fibronectin transcript fractional area due to an 18-fold increase in probe density and 1.3-fold increase in median individual probe focus area. Expression after fWBI was lesser within the parenchymal compartment (glia), with an 18-fold increase in mean probe focus density and 1.3-fold increase in median individual probe focus area.
Transcript aggregates greater than 40 lm2 were present only in animals receiving fWBI and largest within the vascular compartment (median: 1.901 μm2, range: 0.2– 321.1 μm2; P < 0.0001). Fibronectin transcripts were rarely expressed in nonirradiated cerebrovascular cells (geometric mean: 0.4 foci per mm2; 95% CI: 0.0–1.2) and glia (geometric mean: 2.4 foci per mm2; 95% CI: 1.3–3.8).
Microvascular Area Quantification
RIBI was associated with a shift in microvessel crosssectional area distribution (P < 0.0001; KolmogorovSmirnov test; Fig. 5A), resulting in a 57.4% increase in median microvessel cross-sectional profile area (P < 0.0001; Fig. 5D). This corresponded to dilated small caliber blood vessels on histopathology (Fig. 5B and C). There was no significant difference in cumulative microvascular area fraction or microvessel density between groups.
FIG. 5.
fWBI resulted in a shift in microvessel cross-sectional area distribution (panel A); histologically manifested an increase in dilated small caliber vessels in animals receiving fWBI (panel C), as compared to control animals (panel B). Correspondingly, there was a 57.4% increase in median cross-sectional microvessel area per vascular profile, cumulative microvascular area fraction and microvessel density did not differ between groups (panel D). Due to marked right skew, panel A was truncated at 360 μm2 (range: 0.2–7,284.6 μm2). Bars indicate the mean for microvascular area fraction and microvessel density, and median for microvessel profile area. Error bars: 95% CI. KS = Kolmogorov-Smirnov test.
Gene Expression Assessment of Contributing Molecular Pathways
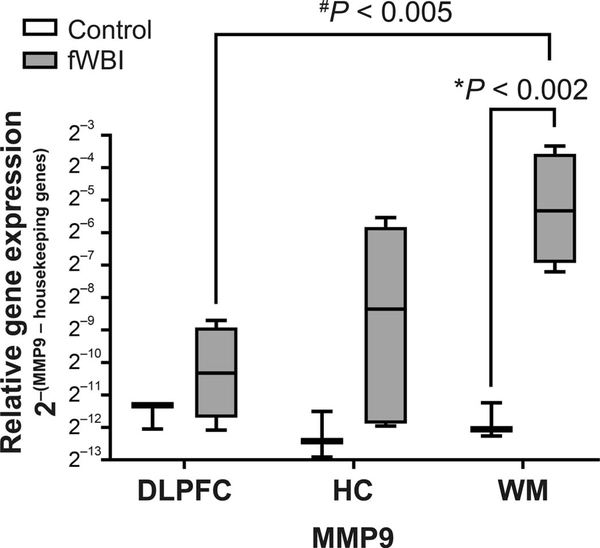
Late-delayed RIBI was associated with an 88-fold increase in MMP9 expression within temporal white matter (P < 0.002; Fig. 6). There were no differences in gene expression in members of the Rho/ROCK (ROCK1, ROCK2, RHOA, CDC42 or RAC1) or RAAS signaling pathways (ACE, ACE2, AGT, AGTR1, MME or MAS1) or other ECM-associated molecules (CTGF, ITGAV, ITGB1, ACTA2 or COL1A1) within any region examined.
FIG. 6.
Late-delayed RIBI was associated with an 88-fold increase in MMP9 expression within temporal white matter (WM; centrum semiovale). The center line denotes the median relative expression value; bar limits indicate the 25th and 75th percentiles. Error bars represent minimum and maximum values. Relative expression was defined as 2–[(MMP9 – geometric mean of housekeeping genes (ACTB and HPRT1)]. *P value indicates with the effect of radiation on relative-gene expression. #P value signifies differences in regional gene expression with respect to irradiation status. DLPFC = dorsolateral prefrontal cortex (Brodmann area 46); HC = hippocampus.
DISCUSSION
Here we have demonstrated that fibronectin is a significant contributor to perivascular ECM in late-delayed RIBI, and after fWBI, expression of the encoding gene FN1 is strongly induced within cerebrovascular endothelial and smooth muscle cells.
