Abstract
This study examined the association between fetal tobacco exposure (FTE) and focused attention at 9 months of child age, and the role of child sex and infant behavioral reactivity as potential moderators of this association. Data were obtained from 203 mothers and their infants (105 fetally exposed and 98 non-exposed) on infant focused attention and behavioral reactivity to a frustration task. FTE was ascertained via nicotine metabolites in infant meconium, reflecting primarily third trimester fetal exposure. Results demonstrated a main effect of FTE on focused attention, such that exposed infants exhibited lower levels of focused attention than non-exposed infants. Behavioral reactivity, but not infant sex, moderated the relationship between FTE and focused attention, such that exposed infants who were highly reactive to frustration had the lowest levels of focused attention. Results suggest that smoking interventions, even in the third trimester, may have a positive impact on attentional outcomes for infants.
Keywords: prenatal tobacco exposure, focused attention, behavioral reactivity, infants
Tobacco is one of the most commonly used drugs during pregnancy and is known to deliver significant amounts of chemical toxins to the fetus via the maternal bloodstream. Despite the known health consequences, 16.4% of American women continue to smoke while pregnant (Substance Abuse and Mental Health Services Administration, 2009), making prenatal tobacco exposure one of the largest preventable causes of poor fetal outcomes such as impaired fetal growth, respiratory problems, and infant mortality in the U.S. (Dietz et al., 2010). There is less consensus regarding other aspects of development, especially basic underlying processes of attention and arousal. One key aspect of attention in infancy that is predictive of both behavioral problems and cognitive abilities in early childhood is focused attention (Lawson & Ruff, 2004).
Focused attention in infancy is a state of attention during which the infant is selectively attending to an object to the exclusion of other things in the environment. Focused attention involves intense concentration, coupled with a quieting of vocalizations, social bids, and irrelevant body movements. In a state of focused attention, the infant is devoting their energy and resources to the exploration of the object, and may remain oriented to the object for longer periods of time than they would in a state of casual attention (Lawson & Ruff, 2001; Ruff, Capozzoli, & Saltarelli, 1996). As their primary source of information, measures of focused attention in infancy are an early index of memory and learning (Lamb, Bornstein, and Teti, 2002). This has been evidenced by the tendency of infants to use focused attention when presented with a novel object (Ruff, 1988; Ruff, Saltarelli, Capozzoli, & Dubiner, 1992; Oakes, Madole, & Cohen, 1991). Once information has been gathered, the infant may move on to more casual behaviors such as mouthing, banging, or shaking, which are characteristic of lower levels of attention. This type of active processing of objects during play is predominant from around 6 to 24 months (Lawson & Ruff, 2001). This study examined the association between FTE and focused attention at 9 months of child age.
Although there is a relatively large literature on the association between prenatal tobacco exposure and ADHD risk (Motlagh et al., 2011), few studies examined the attentional system, specifically. These small number of studies span different developmental periods. For instance, in infancy Espy et al. (2011) reported significant effects of prenatal tobacco exposure on attention/orientation at birth and 2 weeks, but not at 4 weeks. Authors attributed results to withdrawal effects (Espy et al., 2011). Willoughby et al. (2007) noted continued effects beyond the neonatal period, finding lower attention among prenatal tobacco exposed boys compared to control boys, but not girls, during developmental testing at 6-8 months of age. In early childhood, trimester specific effects were noted, with second and third trimester prenatal tobacco exposure associated with greater inattention at 6 years of age (Leech, Richardson, Goldschmidt, & Day, 1999). Finally, in adolescence Jacobsen et al. (2007) found the lowest auditory and visual selective attention among prenatally exposed adolescent girls who were smokers compared to non-exposed girls or those who were non-smokers. Thus, studies examining the association between prenatal tobacco exposure and attentional processes are few in number and to our knowledge none examined the effects of prenatal tobacco exposure on focused attention specifically.
One explanation of the mixed results for attentional processes in general may be the presence of moderators. One such moderator may be behavioral reactivity or arousal, in light of the robust association between attention and arousal. The attention/arousal system is coordinated and attention is often used to regulate arousal (Lyon & Krasnegor, 1996). Attention, arousal, and the ability to maintain behavioral states reflect central nervous system functioning and work inter-dependently to regulate responses to internal and external stimulation (Karmel, Gardner, & Magnano, 1991). Indeed, this dynamic system of attention and arousal is the interface between the infant and their environment, and allows infants to shift and focus attention effectively to external stimuli (Richards, 2008). In this context, high arousal interferes with responses to external stimulation as attentional efforts may be allocated to facilitating emotion regulation and limiting potential distress, and may not be appropriately shifted to other external stimuli (Gardner, Karmel, & Magnano, 1992; Kopp, 2002). Thus, excessive arousal or reactivity may hamper infants’ ability to focus attention effectively, especially among infants who are biologically vulnerable due to prenatal tobacco exposure. Individual differences in reactivity may be indicated by the latency and/or intensity of reactions to frustration. Indeed, Calkins and colleagues (2002) observed easily frustrated infants as less attentive than their less easily frustrated counterparts. Similarly, more intense reactivity to negative events were found previously in children with ADHD as compared to children without ADHD (Jensen & Rosén, 2004).
Infant sex may also have the potential to moderate the association between prenatal tobacco exposure and focused attention. Girls of all ages have fewer attentional problems than boys of the same age (Arnold, 1996). Boys typically receive higher ratings for symptoms of attentional problems than girls (DuPaul et al., 1998). In addition, the attentional abilities of girls often progress at different rates than boys, with sustained attention developing earlier in girls than in boys (Greenberg & Waldman, 1993). Furthermore, boys are more biologically vulnerable to prenatal insult than girls (Lewis & Kestler, 2012), indicating the possibility of the association between prenatal tobacco exposure and poor focused attention to be stronger for boys compared to girls.
The primary purpose of this study was to examine the relationship between prenatal tobacco exposure and focused attention in infancy in a diverse sample of low-income mothers and infants. Based on the literature presented above, we hypothesized that prenatal tobacco exposure would be associated with poorer focused attention, and that behavioral reactivity and infant sex would moderate this relationship. Specifically, we expected that the association between prenatal tobacco exposure and focused attention in infancy would be stronger for infants with higher behavioral reactivity compared to infants with lower behavioral reactivity, and would also be stronger for boys than for girls.