Fibronectin is an approximately 440-kDa glycoprotein and major component of ECM. The majority of fibronectin is produced by the liver and circulates in the plasma as dimeric, soluble fibronectin. Fibronectin may also be secreted locally by endothelial cells, fibroblasts, chondrocytes, synovial cells and myocytes (34, 35). Assembly into an insoluble fibrous matrix is a cell-mediated process, facilitated by binding of fibronectin to integrin α5β1 with subsequent induction of cytoskeletal contraction (36, 37). Resultant clustering of integrins on the cell surface brings multiple fibronectin dimers into close proximity, promoting fibronectin-fibronectin interactions, self-association, fibril elongation and matrix assembly. The assembled fibronectin matrix may then further promote fibrosis through association with collagen fibrils, other ECM contributors and resultant cell-ECM interactions (38). Spontaneous folding and rearrangement of secondary elements within assembled fibronectin matrix intermittently exposes additional binding sites (39, 40), thus providing opportunity to modify deposited ECM and represents an appealing target for interventional therapies.
Fibronectin transcripts were identified with cerebrovascular endothelial cells, vascular smooth muscle and glia. A significant interaction effect between irradiation status and anatomic subcompartment was detected for both fibronectin probe density and fractional area. As the magnitude of change in expression was greatest within the vascular subcompartment, we infer that upregulation of fibronectin occurs with predilection for the cerebral microvasculature (within endothelial cells and vascular smooth muscle cells). This is supportive of a differing radiobiology between the two anatomic subcompartments. Cellular morphology and proximity to areas of necrosis suggest that the parenchymal (glial) contributor to fibronectin expression is the activated microglia/macrophage. Definitive identification of the cell of origin is the focus of future studies. Others have demonstrated that activated macrophages produce fibronectin (41, 42) and that fibronectin induces microglial activation (43, 44). We theorize that fibronectin-induced macrophage/microglial activation may contribute to the persistent neuroinflammation in RIBI via an autocrine signaling loop.
In the current study, we establish that fibronectin is a major contributor to perivascular fibrous ECM with lesser amounts of perivascular collagen-I and IV, as evidenced by immunohistochemistry and supported by TEM. The differential expression of perivascular collagen-I and collagen-IV immunoreactivity between edematous and fibrotic vascular phenotypes suggests transient accumulation of pre-fibrillar collagen precursors during lesion progression. Clearance of these contributors is presumed to be partially mediated by radiation-induced matrix metalloprotease expression, particularly MMP2 and MMP9 (29, 45–48), a matrix metalloprotease which is induced by fibronectin (49, 50), and functions to degrade collagen-IV (51).
Previously published studies in rodent and cell culture models indicate MMP9 expression is increased at 4 h to one week after single doses of radiation (45–48).We reaffirm that radiation exposure induces the expression of MMP9, and furthermore, translate these findings to a clinically relevant, non-human primate model of fractionated wholebrain radiotherapy.
In their published study, Kyrkanides et al. (47) showed that COX2-selective inhibition prior to and after irradiation reduces MMP9 expression. Therefore, persistent neuroinflammation may contribute to ECM remodeling through MMP9-mediated effects. We also hypothesize that MMP9mediated basement membrane degradation facilitates extravasation of soluble fibronectin, providing additional substrate for matrix assembly. Blood-brain-barrier insufficiency as a consequence of fWBI has been demonstrated previously (52–55). While the proportion of extravasated soluble fibronectin to locally produced cellular fibronectin remains undetermined, both are presumed to contribute to perivascular fibronectin accumulation and thus play a role in the pathogenesis of RIBI.
We also affirm that perivascular ECM deposition occurs without corresponding increase in white matter microvascular density, indicating increased accumulation of matrix, rather than a reflection of enhanced microvascular investiture. Interestingly, while overall microvascular area fraction did not differ between irradiation and control comparators, there was a shift in individual microvascular profile distribution, resulting in a greater median individual microvessel profile area. This was unexpected, since fibronectin matrix assembly is typically associated with cytoskeletal contracture rather than dilation, as contraction is necessary for integrin clustering, which facilitates fibronectin-fibronectin interactions and subsequent assembly. Artefactual vasodilatation due to perfusion of the vascular system with saline at the time of necropsy is unlikely, given that the necropsy procedure for animals receiving fWBI and control comparators was identical. We postulate that the difference in vessel size may reflect defects in cytoskeletal coordination or contractile ability within endothelial cells or their adjacent mural contributors (vascular smooth muscle, pericytes), or a vasodilatory compensatory response to a hypoxic environment. Selective capillary loss may also contribute to the shift in microvessel profile area.
Additional irregular, regionally extensive background staining for fibronectin in irradiated animals was suggestive of blood-brain-barrier insufficiency and exudation of plasma-containing fibronectin into the adjacent parenchyma, as mentioned previously. In addition to MMP9-mediated basement membrane degradation, other published studies have indicated that radiation-induced cytoskeletal rearrangement and concomitant contraction of the cell membrane results in disruption of junctional apposition (56–58), a mechanical process that may not be reflected at the transcriptional level. Previously reported studies suggest that radiation-induced cytoskeletal contracture may be mediated by activation of the Rho/ROCK pathway as part of the acute endothelial radiation response (57, 59–62). Radiation-modulated endothelial-fibronectin interactions occur in a RhoA/ROCK-dependent manner (63). While our gene expression analyses did not detect changes in the Rho/ROCK family member mRNAs, activation of RhoA is primarily regulated at the protein level by phosphorylation. Thus, functional alterations may not have been detectable by our transcriptional analyses. A recently published study demonstrated decreased phosphorylated RhoGDI in irradiated endothelial cells (0.5 Gy, 1–7 days postirradiation) (59). RhoGDI functions to sequester RhoA and prevent its activation, and may be a promising target for future mechanistic analyses.
The role of fibronectin in RIBI may be particularly relevant in the tumor-bearing brain. Increased fibronectin extravasation due to the permeability of tumor-associated vasculature (64–68), and local production by glioma cells (69–71) may lead to increased fibronectin substrate available for matrix assembly. This could potentially exacerbate RIBI. The data regarding the influence of fibronectin on glioma cell migration are conflicting (71–75); however, in a mouse glioma model, it was shown that short hairpin RNA (shRNA)-mediated fibronectin knockdown resulted in delayed tumor growth (76). These data suggest that signaling mechanisms that modulate fibronectin deposition and matrix assembly may be relevant targets for therapeutic intervention.
Potential limitations to this study include a history of prior thoracic irradiation in the control comparators, and the relative younger age of the evaluated NHP compared to aged human populations receiving fWBI. Although control comparators received 10 Gy thoracic irradiation eight months prior to necropsy as part of a separate study, the estimated dose to the brain is far lower (≤0.1 Gy). There is little information regarding the long-term consequences of single, low-dose irradiation in the adult brain. Published studies in rodents suggest that doses of ≤0.1 Gy do not significantly impair spatial learning or memory (77) and that cerebrovascular permeability returns to normal by one month postirradiation (78). Retrospective analyses of the Chernobyl and atomic bomb survivor cohorts suggest that increased risk of cerebrovascular injury occurs at doses 0.15–0.5 Sv (79–82). The cerebral consequences of radiation-induced cardiopulmonary injury in these animals are presumed to be minimal. Clinical data regarding cardiac and respiratory function prior to death were carefully reviewed prior to use of archived tissue. Respiratory rate, heart rate, pulse oximetry and ejection fraction were within normal limits until euthanasia.
The young adult NHP used in this study may not fully recapitulate the aged patient population (approximate age: 60 years) (83–85) receiving fWBI. Survival is poorer in aged patient subpopulations than younger comparators; however, these findings are confounded by comorbid neurologic and systemic health conditions, tumor progression and treatment regimen. Data regarding the role of fibronectin in the aging brain are conflicting (86–88) and warrant additional study in the context of neurodegenerative diseases generally.
In conclusion, we have demonstrated that the fibronectin is preferentially produced by cerebrovascular endothelial and smooth muscle cells, and is a major component of perivascular fibrous ECM in late-delayed RIBI. The dynamic nature of the fibronectin extracellular scaffold (39, 40) suggests that even chronically established ECM containing fibronectin may be susceptible to remodeling. Thus, we propose that altering the cellular and molecular dynamics of fibronectin matrix assembly to favor degradation may represent a potential therapeutic target for latedelayed RIBI and other fibrotic conditions in which fibronectin is a contributor.
ACKNOWLEDGMENTS
We thank Lisa O’Donnell and Cathy Mathis for their indispensable enthusiasm, histologic expertise and unending support. This work was supported in part by a National Cancer Institute Cancer Center Support Grant (P30CA012197) to the Comprehensive Cancer Center of Wake Forest Baptist Medical Center, and the North Carolina Biotechnology Center (2015-IDG-1006). We also acknowledge the support of the Comparative Pathology Laboratory Shared Resources of the Comprehensive Cancer Center of Wake Forest Baptist Medical Center. TEM was obtained with the assistance of the WFUSM Cellular Imaging Shared Resource. Additional support was provided by the NCI (grant no. 1R01CA155293–01A1 to SAD) and the National Institutes of Health (NIH grant no. T32 OD010957 to RNA).