Method
Participants
The sample included 258 mother/infant dyads, with 181 infants prenatally exposed to tobacco (99 boys and 82 girls), and 77 not exposed (35 boys and 42 girls). Pregnant women were recruited at their first prenatal appointment in a local area hospital and screened for eligibility. Women were eligible if they met the following criteria: greater than 20 weeks gestation, were not having a multiple birth, were at least 18 years old, were not using illicit drugs (other than cannabis), were not heavy alcohol users (defined as 4 or more drinks in one sitting or drinking an average of more than 1 drink/day), were not heavy marijuana users after pregnancy recognition (defined as smoking an average of more than 1 joint/day), and were English speakers. Women using other substances were excluded in order to disentangle the specific effects of tobacco exposure from those that might be caused by other drugs. At the conclusion of each recruitment month, participating smokers were matched on maternal age and highest educational attainment with the closest eligible nonsmoking woman, who was then invited to participate. The smoking group was oversampled such that one non-smoker was recruited for every two smokers. Oversampling was done to allow for a full range of light to heavy smokers, as well as to allow for the possibility of higher attrition in the smoking group over time. In total, 3,583 women were screened, 1,671 of which were eligible. Of those, 416 (25%) were interviewed prenatally, and 258 women completed at least one postnatal assessment. One mother-infant dyad was dropped from analyses because infant meconium was positive for methamphetamine, two infants were dropped because they had hydrocephaly, one was dropped because of a later diagnosis of autism, one because of binge drinking during pregnancy, four because of maternal diagnosis of bipolar disorder, one because of loss of custody, and one additional participant was excluded due to low maternal cognitive functioning, resulting in a sample size of 247 infants.
Maternal age ranged from 18 to 39 years at the time of their first appointment (M = 24.01, SD = 4.96). The women in the sample were 52% African-American, 30% Caucasian, 18% Hispanic, and 8% other or mixed race, with several identifying as more than one race. Forty-five percent of the expectant mothers were married or living with their partner, 33% were in a relationship, but not living with their partner, 21% were single, and 1% were divorced. Twenty-nine percent of women had less than a high school education, 29% completed high school, 29% completed some college, 9% had a vocational/technical or associates degree, and 4% had a bachelor’s degree. Thus, the sample was largely comprised of single minority women with a high school diploma or less. Women were compensated $20 for each prenatal interview, and $40 for their 9 month appointment, along with a $20 gift card and a toy for the baby (worth an additional $20). Transportation to and from the lab was provided.
Procedures and Instruments
Informed written consent was obtained from all interested and eligible participants at their first laboratory visit, during the first trimester of pregnancy. Assessments were conducted once during each trimester of pregnancy, and at 2 (M = 2.51, SD = .41) and 9 (M = 8.81, SD = .87) months of infant age (corrected for prematurity). Data from prenatal interviews, infant meconium (the first neonatal feces), and the 9 month child assessment were the focus of the present study. Of the 258 mothers and infants who completed the 2 month assessment, 245 completed the 9-month assessment, and of those 245, 203 infants also had results from meconium testing. Meconium was not collected if either (a) milk stool appeared before collection could occur (n = 28), or (b) the participant changed her prenatal care such that she delivered at another hospital (n = 23). For an additional three infants, meconium was collected and sent to the laboratory, but the quantity was not sufficient for analysis. Finally, one infant was excluded because the infant’s meconium was positive for prenatal methamphetamine exposure, leaving 203 infants with fetal exposure results. Mothers of infants missing meconium test results were not significantly different from those with meconium test results on maternal age, t(254) = −1.16 p = .25, education, t(255) = 1.68, p = .10, or prenatal use of cigarettes, t(255) = −0.39, p = .70, alcohol, t(255) = 0.20, p = .84, or marijuana, t(255)= 0.53, p = .60. The infants themselves were not significantly different with regard to birth weight, t(255)= 0.44, p = .66), head circumference, t(232) = 1.85, p = .07), birth length, t (248)= 0.89, p = .37, or gestational age, t(255) = 0.24, p = .81.
Substance use.
Maternal tobacco use in the prenatal period was assessed during each trimester of pregnancy via maternal report using the Timeline Followback Interview (TLFB; Sobell, Sobell, Klajmer, Pavan, & Basian, 1986). The TLFB (Sobell, et al., 1986) assessed self-reported maternal substance use during pregnancy. Participants were provided a calendar and asked to identify events of personal interest (e.g., holidays, birthdays, vacations, etc.) as anchor points to aid recall. This method was established as a reliable and valid method of obtaining longitudinal data on substance-use patterns, has good test-retest reliability, and is highly correlated with other intensive self-report measures (Brown et al., 1998). The TLFB yielded data on average number of cigarettes smoked per day during pregnancy, as well as number of joints per day, and number of standard drinks per day. These variables were used to examine potential dose/response effects.
In addition to maternal self-reports, infant meconium, the first neonatal feces, was collected after birth twice daily until the appearance of milk stools. Meconium was transferred to storage containers and frozen at −80°C until transport to the National Institute on Drug Abuse. Meconium was assayed with a validated quantitative LS-MSMS method (Gray, Shakleya, & Huestis, 2009) for nicotine, cotinine, and trans-3’–hydroxycotinine (OHCOT; see Gray et al. (2010) for further details). Limits of quantification were 2.5 ng/g for nicotine, 1 ng/g for cotinine, and 5 ng/g for OHCOT. As the gold standard for measuring fetal exposure, meconium is a reliable measure of fetal tobacco exposure in the third trimester specifically (Gray et al., 2010). The presence or absence of nicotine or any nicotine metabolites in meconium was used as an indicator of whether tobacco metabolites reached the fetus. Thus while these two measures of maternal tobacco use share some commonalities, each provided some unique information with regard to fetal exposure.
Birth outcomes.
Three measures of growth were examined as potential covariates in this study: birth weight (gm), birth length (cm), and head circumference (cm). All measurements were taken by obstetrical nurses in the delivery room and recorded in the infant’s medical chart. Research staff recorded this information directly from the charts after the birth of the infant. Gestational age was calculated by dates and extracted from medical records (see Table 1).
Table 1.
Bivariate Correlations
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Focused Attention | ||||||||||||
| 2. Fetal Exposure | −.17* | |||||||||||
| 3. Behavioral Reactivity | −.12 | −.01 | ||||||||||
| 4. Maternal Edu. (yrs) | −.04 | −.15* | .05 | |||||||||
| 5. Birth HC | .14 | −.28** | .09 | .15* | ||||||||
| 6. Engagement w/ Toy | .02 | −.02 | .04 | −.03 | .08 | |||||||
| 7. Exp. Effectiveness | .00 | −.05 | .01 | −.06 | .06 | −.04 | ||||||
| 8. Baseline State | −.15 | −.11 | .06 | −.01 | −.07 | −.34** | .04 | |||||
| 9. Sex | .02 | −.05 | .05 | .03 | −.14 | −.07 | .01 | .11 | ||||
| 10. GA | .03 | −.19** | −.09 | .15* | .51** | .09 | .08 | −.10 | −.10 | |||
| 11. Birthweight | −.01 | −.20** | .05 | .19* * |
.67** | .02 | .03 | −.03 | −.11 | .54 | ||
| 12. Prenatal Cigarettes: Maternal Self- Report |
−.09 | .57** | −.07 | −.05 | −.14 | .05 | −.05 | .01 | .04 | −.07 | −.12 |
Note. n = 203. Edu = Education; HC = Head Circumference; Exp = Experimenter; GA = Gestational Age. The pattern of associations for maternal reports of cigarette use per day in each trimester was similar to that of the maternal report of average cigarettes per day across the entire pregnancy. As such the average cigarettes per day across the entire pregnancy was retained as it was more parsimonious.
p < 0.05.
p < 0.01.
Focused attention.
Infants and their mothers came to the laboratory between 34 and 40 weeks of age corrected for prematurity. Infants participated in a brief warm up with the infant assessor, while the interviewer explained the procedures of the visit to the mother. Infants were placed in a high chair and presented with a series of 4 novel toys in a specific order.
Replacement toys were used (n = 10) if mothers indicated that the toys were not novel, as novelty has been related to increases in time spent examining objects (Ruff, 1988; Ruff, et al., 1992; Oakes et al., 1991). Mothers were seated in a chair next to the infant and asked not to interact with the infant during the focused attention procedure. The infant assessor presented each toy, one at a time, for 90 seconds. The procedure was terminated for one infant with repeated toy drops and modified for four infants who became upset during toy administration. For these four infants, the focused attention paradigm was administered at the end of the lab visit, with the child seated in mom’s lap at a card table.
Toy 1 was a plastic block, with various colors and textures. Toy 2 was a blue ball within a yellow casing. When moved, the ball moved and made noise. Toy 3 was a cylinder with small beads inside. When moved, the beads would slide from one side to another. Toy 4 was the most complex; three handles moved in opposite directions of each other and made noise when they were cranked. Video tapes of the focused attention assessments were coded by research assistants who did not participate in the assessments and were blind to group status. The rating of focused attention was a global rating that was based on several dimensions, including duration of focused attention, latency to look at the toy, number of focused looks, vigor of movement, and number of times the toy was thrown or dropped. This global rating, outlined by Lawson and Ruff (2001), is comprised of a 5 point rating scale with a 1 indicating “no focused attention on the object” and a 5 indicating “long periods of focused attention”. This global rating was rated separately for each of the 4 toys, with inter-rater reliabilities calculated on 12% of the tapes. Intra-class correlation were .83 for toy one, .94 for toy two, and .90 for toys three and four.
Behavioral reactivity.
Behavioral reactivity was our index of infant arousal. To assess behavioral reactivity, we used a gentle arm restraint procedure that is well-validated and widely employed as a measure of infant anger and frustration (Goldsmith & Rothbart, 1999; Stifter & Braungart, 1995). This procedure occurred while the infant was still seated at the high chair, immediately after watching a 3 minute neutral video (Spot). Infants were presented with a novel and attractive toy for 30 seconds. The infant assessor facilitated engagement with the toy by saying “Look (baby’s name)”, and demonstrating the movement of the toy at the start of each engagement period. After the 30 second engagement, the infant assessor stood behind the child, moved the toy to the corner of the high chair tray, and placed her hands gently on the child’s forearms, holding them by the child’s side for 30 seconds (trial 1). Infant assessors maintained a neutral expression and did not engage the infant at all during this time. The toy was then moved back in front of the infant, who was again encouraged to play with it for 30 seconds, followed by another 30 seconds of gentle arm restraint (trial 2). These trials were stopped at the request of the caregiver, or if the child reached maximum distress, which was defined as a full cry. This occurred for six infants. Coders blind to group status coded the tapes on how intensely the infant struggled against the restraint, with scores ranging from 0, indicating no struggle, to 4, indicating high intensity struggle. Reliabilities were calculated for 12% of the tapes, and inter-rater reliability for the episode was .93. The maximum intensity of struggle score from the two trials was used.
There were three additional codes for the arm restraint episodes. First, the effectiveness of the experimenter was coded, with codes ranging from 0 (ineffective: not restraining child) to 2 (effective: restrains even when child struggles). Note that there were no cases for which there was a 0 rating on the measure of experimenter effectiveness. Second, child engagement with the toy prior to the episode was coded, with codes ranging from 0 (indifferent) to 2 (fully engaged). Finally, the infant’s baseline state was recorded on a scale ranging from 0 (drowsy) to 5 (crying).
Data Analytic Strategy
We first examined associations between the different prenatal substance exposure variables and our variables of interest using Pearson correlations or ANOVAs as appropriate. These included the fetal exposure variable of meconium positive for tobacco, and the dose response variable of the average number of cigarettes per day across the entire pregnancy. Analyses to guide selection of potential covariates were conducted next using correlations or ANOVAs as appropriate. Demographic or perinatal risk variables that were associated with the independent variables or the dependent variable at p < .10 were treated as covariates in subsequent analyses.
As with any longitudinal study, there were incomplete data for some of the participants on one or more of the variables included in this study. Of these, 28 had missing data for focused attention, 31 had missing data for behavioral reactivity, and four had missing data for head circumference. There were no significant differences between families with complete vs. missing data on maternal education, or on their use of prenatal substances (alcohol, marijuana, and cigarettes), with p values ranging from .08 to .98. Missing cases were deleted listwise from the analysis, leaving an analytic sample of 1711.