REFERENCES
- 1.Maher EA, Mietz J, Arteaga CL, DePinho RA, Mohla S. Brainmetastasis: opportunities in basic and translational research. Cancer Res 2009; 69:6015–20. [DOI] [PubMed] [Google Scholar]
- 2.Crossen JR, Garwood D, Glatstein E, Neuwelt EA. Neurobehav-ioral sequelae of cranial irradiation in adults: a review of radiationinduced encephalopathy. J Clin Oncol 1994; 12:627–42. [DOI] [PubMed] [Google Scholar]
- 3.Meyers CA, Brown PD. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J Clin Oncol 2006; 24:1305–9. [DOI] [PubMed] [Google Scholar]
- 4.Laukkanen E, Klonoff H, Allan B, Graeb D, Murray N. The role of prophylactic brain irradiation in limited stage small cell lung cancer: clinical, neuropsychologic, and CT sequelae. Int J Radiat Oncol Biol Phys 1988; 14:1109–17. [DOI] [PubMed] [Google Scholar]
- 5.Mabbott DJ, Noseworthy MD, Bouffet E, Rockel C, Laughlin S. Diffusion tensor imaging of white matter after cranial radiation in children for medulloblastoma: correlation with IQ. Neuro Oncol 2006; 8:244–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chapman CH, Nagesh V, Sundgren PC, Buchtel H, Chenevert TL, Junck L, et al. Diffusion tensor imaging of normal-appearing white matter as biomarker for radiation-induced late delayed cognitive decline. Int J Radiat Oncol Biol Phys 2012; 82:2033–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanbury DB, Robbins ME, Bourland JD, Wheeler KT, Peiffer AM, Mitchell EL, et al. Pathology of fractionated whole-brain irradiation in rhesus monkeys (Macaca mulatta). Radiat Res 2015; 183:367–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caveness WF. Pathology of radiation damage to the normal brain of the monkey. Natl Cancer Inst Monogr 1977; 46:57–76. [PubMed] [Google Scholar]
- 9.Pennybacker J, Russell DS. Necrosis of the brain due to radiation therapy; clinical and pathological observations. J Neurol Neurosurg Psychiatry 1948; 11:183–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vigliani MC, Duyckaerts C, Hauw JJ, Poisson M, Magdelenat H, Delattre JY. Dementia following treatment of brain tumors with radiotherapy administered alone or in combination with nitrosourea-based chemotherapy: a clinical and pathological study. J Neurooncol 1999; 41:137–49. [DOI] [PubMed] [Google Scholar]
- 11.Price RE, Langford LA, Jackson EF, Stephens LC, Tinkey PT, Ang KK. Radiation-induced morphologic changes in the rhesus monkey (Macaca mulatta) brain. J Med Primatol 2001; 30:81–7. [DOI] [PubMed] [Google Scholar]
- 12.Oi S, Kokunai T, Ijichi A, Matsumoto S, Raimondi AJ. Radiation-induced brain damage in children–histological analysis of sequential tissue changes in 34 autopsy cases. Neurol Med Chir (Tokyo) 1990; 30:36–42. [DOI] [PubMed] [Google Scholar]
- 13.Hopewell JW, Wright EA. The nature of latent cerebral irradiation damage and its modification by hypertension. Br J Radiol 1970; 43:161–7. [DOI] [PubMed] [Google Scholar]
- 14.Kooistra M, Geerlings MI, Mali WP, Vincken KL, van der Graaf Y, Biessels GJ, et al. Diabetes mellitus and progression of vascular brain lesions and brain atrophy in patients with symptomatic atherosclerotic disease. The SMART-MR study. J Neurol Sci 2013; 332:69–74. [DOI] [PubMed] [Google Scholar]
- 15.Robbins ME, Bourland JD, Cline JM, Wheeler KT, Deadwyler SA. A model for assessing cognitive impairment after fractionated whole-brain irradiation in nonhuman primates. Radiat Res 2011; 175:519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakagaki H, Brunhart G, Kemper TL, Caveness WF. Monkey brain damage from radiation in the therapeutic range. J Neurosurg 1976; 44:3–11. [DOI] [PubMed] [Google Scholar]
- 17.Wakisaka S, O’Neill RR, Kemper TL, Verrelli DM, Caveness WF. Delayed brain damage in adult monkeys from radiation in the therapeutic range. Radiat Res 1979; 80:277–91. [PubMed] [Google Scholar]