To test the primary hypotheses, a four step hierarchical multiple regression was employed with SPSS statistical software (Version 24, IBM Corp., 2016). Variables were entered into the regression models, along with their corresponding interaction terms to test moderation. These included centered scores for baseline state, effectiveness of experimenter, engagement with toy, and behavioral reactivity, as well as the dummy coded fetal exposure variable. The outcome variable was the global rating of focused attention for Toy 1 as described in the instruments section. Simple slopes were tested following the guidelines set forth by Aiken and West (1991). Specifically, covariates were entered in Step 1, followed by fetal tobacco exposure in Step 2, moderator in Step 3, and the interaction term in Step 4.
Results
Correlational analyses were conducted with the two indicators of prenatal tobacco exposure (maternal self-report and infant meconium) and focused attention. Results indicated that maternal self-report of cigarettes per day across the entire pregnancy was not related to infant focused attention on any of the 4 toys presented. However, there was a significant association between presence or absence of nicotine metabolites in meconium and infant focused attention for Toy 1 (see Table 1). Thus, all remaining analyses were conducted with infant meconium as the primary independent variable, hereby referred to as FTE, and focused attention during the presentation of Toy 1 as the primary dependent variable.
Demographics
Descriptive statistics for both demographic and substance use variables for mothers and infants in the FTE and non-FTE groups are presented in Table 2. In this predominantly low-income sample, mothers in the FTE group were significantly less educated and less likely to be married or living with their partner. As would be expected, mothers of infants in the FTE group also consumed significantly more alcohol and smoked significantly more cigarettes and marijuana both prenatally and postnatally than mothers of non-exposed infants (see Table 2).
Table 2.
Group Differences for Maternal and Infant Characteristics
| Fetal Exposure (n =105) |
No Fetal Exposure (n =98) |
|||||
|---|---|---|---|---|---|---|
| Variables | M | SD | M | SD | df | t |
| Maternal Characteristics | ||||||
| Demographics | ||||||
| Maternal Age | 24.7 | 5.4 | 23.6 | 5.5 | 200 | −1.38 |
| Education (In Years) | 11.7 | 0.2 | 12.2 | 0.2 | 201 | 2.12* |
| Parity | 2.4 | 1.8 | 2.1 | 1.4 | 199 | −1.41 |
| Married/Living with Partner |
37.1% (n=39) | 54.1% (n=53) | χ2 =5.87* | |||
| Prenatal Substance Use (TLFB) | ||||||
| Cigs/Day | 6.2 | 5.3 | .69 | 2.5 | 173 | −8.80** |
| Joints/Day | 0.4 | 0.7 | .00 | 0.1 | 173 | −4.59** |
| Standard Drinks/Day | 0.1 | 0.2 | .00 | 0.1 | 173 | −2.48* |
| Postnatal Substance Use | ||||||
| Cigs/Day | 7.3 | 5.4 | 1.2 | 3.4 | 173 | −8.80** |
| Joints/Day | 1.2 | 2.1 | 0.1 | 0.4 | 173 | −4.59** |
| Standard Drinks/Day | 2.9 | 3.1 | 1.6 | 2.1 | 173 | −3.25** |
| Infant Characteristics | ||||||
| Perinatal Growth/Risk | ||||||
| % Premature | 9.5% (n = 10) | 4.1% (n = 4) | χ2 = 2.34 | |||
| Birthweight (g) | 3120 | 563.3 | 3340 | 521.0 | 201 | 2.89** |
| Birth Length (cm) | 49.7 | 3.0 | 50.4 | 2.0 | 201 | 2.04* |
| Birth Head | 33.4 | 1.9 | 34.4 | 1.6 | 197 | 4.15** |
| Circumference (cm) | ||||||
| Gestational Age (weeks) | 38.6 | 1.8 | 39.2 | 1.4 | 201 | 2.67** |
| Sex (% male) | 55.2% (n = 58) | 50% (n = 49) | χ2= .56 | |||
| Focused Attention | 2.6 | 0.7 | 2.9 | 0.8 | 173 | 2.39* |
| Behavioral Reactivity | −.05 | 1.0 | .00 | 1.0 | 170 | .33 |
| Baseline State | 2.9 | 0.7 | 3.0 | 0.7 | 170 | 1.18 |
| Engagement with Toy | 1.9 | 0.4 | 1.9 | 0.3 | 170 | .62 |
Note. TLFB = Timeline Follow Back
p < 0.05
p < 0.01
Examination of Potential Covariates
Infants in the FTE group were not significantly different from infants in the non-FTE group in terms of sex, percent premature, or the three variables from the gentle arm restraint procedure (intensity of struggle, baseline state, and engagement with toy). Infants in the FTE group were, however, significantly smaller on all birth outcomes (birth weight, length, and head circumference), had a significantly lower gestational age, and had significantly lower levels of focused attention on Toy 1 than those in the non-FTE group (see Table 1).
We examined the relation of maternal variables (age, education, parity, partner status, prenatal alcohol and marijuana use), and infant variables (birth weight, head circumference at birth, gestational age, and sex) with infant focused attention at 9 months of age. None of the variables that were associated with FTE, including maternal education and measures of fetal growth such as head circumference, birth weight, and gestational age, were associated with focused attention, nor was prenatal alcohol (r = −.07, p = .35) or marijuana (r = −.10, p = .20) use. Consequently, these variables were not considered further as covariates.
Association Between FTE and Focused Attention Controlling for Covariates
ANOVA’s with FTE group status as the independent variable and each of the four focused attention scores (one for each toy) as the dependent variables yielded a significant group difference on Toy 1 only, F(1,173) = 3.08, p = .02, d = −3.28, while Toy 2, F(1,173) = 0.02, p = .85, d = −.26, Toy 3 F(1,173) = 0.82, p = .37, d = −1.28, and Toy 4 F(1,173) = 0.05, p = .82, d = −.33, were not significant (see Table 2). Thus, we utilized the global rating for Toy 1 as the dependent variable in the next set of analyses examining moderation. Bivariate correlations for all variables included in the final regression model are presented in Table 2.
Behavioral Reactivity
We conducted multiple regression analyses to examine whether the association between fetal tobacco exposure and focused attention was moderated by behavioral reactivity. The effectiveness of the experimenter, infant baseline state, and infant engagement with the toy during the arm restraint task were included as covariates in order to account for possible differences in the infant’s experience of the reactivity task. The covariates were entered in the first step, followed by fetal exposure in the second step, behavioral reactivity in the third step, and the interaction term in the final step.
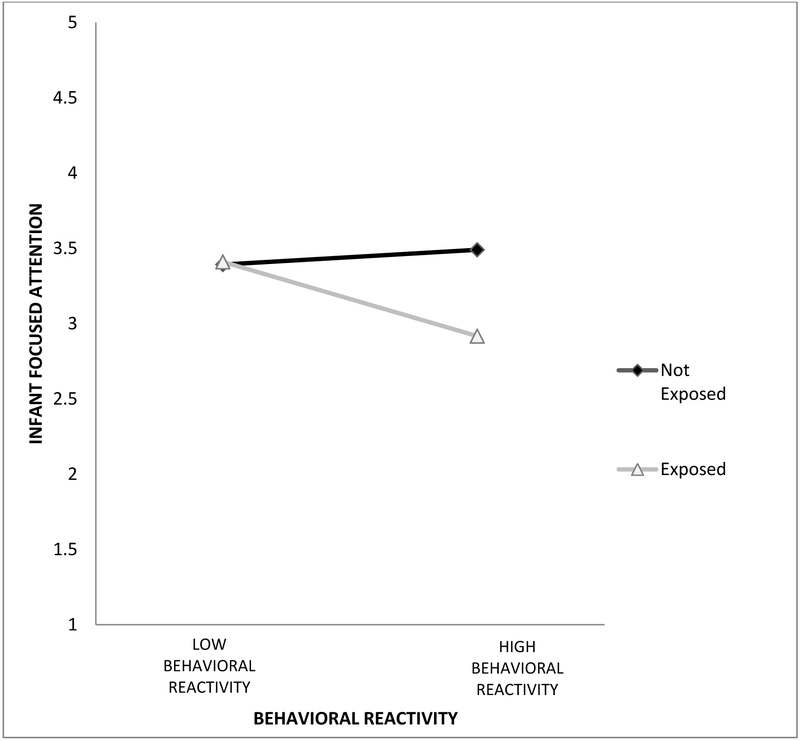
Overall, the model was significant, accounting for 11% of the variability in focused attention, F(6,164)= 3.37, p >.01. Examination of the residuals indicated that the assumptions of normality and homoscedasticity were tenable. Results are presented in Table 3. As noted earlier, there was a main effect of FTE status, such that exposed infants had lower levels of focused attention than non-exposed infants. There were no main effects for the covariates or behavioral reactivity. There was, however, a significant interaction between FTE status and behavioral reactivity (see Table 3). As depicted in Figure 1, there was a significant association, b = −0.57, p < .001, between fetal exposure and focused attention at high levels of reactivity (+1 SD above the mean), but not at low levels of reactivity (−1 SD below the mean), where infants had similar focused attention ratings, b = 0.02, p = .90, regardless of prenatal exposure.
Table 3.
Regression Analysis: DV Focused Attention
| 95% CI | |||||||
|---|---|---|---|---|---|---|---|
| Predictor | R2 | ΔR2 | β | SE | t | LL | UL |
| Step 1 | .022 | .022 | |||||
| Engagement w/ Toy | −0.06 | 0.21 | −0.02 | −0.48 | 0.36 | ||
| Baseline State | −0.17 | 0.09 | −1.92 | −0.35 | 0.01 | ||
| Experimenter Effectiveness |
0.01 | 0.18 | 0.03 | −0.35 | 0.36 | ||
| Step 2 | .061 | .039 | |||||
| Fetal Exposure | −0.15* | 0.06 | −2.65 | −0.26 | −0.04 | ||
| Step 3 | .073 | .013 | |||||
| Behavioral Reactivity | 0.05 | 0.08 | 0.69 | −0.10 | 0.21 | ||
| Step 4 | .110 | .036 | |||||
| Interaction of Arousal and Exposure |
−0.30* | 0.11 | −2.59 | −0.52 | −0.07 | ||
Note. DV = dependent variable.
p < .05.
Figure 1.
Interaction of Fetal Exposure & Behavioral Reactivity. This figure indicates that there is no association between behavioral reactivity and focused attention among non-exposed infants, but there is a significant association between behavioral reactivity and focused attention among fetally exposed infants. Infants with fetal tobacco exposure and high behavioral reactivity have lower focused attention compared to non-exposed infants with high behavioral reactivity.
Sex as a Moderator
We then conducted multiple regression analyses to examine whether the association between fetal tobacco exposure and focused attention was moderated by child sex. The effectiveness of the experimenter, infant baseline state, and infant engagement with the toy during the arm restraint task were again included as covariates. The covariates were entered in the first step, followed fetal exposure in the second step, child sex in the third step, and the interaction term in the final step. Overall, the model was not significant, accounting for only 6.2% of the variability in focused attention, F(6,164) = 1.82, p = .10. As before, there was a significant main effect of FTE status, but there was no main effect of sex, nor was there a significant interaction between FTE status and child sex (see Table 4).
Table 4.
Regression Analysis: DV Focused Attention
| 95% CI | |||||||
|---|---|---|---|---|---|---|---|
| Predictor | R2 | ΔR2 | β | SE | t | LL | UL |
| Step 1 | .022 | .022 | |||||
| Engagement w/ Toy | −0.11 | 0.22 | −0.51 | −0.54 | 0.32 | ||
| Baseline State | −0.20* | 0.09 | −2.19 | −0.38 | −0.02 | ||
| Experimenter Effectiveness |
0.00 | 0.19 | −0.01 | −0.37 | 0.37 | ||
| Step 2 | .061 | .039 | |||||
| Fetal Exposure | −0.35* | 0.17 | −2.08 | −0.67 | −0.02 | ||
| Step 3 | .062 | .001 | |||||
| Child Sex | −0.09 | 0.17 | −0.54 | −0.42 | 0.24 | ||
| Step 4 | .062 | .001 | |||||
| Interaction of Sex and Exposure |
−0.09 | 0.24 | 0.36 | −0.38 | 0.55 | ||
Note. DV = dependent variable.
p < .05.
Discussion
The main goal of this study was to examine the association between fetal tobacco exposure and focused attention, and to see whether this association was moderated by behavioral reactivity and infant sex. Our results indicated that one index of prenatal tobacco exposure that reflects fetal exposure primarily in the third trimester (Gray et al., 2010) was associated with higher risk for poor focused attention in infancy. Results also highlighted the role of infant behavioral reactivity as a significant moderator of this association.