- 18.Zhang K, Sejnowski TJ. A universal scaling law between gray matter and white matter of cerebral cortex. Proc Natl Acad Sci U S A 2000; 97:5621–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karbowski J Scaling of brain metabolism and blood flow in relation to capillary and neural scaling. PloS One 2011; 6:e26709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi L, Linville MC, Iversen E, Molina DP, Yester J, Wheeler KT, et al. Maintenance of white matter integrity in a rat model of radiation-induced cognitive impairment. J Neurol Sci 2009; 285:178–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown WR, Thore CR, Moody DM, Robbins ME, Wheeler KT.Vascular damage after fractionated whole-brain irradiation in rats. Radiat Res 2005; 164:662–8. [DOI] [PubMed] [Google Scholar]
- 22.Di Chiro G, Oldfield E, Wright DC, De Michele D, Katz DA, Patronas NJ, et al. Cerebral necrosis after radiotherapy and/or intraarterial chemotherapy for brain tumors: PET and neuropathologic studies. AJR Am J Roentgenol 1988; 150:189–97. [DOI] [PubMed] [Google Scholar]
- 23.Martins AN, Johnston JS, Henry JM, Stoffel TJ, Di Chiro G. Delayed radiation necrosis of the brain. J Neurosurg 1977; 47:336–45. [DOI] [PubMed] [Google Scholar]
- 24.Reinhold HS, Calvo W, Hopewell JW, van der Berg AP. Development of blood vessel-related radiation damage in the fimbria of the central nervous system. Int J Radiat Oncol Biol Phys 1990; 18:37–42. [DOI] [PubMed] [Google Scholar]
- 25.Dai Z, Aoki T, Fukumoto Y, Shimokawa H. Coronary perivascular fibrosis is associated with impairment of coronary blood flow in patients with non-ischemic heart failure. J Cardiol 2012; 60:416–21. [DOI] [PubMed] [Google Scholar]
- 26.Song JW, Do KH, Kim MY, Jang SJ, Colby TV, Kim DS. Pathologic and radiologic differences between idiopathic and collagen vascular disease-related usual interstitial pneumonia. Chest 2009; 136:23–30. [DOI] [PubMed] [Google Scholar]
- 27.Fukuoka JLK. Practical pulmonary pathology. A diagnostic approach. Philadelphia: Churchill-Livingstone; 2005. [Google Scholar]
- 28.Dellaripa PF. Pulmonary manifestations of rheumatic disease: a comprehensive guide. New York: Springer; 2014. [Google Scholar]
- 29.Andrews RN, Metheny-Barlow LJ, Peiffer AM, Hanbury DB, Tooze JA, Bourland JD, et al. Cerebrovascular remodeling and neuroinflammation is a late effect of radiation-induced brain injury in non-human primates. Radiat Res 2017; 187:599–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pankov R, Yamada KM. Fibronectin at a glance. J Cell Sci 2002;115:3861–3. [DOI] [PubMed] [Google Scholar]
- 31.Euthanasia AP. AVMA Guidelines for the euthanasia of animals.2013 ed. Schaumburg IL: American Veterinary Medical Association; 2013. [Google Scholar]
- 32.Bolon B, Garman RH, Pardo ID, Jensen K, Sills RC, Roulois A, et al. STP position paper: Recommended practices for sampling and processing the nervous system (brain, spinal cord, nerve, and eye) during nonclinical general toxicity studies. Toxicol Pathol 2013; 41:1028–48. [DOI] [PubMed] [Google Scholar]
- 33.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(–Delta Delta C(T)) method. Methods 2001; 25:402–8. [DOI] [PubMed] [Google Scholar]
- 34.To WS, Midwood KS. Plasma and cellular fibronectin: distinct and independent functions during tissue repair. Fibrogenesis Tissue Repair. 2011; 4:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mao Y, Schwarzbauer JE. Fibronectin fibrillogenesis, a cell-mediated matrix assembly process. Matrix Biol 2005; 24:389–99. [DOI] [PubMed] [Google Scholar]
- 36.Ruoslahti E, Obrink B. Common principles in cell adhesion. ExpCell Res 1996; 227:1–11. [DOI] [PubMed] [Google Scholar]
- 37.Danen EH, Sonneveld P, Brakebusch C, Fassler R, Sonnenberg A. The fibronectin-binding integrins alpha5beta1 and alphavbeta3 differentially modulate RhoA-GTP loading, organization of cell matrix adhesions, and fibronectin fibrillogenesis. J Cell Biol 2002; 159:1071–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dallas SL, Sivakumar P, Jones CJ, Chen Q, Peters DM, Mosher DF, et al. Fibronectin regulates latent transforming growth factorbeta (TGF beta) by controlling matrix assembly of latent TGF beta-binding protein-1. J Biol Chem 2005; 280:18871–80. [DOI] [PubMed] [Google Scholar]
- 39.Smith ML, Gourdon D, Little WC, Kubow KE, Eguiluz RA, Luna-Morris S, et al. Force-induced unfolding of fibronectin in the extracellular matrix of living cells. PLoS Biol 2007; 5:e268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohashi T, Augustus AM, Erickson HP. Transient opening of fibronectin type III (FNIII) domains: the interaction of the third FNIII domain of FN with anastellin. Biochemistry 2009; 48:4189–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alitalo K, Hovi T, Vaheri A. Fibronectin is produced by human macrophages. J Exp Med 1980; 151:602–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsukamoto Y, Helsel WE, Wahl SM. Macrophage production of fibronectin, a chemoattractant for fibroblasts. J Immunol 1981; 127:673–8. [PubMed] [Google Scholar]
- 43.Milner R, Crocker SJ, Hung S, Wang X, Frausto RF, del Zoppo GJ. Fibronectin- and vitronectin-induced microglial activation and matrix metalloproteinase-9 expression is mediated by integrins alpha5beta1 and alphavbeta5. J Immunol 2007; 178:8158–67. [DOI] [PubMed] [Google Scholar]
- 44.Kim H, Ahn M, Choi S, Kim M, Sim KB, Kim J, et al. Potential role of fibronectin in microglia/macrophage activation following cryoinjury in the rat brain: an immunohistochemical study. Brain Res 2013; 1502:11–9. [DOI] [PubMed] [Google Scholar]
- 45.Lee WH, Warrington JP, Sonntag WE, Lee YW. Irradiation altersMMP-2/TIMP-2 system and collagen type IV degradation in brain. Int J Radiat Oncol Biol Phys 2012; 82:1559–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nirmala C, Jasti SL, Sawaya R, Kyritsis AP, Konduri SD, Ali-Osman F, et al. Effects of radiation on the levels of MMP-2, MMP9 and TIMP-1 during morphogenic glial-endothelial cell interactions. Int J Cancer 2000; 88:766–71. [DOI] [PubMed] [Google Scholar]
- 47.Kyrkanides S, Moore AH, Olschowka JA, Daeschner JC, Williams JP, Hansen JT, et al. Cyclooxygenase-2 modulates brain inflammation-related gene expression in central nervous system radiation injury. Brain Res Mol Brain Res 2002; 104:159–69. [DOI] [PubMed] [Google Scholar]
- 48.Lee WH, Sonntag WE, Lee YW. Aging attenuates radiation-induced expression of pro-inflammatory mediators in rat brain. Neurosci Lett 2010; 476:89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Esparza J, Vilardell C, Calvo J, Juan M, Vives J, Urbano-Marquez A, et al. Fibronectin upregulates gelatinase B (MMP-9) and induces coordinated expression of gelatinase A (MMP-2) and its activator MT1-MMP (MMP-14) by human T lymphocyte cell lines. A process repressed through RAS/MAP kinase signaling pathways. Blood 1999; 94:2754–66. [PubMed] [Google Scholar]
- 50.Sen T, Dutta A, Maity G, Chatterjee A. Fibronectin induces matrixmetalloproteinase-9 (MMP-9) in human laryngeal carcinoma cells by involving multiple signaling pathways. Biochim 2010; 92:1422–34. [DOI] [PubMed] [Google Scholar]
- 51.Sung HJ, Johnson CE, Lessner SM, Magid R, Drury DN, Galis ZS. Matrix metalloproteinase 9 facilitates collagen remodeling and angiogenesis for vascular constructs. Tissue Eng 2005; 11:267–76. [DOI] [PubMed] [Google Scholar]
- 52.D’Avella D, Cicciarello R, Angileri FF, Lucerna S, La Torre D, Tomasello F. Radiation-induced blood-brain barrier changes: pathophysiological mechanisms and clinical implications. Acta Neurochir Suppl 1998; 71:282–4. [DOI] [PubMed] [Google Scholar]
- 53.d’Avella D, Cicciarello R, Albiero F, Mesiti M, Gagliardi ME, Russi E, et al. Quantitative study of blood-brain barrier permeability changes after experimental whole-brain radiation. Neurosurgery 1992; 30:30–4. [DOI] [PubMed] [Google Scholar]