Previous studies found relationships between infants at high risk and focused attention. Lawson and Ruff (2001) found that high risk infants (in their case very low birthweight preterm infants) had low mean global focused attention ratings at 7 (M = 1.71, SD = .63), and 12 months (M = 2.33, SD = .86). In the current study, the mean global focused attention ratings were higher than the Lawson and Ruff (2001) scores for both the FTE (M = 2.64, SD = .68) and non-FTE infants (M = 2.92, SD = .83). This may be due to differences in the toys, but also may be due to differences in how high risk was determined. Although our sample consisted of infants who were prenatally exposed to tobacco, only a small number were preterm and none qualified as very low birthweight (< 1500 grams). Consequently, our global ratings were higher overall. Despite this, FTE infants in this study exhibited significantly lower focused attention compared to non-FTE infants. Future studies with prospective data may examine how differences in focused attention in infancy may be associated with different developmental trajectories for FTE and non-FTE children.
As hypothesized, behavioral reactivity or reactive arousal was an important moderator of the relationship between FTE and focused attention. Highly reactive children who were fetally exposed to tobacco had the poorest outcomes with regard to focused attention. In other words, the association between fetal tobacco exposure and focused attention was stronger in the context of high behavioral reactivity. The results are generally supportive of studies indicating the coordination between attention and arousal system (Lyon & Krasnegor, 1996; Karmel, Gardner, & Magnano, 1991). Future studies may examine if this is one developmental process linking fetal tobacco exposure to higher risk for disruptive behaviors in later childhood.
Contrary to our hypothesis, the sex of the child was not related to focused attention, nor did sex moderate the association between FTE and focused attention. As noted in a recent review of the literature on sex differences in tobacco exposure (Coles, Kable, & Lynch, 2012), most studies of FTE did not examine the potential for sex differences. However, within this limited literature, our results are supportive of a few studies reporting no sex differences (Pridham, Becker, & Brown, 2000), even when using other measures of focused attention, such as the duration of fixation on an object (Miceli, Whitman, Borkowski, Braungart-Rieker, & Mitchell, 1998).
Implications
A large number of women quit or reduce smoking spontaneously upon pregnancy recognition (Homish, Eiden, Leonard, & Kozlowski, 2012). However, while the majority of women reduce smoking after pregnancy recognition, about 10% of women continue smoking into their third trimester (CDC, 2011). Our results indicated that while infant focused attention was not associated with maternal self-report of tobacco use across the entire pregnancy, it was associated with the presence or absence of nicotine biomarkers in infant meconium. This suggests that fetal tobacco exposure has a significant teratogenic effect on focused attention as evidenced by fetal exposure in the third trimester, and that maternal self-report alone, even when collected prospectively, may not be an adequate index of fetal risk. Given that tobacco metabolites in meconium identifies third trimester tobacco exposure (Gray et al., 2010), results suggest that persistent smoking throughout pregnancy is a stronger risk factor for infant focused attention than smoking only in the early stages of pregnancy. Thus smoking cessation interventions, even in the third trimester, may have a positive impact on infant attentional outcomes.
Also of interest to researchers working to improve observational measures of attention was our finding of group differences on only one of the four toys we presented. Toys two, three, and four were not related to any of our questions of interest. Given that toy one was a simple block and was the only toy that did not have moving parts and did not make any noise, it is possible that the other toys invited the use of more casual attention like shaking and banging as this produced different sounds. It is also possible that the infants may have found the other toys to be too engaging, thus little variability was seen between the groups. Finally, it could be that differences between the FTE and non-FTE infants are only apparent initially, and that they become more similar once the novelty of the task wears off. This perspective is supported by our data, which show a decline in the average focused attention ratings across the four toys. While the focused attention ratings were not significantly different between toys 1 and 2, they were significantly different between toy 1 and toys 3 and 4, indicating significantly lower mean levels of focused attention overall for the last two toys presented. Future studies may want to examine how features of the objects presented, as well as habituation to the task itself, may contribute to, or detract from, infant’s attentional efforts.
Strengths, Limitations, and Conclusion
Our use of meconium to determine fetal tobacco exposure is a strength of the current study. Meconium is the gold standard for assessing fetal substance exposure, and is not prone to the problems of self-reported or retrospective data, making our results more reliable. In addition, while some studies included duration of focused attention as the outcome (Thomas, Whitfield, Oberlander, Synnes, & Grunau, 2012; Lawson & Ruff, 2004; Choudhury & Gorman, 2000), our global attention rating combined several important elements of focused attention, including duration, quieting of motor movements and vocalizations, and object orientation. Finally, Calkins et. al. (2002) discussed the need for studies of frustration and attention in diverse samples. Our study contributes to the literature by exploring this relationship in a sample of primarily single, minority, low-income mothers and their infants.
One limitation of this study may be the lack of repeated assessments of focused attention. There may be changes in focused attention as higher order attentional abilities mature across the second year of life. Future studies exploring focused attention across infancy with multiple assessments points may be better able to answer questions regarding these changes. In addition, the sample was restricted to pregnant smokers with low levels of alcohol use, low to moderate marijuana use after pregnancy recognition, and no other illicit substance use during pregnancy. Thus, the results are generalizable to low-income smokers who met these criteria. It is possible that infants of heavier smokers (who were also more likely to engage in heavier use of other substances) might have different patterns of focused attention than those noted in this more restricted sample, but inclusion of heavy poly-drug users would make it difficult to disentangle the effects of tobacco exposure from those of other drugs. Future studies with less stringent recruitment criteria and including heavy poly-drug users may yield different results.
Despite these limitations, the present findings provide additional support for the deleterious effects of fetal substance exposure on infant focused attention, particularly in the third trimester of pregnancy. Results indicated that this association was stronger in the context of high behavioral reactivity, suggesting greater vulnerability of infants who were more intensely aroused or frustrated. More generally, the results may reflect the fundamental role of emotion regulation for focused attention to occur, although the direction of this association can only be tested with prospective designs. Our findings highlight the potential for positive effects for infants of interventions targeted at persistent pregnancy smokers. Results also suggest the need for prospective data to examine if differences in focused attention in infancy are one developmental mechanism explaining associations between tobacco exposure and poor behavioral outcomes in later childhood.
Highlights.
Examined focused attention in infants with fetal tobacco exposure.