- 54.Zhang J, Tong F, Cai Q, Chen LJ, Dong JH, Wu G, et al. Shenqi fuzheng injection attenuates irradiation-induced brain injury in mice via inhibition of the NF-kappaB signaling pathway and microglial activation. Acta Pharmacol Sin 2015; 36:1288–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tong F, Zhang J, Liu L, Gao X, Cai Q, Wei C, et al. Corilagin attenuates radiation-induced brain injury in mice. Mol Neurobiol 2016; 53:6982–96. [DOI] [PubMed] [Google Scholar]
- 56.Onoda JM, Kantak SS, Diglio CA. Radiation induced endothelial cell retraction in vitro: correlation with acute pulmonary edema. Pathol Oncol Res 1999; 5:49–55. [DOI] [PubMed] [Google Scholar]
- 57.Gabrys D, Greco O, Patel G, Prise KM, Tozer GM, Kanthou C. Radiation effects on the cytoskeleton of endothelial cells and endothelial monolayer permeability. Int J Radiat Oncol Biol Phys 2007; 69:1553–62. [DOI] [PubMed] [Google Scholar]
- 58.Kantak SS, Diglio CA, Onoda JM. Low dose radiation-induced endothelial cell retraction. Int J Radiat Biol 1993; 64:319–28. [DOI] [PubMed] [Google Scholar]
- 59.Azimzadeh O, Subramanian V, Stander S, Merl-Pham J, Lowe D, Barjaktarovic Z, et al. Proteome analysis of irradiated endothelial cells reveals persistent alteration in protein degradation and the RhoGDI and NO signalling pathways. Int J Radiat Biol 2017:1–9. [DOI] [PubMed] [Google Scholar]
- 60.Sriharshan A, Boldt K, Sarioglu H, Barjaktarovic Z, Azimzadeh O, Hieber L, et al. Proteomic analysis by SILAC and 2D-DIGE reveals radiation-induced endothelial response: four key pathways. J Proteomics 2012; 75:2319–30. [DOI] [PubMed] [Google Scholar]
- 61.Pluder F, Barjaktarovic Z, Azimzadeh O, Mortl S, Kramer A, Steininger S, et al. Low-dose irradiation causes rapid alterations to the proteome of the human endothelial cell line EA.hy926. Radiat Environ Biophys 2011; 50:155–66. [DOI] [PubMed] [Google Scholar]
- 62.Sriharshan A, Kraemer A, Atkinson MJ, Moertl S, Tapio S. Radiation-induced crosstalk between micrornas and proteins of the endothelium: In silico analysis. J Proteomics Bioinform 2014; 7:327–31. [Google Scholar]
- 63.Rousseau M, Gaugler MH, Rodallec A, Bonnaud S, Paris F, Corre I. RhoA GTPase regulates radiation-induced alterations in endothelial cell adhesion and migration. Biochem Biophys Res Commun 2011; 414:750–5. [DOI] [PubMed] [Google Scholar]
- 64.Hashizume H, Baluk P, Morikawa S, McLean JW, Thurston G, Roberge S, et al. Openings between defective endothelial cells explain tumor vessel leakiness. Am J Pathol 2000; 156:1363–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morikawa S, Baluk P, Kaidoh T, Haskell A, Jain RK, McDonald DM. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am J Pathol 2002; 160:985–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cai J, Jiang WG, Mansel RE. Phosphorylation and disorganization of vascular-endothelial cadherin in interaction between breast cancer and vascular endothelial cells. Int J Mol Med 1999; 4:191–5. [DOI] [PubMed] [Google Scholar]
- 67.Dvorak HF, Nagy JA, Dvorak JT, Dvorak AM. Identification and characterization of the blood vessels of solid tumors that are leaky to circulating macromolecules. Am J Pathol 1988; 133:95–109. [PMC free article] [PubMed] [Google Scholar]
- 68.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 2005; 307:58–62. [DOI] [PubMed] [Google Scholar]
- 69.Jones TR, Ruoslahti E, Schold SC, Bigner DD. Fibronectin and glial fibrillary acidic protein expression in normal human brain and anaplastic human gliomas. Cancer Res 1982; 42:168–77. [PubMed] [Google Scholar]
- 70.Wei KC, Huang CY, Chen PY, Feng LY, Wu TW, Chen SM, et al. Evaluation of the prognostic value of CD44 in glioblastoma multiforme. Anticancer Res 2010; 30:253–9. [PubMed] [Google Scholar]