Lower focused attention in infants with fetal tobacco exposure.
Gender did not moderate, but behavioral reactivity did.
Infants more highly reactive to frustration had lowest levels of focused attention.
Acknowledgements:
The authors thank the families who participated in this study and the research staff responsible for data collection. Special thanks to Dr. Amol Lele for collaboration on data collection at Women and Children’s Hospital of Buffalo. The study was supported by Award #R01DA019632 from the National Institute on Drug Abuse.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Analyses were also run utilizing multiple imputation with the full 203 participants, using SAS PROC MI (SAS, 1999). These results were identical.
Contributor Information
Shannon Shisler, Research Institute on Addictions, State University of New York at Buffalo, 1021 Main Street, Buffalo, NY 14203. sshisler@ria.buffalo.edu Phone: 716-887-2244.
Rina D. Eiden, Research Institute on Addictions, State University of New York at Buffalo, 1021 Main Street, Buffalo, NY 14203. eiden@ria.buffalo.edu
Danielle S. Molnar, Research Institute on Addictions, State University of New York at Buffalo, 1021 Main Street, Buffalo, NY 14203. dmolnar@ria.buffalo.edu
Pamela Schuetze, Department of Psychology, SUNY Buffalo State, 1300 Elmwood Avenue Buffalo, NY 14222-1095 schuetp@buffalostate.edu.
Claire D. Coles, Psychiatry and Behavioral Sciences, Pediatrics, Emory University, 201 Dowman Drive, Atlanta, GA 30322. ccoles@emory.edu
Marilyn Huestis, Chemistry and Drug Metabolism, IRP, National Institute on Drug Abuse, NIH, Biomedical Research Center Suite 200 Room 05A-721, 251 Bayview Boulevard, Baltimore, MD 21224. MHUESTIS@intra.nida.nih.gov.
Craig R. Colder, Department of Psychology, State University of New York at Buffalo, 227 Park Hall, Buffalo, NY 14260 ccolder@buffalo.edu
References
- Aiken LS, West SG (1991). Multiple regression: testing and interpreting interactions. Sage Publications: Newbury Park, Calif. [Google Scholar]
- Arnold LE (1996). Sex differences in ADHD: Conference summary. Journal of Abnormal Child Psychology, 24(5), 555. [DOI] [PubMed] [Google Scholar]
- Brown RA, Burgess ES, Sales SD, Whiteley JA, Evans DM, & Miller IW (1998). Reliability and validity of a smoking timeline follow-back interview. Psychology of Addictive Behaviors, 12(2), 101–112. [Google Scholar]
- Calkins SD, Dedmon SE, Gill KL, Lomax LE & Johnson LM (2002). Frustration in infancy: Implications for emotion regulation, physiological processes, and temperament. Infancy 3(2), 175–197. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention: Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion (2011). Tobacco use and pregnancy. Retrieved from http://www.cdc.gov/reproductivehealth/tobaccousepregnancy/
- Choudhury N, & Gorman KS (2000). The relationship between sustained attention and cognitive performance in 17–24-month old toddlers. Infant and Child Development, 9(3), 127–146. doi: Doi [DOI] [Google Scholar]
- Coles CD, Kable JA, & Lynch ME (2012). Examination of gender differences in effects of tobacco exposure In Lewis M & Kestler L (Eds.), Gender differences in prenatal substance exposure. (pp. 99–120). Washington, DC US: American Psychological Association. [Google Scholar]
- Dietz PM, England LJ, Shapiro-Mendoza CK, Tong VT, Farr SL, & Callaghan WM (2010). Infant morbidity and mortality attributable to prenatal smoking in the U.S. American Journal of Preventative Medicine, 39(1), 45–52. doi: 10.1016/j.amepre.2010.03.009 [DOI] [PubMed] [Google Scholar]
- DuPaul G, Anastopoulos A, Power T, Reid R, Ikeda M, & McGoey K (1998). Parent ratings of attention-deficit/hyperactivity disorder symptoms: Factor structure and normative data. Journal of Psychopathology and Behavioral Assessment, 20(1), 83–102. doi: 10.1023/a:1023087410712 [DOI] [Google Scholar]
- Espy KA, Fang H, Johnson C, Stoop C, Wiebe SA, Respass J (2011). Prenatal tobacco exposure: Developmental outcomes in the neonatal period. Developmental Psychology 47(1), 153–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner JM, Karmel BZ, & Magnano CL (1992). Arousal/visual preference interactions in high-risk neonates. Developmental Psychology, 28, 821–830. [Google Scholar]
- Gilliland FD, Li Y-F, & Peters JM (2001). Effects of maternal smoking during pregnancy and environmental tobacco smoke on asthma and wheezing in children. American Journal of Respiratory and Critical Care Medicine, 163(2), 429–436. doi: 10.1164/ajrccm.163.2.2006009 [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, & Rothbart MK (1999). Laboratory Temperament Assessment Battery. Unpublished technical manual, University of Wisconsin-Madison. [Google Scholar]
- Gray TR, Shakleya DM & Huestis MA (2009). A liquid chromatography tandem mass spectrometry method for the simultaneous quantification of 20 drugs of abuse and metabolites in human meconium. [Research Support, N.I.H., Intramural]. Analytical and Bioanalytical Chemistry, 393(8), 1977–1990. doi: 10.1007/s00216-009-2680-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray TR, Eiden RD, Leonard KE, Connors G, Shisler S, & Huestis MA (2010). Nicotine and metabolites in meconium as evidence of maternal cigarette smoking during pregnancy and predictors of neonatal growth deficits. Nicotine & Tobacco Research, 12(6), 658–664. doi: 10.1093/ntr/ntq068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg LM, & Waldman ID (1993). Developmental normative data on the test of variables of attention (T.O.V.A.). Journal of Child Psychology and Psychiatry, 34(6), 1019–1030. [DOI] [PubMed] [Google Scholar]
- Homish G, Eiden R, Leonard K & Kozlowski L (2012). Social-environmental factors related to prenatal smoking. Addictive Behaviors, 37(1), 73–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp. [Google Scholar]