- 71.Ohnishi T, Hiraga S, Izumoto S, Matsumura H, Kanemura Y, Arita N, et al. Role of fibronectin-stimulated tumor cell migration in glioma invasion in vivo: clinical significance of fibronectin and fibronectin receptor expressed in human glioma tissues. Clin Exp Metastasis 1998; 16:729–41. [DOI] [PubMed] [Google Scholar]
- 72.Ohnishi T, Arita N, Hiraga S, Taki T, Izumoto S, Fukushima Y, et al. Fibronectin-mediated cell migration promotes glioma cell invasion through chemokinetic activity. Clin Exp Metastasis 1997; 15:538–46. [DOI] [PubMed] [Google Scholar]
- 73.Deryugina EI, Bourdon MA. Tenascin mediates human glioma cell migration and modulates cell migration on fibronectin. J Cell Sci 1996; 109:643. [DOI] [PubMed] [Google Scholar]
- 74.Enam SA, Rosenblum ML, Edvardsen K. Role of extracellular matrix in tumor invasion: migration of glioma cells along fibronectin-positive mesenchymal cell processes. Neurosurgery 1998; 42:599–607; discussion: 8. [DOI] [PubMed] [Google Scholar]
- 75.Sabari J, Lax D, Connors D, Brotman I, Mindrebo E, Butler C, et al. Fibronectin matrix assembly suppresses dispersal of glioblastoma cells. PloS One 2011; 6:e24810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sengupta S, Nandi S, Hindi ES, Wainwright DA, Han Y, Lesniak MS. Short hairpin RNA-mediated fibronectin knockdown delays tumor growth in a mouse glioma model. Neoplasia 2010; 12:837–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang B, Tanaka K, Ji B, Ono M, Fang Y, Ninomiya Y, et al. Total body 100-mGy X-irradiation does not induce Alzheimer’s disease-like pathogenesis or memory impairment in mice. J Radiat Res 2014; 55:84–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sandor N, Walter FR, Bocsik A, Santha P, Schilling-Toth B, Lener V, et al. Low dose cranial irradiation-induced cerebrovascular damage is reversible in mice. PloS One 2014; 9:e112397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ivanov VK, Maksioutov MA, Chekin SY, Petrov AV, Biryukov AP, Kruglova ZG, et al. The risk of radiation-induced cerebrovascular disease in Chernobyl emergency workers. Health Phys 2006; 90:199–207. [DOI] [PubMed] [Google Scholar]
- 80.Shimizu Y, Pierce DA, Preston DL, Mabuchi K. Studies of the mortality of atomic bomb survivors. Report 12, part II. Noncancer mortality: 1950–1990. Radiat Res 1999; 152:374–89. [PubMed] [Google Scholar]
- 81.Preston DL, Shimizu Y, Pierce DA, Suyama A, Mabuchi K. Studies of mortality of atomic bomb survivors. Report 13: Solid cancer and noncancer disease mortality: 1950–1997. Radiat Res 2003; 160:381–407. [DOI] [PubMed] [Google Scholar]
- 82.Loganovsky K Do low doses of ionizing radiation affect the human brain? Data Sci J 2009; 8:BR13–35. [Google Scholar]
- 83.Dyer MA, Arvold ND, Chen YH, Pinnell NE, Mitin T, Lee EQ, et al. The role of whole brain radiation therapy in the management of melanoma brain metastases. Radiat Oncol 2014; 9:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mulvenna P, Nankivell M, Barton R, Faivre-Finn C, Wilson P, McColl E, et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority, randomised trial. Lancet 2016; 388:2004–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Welsh JW, Komaki R, Amini A, Munsell MF, Unger W, Allen PK, et al. Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J Clin Oncol 2013; 31:895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kumazaki T, Kobayashi M, Mitsui Y. Enhanced expression of fibronectin during in vivo cellular aging of human vascular endothelial cells and skin fibroblasts. Exp Cell Res 1993; 205:396–402. [DOI] [PubMed] [Google Scholar]
- 87.Tan FCC, Hutchison ER, Eitan E, Mattson MP. Are there roles for brain cell senescence in aging and neurodegenerative disorders? Biogerontology. 2014; 15:643–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang J, Yin L, Chen Z. New insights into the altered fibronectin matrix and extrasynaptic transmission in the aging brain. J Clin Gerontol Geriatr 2011; 2:35–41. [Google Scholar]