- Jacobsen LK, Slotkin TA, Menci WE, Frost SJ, & Pugh KR (2007). Gender-specific effects of prenatal and adolescent exposure to tobacco smoke on auditory and visual attention. Neuropsychopharmacology, 32(12), 2453–2464. [DOI] [PubMed] [Google Scholar]
- Jansen R, & Oud JHL (1995). Longitudinal LISREL model estimation from incomplete panel data using the EM algorithm and the Kalman smoother. Statistica Neerlandica, 49, 362–377. [Google Scholar]
- Jensen SA, & Rosén LA (2004). Emotional reactivity in children with Attention-Deficit/Hyperactivity Disorder. Journal of Attention Disorders, 8(2), 53–61. doi: 10.1177/108705470400800203 [DOI] [PubMed] [Google Scholar]
- Karmel BZ, Gardner JM, & Magnano CL (1991). Attention and arousal in early infancy In Weiss MJS and Zelazo PR (Eds.), Newborn attention: Biological constraints and the influence of experience (pp. 339–376). Norwood. NJ: Ablex. [Google Scholar]
- Kopp CB (2002). Commentary: The codevelopments of attention and emotion regulation. Infancy, 3(2), 199–208. [DOI] [PubMed] [Google Scholar]
- Lamb ME, Bornstein MH, & Teti DM. (2002). Development in infancy (4th ed.). Mahwah, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Lawson KR, & Ruff HA (Eds.). (2001). Focused attention: Assessing a fundamental cognitive process in infancy. New York: The Guilford Press. [Google Scholar]
- Lawson KR, & Ruff HA (2004). Early focused attention predicts outcome for children born prematurely. Developmental and Behavioral Pediatrics, 25(6), 399–406. [DOI] [PubMed] [Google Scholar]
- Leech SL, Richardson GA, Goldschmidt L, & Day NL (1999). Prenatal substance exposure: Effects on attention and impulsivity of 6-year-olds. Neurotoxicology & Teratology, 21(2), 109–118. [DOI] [PubMed] [Google Scholar]
- Lewis M, & Kestler L (2012). Gender Differences in Prenatal Substance Exposure: American Psychological Association. [Google Scholar]
- Littman A, & Parmelee B (1978). Medical correlation of infant development. Pediatrics, 61, 470–474. [DOI] [PubMed] [Google Scholar]
- Lyon GR, & Krasnegor NA (1996). Attention, memory, and executive function. Baltimore: P.H. Brookes Pub. Co. [Google Scholar]
- Miceli PJ, Whitman TL, Borkowski JG, Braungart-Rieker J, & Mitchell DW (1998). Individual differences in infant information processing: The role of temperamental and maternal factors. Infant Behavior and Development, 21(1), 119–136. doi: 10.1016/S0163-6383(98)90058-3 [DOI] [Google Scholar]
- Motlagh MG, Sukhodolsky DG, Landeros-Weisenberger A, Katsovich L, Thompson N, Scahill L, … Leckman JF (2011). Adverse effects of heavy prenatal maternal smoking on attentional control in children with ADHD. Journal of Attention Disorders, 15(7), 593–603. doi: 10.1177/1087054710374576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes LM, Madole KL, & Cohen LB (1991). Infants' object examining: Habituation and categorization. Cognitive Development, 6, 377–392. [Google Scholar]
- Pridham K, Becker P, & Brown R (2000). Effects of infant and caregiving conditions on an infant's focused exploration of toys. Journal of Advanced Nursing, 31(6), 1439–1448. [DOI] [PubMed] [Google Scholar]
- Richards JE (2008). Attention in young infants: A developmental psychophysiological perspective In Nelson CA, Luciana M, Nelson CA, Luciana M (Eds.), Handbook of developmental cognitive neuroscience (2nd ed.) (pp. 479–497). Cambridge, MA, US: MIT Press. [Google Scholar]
- Royston P (2005). Multiple imputation of missing values: Update of ice. The Strata Journal, 5, 527–536. [Google Scholar]
- Ruff HA (1988). The measurement of attention in high risk infants In Vietze PM, & Vaughan HG (Eds.) Early identification of infants with developmental disabilities, (pp. 282–296), Philadelphia: Grune & Stratton. [Google Scholar]
- Ruff HA, Capozzoli M, & Saltarelli LM (1996). Focused visual attention and distractibility in 10-month-old infants. Infant Behavior & Development, 19(3), 281–293. [Google Scholar]
- Ruff HA, Saltarelli LM, Capozzoli M, & Dubiner K (1992). The differentiation of activity in infants' exploration of objects. Developmental Psychology, 28(5), 851–861. doi: 10.1037/0012-1649.28.5.851 [DOI] [Google Scholar]
- Salihu H, Aliyu M, Pierre-Louis B, & Alexander G (2003). Levels of excess infant deaths attributable to maternal smoking during pregnancy in the United States. Maternal and Child Health Journal, 7(4), 219–227. doi: 10.1023/a:1027319517405 [DOI] [PubMed] [Google Scholar]
- Schafer JL, & Graham JW (2002). Missing data: Our view of the state of art. Psychology Methods, 7, 147–177. [PubMed] [Google Scholar]
- Sobell MB, Sobell LC, Klajmer F, Pavan D, & Basian E (1986). The reliability of a timeline method for assessing normal drinker college students' recent drinking history. Addict Behav, 11, 149–162. [DOI] [PubMed] [Google Scholar]
- Stifter CA & Braungart JM (1995). The regulation of negative reactivity in infancy: function and development, Developmental Psychology, 31, 448–455. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. (2009). Results from the 2008 National Survey on Drug Use and Health: National findings (No. DHHS Publication No. SMA 09–4434). Rockville, MD: Department of Health and Human Services. [Google Scholar]
- Thomas J, Whitfield M, Oberlander T, Synnes A, & Grunau R (2012). Focused attention, heart rate deceleration, and cognitive development in preterm and full-term infants. Developmental Psychobiology, 54, 383–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JMD, Clark PM, Robinson E, Becroft DMO, Pattison NS, Glavish N, … Mitchell EA (2001). Risk factors for small-for-gestational-age babies: The Auckland Birthweight Collaborative Study. Journal of Paediatrics and Child Health, 37(4), 369–375 doi: 10.1046/j.1440-1754.2001.00684.x [DOI] [PubMed] [Google Scholar]
- Willoughby M, Greenberg M, Blair C, Stifter C, & Group TFLI (2007). Neurobehavioral consequences of prenatal exposure to smoking at 6 to 8 months of age. Infancy, 12(3), 273–301. [Google Scholar]